Submitted:
06 June 2023
Posted:
06 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
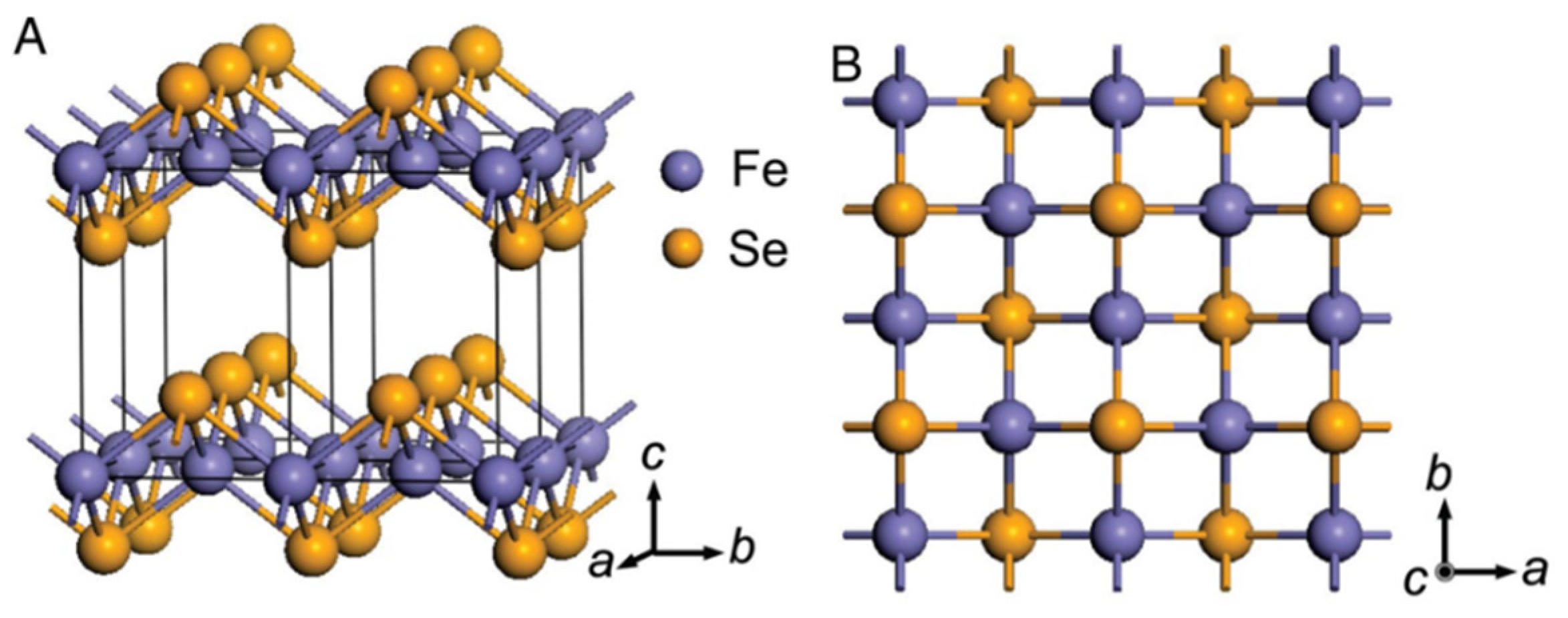
2. Single Crystal Growth and Superconductivity of FeSe
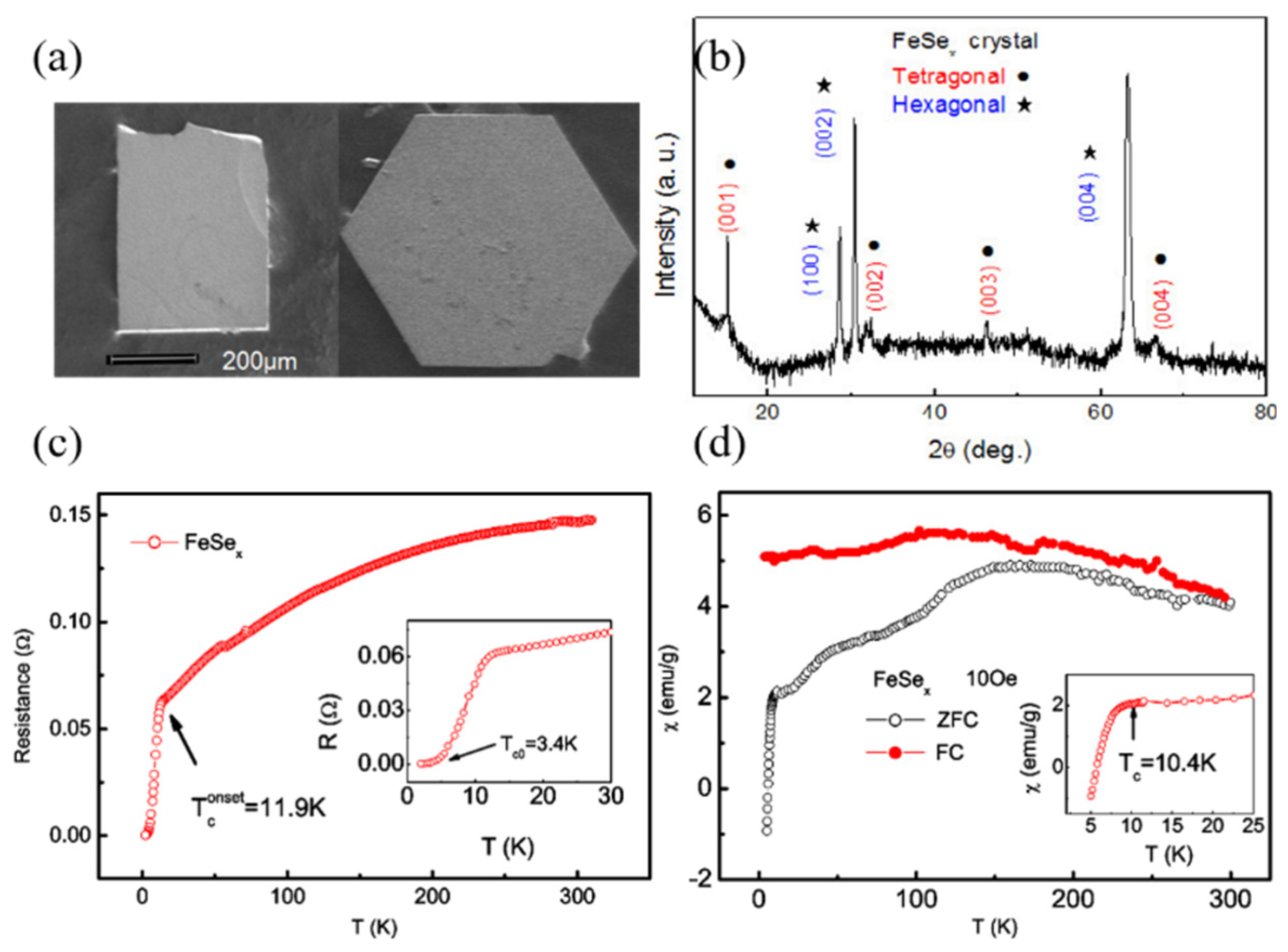
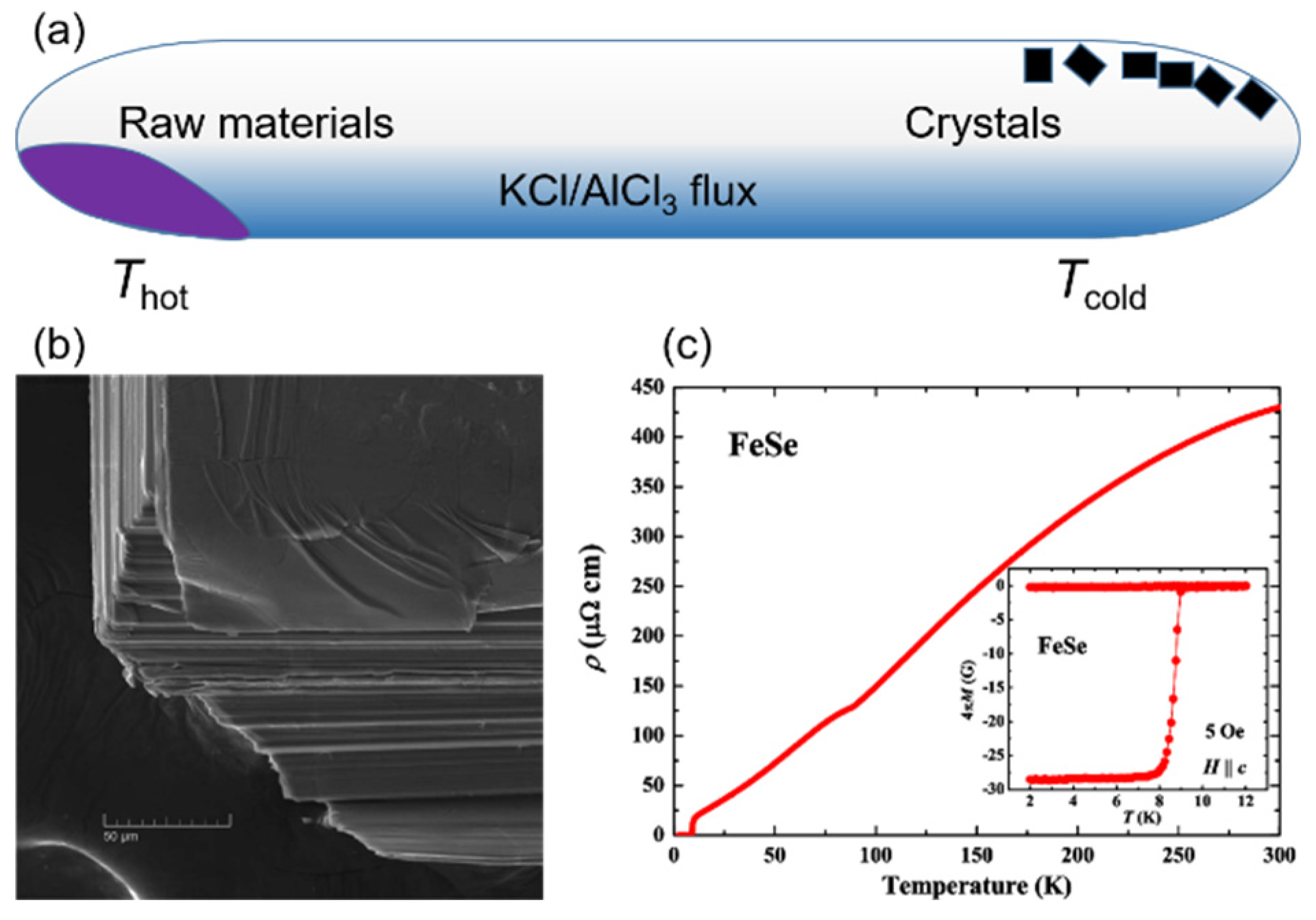
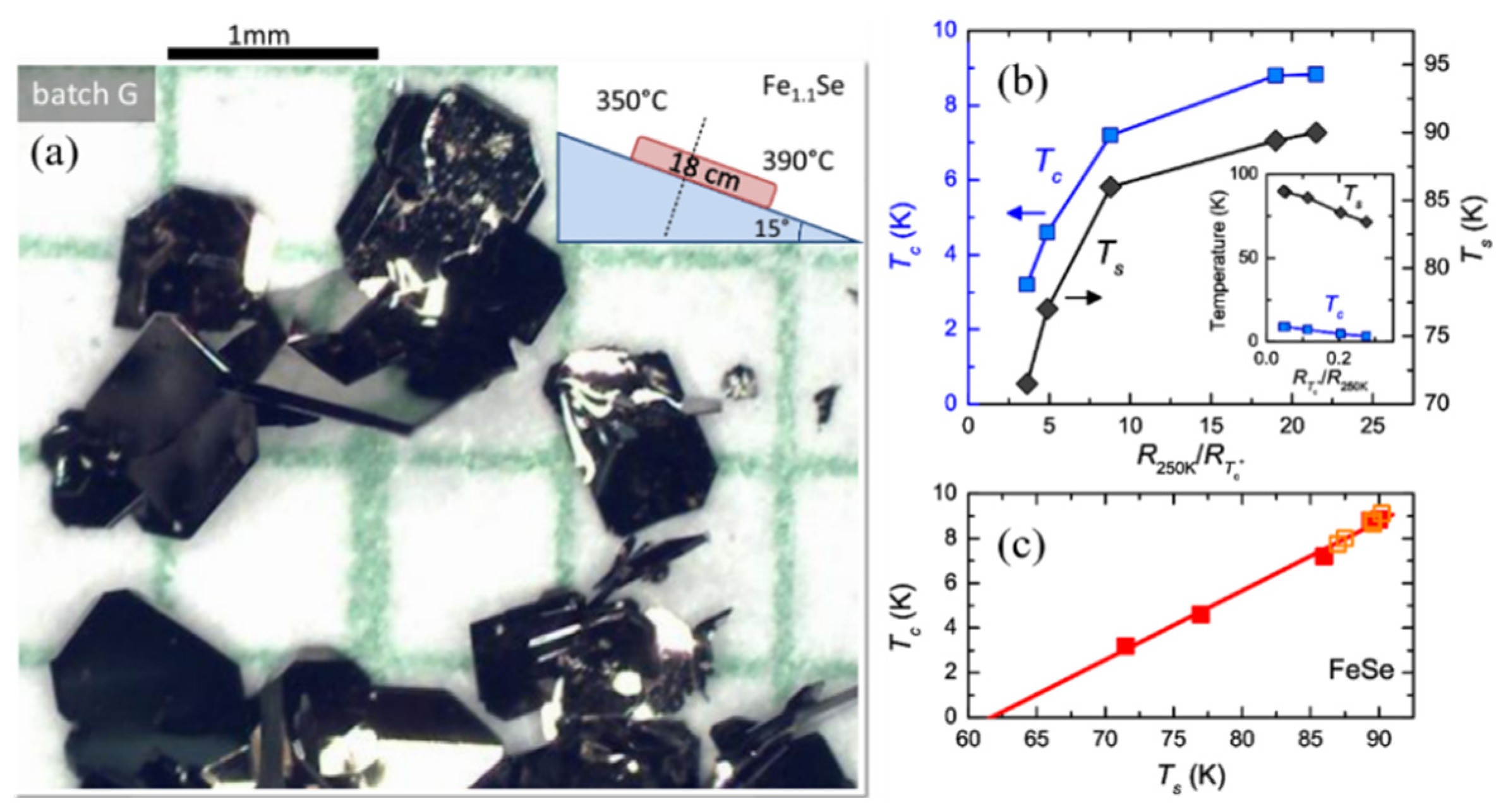
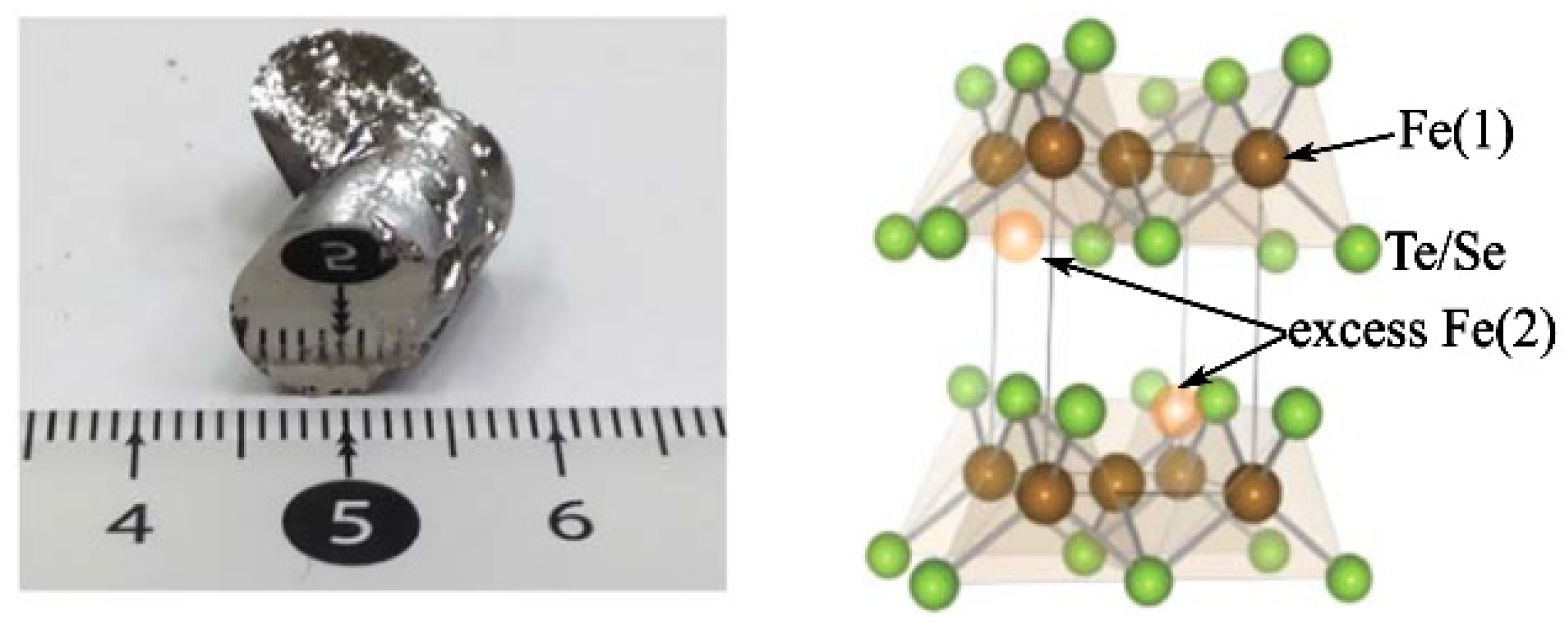
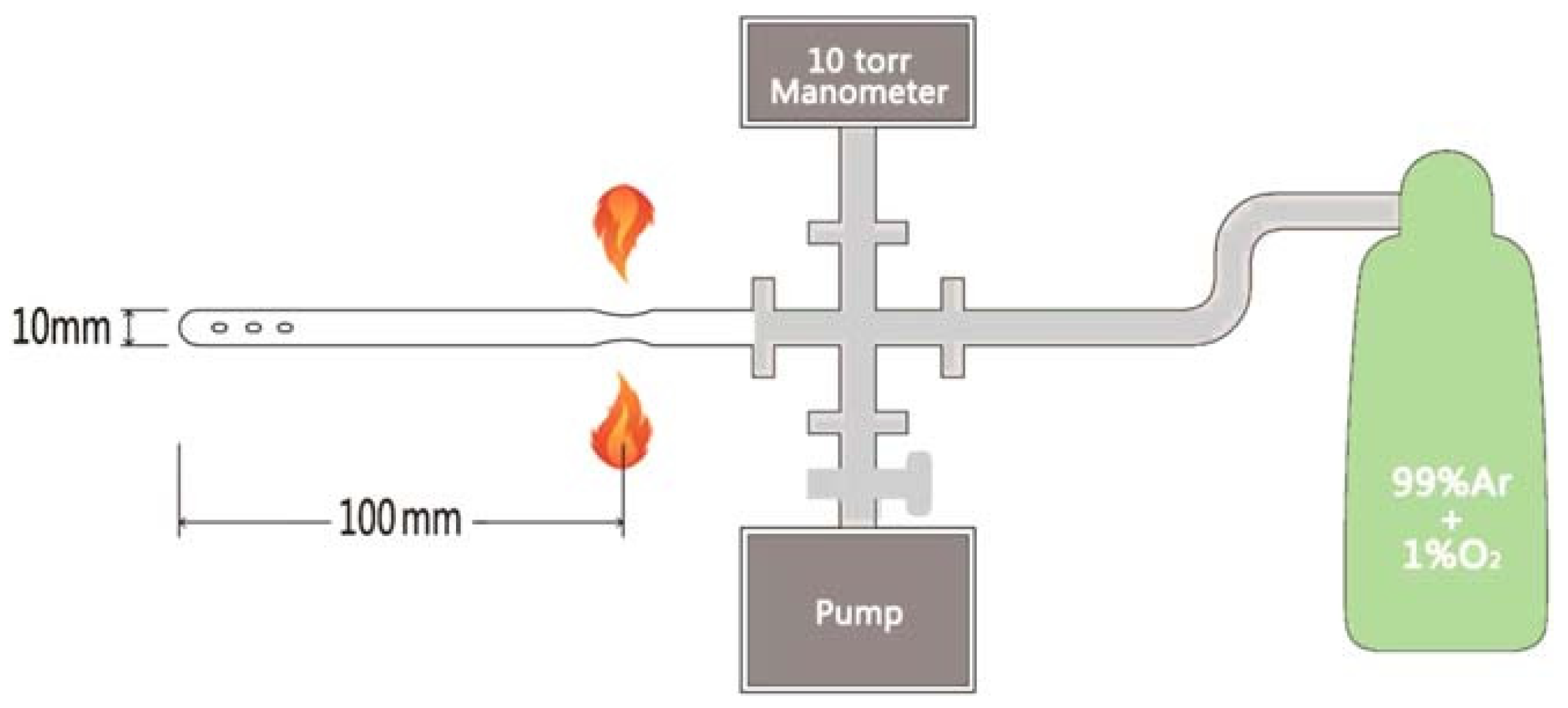
2.1. Flux Method for Growing FeSe Single Crystals
2.2. Chemical Vapor Transport (CVT) Method for Growing FeSe Single Crystals
3. Single Crystal Growth and Superconductivity of FeSe1-xSx
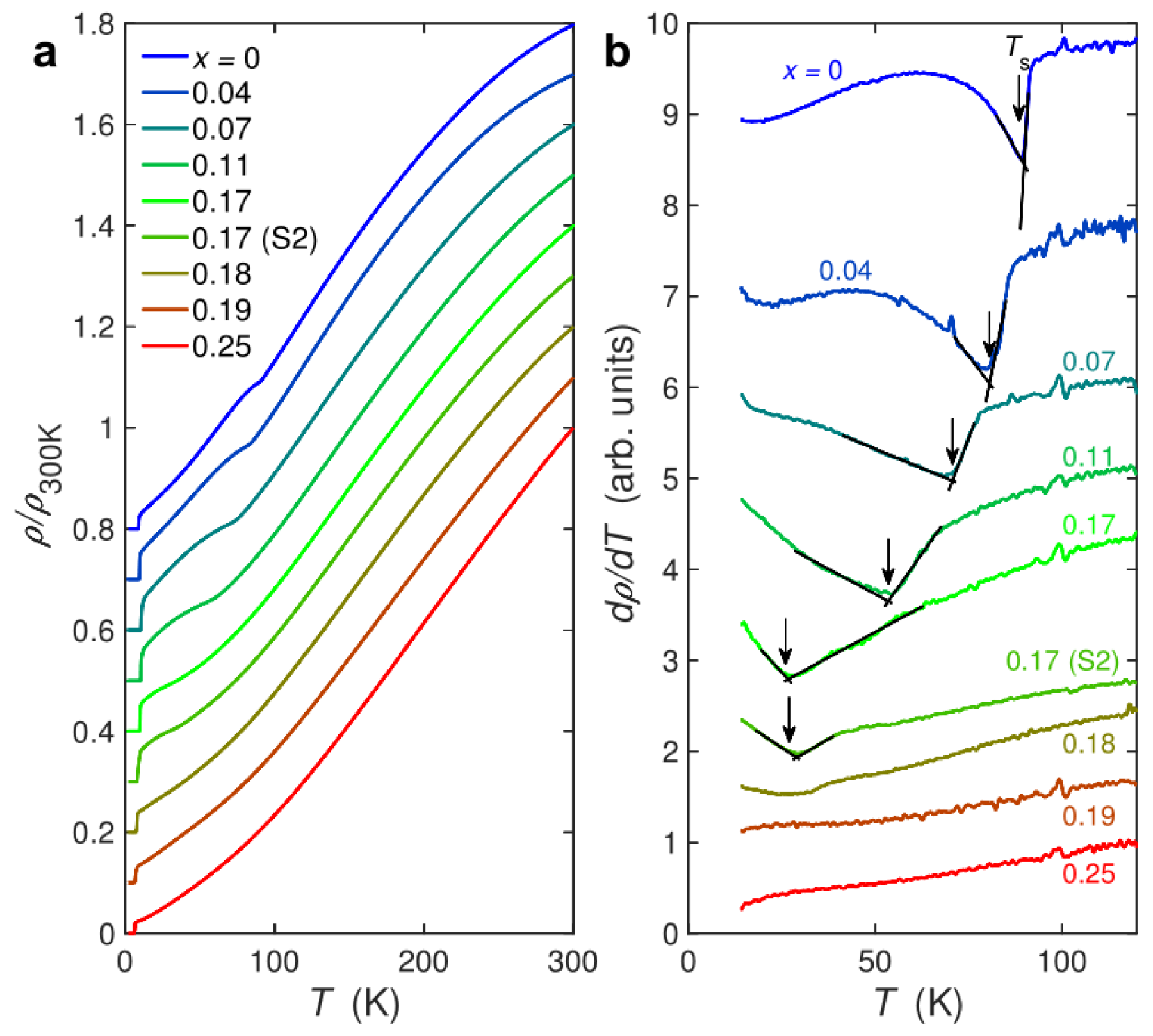
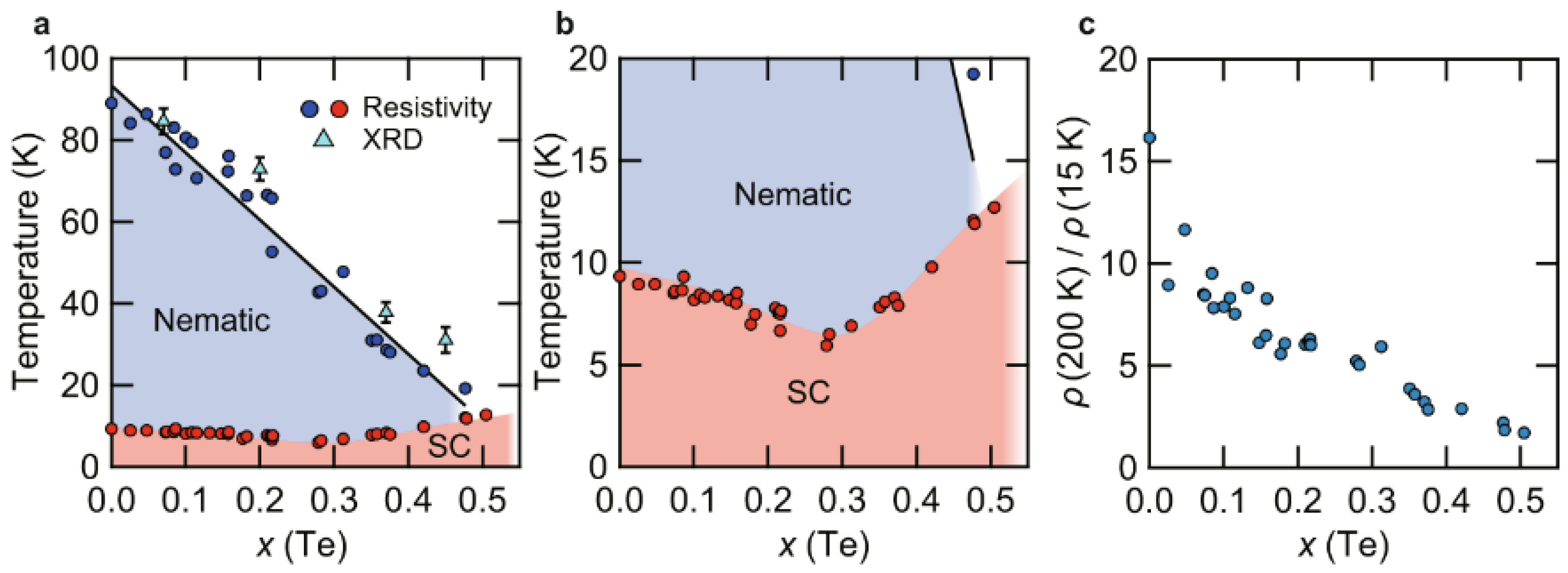
3.1. CVT Growth of FeSe1-xSx single crystals with Low S Doping
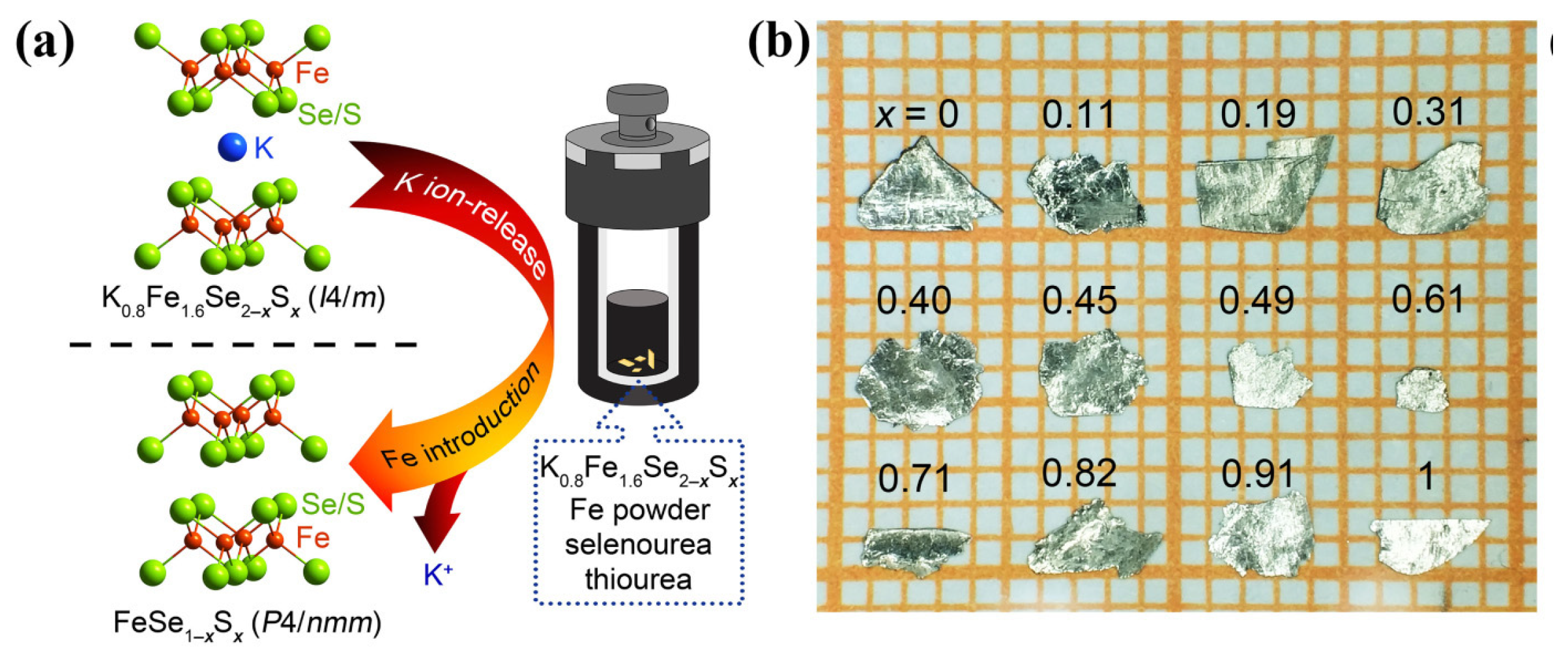
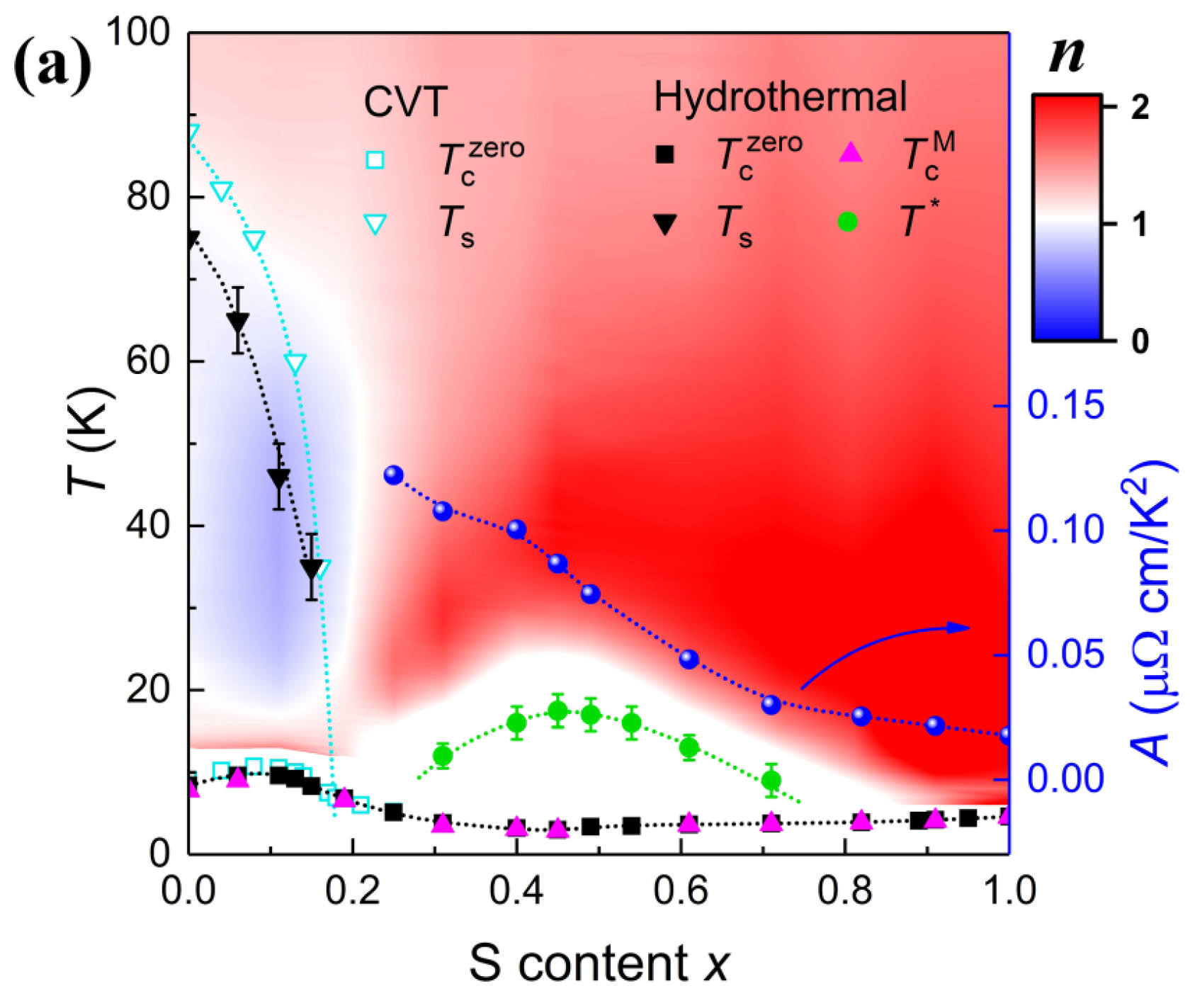
3.2. Hydrothermal Method for Growing FeSe1−xSx single Crystals across the Entire Doping Range
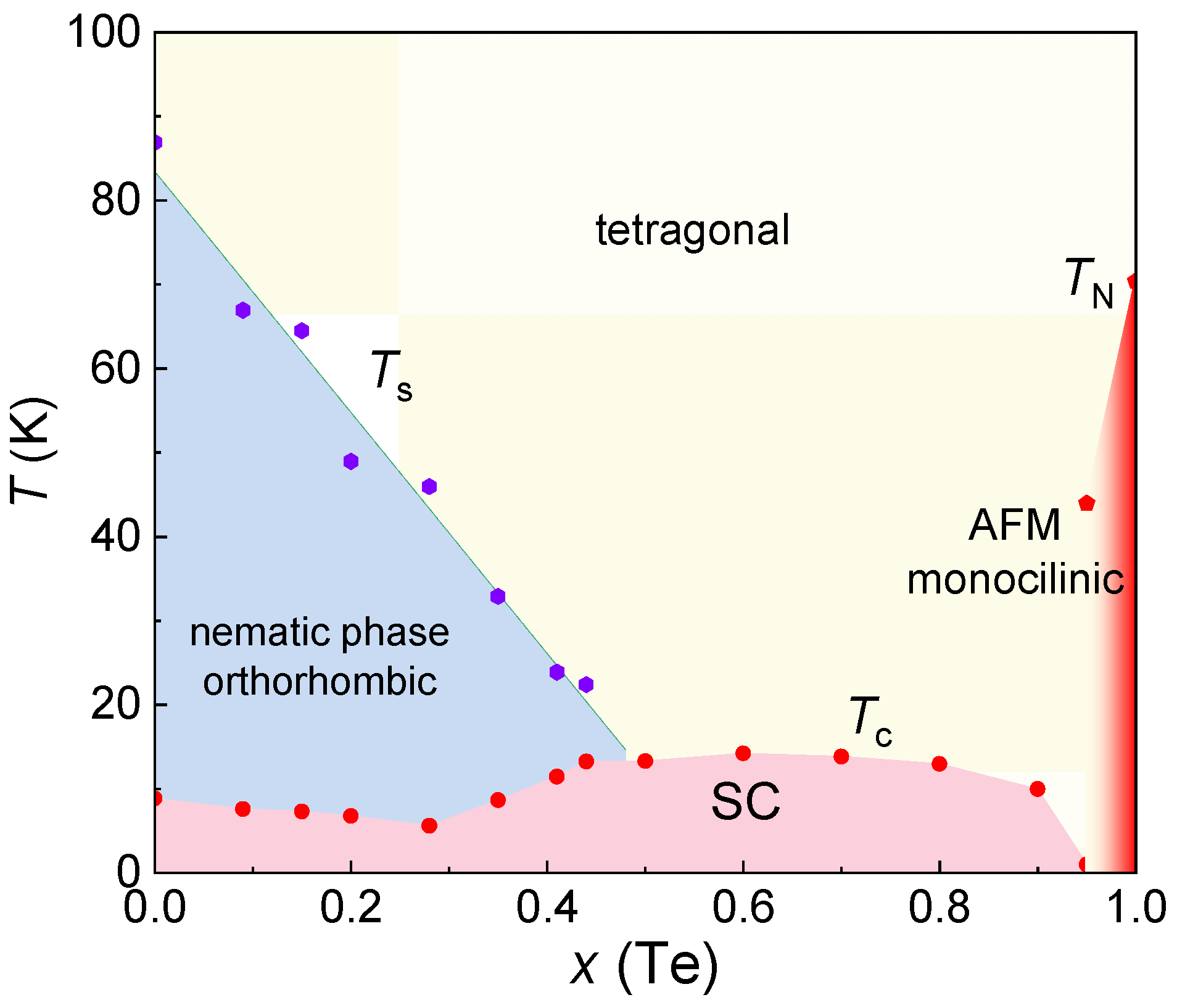
4. Single Crystal Growth and Superconductivity of FeSe1-xTex
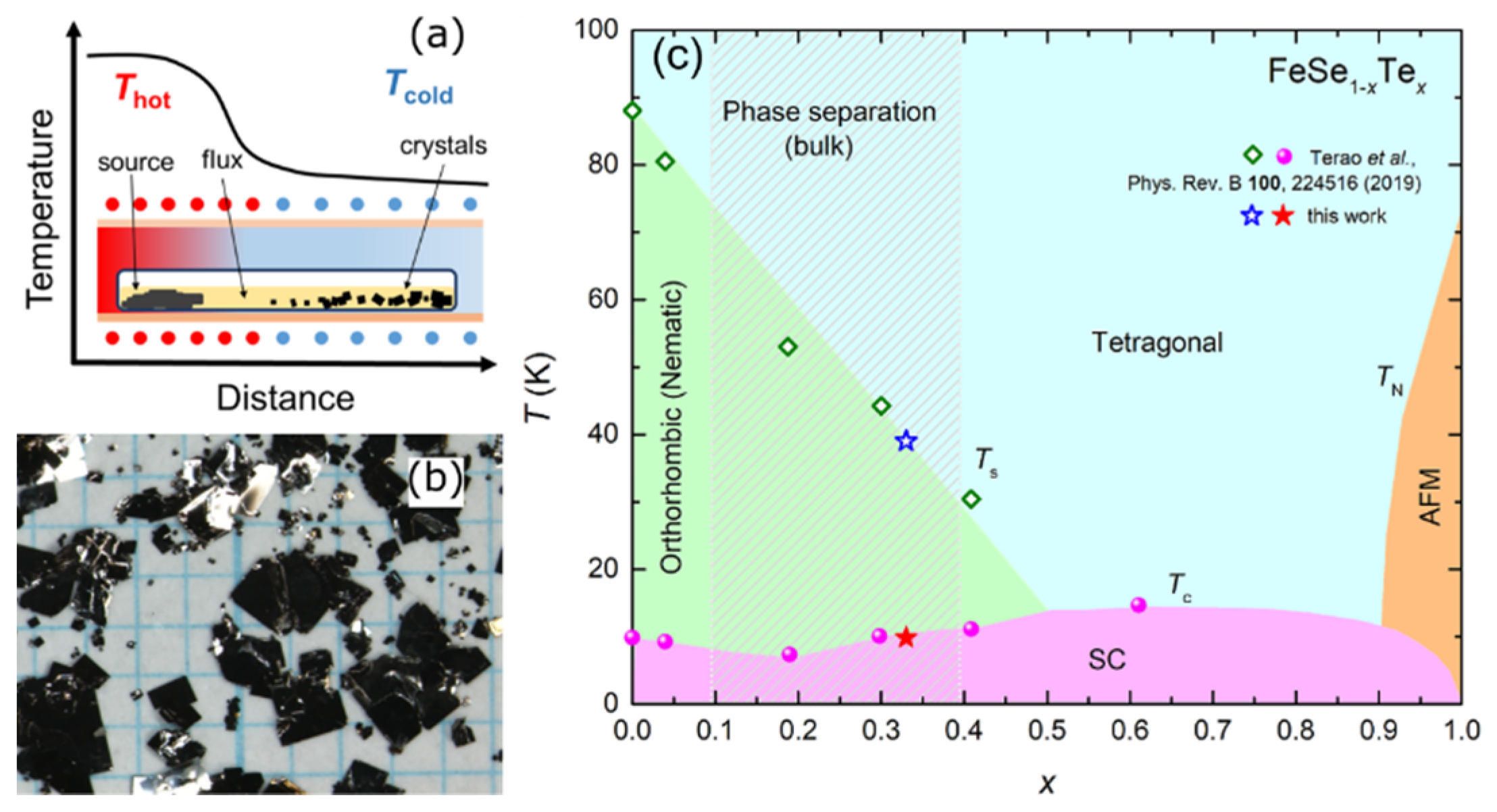
4.1. CVT Growth of FeSe1-xTex (0 < x < 0.5) Single Crystals
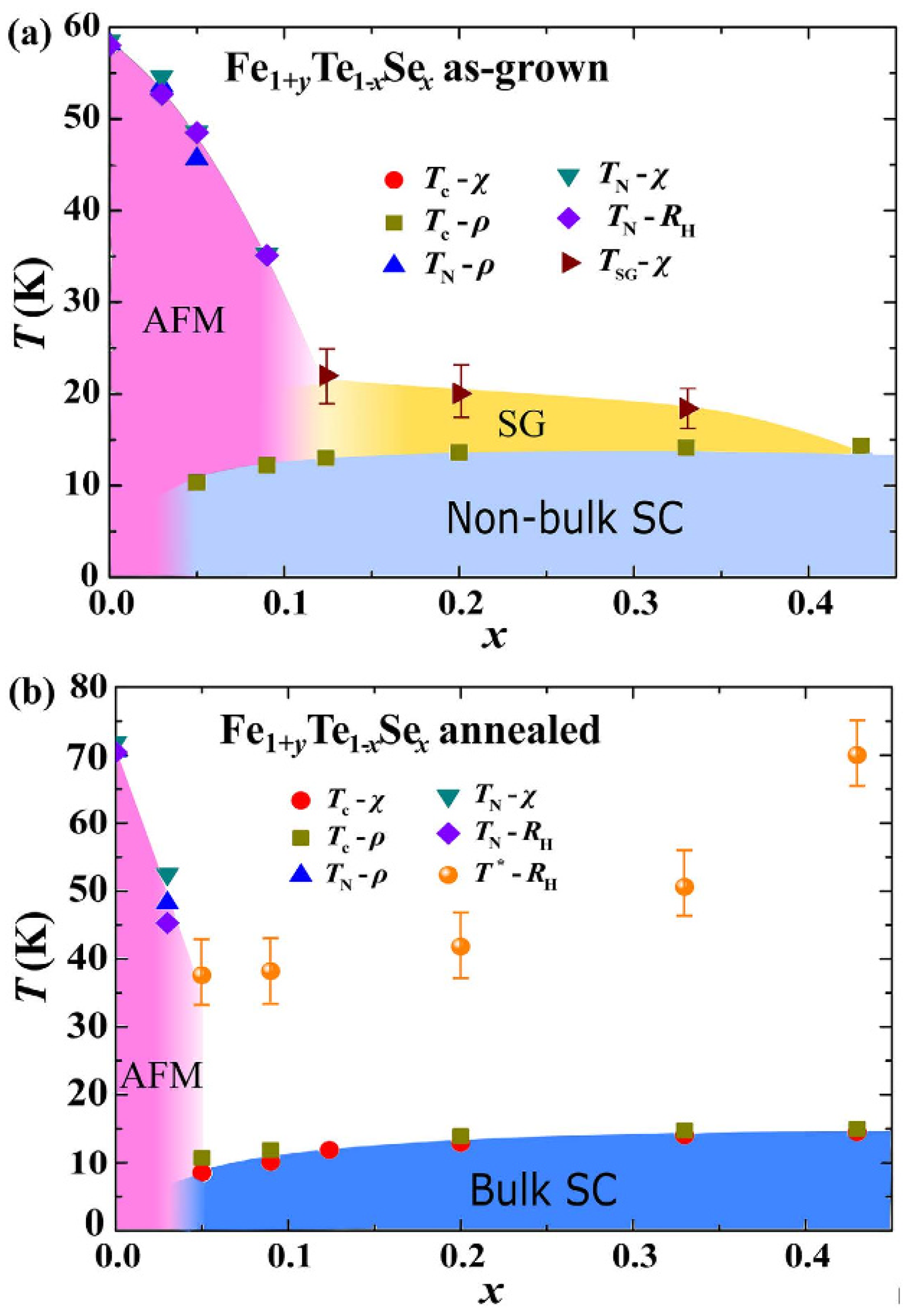
4.2. Self-Flux plus Annealing Method for Growing FeSe1-xTex (0.5 < x ≤ 1) Single Crystals
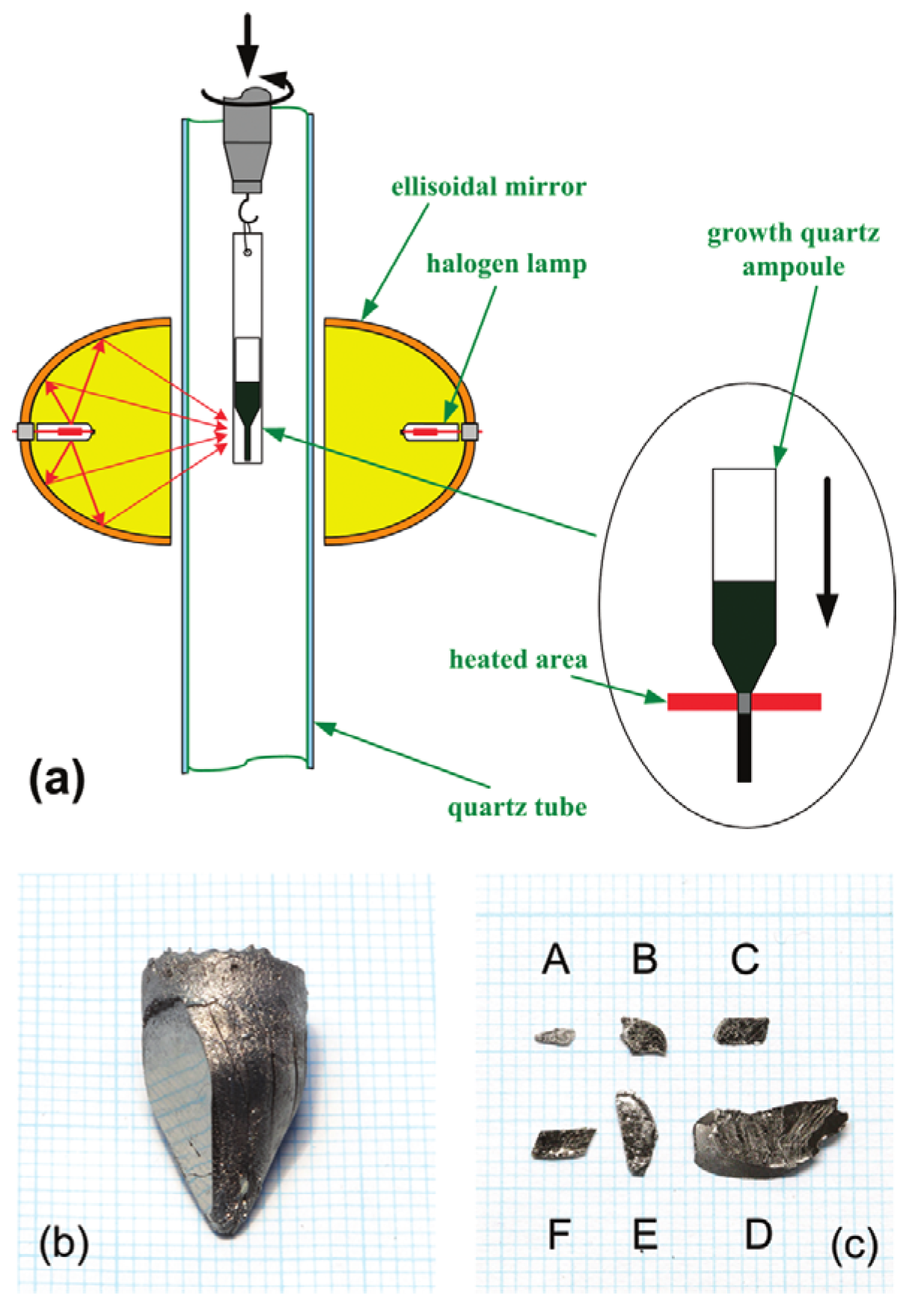
4.3. Optical Zone-Melting Technique for Growing FeSe1-xTex Single Crystals
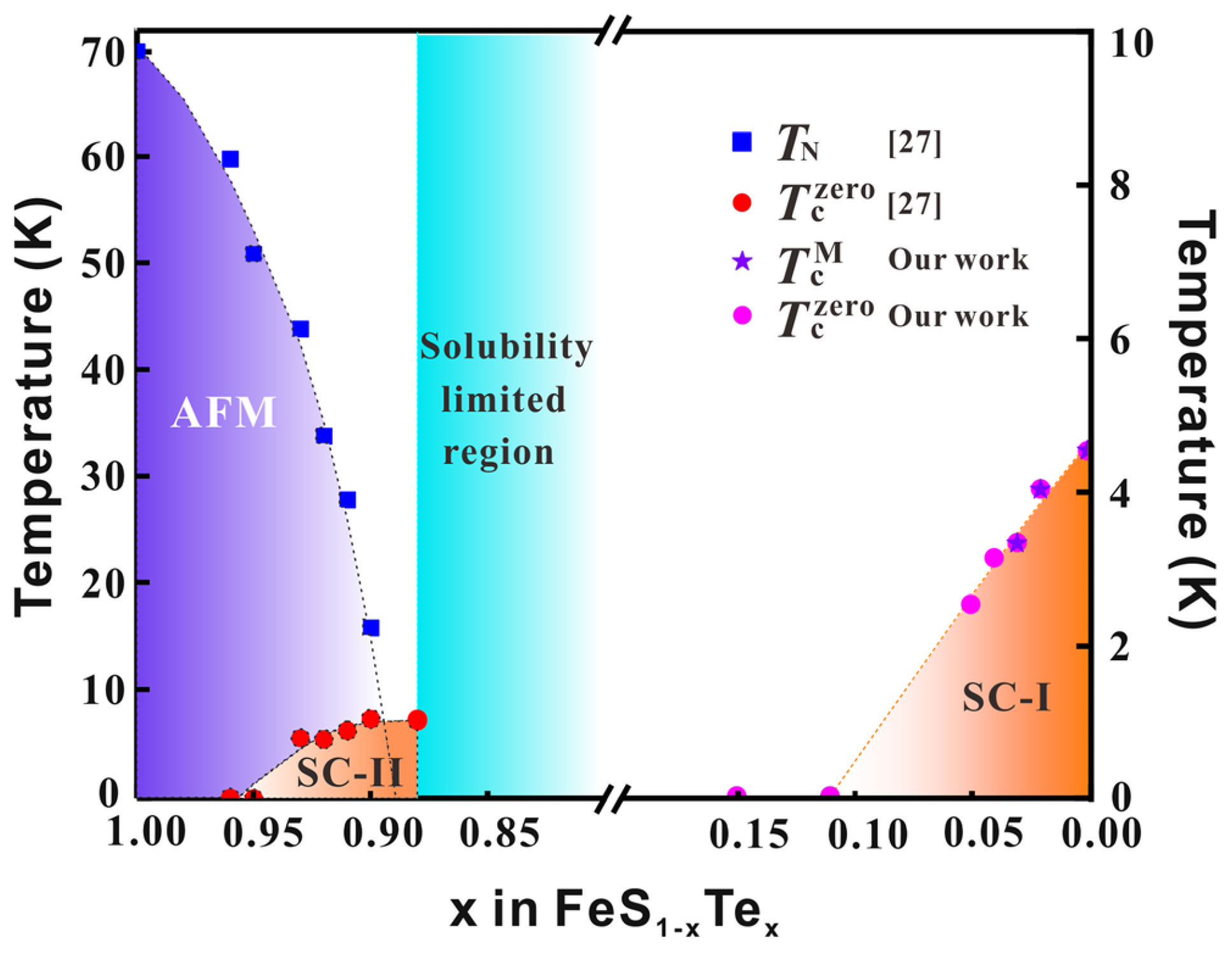
5. Single Crystal Growth and Superconductivity of FeTe1-xSx
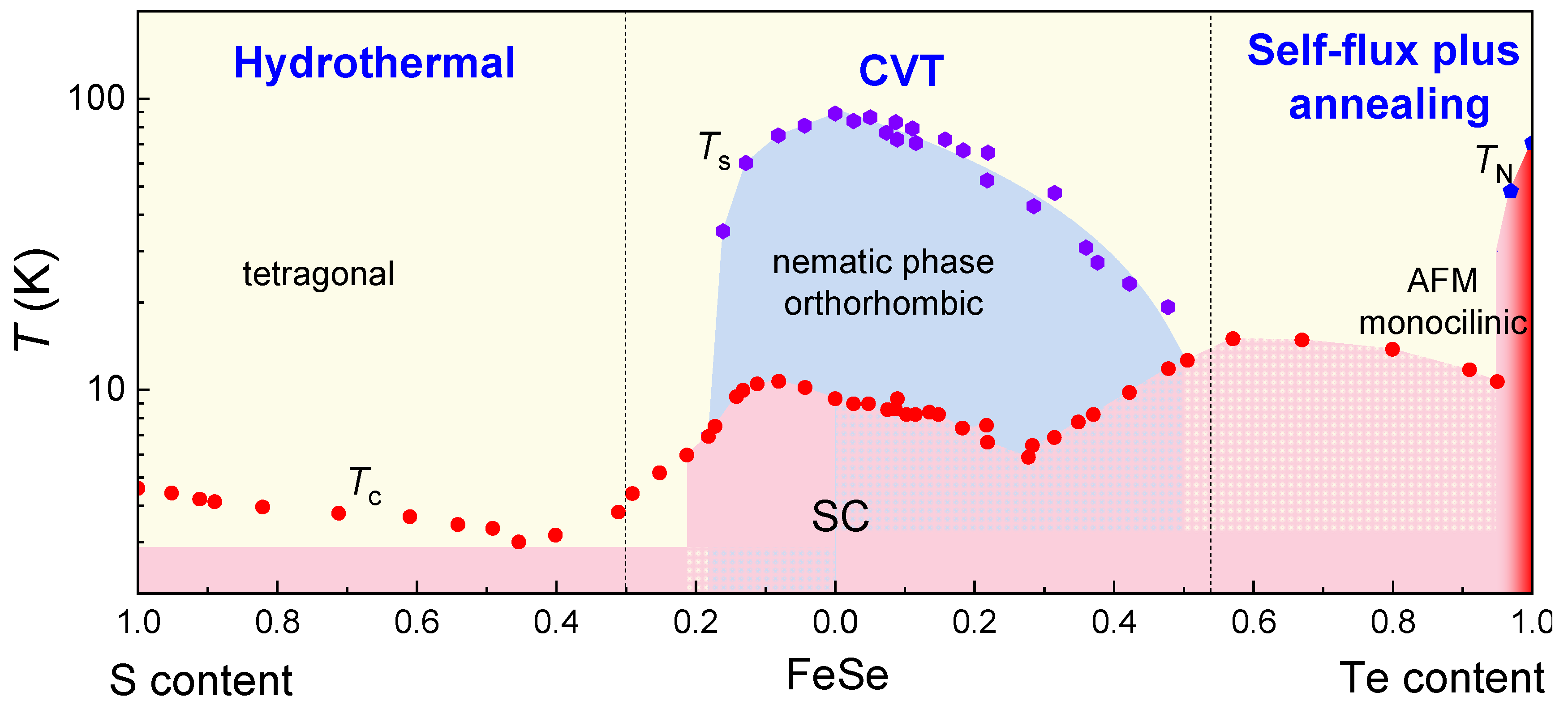
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsu, F.-C.; Luo, J.-Y.; Yeh, K.-W.; Chen, T.-K.; Huang, T.-W.; Wu, P.M.; Lee, Y.-C.; Huang, Y.-L.; Chu, Y.-Y.; Yan, D.-C.; et al. Superconductivity in the PbO-Type Structure α-FeSe. Proceedings of the National Academy of Sciences 2008, 105, 14262–14264. [Google Scholar] [CrossRef] [PubMed]
- Shibauchi, T.; Hanaguri, T.; Matsuda, Y. Exotic Superconducting States in FeSe-Based Materials. J. Phys. Soc. Jpn. 2020, 89, 102002. [Google Scholar] [CrossRef]
- Chen, T.-K.; Chang, C.-C.; Chang, H.-H.; Fang, A.-H.; Wang, C.-H.; Chao, W.-H.; Tseng, C.-M.; Lee, Y.-C.; Wu, Y.-R.; Wen, M.-H.; et al. Fe-Vacancy Order and Superconductivity in Tetragonal β-Fe1-xSe. Proceedings of the National Academy of Sciences 2014, 111, 63–68. [Google Scholar] [CrossRef] [PubMed]
- McQueen, T.M.; Williams, A.J.; Stephens, P.W.; Tao, J.; Zhu, Y.; Ksenofontov, V.; Casper, F.; Felser, C.; Cava, R.J. Tetragonal-to-Orthorhombic Structural Phase Transition at 90 K in the Superconductor Fe1.01Se. Phys. Rev. Lett. 2009, 103, 057002. [Google Scholar] [CrossRef] [PubMed]
- Böhmer, A.E.; Hardy, F.; Eilers, F.; Ernst, D.; Adelmann, P.; Schweiss, P.; Wolf, T.; Meingast, C. Lack of Coupling between Superconductivity and Orthorhombic Distortion in Stoichiometric Single-Crystalline FeSe. Phys. Rev. B 2013, 87, 180505. [Google Scholar] [CrossRef]
- Fernandes, R.M.; Chubukov, A.V.; Schmalian, J. What Drives Nematic Order in Iron-Based Superconductors? Nature Phys 2014, 10, 97–104. [Google Scholar] [CrossRef]
- Rößler, S.; Coduri, M.; Tsirlin, A.A.; Ritter, C.; Cuello, G.; Koz, C.; Muzica, L.; Schwarz, U.; Rößler, U.K.; Wirth, S.; et al. Nematic State of the FeSe Superconductor. Phys. Rev. B 2022, 105, 064505. [Google Scholar] [CrossRef]
- Medvedev, S.; McQueen, T.M.; Troyan, I.A.; Palasyuk, T.; Eremets, M.I.; Cava, R.J.; Naghavi, S.; Casper, F.; Ksenofontov, V.; Wortmann, G.; et al. Electronic and Magnetic Phase Diagram of β-Fe1.01Se with Superconductivity at 36.7 K under Pressure. Nature Mater 2009, 8, 630–633. [Google Scholar] [CrossRef]
