Submitted:
07 June 2023
Posted:
07 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
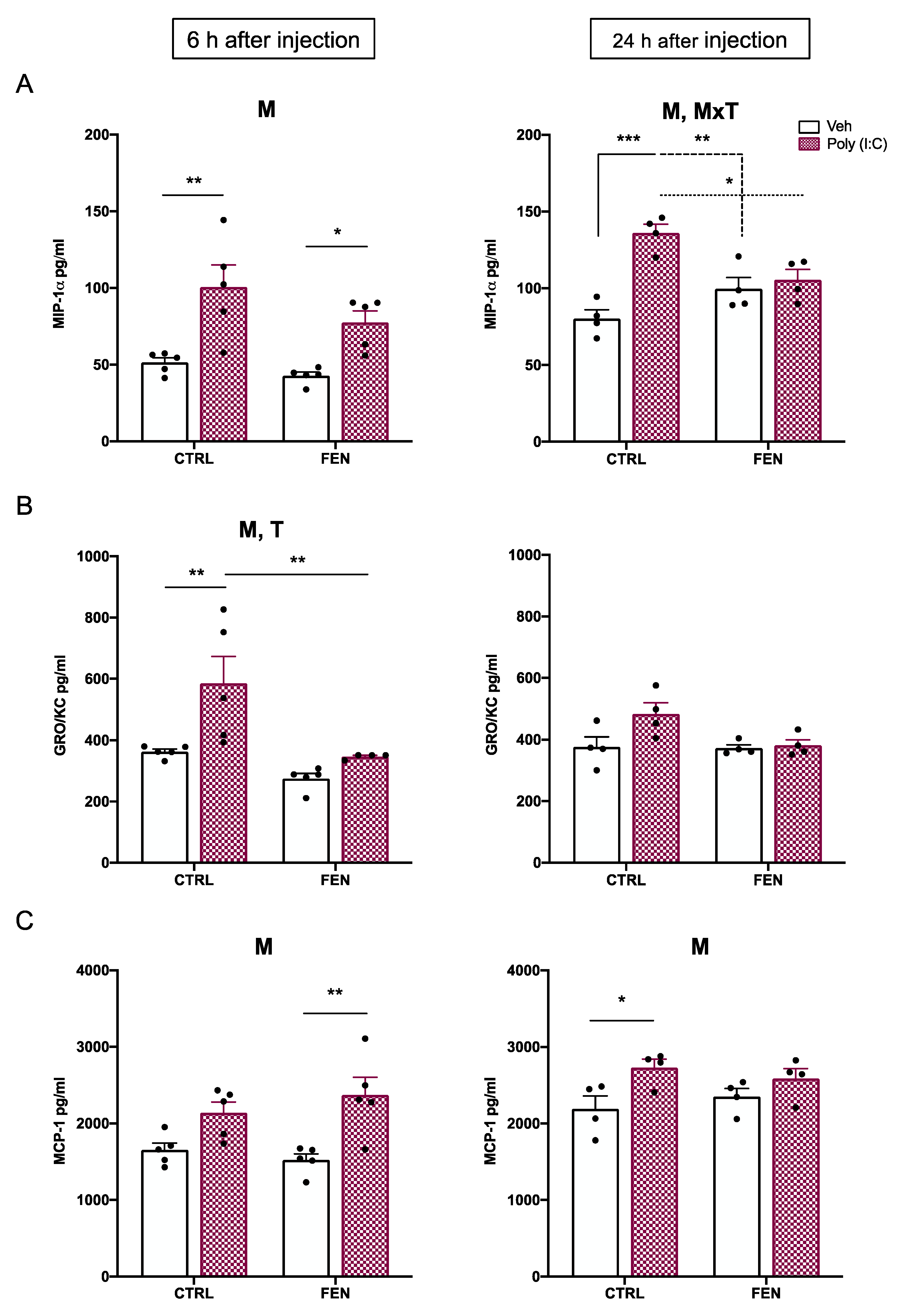
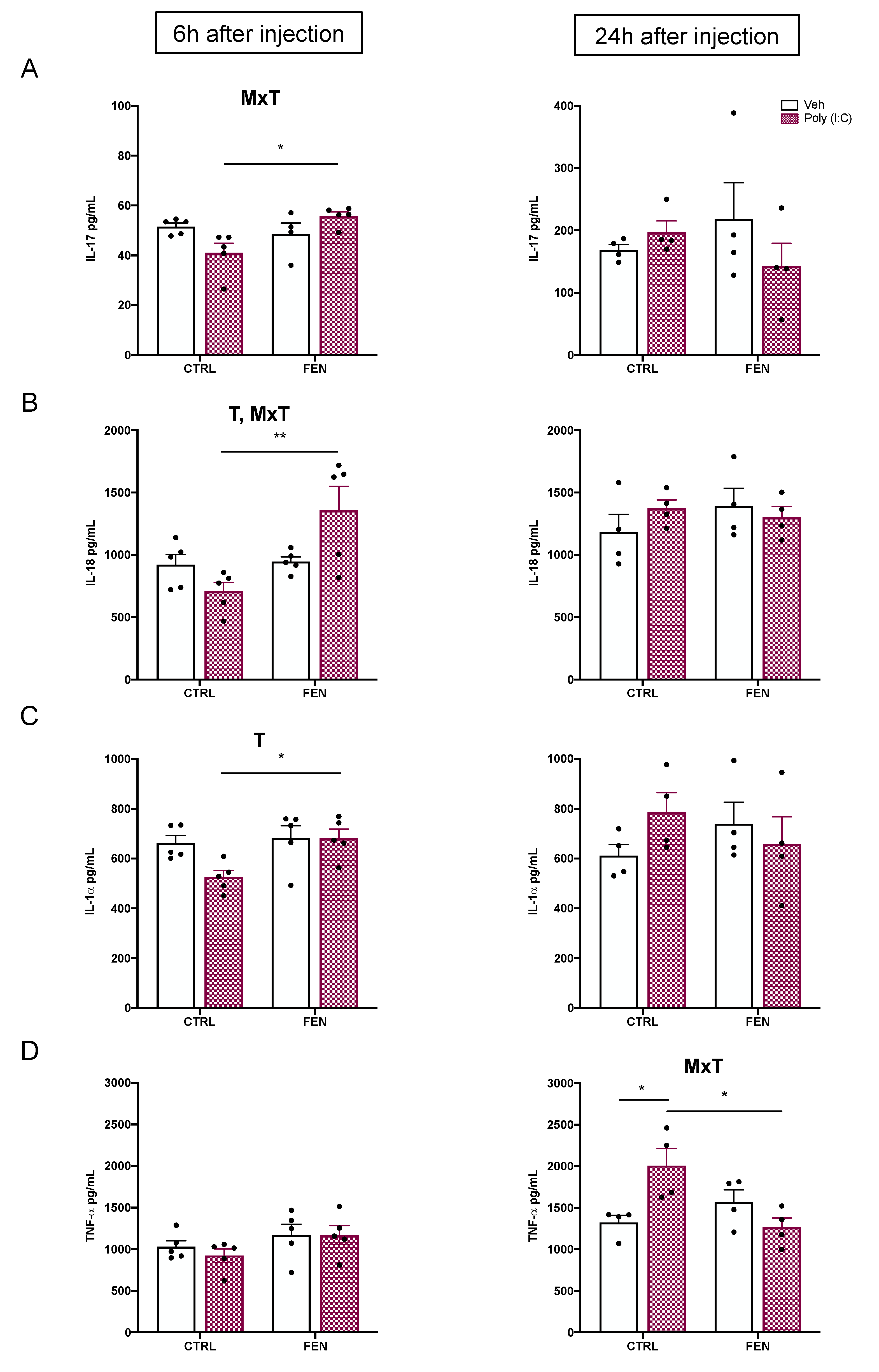
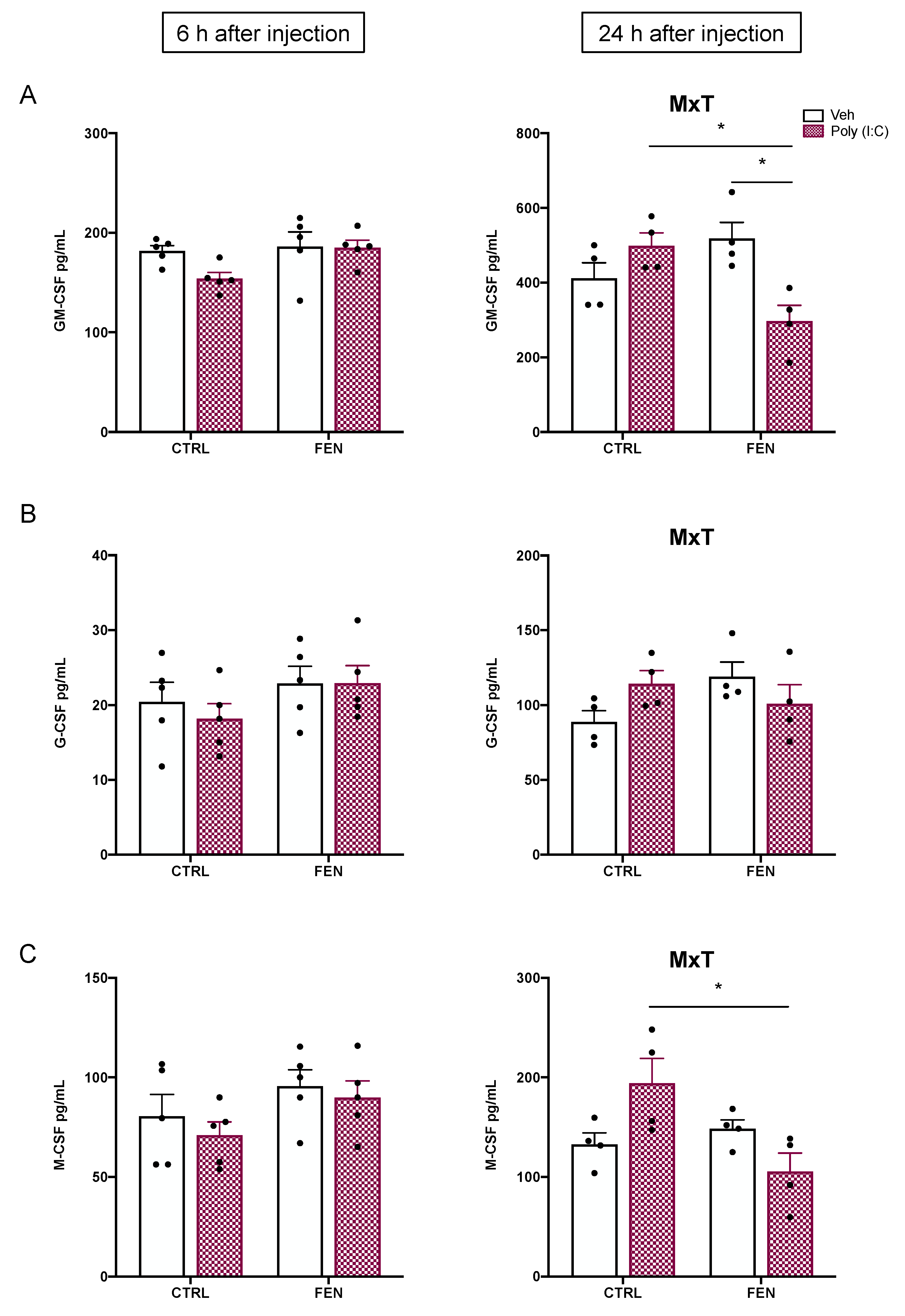
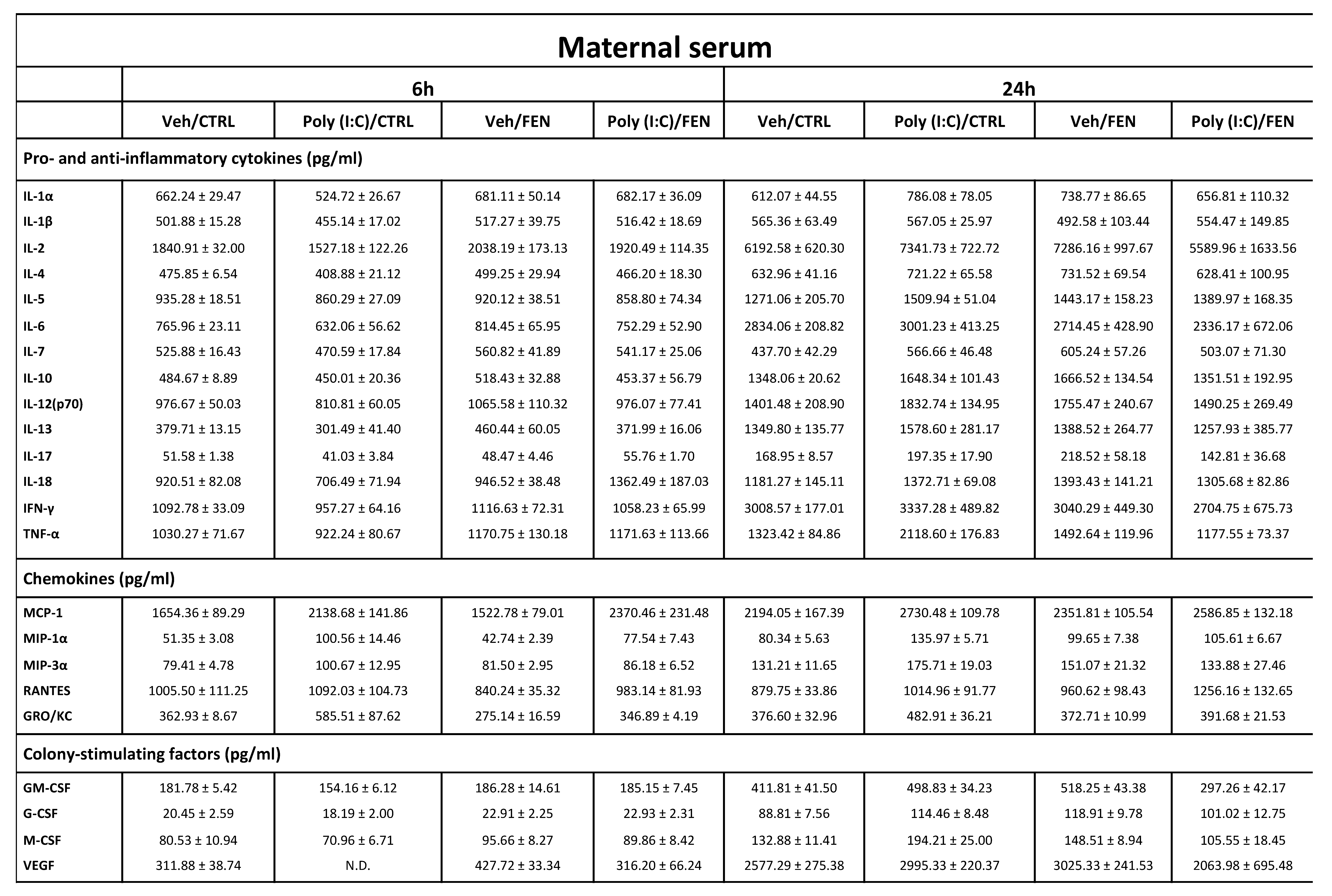
2.1. Effect of fenofibrate treatment on MIA-induced inflammation in the maternal serum.
2.1.1. Chemokines
2.1.2. Cytokines
2.1.3. Colony-stimulating factors
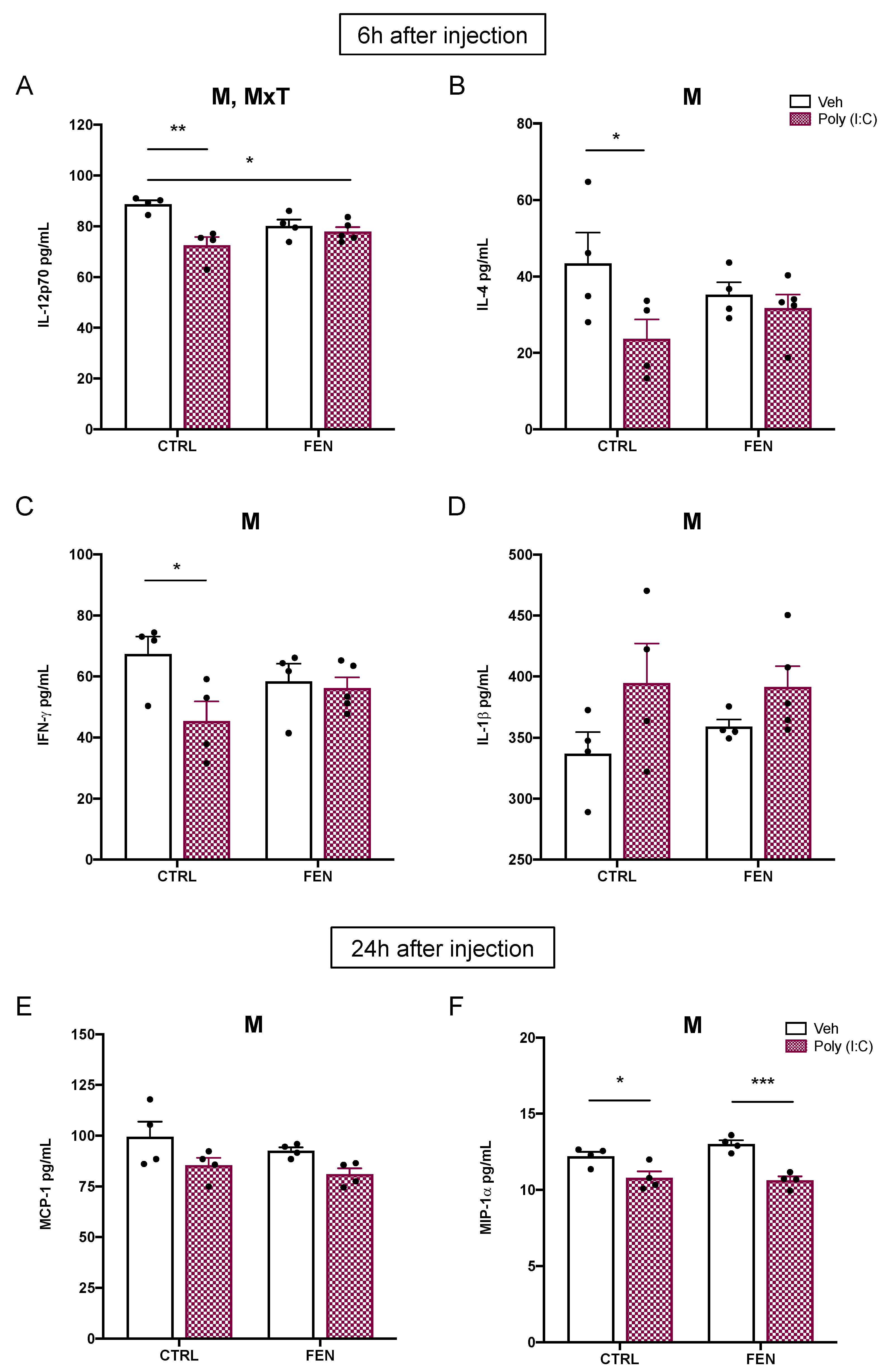
