Submitted:
06 June 2023
Posted:
06 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Body Composition
1.2. Body Parameters
| Authors - Year | Dependent variables | Independent variables (R2) | Statistical method |
|---|---|---|---|
| Yan et al. – 2019 [21] | LW of yaks measured by digital scale | Angle length (0.90), height (0.94), Side area (0.74) | Multiple linear regression |
| Zhang et al. – 2020 [22] | LW yaks measured by digital scale | Height (0.92), body length (0.88), body depth (0.95), circumference (0.91) | K-Nearest Neighbour |
| Ribeiro et al. – 2014 [23] | Back fat thickness and longissimus muscle area – Beef | Back fat thickness in cm and longissimus muscle area cm2 (0.68 to 0.95) | Multiple linear regression |
| Dias et al. – 2020 [24] | Fat Lean |
LW, sternum fat depth multiplied by LW, lumbar subcutaneous fat depth multiplied by sternum fat depth, sternum fat depth, lumbar subcutaneous fat depth and dependent variable the total body fat (0.68 to 0.95) LW and sternum fat depth (0.96) |
Multiple linear regression |
| Morales-Martinez et al. – 2020 [25] | fat | measuring kidney adipose thickness (0.77) | Multiple linear regression |
| Johnson et al. – 2020 [26] | Internal fat | Forequarter, loin, rack and the hindleg (0.92) | Multiple linear regression |
| Yilmaz et al. – 2013 [27] | LW sheep measured by digital scale | Body length (0.79) | Multiple linear regression |
| Iqbal et al. – 2019 [28] | LW sheep measured by digital scale | Body weight, withers height, body length, chest girth, paunch circumference, face length, the length between ears, ear length, fat tail width, tail length (0.92) | Penalized regression |