- Sun, J.P.; Matsuura, K.; Ye, G.Z.; Mizukami, Y.; Shimozawa, M.; Matsubayashi, K.; Yamashita, M.; Watashige, T.; Kasahara, S.; Matsuda, Y.; et al. Dome-Shaped Magnetic Order Competing with High-Temperature Superconductivity at High Pressures in FeSe. Nat Commun 2016, 7, 12146. [Google Scholar] [CrossRef]
- Sun, J.P.; Ye, G.Z.; Shahi, P.; Yan, J.-Q.; Matsuura, K.; Kontani, H.; Zhang, G.M.; Zhou, Q.; Sales, B.C.; Shibauchi, T.; et al. High-Tc Superconductivity in FeSe at High Pressure: Dominant Hole Carriers and Enhanced Spin Fluctuations. Phys. Rev. Lett. 2017, 118, 147004. [Google Scholar] [CrossRef]
- Gati, E.; Böhmer, A.E.; Bud’ko, S.L.; Canfield, P.C. Bulk Superconductivity and Role of Fluctuations in the Iron-Based Superconductor FeSe at High Pressures. Phys. Rev. Lett. 2019, 123, 167002. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.F.; Wang, N.Z.; Wu, H.; Wu, Y.P.; Zhao, D.; Zeng, X.Z.; Luo, X.G.; Wu, T.; Bao, W.; Zhang, G.H.; et al. Coexistence of Superconductivity and Antiferromagnetism in (Li0.8Fe0.2)OHFeSe. Nature Mater 2015, 14, 325–329. [Google Scholar] [CrossRef]
- Shi, M.Z.; Wang, N.Z.; Lei, B.; Ying, J.J.; Zhu, C.S.; Sun, Z.L.; Cui, J.H.; Meng, F.B.; Shang, C.; Ma, L.K.; et al. FeSe-Based Superconductors with a Superconducting Transition Temperature of 50 K. New J. Phys. 2018, 20, 123007. [Google Scholar] [CrossRef]
- Lei, B.; Cui, J.H.; Xiang, Z.J.; Shang, C.; Wang, N.Z.; Ye, G.J.; Luo, X.G.; Wu, T.; Sun, Z.; Chen, X.H. Evolution of High-Temperature Superconductivity from a Low-Tc Phase Tuned by Carrier Concentration in FeSe Thin Flakes. Phys. Rev. Lett. 2016, 116, 077002. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Xing, X.; Yi, X.; Li, B.; Zhou, N.; Li, M.; Zhang, Y.; Wei, W.; Feng, J.; Terashima, K.; et al. Protonation-Induced Discrete Superconducting Phases in Bulk FeSe Single Crystals. Phys. Rev. B 2022, 105, 134506. [Google Scholar] [CrossRef]
- Meng, Y.; Wei, W.; Xing, X.; Yi, X.; Zhou, N.; Zhang, Y.; Liu, W.; Sun, Y.; Shi, Z. Significant Enhancement of Critical Current Density in H+-Intercalated FeSe Single Crystal. Supercond. Sci. Technol. 2022, 35, 075012. [Google Scholar] [CrossRef]
- Shi, X.; Han, Z.-Q.; Peng, X.-L.; Richard, P.; Qian, T.; Wu, X.-X.; Qiu, M.-W.; Wang, S.C.; Hu, J.P.; Sun, Y.-J.; et al. Enhanced Superconductivity Accompanying a Lifshitz Transition in Electron-Doped FeSe Monolayer. Nat Commun 2017, 8, 14988. [Google Scholar] [CrossRef]
- Wen, C.H.P.; Xu, H.C.; Chen, C.; Huang, Z.C.; Lou, X.; Pu, Y.J.; Song, Q.; Xie, B.P.; Abdel-Hafiez, M.; Chareev, D.A.; et al. Anomalous Correlation Effects and Unique Phase Diagram of Electron-Doped FeSe Revealed by Photoemission Spectroscopy. Nat Commun 2016, 7, 10840. [Google Scholar] [CrossRef]
- Ge, J.-F.; Liu, Z.-L.; Liu, C.; Gao, C.-L.; Qian, D.; Xue, Q.-K.; Liu, Y.; Jia, J.-F. Superconductivity above 100 K in Single-Layer FeSe Films on Doped SrTiO3. Nature Mater 2015, 14, 285–289. [Google Scholar] [CrossRef]
- Qing-Yan, W.; Zhi, L.; Wen-Hao, Z.; Zuo-Cheng, Z.; Jin-Song, Z.; Wei, L.; Hao, D.; Yun-Bo, O.; Peng, D.; Kai, C.; et al. Interface-Induced High-Temperature Superconductivity in Single Unit-Cell FeSe Films on SrTiO3. Chinese Phys. Lett. 2012, 29, 037402. [Google Scholar]
- Reiss, P.; Watson, M.D.; Kim, T.K.; Haghighirad, A.A.; Woodruff, D.N.; Bruma, M.; Clarke, S.J.; Coldea, A.I. Suppression of Electronic Correlations by Chemical Pressure from FeSe to FeS. Phys. Rev. B 2017, 96, 121103. [Google Scholar] [CrossRef]
