Submitted:
06 June 2023
Posted:
07 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Instrumental Analytical Techniques
2.1. UV-Vis Spectrophotometry
2.2. X-ray Fluorescence Spectrometry (Both WD-XRF & ED-XRF)
2.3. Instrumental Neutron Activation Analysis (INAA)
2.4. Indirect Measurement of REE by the Radiometric Method
2.5. Atomic Absorption Spectrometry (Both Flame-AAS & GF-AAS)
2.6. Microwave Plasma Atomic Emission Spectrometry (MP-AES)
2.7. Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES)
2.8. Laser-Induced Breakdown Spectroscopy (LIBS)
2.9. Inductively Coupled Plasma Mass Spectrometry Techniques (All Forms of ICP-MS, ICP-MS/MS, ICP-TOF-MS, HR-ICP-MS, MH-ICP-MS & MC-ICP-MS with Both Solution and Direct Solid Sampling by Laser Ablation)
2.9.1. Inductively Coupled Mass Spectrometry (ICP-MS)
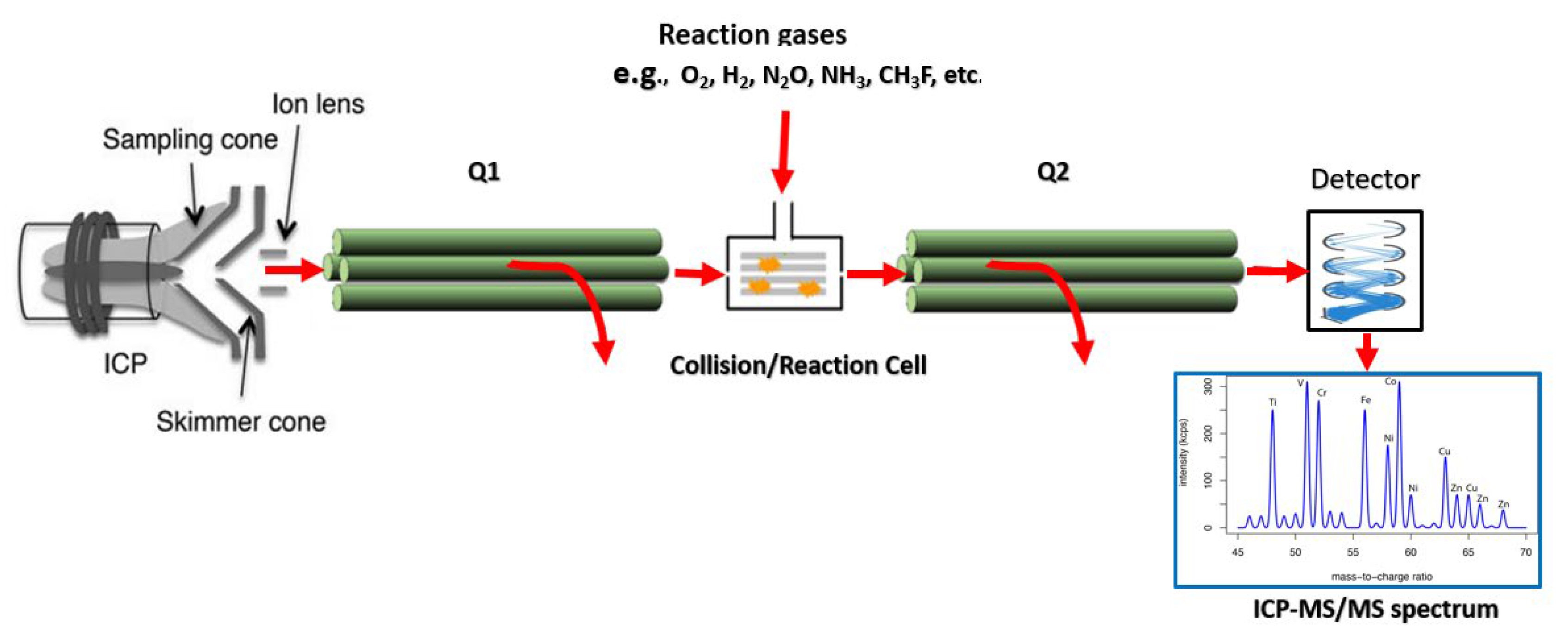
2.9.2. ICP-Tandem Mass Spectrometry (ICP-MS/MS)
2.9.3. ICP-TOF-MS
2.9.4. Magnetic Sector or High Resolution -ICP-MS (HR-ICP-MS)
2.9.5. MH-ICP-MS
3. Isotopic Studies
3.1. Multi-Collector ICP-MS (MC-ICP-MS)
3.2. Thermal Ionization Mass Spectrometry (TIMS)
3.3. Sensitive High-Resolution Ion Micro Probe (SHRIMP)
4. Mineralogical Studies and In-Situ Analytical Techniques
4.1. X-Ray Diffractometry (XRD)
4.2. Electron Probe Micro Analyser (EPMA)
4.3. Ion-Microprobe (SIMS)
4.4. Scanning Electron Microprobe (SEM-EDS)
5. Laser Ablation-ICP-MS (LA-ICP-MS)
5.1. LA-ICP-MS/MS
5.2. Laser Ablation Split Stream (LASS) Technique
6. Portable Miniatured Analytical Techniques
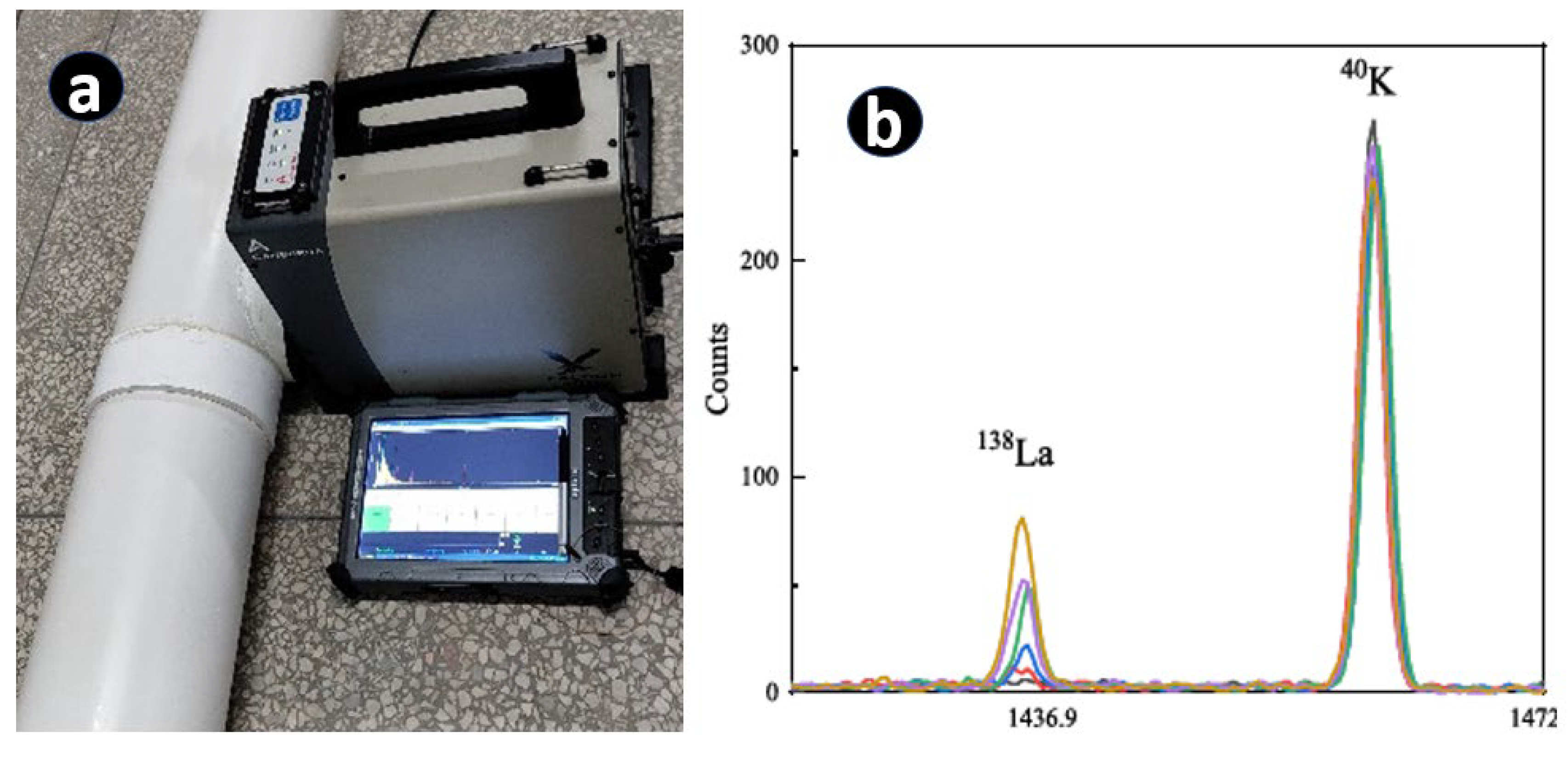
6.1. Portable XRF (pXRF or µXRF)
6.2. pLIBS
6.3. pXRD
6.4. Portable Raman Spectrometer
6.5. Fourier Transform Infrared (FTIR) Spectrometry
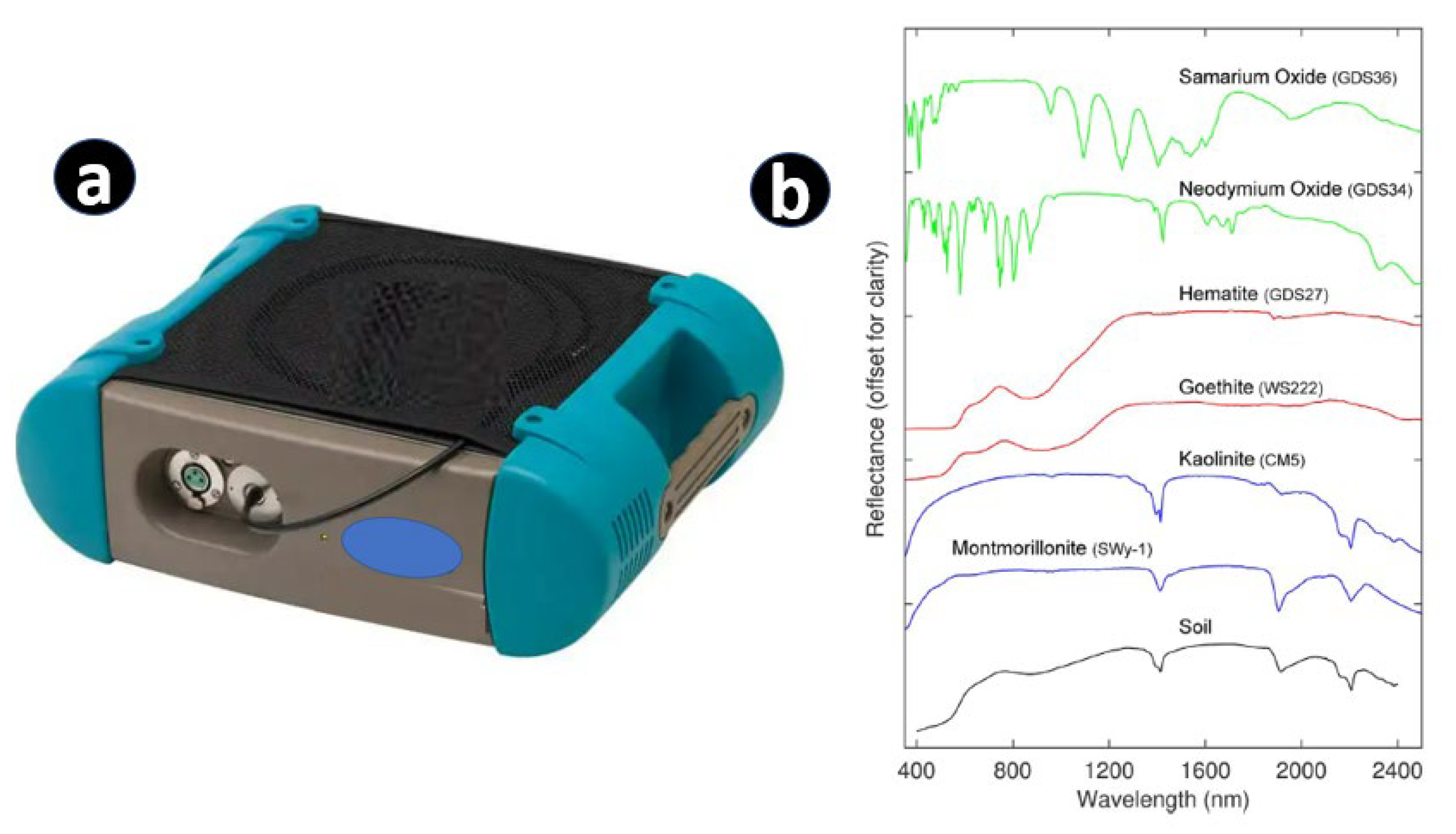
7. Hyperspectral Remote Sensing Techniques (Handheld, Drone, and Satellite-Based)
8. Electrochemical and Biosensors for the Detection of REE
9. Miscellaneous Analytical Techniques
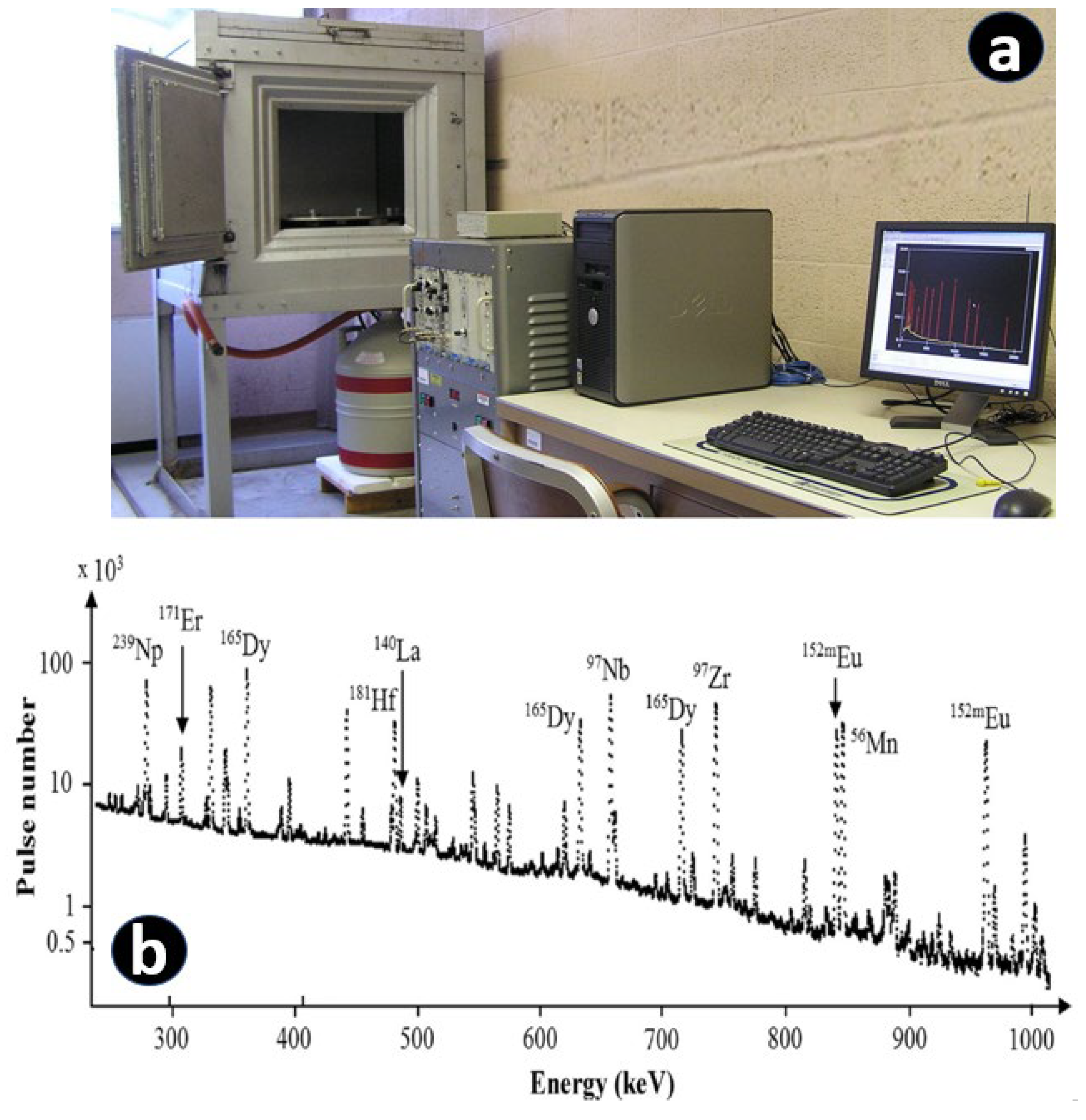
10. Online analysis of La by In-Situ Gamma Spectrometry during the Industrial Extraction Process
11. Comparison of Different Analytical Techniques for REE Analysis
12. Sample Preparation Methods for REE Studies (Acid, Fusion, and Microwave)
12.1. Acid Dissolution Methods
12.2. Fusion Dissolution Methods
12.3. Microwave, Ultrasound-Assisted, High-Pressure Digestion, and Infrared Heating Methods
13. Quality Assurance and Quality Control during Analysis
14. Conclusions
Acknowledgments
Conflicts of Interest
References
- Balaram, V. Recent trends in the instrumental analysis of rare earth elements in geological and industrial materials. Trends in Analytical Chemistry 1996, 15, 475-486. [CrossRef]
- Bandyopadhyay, D. K.; Ghosh, S.; Mondal, A.; Das, D. K. Role of rare earth elements as provenance indicator in coal seams: A case study from IB-River Coalfield, Orissa. Indian Minerals 2006, 60, 3, 171-180.
- Drobniak, A.; Mastalerz, M. Rare Earth Elements—A brief overview: Indiana Geological and Water Survey, Indiana Journal of Earth Sciences 2022, 4. [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2018. U.S. Geological Survey, Reston, Virginia 2018, 132e133. [CrossRef]
- European Commission. Critical materials for strategic technologies and sectors in the EU - a foresight study 2020, 100 . https://ec.europa.eu/docsroom/documents/42882.
- Fedele, L.; Plant, J. A.; De Vivo, B.; Lima, A. The rare earth element distribution over Europe: geogenic and anthropogenic sources. Geochemistry: Exploration, Environment, Analysis 2008, 8, 3–18.
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geoscience Frontiers 2019, 10, 4, 1285-1303. [CrossRef]
- Wagh, A. S.; Pinnock, W. R. Occurrence of scandium and rare earth elements in Jamaican bauxite waste. Economic Geology 1987, 82, 3, 757–761. [CrossRef]
- Cocker, M. D. Lateritic supergene rare earth element (REE) deposits. Arizona Geological Survey, Special Paper 9 2012, Chapter # 4, 1-20.
- Lister, T. E.; Diaz, L. A.; Clark, G. G.; Keller, P. Process development for the recovery of critical materials from electronic waste. United States 2016. https://www.osti.gov/biblio/1358185.
- Robert, Z.; Andrew, F.; Mark.; Jim, P. Maximizing REE Recovery in Geothermal Systems. United States 2018. [CrossRef]