| Sabbioni et al. – 2020 [29] | LW sheep measured by digital scale | Height at withers, chest circumference, body length, height at croup, chest width, chest depth and croup width (0.96) | Multiple linear regression |
| Topai and Macit – 2004 [32] | LW Sheep measured by digital scale | Heart girth (0.75) | Multiple linear regression |
| Doeschl et al. – 2004 [35] | fat weight Pigs measured by slaughtering and dissection | Rump width (0.69) | Multiple linear regression |
| Zhang et al., 2018 [36] | LW sheep measured by digital scale | Withers height, back height, rump height, body length, chest depth, chest width, abdominal width and rump width (0.99) | SMV |
| Abdelhady et al. – 2019 [37] | Live Weight Sheep measured by digital scale | Body breadth and Body length (0.98) | Multiple linear regression |
| Diaz et al. – 2020 [38] | Muscle&fat weight by slaughtering Bone weight by slaughtering |
shrunk body weight, rump depth, abdomen circumference and hook bone width (0.91) shrunk body weight, rib depth and girth (0.86) |
Multiple linear regression |
2. Materials and Methods
2.1. Experimental approach
2.2. Data collection
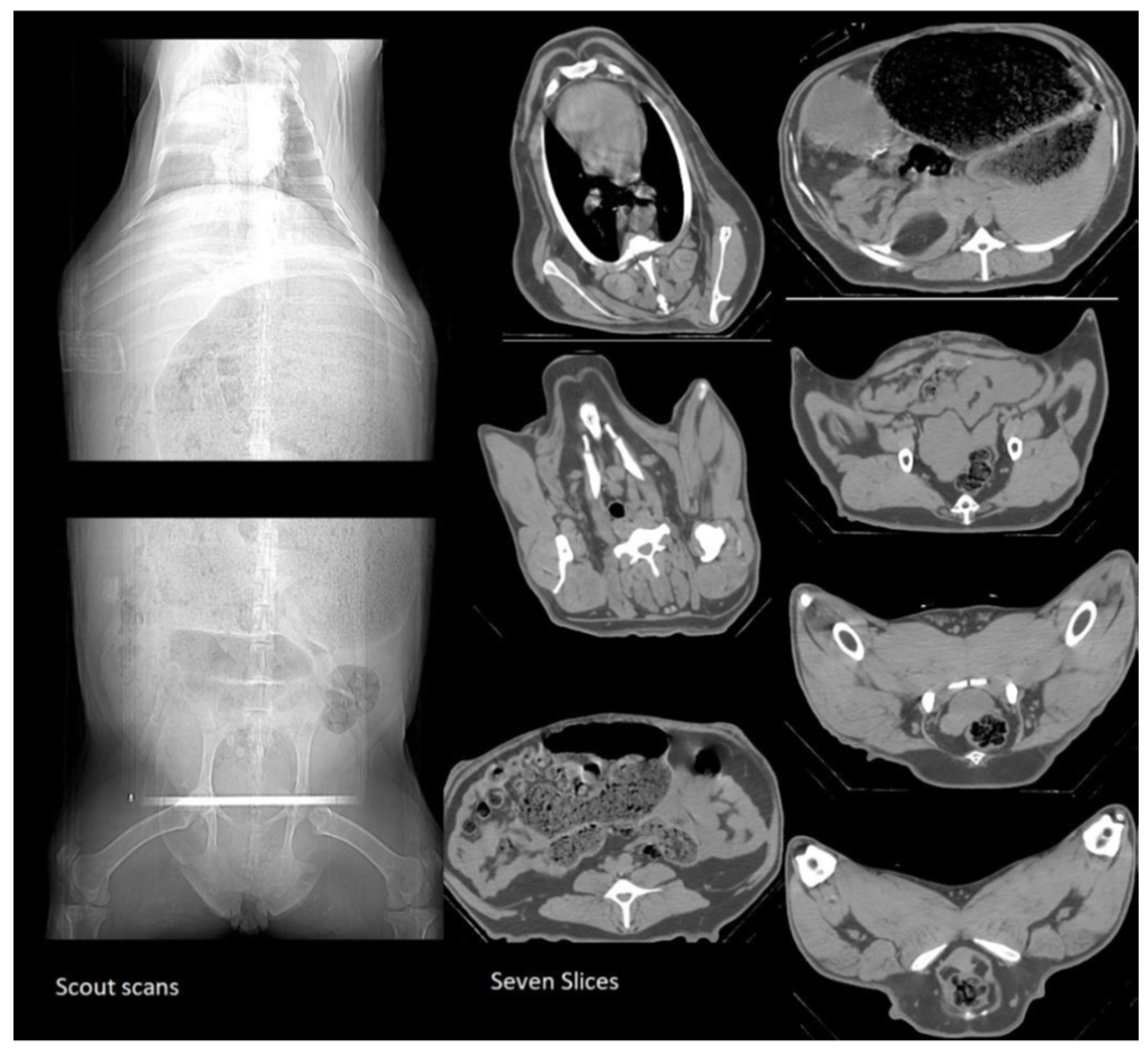
2.2.1. CT scans
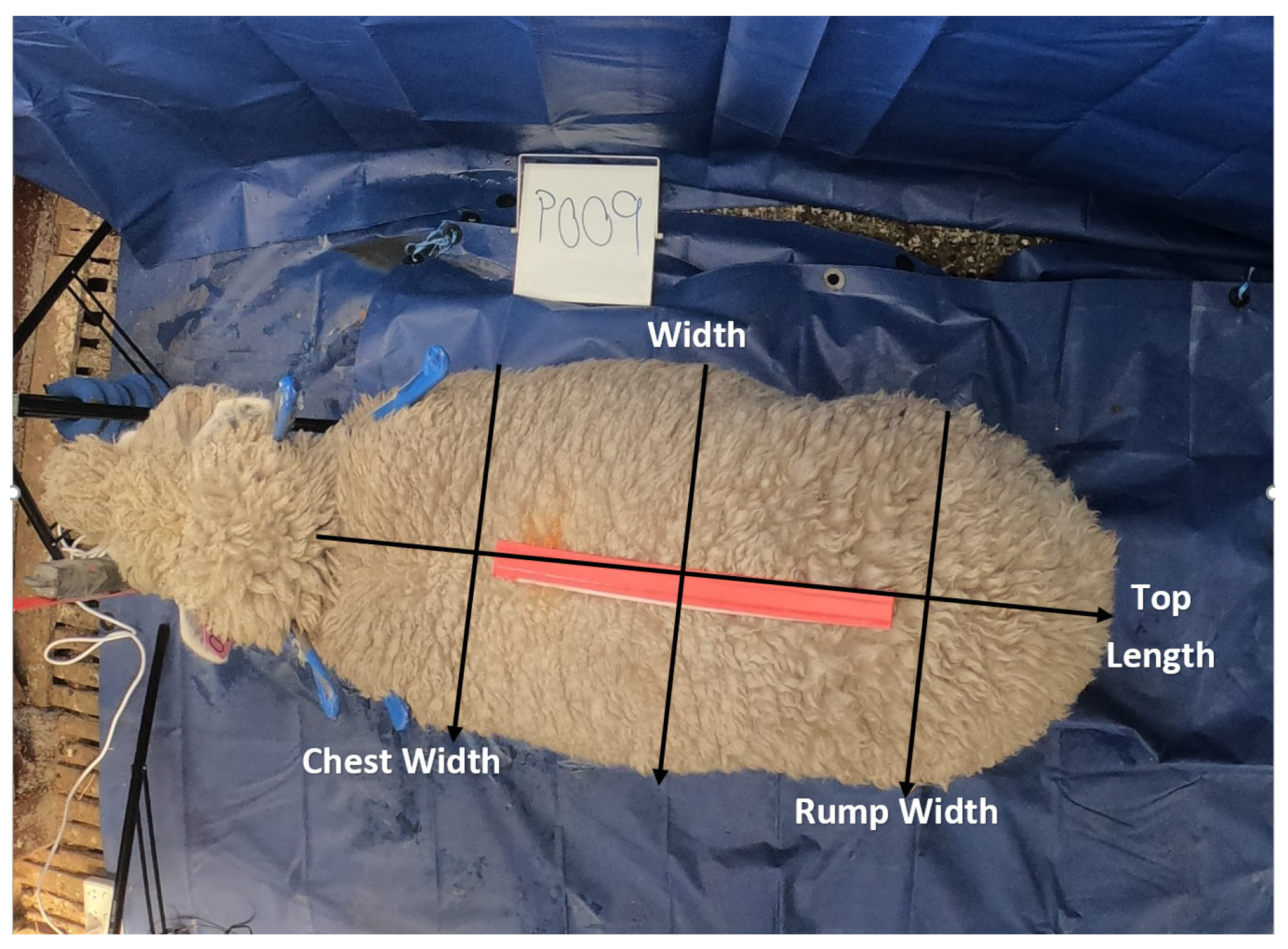
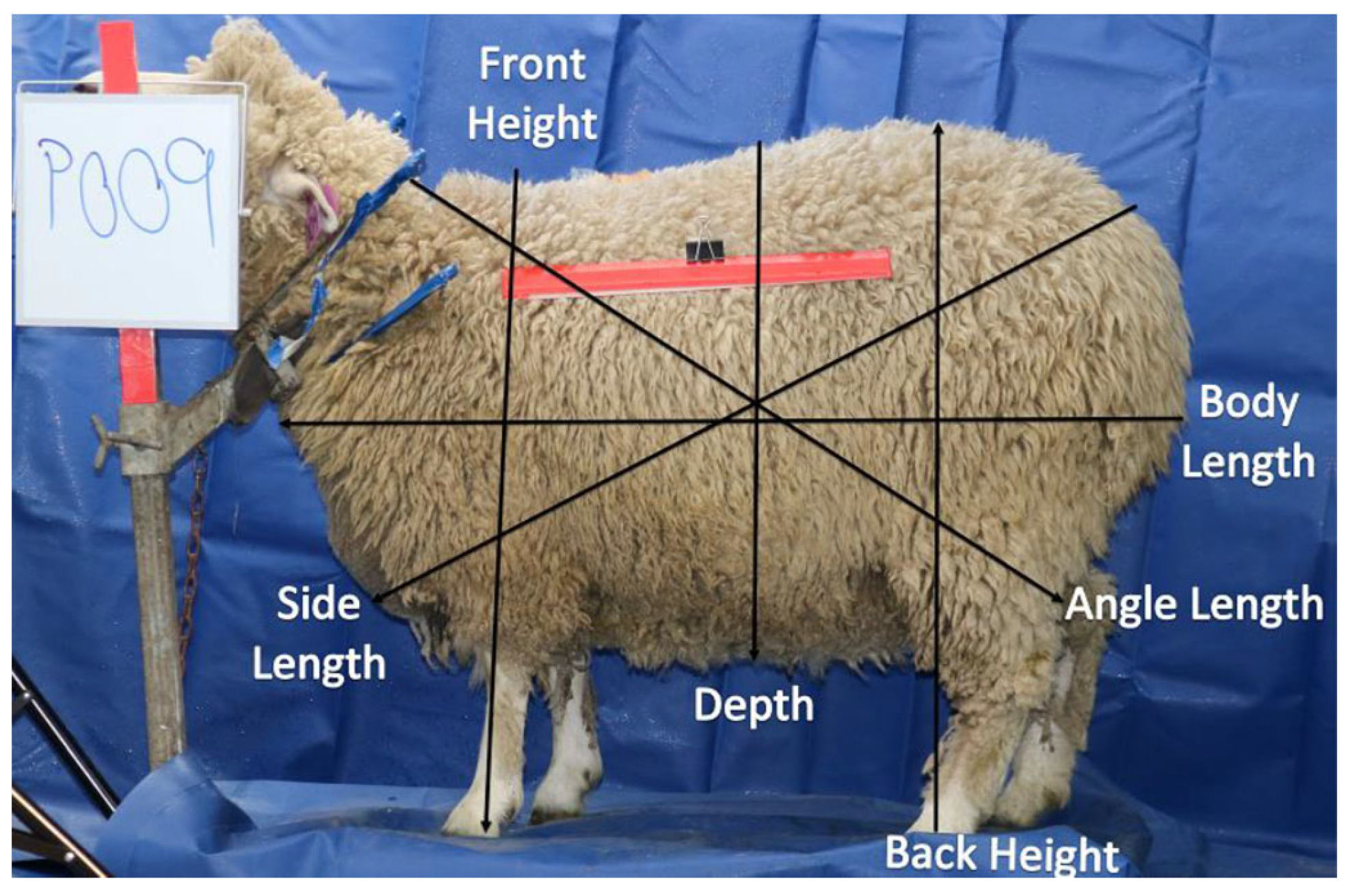
2.2.2. Body measurements
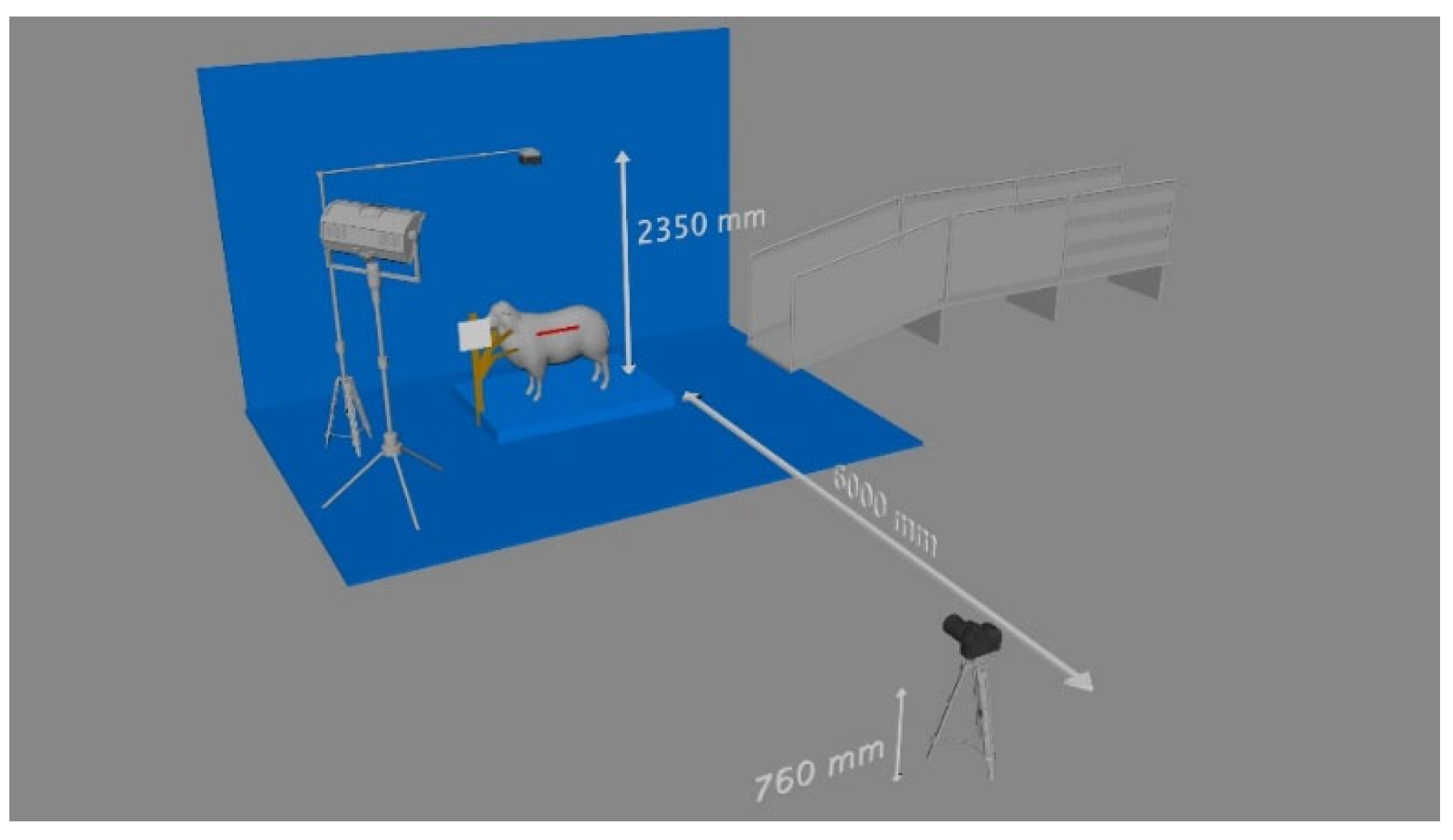
2.2.3. Visual image capture
2.3. Wool test
2.4. Analysis
3. Results
3.1. Descriptive statistics
3.2. Error estimation
3.3. Application accuracy
3.4. Factor analysis
3.5. Fat
