Submitted:
07 June 2023
Posted:
08 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Ambulatory ECG Recordings
2.3. Measurement of Holter-Based LPs
2.4. Heart Rate Variability Analysis
2.5. Statistical Analyses
3. Results
3.1. Patient Demographics
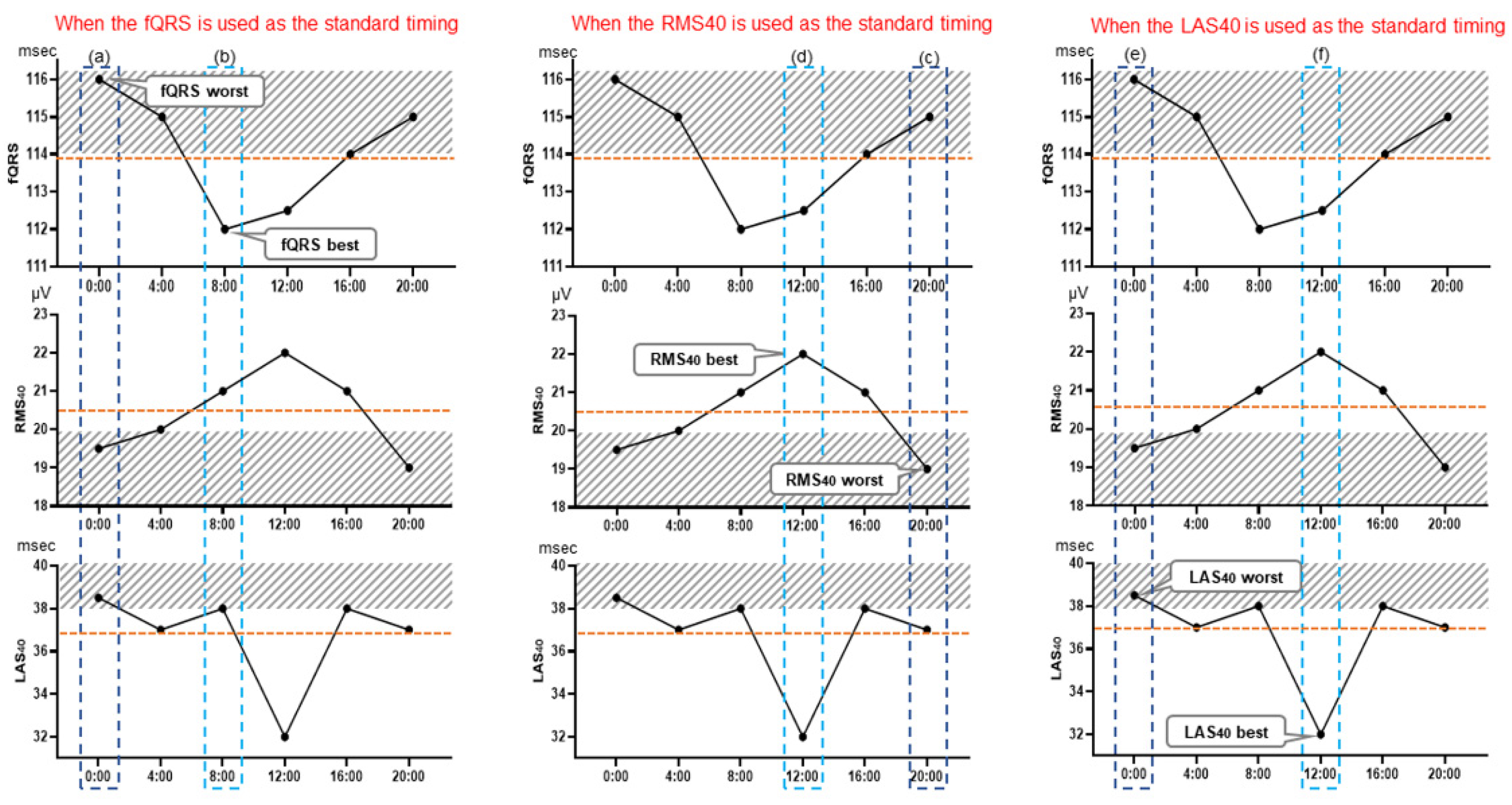
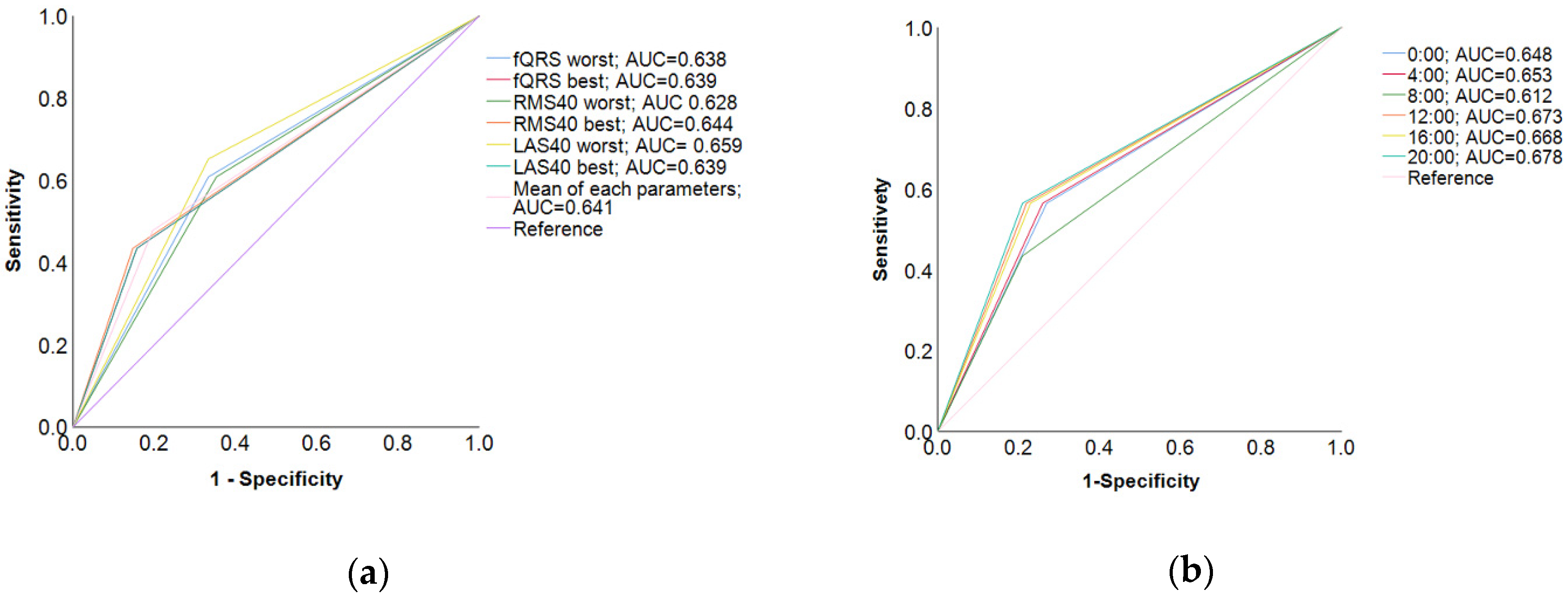
3.2. Optimal Measurement Timing for Assessment of Holter-Based LPs
3.3. Factors Influencing Diurnal Variability in Holter-Based LP
4. Discussion
4.1. Optimal Measurement Timing of LP for Predicting VT
4.2. Diurnal Variation of LP and Factors Influencing LP Values
4.3. Clinical Implications
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomes, J.A.; Winters, S.L.; Stewart, D.; Horowitz, S.; Milner, M.; Barreca, P. A new noninvasive index to predict sustained ventricular tachycardia and sudden death in the first year after myocardial infarction: based on signal-averaged electrocardiogram, radionuclide ejection fraction and Holter monitoring. J Am Coll Cardiol 1987, 10, 349–357. [Google Scholar] [CrossRef]
- Ikeda, T.; Sakata, T.; Takami, M.; Kondo, N.; Tezuka, N.; Nakae, T.; Noro, M.; Enjoji, Y.; Abe, R.; Sugi, K.; et al. Combined assessment of T-wave alternans and late potentials used to predict arrhythmic events after myocardial infarction. A prospective study. J Am Coll Cardiol 2000, 35, 722–730. [Google Scholar] [CrossRef]
- Mancini, D.M.; Wong, K.L.; Simson, M.B. Prognostic value of an abnormal signal-averaged electrocardiogram in patients with nonischemic congestive cardiomyopathy. Circulation 1993, 87, 1083–1092. [Google Scholar] [CrossRef]
- Kamath, G.S.; Zareba, W.; Delaney, J.; Koneru, J.N.; McKenna, W.; Gear, K.; Polonsky, S.; Sherrill, D.; Bluemke, D.; Marcus, F.; et al. Value of the signal-averaged electrocardiogram in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm 2011, 8, 256–262. [Google Scholar] [CrossRef]
- Yodogawa, K.; Seino, Y.; Ohara, T.; Iwasaki, Y.K.; Hayashi, M.; Miyauchi, Y.; Azuma, A.; Shimizu, W. Prognostic significance of ventricular late potentials in patients with pulmonary sarcoidosis. Heart Rhythm 2018, 15, 798–802. [Google Scholar] [CrossRef]
- Hashimoto, K.; Takase, B.; Nagashima, M.; Kasamaki, Y.; Shimabukuro, H.; Soma, M.; Nakayama, T. A novel signal-averaged electrocardiogram and an ambulatory-based signal-averaged electrocardiogram show strong correlations with conventional signal-averaged electrocardiogram in healthy subjects: A validation study. J Electrocardiol 2018, 51, 1145–1152. [Google Scholar] [CrossRef]