- Hartzler, D.; Bhatt, C.; Jain, J.; McIntyre, D. L. Evaluating laser induced breakdown spectroscopy sensor technology for rapid source characterization of rare earth elements. Journal of Energy Resources Technology 2019. [CrossRef]
- Balaram, V. Rare Earth Element Deposits - Sources, and Exploration Strategies. Journal of Geological Society of India 2022, 98, 1210-1216. [CrossRef]
- Valetich, M.; Zivak, D.; Spandler, C.; Degeling, H.; Grigorescu, M. REE enrichment of phosphorites: An example of the Cambrian Georgina Basin of Australia, Chemical Geology 2022, 588, 120654. [CrossRef]
- Borsato, N. W.; Hoeijmakers, H. J.; Prinoth1, B.; Thorsbro, B.; Forsberg, R.; Kitzmann, D.; Jones, K.; Heng, K. The Mantis Network III: Expanding the limits of chemical searches within ultra-hot Jupiters - New detections of Ca, V, Ti, Cr, Ni, Sr, Ba, and Tb in KELT-9 b. Astronomy & Astrophysics 2023, 1-31. [CrossRef]
- Dai, S.; Finkelman, R. B.; French, D.; Hower, J. C.; Graham, I. T.; Zhao, F. Modes of occurrence of elements in coal: A critical evaluation. Earth-Science Reviews 2021, 222, 103815. [CrossRef]
- Cheatham, M. M.; Sangrey, W. F.; White, W. M. Sources of error in external calibration ICP-MS analysis of geological samples and an improved non-linear drift correction procedure. Spectrochimica Acta 1993, 48B, 3, E467-E506.
- Pu, Q.; Liu, P.; Hu, Z.; Su, Z. Spectrophotometric determination of the sum of rare earth elements by flow-injection on-line preconcentration with a novel aminophosphonic–carboxylic acid resin. Analytical Letters 2002, 35, 8, 1401–1414. [CrossRef]
- Saputra, H. A.; Anggraeni, A.; Mutalib, A.; Bahti, H. H. Development of a Fast Simultaneous Analysis Method for Determination of Middle Rare-Earth Elements in Monazite Samples. Jurnal Kimia Sains dan Aplikasi 2021, 24, 5, 177-184. [CrossRef]
- Potts, P.J. X-ray fluorescence analysis. In: Geochemistry. Encyclopedia of Earth Science 1998, Springer, Dordrecht. [CrossRef]
- De Vito, I. E.; Olsina, R. A.; Masi, A. N. Enrichment method for trace amounts of rare earth elements using chemofiltration and XRF determination. Fresenius’ Journal of Analytical Chemistry 2000, 368, 4, 392–396. [CrossRef]
- Juras, S. J.; Hickson, C. J.; Horsky, S. J.; Godwin, C. I.; Mathews, W. H. A practical method for the analysis of rare-earth elements in geological samples by graphite furnace atomic absorption and X-ray fluorescence, Chemical Geology 1987, 64, 1–2, 143-148. [CrossRef]
- Wu, W.; Xu, T.; Hao, Q.; Wang, Q.; Zhang, S.; Zhao, C. Applications of X-ray fluorescence analysis of rare earths in China. Journal of Rare Earths, 2010, 28, 30–36. [CrossRef]
- Pauw, E. D.; Tack, P.; Lindner, M.;. Ashauer, A. et al. Highly Sensitive Nondestructive Rare Earth Element Detection by Means of Wavelength-Dispersive X-ray Fluorescence Spectroscopy Enabled by an Energy Dispersive pn-Charge-Coupled-Device Detector. Anal. Chem. 2020, 92, 1, 1106–1113. [CrossRef]
- Adeti, PJ.; Amoako, G.; Tandoh,J. B.; Gyampo,O.; Ahiamadjie,H.; Amable, A.S.K.; Kansaana, C.; Annan, R. A.T.; Bamford, A. Rare-earth element comparative analysis in chosen geological samples using nuclear-related analytical techniques, Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 2023, 540, 122-128. [CrossRef]
- Sarker, S. K.; Bruckard, W.; Haque, N.; Roychand, R.; et al. Characterization of a carbonatite-derived mining tailing for the assessment of rare earth potential. Process Safety and Environmental Protection 2023, 173, 154-162. [CrossRef]
- Kurniawati, S.; Santoso, M.; Lestiani, D. D.; Adventini, N.; Yatu, W.; Syahfitri, N. Analytical Capabilities of EDXRF for Determination of Rare Earth Elements. Indonesian Journal of Nuclear Science and Technology 2021, 22, 1, 1-6. [CrossRef]
- Wenqi, W.U.; Tao, X. U.; Qian, H.; Qiang, W.; Shujie, Z.; Changyu, Z. Applications of X-ray fluorescence analysis of rare earths in China. Journal of Rare Earths 2013, 28, Spec. Issue, 30-33.
- Taam, I.; Jesus, C.S.; Mantovano J, L.; Gante, V. Quantitative Analysis or Rare Earths By X-Ray Fluorescence Spectrometry. International Nuclear Atlantic Conference - INAC 2013Recife, PE, Brazil, Associação Brasileira De Energia Nuclear – ABEN ISBN 2013, 978-85-99141-05-2.