3.6. Lean
3.7. Bone
3.8. Summary of results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, S.; Cadavez, V. Real-time ultrasound (RTU) imaging methods for quality control of meats. Computer Vision Technology in the Food and Beverage Industries, 2012: P. 277-329.
- Keinprecht, H.; et al. Short term repeatability of body fat thickness measurement and body condition scoring in sheep as assessed by a relatively small number of assessors. Small Ruminant Research 2016, 139, 30–38. [Google Scholar] [CrossRef]
- Kenyon, P.R. Maloney, S.K.;Blache, D., Review of sheep body condition score in relation to production characteristics. New Zealand journal of agricultural research 2014, 57, 38–64. [Google Scholar] [CrossRef]
- Russel, A. Body condition scoring of sheep. In Practice 1984, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Tait, I.; et al. Associations of body condition score and change in body condition score with lamb production in New Zealand Romney ewes. New Zealand Journal of Animal Science and Production 2019, 79, 91–94. [Google Scholar]
- van Burgel, A.J.; et al. The merit of condition score and fat score as alternatives to liveweight for managing the nutrition of ewes. Animal Production Science 2011, 51, 834–841. [Google Scholar] [CrossRef]
- Ducker, M.J.; Boyd, J.S. The effect of body size and body condition on the ovulation rate of ewes. Animal Production 2010, 24, 377–385. [Google Scholar] [CrossRef]
- Wuliji, T.; Dodds, K.G. Effect of fleece weight selection on reproduction, live weight and wool characteristics in mature Romney ewes. Small Ruminant Research 2011, 97, 55–59. [Google Scholar] [CrossRef]