- Gatzoulis, K.A.; Tsiachris, D.; Arsenos, P.; Antoniou, C.K.; Dilaveris, P.; Sideris, S.; Kanoupakis, E.; Simantirakis, E.; Korantzopoulos, P.; Goudevenos, I.; et al. Arrhythmic risk stratification in post-myocardial infarction patients with preserved ejection fraction: the PRESERVE EF study. Eur Heart J 2019, 40, 2940–2949. [Google Scholar] [CrossRef]
- Hashimoto, K.; Amino, M.; Yoshioka, K.; Kasamaki, Y.; Kinoshita, T.; Ikeda, T. Combined evaluation of ambulatory-based late potentials and nonsustained ventricular tachycardia to predict arrhythmic events in patients with previous myocardial infarction: A Japanese noninvasive electrocardiographic risk stratification of sudden cardiac death (JANIES) substudy. Ann Noninvasive Electrocardiol 2021, 26, e12803. [Google Scholar] [CrossRef]
- Hashimoto, K.; Kinoshita, T.; Miwa, Y.; Amino, M.; Yoshioka, K.; Yodogawa, K.; Nakagawa, M.; Nakamura, K.; Watanabe, E.; Nakamura, K.; et al. Ambulatory electrocardiographic markers predict serious cardiac events in patients with chronic kidney disease: The Japanese Noninvasive Electrocardiographic Risk Stratification of Sudden Cardiac Death in Chronic Kidney Disease (JANIES-CKD) study. Ann Noninvasive Electrocardiol 2022, 27, e12923. [Google Scholar] [CrossRef]
- Abe, A.; Kobayashi, K.; Yuzawa, H.; Sato, H.; Fukunaga, S.; Fujino, T.; Okano, Y.; Yamazaki, J.; Miwa, Y.; Yoshino, H.; et al. Comparison of late potentials for 24 hours between Brugada syndrome and arrhythmogenic right ventricular cardiomyopathy using a novel signal-averaging system based on Holter ECG. Circ Arrhythm Electrophysiol 2012, 5, 789–795. [Google Scholar] [CrossRef]
- Yoshioka, K.; Amino, M.; Zareba, W.; Shima, M.; Matsuzaki, A.; Fujii, T.; Kanda, S.; Deguchi, Y.; Kobayashi, Y.; Ikari, Y.; et al. Identification of high-risk Brugada syndrome patients by combined analysis of late potential and T-wave amplitude variability on ambulatory electrocardiograms. Circ J 2013, 77, 610–618. [Google Scholar] [CrossRef]
- Nakagawa, M.; Iwao, T.; Ishida, S.; Yonemochi, H.; Fujino, T.; Saikawa, T.; Ito, M. Circadian rhythm of the signal averaged electrocardiogram and its relation to heart rate variability in healthy subjects. Heart 1998, 79, 493–496. [Google Scholar] [CrossRef]
- Kinoshita, T.; Hashimoto, K.; Yoshioka, K.; Miwa, Y.; Yodogawa, K.; Watanabe, E.; Nakamura, K.; Nakagawa, M.; Nakamura, K.; Watanabe, T.; et al. Risk stratification for cardiac mortality using electrocardiographic markers based on 24-hour Holter recordings: the JANIES-SHD study. J Cardiol 2020, 75, 155–163. [Google Scholar] [CrossRef]