- Balaram, V. Current and emerging analytical techniques for geochemical and geochronological studies. Geol. Jour. 2021, 56, 5, 2300-2359. [CrossRef]
- Rethfeldt, N.; Brinkmann, P.; Riebe, D.; Beitz, T.; Köllner, N.; Altenberger, U.; Löhmannsröben, H.-G. Detection of Rare Earth Elements in Minerals and Soils by Laser-Induced Breakdown Spectroscopy (LIBS) Using Interval PLS. Minerals 2021, 11, 1379. [CrossRef]
- Harikrishnan, S.; Ananthachar, A.; Choudhari, K.S.; George, S.D.; Chidangil, S.; Unnikrishnan, V.K. Laser-Induced Breakdown Spectroscopy (LIBS) for the Detection of Rare Earth Elements (REEs) in Meteorites. Minerals 2023, 13, 182. [CrossRef]
- Korotkova, N.A.; Baranovskaya, V.B.; Petrova, K.V. Microwave Digestion and ICP-MS Determination of Major and Trace Elements in Waste Sm-Co Magnets. Metals 2022, 12, 1308. [CrossRef]
- Nakayama, K.; Nakamura, T.; X-ray Fluorescence Analysis of Rare Earth Elements in Rocks Using Low Dilution Glass Beads. Anal. Sci. 2005, 21, 815–822. [CrossRef]
- Ndjama, J.; Mafany, G.; Ndondo, R.G.N.; Belmond, B. E.; Bessa, A. Z. E. Rare earth elements in surface waters and sediments of the Mgoua watershed, south western Cameroon. Arabian Journal of Geosciences 2022, 15, 1001. [CrossRef]
- Tanaka, T.; Lee, S. G.; Kim, T.; Han, S; et al. Precise determination of 14 REEs in GSJ/AIST geochemical reference materials JCp-1 (coral) and JCt-1 (giant clam) using isotope dilution ICP-quadrupole mass spectrometry. Geochemical Journal 2017, 51, 1-5. [CrossRef]
- Oliveira, S.M. B.; Larizzatti, F. E.; Fávaro, D.I.T.; Moreira, S. R. D.; Mazzilli,B. P.; Piovano, E. L. Rare earth element patterns in lake sediments as studied by neutron activation analysis. Journal of Radioanalytical and Nuclear Chemistry 2003, 258, 3, 531-535. [CrossRef]
- Awad, H.; Zakaly, H.M.H.; El-Taher, A.; Sebak, M. Determination of lanthanides in phosphate rocks by instrumental neutron activation analysis. June 2022AIP Conference Proceedings 2022, 2466, 1, 050002. [CrossRef]
- Figuelredo, A. M. G.; Marques, L. S. Determination of rare earths and other trace elements in the Brazilian geological standards, BB-1 and GB-1 by neutron activation analysis. Geochimica Brasiliensis 1989, 3, 1, 1-8.
- Bounouira, H.; Choukri, A.; Elmoursli, R. C.; Hakam, O.; Chakiri, S. Distribution of the rare earth elements in the sediments of the Bouregreg river (Morocco) using the instrumental neutron activation analysis (INAA). J. Appl. Sci. Environ. Manage. 2007, 11, 1, 57 - 60.
- Sitko, R.; Zawisza, B.; Czaja, M. Fundamental parameters method for determination of rare earth elements in apatites by wavelength-dispersive X-ray fluorescence spectrometry. Journal of Analytical Atomic Spectrometry 2005, 20, 8, 741. [CrossRef]
- Manard, B. T.; Wylie, E. M.; Willson, S. P. Analysis of Rare Earth Elements in Uranium Using Handheld Laser-Induced Breakdown Spectroscopy (HH LIBS). Applied Spectroscopy 2018, 000370281877543. [CrossRef]
- Alamoudi, Z.; El-Taher, A. Application of Nuclear Analytical Techniques in Elemental Characterization of Wadi El-Nakhil Alabaster, Central EasternDesert, Egypt. Science and Technology of Nuclear Installations, 2016, 2892863. [CrossRef]
- Klinkhammer, G.; German, C. R.; Elderfield, H.; Greaves, M. J.; Mitra, A. Rare earth elements in hydrothermal fluids and plume particulates by inductively coupled plasma mass spectrometry. Marine Chemistry, 1994, 45, 3, 179–186. [CrossRef]
- Wu, C. C. Advanced and Applied Studies on Ultra-Trace Rare Earth Elements (REEs) in Carbonates Using SN-ICPMS and LA-ICPMS. Ph.D Thesis. National Taiwan University, Taipei, Taiwan. Springer Nature Singapore Pte Ltd. 2021, 1-74.
- Vukotić, P. Determination of rare earth elements in bauxites by instrumental neutron activation analysis. J. Radioanal. Chem. 1983, 78, 105–115. [CrossRef]
- Baidya, T.K.; Mondal, S.K.; Balaram, V.; Parthasarathi, R.; Verma, R.; Mathur, P. K. PGE-Ag-Au mineralisations in a Cu-Fe-Ni sulphide-rich breccia zone of the Precambrian Nuasahi ultramafic-mafic complex, Orissa, India. Journal of the Geological Society of India 1999, 54, 473e482.
- Silachyov, I. Zircon concentrate analysis for sixteen rare earth elements by the complex of nuclear analytical methods. Journal of Radioanalytical and Nuclear Chemistry 2023, 332, 2017–2026. [CrossRef]
- Ravisankar, R.; Manikandan, E.; Dheenathayalu, M.; Rao, B.; Seshadreesan, N.P.; Nair, K.G.M. Determination and distribution of rare earth elements in beach rock samples using instrumental neutron activation analysis (INAA). Nuclear Instruments and Methods in Physics Research B 2006, 251, 496–500. [CrossRef]
- Krishnan, K.; Saion, E. Distributions of Rare Earth Element (REE) in Mangrove Surface Sediment by Nuclear Technique. International Journal 2022, 03, eISSN:2600-7320.
- Ahmed, M. E.; Bounouira, H.; Abbo, M. A.; et al. Utilizing the k0-IAEA program to determine rare earth elements in soil samples from gold-mining areas in Sudan. J Radioanal Nucl Chem. 2023, 332, 9. [CrossRef]
- Kin, F. D.; Prudêncio, M. I.; Gouveia, M. Â.; Magnusson, E. Determination of Rare Earth Elements in Geological Reference Materials: A Comparative Study by INAA and ICP-MS. Geostandards and Geoanalytical Research 1999, 23, 1, 47–58. [CrossRef]
- El-Taher, A. Nuclear Analytical Techniques for Detection of Rare Earth Elements, J. Rad. Nucl. Appl. 2018, 3, 1, 53-64. [CrossRef]
- Ghannadpour, S.S.; Hezarkhani, A. Prospecting rare earth elements (REEs) using radiation measurement: case study of Baghak mine, Central Sangan iron ore mine, NE of Iran. Environ Earth Sci. 2022, 81, 363. [CrossRef]
- Huang, Y.;Wen,W.; Liu, J.; Liang, X.; Yuan,W.; Ouyang, Q.; Liu, S.; Gok, C.;Wang, J.; Song, G. Preliminary Screening of Soils Natural Radioactivity and Metal(loid) Content in a Decommissioned Rare Earth Elements Processing Plant, Guangdong, China. Int. J. Environ. Res. Public Health 2022, 19, 14566. [CrossRef]
- Walsh, A. (1955). The application of atomic absorption spectra to chemical analysis. Spectrochimica Acta, 7, 108–117.
- L’vov, B. V. A continuum source vs. line source on the way toward absolute graphite furnace atomic absorption spectrometry, Spectrochimica Acta Part B: Atomic Spectroscopy 1999, 54, 11, 1637-1646. [CrossRef]
- Balaram, V.; Sunder Raju, P.V.; Ramesh, S.L.; Anjaiah, K.V.; Dasaram, B.; Manikyamba, C.; Ram Mohan, M.; Sarma, D. S. Rapid partial dissolution method in combination with atomic absorption spectroscopy techniques for use in geochemical exploration. Atomic Spectroscopy 1999, 20, 4, 155-160.
- Hammer, M. R. A magnetically excited microwave plasma source for atomic emission spectroscopy with performance approaching that of the inductively coupled plasma, Spectrochimica Acta Part B: Atomic Spectroscopy 2008, 63, 4, 456-464. [CrossRef]
- Balaram, V. Microwave plasma atomic emission spectrometry (MPAES) and its applications: A critical review. Microchemical Jour. 2020,159,18. [CrossRef]
- Balaram, V.; Dharmendra, V.; Roy, P.; Taylor, C.; Kar, P.; Raju, A. K.; Krishnaiah, A. Determination of Precious Metals in Rocks and Ores by Microwave Plasma- Atomic Emission Spectrometry (MP-AES) for Geochemical Prospecting, Current Science 2013, 104, 9, 1207-1215.
- Balaram V.; Dharmendra, V.; Roy, P.; Taylor, C.; Kamala, C. T.; Satyanarayanan, M.; Kar, P.; Subramanyam, K. S. V.; Raju, A. K.; Krishnaiah, A. Analysis of Geochemical Samples by Microwave Plasma-AES, Atomic Spectroscopy 2014, 35, 2, 65-78.
- Kamala, C. T.; Balaram, V.; Dharmendra, V.; Roy, P.; Satyanarayanan, M.; Subramanyam, K. S. V. Application of Microwave Plasma Atomic Emission Spectrometry (MP-AES) for Environmental Monitoring of Industrially Contaminated sites in Hyderabad City", Environmental Monitoring and Assessment 2014, 186, 7097–7113. [CrossRef]
- Helmeczi, E.; Wang, Y.; Brindle, I. D. A novel methodology for rapid digestion of rare earth element ores and determination by microwave plasma-atomic emission spectrometry and dynamic reaction cell-inductively coupled plasma-mass spectrometry. Talanta 2016, 160, 521–527. [CrossRef]
- Varbanova, E.; Stefanova, V. A comparative study of inductively coupled plasma optical emission spectrometry and microwave plasma atomic emission spectrometry for the direct determination of lanthanides in water and environmental samples. Ecology & Safety 2015, 9, 362-374.
- Greenfield, S.; Jones, I. L. I.; Berry, C. T. High pressure plasmas as spectroscopic emission sources. Analyst 1964, 89, 713–720.
- Wendt, R. H.; Fassel, V. Inductively-coupled plasma spectrometric excitation source. Analytical Chemistry 1965, 37, 920e922.
- Balaram, V.; Anjaiah, K.V.; Reddy, M.R.P. A comparative study o the trace and rare earth element analysis o an Indian Polymetallic Nodule Reference Sample by Inductively Coupled Plasma Atomic Emission Spectrometry and Inductively Coupled Plasma Mass Spectrometry. Analyst 1995, 120, 1401-1406.
- Kumar, N. S.; Dharmendra, V.; Sreenivasulu, V.; Asif, M.; Balaram, V. Separation and Preconcentration of Pb and Cd in Water Samples using 3-(2-hydroxyphenyl)-1H-1,2,4-triazole-5(4H)-thione (HTT) and their Determination by Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES), Metals, 2017, 7, 240, 1-12. [CrossRef]
- Makombe, M.; van der Horst, C.; Silwana, B.; Iwuoha, E.; Somerset, V. Optimization of Parameters for Spectroscopic Analysis of Rare Earth Elements in Sediment Samples, INTECH, Open Science 2017, Chapter 3: . [CrossRef]
- Gorbatenko, A. A.; Revina, E. I. A review of instrumental methods for determination of rare earth elements. Inorganic Materials 2015, 51,14, 1375–1388. [CrossRef]
- Pradhan, S. R.; Ambade, B. Extractive separation of rare earth elements and their determination by inductively coupled plasma optical emission spectrometry in geological samples. J. Anal. At. Spectrom. 2020, 35, 1395-1404. [CrossRef]
- Amaral, C.D.B.; Machado, R.C.; Barros, J. A. V. A..; Virgilio, A. et al. Determination of rare earth elements in geological and agricultural samples by ICP-OES, Spectroscopy 2017, 32, 10, 32-36.