- Sato, Y.; Kasahara, S.; Taniguchi, T.; Xing, X.; Kasahara, Y.; Tokiwa, Y.; Yamakawa, Y.; Kontani, H.; Shibauchi, T.; Matsuda, Y. Abrupt Change of the Superconducting Gap Structure at the Nematic Critical Point in FeSe1-xSx. Proceedings of the National Academy of Sciences 2018, 115, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Wiecki, P.; Rana, K.; Böhmer, A.E.; Lee, Y.; Bud’ko, S.L.; Canfield, P.C.; Furukawa, Y. Persistent Correlation between Superconductivity and Antiferromagnetic Fluctuations near a Nematic Quantum Critical Point in FeSe1-xSx. Phys. Rev. B 2018, 98, 020507. [Google Scholar] [CrossRef]
- Licciardello, S.; Buhot, J.; Lu, J.; Ayres, J.; Kasahara, S.; Matsuda, Y.; Shibauchi, T.; Hussey, N.E. Electrical Resistivity across a Nematic Quantum Critical Point. Nature 2019, 567, 213–217. [Google Scholar] [CrossRef]
- Yi, X.; Xing, X.; Qin, L.; Feng, J.; Li, M.; Zhang, Y.; Meng, Y.; Zhou, N.; Sun, Y.; Shi, Z. Hydrothermal Synthesis and Complete Phase Diagram of FeSe1-xSx (0 ≤ x ≤ 1) Single Crystals. Phys. Rev. B 2021, 103, 144501. [Google Scholar] [CrossRef]
- Lai, X.; Zhang, H.; Wang, Y.; Wang, X.; Zhang, X.; Lin, J.; Huang, F. Observation of Superconductivity in Tetragonal FeS. J. Am. Chem. Soc. 2015, 137, 10148–10151. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Mizukami, Y.; Arai, Y.; Sugimura, Y.; Maejima, N.; Machida, A.; Watanuki, T.; Fukuda, T.; Yajima, T.; Hiroi, Z.; et al. Maximizing T c by Tuning Nematicity and Magnetism in FeSe1-xSx Superconductors. Nat Commun 2017, 8, 1143. [Google Scholar] [CrossRef] [PubMed]
- Terao, K.; Kashiwagi, T.; Shizu, T.; Klemm, R.A.; Kadowaki, K. Superconducting and Tetragonal-to-Orthorhombic Transitions in Single Crystals of FeSe1-xTex (0≤x≤0.61). Phys. Rev. B 2019, 100, 224516. [Google Scholar] [CrossRef]
- Mukasa, K.; Matsuura, K.; Qiu, M.; Saito, M.; Sugimura, Y.; Ishida, K.; Otani, M.; Onishi, Y.; Mizukami, Y.; Hashimoto, K.; et al. High-Pressure Phase Diagrams of FeSe1−xTex: Correlation between Suppressed Nematicity and Enhanced Superconductivity. Nat Commun 2021, 12, 1–7. [Google Scholar] [CrossRef]
- Xing, X.; Sun, Y.; Yi, X.; Li, M.; Feng, J.; Meng, Y.; Zhang, Y.; Li, W.; Zhou, N.; He, X.; et al. Electronic Transport Properties and Hydrostatic Pressure Effect of FeSe0.67Te0.33 Single Crystals Free of Phase Separation. Supercond. Sci. Technol. 2021, 34, 055006. [Google Scholar] [CrossRef]
- Sun, Y.; Yamada, T.; Pyon, S.; Tamegai, T. Influence of Interstitial Fe to the Phase Diagram of Fe1+yTe1-xSex Single Crystals. Sci Rep 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.J.; Hu, J.; Qian, B.; Fobes, D.; Mao, Z.Q.; Bao, W.; Reehuis, M.; Kimber, S. a. J.; Prokeš, K.; Matas, S.; et al. From (π,0) Magnetic Order to Superconductivity with (π,π) Magnetic Resonance in Fe1.02Te1-xSex. Nature Mater 2010, 9, 718–720. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shi, Z.; Tamegai, T. Review of Annealing Effects and Superconductivity in Fe1+yTe1-xSex Superconductors. Supercond. Sci. Technol. 2019, 32, 103001. [Google Scholar] [CrossRef]
- Bao, W.; Qiu, Y.; Huang, Q.; Green, M.A.; Zajdel, P.; Fitzsimmons, M.R.; Zhernenkov, M.; Chang, S.; Fang, M.; Qian, B.; et al. Tunable (Δπ, Δπ,)-Type Antiferromagnetic Order in α-Fe(Te,Se) Superconductors. Phys. Rev. Lett. 2009, 102, 247001. [Google Scholar] [CrossRef]
- Li, S.; de la Cruz, C.; Huang, Q.; Chen, Y.; Lynn, J.W.; Hu, J.; Huang, Y.-L.; Hsu, F.-C.; Yeh, K.-W.; Wu, M.-K.; et al. First-Order Magnetic and Structural Phase Transitions in Fe1+ySexTe1-x. Phys. Rev. B 2009, 79, 054503. [Google Scholar] [CrossRef]
