Submitted:
05 June 2023
Posted:
06 June 2023
You are already at the latest version
Abstract
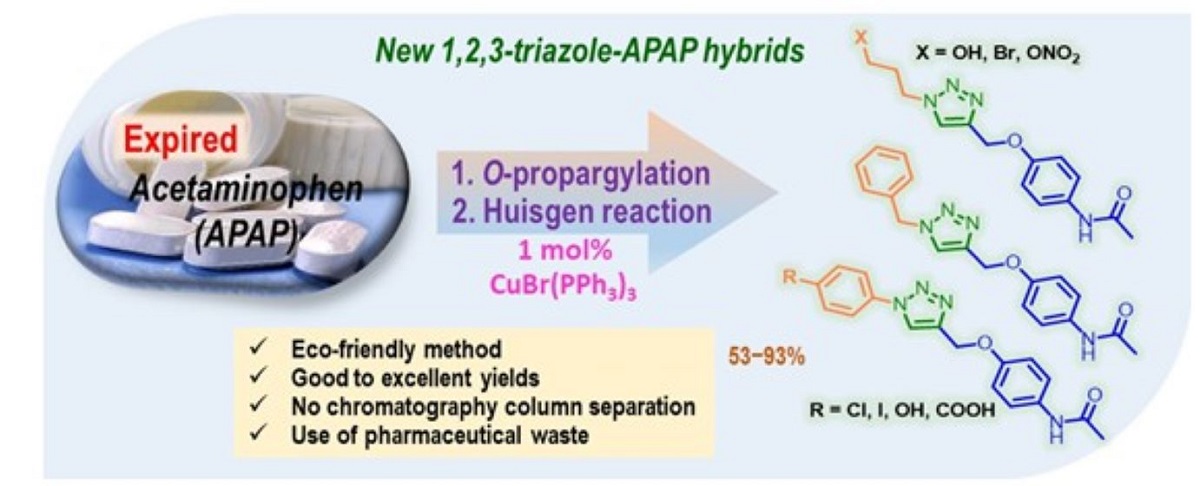
Keywords:
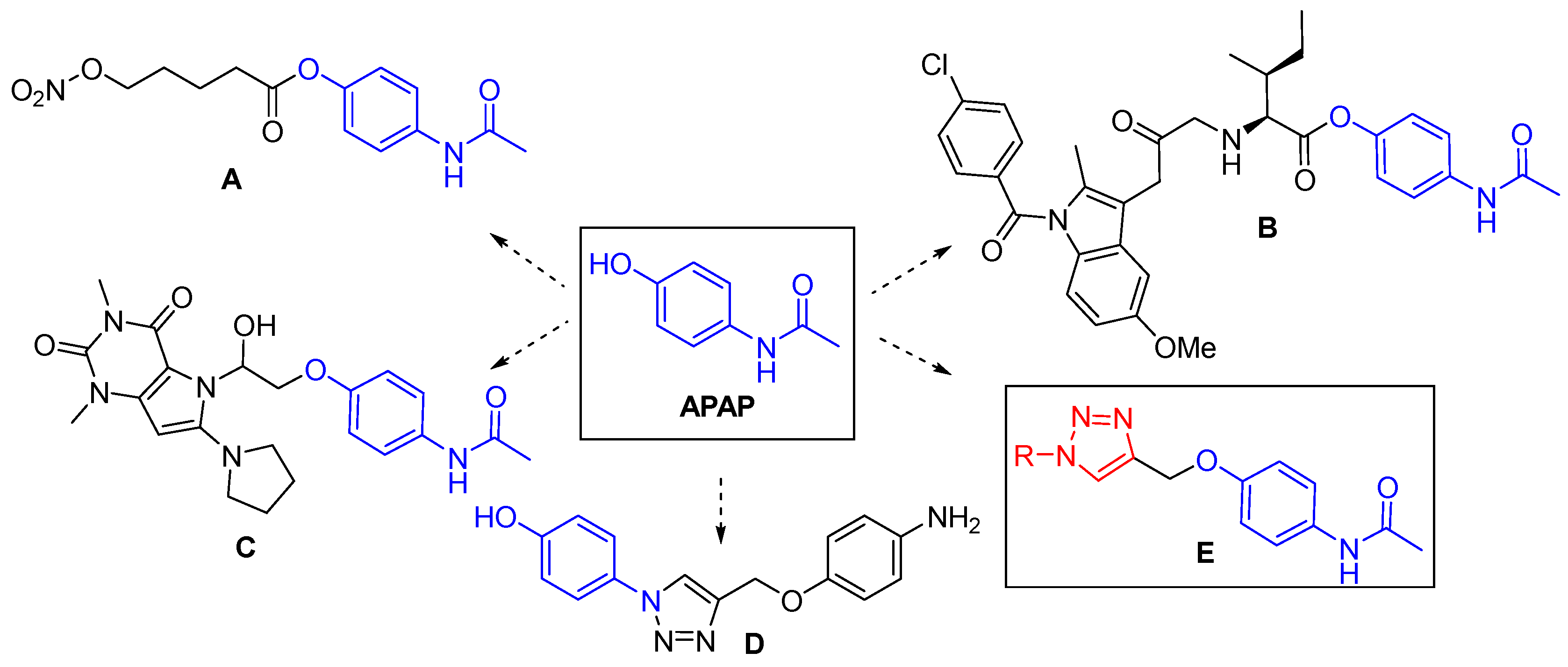
1. Introduction
2. Materials and Methods
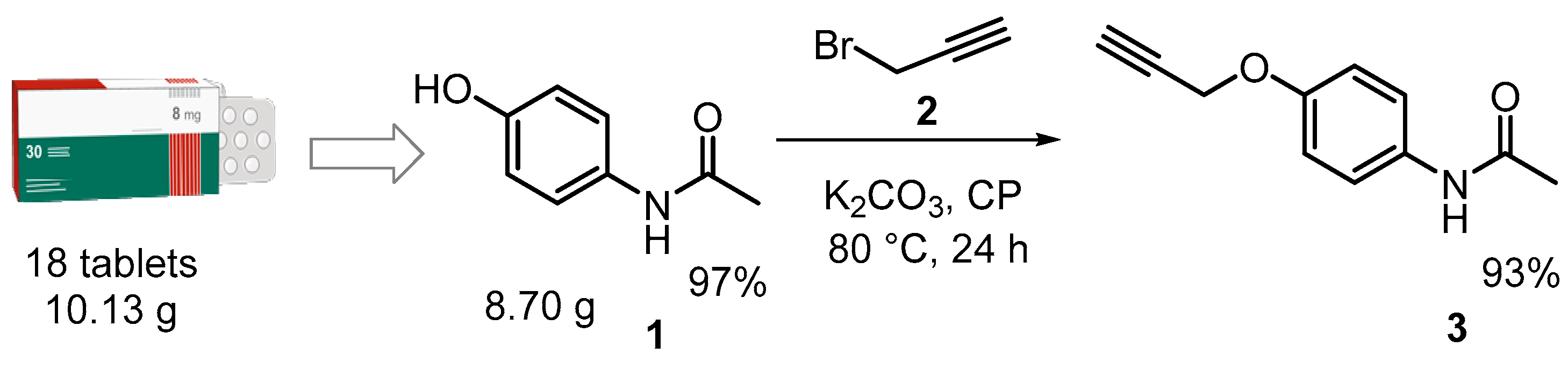
2.1. Materials and Instruments
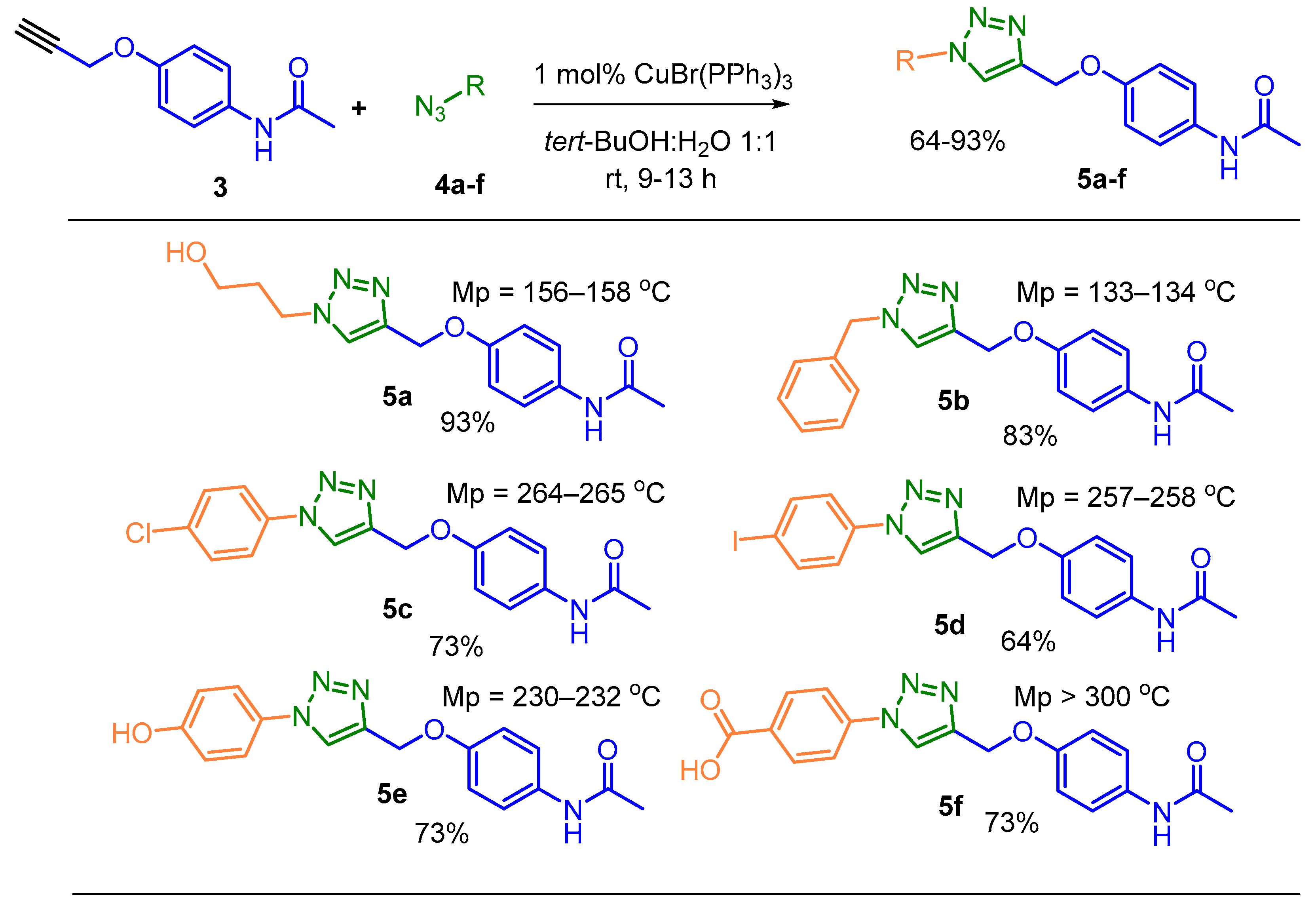
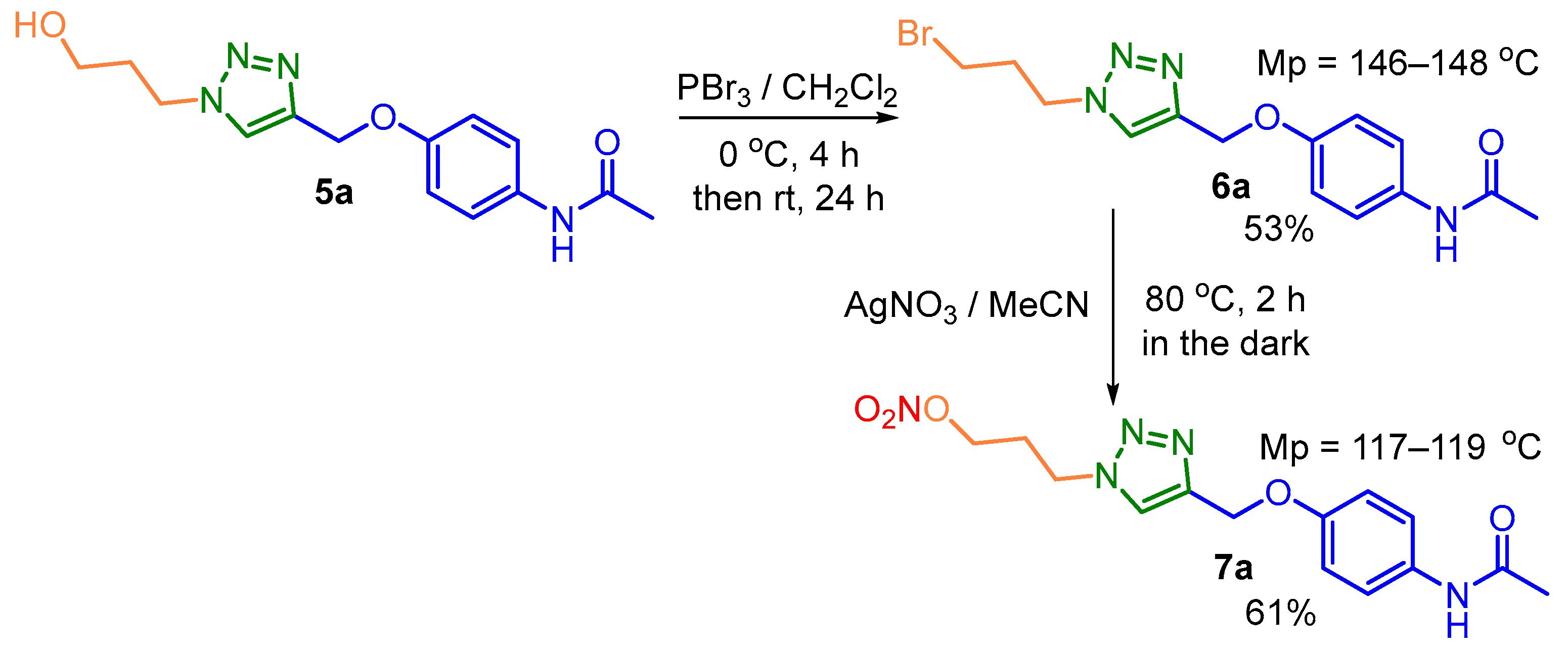
2.2. General Procedure for the Synthesis of 1,2,3-Triazole-Based APAP Derivatives 5a-f
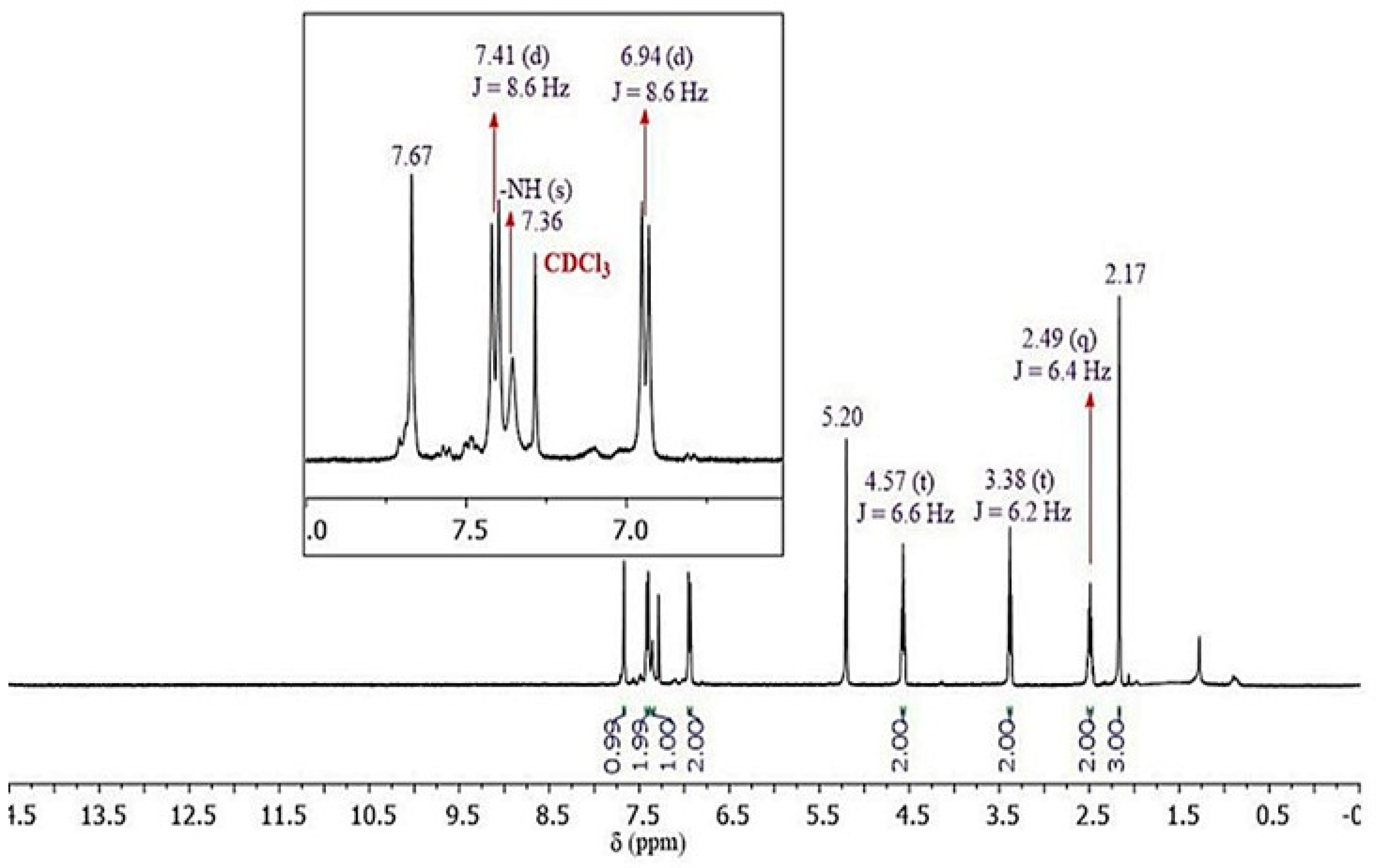
2.3. Spectroscopic Characterization
3. Results and Discussion
3.1. Chemistry

| Entry | Solvent | Catalyst | Additive: mol % NaAsCb |