- Cottle, D. International Sheep and Wool Handbook. 1 ed. 2010: Nottingham University Press. 766.
- Termatzidou, S.A.; et al. Association of body condition score with ultrasound backfat and longissimus dorsi muscle depth in different breeds of dairy sheep. Livestock Science 2020, 236, 104019. [Google Scholar] [CrossRef]
- Moro; et al. Prediction of physical characteristics of the lamb carcass using in vivo bioimpedance analysis. Animal 2019, 13, 1744–1749. [Google Scholar] [CrossRef]
- Miller, D.; et al. Dual-energy X-ray absorptiometry scans accurately predict differing body fat content in live sheep. Journal of animal science and biotechnology 2019, 10, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Cam, M.A. Garipoglu, A.V.;Koray, K., Body condition status at mating affects gestation length, offspring yield and return rate in ewes. Archiv für Tierzucht 2018, 61, 221–228. [Google Scholar]
- MacLaughlin, S.M.; et al. Periconceptional nutrition and the relationship between maternal body weight changes in the periconceptional period and feto-placental growth in the sheep. The Journal of Physiology 2005, 565, 111–124. [Google Scholar] [CrossRef]
- Borg, R.C. Notter, D.R.;Kott, R.W., Phenotypic and genetic associations between lamb growth traits and adult ewe body weights in western range sheep. Journal of Animal Science 2009, 87, 3506–14. [Google Scholar] [CrossRef]
- Fthenakis, G.C.; et al. Interactions between parasitic infections and reproductive efficiency in sheep. Veterinary parasitology, 2015. 208, 56-66.