- Amino, M.; Yoshioka, K.; Ichikawa, T.; Watanabe, E.; Kiyono, K.; Nakamura, M.; Sakama, S.; Ayabe, K.; Fujii, T.; Hashida, T.; et al. The presence of late potentials after percutaneous coronary intervention for the treatment of acute coronary syndrome as a predictor for future significant cardiac events resulting in re-hospitalization. J Electrocardiol 2019, 53, 71–78. [Google Scholar] [CrossRef]
- Breithardt, G.; Cain, M.E.; el-Sherif, N.; Flowers, N.C.; Hombach, V.; Janse, M.; Simson, M.B.; Steinbeck, G. Standards for analysis of ventricular late potentials using high-resolution or signal-averaged electrocardiography. A statement by a Task Force Committee of the European Society of Cardiology, the American Heart Association, and the American College of Cardiology. Circulation 1991, 83, 1481–1488. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology, the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Steinbigler, P.; Haberl, R.; Jilge, G.; Steinbeck, G. Circadian variability of late potential analysis in Holter electrocardiograms. Pacing Clin Electrophysiol 1999, 22, 1448–1456. [Google Scholar] [CrossRef]
- Nakamura, M.; Yoshioka, K.; Amino, M.; Watanabe, E.; Fujii, T.; Hashida, T.; Fujibayashi, D.; Kanda, S.; Kobayashi, Y.; Tanabe, T.; Ikari, Y. Late potential as a predictor of re-hospitalization after percutaneous coronary intervention for acute coronary syndrome. Tokai J Exp Clin Med 2016, 41, 172–180. [Google Scholar] [PubMed]
- Kremers, M.S.; Black, W.H.; Lange, R.; Wells, P.J.; Solo, M. Electrocardiographic signal-averaging during atrial pacing and effect of cycle length on the terminal QRS in patients with and without inducible ventricular tachycardia. Am J Cardiol 1990, 66, 1095–1098. [Google Scholar] [CrossRef]
- Goldberger, J.J.; Ahmed, M.W.; Parker, M.A.; Kadish, A.H. Assessment of effects of autonomic stimulation and blockade on the signal-averaged electrocardiogram. Circulation 1994, 89, 1656–64. [Google Scholar] [CrossRef]
- Yoshioka, K.; Amino, M.; Nakamura, M.; Kanda, S.; Kobayashi, Y.; Ikari, Y.; Shima, M.; Tanabe, T. Incidence of positive ventricular late potentials differs in postural changes among supine, left, and right lateral decubitus, and prone and sitting positions in Brugada syndrome. Ann Noninvasive Electrocardiol 2015, 20, 488–497. [Google Scholar] [CrossRef]
