Submitted:
02 June 2023
Posted:
06 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. MBBR-MBR Set-up and Operating Conditions
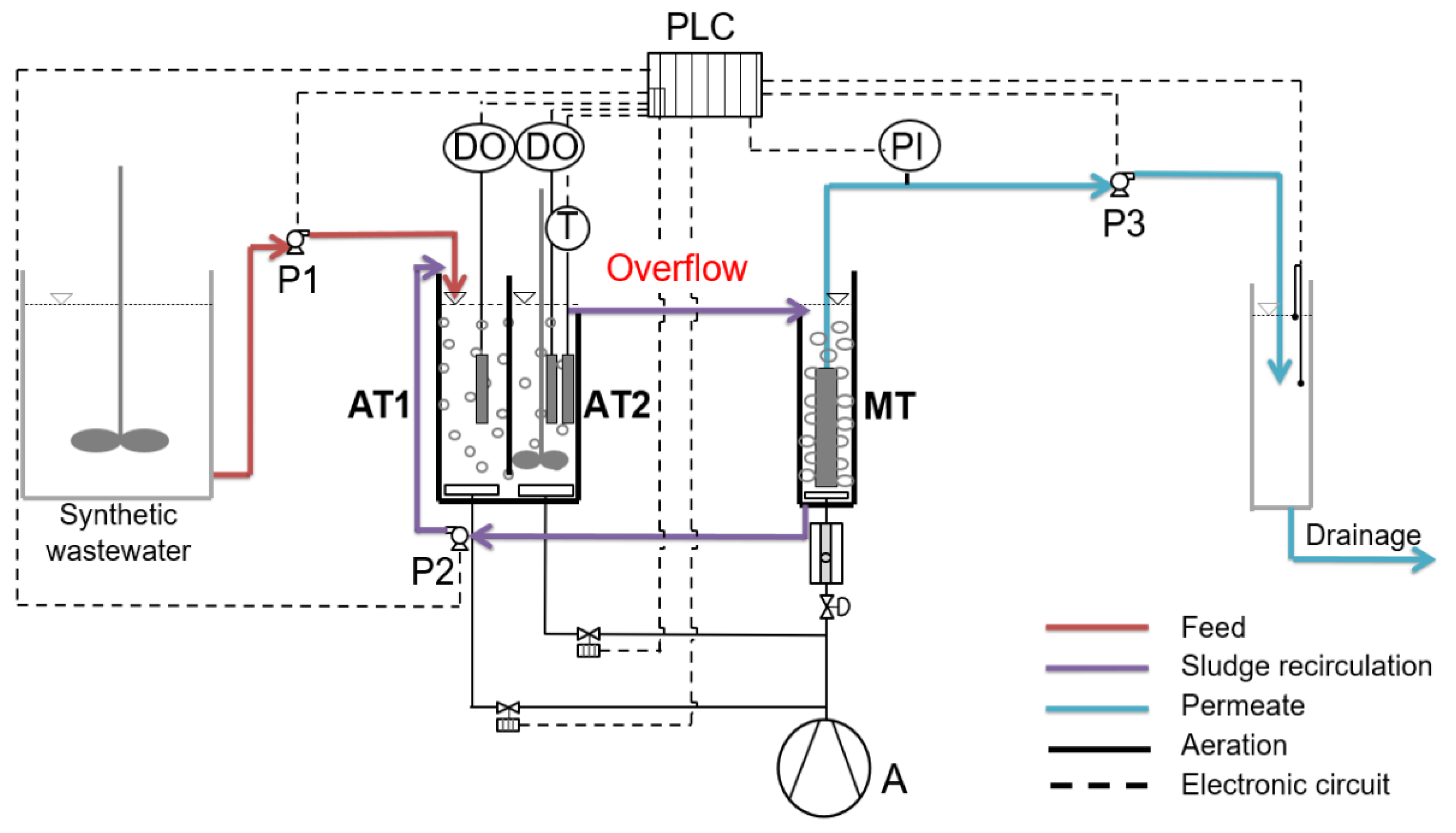
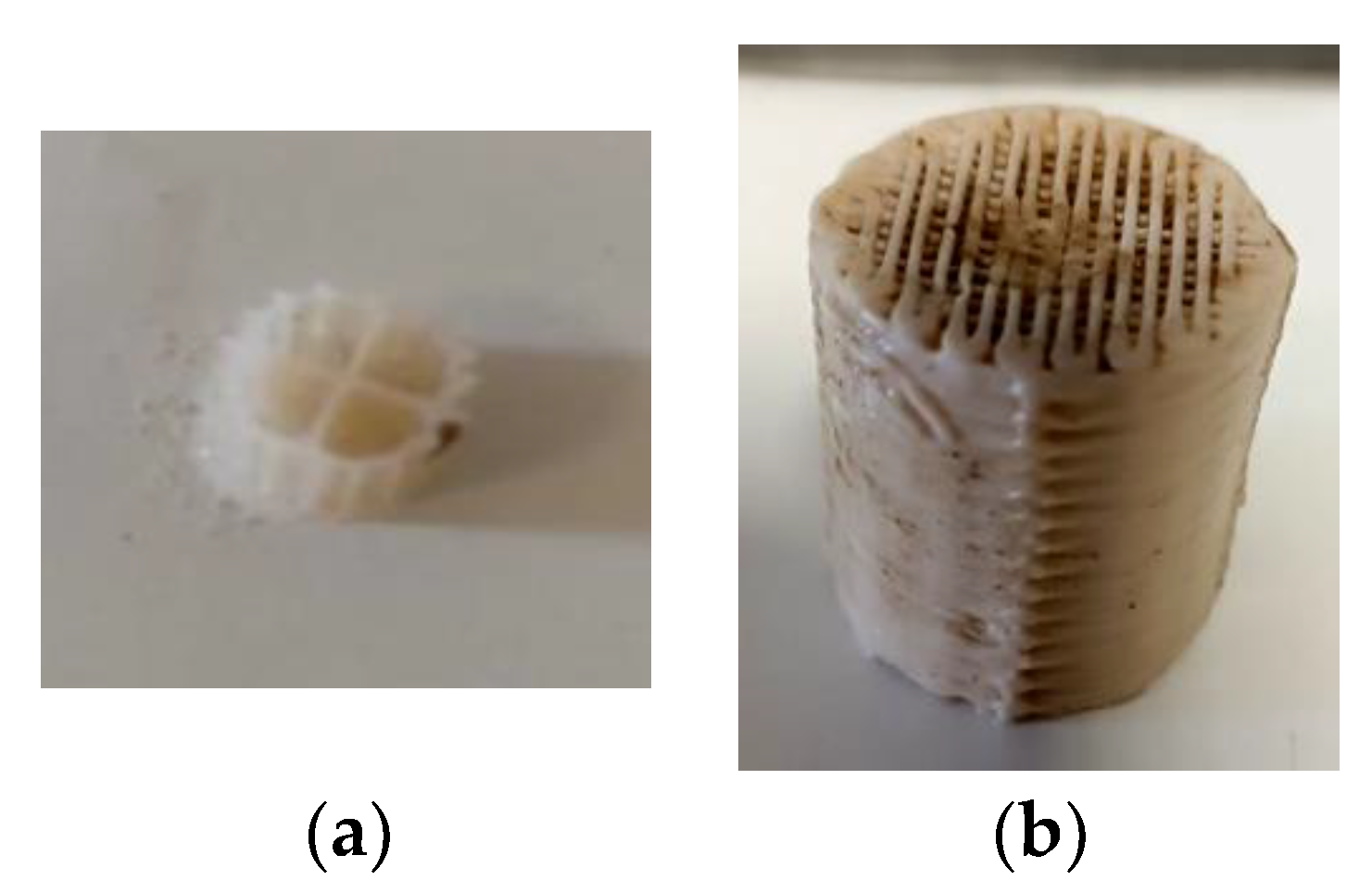
2.2. Biofilm Extraction Method
2.3. Printing Methodology of the 3D-Printed Biocarriers with 13X with Halloysite
| Material | Paste content | Zeolite/clay percentage | |
|---|---|---|---|
| Zeolite | 13X | 50% | 89% |
| Inorganic binder | Halloysite nanotubes | 6% | 11% |
| Colloidal silica | Ludox AS-40 | 16% | |