- Lee, P.A.; Nagaosa, N.; Wen, X.-G. Doping a Mott Insulator: Physics of High-Temperature Superconductivity. Rev. Mod. Phys. 2006, 78, 17–85. [Google Scholar] [CrossRef]
- Liu, Z.K.; He, R.-H.; Lu, D.H.; Yi, M.; Chen, Y.L.; Hashimoto, M.; Moore, R.G.; Mo, S.-K.; Nowadnick, E.A.; Hu, J.; et al. Measurement of Coherent Polarons in the Strongly Coupled Antiferromagnetically Ordered Iron-Chalcogenide Fe1.02Te Using Angle-Resolved Photoemission Spectroscopy. Phys. Rev. Lett. 2013, 110, 037003. [Google Scholar] [CrossRef]
- Fobes, D.; Zaliznyak, I.A.; Xu, Z.; Zhong, R.; Gu, G.; Tranquada, J.M.; Harriger, L.; Singh, D.; Garlea, V.O.; Lumsden, M.; et al. Ferro-Orbital Ordering Transition in Iron Telluride Fe1+yTe. Phys. Rev. Lett. 2014, 112, 187202. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Tomioka, F.; Tsuda, S.; Yamaguchi, T.; Takano, Y. Substitution Effects on FeSe Superconductor. J. Phys. Soc. Jpn. 2009, 78, 074712. [Google Scholar] [CrossRef]
- McQueen, T.M.; Huang, Q.; Ksenofontov, V.; Felser, C.; Xu, Q.; Zandbergen, H.; Hor, Y.S.; Allred, J.; Williams, A.J.; Qu, D.; et al. Extreme Sensitivity of Superconductivity to Stoichiometry in Fe,1+δSe. Phys. Rev. B 2009, 79, 014522. [Google Scholar] [CrossRef]
- Wen, J.; Xu, G.; Gu, G.; Tranquada, J.M.; Birgeneau, R.J. Interplay between Magnetism and Superconductivity in Iron-Chalcogenide Superconductors: Crystal Growth and Characterizations. Rep. Prog. Phys. 2011, 74, 124503. [Google Scholar] [CrossRef]
- Fang, M.H.; Pham, H.M.; Qian, B.; Liu, T.J.; Vehstedt, E.K.; Liu, Y.; Spinu, L.; Mao, Z.Q. Superconductivity Close to Magnetic Instability in Fe(Se1−xTex)0.82. Phys. Rev. B 2008, 78, 224503. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Takano, Y. Review of Fe Chalcogenides as the Simplest Fe-Based Superconductor. J. Phys. Soc. Jpn. 2010, 79, 102001. [Google Scholar] [CrossRef]
- Liu, T.J.; Ke, X.; Qian, B.; Hu, J.; Fobes, D.; Vehstedt, E.K.; Pham, H.; Yang, J.H.; Fang, M.H.; Spinu, L.; et al. Charge-Carrier Localization Induced by Excess Fe in the Superconductor Fe1+yTe1-xSex. Phys. Rev. B 2009, 80, 174509. [Google Scholar] [CrossRef]
- Ieki, E.; Nakayama, K.; Miyata, Y.; Sato, T.; Miao, H.; Xu, N.; Wang, X.-P.; Zhang, P.; Qian, T.; Richard, P.; et al. Evolution from Incoherent to Coherent Electronic States and Its Implications for Superconductivity in FeTe1-xSex. Phys. Rev. B 2014, 89, 140506. [Google Scholar] [CrossRef]
- Sun, Y.; Taen, T.; Yamada, T.; Pyon, S.; Nishizaki, T.; Shi, Z.; Tamegai, T. Multiband Effects and Possible Dirac Fermions in Fe1+yTe0.6Se0.4. Phys. Rev. B 2014, 89, 144512. [Google Scholar] [CrossRef]
- Katayama, N.; Ji, S.; Louca, D.; Lee, S.; Fujita, M.; J. Sato, T.; Wen, J.; Xu, Z.; Gu, G.; Xu, G.; et al. Investigation of the Spin-Glass Regime between the Antiferromagnetic and Superconducting Phases in Fe1+ySexTe1-x. J. Phys. Soc. Jpn. 2010, 79, 113702. [Google Scholar] [CrossRef]
- Otsuka, T.; Hagisawa, S.; Koshika, Y.; Adachi, S.; Usui, T.; Sasaki, N.; Sasaki, S.; Yamaguchi, S.; Nakanishi, Y.; Yoshizawa, M.; et al. Incoherent-Coherent Crossover and the Pseudogap in Te-Annealed Superconducting Fe1+yTe1-xSex Revealed by Magnetotransport Measurements. Phys. Rev. B 2019, 99, 184505. [Google Scholar] [CrossRef]
- Zhang, S.B.; Sun, Y.P.; Zhu, X.D.; Zhu, X.B.; Wang, B.S.; Li, G.; Lei, H.C.; Luo, X.; Yang, Z.R.; Song, W.H.; et al. Crystal Growth and Superconductivity of FeSex. Supercond. Sci. Technol. 2008, 22, 015020. [Google Scholar] [CrossRef]