- Manly, H.; et al. The association of ewe body condition with weight of lamb weaned. Proceedings of the New Zealand Society of Animal Production 2011, 71, 62–65. [Google Scholar]
- Kvame, T.; Vangen, O. Selection for lean weight based on ultrasound and CT in a meat line of sheep. Livestock Science 2007, 106, 232–242. [Google Scholar] [CrossRef]
- Macfarlane, J.M.; et al. Predicting carcass composition of terminal sire sheep using X-ray computed tomography. British Society of Animal Science, 2006.
- Riva, J.; et al. Body measurements in Bergamasca sheep. Small Ruminant Research 2004, 55, 221–227. [Google Scholar] [CrossRef]
- Yan, Q.; et al. Body weight estimation of yaks using body measurements from image analysis. Measurement, 2019. 140.
- Zhang; et al., Body Weight Estimation of Yak Based on Cloud Edge Computing. 2020.
- Ribeiro, F.; et al. Comparison of real-time ultrasound measurements for body composition traits to carcass and camera data in feedlot steers. The Professional Animal Scientist 2014, 30, 597–601. [Google Scholar] [CrossRef]
- Dias, L. Silva, S.;Teixeira, A., Simultaneously prediction of sheep and goat carcass composition and body fat depots using in vivo ultrasound measurements and live weight. Research in Veterinary Science 2020, 133, 180–187. [Google Scholar] [CrossRef]
- Morales-Martinez, M.A.; et al. Developing equations for predicting internal body fat in Pelibuey sheep using ultrasound measurements. Small Ruminant Research 2020, 183, 106031. [Google Scholar] [CrossRef]
- Johnson, P. Juengel, J.;Bain, W., Predicting internal adipose from selected computed tomography images in sheep. New Zealand Journal of Animal Science and Production 2020, 80, 113–116. [Google Scholar]
- Yilmaz, O. Cemal, I.;Karaca, O., Estimation of mature live weight using some body measurements in Karya sheep. Tropical Animal Health and Production 2013, 45, 397–403. [Google Scholar] [CrossRef]
- Iqbal, F.; et al. PREDICTING LIVE BODY WEIGHT OF HARNAI SHEEP THROUGH PENALIZED REGRESSION MODELS. Journal of Animal and Plant Sciences 2019, 29, 1541–1548. [Google Scholar]
- Sabbioni, A.; et al. Body weight estimation from body measures in Cornigliese sheep breed. Italian Journal of Animal Science 2020, 19, 25–30. [Google Scholar] [CrossRef]
- Zhang; et al. Development and validation of a visual image analysis for monitoring the body size of sheep. Journal of Applied Animal Research 2018, 46, 1004–1015. [Google Scholar] [CrossRef]
- Burke, J. Nuthall, P.L.;McKinnon, A.E., An Analysis of the Feasibility Of Using Image Processing To Estimate the Live Weight of Sheep. Farm and Horticultural Management Group Applied Management and Computing Division Lincoln University 2004.
- Topai, M.; Macit, M. Prediction of Body Weight from Body Measurements in Morkaraman Sheep. Journal of Applied Animal Research 2004, 25, 97–100. [Google Scholar] [CrossRef]
- Khojastehkey, M.; et al. Body size estimation of new born lambs using image processing and its effect on the genetic gain of a simulated population. Journal of Applied Animal Research 2016, 44, 326–330. [Google Scholar] [CrossRef]
- Puth, M.-T. Neuhäuser, M.;Ruxton, G.D., Effective use of Spearman's and Kendall's correlation coefficients for association between two measured traits. Animal Behaviour 2015, 102, 77–84. [Google Scholar] [CrossRef]
- Doeschl, A.B.; et al. The relationship between the body shape of living pigs and their carcass morphology and composition. Animal Science 2004, 79, 73–83. [Google Scholar] [CrossRef]
- Zhang, A.L.N.; et al. Development and validation of a visual image analysis for monitoring the body size of sheep. Journal of Applied Animal Research 2018, 46, 1004–1015. [Google Scholar] [CrossRef]
- Abdelhady, A.; et al. Automatic Sheep Weight Estimation Based on K-Means Clustering and Multiple Linear Regression. 2019. p. 546-555.
- Bautista-Díaz, E.;et al. Prediction of Carcass Traits of Hair Sheep Lambs Using Body Measurements. Animals 2020, 10. [CrossRef]
- Zhang, A.; et al. Algorithm of sheep body dimension measurement and its applications based on image analysis. Computers and Electronics in Agriculture 2018, 153, 33–45. [Google Scholar] [CrossRef]
- Maeno, H. Oishi, K.;Hirooka, H., Interspecies differences in the empty body chemical composition of domestic animals. 2013. 7(7).