| Water | 27% | ||
| Organic binder | Methyl cellulose | 1% |
2.4. Determination of the Physicochemical Parameters
2.5. DNA Extraction and 16S rRNA Gene Amplicon Sequencing
2.6. Bioinformatics
3. Results
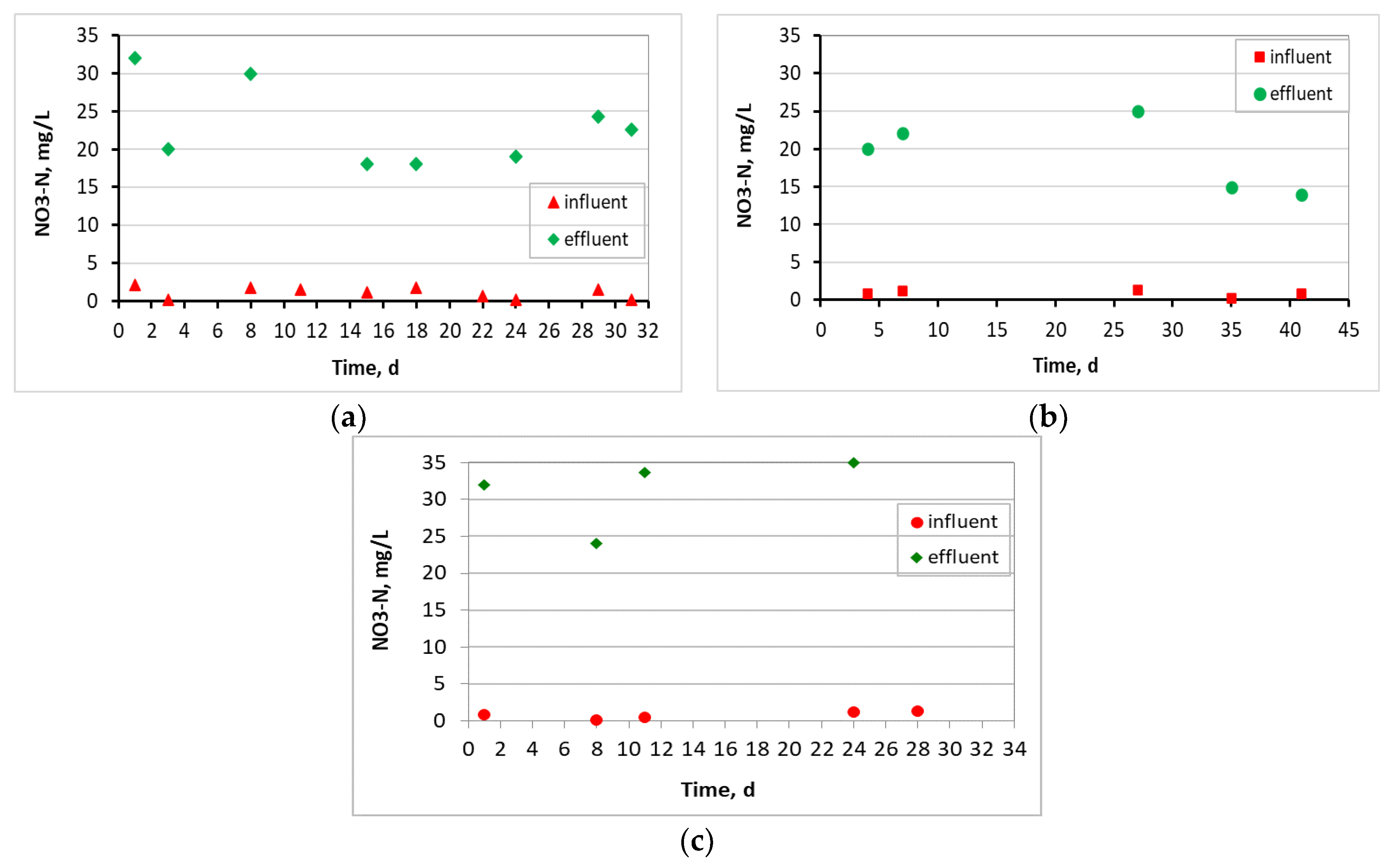
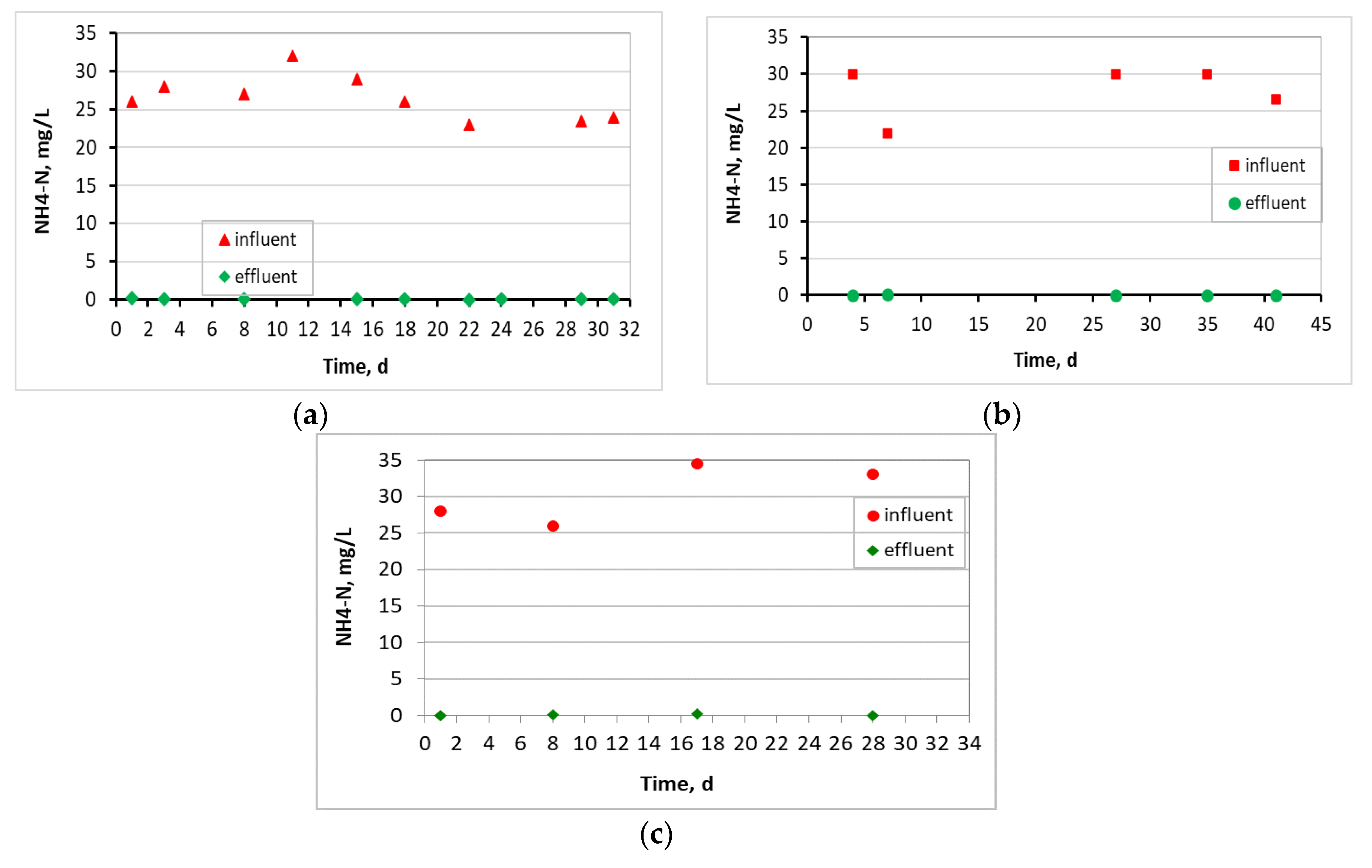
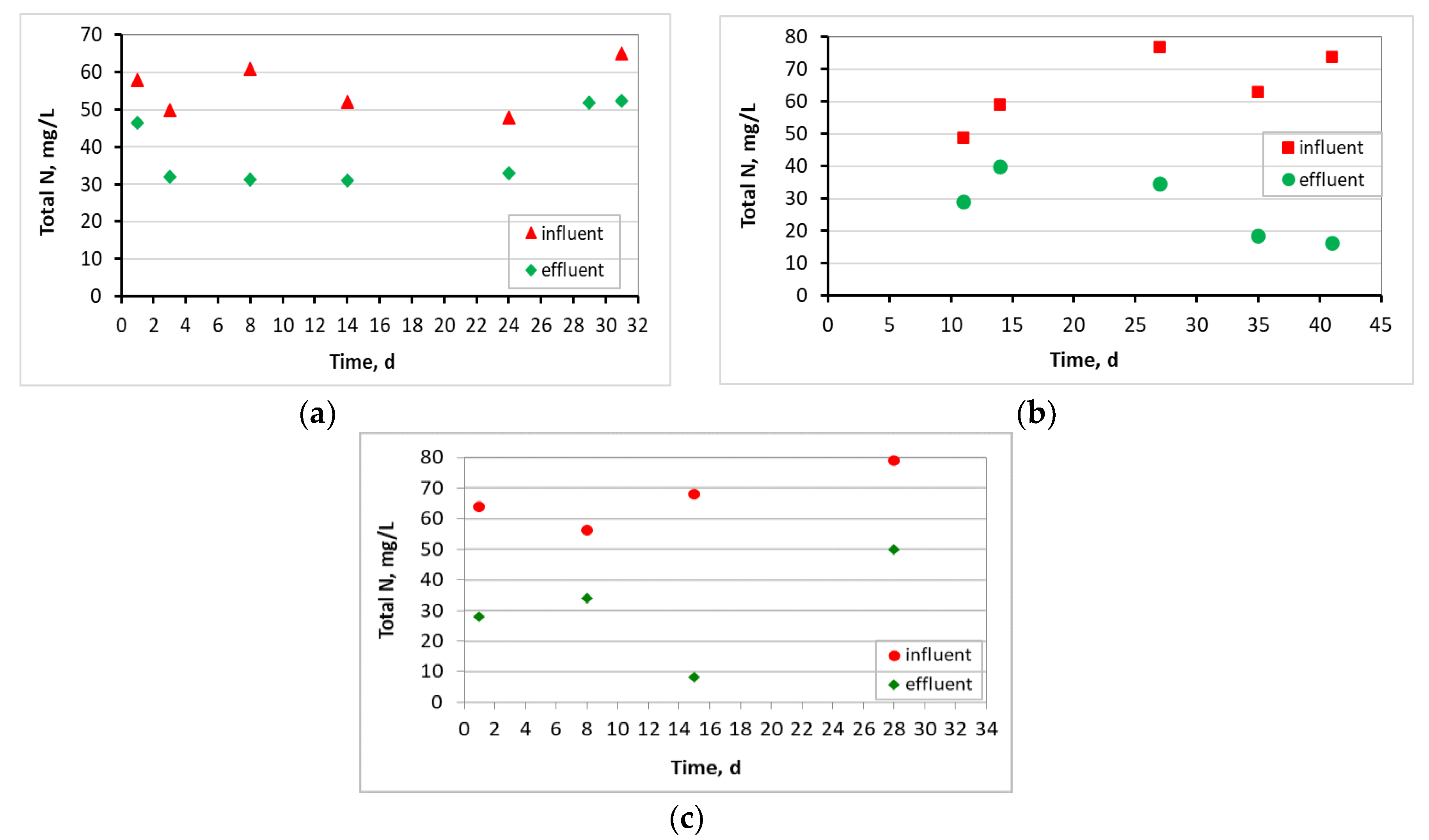
3.1. Evaluation of the MBBR-MBR Performance for the 3 Units
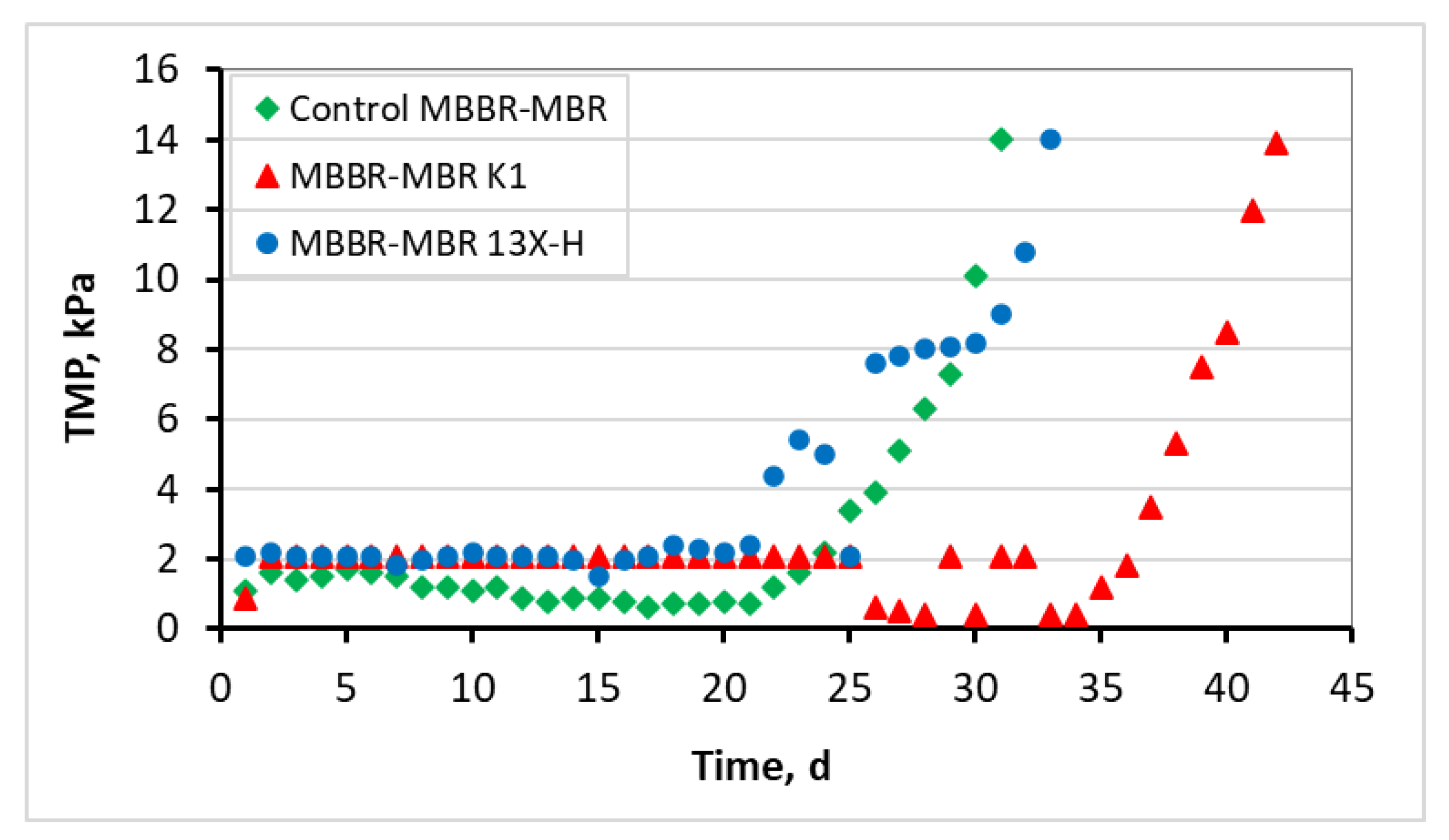
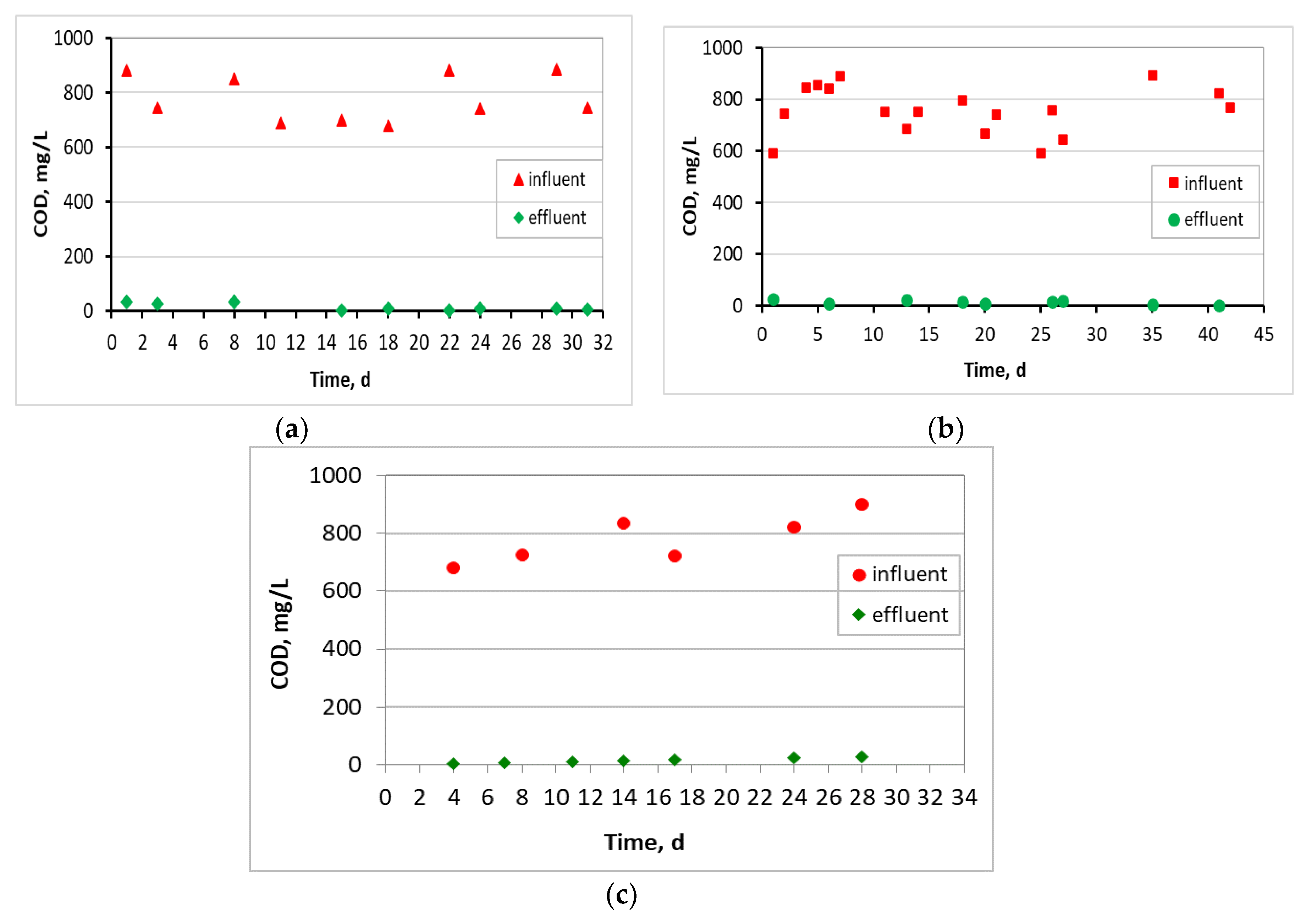
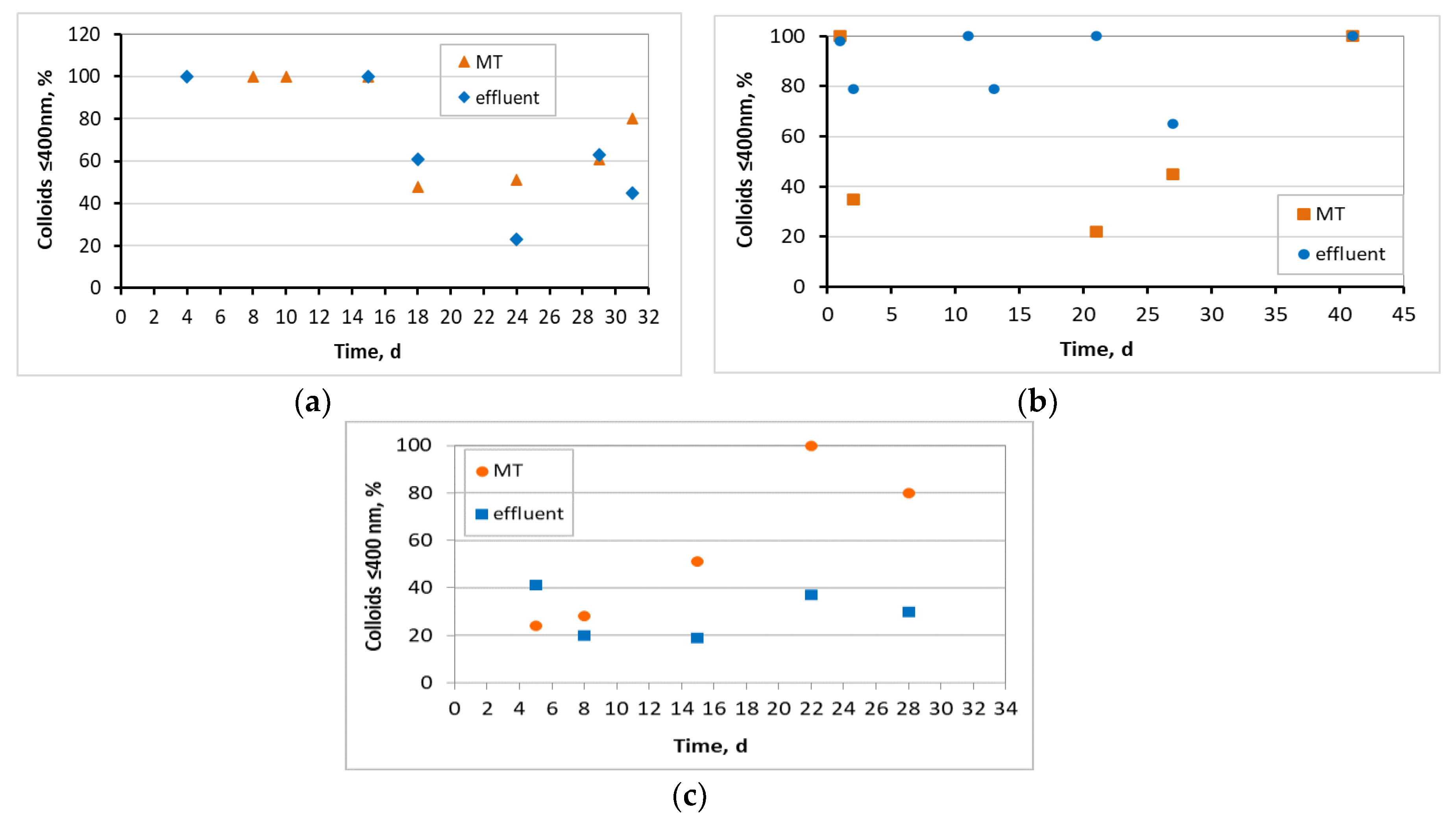