Load catalyst (mol%) |
Time (h) | Yield (%)c |
|---|---|---|---|---|---|---|
| 1 | tert-BuOH:H2O 1:1 | CuSO4.5H2O | 15 | 10 | 18 | 83 |
| 2 | tert-BuOH:H2O 1:1 | CuSO4.5H2O | 10 | 5 | 18 | 86 |
| 3 | tert-BuOH:H2O 1:1 | CuSO4.5H2O | 1 | 0.5 | 13 | 85 |
| 4 | tert-BuOH:H2O 1:1 | [CuBr(PPh3)3] | - | 5 | 18 | 90 |
| 5 | tert-BuOH:H2O 1:1 | [CuBr(PPh3)3] | - | 1 | 18 | 92 |
| 6 | tert-BuOH:H2O 1:1 | [CuBr(PPh3)3] | - | 1 | 12 | 90 |
| 7 | tert-BuOH:H2O 1:1 | [CuBr(PPh3)3] | - | 1 | 9 | 93 |
| 8 | tert-BuOH:H2O 1:1 | [CuBr(PPh3)3] | - | 0.5 | 11 | 87 |
| 9 | CH3CN | [CuBr(PPh3)3] | - | 1 | 12 | 73 |
| 10 | CP | [CuBr(PPh3)3] | - | 1 | 18 | NRd |