- Chamiec, T.; Kułakowski, P.; Ceremuzyński, L. Exercise producing alterations in the signal-averaged electrocardiogram in patients after myocardial infarction. Eur Heart J 1995, 16, 354–359. [Google Scholar] [CrossRef]
- Shimizu, M.; Suzuki, M.; Fujii, H.; Kimura, S.; Nishizaki, M.; Sasano, T. Machine learning of microvolt-level 12-lead electrocardiogram can help distinguish takotsubo syndrome and acute anterior myocardial infarction. Cardiovasc Digit Health J 2022, 3, 179–188. [Google Scholar] [CrossRef]
| Demographics | MI-VT group (n=23) | MI-non-VT group (n=103) | p value | Control group (n=60) | ||||
|---|---|---|---|---|---|---|---|---|
| Age (years) | 66.9±12.4 | 66.9±13.1 | 0.994 | 56.7±20.5 | ||||
| Sex: male, n (%) | 22 (96) | 83 (81) | 0.195 | 33 (55) | ||||
| Hypertension, n (%) | 18 (23) | 87 (84) | 0.758 | ― | ||||
| Dyslipidemia, n (%) | 14 (61) | 68 (66) | 0.831 | ― | ||||
| Diabetes mellitus, n (%) | 17 (74) | 41 (40) | 0.002 | ― | ||||
| Coronary culprit lesion | ||||||||
| RCA | 3 (13) | 39 (38) | 0.023 | ― | ||||
| LAD | 17 (73) | 43 (42) | 0.04 | ― | ||||
| Cx | 2 (13) | 10 (20) | 0.562 | ― | ||||
| Echocardiographic data | ||||||||
| LVEF (%) | 48.5±16.0 | 58.4±11.9 | <0.001 | 70.8±6.5 | ||||
| LVDd (mm) | 57.1±11.6 | 50.1±7.4 | <0.001 | 44.4±4.6 | ||||
| Renal function | ||||||||
| Estimate GFR (mL/min per 1.73 m2) | 46.9 [34.7, 68.5] | 61.3 [37.7, 76.1] | 0.146 | 78.5±18.2 | ||||
| Creatine (mg/dL) | 1.1 [0.8, 1.5] | 0.93 [0.7, 1.2] | 0.152 | 0.69 [0.63, 0.79] | ||||
| Therapy | ||||||||
| β-Blocker (%) | 19 (83) | 77 (75) | 0.424 | ― | ||||
| RAS inhibitor (%) | 14 (61) | 66 (64) | 0.729 | ― | ||||
| CCB (%) | 11(48) | 32 (31) | 0.125 | ― | ||||
| Diuretic (%) | 12 (52) | 42 (41) | 0.581 | ― | ||||
| Amiodarone (%) | 8 (34) | 6 (6) | <0.001 | ― | ||||
| Ib (%) | 1 (4.3) | 5 (4.8) | 0.918 | ― | ||||
| Ic (%) | 0 (0) | 0 (0) | ― | ― | ||||
| MI-VT group (n=23) | |||||||||
| 0:00 | 4:00 | 8:00 | 12:00 | 16:00 | 20:00 | p value | |||
| fQRS (ms) | median | 115.0 | 116.0 | 116.0 | 116.0 | 114.0 | 118.0 | 0.005 | |
| [interquartile range] | [108.0,134.8] | [108.0, 131.0] | [101.0, 135.0] | [102.0, 135.0] | [107.0, 132.0] | [107.0, 134.0] | |||
| RMS40 (µV) | median | 14.0 | 14.0 | 21.0 | 18.0 | 16.0 | 16.0 | 0.04 | |
| [interquartile range] | [10.3, 54.8] | [10.0, 43.0] | [11.0, 55.0] | [8.0, 57.0] | [9.0, 43.0] | [6.6, 52.0] | |||
| LAS40 (ms) | median | 43.5 | 41.0 | 37.0 | 40.0 | 40.0 | 39.0 | 0.02 | |
| [interquartile range] | [29.0, 53.0] | [31.0, 48.0] | [27.0, 46.0] | [27.0, 46.0] | [26.0, 51.0] | [30.0, 50.0] | |||