- Mok, B.H.; Rao, S.M.; Ling, M.C.; Wang, K.J.; Ke, C.T.; Wu, P.M.; Chen, C.L.; Hsu, F.C.; Huang, T.W.; Luo, J.Y.; et al. Growth and Investigation of Crystals of the New Superconductor α-FeSe from KCl Solutions. Crystal Growth & Design 2009, 9, 3260–3264. [Google Scholar]
- Patel, U.; Hua, J.; Yu, S.H.; Avci, S.; Xiao, Z.L.; Claus, H.; Schlueter, J.; Vlasko-Vlasov, V.V.; Welp, U.; Kwok, W.K. Growth and Superconductivity of FeSex Crystals. Applied Physics Letters 2009, 94, 082508. [Google Scholar] [CrossRef]
- Tissen, V.G.; Ponyatovsky, E.G.; Nefedova, M.V.; Titov, A.N.; Fedorenko, V.V. Effects of Pressure-Induced Phase Transitions on Superconductivity in Single-Crystal Fe1.02Se. Phys. Rev. B 2009, 80, 092507. [Google Scholar] [CrossRef]
- Wu, M.K.; Hsu, F.C.; Yeh, K.W.; Huang, T.W.; Luo, J.Y.; Wang, M.J.; Chang, H.H.; Chen, T.K.; Rao, S.M.; Mok, B.H.; et al. The Development of the Superconducting PbO-Type β-FeSe and Related Compounds. Physica C: Superconductivity 2009, 469, 340–349. [Google Scholar] [CrossRef]
- Malavasi, L.; Margadonna, S. Structure–Properties Correlations in Fe Chalcogenide Superconductors. Chem. Soc. Rev. 2012, 41, 3897–3911. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Lei, H.; Abeykoon, M.; Bozin, E.S.; Billinge, S.J.L.; Warren, J.B.; Siegrist, T.; Petrovic, C. Synthesis, Crystal Structure, and Magnetism of β-Fe1.00(2)Se1.00(3) Single Crystals. Phys. Rev. B 2011, 83, 224502. [Google Scholar] [CrossRef]
- Chareev, D.; Osadchii, E.; Kuzmicheva, T.; Lin, J.-Y.; Kuzmichev, S.; Volkova, O.; Vasiliev, A. Single Crystal Growth and Characterization of Tetragonal FeSe1−x Superconductors. CrystEngComm 2013, 15, 1989–1993. [Google Scholar] [CrossRef]
- Wu, M.K.; Wu, P.M.; Wen, Y.C.; Wang, M.J.; Lin, P.H.; Lee, W.C.; Chen, T.K.; Chang, C.C. An Overview of the Fe-Chalcogenide Superconductors. J. Phys. D: Appl. Phys. 2015, 48, 323001. [Google Scholar] [CrossRef]
- Yu, R.; Zhu, J.-X.; Si, Q. Orbital Selectivity Enhanced by Nematic Order in FeSe. Phys. Rev. Lett. 2018, 121, 227003. [Google Scholar] [CrossRef]
- Massat, P.; Farina, D.; Paul, I.; Karlsson, S.; Strobel, P.; Toulemonde, P.; Méasson, M.-A.; Cazayous, M.; Sacuto, A.; Kasahara, S.; et al. Charge-Induced Nematicity in FeSe. Proceedings of the National Academy of Sciences 2016, 113, 9177–9181. [Google Scholar] [CrossRef]
- Farrar, L.S.; Zajicek, Z.; Morfoot, A.B.; Bristow, M.; Humphries, O.S.; Haghighirad, A.A.; McCollam, A.; Bending, S.J.; Coldea, A.I. Unconventional Localization of Electrons inside of a Nematic Electronic Phase. Proceedings of the National Academy of Sciences 2022, 119, e2200405119. [Google Scholar] [CrossRef]
- Sun, Y.; Kittaka, S.; Nakamura, S.; Sakakibara, T.; Irie, K.; Nomoto, T.; Machida, K.; Chen, J.; Tamegai, T. Gap Structure of FeSe Determined by Angle-Resolved Specific Heat Measurements in Applied Rotating Magnetic Field. Phys. Rev. B 2017, 96, 220505. [Google Scholar] [CrossRef]
- Sun, Y.; Pyon, S.; Tamegai, T.; Kobayashi, R.; Watashige, T.; Kasahara, S.; Matsuda, Y.; Shibauchi, T.; Kitamura, H. Enhancement of Critical Current Density and Mechanism of Vortex Pinning in H+-Irradiated FeSe Single Crystal. Appl. Phys. Express 2015, 8, 113102. [Google Scholar] [CrossRef]
- Sun, Y.; Pyon, S.; Tamegai, T.; Kobayashi, R.; Watashige, T.; Kasahara, S.; Matsuda, Y.; Shibauchi, T. Critical Current Density, Vortex Dynamics, and Phase Diagram of Single-Crystal FeSe. Phys. Rev. B 2015, 92, 144509. [Google Scholar] [CrossRef]