3.2. In-Silico Prediction of Physicochemical Properties (Lipinski Descriptors) of Prepared Triazole-APAP Hybrids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, B.J. Paracetamol (Acetaminophen): mechanisms of action. Paediatr. Anaesth. 2008, 18, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, S.S. Paracetamol (acetaminophen): A familiar drug with an unexplained mechanism of action. Temp. 2021, 8, 351–371. [Google Scholar] [CrossRef]
- Klotz, U. Paracetamol (acetaminophen)–a popular and widely used nonopioid analgesic. Arzneimittelforschung 2012, 62, 355–359. [Google Scholar] [CrossRef]
- Brune, K.; Renner, B.; Tiegs, G. Acetaminophen/paracetamol: a history of errors, failures and false decisions. Eur. J. Pain 2015, 19, 953–965. [Google Scholar] [CrossRef]
- Freo, U.; Ruocco, C.; Valerio, A.; Scagnol, I.; Nisoli, E. Paracetamol: a review of guideline recommendations. J. Clin. Med. 2021, 10, 3420. [Google Scholar] [CrossRef]
- FDA drug safety communication: prescription acetaminophen products are to be limited to 325 mg per dosage unit; a boxed warning will highlight the potential for severe liver failure. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-prescription-acetaminophen-products-be-limited-325-mg-dosage-unit.