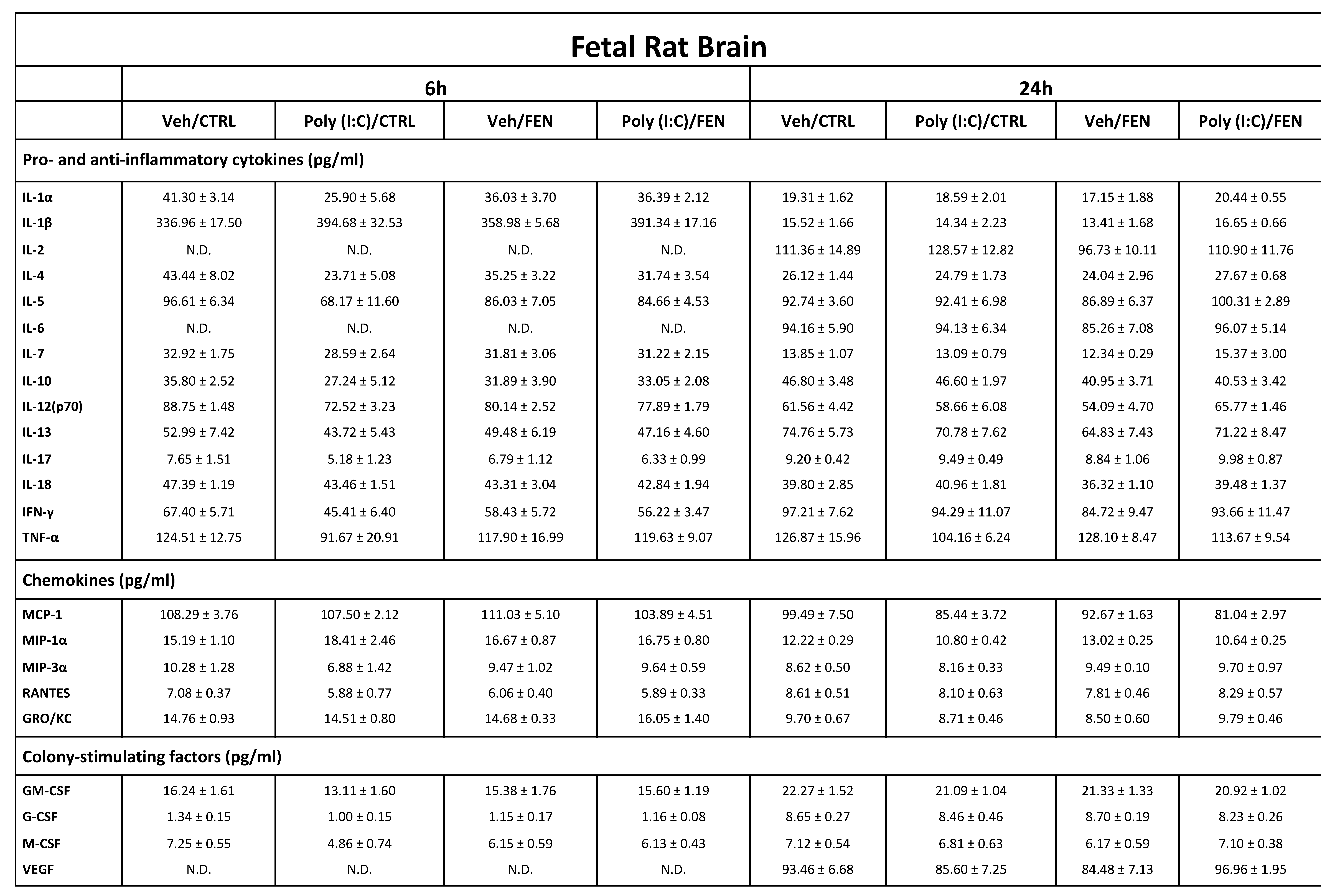
2.2. Effect of fenofibrate treatment on MIA-induced neuroinflammation in the fetal brain.
2.2.1. Cytokines
2.2.2. Chemokines and colony-stimulating factors
3. Discussion
4. Materials and Methods
4.1. Animals.
4.2. Drugs and Treatments.
4.3. Maternal blood and fetal brain collection.
4.4. Blood and brain tissue processing.