- Tupaz, C.A.J.; Gregorio, C. G. C.; Arcilla, C. Determination of Scandium (Sc), Yttrium (Y), and Rare-Earth Elements (REEs) in Mafic and Ultramafic Rock Powder by a Modified and Validated Digestion Protocol and Inductively Coupled Plasma – Mass Spectrometry (ICP-MS). Analytical Letters 2022, 56, 6, 932-943. [CrossRef]
- Zhu, Y. Determination of rare earth elements in seawater samples by inductively coupled plasma tandem quadrupole mass spectrometry after coprecipitation with magnesium hydroxide. Talanta 2020, 209, 120536. [CrossRef]
- Robinson, P.; Townsend, A. T.; Yu, Z.; Münker, C. Determination of Scandium, Yttrium and Rare Earth Elements in Rocks by High Resolution Inductively Coupled Plasma-Mass Spectrometry. Geostandards and Geoanalytical Research 1999, 23, 1, 31–46. [CrossRef]
- Bauchle, M.; Ludecke, T.; Rabieh, S.; Calnek, K.; Bromage, T. G. Quantification of 71 detected elements from Li to U for aqueous samples by simultaneous-inductively coupled plasma-mass spectrometry. RSC Adv. 2018, 8, 8, 37008–37020. [CrossRef]
- Leitzke, F. P.; Wegner, A. C.; Porcher, C. C.; Vieira, N.I.M.; Berndt, J.; Klemme, J.; Conceição, R. V. Whole-rock trace element analyses via LA-ICP-MS in glasses produced by sodium borate flux fusion. Braz. J. Geol. 2021, 51, 2. [CrossRef]
- Kin, F. D.; Prudêncio, M. I.; Gouveia, M. Â.; Magnusson, E. Determination of Rare Earth Elements in Geological Reference Materials: A Comparative Study by INAA and ICP-MS. Geostandards and Geoanalytical Research 1999, 23, 1, 47–58. [CrossRef]
- Hirose, F.; Itoh, S.; Okochi, H. Determination of Rare-earth Elements in Metallic La, Pr, Nd, Gd and Tb by Glow Discharge Mass Spectrometry. Tetsu-to-Hagane 1991, 77, 4, 598–604. [CrossRef]
- Pedarnig, J.D.; Trautner, S.; Grünberger, S.; Giannakaris, N.; Eschlböck-Fuchs, S.; Hofstadler, J. Review of Element Analysis of Industrial Materials by In-Line Laser—Induced Breakdown Spectroscopy (LIBS). Appl. Sci. 2021, 11, 9274. [CrossRef]
- Harmon, R. S; Senesi, G. S. Laser-Induced Breakdown Spectroscopy – A geochemical tool for the 21st century. Applied Geochemistry 2021, 128, 104929. [CrossRef]
- Alamelu, D.; Sarkar, A.; Aggarwal, S. K. Laser-induced breakdown spectroscopy for simultaneous determination of Sm, Eu and Gd in aqueous solution. Talanta 2008, 77, 1, 256–261. [CrossRef]
- Abedin, K. M.; Haider, A. F. M. Y.; Rony, M. A.; Khan, Z. H. Identification of multiple rare earths and associated elements in raw monazite sands by laser-induced breakdown spectroscopy. Optics & Laser Technology 2011, 43, 1, 45–49. [CrossRef]
- Bhatt, C. R.; Jain, J. C.; Goueguel, C. L.; McIntyre, D. L.; Singh, J. P. Determination of Rare Earth Elements in Geological Samples Using Laser-Induced Breakdown Spectroscopy (LIBS). Applied Spectroscopy 2017, 72, 1, 114–121. [CrossRef]
- Unnikrishnan, V. K.; Nayak, R.; Devangad, P.; Tamboli, M. M.; Santhosh, C.; Kumar, G. A.; Sardar, D. K. Calibration based laser-induced breakdown spectroscopy (LIBS) for quantitative analysis of doped rare earth elements in phosphors. Materials Letters 2013, 107, 322–324. [CrossRef]
- Long, J.; Song, W.R.; Hou, Z.Y.; Wang, Z. A data selection method for matrix effects and uncertainty reduction for laser-induced breakdown spectroscopy, Plasma Science & Technology 2023, 25, 7, 075501. [CrossRef]
- Haider, A. F. M. Y.; Khan, Z. H. Identification of multiple rare earths and other associated elements in zircon by laser-induced breakdown spectroscopy. J. Bangladesh Acad. Sci. 2020, 44, 1, 59-68. [CrossRef]
- Liu, C.; Jiang, J.; Jiang, J.; Zhou, Z.; Ye, S. Automatic coal-rock recognition by laser-induced breakdown spectroscopy combined with an artificial neutral network. Spectroscopy, 2023, 38, 2, 23-28.
- Gaft, M.; Raichlin, Y.; Pelascini, F.; Panzer, G.; Motto Ros, V. Imaging rare-earth elements in minerals by laser-induced plasma spectroscopy: Molecular emission and plasma-induced luminescence. Spectrochimica Acta Part B: Atomic Spectroscopy 2019, 151, 12-19. [CrossRef]
- Afgan, M.S.; Hou, Z.; Song,W.; Liu, J.; Song, Y.; Gu, W.; Wang, Z. On the Spectral Identification and Wavelength Dependence of Rare-Earth Ore Emission by Laser-Induced Breakdown Spectroscopy. Chemosensors 2022, 10, 350. [CrossRef]
- Houk, R. S.; Fassel, V. A.; Flesch, G. D.; Svec, H. J.; Gray, A. L.; Taylor, C. E. Inductively coupled argon plasma as an ion source for mass spectrometric determination of trace elements. Analytical Chemistry 1980, 52, 2283–2289. [CrossRef]
- Atsunori, N.; Ran, K.; Atsuyuki, O. Multi-element analysis of geological samples using ICP-MS equipped with integrated sample introduction and aerosol dilution systems. Bulletin of the Geological Survey of Japan 2023, 74, 2, 71–85.
- Mnculwane, H.T. Rare Earth Elements Determination by Inductively Coupled Plasma Mass Spectrometry after Alkaline Fusion Preparation. Analytica 2022, 3, 135–143. [CrossRef]
- Veerasamy, N.; Sahoo, S.K.; Murugan, R.; Kasar, S.; Inoue, K.; Fukushi, M.; Natarajan, T. ICP-MS Measurement of Trace and Rare Earth Elements in Beach Placer-Deposit Soils of Odisha, East Coast of India, to Estimate Natural Enhancement of Elements in the Environment. Molecules 2021, 26, 7510. [CrossRef]
- Lin, R.; Bank, T. L.; Roth, E. A.; Granite, E. J.; Soong, Y. Organic and inorganic associations of rare earth elements in central Appalachian coal. International Journal of Coal Geology 2017, 179, 295–301. [CrossRef]
- Nguyen,V. H.; Ramzan, M.; Kifle D.; Wibetoe, G. A simple separation system for elimination of molecular interferences for purity determination of europium and ytterbium oxides by HPLC-ICP-MS. J. Anal. At. Spectrom. 2020, 35, 11, 2594–2599. [CrossRef]
- Liu, W.; An, Y.; Qu, Q.; Li, P. et al. An efficient method for separation of REEs from Ba for accurate determination of REEs contents in Ba-rich samples by ICP-MS. Journal of Analytical Atomic Spectrometry 2023, 38, 449-456. [CrossRef]
- Wysocka, I. Determination of rare earth elements concentrations in natural waters – A review of ICP-MS measurement approaches. Talanta 2021, 221, 121636. [CrossRef]
- Palozzi, J.; Bailey, J. G.; Tran, Q. A.; Stanger, R. A characterization of rare earth elements in coal ash generated during the utilization of Australian coals. 2023. [CrossRef]
- El-Taher, A.; Ashry, A.; Ene, A.; Almeshari, M.; Zakaly, H. Determination of phosphate rock mines signatures using XRF and ICP-MS elemental analysis techniques: Radionuclides, oxides, rare earth and trace elements. Romanian Reports in Physics 2022 xx, xyz.
- Balaram, V. Inductively Coupled Plasma-Tandem Mass Spectrometry (ICP-MS/MS) and Its Applications, Journal of ISAS 2022, 1, 1, 1-26.
- Jason, P.; Bailey, J. G.; Tran, Q. A.; Stanger, R. A characterization of rare earth elements in coal ash generated during the utilization of Australian coals. International Journal of Coal Preparation and Utilization 2023. [CrossRef]
- Krasavtseva, E.; Sandimirov, S.; Elizarova, I.; Makarov, D. Assessment of Trace and Rare Earth Elements Pollution in Water Bodies in the Area of Rare Metal Enterprise Influence: A Case Study—Kola Subarctic Water, 2022, 14, 3406. [CrossRef]
- Wencai, X.; Zhigang, Z.; Xiaoyun, S.; et al. On pretreatment method for the determination of rare earth elements in deep sea REY-rich sediments by inductively coupled plasma-mass spectrometry. Marine Geology Frontiers 2022, 38, 9, 92-96. [CrossRef]
- Li, H.; Tong, R.; Guo, W.; Xu, Q.; Tao, D.; Lai,Y.; Jina, L.; Hu, S. Development of a fully automatic separation system coupled with online ICP-MS for measuring rare earth elements in seawater.: RSC Adv. 2022, 12, 24003. [CrossRef]
- Wysocka, I. A; Kurzawa, D. K.; Porowski, A. Development and validation of seaFAST-ICP-QMS method for determination of rare earth elements total concentrations in natural mineral waters. Food Chemistry 2022, 388, 133008. [CrossRef]
- Li D.; Wang X.; Huang K.; Wang Z. Multielemental Determination of Rare Earth Elements in Seawater by Inductively Coupled Plasma Mass Spectrometry (ICP-MS) After Matrix Separation and Pre-concentration With Crab Shell Particles. Front. Environ. Sci. 2021, 9,781-996. [CrossRef]
- Balaram, V. Strategies to overcome interferences in elemental and isotopic geochemical studies by quadrupole ICP-MS: A critical evaluation of the recent developments. Rapid Commun. Mass Spectrom. 2021, 1-29, e9065. [CrossRef]
- Zhu, Y. Determination of Rare Earth Elements by Inductively Coupled Plasma–Tandem Quadrupole Mass Spectrometry with Nitrous Oxide as the Reaction Gas. Front. Chem. 2022, 10:912938. [CrossRef]
- Santoro, A.; Thoss, V.; Ribeiro Guevara, S.; Urgast, D.; Raab, A.; Mastrolitti, S.; Feldmann, J. Assessing rare earth elements in quartz rich geological samples. Applied Radiation and Isotopes, 2016, 107, 323–329. [CrossRef]
- Lancaster, S. T.; Prohaska, T.; Irrgeher, J. Characterisation of gas cell reactions for 70+ elements using N2O for ICP tandem mass spectrometry measurements. J. Anal. At. Spectrom. 2023. 38, 1135-1145. [CrossRef]
- Ntiharirizwa, S.; Boulvais, P.; Poujol, M.; Branquet, Y.; et al. Geology and U-Th-Pb Dating of the Gakara REE Deposit, Burundi. Minerals 2018, 8, 394. [CrossRef]
- Myers, P.; Li, G.; Yang, P.; Hieftje, G. M. An inductively coupled plasma-time-of-flight mass spectrometer for elemental analysis. Part I: Optimization and characteristics. Journal of the American Society for Mass Spectrometry 1994, 5, 1008–1016.
- Mahoney, P. P.; Ray, S. J.; Hieftje, G. M.; Li, G. Continuum background reduction in orthogonal-acceleration time-of-flight mass spectrometry with continuous ion source. Journal of the American Society for Mass Spectrometry 1997, 125, 125–131.
- Balaram, V.; Satyanarayanan, M.; Murthy, P. K.; Mohapatra, C.; Prasad, K. L. Quantitative multi-element analysis of cobalt crust from Afanasy-Nikitin seamount in the North Central Indian Ocean by inductively coupled plasma time-of-flight mass spectrometry. MAPAN-J. Metrology Society of India 2013, 28, 2, 63–77.
- Dick, D.; Wegner, A.; Gabrielli, P.; Ruth, U.; Barbante, C.; Kriews, M. Rare earth elements determined in Antarctic ice by inductively coupled plasma—Time of flight, quadrupole and sector field-mass spectrometry: An inter-comparison study. Analytica Chimica Acta 2008, 621, 2, 140–147. [CrossRef]
- Nakazato, M.; Asanuma, H.; Niki, S.; Iwano, H.; Hirata, T. Depth-Profiling Determinations of Rare Earth Element Abundances and U-Pb Ages from Zircon Crystals Using Sensitivity-Enhanced Inductively Coupled Plasma-Time of Flight-Mass Spectrometry. Geostandards and Geoanalytical Research 2022, 46, 4, 603-620. [CrossRef]
- Peng, J.; Li, D.; Hollings, P.; Fu, Y.; Sun, X. Visualization of critical metals in marine nodules by rapid and high-resolution LA-ICP-TOF-MS mapping. Ore Geology Reviews 2023, 154, 105342. [CrossRef]
- Chew, D.; Drost, K.; Marsh, H.; Petrus, J. A. LA-ICP-MS imaging in the geosciences and its applications to geochronology, Chemical Geology 2021, 559, 119917. [CrossRef]
- Satyanarayanan, M.; Balaram, V.; Sawant, S. S.; Subramanyam, K. S. V.; Krishna, V.; Dasaram, B..; Manikyamba, C. Rapid determination of REE, PGE and other trace elements in geological and environmental materials by HR-ICP-MS2018Atomic Spectroscopy 2018, 39, 1, 1–15.
- Nath, B. N.; Balaram, V.; Sudhakar, M.; Pluger, W. L. Rare earth element geochemistry of ferromanganese deposits from the Indian Ocean. Marine Chemistry 1992, 38, 185–208. [CrossRef]
- Charles, C.; Barrat, J. A .; Pelleter, E. Trace element determinations in Fe–Mn oxides by high resolution ICP-MS after Tm addition. Talanta 2021, 233, 122446. [CrossRef]
- Balaram, V.; Roy, P.; Subramanyam, K. S. V.; Durai, L.; Mohan, M. R.; Satyanarayanan, M.; Vani, K. REE geochemistry of seawater from Afanasy-Nikitin seamount in the eastern equatorial Indian Ocean by high resolution inductively coupled plasma mass spectrometry. Indian Journal of Geo-Marine Science 2015, 44, 3, 339–347.