- Aminoshariae, A.; Khan, A. Acetaminophen: old drug, new issues. J. End. 2015, 41, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Bunchorntavakul, C.; Reddy, K.R. Acetaminophen (APAP or N-acetyl-p-aminophenol) and acute liver failure. Clin. Liver Dis. 2018, 22, 325–346. [Google Scholar] [CrossRef] [PubMed]
- Hanel, A.M.; Lands, W.E. Modification of anti-inflammatory drug effectiveness by ambient lipid peroxides. Biochem. Pharmacol. 1982, 31, 3307–3311. [Google Scholar] [CrossRef]
- Boutaud, O.; Aronoff, D.M.; Richardson, J.H.; Marnett, L.J.; Oates, J.A. Determinants of the cellular specificity of acetaminophen as an inhibitor of prostaglandin H2 synthases. Proc. Natl. Acad. Sci. USA 2002, 99, 7130–7135. [Google Scholar] [CrossRef]
- Schildknecht, S.; Daiber, A.; Ghisla, S.; Cohen, R.A.; Bachschmid, M.M. Acetaminophen inhibits prostanoid synthesis by scavenging the PGHS-activator peroxynitrite. FASEB J. 2008, 22, 215–224. [Google Scholar] [CrossRef]
- Graham, G.G.; Davies, M.J.; Day, R.O.; Mohamudally, A.; Scott, K.F. The modern pharmacology of paracetamol: therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacol. 2013, 21, 201–232. [Google Scholar] [CrossRef] [PubMed]
- Bessems, J.G.; Gaisser, H.D.; Te Koppele, J.M.; Van Bennekom, W.P.; Commandeur, J.N.; Vermeulen, N.P. 3, 5-Disubstituted analogues of paracetamol. Synthesis, analgesic activity and cytotoxicity. Chem. Biol. Interact. 1995, 98, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Tavakolinejad-Kermani, E.; Saidi, K.; Islami, M.R. New synthesis of acetaminophen derivatives containing a phosphorus atom. Phosphorus Sulfur Silicon Relat. Elem. 2005, 180, 1879–1884. [Google Scholar] [CrossRef]
- Santos, C.; Mateus, M.L.; dos Santos, A.P.; Moreira, R.; de Oliveira, E.; Gomes, P. Cyclization-activated prodrugs. Synthesis, reactivity and toxicity of dipeptide esters of paracetamol. Bioorg. Med. Chem. Lett. 2005, 15, 1595–1598. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Tsukuda, Y.; Kawashima, R.; Ishiki, T.; Matsumoto, A.; Nakaniwa, A.; Takagi, M.; Noguchi, T.; Imai, N. Convenient synthesis of acetaminophen analogues containing α-amino acids and fatty acids via their mixed carbonic carboxylic anhydrides in aqueous organic solvent. Tetrahedron Lett. 2013, 54, 5718–5720. [Google Scholar] [CrossRef]
- Tiwari, A.D.; Panda, S.S.; Girgis, A.S.; Sahu, S.; George, R.F.; Srour, A.M.; La Starza, B.; Hall, C.D.; Asiri, A.M.; Katritzky, A.R. Microwave assisted synthesis and QSAR study of novel NSAID acetaminophen conjugates with amino acid linkers. Org. Biomol. Chem. 2014, 12, 7238–7249. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Khalili, M.; Sadeghi, S.; Soleimani, N.; Nahri-Niknafs, B. Synthesis of New Acetaminophen Analogs and Their Ibuprofen Conjugates as Novel Analgesic Drugs. Pharm. Chem. J. 2016, 50, 369–376. [Google Scholar] [CrossRef]
- Vaccarino, A.L.; Paul, D.; Mukherjee, P.K.; de Turco, E.B.R.; Marcheselli, V.L.; Xu, L.; Trudell, M.L.; Minguez, J.M.; Matía, M.P.; Sunkel, C.; Alvarez-Builla, J.; Bazan, N.G. Synthesis and in vivo evaluation of non-hepatotoxic acetaminophen analogs. Bioorg. Med. Chem. 2007, 15, 2206–2215. [Google Scholar] [CrossRef]
- Queiroz, L.M.; Rocha, J.R.; Leitão, A.; Montanari, C.A.; da Silva, A.B.; Sousa, P.J.; Borges, R.S. A Combined Study Using Ligand-Based Design, Synthesis, and Pharmacological Evaluation of Analogues of the Acetaminophen Ortho-Regioisomer with Potent Analgesic Activity. Chem. Biol. Drug Des. 2012, 80, 99–105. [Google Scholar] [CrossRef]
- Alisi, M.A.; Brufani, M.; Cazzolla, N.; Ceccacci, F.; Dragone, P.; Felici, M.; Furlotti, G.; Garofalo, B.; La Bella, A.; Dragone, P.; Lanzalung, O.; Leonelli, F.; Bettolo, R.M.; Maugeri, C.; Maria Migneco, L.M.; Russo, V. DPPH radical scavenging activity of paracetamol analogues. Tetrahedron 2012, 68, 10180–10187. [Google Scholar] [CrossRef]
