Submitted:
11 June 2023
Posted:
12 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
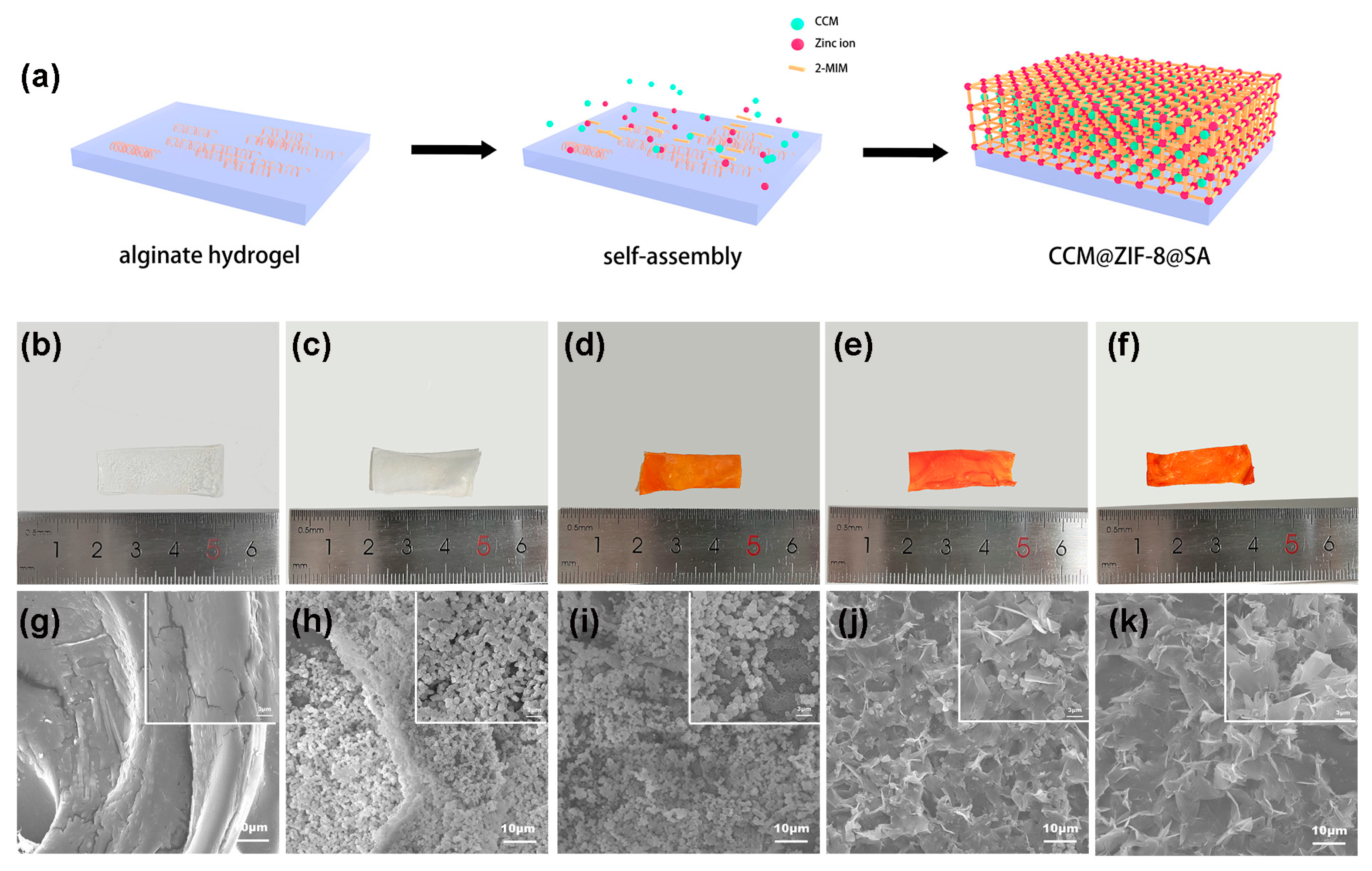
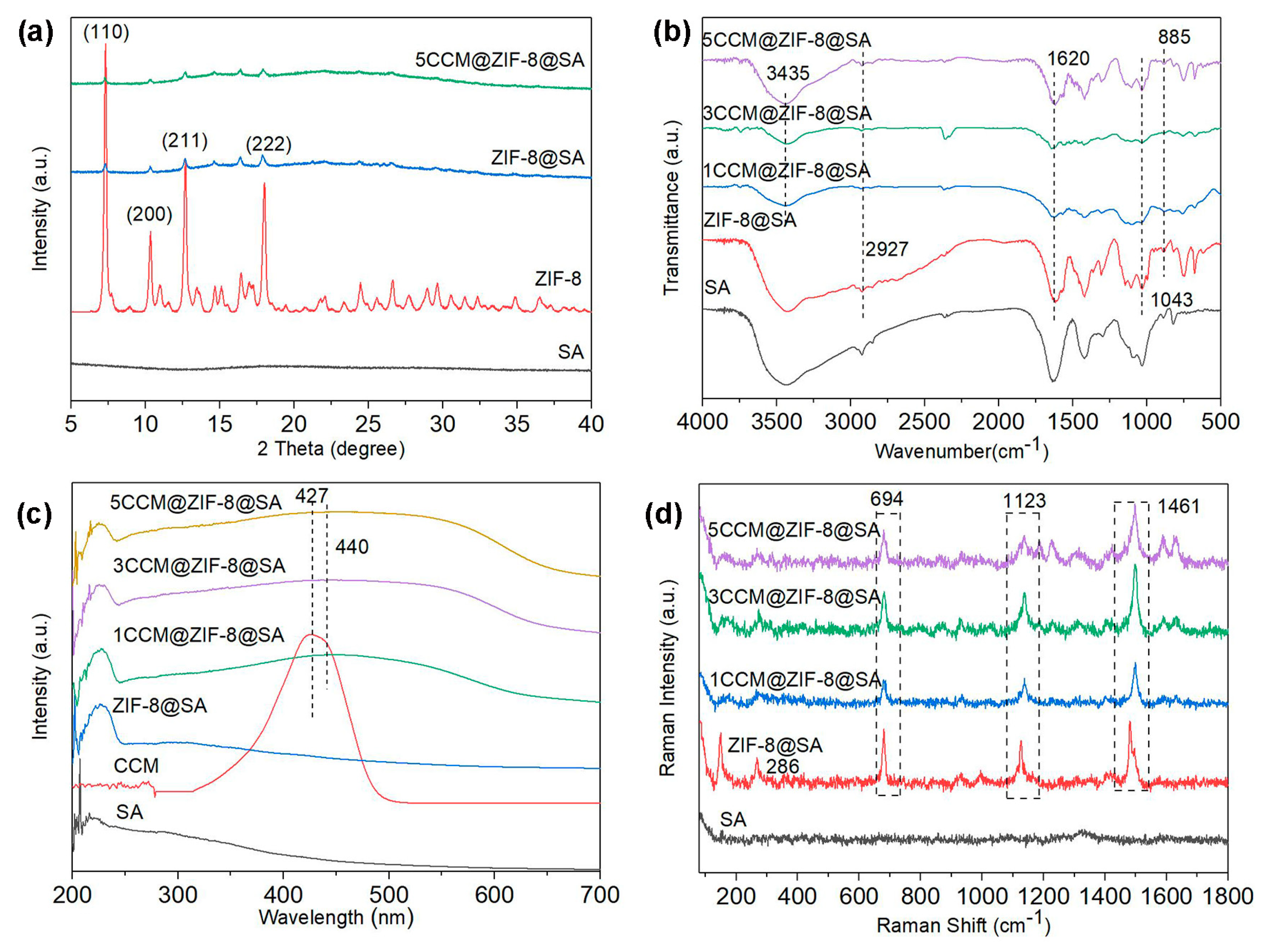
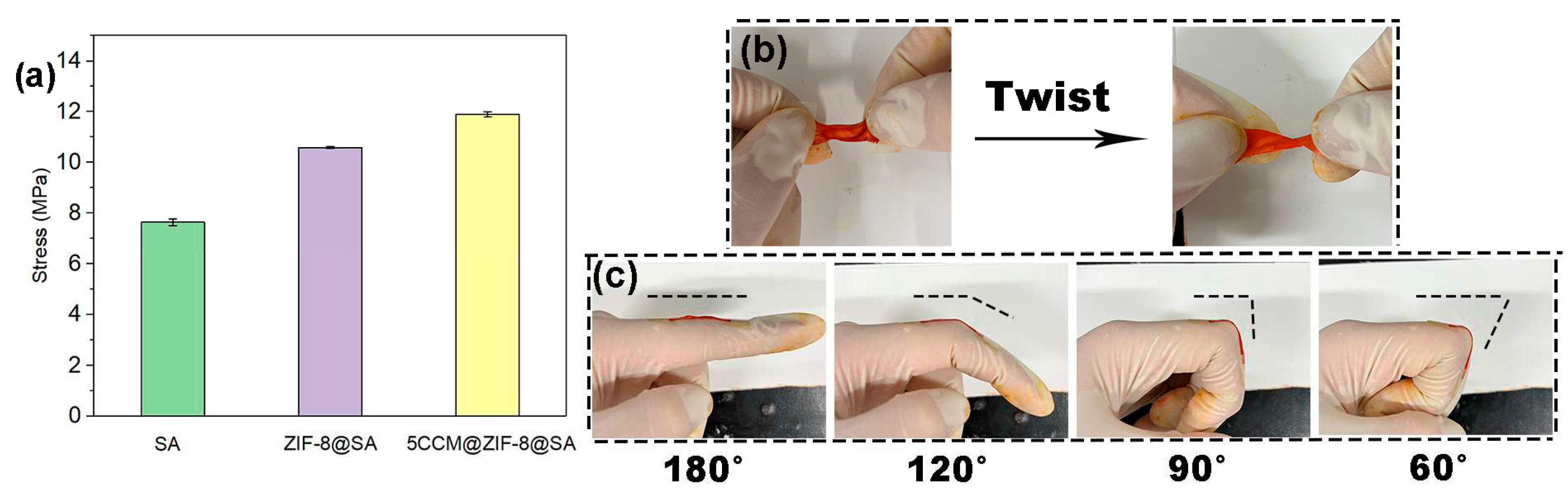
2.1. Characterization of CCM@ZIF-8@SA composite hydrogels
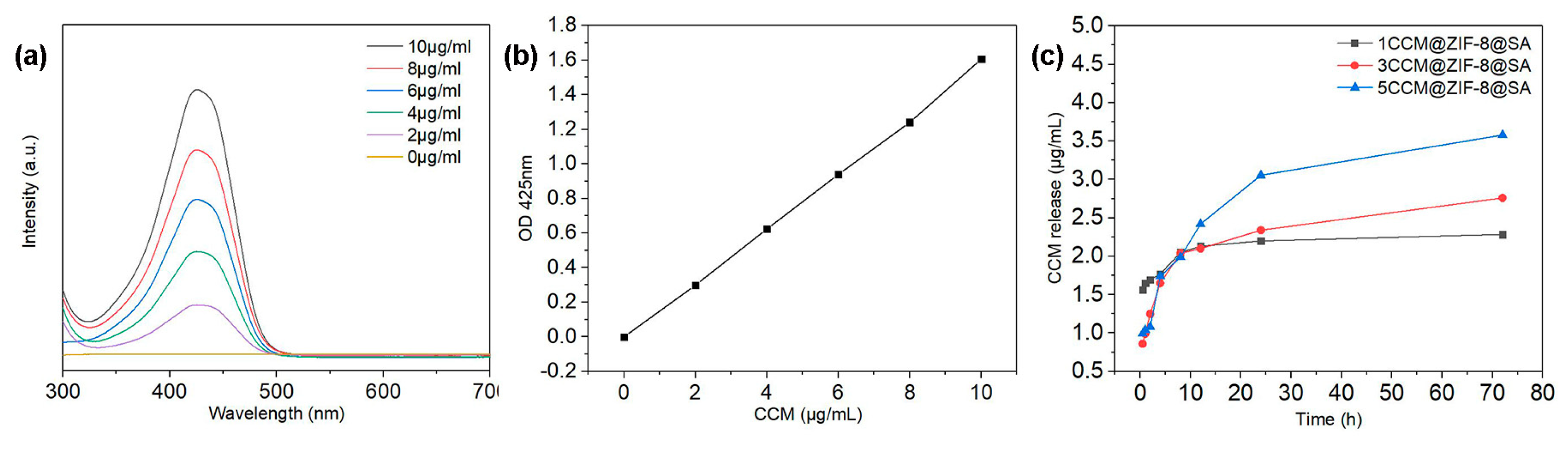
2.2. Drug release behavior of CCM@ZIF-8@SA composite hydrogel
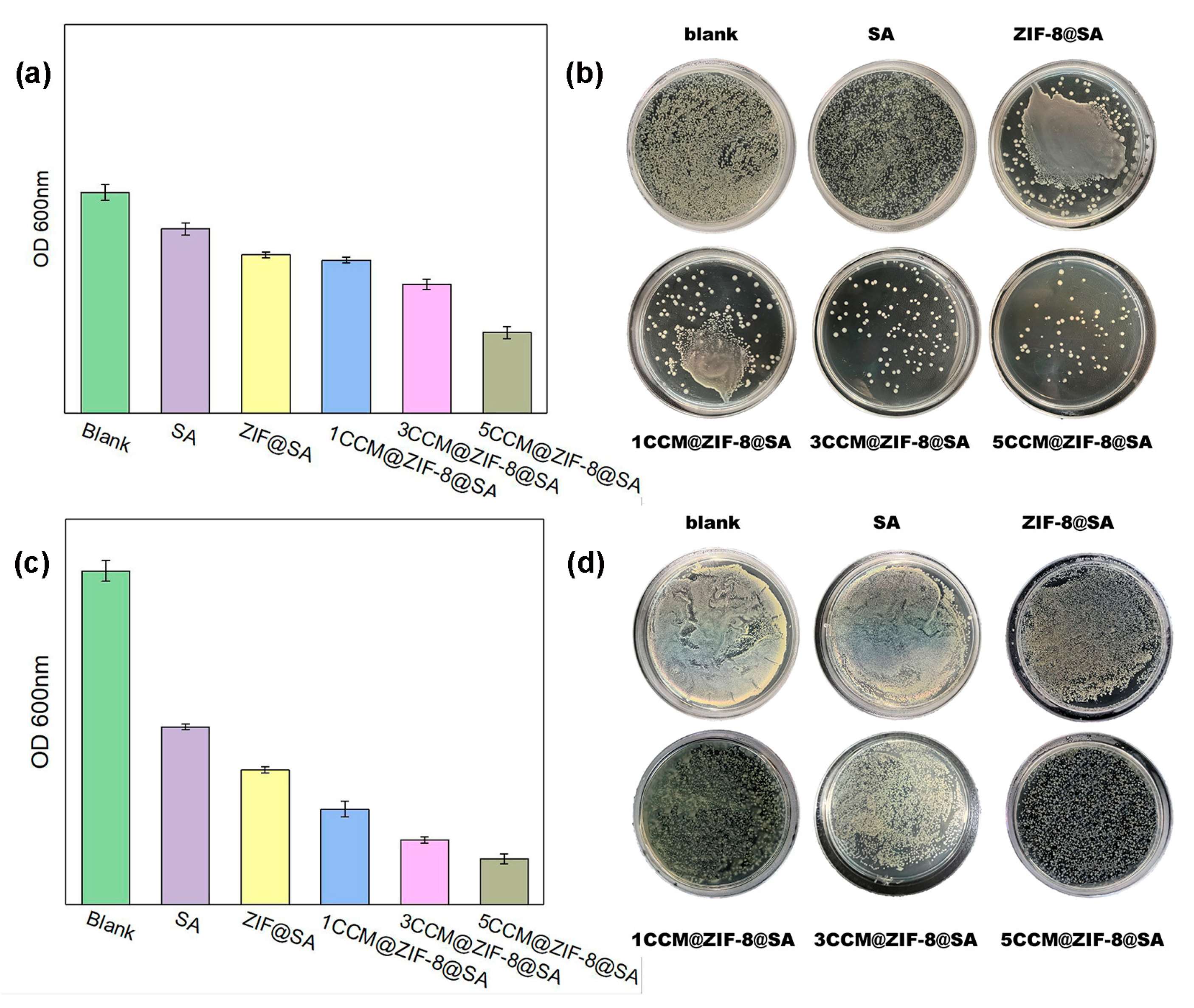
2.3. Antibacterial activities of CCM@ZIF-8@SA composite hydrogels
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.3. Characterizations
3.4. Drug release tests
-