- Gao, J., L. D.; Tom van Loon, A. J.; Hower, J. C. et al. Reconstruction of provenance and tectonic setting of the Middle Jurrasic Yan’an Formation (Ordos Basin, North China) by analysis of major, trace and rare earth elements in the coals. Ore Geology Reviews 2022, 151, 105218. [CrossRef]
- Pedreira, W. R.; Sarkis, J. E. S.; Rodrigues, C.; Queirozb, C. A. D. T,; Abrao, A. Determination of trace amounts of rare earth elements in highly pure praseodymium oxide by double focusing inductively coupled plasma mass spectrometry and high-performance liquid chromatography. Journal of Alloys and Compounds 2001, 323–324, 49–52. [CrossRef]
- Soto-Jiménez, M. F.; Martinez-Salcido, A. I.; Morton-Bermea, O.; Ochoa-Izaguirre, M. J. Lanthanoid analysis in seawater by seaFAST-SP3™ system in off-line mode and magnetic sector high-resolution inductively coupled plasma source mass spectrometer. MethodsX. 2022, 9, 101625. [CrossRef]
- Zhu, Y.; Nakano, K.; Shikamori, Y.; Itoh, A. Direct determination of rare earth elements in natural water samples by inductively coupled plasma tandem quadrupole mass spectrometry with oxygen as the reaction gas for separating spectral interferences. Spectrochimica Acta Part B: Atomic Spectroscopy 2021, 179, 106100. [CrossRef]
- Lawrence, M. G.; Greig, A.; Collerson, K.D.; Kamber, B. S. Direct quantification of rare earth element concentrations in natural waters by ICP-MS, Appl. Geochem. 2006, 21, 839–848.
- Chung, C.-H.; Brenner, I.; You, C. F. Comparison of micro-concentric and membrane desolvation sample introduction systems for determination of low rare earth element concentrations in surface and subsurface waters using sector field inductively coupled plasma mass spectrometry, Spectrochim. Acta Part B 2009, 64, 849–856.
- Rousseau, T.C.C.; Sonke, J.E.; Chmeleff, F.; Candaudap, J.; Lacan, F.; Boaventura, G.; Seyler, P.; Jeandel, C. Rare earth element analysis in natural waters by multiple isotope dilution – sector field ICP-MS, J. Anal. At. Spectrom. 2013, 28, 573–584.
- Yeghicheyan, D.; Carignan, J.; Valladon, M.; Coz, M. B,; Cornec, F. L.; Castrec-Rouelle, M.; Serrat, E. A Compilation of Silicon and Thirty-one Trace Elements Measured in the Natural River Water Reference Material SLRS-4 (NRC-CNRC). Geostandards and Geoanalytical Research 2001, 25, 2-3, 465–474. [CrossRef]
- Bäuchle, M.; Lüdecke, T.; Rabieh, S.; Calnek, K.; Bromage, T.G. Quantification of 71 detected elements from Li to U for aqueous samples by simultaneous-inductively coupled plasma-mass spectrometry. RSC Adv. 2018, 8, 37008–37020. [CrossRef]
- Rabieh, S.; Bayaraa, O.; Romeo, E.; Amosa, P. et al. MH-ICP-MS Analysis of the Freshwater and Saltwater Environmental Resources of Upolu Island, Samoa. Molecules 2020, 25, 4871. [CrossRef]
- Walder, A.J.; Freedman, P.A. Communication. Isotopic ratio measurement using a double focusing magnetic sector mass analyzer with an inductively coupled plasma as an ion source. J. Anal. At. Spectrom. 1992, 7 (3), 571. [CrossRef]
- Balaram, V.; Rahaman, W.; Roy, P. Recent Advances in MC-ICPMS Applications in the Earth, Environmental Sciences: Challenges and Solution, Geosystems and Geoenvironment 2022, 1, 2, 100019. [CrossRef]
- Bai, J. H.; Liu, F.; Zhang, Z. F.; Ma, J. L.; et al. Simultaneous measurement stable and radiogenic Nd isotopic compositions by MC-ICP-MS with a single-step chromatographic extraction technique. J. Anal. At. Spectrom. 2021, 36, 2703-2695. [CrossRef]
- Bai, J. H.; Lin, M.; Zhong, S. X.; Deng, Y, N.; Zhang, L.; Kai, L.; Wu, H.; Ma, J.; Wei, G, High intermediate precision Sm isotope measurements in geological samples by MC-ICP-MS. . Anal. At. Spectrom. 2023, 38, 629-637. [CrossRef]
- Lee, S. G.; Ko, K. S. Development of an analytical method for accurate and precise determination of rare earth element concentrations in geological. materials using an MC-ICP-MS and group separation. Front. Chem. 2023. 10:906160. [CrossRef]
- Kent, A. J. R.; Jacobsen, B.; Peate, D. W.; Waight, T. E.; Baker, J. A. Isotope Dilution MC-ICP-MS Rare Earth Element Analysis of Geochemical Reference Materials NIST SRM 6 10, NIST SRM 6 12, NIS T SRM 6 14, BHVO-2G, BHVO-2, BCR-2G, JB-2, WS-E, W-2, AGV-1 and AGV-2. Geostandards and Geoanalytical Research 2004, 28, 3, 417-429. [CrossRef]
- Pourmand, A.; Dauphas, N.; Ireland, T. J. A novel extraction chromatography and MC-ICP-MS technique for rapid analysis of REE, Sc and Y: Revising CI-chondrite and Post-Archean Australian Shale (PAAS) abundances. Chemical Geology 2012, 291, 38–54. [CrossRef]
- Baker, J.; Waight, T.; Ulfbeck, D. Rapid and highly reproducible analysis of rare earth elements by multiple-collector inductively coupled plasma mass spectrometry. Geochimica et Cosmochimica Acta 2002, 66, 20, 3635–3646. [CrossRef]
- Yang, X.; Kozar, D.; Gorski, D. et al. Using yttrium as an indicator to estimate total rare earth element concentration: a case study of anthracite-associated clays from northeastern Pennsylvania. Int J Coal Sci Technol 2020, 7, 652–661. [CrossRef]
- Li, X. C.; Yang, K. F.; Spandler, C.; Fan, H. R.; Zhou, M. F.; Hao, J. L.; Yang, Y.H. The effect of fluid-aided modification on the Sm-Nd and Th-Pb geochronology of monazite and bastnäsite: Implication for resolving complex isotopic age data in REE ore systems. Geochimica et Cosmochimica Acta 2021, 300, 1–24. [CrossRef]
- Guerra-Sommer, M.; Cazzulo-Klepzig, M.; Menegat, R et al. Geochronological data from the Faxinal coal succession, southern Paraná Basin, Brazil: A preliminary approach combining radiometric U-Pb dating and palynostratigraphy. Journal of South American Earth Sciences 2008, 25, 2, 246-256, . [CrossRef]
- Chafe, A. N.; Hanchar, J. M.; Fisher, C.; Piccoli, P. M.; Crowley, J. L.; Dimmell, P. M. Direct dating and characterization of the Pope's Hill REE Deposit, Labrador. American Geophysical Union, Fall Meeting 2012, abstract id. V43C-2845.
- Ramesh, R.; Ramanathan, A.; Ramesh, S.; Purvaja, R.; Subramanian, V. Distribution of rare earth elements and heavy metals in the surficial sediments of the Himalayan River system. Geochem. J. 2000, 34, 295–319. [CrossRef]
- Natarajan, T.; Inoue, K.; Sahoo, S. K. Rare earth elements geochemistry and 234U/238U, 235U/238U 2 isotope ratios of the Kanyakumari beach placer deposits: Occurrence and provenance. Minerals 2023, 13.
- Compston, W., Pidgeon, R. Jack Hills, evidence of more very old detrital zircons in Western Australia. Nature 1986, 321, 766–769. [CrossRef]
- Sindern, S. Analysis of Rare Earth Elements in Rock and Mineral Samples by ICP-MS and LA-ICP-MS. Physical Sciences Reviews 2017, 2 , 2. [CrossRef]
- Campbell, L.S.; Compston, W.; Sircombe, K.N. et al. Zircon from the East Orebody of the Bayan Obo Fe–Nb–REE deposit, China, and SHRIMP ages for carbonatite-related magmatism and REE mineralization events. Contrib Mineral Petrol. 2014, 168, 1041. [CrossRef]
- Bhunia, S.; Rao, N.V.C.; Belyatsky, B.; Talukdar, D.; Pandey, R.; Lehmann, B. U-Pb Zircon SHRIMP dating of the Carbonatite hosted REE deposit (Kamthai), Late Cretaceous polychronous Sarnu Dandali alkaline Complex, NW India: links to the Plume-related metallogeny and CO2 outgassing at the K-Pg boundary. Gondwana Research 2022, 112, 116-125. [CrossRef]
- Sano, Y.; Terada, K.; Fukuoka, T. High mass resolution ion microprobe analysis of rare earth elements in silicate glass, apatite and zircon: lack of matrix dependency. Chemical Geology 2002, 184, 3-4, 217–230. [CrossRef]
- Kolker, A.; Scott, C.; Hower, J. C.; Vazquez, J. A.; Lopano, C. L.; Dai, S. Distribution of rare earth elements in coal combustion fly ash, determined by SHRIMP-RG ion microprobe. International Journal of Coal Geology 2017, 184, 1-10. [CrossRef]
- Hong, J.; Khan, T.; Li, W.; Khalil, Y. S.; Narejo, A. A.; Rashid, M. U.; Zeb, M. J. SHRIMP U–Pb ages, mineralogy, and geochemistry of carbonatite–alkaline complexes of the Sillai Patti and Koga areas, NW Pakistan: Implications for petrogenesis and REE mineralization. Ore Geology Reviews 2021, 139, 104547. [CrossRef]
- Balaram, V.; Sawant, S. S. Indicator Minerals, Pathfinder Elements, and Portable Analytical Instruments in Mineral Exploration Studies. Minerals 2022, 12, 394. [CrossRef]
- Jo, J.; Shin, D. Geochemical characteristics of REE-enriched weathered anorthosite complex in Hadong district, South Korea. Geochemical Journal 2023, 57, 1, 13–27. [CrossRef]
- Villanova-de-Benavent, C.; Proenza, J. A.; Torro, L.; Aiglsperger, T.; et al. REE ultra-rich karst bauxite deposits in the Pedernales Peninsula, Dominican Republic: Mineralogy of REE phosphates and carbonates. Ore Geology Reviews 2023, 157, 105422. [CrossRef]
- Reed, S. J. B.; Buckley, A. Rare-earth element determination in minerals by electron-probe microanalysis: application of spectrum synthesis. Mineralogical Magazine 1998, 62, 1, 1-8.
- Wu, L.; Ma, L.; Huang, G.; Li, J.; Xu, H. Distribution and Speciation of Rare Earth Elements in Coal Fly Ash from the Qianxi Power Plant, Guizhou Province, Southwest China. Minerals 2022, 12, 1089. [CrossRef]
- Balaram, V. Potential Future Alternative Resources for Rare Earth Elements: Opportunities and Challenges. Minerals 2023, 13, 425. [CrossRef]
- Sano, Y.; Terada, K.; Hidaka, H.; Nishio, Y.; Amakawa, H.; Nozaki, Y. Ion-Microprobe Analysis of Rare Earth Elements in Oceanic Basalt Glass. Analytical Sciences 1999, 15,743-748.
- Zinner, E.; Crozaz, G. A method for the quantitative measurement of rare earth elements in the ion microprobe. International Journal of Mass Spectrometry and Ion Processes 1986, 69, 1, 17–38. [CrossRef]
- Shi, L.; Sano, Y.; Takahata, N.; Koike, M.; Morita. T.; Koyama, Y.; Kagoshima. T.; Li, Y.; Xu, S.; Liu, C. NanoSIMS Analysis of Rare Earth Elements in Silicate Glass and Zircon: Implications for Partition Coefficients. Front. Chem. 2022, 10, 844953. [CrossRef]
- Ling, X. X.; Li, Q.L.; Liu, Y.; Yang, Y. H et al. In situ SIMS Th–Pb dating of bastnaesite: constraint on the mineralization time of the Himalayan Mianning–Dechang rare earth element deposits. J. Anal. At. Spectrom. 2016, 31, 1680. [CrossRef]
- Sahijpal, S.; Marhas, K. K.; Goswami, J. N.; analytical procedures devised for measurement of rare earth element (REE) abundances using a secondary ion mass spectrometer (ion microprobe) are described. Proc. Indian Acad. Sci. (Earth Planet. Sci.) 2003, 112, 4, 485-498.