| MI-non-VT group (n=103) | |||||||||
| 0:00 | 4:00 | 8:00 | 12:00 | 16:00 | 20:00 | p value | |||
| fQRS (ms) | median | 101.0 | 102.5 | 100.5 | 98.0 | 99.0 | 99.0 | <0.001 | |
| [interquartile range] | [93.0, 115.0] | [94.0, 113.5] | [91.8, 112.3] | [93.0, 114.0] | [90.0, 110.5] | [94.0, 113.5] | |||
| RMS40 (µV) | median | 30.5 | 30.5 | 32.5 | 34.0 | 36.0 | 30.0 | <0.001 | |
| [interquartile range] | [16.0, 45.8] | [16.0, 45.8] | [20.0, 48.3] | [18.5, 47.0] | [19.5, 50.5] | [20.8, 48.5] | |||
| LAS40 (ms) | median | 30.0 | 32.0 | 30.0 | 30.0 | 29.0 | 31.0 | 0.03 | |
| [interquartile range] | 24.0, 41.5] | [24.0, 39.5] | [24.0, 36.5] | [24.0, 36.5] | [24.5, 36.0] | [25.0, 36.0] | |||
| Control group (n=60) | |||||||||
| 0:00 | 4:00 | 8:00 | 12:00 | 16:00 | 20:00 | p value | |||
| fQRS (ms) | median | 90.0 | 90.0 | 87.5 | 85.0 | 87.0 | 88.0 | ||
| [interquartile range] | [86.0, 95.3] | [87.0, 96.0] | [83.0, 93.3] | [83.8, 90.0] | [83.0, 91.0] | [83.0, 93.0] | <0.001 | ||
| RMS40 (µV) | median | 45.5 | 44.5 | 49.5 | 55.5 | 53.0 | 47.0 | ||
| [interquartile range] | [29.5,64.0] | [28.8, 65.8] | [31.0, 81.8] | [33.0, 81.5] | [38.3, 78.8] | [33.0, 79.6] | <0.001 | ||
| LAS40 (ms) | median | 28.0 | 27.0 | 27.0 | 26.0 | 26.0 | 25.0 | ||
| [interquartile range] | [23.0, 32.0] | [24.0, 31.3] | [21.0, 33.0] | [20.0, 30.3] | [21.0, 29.0] | [22.0, 31.3] | 0.03 | ||
| MI-VT group (n=23) | |||||||
| 0:00 | 4:00 | 8:00 | 12:00 | 16:00 | 20:00 | p value | |
| Number of patients | 13 (57) |
13 (57) |
10§ (43) |
11# (48) |
13 (57) |
12 (52) |
0.009 |
| (%) | |||||||
| MI-non-VT group (n=103) | |||||||
| 0:00 | 4:00 | 8:00 | 12:00 | 16:00 | 20:00 | p value | |
| Number of patients | 24 (23) |
23 (22) |
18§ (17) |
19§ (18) |
21 (20) |
21 (20) |
0.002 |
| (%) | |||||||
| Control group (n=60) | |||||||
| 0:00 | 4:00 | 8:00 | 12:00 | 16:00 | 20:00 | p value | |
| Number of participants | 7 (12) |
4# (7) |
4# (7) |
3§ (5) |
2§ (3) |
3§ (5) |
0.009 |
| (%) | |||||||
| Sensitivity | Specificity | PPV | NPV | Sensitivity | Specificity | PPV | NPV | ||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Time point | ||||||||
| fQRS worst | 61 | 67 | 61 | 89 | 0:00 | 57 | 74 | 57 | 88 |
| fQRS best | 43 | 80 | 43 | 86 | 4:00 | 57 | 75 | 57 | 89 |
| RMS40 worst | 61 | 65 | 61 | 88 | 8:00 | 43 | 75 | 43 | 86 |
| RMS40 best | 43 | 85 | 43 | 87 | 12:00 | 52 | 57 | 52 | 76 |
| LAS40 worst | 65 | 63 | 65 | 87 | 16:00 | 61 | 78 | 61 | 90 |
| LAS40 best | 43 | 84 | 43 | 87 | 20:00 | 57 | 80 | 57 | 90 |