(i)The standard curve of CCM:120 mg of CCM was dissolved in 10 mL of ethanol as the stock solution. Then 1 mL was extracted from the above solution and diluted to give the CCM solution gradients of 10, 8, 6, 4, 2, and 0 μg/mL. Subsequent Measurement of the UV-Vis absorption of these solutions was conducted in the wavelength range of 200-800 nm, and the absorbance intensities at 427 nm were recorded and were plotted as a function of CCM concentrations.
-
(ii)The drug release test of CCM@ZIF-8@SA composite hydrogels:The as-prepared CCM@ZIF-8@SA composite hydrogel was soaked in 15 mL of phosphate buffer solution (pH=7.4). 3 mL was taken out every time in an interval of 0.5, 1, 2, 4, 8, 24, and 72 h, and the absorbance intensities at 427 nm were measured. Finally, the release amount of CCM was calculated according to the standard curve. In this experiment, three sets of parallel experiments were performed, and the results of the three groups of experiments were averaged and then processed for data processing.
3.5. Antimicrobial properties
-
(i)Sample processing:The as-prepared hydrogels were put into a centrifuge tube, and were irradiated under ultraviolet lamp for 30 min.
-
(ii)Medium configuring:4 g of Luria-Bertani (LB) broth was added into 200 mL of deionized water and stirred to obtain liquid medium, and 9.6 g of agar medium was added into 300 mL of deionized water and stirred well to obtain solid medium. Then two different media were put into into the LX-B50L autoclave for sterilization(120 °C, 30 min) and then cooled down. At last, the solid medium was poured into the Petri dish in an ultra-clean stage and stored.
-
(iii)Bacterial resuscitation:The strains used in this experiment are Gram-positive S. aureus (BNCC186335) and Gram-negative E. coli (BNCC336902). Before the experiment, 50 mL of centrifugal tube, parafilm and other experimental consumables were subjected to ultraviolet irradiation for 30 minutes on the ultra-clean table for sterilization. During the experiment, a small amount of bacteria was placed in aliquot liquid medium (30 mL) with an overheated inoculation ring, sealed with a breathing membrane, and cultured in a shaker at a temperature of 37 °C for 18 h.
-
(IV)Bacterial proliferation:The bacterial proliferation was analyzed by measuring OD values and coating method. After 24 h of bacterial recovery, removed from the shaker, the bacteria solution was diluted 3-fold by a factor with liquid medium and was measured with a UV spectrophotometer. Under the condition of bacterial concentration of 106 cfu·mL-1, 5 mL of bacterial liquid was extracted and put into a centrifuge tube where the hydrogel samples were added. The bacterial solution was then sealed with a breathing membrane, and put into the incubator at a constant temperature of 37 °C for a total of 24h.