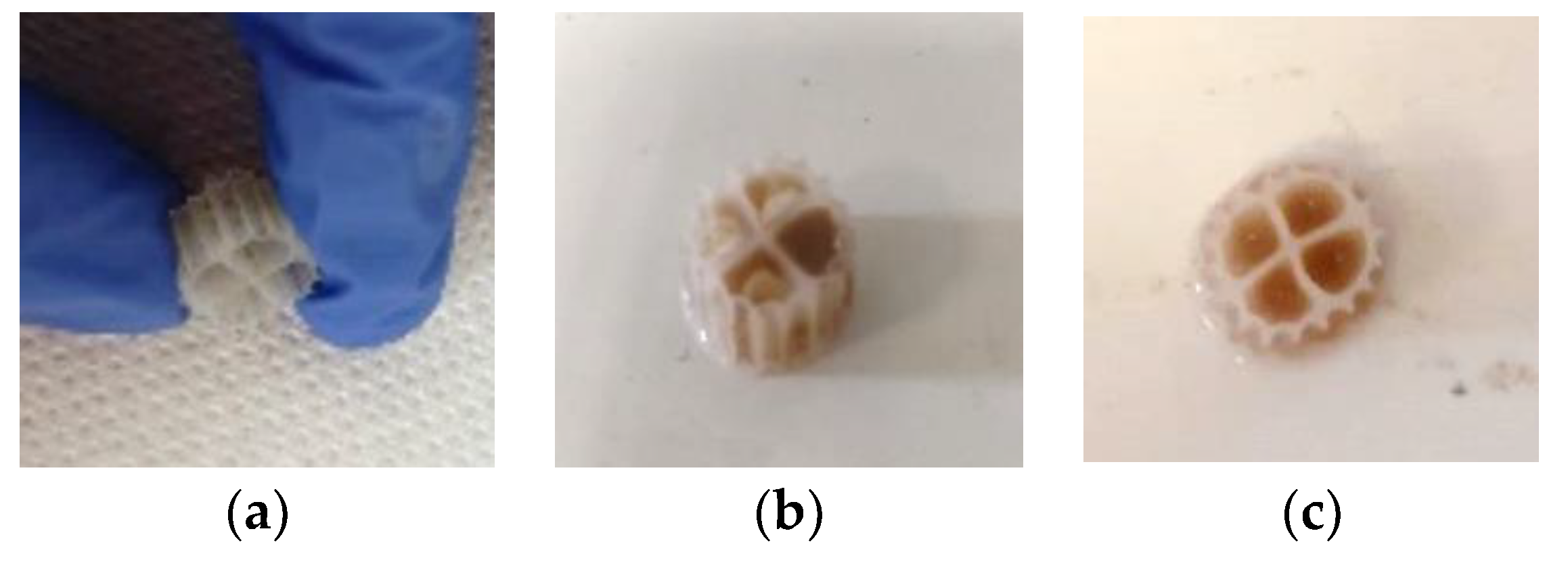
3.2. Biofilm Evaluation for the Kaldnes K1 Biocarriers
| t, d | Dry Mass of Biofilm, mg | MLSS, mg/L |
|---|---|---|
| 6 | 3.2 | 40 |
| 20 | 3.5 | 240 |
| 32 | 4.6 | 360 |
| 41 | 2.9 | 20 |
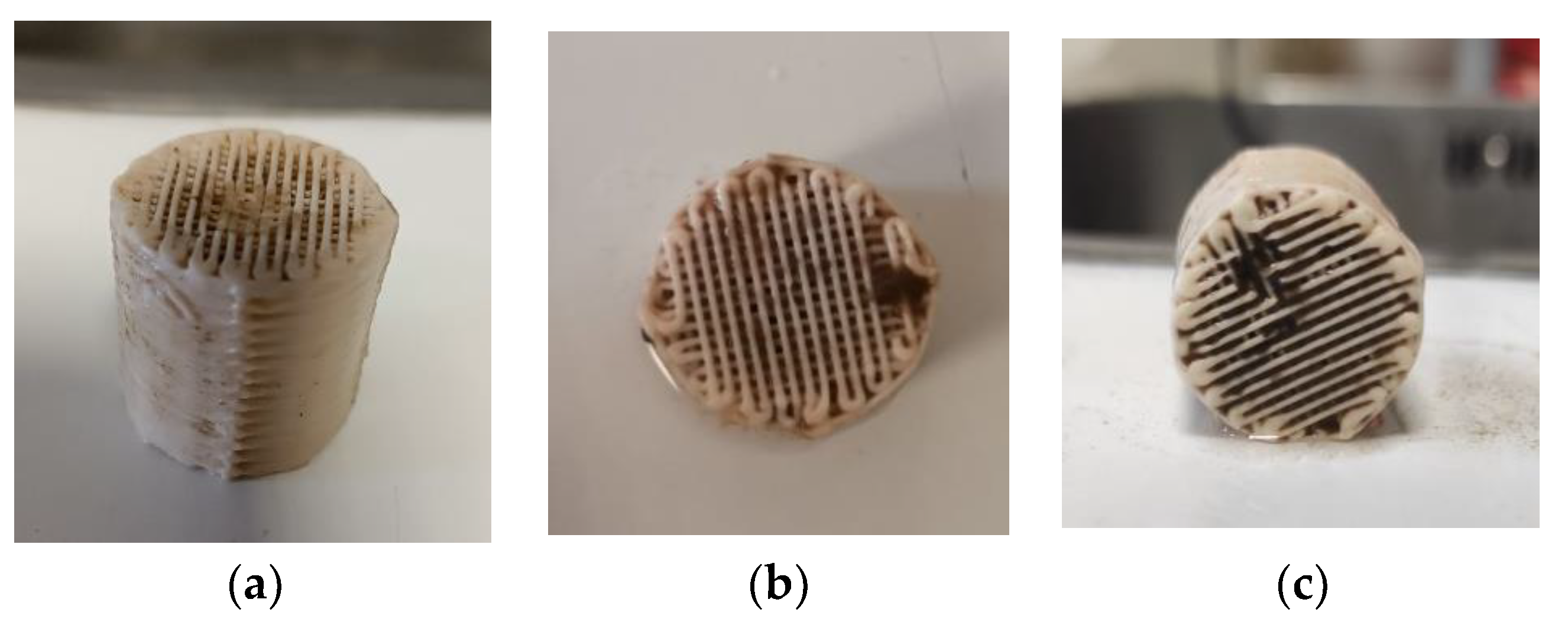
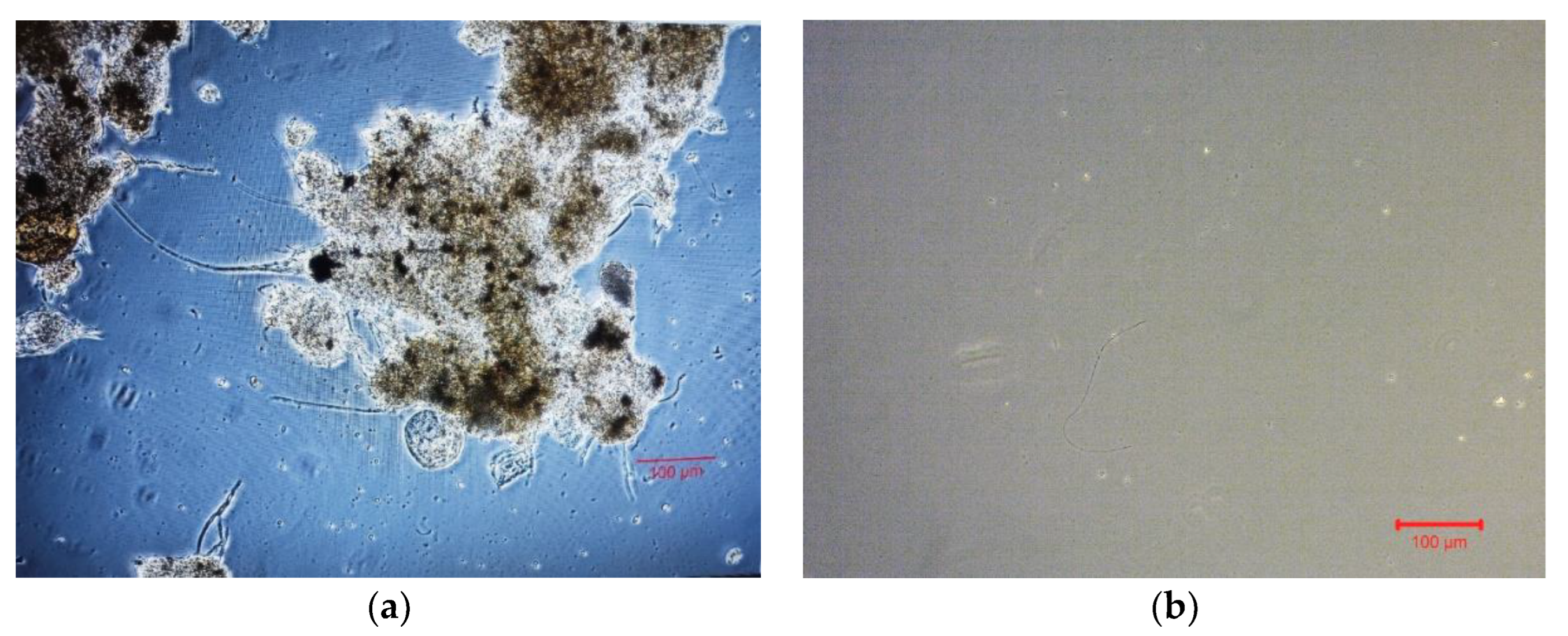
3.3. Biofilm Evaluation for the 3D-Printed 13X and Halloysite Biocarriers
| t, d | Dry mass, mg | MLSS, mg/L |
|---|---|---|
| 11 | 4,980 | 863 |
| 14 | 5,426 | 1,875 |
| 24 | 5,210 | 1,038 |
| 28 | 5,711 | 1,250 |