| Mean values of 3 LP parameters |
48 | 78 | 48 | 85 |
| For each LP parameter | Univariate | Multivariate | Multivariate (stepwise) | ||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| fQRS worst | 3.11 | 1.22–7.91 | <0.001 | 1.00 | 0.87–11.56 | 0.998 | |||
| fQRS best | 4.13 | 1.55–11.03 | <0.001 | ||||||
| RMS40 worst | 2.85 | 1.12–7.23 | <0.001 | 0.332 | 0.021–5.36 | 0.437 | |||
| RMS40 best | 4.46 | 1.66–12.0 | <0.001 | ||||||
| LAS40 worst | 3.75 | 1.45–9.71 | 0.006 | 10.41 | 0.58–185.46 | 0.111 | 3.75 | 1.45–9.71 | 0.006 |
| LAS40 best | 4.14 | 1.55–11.04 | <0.001 | ||||||
| Mean values of three LP parameters | 3.76 | 1.45–9.75 | <0.001 | ||||||
| For each timepoint | Univariate | Multivariate | Multivariate (stepwise) | ||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| 0:00 | 3.61 | 1.42–9.19 | 0.007 | 0.66 | 0.75–5.81 | 0.710 | |||
| 4:00 | 3.80 | 1.49–9.70 | <0.001 | 0.93 | 0.084–10.27 | 0.953 | |||
| 8:00 | 2.97 | 1.14–7.70 | <0.001 | 0.21 | 0.024–1.75 | 0.148 | |||
| 12:00 | 4.67 | 1.80–12.07 | <0.001 | 3.16 | 0.29–33.91 | 0.342 | |||
| 16:00 | 4.41 | 1.11–11.36 | <0.001 | 2.74 | 0.39–19.26 | 0.310 | |||
| 20:00 | 5.00 | 1.93–13.02 | <0.001 | 4.40 | 0.52–37.25 | 0.174 | 4.89 | 1.88–12.7 | 0.001 |
| (a) | |||||||
| fQRS | R=0.490 | R=0.448a | |||||
| β | p | VIF | β | p | VIF | ||
| body position | 0.031 | 0.770 | 1.527 | ||||
| log Noise (μV) | 0.081 | 0.484 | 1.812 | ||||
| log HR (bpm) | -0.188 | 0.085 | 1.599 | -0.180 | 0.037 | 1.016 | |
| log PNN50 (%) | 0.256 | 0.270 | 7.296 | 0.433 | <0.001 | 1.016 | |
| log RMSSD (ms) | 0.212 | 0.417 | 9.246 | ||||
| log ASDNN (ms) | -0.180 | 0.382 | 5.771 | ||||
| log SDANN (ms) | -0.021 | 0.832 | 1.325 | ||||
| log VLF (ms2) | 0.187 | 0207 | 2.977 | ||||
| log HFnu (TP) | 0.183 | 0.140 | 2.070 | ||||
| log LF/HF | 0.086 | 0.400 | 1.399 | ||||
| RMS40 | R=0.500 | R=0.305a | |||||
| β | p | VIF | β | p | VIF | ||
| body position | -0.092 | 0.417 | 1.397 | ||||
| log Noise (μV) | -0.018 | 0.881 | 1.550 | ||||
| log HR (bpm) | 0.422 | 0.000 | 1.441 | 0.305 | 0.003 | 1.000 | |
| log PNN50 (%) | -0.230 | 0.336 | 6.211 | ||||
| log RMSSD (ms) | -0.066 | 0.790 | 6.619 | ||||
| log ASDNN (ms) | 0.180 | 0.415 | 5.264 | ||||
| log SDANN (ms) | 0.077 | 0.509 | 1.483 | ||||
| log VLF (ms2) | 0.076 | 0.648 | 2.971 | ||||
| log HFnu (TP) | 0.796 | 0.002 | 7.084 | ||||
| log LF/HF | 0.733 | 0.007 | 7.566 | ||||
| LAS40 | R=0.392 | R=0.292a | |||||
| β | *p | VIF | β | p | VIF | ||
| body position | 0.013 | 0.916 | 1.492 | ||||