| Item | Minimum | Maximum | Mean | Std deviation |
|---|---|---|---|---|
| Fat (kg) | 0.88 | 17.65 | 5.26 | 3.00 |
| Lean (kg) | 12.65 | 20.78 | 16.22 | 1.49 |
| Bone (kg) | 2.03 | 3.77 | 2.68 | .32 |
| BCS | 2.0 | 4.5 | 2.72 | .52 |
| Weight (kg) | 44.00 | 88.50 | 58.92 | 7.83 |
| Chest width (mm) | 220.3 | 360.2 | 270.2 | 20.8 |
| Angle length (mm) | 670.1 | 870.6 | 770.9 | 40.2 |
| Body length (mm) | 600.7 | 810.6 | 710.7 | 40.5 |
| Side length (mm) | 640.7 | 870.1 | 760.6 | 40.4 |
| Front height (mm) | 540.9 | 670.8 | 620.2 | 20.7 |
| back height (mm) | 560.1 | 710.6 | 640.0 | 30.0 |
| Depth (mm) | 320.6 | 470.0 | 380.2 | 20.6 |
| Top length (mm) | 670.5 | 950.7 | 780.6 | 50.4 |
| Width (mm) | 270.8 | 370.3 | 310.9 | 20.1 |
| Back width (mm) | 200.0 | 380.5 | 300.7 | 20.9 |
| Top area (mm2) | 13543 | 288061 | 197304.6 | 28909.4 |
| Side area (mm2) | 216830 | 316730 | 283703.6 | 31401.9 |
| Values | Body length | Angle length | Height | Depth | Abdominal width |
|---|---|---|---|---|---|
| Max, min | +6, -15 | +8, -11 | +17, -12 | +15, -4 | +10, -13 |
| Mean diff. | 1% | 1% | 1% | 1% | 1% |
| Values | Angle length | Body Length | Height | Depth | Top length | Width | Side length |
|---|---|---|---|---|---|---|---|
| Weaning | 5% | 4% | 4% | 3% | 5% | 4% | n/a |
| Pre-mating | 7% | 3% | 3% | 4% | 6% | 5% | 4% |
| Component matrixa | |||
|---|---|---|---|
| Component | |||
| 1 | 2 | 3 | |
| BCS | .789 | ||
| LW | .707 | ||
| Chest width | .827 | ||
| Angle length | .805 | ||
| Body length | .834 | ||
| Side length | .859 | ||
| Height | .779 | ||
| Back height | .769 | ||
| Depth | .708 | ||
| Top length | .736 | ||
| Width | .745 | ||
| Rump width | .726 | ||
| Top area | .916 | ||
| Side area | .935 | ||
| Extraction method: Principal component analysis. Rotation method: Varimax with Kaiser normalisation. | |||
| a. 3 components extracted | |||
| Independent Variables | R2 | Equation | RMSE |
|---|---|---|---|
| LW, Chest width | 0.79 | -20.043 + 0.244LW + 0.401CH | 1.34 |
| LW, Angle length | 0.71 | -23.159 + 0.296LW + 0.141AL | 1.59 |
| LW, Body length | 0.72 | -21.733 + 0.301LW+ 0.129BL | 1.59 |
| LW, Side length | 0.71 | -21.119 + 0.293LW + 0.119SL | 1.62 |
| LW, Front height | 0.68 | -14.670 + 0.315LW+ 0.022FH | 1.69 |
| LW, Back height | 0.68 | -13.195 + 0.318LW + -0.004BH | 1.69 |
| LW, Depth | 0.70 | -18.764 + 0.288LW + 0.185D | 1.64 |
| LW, Top length | 0.69 | -17.092 + 0.310LW + 0.052TL | 1.67 |
| LW, Width | 0.73 | -22.113 + 0.247LW + 0.402W | 1.56 |
| LW, Rump width | 0.71 | -17.949 + 0.303LW + 0.175RW | 1.62 |
| LW, Top area | 0.73 | -16.688 + 0.291LW + 0.002TA | 1.55 |
| LW, Side area | 0.72 | -17.402 + 0.285LW + 0.002SA | 1.58 |