-
(V) Determination of bacterial bacteriostatic rate:The OD values at 600 nm were measured with the ultraviolet spectrophotometer after diluting the bacterial solution after 24 h of total culture, and then the bacterial solution was diluted by 4 concentration gradients, coated with a plate, and then put into the incubator for culture. The dish was then taken out for colony counting after 24 h to observe the growth of bacteria. The relative bacteriostatic rate was calculated according to the following formula:Relative bacteriostatic rate (%) = × 100%A - the OD values of the colonies in the blank group;B - the OD values of the colonies in the experimental group.
4. Conclusions
Author Contributions
Acknowledgments
References
- Liu, J. Z.; Dong, J.; Zhang, T.; Peng, Q. , Graphene-based nanomaterials and their potentials in advanced drug delivery and cancer therapy. J Control Release 2018, 286, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Naghdi, T.; Golmohammadi, H.; Vosough, M.; Atashi, M.; Saeedi, I.; Maghsoudi, M. T. , Lab-on-nanopaper: An optical sensing bioplatform based on curcumin embedded in bacterial nanocellulose as an albumin assay kit. Anal. Chim. Acta 2019, 1070, 104–111. [Google Scholar] [CrossRef]
- Fan, Y. T.; Yi, J.; Zhang, Y. Z.; Yokoyama, W. , Fabrication of curcumin-loaded bovine serum albumin (BSA)-dextran nanoparticles and the cellular antioxidant activity. Food Chemistry 2018, 239, 1210–1218. [Google Scholar] [CrossRef]
- Ghosh, S.; Banerjee, S.; Sil, P. C. , The beneficial role of curcumin on inflammation; diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol 2015, 83, 111–124. [Google Scholar] [CrossRef]
- Zheng, B. J.; McClements, D. J. , Formulation of More Efficacious Curcumin Delivery Systems Using Colloid Science: Enhanced Solubility, Stability, and Bioavailability. Molecules 2020, 25, 2791. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Y.; Fan, Y. L.; Yan, J. J.; Yang, M. , Engineering polyphenol-based polymeric nanoparticles for drug delivery and bioimaging. Chem. Eng. J 2022, 439, 135661. [Google Scholar] [CrossRef]
- Almawash, S. , Solid lipid nanoparticles, an effective carrier for classical antifungal drugs. Saudi Pharmaceutical Journal 2023, 31, 1167–1180. [Google Scholar] [CrossRef]
- Zhu, H. J.; Li, B. F.; Chan, C. Y.; Ling, B. L. Q.; Tor, J.; Oh, X. Y.; Jiang, W. B.; Ye, E. Y.; Li, Z. B.; Loh, X. J. , Advances in Single-component inorganic nanostructures for photoacoustic imaging guided photothermal therapy. Adv. Drug Deliv. Rev 2023, 192, 114644. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Abbasi, P.; Eshaghi, M. M.; Bakhshi, A.; Manicum, A. L. E.; Rahdar, A.; Pandey, S.; Jadoun, S.; Diez-Pascual, A. M. , Curcumin delivery and co-delivery based on nanomaterials as an effective approach for cancer therapy. J Drug Deliv Sci Technol 2022, 78, 103982. [Google Scholar] [CrossRef]
- Ban, E.; Kim, A. , Coacervates: Recent developments as nanostructure delivery platforms for therapeutic biomolecules. Int J Pharm 2022, 624, 122058. [Google Scholar] [CrossRef] [PubMed]
- Hirschbiegel, C. M.; Zhang, X.; Huang, R.; Cicek, Y. A.; Fedeli, S.; Rotello, V. M. , Inorganic nanoparticles as scaffolds for bioorthogonal catalysts. Adv Drug Deliv Rev 2023, 195, 114730. [Google Scholar] [CrossRef] [PubMed]