- Reddy, Y.D.; Kumari, Y.B.; Dubey, P.K. Synthesis of a novel water soluble phthalimide derivative of acetaminophen as potential analgesic and antipyretic agent. Indian J. Chem. 2013, 52B, 691–693. [Google Scholar] [CrossRef]
- Raj, N.U.I. Synthesis, single crystal XRD and CT DNA/BSA binding studies of new paracetamol derivatives. J. Mol. Struct. 2020, 1208, 127911. [Google Scholar]
- Asghari, A.; Ameri, M.; Taghipour, S.; Ghaderi, O. Facile and clean electrochemical synthesis of new acetaminophen derivatives through electrochemical oxidation of acetaminophen in the presence of thiouracil derivatives. J. Sulphur Chem. 2017, 38, 163–172. [Google Scholar] [CrossRef]
- Profire, L.; Sunel, V.; Lupascu, D.; Baican, M.C.; Bibire, N.; Vasile, C. New theophylline derivatives with potential pharmacological activity. Farmacia 2010, 58, 170–176. [Google Scholar]
- Das, M.; Bhattacharjee, S.; Fronczek, F.R.; Bazan, N.G.; Trudell, M.L. Synthesis, hepatotoxic evaluation and antipyretic activity of nitrate ester analogs of the acetaminophen derivative SCP-1. Bioorg. Med. Chem. Lett. 2018, 28, 3798–3801. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Das, D.; Sahu, P.; Mishra, S.; Sakthivel, A.; Gajbhiye, A.; Agrawal, R. Bioisosteric replacement of amide group with 1, 2, 3-triazoles in acetaminophen addresses reactive oxygen species-mediated hepatotoxic insult in Wistar albino rats. Chem. Res. Toxicol. 2020, 33, 522–535. [Google Scholar] [CrossRef] [PubMed]
- Al-Swayeh, O.A.; Futter, L.E.; Clifford, R.H.; Moore, P.K. Nitroparacetamol exhibits anti-inflammatory and anti-nociceptive activity. Br. J. Pharmacol. 2000, 130, 1453–1456. [Google Scholar] [CrossRef]
- Proschak, E.; Stark, H.; Merk, D. Polypharmacology by design: a medicinal chemist’s perspective on multitargeting compounds. J. Med. Chem. 2018, 62, 420–444. [Google Scholar] [CrossRef]
- Bansal, Y.; Silakari, O. Multifunctional compounds: Smart molecules for multifactorial diseases. Eur. J. Med. Chem. 2014, 76, 31–42. [Google Scholar] [CrossRef]
- Bérubé, G. An overview of molecular hybrids in drug discovery. Expert Opin. Drug Discov. 2016, 11, 281–305. [Google Scholar] [CrossRef]
- Abdolmaleki, A.; Ghasemi, J.B. Dual-acting of hybrid compounds-a new dawn in the discovery of multi-target drugs: lead generation approaches. Curr. Top. Med. Chem. 2017, 17, 1096–1114. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.P. NCX-701. NCX-701. NicOx. Curr. Opin. Investig. 2004, 5, 755–759. [Google Scholar]
- Romero-Sandoval, E.A.; Curros-Criado, M.M.; Gaitan, G.; Molina, C.; Herrero, J.F. Nitroparacetamol (NCX-701) and Pain: First in a Series of Novel Analgesics. CNS Drug Reviews, 2007, 13, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Massarotti, A.; Aprile, S.; Mercalli, V.; Del Grosso, E.; Grosa, G.; Sorba, G.; Tron, G.C. Are 1, 4-and 1, 5-Disubstituted 1, 2, 3-Triazoles Good Pharmacophoric Groups? ChemMedChem 2014, 9, 2497–2508. [Google Scholar] [CrossRef] [PubMed]
- Lengerli, D.; Ibis, K.; Nural, Y.; Banoglu, E. The 1,2,3-triazole ‘all-in-one’ring system in drug discovery: a good bioisostere, a good pharmacophore, a good linker, and a versatile synthetic tool. Expert Opin. Drug Discov. 2022, 17, 1209–1236. [Google Scholar] [CrossRef]
- Bonandi, E.; Christodoulou, M.S.; Fumagalli, G.; Perdicchia, D.; Rastelli, G.; Passarella, D. The 1, 2, 3-triazole ring as a bioisostere in medicinal chemistry. Drug Discov. Today 2017, 22, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Singh, G.; Singh, A.; Maqbool, U.; Kaur, G.; Singh, J. CuAAC-ensembled 1, 2, 3-triazole-linked isosteres as pharmacophores in drug discovery. RSC Adv. 2020, 10, 5610–5635. [Google Scholar] [CrossRef]