- Okamoto, H. The Fe-Se (Iron-Selenium) System. JPE 1991, 12, 383–389. [Google Scholar] [CrossRef]
- Böhmer, A.E.; Taufour, V.; Straszheim, W.E.; Wolf, T.; Canfield, P.C. Variation of Transition Temperatures and Residual Resistivity Ratio in Vapor-Grown FeSe. Phys. Rev. B 2016, 94, 024526. [Google Scholar] [CrossRef]
- Watson, M.D.; Kim, T.K.; Haghighirad, A.A.; Blake, S.F.; Davies, N.R.; Hoesch, M.; Wolf, T.; Coldea, A.I. Suppression of Orbital Ordering by Chemical Pressure in FeSe1-xSx. Phys. Rev. B 2015, 92, 121108. [Google Scholar] [CrossRef]
- Hosoi, S.; Matsuura, K.; Ishida, K.; Wang, H.; Mizukami, Y.; Watashige, T.; Kasahara, S.; Matsuda, Y.; Shibauchi, T. Nematic Quantum Critical Point without Magnetism in FeSe1-xSx Superconductors. Proceedings of the National Academy of Sciences 2016, 113, 8139–8143. [Google Scholar] [CrossRef] [PubMed]
- Bristow, M.; Reiss, P.; Haghighirad, A.A.; Zajicek, Z.; Singh, S.J.; Wolf, T.; Graf, D.; Knafo, W.; McCollam, A.; Coldea, A.I. Anomalous High-Magnetic Field Electronic State of the Nematic Superconductors FeSe1-xSx. Phys. Rev. Res. 2020, 2, 013309. [Google Scholar] [CrossRef]
- Sun, Y.; Pyon, S.; Tamegai, T. Electron Carriers with Possible Dirac-Cone-like Dispersion in FeSe1-xSx (x = 0 and 0.14) Single Crystals Triggered by Structural Transition. Phys. Rev. B 2016, 93, 104502. [Google Scholar] [CrossRef]
- Licciardello, S.; Maksimovic, N.; Ayres, J.; Buhot, J.; Čulo, M.; Bryant, B.; Kasahara, S.; Matsuda, Y.; Shibauchi, T.; Nagarajan, V.; et al. Coexistence of Orbital and Quantum Critical Magnetoresistance in FeSe1-xSx. Phys. Rev. Res. 2019, 1, 023011. [Google Scholar] [CrossRef]
- Lin, H.; Li, Y.; Deng, Q.; Xing, J.; Liu, J.; Zhu, X.; Yang, H.; Wen, H.-H. Multiband Superconductivity and Large Anisotropy in FeS Crystals. Phys. Rev. B 2016, 93, 144505. [Google Scholar] [CrossRef]
- Ying, T.P.; Lai, X.F.; Hong, X.C.; Xu, Y.; He, L.P.; Zhang, J.; Wang, M.X.; Yu, Y.J.; Huang, F.Q.; Li, S.Y. Nodal Superconductivity in FeS: Evidence from Quasiparticle Heat Transport. Phys. Rev. B 2016, 94, 100504. [Google Scholar] [CrossRef]
- Borg, C.K.H.; Zhou, X.; Eckberg, C.; Campbell, D.J.; Saha, S.R.; Paglione, J.; Rodriguez, E.E. Strong Anisotropy in Nearly Ideal Tetrahedral Superconducting FeS Single Crystals. Phys. Rev. B 2016, 93, 094522. [Google Scholar] [CrossRef]
- Guo, Z.; Sun, F.; Chen, Y.; Mao, Y.; Wan, L.; Yan, X.; Yang, Y.; Yuan, W. Synthesis, Structure and Superconductivity of FeSe1-xSx (0 ≤ x ≤ 1) Solid Solution Crystals. CrystEngComm 2019, 21, 2994–2999. [Google Scholar] [CrossRef]
- Yuan, D.; Huang, Y.; Ni, S.; Zhou, H.; Mao, Y.; Hu, W.; Yuan, J.; Jin, K.; Zhang, G.; Dong, X.; et al. Synthesis of Large FeSe Superconductor Crystals via Ion Release/Introduction and Property Characterization*. Chinese Phys. B 2016, 25, 077404. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, A.; Ivanovski, V.N.; Du, Q.; Koteski, V.; Petrovic, C. Thermoelectricity and Electronic Correlation Enhancement in FeS by Light Se Doping. Phys. Rev. B 2022, 105, 045133. [Google Scholar] [CrossRef]
- Pachmayr, U.; Fehn, N.; Johrendt, D. Structural Transition and Superconductivity in Hydrothermally Synthesized FeX (X = S, Se). Chem. Commun. 2015, 52, 194–197. [Google Scholar] [CrossRef]
- Noji, T.; Suzuki, T.; Abe, H.; Adachi, T.; Kato, M.; Koike, Y. Growth, Annealing Effects on Superconducting and Magnetic Properties, and Anisotropy of FeSe1-xTex (0.5≤x≤1) Single Crystals. J. Phys. Soc. Jpn. 2010, 79, 084711. [Google Scholar] [CrossRef]