- Jang, E. H.; Kim, G. L.; Park, M. G.; Shim, M. K.; Kim, J.-H. , Hypoxia-responsive, organic-inorganic hybrid mesoporous silica nanoparticles for triggered drug release. Journal of Drug Delivery Science and Technology 2020, 56. [Google Scholar] [CrossRef]
- Mohan, B.; Kamboj, A. ; Virender; Singh, K.; Priyanka; Singh, G.; Pombeiro, A. J. L.; Ren, P., Metal-organic frameworks (MOFs) materials for pesticides, heavy metals, and drugs removal: Environmental safety. Separation and Purification Technology 2023, 310. [Google Scholar] [CrossRef]
- Acharya, A. P.; Sezginel, K. B.; Gideon, H. P.; Greene, A. C.; Lawson, H. D.; Inamdar, S.; Tang, Y.; Fraser, A. J.; Patel, K. V.; Liu, C.; Rosi, N. L.; Chan, S. Y.; Flynn, J. L.; Wilmer, C. E.; Little, S. R. , In silico identification and synthesis of a multi-drug loaded MOF for treating tuberculosis. J Control Release 2022, 352, 242–255. [Google Scholar] [CrossRef]
- Gupta, A.; Keddie, D. J.; Kannappan, V.; Gibson, H.; Khalil, I. R.; Kowalczuk, M.; Martin, C.; Shuai, X.; Radecka, I. , Production and characterisation of bacterial cellulose hydrogels loaded with curcumin encapsulated in cyclodextrins as wound dressings. Eur. Polym. J 2019, 118, 437–450. [Google Scholar] [CrossRef]
- Norahan, M. H.; Pedroza-Gonz, S. C.; Sanchez-Salazar, M. G.; Alvarez, M. M.; Santiago, G. T. D. , Structural and biological engineering of 3D hydrogels for wound healing. Bioact. Mater 2023, 24, 197–235. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, Y. Y.; Zha, X. Q.; Li, Q. M.; Pan, L. H.; Luo, J. P. , Research progress on polysaccharide/protein hydrogels: Preparation method, functional property and application as delivery systems for bioactive ingredients. Food Res. Int 2021, 147, 110542. [Google Scholar] [CrossRef] [PubMed]
- Li, H. B.; Cheng, F.; Wei, X. J.; Yi, X. T.; Tang, S. Z.; Wang, Z. Y.; Zhang, Y. S.; He, J. M.; Huang, Y. D. , Injectable, self-healing, antibacterial, and hemostatic N,O-carboxymethyl chitosan/oxidized chondroitin sulfate composite hydrogel for wound dressing. Mater. Sci. Eng. C 2021, 118, 111324. [Google Scholar] [CrossRef]
- Zhang, X. J.; Lin, G.; Kumar, S. R.; Mark, J. E. , Hydrogels prepared from polysiloxane chains by end linking them with trifunctional silanes containing hydrophilic groups. Polymer 2009, 50, 5414–5421. [Google Scholar] [CrossRef]
- Badsha, M. A. H.; Khan, M.; Wu, B. L.; Kumar, A.; Lo, I. M. C. , Role of surface functional groups of hydrogels in metal adsorption: From performance to mechanism. J. Hazard. Mater 2021, 408, 124463. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Ferrer, M. L.; Mateo, C. R.; Monte, F. D. , Freeze-drying of aqueous solutions of deep eutectic solvents: a suitable approach to deep eutectic suspensions of self-assembled structures. Langmuir 2009, 25, 5509–5515. [Google Scholar] [CrossRef]
- Su, H.; Sun, F.; Jia, J.; He, H.; Wang, A.; Zhu, G. , A highly porous medical metal-organic framework constructed from bioactive curcumin. Chem Commun (Camb) 2015, 51, 5774–5777. [Google Scholar] [CrossRef]
- Wu, C. S.; Xiong, Z. H.; Li, C.; Zhang, J. M. , Zeolitic imidazolate metal organic framework ZIF-8 with ultra-high adsorption capacity bound tetracycline in aqueous solution. RSC Adv 2015, 5, 82127–82137. [Google Scholar] [CrossRef]