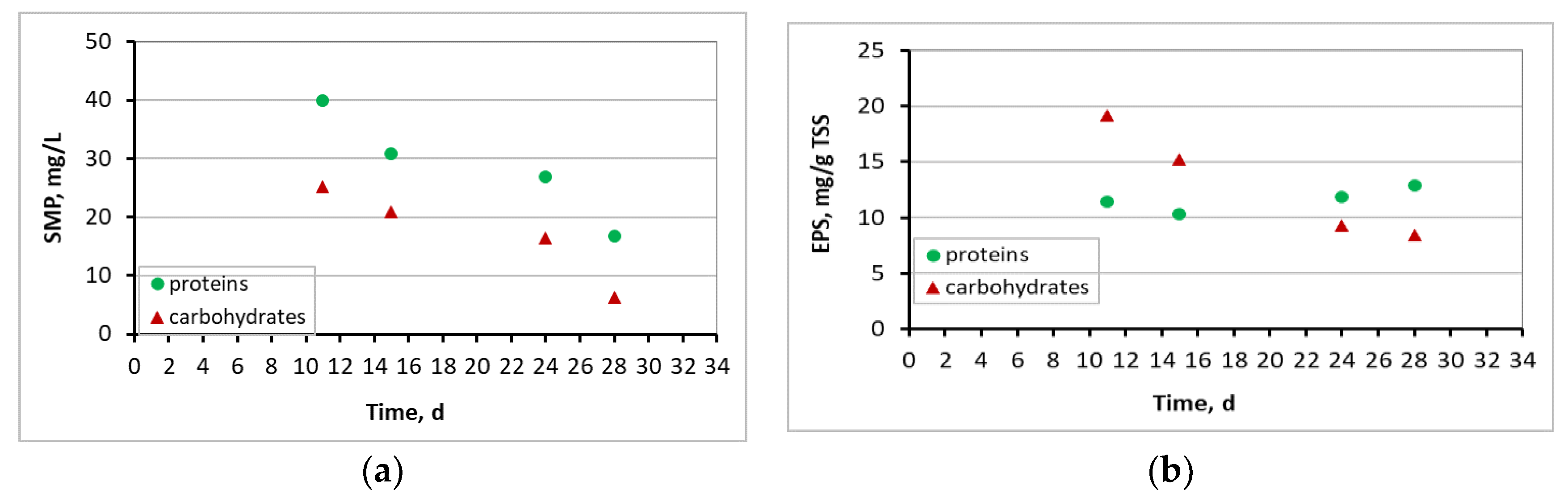

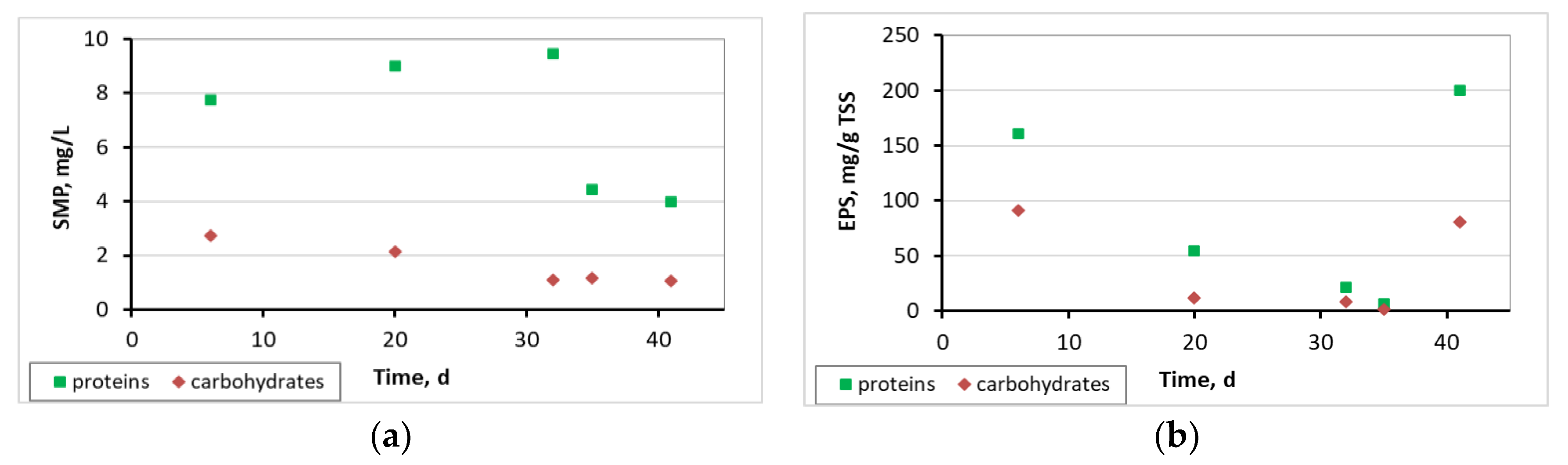
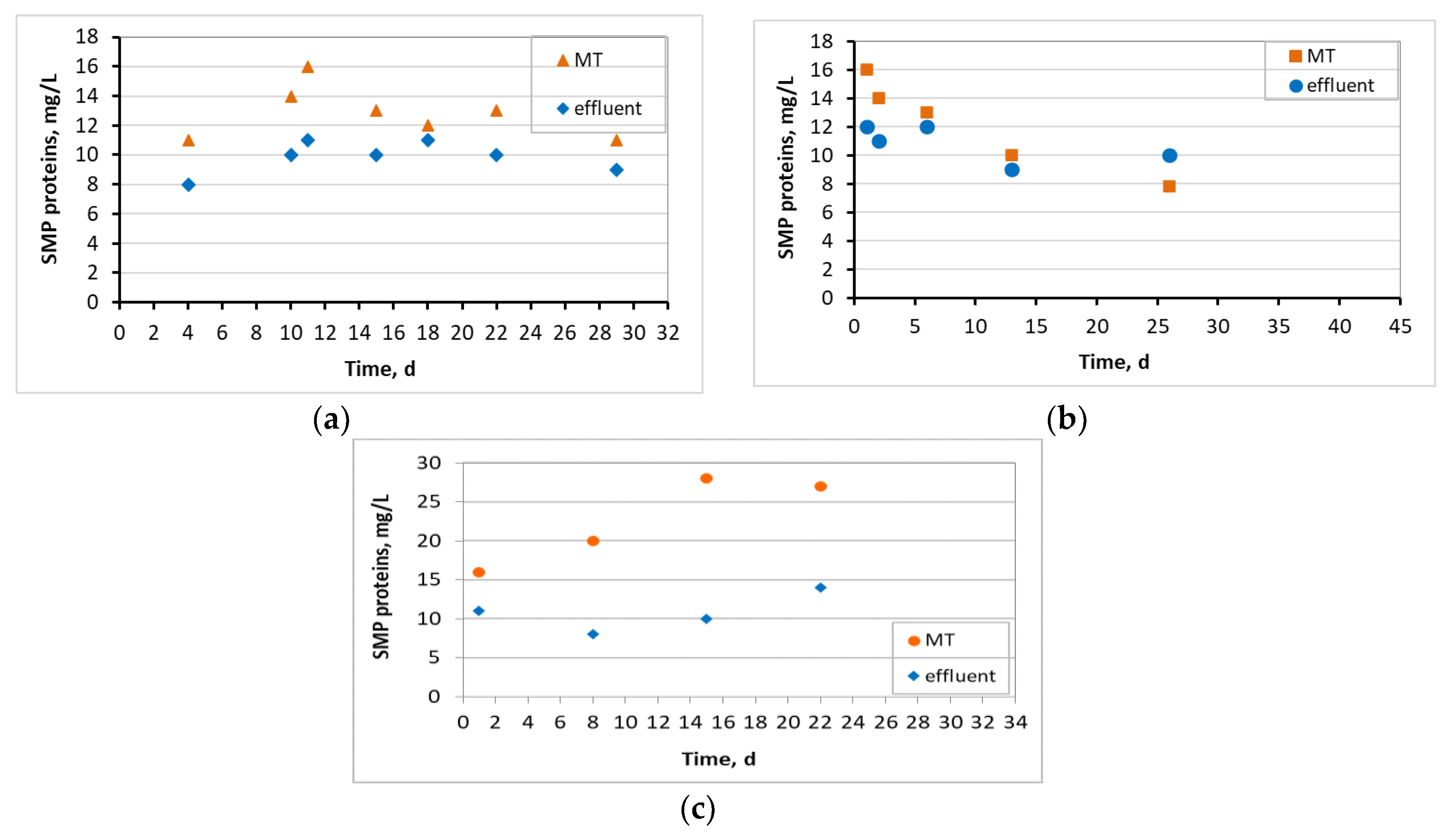
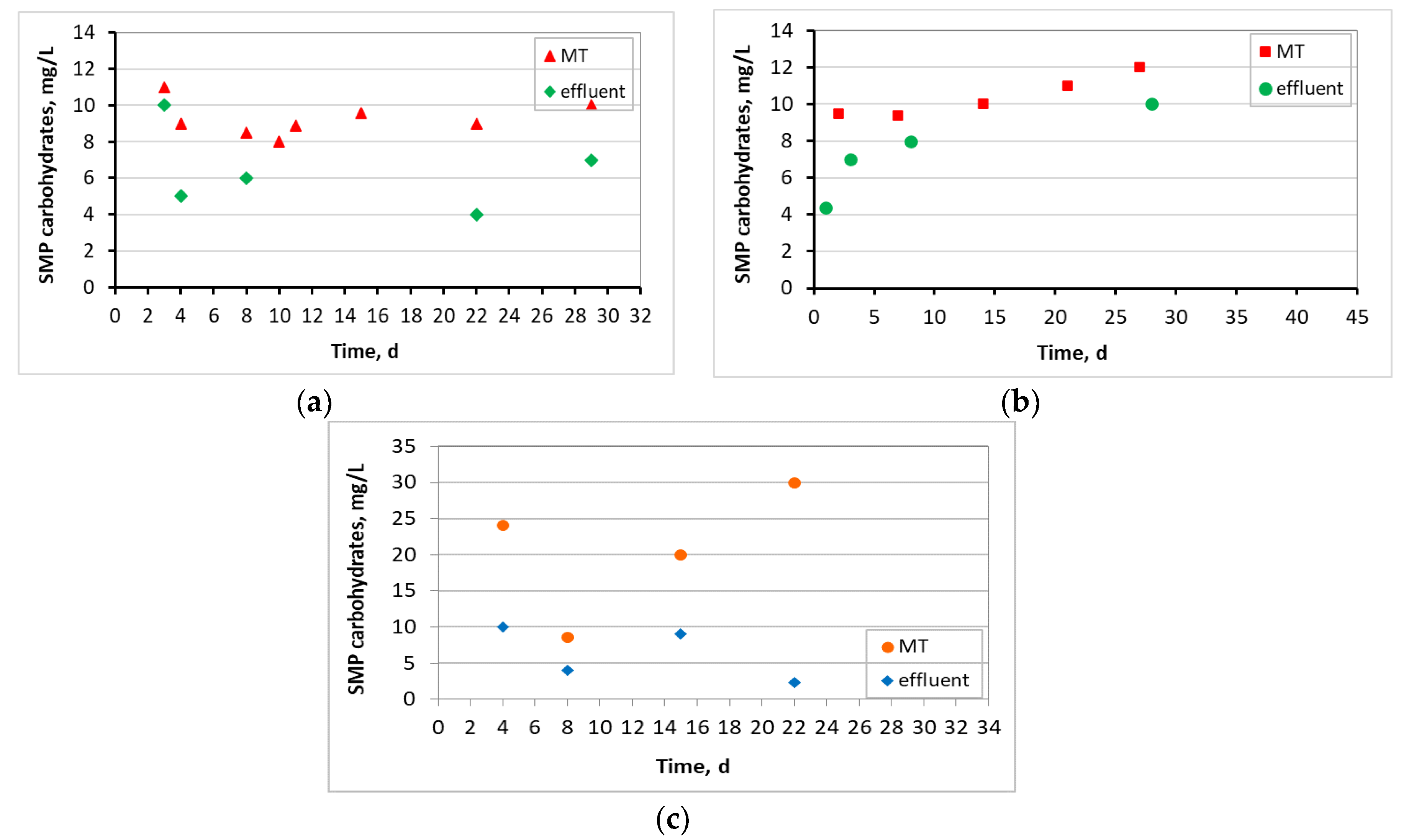
3.4. Microbiome Analysis on Biofilm of Biocarriers via 16S rRNA Sequencing
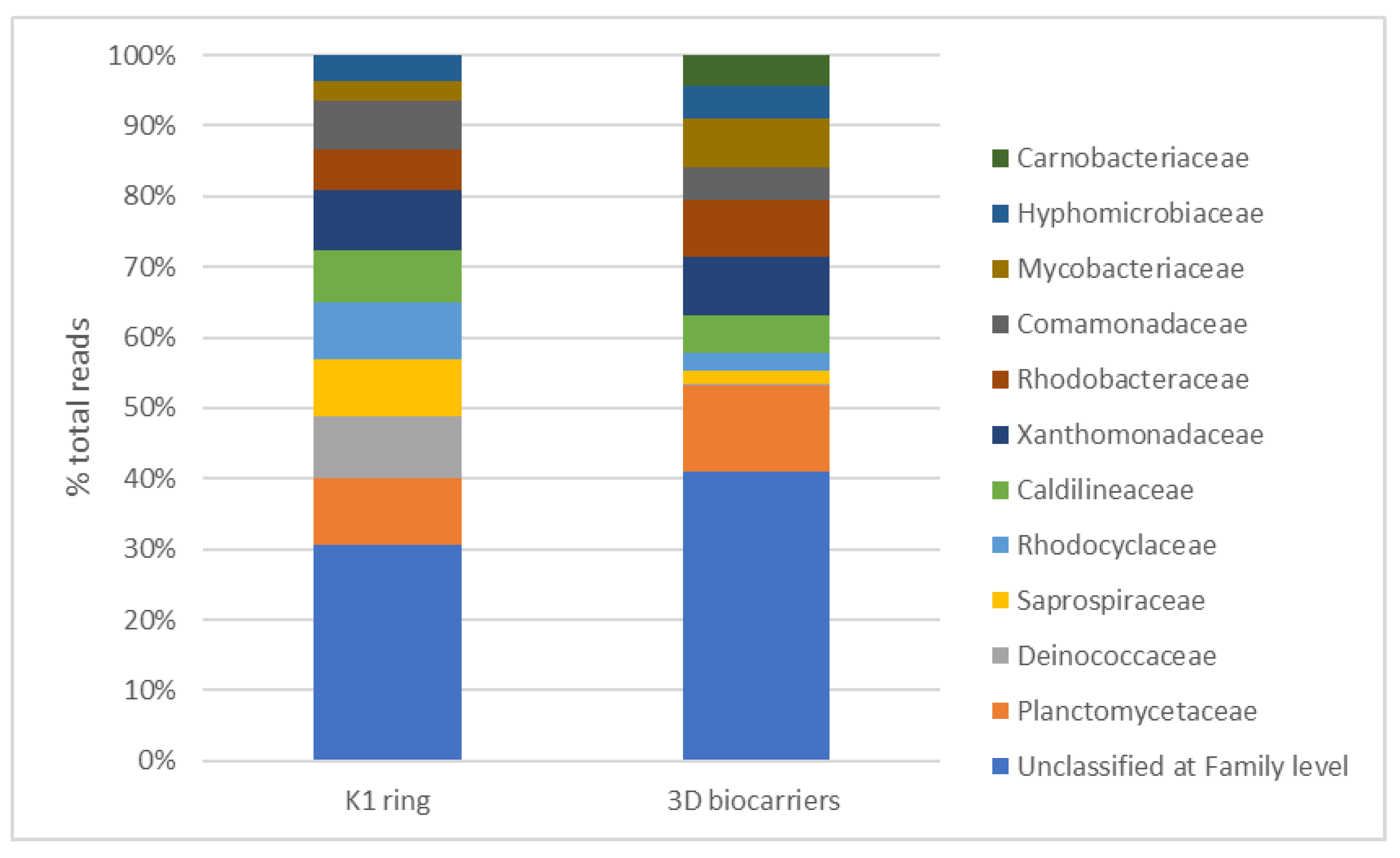
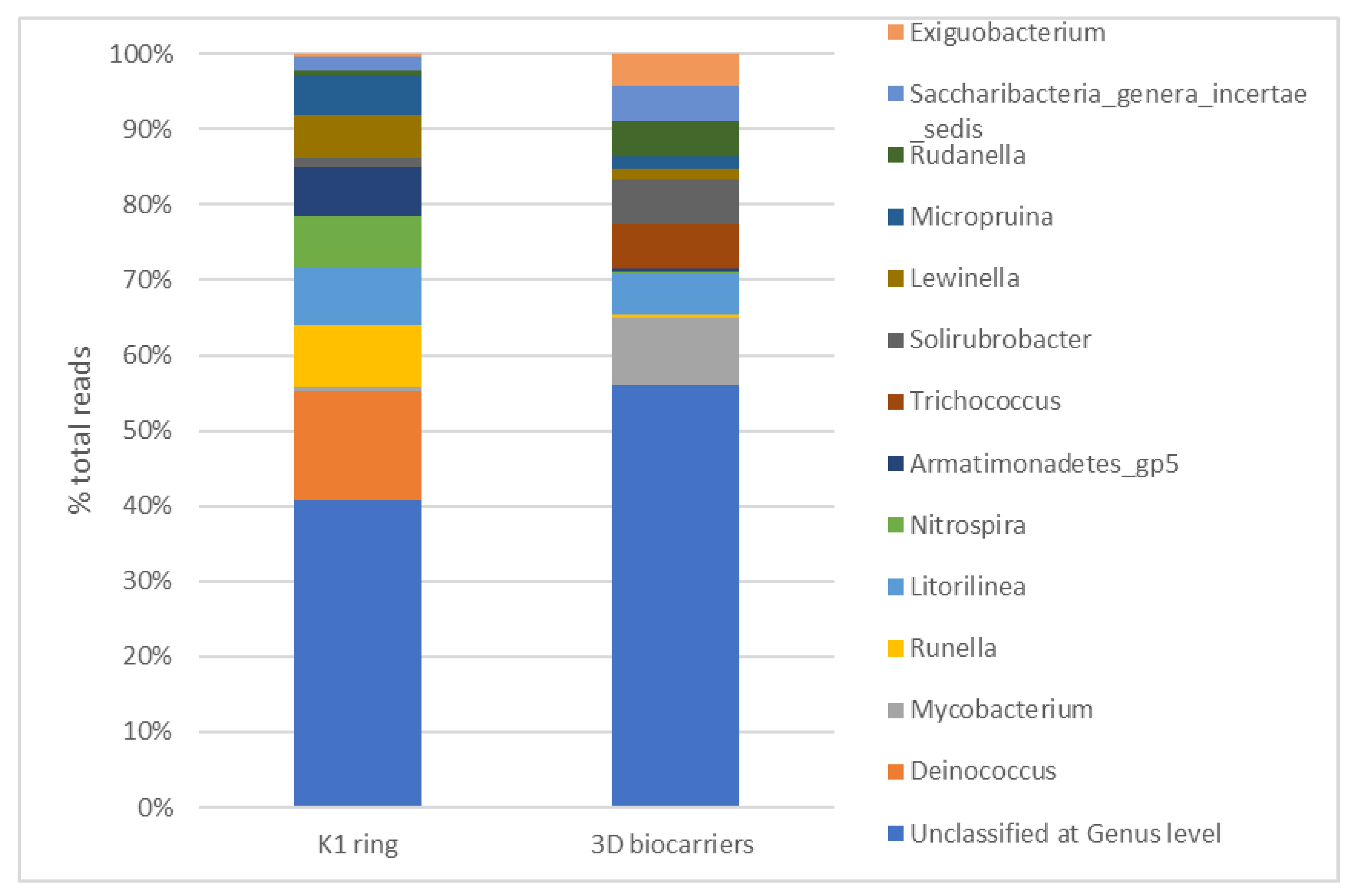
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boltz, J.; Boltz, J.P.; Smets, B.F.; Rittmann, B.E.; Loosdrecht, M.C.M. Van; Morgenroth, E.; Daigger, G.T. From biofilm ecology to reactors : A focused review From bio fi lm ecology to reactors : a focused review. 2017. [CrossRef]
- Saidulu, D.; Majumder, A.; Gupta, A.K. A systematic review of moving bed biofilm reactor, membrane bioreactor, and moving bed membrane bioreactor for wastewater treatment: Comparison of research trends, removal mechanisms, and performance. Journal of Environmental Chemical Engineering 2021, 9, 106112. [CrossRef]
- Leyva-Díaz, J.C.; Monteoliva-García, A.; Martín-Pascual, J.; Munio, M.M.; García-Mesa, J.J.; Poyatos, J.M. Moving bed biofilm reactor as an alternative wastewater treatment process for nutrient removal and recovery in the circular economy model. Bioresource Technology 2020, 299, 122631. [CrossRef]
- Liu, J.; Sun, F.; Zhang, P.; Zhou, Y. Integrated powdered activated carbon and quorum quenching strategy for biofouling control in industrial wastewater membrane bioreactor. Journal of Cleaner Production 2021, 279, 123551. [CrossRef]
- Banti, D.C.; Tsangas, M.; Samaras, P.; Zorpas, A. LCA of a membrane bioreactor compared to activated sludge system for municipal wastewater treatment. Membranes 2020, 10, 1–15. [CrossRef]
- Lemonidis, I.; Banti, D.C.; Tzenos, C.A.; Kalamaras, S.D.; Kotsopoulos, T.A.; Samaras, P. Energy Valorization of Fine Screenings from a Municipal Wastewater Treatment Plant. Energies 2022, 15. [CrossRef]
- Kawan, J.A.; Abu Hasan, H.; Suja, F.; Jaafar, O.; Abd-Rahman, R. A review on sewage treatment and polishing using moving bed bioreactor (Mbbr). Journal of Engineering Science and Technology 2016, 11, 1098–1120.