- Okazaki, K.; Ito, Y.; Ota, Y.; Kotani, Y.; Shimojima, T.; Kiss, T.; Watanabe, S.; Chen, C.-T.; Niitaka, S.; Hanaguri, T.; et al. Evidence for a Cos(4φ) Modulation of the Superconducting Energy Gap of Optimally Doped FeTe0.6Se0.4 Single Crystals Using Laser Angle-Resolved Photoemission Spectroscopy. Phys. Rev. Lett. 2012, 109, 237011. [Google Scholar] [CrossRef]
- Sales, B.C.; Sefat, A.S.; McGuire, M.A.; Jin, R.Y.; Mandrus, D.; Mozharivskyj, Y. Bulk Superconductivity at 14 K in Single Crystals of Fe1+yTexSe1-x. Phys. Rev. B 2009, 79, 094521. [Google Scholar] [CrossRef]
- Yeh, K.W.; Ke, C.T.; Huang, T.W.; Chen, T.K.; Huang, Y.L.; Wu, P.M.; Wu, M.K. Superconducting FeSe1−xTex Single Crystals Grown by Optical Zone-Melting Technique. Crystal Growth & Design 2009, 9, 4847–4851. [Google Scholar]
- Sun, Y.; Taen, T.; Yamada, T.; Tsuchiya, Y.; Pyon, S.; Tamegai, T. Evolution of Superconducting and Transport Properties in Annealed FeTe1−xSex (0.1 ≤ x ≤ 0.4) Multiband Superconductors. Supercond. Sci. Technol. 2015, 28, 044002. [Google Scholar] [CrossRef]
- Taen, T.; Tsuchiya, Y.; Nakajima, Y.; Tamegai, T. Superconductivity at Tc~14 K in Single-Crystalline FeTe0.61Se0.39. Phys. Rev. B 2009, 80, 092502. [Google Scholar] [CrossRef]
- Komiya, S.; Hanawa, M.; Tsukada, I.; Maeda, A. Effect of Vacuum Annealing on Superconductivity in Fe(Se,Te) Single Crystals. J. Phys. Soc. Jpn. 2013, 82, 064710. [Google Scholar] [CrossRef]
- Hu, J.; Wang, G.C.; Qian, B.; Mao, Z.Q. Inhomogeneous Superconductivity Induced by Interstitial Fe Deintercalation in Oxidizing-Agent-Annealed and HNO3-Treated Fe1+y(Te1-xSex). Supercond. Sci. Technol. 2012, 25, 084011. [Google Scholar] [CrossRef]
- Sun, Y.; Taen, T.; Tsuchiya, Y.; Shi, Z.X.; Tamegai, T. Effects of Annealing, Acid and Alcoholic Beverages on Fe1+yTe0.6Se0.4. Supercond. Sci. Technol. 2012, 26, 015015. [Google Scholar] [CrossRef]
- Sun, Y.; Tsuchiya, Y.; Taen, T.; Yamada, T.; Pyon, S.; Sugimoto, A.; Ekino, T.; Shi, Z.; Tamegai, T. Dynamics and Mechanism of Oxygen Annealing in Fe1+yTe0.6Se0.4 Single Crystal. Sci Rep 2014, 4, 4585. [Google Scholar] [CrossRef]
- Sun, Y.; Taen, T.; Tsuchiya, Y.; Ding, Q.; Pyon, S.; Shi, Z.; Tamegai, T. Large, Homogeneous, and Isotropic Critical Current Density in Oxygen-Annealed Fe1+yTe0.6Se0.4 Single Crystal. Appl. Phys. Express 2013, 6, 043101. [Google Scholar] [CrossRef]
- Sun, Y.; Tsuchiya, Y.; Yamada, T.; Taen, T.; Pyon, S.; Shi, Z.; Tamegai, T. Evolution of Superconductivity in Fe1+yTe1-xSex Annealed in Te Vapor. J. Phys. Soc. Jpn. 2013, 82, 093705. [Google Scholar] [CrossRef]
- Rodriguez, E.E.; Stock, C.; Hsieh, P.-Y.; Butch, N.P.; Paglione, J.; Green, M.A. Chemical Control of Interstitial Iron Leading to Superconductivity in Fe1+xTe0.7Se0.3. Chem. Sci. 2011, 2, 1782–1787. [Google Scholar] [CrossRef]
- Koshika, Y.; Usui, T.; Adachi, S.; Watanabe, T.; Sakano, K.; Simayi, S.; Yoshizawa, M. Effects of Annealing under Tellurium Vapor for Fe1.03Te0.8Se0.2 Single Crystals. J. Phys. Soc. Jpn. 2013, 82, 023703. [Google Scholar] [CrossRef]
- Sun, Y.; Tsuchiya, Y.; Yamada, T.; Taen, T.; Pyon, S.; Shi, Z.; Tamegai, T. Bulk Superconductivity in Fe1+yTe1-xSex Induced by Annealing in Se and S Vapor. J. Phys. Soc. Jpn. 2013, 82, 115002. [Google Scholar] [CrossRef]
- Zhou, W.; Sun, Y.; Zhang, S.; Zhuang, J.; Yuan, F.; Li, X.; Shi, Z.; Yamada, T.; Tsuchiya, Y.; Tamegai, T. Bulk Superconductivity in Fe1+yTe0.6Se0.4 Induced by Removal of Excess Fe. J. Phys. Soc. Jpn. 2014, 83, 064704. [Google Scholar] [CrossRef]