- Devarayapalli, K. C.; Vattikuti, S. V. P.; Yoo, K. S.; Nagajyothi, P. C.; Shim, J. , Rapid microwave-assisted construction of ZIF-8 derived ZnO and ZnO@Ta2O5 nanocomposite as an efficient electrode for methanol and urea electro-oxidation. J. Electroanal. Chem 2020, 878, 114634. [Google Scholar] [CrossRef]
- Geng, C.; Liu, X.; Ma, J.; Ban, H.; Bian, H.; Huang, G. , High strength, controlled release of curcumin-loaded ZIF-8/chitosan/zein film with excellence gas barrier and antibacterial activity for litchi preservation. Carbohydr Polym 2023, 306, 120612. [Google Scholar] [CrossRef]
- Moussawi, R. N.; Patra, D. , Modification of nanostructured ZnO surfaces with curcumin: fluorescence-based sensing for arsenic and improving arsenic removal by ZnO. RSC Adv 2016, 6, 17256–17268. [Google Scholar] [CrossRef]
- Kurkcuoglu, G. S.; Kavlak, I.; Kinik, B.; Sahin, O. , Experimental and theoretical studies on the molecular structures and vibrational spectra of cyanide complexes with 1,2-dimethylimidazole: M(dmi)(2)Ni(mu-CN)(4) (M = Cu, Zn or Cd). J. Mol. Struct 2020, 1199, 126892. [Google Scholar] [CrossRef]
- Kumari, G.; Jayaramulu, K.; Maji, T. K.; Narayana, C. , Temperature Induced Structural Transformations and Gas Adsorption in the Zeolitic Imidazolate Framework ZIF-8: A Raman Study. Journal of Physical Chemistry A 2013, 117, 11006–11012. [Google Scholar] [CrossRef]
- Radhakrishnan, D.; Narayana, C. , Guest dependent Brillouin and Raman scattering studies of zeolitic imidazolate framework-8 (ZIF-8) under external pressure. J. Chem. Phys 2016, 144, 134704. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, S.; Guan, X. G.; Xie, Z. G. , One-Step Synthesis of Nanoscale Zeolitic Imidazolate Frameworks with High Curcumin Loading for Treatment of Cervical Cancer. ACS Appl. Mater. Interfaces 2015, 7, 22181–22187. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Z.; Wang, F.; Li, Y.; Ou, Z.; Jiang, J. , A novel strategy to reinforce double network hydrogels with enhanced mechanical strength and swelling ratio by nano cement hydrates. Polymer 2023, 269. [Google Scholar] [CrossRef]
- Xu, P.; Shang, Z.; Yao, M.; Li, X. , Mechanistic insight into improving strength and stability of hydrogels via nano-silica. Journal of Molecular Liquids 2022, 357. [Google Scholar] [CrossRef]
- Nikpour, S.; Ansari-Asl, Z.; Sedaghat, T.; Hoveizi, E. , Curcumin-loaded Fe-MOF/PDMS porous scaffold: Fabrication, characterization, and biocompatibility assessment. Journal of Industrial and Engineering Chemistry 2022, 110, 188–197. [Google Scholar] [CrossRef]
- Kang, L.; Liang, Q.; Abdul, Q.; Rashid, A.; Ren, X.; Ma, H. , Preparation technology and preservation mechanism of gamma-CD-MOFs biaological packaging film loaded with curcumin. Food Chem 2023, 420, 136142. [Google Scholar] [CrossRef] [PubMed]
- Phan, T. N.; Buckner, T.; Sheng, J.; Baldeck, J. D.; Marquis, R. E. , Physiologic actions of zinc related to inhibition of acid and alkali production by oral streptococci in suspensions and biofilms. Oral Microbiology and Immunology 2010, 19, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tan, L.; Liu, X.; Liang, Y.; Zheng, Y.; Yeung, K. W. K.; Cui, Z.; Zhu, S.; Li, Z.; Wu, S. , Zn(2+)-assisted photothermal therapy for rapid bacteria-killing using biodegradable humic acid encapsulated MOFs. Colloids Surf B Biointerfaces 2020, 188, 110781. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