- Sahu, A.; Sahu, P.; Agrawal, R. A recent review on drug modification using 1, 2, 3-triazole. Curr. Chem. Biol. 2020, 14, 71–87. [Google Scholar] [CrossRef]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1, 2, 3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef]
- Keri, R.S.; Patil, S.A.; Budagumpi, S.; Nagaraja, B.M. Triazole: a promising antitubercular agent. Chem. Biol. Drug. Des. 2015, 86, 410–423. [Google Scholar] [CrossRef]
- Song, M.X.; Deng, X.Q. Recent developments on triazole nucleus in anticonvulsant compounds: a review. J. Enzyme Inhib. Med. Chem. 2018, 33, 453–478. [Google Scholar] [CrossRef] [PubMed]
- Lal, K.; Yadav, P. Recent advancements in 1, 4-disubstituted 1H-1, 2, 3-triazoles as potential anticancer agents. Anti-Cancer Agents Med. Chem. 2018, 18, 21–37. [Google Scholar] [CrossRef]
- Alam, M.M. 1,2,3-Triazole hybrids as anticancer agents: A review. Arch. Pharm. 2022, 355, 2100158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B. Comprehensive review on the anti-bacterial activity of 1, 2, 3-triazole hybrids. Eur. J. Med. Chem. 2019, 168, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Nemallapudi, B.R.; Guda, D.R.; Ummadi, N.; Avula, B.; Zyryanov, G.V.; Reddy, C.S.; Gundala, S. New methods for synthesis of 1,2,3-triazoles: A review. Polycycl. Aromat. Compd. 2022, 42, 3874–3892. [Google Scholar] [CrossRef]
- Vala, D.P.; Vala, R.M.; Patel, H.M. Versatile Synthetic Platform for 1,2,3-Triazole Chemistry. ACS Omega 2022, 7, 36945–36987. [Google Scholar] [CrossRef] [PubMed]
- Huisgen, R. Cycloadditions—definition, classification, and characterization. Angew. Chem. Int. Ed. 1968, 7, 321–328. [Google Scholar] [CrossRef]
- Breugst, M.; Reissig, H.U. The Huisgen Reaction: Milestones of the 1, 3-Dipolar Cycloaddition. Angew. Chem. Int. Ed. 2020, 59, 12293–12307. [Google Scholar] [CrossRef] [PubMed]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Haldón, E.; Nicasio, M.C.; Pérez, P.J. Copper-catalysed azide−alkyne cycloadditions (CuAAC): an update. Org. Biomol. Chem. 2015, 13, 9528–9550. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Balderas, F.; Ortega-Munoz, M.; Morales-Sanfrutos, J.; Hernández-Mateo, F.; Calvo-Flores, F.G.; Calvo-Asín, J.A.; Isac-García, J.; Santoyo-González, F. Multivalent neoglycoconjugates by regiospecific cycloaddition of alkynes and azides using organic-soluble copper catalysts. Org. Lett. 2003, 5, 1951–1954. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Diez-Gonzalez, S. [CuBr (PPh3)3] for azide− alkyne cycloaddition reactions under strict click conditions. J. Org. Chem. 2011, 76, 2367–2373. [Google Scholar] [CrossRef]
- Han, J. Barcoding drug information to recycle unwanted household pharmaceuticals: a review. Environ. Chem. Lett. 2022, 20, 2989–3003. [Google Scholar] [CrossRef]
- Pratama, D.E.; Hsieh, W.C.; Elmaamoun, A.; Lee, H.L.; Lee, T. Recovery of active pharmaceutical ingredients from unused solid dosage-form drugs. ACS Omega 2020, 5, 29147–29157. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, D.S.; Lindrud, M.; Lu, X.; Zordan, C.; Tang, L.; Davies, M. A process for active pharmaceutical ingredient recovery from tablets using green engineering technology. Org. Process Res. Dev. 2017, 21, 1272–1285. [Google Scholar] [CrossRef]
- Messina, L.C.; de Espindola, C.S.; Omori, A.T. Direct One-Pot Synthesis of Propofol from Paracetamol Tablets. ACS Sustainable Chem. Eng. 2023, 11, 1638–1642. [Google Scholar] [CrossRef]
- Kouznetsov, V.V.; Vargas-Méndez, L.Y.; Zubkov, F.I. Recent Advances in Synthesis of Bioactive Quinoline-based 1,2,3-Triazoles via Cu-catalyzed Huisgen 1,3-Dipolar Cycloaddition (“Click reaction”). Mini-Rev. Org. Chem. 2016, 13, 488–503. [Google Scholar] [CrossRef]