- Yamada, T.; Sun, Y.; Pyon, S.; Tamegai, T. Effects of Pnictogen Atmosphere Annealing on Fe1+yTe0.6Se0.4. J. Phys. Soc. Jpn. 2016, 85, 024712. [Google Scholar] [CrossRef]
- Chen, J.; Sun, Y.; Yamada, T.; Pyon, S.; Tamegai, T. Effects of Iodine Annealing on Fe1+yTe0.6Se0.4. J. Phys. Soc. Jpn. 2016, 85, 104714. [Google Scholar] [CrossRef]
- Ge, J.; Cao, S.; Shen, S.; Yuan, S.; Kang, B.; Zhang, J. Superconducting Properties of Highly Oriented Fe1.03Te0.55Se0.45 with Excess Fe. Solid State Communications 2010, 150, 1641–1645. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Tomioka, F.; Tsuda, S.; Yamaguchi, T.; Takano, Y. Superconductivity in S-Substituted FeTe. Applied Physics Letters 2009, 94, 012503. [Google Scholar] [CrossRef]
- Hu, R.; Bozin, E.S.; Warren, J.B.; Petrovic, C. Superconductivity, Magnetism, and Stoichiometry of Single Crystals of Fe1+y(Te1-xSx)z. Phys. Rev. B 2009, 80, 214514. [Google Scholar] [CrossRef]
- Lei, H.; Hu, R.; Choi, E.S.; Warren, J.B.; Petrovic, C. Effects of Excess Fe on Upper Critical Field and Magnetotransport in Fe1+y(Te1-xSx)z. Phys. Rev. B 2010, 81, 184522. [Google Scholar] [CrossRef]
- Lei, H.; Hu, R.; Choi, E.S.; Petrovic, C. Thermally Activated Energy and Flux-Flow Hall Effect of Fe1+y(Te1-xSx)z. Phys. Rev. B 2010, 82, 134525. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Deguchi, K.; Tsuda, S.; Yamaguchi, T.; Takano, Y. Moisture-Induced Superconductivity in FeTe0.8S0.2. Phys. Rev. B 2010, 81, 214510. [Google Scholar] [CrossRef]
- Wang, A.; Kampert, E.; Saadaoui, H.; Luetkens, H.; Hu, R.; Morenzoni, E.; Wosnitza, J.; Petrovic, C. Normal State above the Upper Critical Field in Fe1+yTe1-x(Se, S)x. Phys. Rev. B 2017, 95, 184504. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Deguchi, K.; Kawasaki, Y.; Ozaki, T.; Nagao, M.; Tsuda, S.; Yamaguchi, T.; Takano, Y. Superconductivity in Oxygen-Annealed FeTe1-xSx Single Crystal. Journal of Applied Physics 2011, 109, 013914. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Yang, Z.R.; Li, L.; Pi, L.; Tan, S.; Zhang, Y.H. Annealing Effects on Superconductivity and Magnetism in Fe1+yTe1-xSx Single Crystals. Journal of Applied Physics 2012, 111, 07E118. [Google Scholar] [CrossRef]
- Awana, V.P.S.; Pal, A.; Vajpayee, A.; Gahtori, B.; Kishan, H. Superconductivity and Thermal Properties of Sulphur Doped FeTe with Effect of Oxygen Post Annealing. Physica C: Superconductivity 2011, 471, 77–82. [Google Scholar] [CrossRef]
- Dong, C.; Wang, H.; Mao, Q.; Khan, R.; Zhou, X.; Li, C.; Yang, J.; Chen, B.; Fang, M. Phase Diagram and Annealing Effect for Fe1+δTe1-xSx Single Crystals. J. Phys.: Condens. Matter 2013, 25, 385701. [Google Scholar]
- Yamazaki, T.; Sakurai, T.; Yaguchi, H. Size Dependence of Oxygen-Annealing Effects on Superconductivity of Fe1+yTe1-xSx. J. Phys. Soc. Jpn. 2016, 85, 114712. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yamazaki, T.; Yamanaka, T.; Ueta, D.; Yoshizawa, H.; Yaguchi, H. Anisotropic Pressure Effects on Superconductivity in Fe1+yTe1-xSx. J. Phys. Soc. Jpn. 2018, 87, 054705. [Google Scholar] [CrossRef]
- Dong, C.; Wang, H.; Yang, J.; Qian, B.; Chen, J.; Li, Z.; Yuan, H.; Fang, M. Effect of Annealing on Superconductivity in Fe1+y(Te1-xSx) System. Sci. China Phys. Mech. Astron. 2010, 53, 1216–1220. [Google Scholar] [CrossRef]
- Zhao, C.; Yi, X.; Hou, Q.; Feng, J.; Zhang, Y.; Xu, M.; Shi, Z. Hydrothermal Synthesis and Transport Properties of FeS1-xTex (0 ≤ x ≤ 0.15) Single Crystals. J Supercond Nov Magn 2021, 34, 2565–2572. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).