- Singh, S.P.; Balaram, V.; Satyanarayanan, M.; Sarma D. S.; Subramanyam, K.S.V; Anjaiah, K. V.; Kharia, A. Platinum group minerals from the Madawara ultramafic – mafic complex, Bundelkhand Massif, Central India: A preliminary note, Journal of Geological Society of India 2011, 78: 281-283.
- Pan, J.; Zhang, L.; Wen, Z.; Nie, T; Zhang, T.; Zhou, C. The Mechanism Study on the Integrated Process of NaOH Treatment and Citric Acid Leaching for Rare Earth Elements Recovery from Coal Fly Ash. Journal of Environmental Chemical Engineering, 2023, 109921. [CrossRef]
- Li, X.; Qiao, X.; Chen, D.; Wu, P.; Xie, Y.; Chen, X. Anomalous concentrations of rare earth elements in acid mine drainage and implications for rare earth resources from late Permian coal seams in northern Guizhou, Science of The Total Environment 2023, 879, 163051. [CrossRef]
- Van Rythoven, A. D.; Pfaff, K.; Clark, J. G. Use of QEMSCAN R ○to characterize oxidized REE ore from the Bear Lodge carbonatite, Wyoming, USA. Ore and Energy Resource Geology 2020, 2–3, 100005. [CrossRef]
- Gray, A. L. Solid sample introduction by laser ablation for inductively coupled plasma source mass spectrometry. Analyst 1985, 110 (5), 551–556.
- Liu, S. Q.; Jiang, S. Y.; Chen, W.; C. Y.; et al. Precise determination of major and trace elements in micrometer-scale ilmenite lamellae in titanomagnetite using LA-ICP-MS technique: application of regression analysis to time-resolved signals. RSC Adv. 2023, 13, 13303. [CrossRef]
- Guo, Z.; Li, J.; Xu, X.; Song, Z.; Dong, X.; Tian, J.; Yang, Y.; She, H.; Xiang, A.; Kang, Y. Sm-Nd dating and REE Composition of scheelite for the Honghuaerji scheelite deposit, Inner Mongolia, Northeast China, Lithos 2016, 2016261, 307-321. [CrossRef]
- Mohanty, S.; Papadopoulos, A.; Petreli, M.; Papadopoulou, L.; Sengupta, D. Geochemical Studies of Detrital Zircon Grains from River Bank and Beach Placers of Coastal Odisha, India. Minerals 2023, 13, 192. [CrossRef]
- Jiu, B.; Huang, W.; Spiro, B.; Hao, R.; Mu, N.; Wen, L.; Hao, H. Distribution of Li, Ga, Nb, and REEs in coal as determined by LA-ICP-MS imaging: A case study from Jungar coalfield, Ordos Basin, China. International Journal of Coal Geology 2023, 267, 104184. [CrossRef]
- Chi, G.; Potter, E.G.; Petts, D.C.; Jackson, S.; Chu, H. LA-ICP-MS Mapping of Barren Sandstone from the Proterozoic Athabasca Basin (Canada)—Footprint of U- and REE-Rich Basinal Fluids. Minerals 2022, 12, 733. [CrossRef]
- Oostingh, K. Analysis of Rare Earth Element concentrations in barite (BaSO4). MSc-thesis, Department Earth Sciences, Utrecht University, The Netherlands, 2011, 1- 96.
- Liu, Y.S.; Hu Z. C.; Li, M.; Gao, S. Applications of LA-ICP-MS in the elemental analyses of geological samples. Chinese Science Bulletin 2013. 58, 32, 3863-3878. [CrossRef]
- Maruyama, S.; Hattori, K.; Hirata, T.; Suzuki, T.; Danhara, T. Simultaneous determination of 58 major and trace elements in volcanic glass shards from the INTAV sample mount using femtosecond laser ablation-inductively coupled plasma-mass spectrometry. Geochemical Journal 2016, 50, 403 - 422. [CrossRef]
- Wu, S. T.; Wang, H.; Yang, Y. H.; Niu, J.; et al. Lu-Hf geochronology with LA-ICP-MS/MS analysis. J. Anal. At. Spectrom. 2023. [CrossRef]
- Ham-Meert, A. V.; Bolea-Fernandez, E.; Belza, J.; Bevan, D.; Jochum, K. P.; Neuray, B.; Stoll, B.; Vanhaecke, F.; Van Wersch, L. Comparison of Minimally Invasive Inductively Coupled Plasma–Mass Spectrometry Approaches for Strontium Isotopic Analysis of Medieval Stained Glass with Elevated Rubidium and Rare-Earth Element Concentrations. ACS Omega 2021, 6(28), 18110–18122. [CrossRef]
- Yuan, H. L.; Gao, S.; Dai, M. N.; Zong, C. L.; Gunther, D.; Fontaine, G. H.; Diwu, C. Simultaneous determinations of U–Pb age, Hf isotopes and trace element com- positions of zircon by excimer laser ablation quadrupole and multiple-collector ICP-MS. Chem. Geol. 2008, 247, 1–2, 100–118. [CrossRef]
- Qian, S. P.; Zhang, L. Simultaneous in situ determination of rare earth element concentrations and Nd isotope ratio in apatite by laser ablation ICP-MS. Geochemical Journal 2019, 53, 319 – 328. [CrossRef]
- Kylander-Clark, A.R.C.; Hacker, B. R.; Cottle, J. M. Laser-ablation split-stream ICP petrochronology, Chemical Geology 2013, 345, 99-112. [CrossRef]
- Simandl, G. J.; Fajber, R.; Paradis, S. Portable X-ray fluorescence in the assessment of rare earth element enriched sedimentary phosphate deposits. Geochemistry: Exploration, Environment, Analysis 2014, 14, 161–169. [CrossRef]
- Fajber, R,; Simandl, G. J. Evaluation of Rare Earth Element-enriched Sedimentary Phosphate Deposits Using Portable X-ray Fluorescence (XRF) Instruments, Geological Survey of Canada, Sidney, British Columbia, Geological Fieldwork 2011, 2012, Paper -1, 199-210.
- Sukadana, I. G.; Warmada, W. I.; Pratiwi, F.; Harijoko, A.; Adimedha, T. B.; Yogatama, A. W. Elemental Mapping for Characterizing of Thorium and Rare Earth Elements (REE) Bearing Minerals Using μXRF. Atom Indonesia 2022, 48, 2. [CrossRef]
- Gibaga, C.R.L.; Montano, M. O.; Samaniego, J. O.; Tanciongco, A. M.; Quierrez, R. N. M. Comparative Study on Determination of Selected Rare Earth Elements (REEs) in Ion Adsorption Clays Using Handheld LIBS and ICP-MS. Philippine Journal of Science 2022, 151 (5): 1599-1604.
- Bellie, V.; Gokulraju, R.; Rajasekar, C.; Vinoth, S.; Mohankumar, V.; Gunapriya, B. Laser induced Breakdown Spectroscopy for new product development in mining industry. Materials Today: Proceedings 2021, 45, 9, 8157-8161. [CrossRef]
- Gerardo, S.; Davletshin, A. R.; Loewy, S. L.; Song, W. From Ashes to Riches: Microscale Phenomena Controlling Rare Earths Recovery from Coal Fly Ash, Environ. Sci. Technol. 2022, 56, 22, 16200–16208. [CrossRef]
- Brewer, P. G.; Malby, G.; Pasteris,J. D. Development of a laser Raman spectrometer for deep-ocean science, Deep Sea Research Part I, Oceanographic Research Papers 2004, 51, 5, 739-753. [CrossRef]
- Moroz, T. N.; Edwards, H. G. M.; Zhmodik, S. M. Detection of carbonate, phosphate minerals and cyanobacteria in rock from the Tomtor deposit, Russia, by Raman spectroscopy. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2021, 250, 119372. [CrossRef]
- Liu, H.; Yu, T.; Hu, B.; Hou, X.; Zhang, Z.; Liu, X.; Liu, J.;Wang, X.; Zhong, J.; Tan, Z.; et al. UAV-Borne Hyperspectral Imaging Remote Sensing System Based on Acousto-Optic Tunable Filter for Water Quality Monitoring. Remote Sens. 2021, 13, 4069. [CrossRef]
- Qasim, M.; Khan, S.D. Detection and Relative Quantification of Neodymium in Sillai Patti Carbonatite Using Decision Tree Classification of the Hyperspectral Data. Sensors 2022, 22, 7537. [CrossRef]
- Ye, X.; Bai, F. Spectral Characteristics, Rare Earth Elements, and Ore-Forming Fluid Constrains on the Origin of Fluorite Deposit in Nanlishu, Jilin Province, China. Minerals 2022, 12, 1195. [CrossRef]
- Wang, C.; Zhang, T.; Pan, X. Potential of visible and near-infrared reflectance spectroscopy for the determination of rare earth elements in soil. Geoderma 2017, 306, 120–126. [CrossRef]
- Boesche, N. K.; Rogass, C.; Lubitz, C.; Brell, M.; et al. Hyperspectral REE (Rare Earth Element) Mapping of Outcrops—Applications for Neodymium Detection. Remote Sens. 2015, 7, 5160-5186. [CrossRef]
- Karimzadeh, S.; Tangestani, M. H. Potential of Sentinel-2 MSI data in targeting rare earth element (Nd3+) bearing minerals in Esfordi phosphate deposit, Iran. The Egyptian Journal of Remote Sensing and Space Sciences 2022, 25, 697–710. [CrossRef]
- Booysen, R.; Jackisch, R.; Lorenz, S.; Zimmermann, R.; et al. Detection of REEs with lightweight UAV-based hyperspectral imaging. Sci Rep. 2020, 10, 1, 17450. [CrossRef]
- Maia, A. J.; da Silva, Y.J.A.B.; do Nascimento, C.W.A;, Veras. G.; Escobar M.; Cunha, C.S,M,; da Silva, Y.J.A.B.; Nascimento, R.C.; de Souza, P. L. H. Near-infrared spectroscopy for the prediction of rare earth elements in soils from the largest uranium-phosphate deposit in Brazil using PLS, iPLS, and iSPA-PLS models. Environ Monit Assess. 2020,192,11, 675. [CrossRef]
- Turner, D. J.; Rivard, B,; Groat, L. Visible and short-wave infrared reflectance spectroscopy of selected REE-bearing silicate minerals. American Mineralogist 2018, 103, 927–943. [CrossRef]
- Featherston, E.R.; Issertell, E. J.; Cotruvo, Jr. J. A. Probing Lanmodulin’s Lanthanide Recognition via Sensitized Luminescence Yields a Platform for Quantification of Terbium in Acid Mine Drainage. J. Am. Chem. Soc. 2021, 143, 35, 14287–14299. [CrossRef]
- Cruickshank, L.; Officer, S.; Pollard, P.; Prabhu, R.; Stutter, M.; Fernandez, C. Rare elements electrochemistry: the development of a novel electrochemical sensor for the rapid detection of europium in environmental samples using gold electrode modified with 2-pyridinol-1-oxide. Analytical Sciences 2015, 31, 623-627. [CrossRef]
- Shehu, G.; Bagudo, I. M. Mineralogical and Structural Analyses of Natural Fluorite from Yantuwaru Mining Site, Nigeria. UMYU Scientifica 2023, 2, 1, 43 – 51. https://scientifica.umyu.edu.ng/.
- Xiong, X.; Jiang, T.; Qi, W.; Zuo,J.; et al. Some Rare Earth Elements Analysis by Microwave Plasma Torch Coupled with the Linear Ion Trap Mass Spectrometry International Journal of Analytical Chemistry 2015, 156509. [CrossRef]
- Yuan, L.; Zhou, X.; Cao, Y.; Yan, N.; Peng, L.; Lai, X..; Tao, H.; Jiang, T.; Li, L.; Zhu, Z. Microwave Plasma Torch Mass Spectrometry for some Rare Earth Elements, Arabian Journal of Chemistry 2022, 15, 12, 104379. [CrossRef]
- Maia, A. J.; Nascimento, R. C.; da Silva, Y. J. A. B.; et al. Near-infrared spectroscopy for prediction of potentially toxic elements in soil and sediments from a semiarid and coastal humid tropical transitional river basin. Microchemical Journal 2022, 179, 107544. [CrossRef]
- Imashuku, S. Rapid determination of the approximate content of bastnäsite in ores using cathodoluminescence imaging, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2023, 287,1, 122055. [CrossRef]
- Duplouy, C. Preliminary Investigation of Rare Earth Elements Ion Exchange on Zeolites. Master Thesis. Department of Chemistry, University of Helsinki, Finland, 2016, 1- 57.