- Luna-Parada, L.K.; Vargas-Méndez, L.Y.; Kouznetsov, V.V. Quinoline-Substituted 1,2,3-Triazole-Based Molecules, As Promising Conjugated Hybrids in Biomedical Research. Organic Medicinal Chem. IJ. 2018, 7, 555708. [Google Scholar] [CrossRef]
- Luna-Parada, L.K.; Kouznetsov, V.V. 5-Chloro-8-{[1-(2-chlorobenzyl)-1H-1,2,3-triazol-4-yl] methoxy}quinoline. Molbank 2019, 2019, M1038. [Google Scholar] [CrossRef]
- Rosado-Solano, D.N.; Barón-Rodríguez, M.A.; Sanabria-Florez, P.L.; Luna-Parada, L.K.; Puerto-Galvis, C.E.; Zorro-González, A.F.; Kouznetsov, V.V.; Vargas-Méndez, L.Y. Synthesis, Biological Evaluation and in silico Computational Studies of 7-Chloro-4-(1H-1,2,3-triazol-1-yl)quinoline Derivatives. Search for new controlling agents against Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae. J. Agric. Food Chem. 2019, 67, 9210–9219. [Google Scholar] [CrossRef]
- Calderón Lamus, D.; Puerto Galvis, C.E.; Kouznetsov, V.V. Cu(PPh3)3Br-catalyzed synthesis of new paracetamol-1,2,3-triazole molecular hybrids from expired commercial tablets and their in silico assessment to study their pharmacological properties. Med. Sci. Forum 2022, 14, 85, (8th International Electronic Conference on Medicinal Chemistry Session Small molecules as drug candidates, 01-30 November 2022, MDPI). [Google Scholar]
- Pak, J.K.; Hesse, M. Synthesis of penta-N-protected homocaldopentamine and its selective acylation. J. Org. Chem. 1998, 63, 8200–8204. [Google Scholar] [CrossRef]
- Kutonova, K.V.; Trusova, M.E.; Postnikov, P.S.; Filimonov, V.D.; Parello, J. A simple and effective synthesis of aryl azides via arenediazonium tosylates. Synthesis 2013, 45, 2706–2710. [Google Scholar]
- Vong, L.B.; Nagasaki, Y. Nitric oxide nano-delivery systems for cancer therapeutics: Advances and challenges. Antioxidants 2020, 9, 791. [Google Scholar] [CrossRef]
- Serafim, R.A.; Pernichelle, F.G.; Ferreira, E.I. The latest advances in the discovery of nitric oxide hybrid drug compounds. Expert Opin. Drug Discov. 2017, 12, 941–953. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.K.; Bhardwaj, T.R. Therapeutic role of nitric oxide as emerging molecule. Biomed. Pharmacother. 2017, 85, 182–201. [Google Scholar] [CrossRef]
- Molinspiration Cheminformatics. Available online: http://www.molinspiration.com (accessed on 15 January 2023).
- Lipinski, C.A. Lead-and drug-like compounds: the rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Organic Chemistry Portal. The OSIRIS Property Explorer open-source program. Available online: http://www.organic-chemistry.org/prog/peo/ (accessed on 15 January 2023).
| Comp. | MWb | cLogPc | HBAd | HBDe | ROTBf | TPSAg | n violations |
|---|---|---|---|---|---|---|---|
| 5a | 290.32 | 0.60 | 7 | 2 | 7 | 89.28 | 0 |
| 5b | 322.37 | 2.56 | 6 | 1 | 6 | 69.05 | 0 |
| 5c | 342.79 | 2.92 | 6 | 1 | 5 | 69.05 | 0 |
| 5d | 434.24 | 3.32 | 6 | 1 | 5 | 69.05 | 0 |
| 5e | 324.34 | 1.76 | 7 | 2 | 5 | 89.28 | 0 |
| 5f | 352.35 | 2.15 | 8 | 2 | 6 | 106.35 | 0 |
| 6a | 353.22 | 1.97 | 6 | 1 | 7 | 69.05 | 0 |
| 7a | 335.32 | 1.86 | 10 | 1 | 9 | 124.11 | 0 |
| APAP | 151.16 | 0.68 | 3 | 2 | 1 | 49.33 | 0 |
| APAP-ONO2 | 296.28 | 2.17 | 8 | 1 | 9 | 110.46 | 0 |
| Comp. | Mut | Tum | Irr | Rep | Drug-likeness | Drug-score |
|---|---|---|---|---|---|---|
| 5a |  |
 |
 |
 |
-0.75 | 0.62 |
| 5b |  |
 |
 |
 |
1.72 | 0.78 |
| 5c |  |
 |
 |
 |
1.06 | 0.70 |
| 5d |  |
 |
 |
 |
0.65 | 0.59 |
| 5e |  |
 |
 |
 |
0.46 | 0.72 |
| 5f |  |
 |
 |
 |
-0.52 | 0.59 |
| 6a |  |
 |
 |
 |
-8.16 | 0.12 |
| 7a |  |
 |
 |
 |
-0.71 | 0.59 |
| APAP |  |
 |
 |
 |
1.93 | 0.20 |
| APAP-ONO2 |  |
 |
 |
 |
-8.25 | 0.45 |
 = No risk,
= No risk,  = Moderated risk,
= Moderated risk,  = High risk
= High risk
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