| All variables | 0.80 | -21.115 + 0.235LW + 0.522CH + 0.101AL + -0.042BL + 0.008SL + 0.034FH + 0.013BH + -0.067D + -0.053TL + -0.021W + -0.090RW |
1.34 |
| Independent variables | R2 | RMSE |
|---|---|---|
| LW, Chest width | 0.88 | 1.17 |
| LW, Angle length | 0.84 | 1.61 |
| LW, Body length | 0.83 | 1.36 |
| LW, Side length | 0.82 | 1.33 |
| LW, Front height | 0.80 | 1.81 |
| LW, Back height | 0.81 | 1.78 |
| LW, Depth | 0.85 | 1.47 |
| LW, Top length | 0.85 | 1.44 |
| LW, Width | 0.84 | 2.18 |
| LW, Rump width | 0.84 | 2.21 |
| LW, Top area | 0.84 | 2.21 |
| LW, Side area | 0.85 | 1.28 |
| All variables | 0.95 | 1.22 |
| Independent variables | R2 | Equation | RMSE |
|---|---|---|---|
| LW, Chest width | 0.51 | 10.773 + 0.156LW + -0.138CH | 1.04 |
| LW, Angle length | 0.47 | 9.564 + 0.134LW + -0.015AL | 1.09 |
| LW, Body length | 0.47 | 9.700 + 0.134LW + -0.019BL | 1.09 |
| LW, Side length | 0.47 | 9.778 + 0.135LW+ -0.045SL | 1.09 |
| LW, Front height | 0.47 | 5.919 + 0.127LW + 0.045FH | 1.08 |
| LW, Back height | 0.47 | 7.476 + 0.129LW + 0.018BH | 1.09 |
| LW, Depth | 0.47 | 10.112 + 0.140LW + -0.056D | 1.08 |
| LW, Top length | 0.47 | 8.345 + 0.131LW + 0.002TL | 1.09 |
| LW, Width | 0.52 | 12.588 + 0.164LW + -0.189W | 1.03 |
| LW, Rump width | 0.48 | 10.146 + 0.137LW + -0.064BW | 1.07 |
| LW, Top area | 0.49 | 9.500 + 0.139LW + -0.001TA | 1.07 |
| LW, Side area | 0.47 | 9.303 + 0.138LW + 0.000SA | 1.58 |
| All variables | 0.52 | -5.109 + 0.151LW + -0.082CH + -0.004AL + 0.020BL + -0.044SL + 0.010FH + -0.004BH + -0.046D + -0.102TL + -0.004W + 0.072RW + -0.003TA + 0.001SA |
1.4 |
| Independent variables | R2 | RMSE |
|---|---|---|
| LW, Chest width | 0.76 | 1.13 |
| LW, Angle length | 0.63 | 1.87 |
| LW, Body length | 0.62 | 1.01 |
| LW, Side length | 0.73 | 1.11 |
| LW, Front height | 0.74 | 1.03 |
| LW, Back height | 0.73 | 1.33 |
| LW, Depth | 0.71 | 2.42 |
| LW, Top length | 0.63 | 1.09 |
| LW, Width | 0.65 | 1.11 |
| LW, Rump width | 0.66 | 1.07 |
| LW, Top area | 0.71 | 1.01 |
| LW, Side area | 0.72 | 1.09 |
| All variables | 0.79 | 1.20 |
| LW, Rump width, Front height | 0.77 | 1.26 |
| Independent variables | R2 | Equation | RMSE |
|---|---|---|---|
| LW, Chest width | 0.22 | 1.616 + 0.021LW + -0.006CH | 0.89 |
| LW, Angle length | 0.24 | 0.861 + 0.018LW + 0.010AL | 0.88 |
| LW, Body length | 0.24 | 0.952 + 0.019LW + 0.009BL | 0.88 |
| LW, Side length | 0.24 | 0.947 + 0.018LW + 0.009SL | 0.88 |
| LW, Front height | 0.23 | 1.133 + 0.019LW + 0.007FH | 0.89 |
| LW, Back height | 0.22 | 1.317 + 0.019LW + 0.004BH | 0.89 |
| LW, Depth | 0.25 | 2.152 + 0.023LW + -0.022D | 0.88 |
| LW, Top length | 0.25 | 0.808 + 0.018LW + 0.010TL | 0.88 |