- Zhao, J.; Xing, Y.; Ge, L.; Wang, L.; Li, T.; Zhang, Q.; Wu, H.; Li, W.; Liu, Y. Direct analysis of lanthanum in extraction process by in-situ gamma spectrometry. Journal of Radioanalytical and Nuclear Chemistry 2022, 331, 9, 3807-3817. [CrossRef]
- Borst, A. M.; Smith, M. P.; Finch, A. A.; Estrade, G.; Villanova-de-Benavent, C.; Nason , P .; Marquis , E .; Horsburgh, N. J.; Goodenough , K .M .; Xu , C.; Kynický, J.; Geraki , K. 2020, ' Adsorption of rare earth elements in regolith-hosted clay deposits ' , Nature Communications 2022, 11, 4386. [CrossRef]
- Obhodaš, J.; Sudac, D.; Meric, I.; Pettersen, H.E.S.; Uroic´, M.; Nad¯, K.; Valkovic´, V. In-situ measurements of rare earth elements in deep sea sediments using nuclear methods. Sci. Rep. 2018, 8, 4925. [CrossRef]
- Balaram, V. Deep-sea mineral deposits as a source of critical metals for high-and green-technology applications. Minerals and Mineral Materials 2023, 2, 5. [CrossRef]
- Stuckman, M. Y.; Lopano, C. L.; Granite, E. J. Distribution and speciation of rare earth elements in coal combustion by-products via synchrotron microscopy and spectroscopy, International Journal of Coal Geology 2018, 195, 125-138. [CrossRef]
- Jochum, K. P.; Seufert, H. M.; Midinet-Best, S.; Rettmann, E.; Schiinberger, K.; Zimmer, M. Multi-element analysis by isotope dilution-spark source mass spectrometry (ID-SSMS) Fresenius Z Anal Chem. 1988, 331: 104-110.
- Folkedahl, B.; Nyberg, C.; Biswas, S.; Zhang, X. Round-Robin Interlaboratory Study on Rare-Earth Elements in 2 U.S.-Based Geologic Materials Minerals 2023, 13, x.
- Balaram, V.; Subramanyam, K.S. V. Sample Preparation for Geochemical Analysis: Strategies and Significance Advances in Sample Preparation 2022, 1, 100010. [CrossRef]
- Xuan, H., Zi-hui, C.; Shu-chao, Z.; Lei, S. Matrix Separation-Determination of Rare Earth Oxides in Bauxite by Inductively Coupled Plasma-Atomic Emission Spectrometry. Spectroscopy and Spectral Analysis 2022, 42, 10, 3130-3134. [CrossRef]
- Schramm, R. Use of X-ray Fluorescence Analysis for the Determination of Rare Earth Elements. Physical Sciences Reviews 2016, 9. [CrossRef]
- Lett, R. E.; Paterson, K. A Comparison of Several Commercially Available Methods for the Geochemical Analysis of Rare Earth, Rare Metal and High Field Strength Elements in Geological Samples. Geological Fieldwork 2010, British Columbia Geological Survey Paper 2011-1, 181-188.
- Hanchar, J. M.; Finc, R. J.; Hoskin, W.O.; et al. Rare earth elements in synthetic zircon: Part 1. Synthesis, and rare earth element and phosphorus doping American Mineralogist 2001, 86, 5. [CrossRef]
- Udayakumar, S.; Baharun, N.; Rezan, S. A. Microwave-Assisted Acid Digestion of Malaysian Monazite for Determination of REEs Using ICP-MS. Key Engineering Materials, 2022, 908, 481-486. [CrossRef]
- He, H.; Zhao, X.; hang, Y.; Zhao, L.; Hu, R.; Li, L. Determination of rare earth elements in uranium ores by ICP-MS after total dissolution with NH4F and matrix separation with TRU resin. Journal of Radioanalytical and Nuclear Chemistry 2023. [CrossRef]
- Kasar, S.; Murugan, R.; Arae, H.; Aono, T.; Sahoo, S. K. A Microwave Digestion Technique for the Analysis of Rare Earth Elements, Thorium and Uranium in Geochemical Certified Reference Materials and Soils by Inductively Coupled Plasma Mass Spectrometry. Molecules 2020, 25, 5178. [CrossRef]
- Zuma, M. C.; Nomngongo, P.N.; Mketo, N. Simultaneous Determination of REEs in Coal Samples Using the Combination of Microwave-Assisted Ashing and Ultrasound-Assisted Extraction Methods Followed by ICP-OES Analysis. Minerals 2021, 11, 1103. [CrossRef]
- Balaram, V.; Satyanarayanan, M. Data Quality in Geochemical Elemental and Isotopic Analysis. Minerals 2022, 12, 999. [CrossRef]
- Zhang, Y.; Sun, Y.; Zhou, J.; Yang, J.; et al. Preparation of REE-doped NaY(WO4)2 single crystals for quantitative determination of rare earth elements in REE:NaY(WO4)2 laser crystals by LA-ICP-MS. Anal. Methods 2022,14, 4085-4094. [CrossRef]
- Akhmetzhanova, T. F.; Popov, A. M. Direct determination of lanthanides by LIBS in REE-rich ores: comparison between univariate and DoE based multivariate calibrations with respect to spectral resolution. J. Anal. At. Spectrom. 2022, 37, 2330-2339. [CrossRef]
- Verplanck, P.L.; Antweiler, R.; CNordstrom, D. K.; Taylor, H. E. Standard reference water samples for rare earth element determinations. Applied Geochemistry 2001. 16, 231-244.






| Source | ∑ REE |
|---|---|
| Earth's crust | 150 to 220 µg/g |
| REE ore | 0.1 – 10% |
| Surface and groundwater | 0.1 – 100 pg/g |
| Geothermal fluids | Up to 21.76 µg/g |
| Acid mine drainage | 1- 1000 ng/g |
| Coal and pre-combustion by-products | 10 – 1000 µg/g |
| E-waste | ~ 600 µg/g |
| Coal ash | 10 – 1000 µg/g |
| Ferromanganese crust from the Indian Ocean | 1727 to 2511 μg/g |
| Laterites | 0.021 to 0.099 |
| Red Mud | 0.23 to 0.38 |
| Phosphorites | up to 0.5 wt% |
| Bauxite mine waste ponds | 1,900 to 2,600 µg/g |
| Element | ED-XRF value (µg/g) | Certified value |
|---|---|---|
| La | 10.78 | 11.23 |
| Ce | 26.44 | 25.65 |
| Nd | 9.59 | 9.45 |
| Sm | 1.72 | 1.69 |
| Y | 3.14 | 3.10 |
| Nature of material | Analytes | Sample preparation / decomposition method | Analytical technique | Remarks | Reference |
|---|---|---|---|---|---|
| REE-bearing rock and soil samples | La, Ce, Nd and Y | Pressed pellets of homogenized soil samples | LIBS | Portable LIB spectrometers are useful in exploration of new REE deposits. | [31] |
| Lunar meteorites | REE | Direct ablating the sample | LIBS | Information on the constituents in sample drawn from spectral details |
[32] |
| Waste Sm-Co Magnets | REE and several other major, minor, and trace elements | Microwave digestion procedure using HNO3, H2SO4, HCl, & HF | ICP-MS & ICP-OES | Recoveries were between 99–100% and RSD was < 5%. | [33] |
| Rocks | REE | Low dilution glass beads made with sample to lithium borate (1:1) heating twice at 1200oC with agitation | XRF | Using this method Y, La, Ce, Pr, Nd, Sm. Gd, Dy and several other elements were determined in rhyolitic and granitic rocks | [34] |
| Surface waters and sediments of the Mgoua watershed, Cameroon |
REE | Acidified water samples analysed directly. Sediments were dissolved using a mixture of acids before analysis | ICP-MS | REE concentrations in waters 0.11 to 6.60 ng/ml and 282.12 to 727.67 µg/g in sediments | [35] |
| JCp-1 (coral) and JCt-1 (giant clam) CRMs | REE | Two methods: i) simple dissolution by HCl, and; ii) HF+HNO3+HClO4 digestion and a further fusion process with Na2CO3 and H3BO3 in a Pt-crucible. |
ID-ICP-MS | No significant differences in REE results were found between the two decomposition methods |
[36] |
| Sedimentary cores from Laguna Mar Chiquita, Argentina | La, Ce, Nd, Sm, Eu, Tb, Yb & Lu | 200 mg of sediment samples in polyethylene bags were irradiated |
INAA | Global REE averages show higher REE contents in clastic than in chemical sediments |
[37] |
| Phosphate rocks from Egypt and Saudi Arabia | REE | Thirty grams aliquots encapsulated in a polyethylene vial and irradiated |
INAA | Choice of the nuclear reaction, irradiation and decay times and of the proper gamma radiation are important | [38] |
| Brazilian geological CRMs | REE | For each sample, one CRM was simultaneously processed in exactly the same way | INAA | Geological CRMs, GB-1 & BB-1 were provided new trace element data | [39] |
| Sediments of Bouregreg river, Morocco | REE | 100 mg sample of CRMs and samples were irradiated about 7 hours | INAA | INAA offers good sensitivity and selectivity for analysis of sediments |
[40] |
| Apatite mineral | La, Ce, Pr, Nd, Sm, Eu, Gd and Dy | About 25 mg digested in 25 ml HNO3 and 6ml HCl. Then 1 mL of the solution was pipetted onto a Millipore membrane filter (1.2 mm pore size) and dried under an IR heater at 50oC. |
WD-XRF | Determination in emission–transmission method. Precisions are ~ 3% RSD with comparable accuracies | [41] |
| Uranium oxide | Eu, Nd, and Yb | Sample powders were encapsulated in clear tape and analysed directly |
pLIBS | REE constituents in sub-percent levels detected | [42] |
| Alabaster rocks (crystalline CaCO 3) | Sc, Lu, Ce, Sm, La, Yb, and Eu | 100mg powder in polyethylene capsules irradiated |
INAA | Technique is useful for geochemical and mineral exploration studies | [43] |