| log Noise (μV) | -0.010 | 0.942 | 1.788 | ||||
| log HR (bpm) | -0.330 | 0.010 | 1.524 | -0.261 | 0.011 | 1.000 | |
| log PNN50 (%) | 0.081 | 0.749 | 6.233 | ||||
| log RMSSD (ms) | 0.148 | 0.568 | 6.483 | ||||
| log ASDNN (ms) | -0.032 | 0.890 | 5.196 | ||||
| log SDANN (ms) | -0.008 | 0.950 | 1.475 | ||||
| log VLF (ms2) | -0.134 | 0.448 | 2.970 | ||||
| log HFnu (TP) | -0.525 | 0.057 | 7.175 | ||||
| log LF/HF | -0.402 | 0.154 | 7.582 | ||||
| log ASDNN=logarithm of mean of the standard deviations of all NN intervals for all 5-min segments in 24-h HF; log HR=logarithm of heart rate; log HFnu=logarithm of power in the high-frequency area normalized unit; log LF/HF=logarithm of power in the low-frequency/power in the high-frequency ratio; log pNN50=logarithm of percent of difference between adjacent normal RR intervals greater than 50 ms; log RMSSD=logarithm of root mean square successive difference; log SDANN=logarithm of standard deviation of 5-min average NN intervals; VIF=variance inflation factor; log VLF=logarithm of low frequency area. a=variables by multiple linear regression with stepwise selection. | |||||||
| (b) | |||||||
| fQRS | R=0.366 | R=0.353a | |||||
| β | p | VIF | β | p | VIF | ||
| body position | -0.054 | 0.348 | 1.287 | ||||
| log Noise (μV) | -0.036 | 0.529 | 1.308 | ||||
| log HR (bpm) | -0.021 | 0.725 | 1.436 | ||||
| log PNN50 (%) | 0.305 | 0.001 | 3.092 | 0.298 | 0.001 | 2.945 | |
| log ASDNN (ms) | -0.235 | 0.028 | 4.480 | -0.222 | 0.029 | 4.047 | |
| log SDANN (ms) | 0.005 | 0.934 | 1.406 | ||||
| log VLF (ms2) | -0.184 | 0.037 | 3.027 | -0.180 | 0.030 | 2.684 | |
| log HFnu (TP) | -0.038 | 0.692 | 3.680 | ||||
| log LF/HF | 0.190 | 0.071 | 4.291 | 0.209 | 0.002 | 1.822 | |
| RMS40 | R=0.367 | R=0.327a | |||||
| β | p | VIF | β | p | VIF | ||
| body position | -0.039 | 0.493 | 1.287 | ||||
| log Noise (μV) | 0.155 | 0.007 | 1.308 | 0.156 | 0.002 | 1.000 | |
| log HR (bpm) | 0.046 | 0.446 | 1.436 | ||||
| log PNN50 (%) | -0.241 | 0.007 | 3.092 | -0.208 | 0.003 | 1.903 | |
| log ASDNN (ms) | 0.136 | 0.203 | 4.480 | 0.206 | 0.003 | 1.902 | |
| log SDANN (ms) | 0.075 | 0.209 | 1.406 | ||||
| log VLF (ms2) | 0.119 | 0.175 | 3.027 | ||||
| log HFnu (TP) | -0.027 | 0.777 | 3.680 | ||||
| log LF/HF | -0.157 | 0.134 | 4.291 | ||||
| LAS40 | R=0.344 | R=0.314a | |||||
| β | p | VIF | β | p | VIF | ||
| body position | 0.029 | 0.617 | 1.287 | ||||
| log Noise (μV) | -0.119 | 0.041 | 1.308 | -0.122 | 0.017 | 1.000 | |
| log HR (bpm) | -0.008 | 0.890 | 1.436 | ||||