| LW, Width | 0.26 | 2.321 + 0.026LW + -0.037W | 0.87 |
| LW, Rump width | 0.22 | 1.553 + 0.020LW + -0.001BW | 0.89 |
| LW, Top area | 0.22 | 1.474 + 0.019LW + -0.005TA | 0.89 |
| LW, Side area | 0.22 | 1.458 + 0.019LW + 0.005SA | 0.89 |
| All variables | 0.36 | 1.678 + 0.029LW + -0.035CH + -0.008AL + 0.0006BL + -0.013SL + -0.017FH + 0.019BH + -0.063D + -0.026TL + -0.067W + 0.023RW + -0.001TA + 0.000SA | 0.25 |
| Independent variables | R2 | RMSE |
|---|---|---|
| LW, Chest width | 0.45 | 2.3 |
| LW, Angle length | 0.50 | 2.31 |
| LW, Body length | 0.43 | 1.03 |
| LW, Side length | 0.41 | 1.05 |
| LW, Front height | 0.60 | 1.82 |
| LW, Back height | 0.42 | 1.04 |
| LW, Depth | 0.57 | 1.94 |
| LW, Top length | 0.65 | 1.15 |
| LW, Width | 0.65 | 1.05 |
| LW, Rump width | 0.50 | 1.03 |
| LW, Top area | 0.58 | 1.01 |
| LW, Side area | 0.56 | 2.22 |
| All variables | 0.75 | 2.40 |
| LW, Chest width, Front height | 0.59 | 1.2 |
| LW, Angle length, Front height | 0.46 | 1.12 |
| LW, Body length, Front height | 0.53 | 1.19 |
| LW, Side length, Front height | 0.52 | 2.17 |
| LW, Depth, Front height | 0.61 | 1.0 |
| LW, Top length, Front height | 0.53 | 2.36 |
| LW, Width, Front height | 0.72 | 1.11 |
| Dependent variables | Independent variables | ||
|---|---|---|---|
| MLR – R2 | ANNs – R2 | RT – R2 | |
| Fat | 0.87 (LW, chest width) | 0.90 (LW, chest width) | 0.74 (LW, chest width) |
| Lean | 0.41 (LW and width) | 0.72 (LW, Rump width, Front height) | 0.21 (LW, width and chest width) |
| Bone | 0.34 (LW and width) | 0.50 (LW, Width, Front height) | 0.03 (LW, rump width and chest width) |
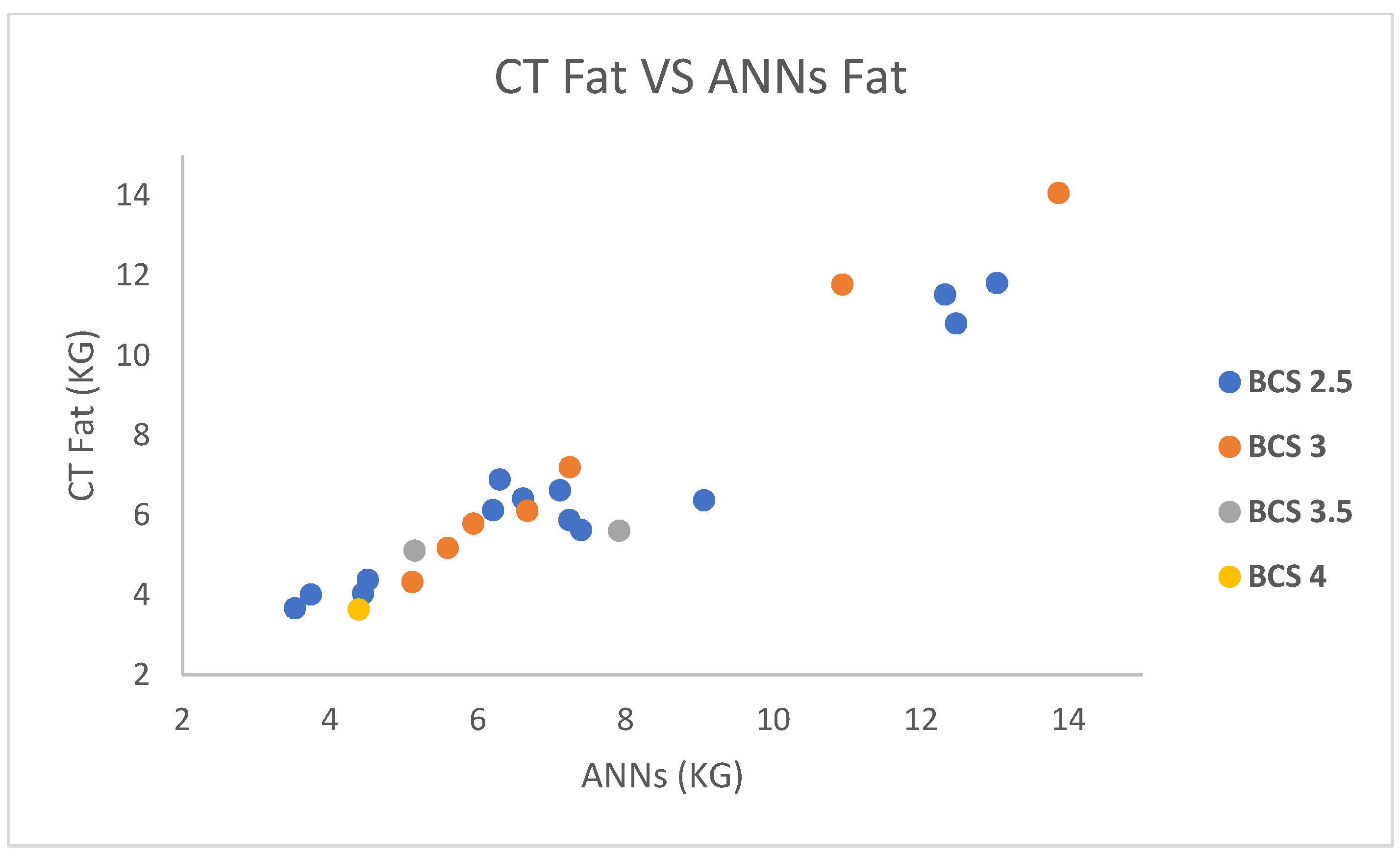
| CT Fat | MLR | ANNs | RT | BCS |
|---|---|---|---|---|
| 13.867 | 11.240 | 14.062 | 6.92 | 3 |
| 12.479 | 9.588 | 10.800 | 6.5 | 2.5 |
| 7.249 | 7.680 | 7.196 | 5.93 | 3 |
| 4.390 | 3.631 | 3.639 | 11.51 | 4 |
| 6.674 | 6.035 | 6.105 | 8.59 | 3 |
| 9.066 | 7.157 | 6.372 | 3.5 | 2.5 |
| 6.209 | 6.974 | 6.125 | 5.53 | 2.5 |
| 7.919 | 6.230 | 5.608 | 14.12 | 3.5 |
| 12.329 | 9.850 | 11.521 | 3.98 | 2.5 |
| 7.242 | 6.364 | 5.874 | 3.83 | 2.5 |
| 5.117 | 4.656 | 4.324 | 4.05 | 3 |
| 5.943 | 6.084 | 5.790 | 8.99 | 3 |
| 13.034 | 9.876 | 11.809 | 6.1 | 2.5 |
| 4.451 | 4.185 | 4.036 | 6.63 | 2.5 |
| 3.742 | 4.141 | 4.007 | 8.27 | 2.5 |
| 4.513 | 4.518 | 4.379 | 5.16 | 2.5 |
| 10.940 | 10.129 | 11.774 | 6.62 | 3 |
| 7.403 | 6.486 | 5.627 | 6.66 | 2.5 |
| 6.301 | 7.687 | 6.893 | 5.71 | 2.5 |
| 5.148 | 5.177 | 5.114 | 10.34 | 3.5 |
| 5.595 | 5.771 | 5.176 | 8.41 | 3 |
| 7.114 | 7.567 | 6.620 | 8.12 | 2.5 |
| 6.616 | 7.314 | 6.411 | 2.67 | 2.5 |
| 3.525 | 3.653 | 3.666 | 7.687 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