| Fluids from deep-sea hydrothermal vents | REE | REE are isolated from other elements on miniature cation exchange columns | ICP-MS | ID-TIMS results compare favourably with |CP-MS results and accurate at the 6% (2a) level | [44] |
| Natural carbonates | REE | Samples dissolved in HNO3 | ICP-MS | The carbonate REE-related studies useful in climate change, paleoceanography, and environmental research |
[45] |
| Drilling subsamples of 50–100 mg analysed directly | LA-ICP-MS and LA-HR-ICP-MS |
| REE | Concentration (µg/g) | |
|---|---|---|
| INAA value | Certified value | |
| La | 264±25 | 260±9 |
| Ce | 460±45 | 463±20 |
| Pr | 47.6±5.7 | 47.1±2.4 |
| Nd | 145±14 | 152±8 |
| Sm | 24.0±2.3 | 23.6±0.4 |
| Eu | 3.64±0.43 | 3.71±0.23 |
| Gd | 23.6±3.0 | 23.6±1.4 |
| Tb | 3.71±0.32 | 3.80±0.23 |
| Dy | 23.7±2.8 | 23.2±0.4 |
| Ho | 4.89±0.45 | 4.81±0.14 |
| Er | 15.4±1.9 | 14.9±0.5 |
| Tm | 2.33±0.21 | 2.31±0.11 |
| Yb | 15.7±1.3 | 14.9±0.4 |
| Lu | 2.30±0.20 | 2.26±0.11 |
| Sc | 6.68±0.50 | 6.10 |
| Y (ED-XRF) | 132±23 | 142±3 |
| REE | ICP-MS [74] (ng/ml) |
ICP-MS/MS [75] (pg/ml) |
HR-ICP-MS [76] (pg/ml) |
MH-ICP-MS [77] (ng/ml) |
ICP-OES [70] (µg/ml) |
LA-HR-ICP-MS [78] (µg/g) |
INAA [79] (µg/g) |
LIBS [31] (µg/g) |
GD-MS [80] (ng/g) |
|---|---|---|---|---|---|---|---|---|---|
| La | 910 | 0.12 | 0.15 | 0.005 | 1.1 | 0.002 | 0.3 | 160 | 5.6 |
| Ce | 260 | 0.15 | 0.33 | 0.007 | 1.6 | 0.01 | 0.9 | 285 | 1.5 |
| Pr | 3 | 0.16 | 0.09 | <0.001 | 1.2 | 0.003 | - | - | 5.6 |
| Nd | 10 | 0.14 | 1.06 | 0.003 | 2.4 | 0.005 | 0.6 | 414 | 4.5 |
| Sm | 5 | 0.16 | 0.50 | 0.005 | 2.8 | 0.002 | 0.04 | - | 4.6 |
| Eu | 10 | 0.19 | 0.35 | 0.003 | 0.8 | 0.001 | 0.02 | - | 2.0 |
| Gd | 4 | 0.13 | 0.97 | 0.009 | 1.1 | 0.006 | - | - | - |
| Tb | 1 | 0.17 | 0.09 | 0.001 | 2.3 | - | 0.05 | - | - |
| Dy | 5 | 0.08 | 0.16 | 0.006 | 1.4 | 0.006 | - | - | 3.6 |
| Ho | 3 | 0.18 | 0.04 | 0.003 | 0.8 | 0.0009 | - | - | 0.4 |
| Er | 4 | 0.15 | 0.10 | 0.001 | 0.5 | 0.006 | - | - | 0.7 |
| Tm | 2 | 0.15 | 0.05 | 0.001 | 0.5 | - | - | - | 0.3 |
| Yb | 10 | 0.13 | 0.12 | 0.006 | 0.1 | 0.008 | 0.08 | - | - |
| Lu | 1 | 0.15 | 0.05 | 0.002 | 0.1 | 0.001 | 0.03 | - | 1.4 |
| Sc | 60 | - | 17.9 | 0.006 | - | - | - | - | 0.5 |
| Y | 170 | - | 0.38 | 0.003 | 0.1 | 0.003 | - | 227 | 0.6 |
| REE | Na2O2 Fusion/ICP-MS/MS | INAA | Certified value |
|---|---|---|---|
| La | 26±4 | 29.4±0.8 | 27±2 |
| Ce | 50±8 | 58±3 | 54±6 |
| Pr | 5.7±0.9 | - | - |
| Nd | 21±3 | 28±2 | 26 |
| Sm | 3.9±0.5 | 4.9±0.3 | 4.9±0.2 |
| Eu | 1.2±0.2 | 1.38±0.06 | 1.43±0.12 |
| Gd | 3.7±0.5 | - | - |
| Tb | 0.7±0.1 | 0.67±0.05 | 0.71±0.07 |
| Dy | 3.3±0.4 | 4.8±0.5 | 3.8±0.3 |
| Ho | 0.73±0.15 | - | - |
| Er | 2.0±0.2 | - | - |
| Tm | 0.30±0.06 | - | 0.37±0.04 |
| Yb | 2.1±0.3 | 2.7±0.3 | 2.3±0.2 |
| Lu | 0.31±0.07 | 0.37±0.03 | 0.37±0.4 |
| Sc | - | 9.7±0.3 | - |
| Y | 22±4 | - | 24±3 |
| REE | Nod A-1 (µg/g) | Nod P1 (µg/g) | |||||
|---|---|---|---|---|---|---|---|
| ICP-MS value [122] | HR-ICP-MS value [123] | ICP-MS value [122] Nath et al. 1992 |
HR-ICP-MS value [123] |
||||
| LR | HR | LR | HR | ||||
| La | 115 | 111.2 | 110.0 | 105 | 106.4 | 107.1 | |
| Ce | 656 | 745 | 740 | 318 | 319 | 321 | |
| Pr | 21.7 | 23.85 | 23.79 | 27.5 | 23.85 | 23.79 | |
| Nd | 94 | 99.55 | 99.45 | 114 | 132.2 | 134.5 | |
| Sm | 20.4 | 21.79 | 21.73 | 27.2 | 31.87 | 32.41 | |
| Eu | 6.10 | 5.28 | 5.41 | 7.44 | 7.68 | 7.97 | |
| Gd | 23.6 | 24.28 | 24.75 | 33.8 | 30.28 | 30.85 | |
| Tb | 4.20 | 3.84 | 3.90 | 4.53 | 4.71 | 4.74 | |
| Dy | 25.80 | 23.06 | 22.92 | 25.99 | 26.29 | 27.03 | |
| Ho | 5.09 | 4.96 | 5.02 | 4.73 | 5.00 | 5.16 | |
| Er | 15.6 | 14.31 | 14.52 | 13.3 | 13.42 | 12.70 | |
| Tm | 2.19 | - | - | 1.72 | - | - | |
| Yb | 15.40 | 13.48 | 13.69 | 13.26 | 12.70 | 13.08 | |
| Lu | 2.21 | 2.08 | 2.14 | 1.75 | 1.82 | 1.93 | |
| %RSD | < 5.0 | <2.9 | < 5.37 | <5.0 | <1.4 | <5.73 | |
| Analyte | 1A | 2A | 3A | 4A | 5A | 6A | 7A | 8A | 9A | 10A | 11A | 12A | 13A |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | Puga drill hole | Puga river | Chumathang | Chumathang | Chumathang | Kiagor-Tso lake | Tso-Morari | Yan river side | Kalra nala | Ribil | Sundo confluence | Indus river | Indus river |
| Type | Spring water | River water | Spring water | River water | River water | Lake water | Lake water | River water | River water | River water | River water | River water | River water |
| Sc | 23.1 | 5.6 | 19.7 | 5.3 | 3.5 | 1.5 | 4.7 | 1.1 | 1.4 | 4.7 | 2.6 | 5.6 | 4.7 |
| Y | 53.8 | 153.8 | 21.2 | 626.3 | 393.6 | 91.6 | 90.6 | 77.5 | 23.3 | 433.9 | 222.3 | 128.6 | 49.0 |
| La | 46.2 | 303.4 | 27.7 | 1441.9 | 847.1 | 149.5 | 177.5 | 196.4 | 31.6 | 1176.3 | 443.1 | 176.4 | 67.3 |
| Ce | 74.5 | 482.0 | 40.2 | 2273.0 | 1321.6 | 254.7 | 287.3 | 278.8 | 48.9 | 1879.7 | 695.8 | 274.3 | 99.8 |
| Pr | 5.9 | 35.7 | 4.1 | 147.9 | 86.4 | 16.5 | 20.2 | 22.0 | 5.0 | 116.0 | 46.7 | 21.2 | 8.6 |
| Nd | 19.7 | 71.8 | 18.0 | 277.4 | 169.9 | 36.9 | 44.1 | 50.0 | 21.7 | 227.6 | 100.8 | 50.9 | 31.2 |
| Sm | 6.1 | 19.7 | 4.9 | 73.1 | 43.1 | 11.3 | 11.6 | 13.2 | 4.4 | 54.0 | 25.1 | 13.1 | 6.4 |
| Eu | 0.6 | 0.9 | 0.6 | 3.9 | 2.5 | 0.6 | 0.7 | 0.6 | 0.3 | 3.5 | 1.8 | 0.7 | 0.4 |
| Gd | 3.2 | 8.0 | 2.2 | 27.5 | 16.2 | 4.9 | 5.2 | 4.6 | 1.9 | 21.2 | 10.2 | 5.6 | 2.4 |
| Tb | 1.0 | 2.2 | 0.5 | 6.8 | 4.3 | 1.3 | 1.4 | 1.2 | 0.5 | 4.8 | 2.4 | 1.4 | 0.7 |
| Dy | 3.6 | 8.8 | 1.9 | 29.7 | 19.5 | 5.7 | 5.3 | 5.1 | 2.2 | 21.1 | 10.9 | 7.1 | 3.3 |
| Ho | 1.8 | 1.6 | 0.8 | 4.8 | 3.2 | 0.9 | 1.0 | 0.8 | 0.5 | 3.7 | 1.9 | 1.3 | 0.7 |
| Er | 7.4 | 5.2 | 3.2 | 14.3 | 8.5 | 2.5 | 2.4 | 2.4 | 1.3 | 11.3 | 5.6 | 4.1 | 2.5 |
| Tm | 0.5 | 3.4 | 0.4 | 1.5 | 1.1 | 0.5 | 0.5 | 0.4 | 0.3 | 1.4 | 0.8 | 0.5 | 0.4 |
| Yb | 1.7 | 2.4 | 1.4 | 6.1 | 4.1 | 1.8 | 1.7 | 1.3 | 1.1 | 5.6 | 3.2 | 2.0 | 1.3 |
| Lu | 0.6 | 0.9 | 0.4 | 2.8 | 2.0 | 0.7 | 0.7 | 0.5 | 0.4 | 2.6 | 1.3 | 0.8 | 0.4 |
| REE | Concentration (pg/ml) | |||||
|---|---|---|---|---|---|---|
| ICP-MS/MS [128] | ICP-MS [129] | HR-ICP-MS [130] |
ID-HR-ICP-MS [131] | Compiled values [128] |
Compiled value [132] |
|
| La | 294.5 ± 3.2 | 302.2 ± 7.3 | 279±12 | 290.3±6.4 | 291±9 | 287±8 |
| Ce | 357.5 ± 3.2 | 378.4 ± 8.2 | 369±15 | 364.1±3.5 | 363±9 | 360±12 |
| Pr | 70.9 ± 0.4 | 73.6 ± 1.5 | 75.4±8.0 | 70.6±2.3 | 71±2.4 | 69.3±1.8 |
| Nd | 274.2 ± 3.2 | 277.4 ± 5.7 | 261±9 | 270.3±2.8 | 271±6 | 269±14 |
| Sm | 58.5 ± 1.9 | 59.3 ± 1.4 | 54.3±5.0 | 57.2±0.3 | 57.6±1.8 | 57.4±2.8 |
| Eu | 8.06 ± 0.41 | 8.09 ± 0.61 | 8.4±0.8 | 8.00±0.7 | 8.44±0.57 | 8.0±0.6 |
| Gd | 33.86 ± 1.46 | 35.13 ± 1.01 | 38.3±6.0 | 33.80±0.36 | 34.2±1.8 | 34.2±2.0 |
| Tb | 4.27 ± 0.20 | 4.50 ± 0.23 | 4.1±0.5 | 4.30±0.12 | 4.32±0.14 | 4.3±0.4 |
| Dy | 22.82 ± 0.75 | 23.91 ± 0.66 | 21.7±3.0 | 23.60±0.16 | 23.6±1.0 | 24.2±1.6 |
| Ho | 4.39 ± 0.19 | 4.86 ± 0.11 | 4.2±0.5 | 4.60±0.18 | 4.66±0.27 | 4.7±0.3 |
| Er | 13.21 ± 0.46 | 13.53 ± 0.70 | 11.4±3.0 | 13.10±0.06 | 13.2±0.8 | 13.4±0.6 |
| Tm | 1.75 ± 0.11 | 1.91 ± 0.04 | 1.8±0.2 | 1.80±0.02 | 1.82±0.08 | 1.7±0.2 |
| Yb | 11.73 ± 0.36 | 12.03 ± 0.51 | 10.6±2.0 | 12.30±0.07 | 12.2±0.7 | 12.0±0.4 |
| Lu | 1.76 ±0.09 | 1.86 ± 0.11 | 1.7±0.4 | 1.95±0.02 | 1.91±0.10 | 1.9±0.10 |
| REE | Mean concentrations (ng/ml) |
|---|---|
| La | 0.075 |
| Ce | 0.17 |
| Pr | 0.021 |
| Nd | 0.064 |
| Sm | 0.053 |
| Eu | 0.012 |
| Gd | 0.026 |
| Tb | 0.0083 |
| Dy | 0.017 |
| Ho | 0.012 |
| Er | 0.014 |
| Tm | 0.0025 |
| Yb | 0.025 |
| Lu | 0.0025 |
| Sc | - |
| Y | 0.094 |
| REE | AS3 Zircon (µg/g) | |
|---|---|---|
| Nano SIMS value | Certified value | |
| La | 0.250 ± 0.147 | 0.096 ± 0.063 |
| Ce | 11.56 ± 0.362 | 7.69 ± 1.07 |
| Pr | 0.544 ± 0.295 | 0.578 ± 0/173 |
| Nd | 7.34 ± 3.07 | 7.60 ± 2.09 |
| Sm | 12.77 ± 4.45 | 9.21 ± 2.24 |
| Eu | 0.399 ± 0.159 | 0.331 ± 0.073 |
| Gd | 42.7 ± 11.4 | 40.9 ± 8.5 |
| Tb | 15.63 ± 3.65 | 14.94 ± 3.18 |
| Dy | 165,6 ± 34.5 | 168.5 ± 30.0 |
| Ho | 53.2 ± 9.9 | 63,3 ± 10.7 |
| Er | 222.5 ± 45.8 | 261.0 ± 41.7 |
| Tm | 40.5 ± 6.7 | 54.2 ± 8.2 |
| Yb | 332.5 ± 50.8 | 408.6 ± 57.3 |
| Lu | 62.7 ± 10.3 | 89.6 ± 12.2 |
| Selected REE | Average concentration (µg/g) | |
|---|---|---|
| pLIBS | ICP-MS | |
| La | 54.3 | 53.99 |
| Ce | 99.5 | 94.77 |
| Pr | 13.1 | 13.72 |
| Nd | 36.4 | 52.62 |
| Sm | 15.6 | 11.38 |
| Gd | 7.5 | 10.40 |
| Dy | 6.4 | 9.58 |
| Yb | 8.7 | 4.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