| log PNN50 (%) | 0.265 | 0.003 | 3.092 | 0.219 | 0.002 | 1.903 | |
| log ASDNN (ms) | -0.221 | 0.041 | 4.480 | -0.224 | 0.001 | 1.902 | |
| log SDANN (ms) | -0.086 | 0.154 | 1.406 | ||||
| log VLF (ms2) | -0.008 | 0.929 | 3.027 | ||||
| log HFnu (TP) | 0.070 | 0.472 | 3.680 | ||||
| log LF/HF | 0.155 | 0.142 | 4.291 | ||||
| Abbreviations as in Table 6 (MI-VT group). a=Variables identified by multiple linear regression with stepwise selection. ※Logarithm of root mean square successive difference (log RMSSD) was removed from analysis because of multicollinearity. | |||||||
| (c) | |||||||
| fQRS | R=0.458 | R=0.452a | |||||
| β | p | VIF | β | p | VIF | ||
| body position | -0.035 | 0.556 | 1.352 | ||||
| log Noise (μV) | -0.473 | <0.001 | 1.271 | -0.484 | <0.001 | 1.179 | |
| log HR (bpm) | 0.139 | 0.050 | 1.948 | 0.141 | 0.022 | 1.473 | |
| log PNN50 (%) | -0.048 | 0.631 | 3.860 | ||||
| log ASDNN (ms) | 0.118 | 0.332 | 5.753 | ||||
| log SDANN (ms) | -0.024 | 0.705 | 1.530 | ||||
| log VLF (ms2) | -0.105 | 0.298 | 3.985 | ||||
| log HFnu (TP) | -0.150 | 0.319 | 8.789 | -0.129 | 0.028 | 1.356 | |
| log LF/HF | 0.004 | 0.982 | 9.626 | ||||
| RMS40 | R=0.396 | R=0.356a | |||||
| β | p | VIF | β | p | VIF | ||
| body position | 0.112 | 0.078 | 1.385 | ||||
| log Noise (μV) | 0.138 | 0.042 | 1.588 | 0.147 | 0.008 | 1.049 | |
| log HR (bpm) | -0.081 | 0.265 | 1.840 | ||||
| log PNN50 (%) | 0.123 | 0.249 | 3.925 | 0.094 | 0.089 | 1.049 | |
| log ASDNN (ms) | -0.013 | 0.911 | 4.489 | ||||
| log SDANN (ms) | 0.035 | 0.552 | 1.227 | ||||
| log VLF (ms2) | -0.075 | 0.407 | 2.837 | ||||
| log HFnu (TP) | -0.027 | 0.768 | 2.795 | ||||
| log LF/HF | 0.001 | 0.987 | 1.523 | ||||
| LAS40 | R=0.575 | R=0.563a | |||||
| β | p | VIF | β | p | VIF | ||
| body position | 0.032 | 0.558 | 1.352 | ||||
| log Noise (μV) | -0.633 | <0.001 | 1.271 | -0.609 | <0.001 | 1.169 | |
| log HR (bpm) | 0.240 | <0.001 | 1.948 | 0.245 | <0.001 | 1.169 | |
| log PNN50 (%) | 0.100 | 0.278 | 3.860 | ||||
| log ASDNN (ms) | -0.008 | 0.946 | 5.753 | ||||
| log SDANN (ms) | 0.035 | 0.548 | 1.530 | ||||
| log VLF (ms2) | -0.026 | 0.781 | 3.985 | ||||
| log HFnu (TP) | 0.051 | 0.715 | 8.789 | ||||
| log LF/HF | 0.152 | 0.295 | 9.626 | ||||
| Abbreviations as in Table 6 (MI-VT group). a=Variables by multiple linear regression with stepwise selection. ※Logarithm of root mean square successive difference (log RMSSD) was removed because of multicollinearity. | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