- Banti, D.C.; Samaras, P.; Tsioptsias, C.; Zouboulis, A.; Mitrakas, M. Mechanism of SMP aggregation within the pores of hydrophilic and hydrophobic MBR membranes and aggregates detachment. Separation and Purification Technology 2018. [CrossRef]
- Banti, D.C.; Karayannakidis, P.D.; Samaras, P.; Mitrakas, M.G. An innovative bioreactor set-up that reduces membrane fouling by adjusting the filamentous bacterial population. Journal of Membrane Science 2017. [CrossRef]
- Kampouris, I.D.; Karayannakidis, P.D.; Banti, D.C.; Sakoula, D.; Konstantinidis, D.; Yiangou, M.; Samaras, P.E. Evaluation of a novel quorum quenching strain for MBR biofouling mitigation. Water Research 2018, 143, 56–65. [CrossRef]
- Gkotsis, P.; Banti, D.; Pritsa, A.; Mitrakas, M.; Samaras, P.; Peleka, E.; Zouboulis, A. Effect of operating conditions on membrane fouling in pilot-scale mbrs; filaments growth, diminishing dissolved oxygen and recirculation rate of the activated sludge. Membranes 2021, 11, 1–16. [CrossRef]
- Lin, H.; Zhang, M.; Wang, F.; Meng, F.; Liao, B.Q.; Hong, H.; Chen, J.; Gao, W. A critical review of extracellular polymeric substances (EPSs) in membrane bioreactors: Characteristics, roles in membrane fouling and control strategies. Journal of Membrane Science 2014, 460, 110–125. [CrossRef]
- Banti, D.; Mitrakas, M.; Fytianos, G.; Tsali, A.; Samaras, P. Combined effect of colloids and SMP on membrane fouling in MBRs. Membranes 2020, 10, 1–15. [CrossRef]
- Mcquarriev, J.P.; Boltz, J.P. Moving Bed Biofilm Reactor Technology ; Process Applications , Design , and Performance. 2010.
- Dong, Y.; Fan, S.Q.; Shen, Y.; Yang, J.X.; Yan, P.; Chen, Y.P.; Li, J.; Guo, J.S.; Duan, X.M.; Fang, F.; et al. A Novel Bio-carrier Fabricated Using 3D Printing Technique for Wastewater Treatment. Scientific Reports 2015, 5. [CrossRef]
- Tang, B.; Song, H.; Bin, L.; Huang, S.; Zhang, W.; Fu, F.; Zhao, Y.; Chen, Q. Bioresource Technology Determination of the profile of DO and its mass transferring coefficient in a biofilm reactor packed with semi-suspended bio-carriers. Bioresource Technology 2017, 241, 54–62.
- Lee, S.; Badoux, G.O.; Wu, B.; Chong, T.H. Enhancing performance of biocarriers facilitated gravity-driven membrane (GDM) reactor for decentralized wastewater treatment: Effect of internal recirculation and membrane packing density. Science of the Total Environment 2021, 762, 144104. [CrossRef]
- Sun, H.; Liu, H.; Zhang, M.; Liu, Y. A novel single-stage ceramic membrane moving bed biofilm reactor coupled with reverse osmosis for reclamation of municipal wastewater to NEWater-like product water. Chemosphere 2021, 268, 128836. [CrossRef]
- Barwal, A.; Chaudhary, R. To study the performance of biocarriers in moving bed biofilm reactor ( MBBR ) technology and kinetics of biofilm for retrofitting the existing aerobic treatment systems : a review. 2014, 285–299. [CrossRef]
- Felföldi, T.; Jurecska, L.; Vajna, B.; Barkács, K.; Makk, J.; Cebe, G.; Szabó, A.; Záray, G.; Márialigeti, K. Texture and type of polymer fiber carrier determine bacterial colonization and biofilm properties in wastewater treatment. 2015, 264, 824–834. [CrossRef]
- Elliott, O.; Gray, S.; Mcclay, M.; Nassief, B.; Nunnelley, A.; Ekong, J.; Kardel, K.; Khoshkhoo, A.; Proaño, G.; David, M.; et al. Design and Manufacturing of High Surface Area 3D-Printed Media for Moving Bed Bioreactors for Wastewater Treatment. 2017, 144–156. [CrossRef]
- Zhang, Y.; Hsu, H.H.; Wheeler, J.J.; Tang, S.; Jiang, X. Emerging investigator series: Emerging biotechnologies in wastewater treatment: From biomolecular engineering to multiscale integration. Environmental Science: Water Research and Technology 2020, 6, 1967–1985. [CrossRef]
- Tang, B.; Zhao, Y.; Bin, L.; Huang, S.; Fu, F. Bioresource Technology Variation of the characteristics of bio fi lm on the semi-suspended bio-carrier produced by a 3D printing technique : Investigation of a whole growing cycle. Bioresource Technology 2017, 244, 40–47. [CrossRef]
- Chioti, A.G.; Tsioni, V.; Patsatzis, S.; Filidou, E.; Banti, D.; Samaras, P.; Economou, E.A.; Kostopoulou, E.; Sfetsas, T. Characterization of Biofilm Microbiome Formation Developed on Novel 3D-Printed Zeolite Biocarriers during Aerobic and Anaerobic Digestion Processes. 2022. [CrossRef]
- APHA (American Public Health Association) Standard Methods for the Examination of Water and Wastewater; Washington, DC, 1998;
- Hwang, B.K.; Kim, J.H.; Ahn, C.H.; Lee, C.H.; Song, J.Y.; Ra, Y.H. Effect of disintegrated sludge recycling on membrane permeability in a membrane bioreactor combined with a turbulent jet flow ozone contactor. Water Research 2010, 44, 1833–1840. [CrossRef]
- Hartree, E.F. Determination of protein: A modification of the Lowry method that gives a lineart photometric response. Analytical Biochemistry 1972, 48. [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. 1956, 350–356. [CrossRef]
- Eikelboom, D.H. Process control of activated sludge plants by microscopic investigation; IWA Publishing: Zutphen, 2000;
- Banti, D.C.; Mitrakas, M.; Samaras, P. Membrane fouling controlled by adjustment of biological treatment parameters in step-aerating MBR. Membranes 2021, 11, 1–15. [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nature Biotechnology 2019, 37, 852–857. [CrossRef]
- Benjamin, C.; McMurdie, P.; Rosen, M.; Han, A.; Johnson, A.; Holmes, S. DADA2:High resolution sample inference from Illumina amplicon data. Encyclopedia of Medical Immunology 2020, 13, 1–7. [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Research 2013, 41, 590–596. [CrossRef]
- Borea, L.; Naddeo, V.; Belgiorno, V. Frontiers in wastewater treatment and modelling-An electro moving bed membrane bioreactore (eMB-MBR) as a novel technology for wastewater treatment and reuse; Mannina, G., Ed.; Springer Netherlands, 2017; ISBN 9783319584201.
- Abdelfattah, A.; Hossain, M.I.; Cheng, L. High-strength wastewater treatment using microbial biofilm reactor: a critical review. World Journal of Microbiology and Biotechnology 2020, 36. [CrossRef]
- Shreve, M.J.; Brennan, R.A. Trace organic contaminant removal in six full-scale integrated fixed-film activated sludge (IFAS) systems treating municipal wastewater. Water Research 2019, 151, 318–331. [CrossRef]
- Mazioti, A.A.; Koutsokeras, L.E.; Constantinides, G.; Vyrides, I. Untapped potential of moving bed biofilm reactors with different biocarrier types for bilge water treatment: A laboratory-scale study. Water (Switzerland) 2021, 13. [CrossRef]
- Zhou, Y.; Kiely, P.D.; Kibbee, R.; Ormeci, B. Effect of polymeric support material on biofilm development, bacterial population, and wastewater treatment performance in anaerobic fixed-film systems. Chemosphere 2021, 264, 128477. [CrossRef]
- Qin, S.; Wainaina, S.; Liu, H.; Soufiani, A.M.; Pandey, A.; Zhang, Z.; Awasthi, M.K.; Taherzadeh, M.J. Microbial dynamics during anaerobic digestion of sewage sludge combined with food waste at high organic loading rates in immersed membrane bioreactors. Fuel 2021, 303, 121276. [CrossRef]
- Gerber, E.; Bernard, R.; Castang, S.; Chabot, N.; Coze, F.; Dreux-Zigha, A.; Hauser, E.; Hivin, P.; Joseph, P.; Lazarelli, C.; et al. Deinococcus as new chassis for industrial biotechnology: Biology, physiology and tools. Journal of Applied Microbiology 2015, 119, 1–10. [CrossRef]
- Gielnik, A.; Pechaud, Y.; Huguenot, D.; Esposito, G.; Guibaud, G.; van Hullebusch, E.D. Potential Use of Waste-to-Bioenergy By-Products in Bioremediation of Total Petroleum Hydrocarbons (TPH)-Contaminated Soils; 2020; ISBN 9783030403485.
- Tong, J.; Cui, L.; Wang, D.; Wang, X.; Liu, Z. Assessing the performance and microbial structure of biofilms in membrane aerated biofilm reactor for high p-nitrophenol concentration treatment. Journal of Environmental Chemical Engineering 2022, 10, 108635. [CrossRef]
- Wang, X.; Gao, C.; Jin, P.; Zhang, Y.; Xie, Y.; Chen, T.; Zhang, A. Nitrogen removal performance and bacterial community in a full-scale modified Orbal oxidation ditch with internal nitrate recycle and biocarriers. Journal of Water Process Engineering 2021, 40, 101791. [CrossRef]
- Wu, X.; Wang, C.; Wang, D.; Huang, Y.X.; Yuan, S.; Meng, F. Simultaneous methanogenesis and denitrification coupled with nitrifying biofilm for high-strength wastewater treatment: Performance and microbial mechanisms. Water Research 2022, 225, 119163. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