4.5. Cytokines, chemikines and CSFs measurements.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Estes, M.L.; McAllister, A.K. Maternal Immune Activation: Implications for Neuropsychiatric Disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Han, V.X.; Patel, S.; Jones, H.F.; Nielsen, T.C.; Mohammad, S.S.; Hofer, M.J.; Gold, W.; Brilot, F.; Lain, S.J.; Nassar, N.; et al. Maternal Acute and Chronic Inflammation in Pregnancy Is Associated with Common Neurodevelopmental Disorders: A Systematic Review. Transl Psychiatry 2021, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Han, V.X.; Patel, S.; Jones, H.F.; Dale, R.C. Maternal Immune Activation and Neuroinflammation in Human Neurodevelopmental Disorders. Nat Rev Neurol 2021, 17, 564–579. [Google Scholar] [CrossRef]
- Shook, L.L.; Sullivan, E.L.; Lo, J.O.; Perlis, R.H.; Edlow, A.G. COVID-19 in Pregnancy: Implications for Fetal Brain Development. Trends Mol Med 2022, 28, 319–330. [Google Scholar] [CrossRef]
- Edlow, A.G.; Castro, V.M.; Shook, L.L.; Kaimal, A.J.; Perlis, R.H. Neurodevelopmental Outcomes at 1 Year in Infants of Mothers Who Tested Positive for SARS-CoV-2 During Pregnancy. JAMA Netw Open 2022, 5, e2215787. [Google Scholar] [CrossRef]
- Patterson, P.H. Immune Involvement in Schizophrenia and Autism: Etiology, Pathology and Animal Models. Behav Brain Res 2009, 204, 313–321. [Google Scholar] [CrossRef]
- Meyer, U. Developmental Neuroinflammation and Schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2013, 42, 20–34. [Google Scholar] [CrossRef]
- Kreitz, S.; Zambon, A.; Ronovsky, M.; Budinsky, L.; Helbich, T.H.; Sideromenos, S.; Ivan, C.; Konerth, L.; Wank, I.; Berger, A.; et al. Maternal Immune Activation during Pregnancy Impacts on Brain Structure and Function in the Adult Offspring. Brain Behav Immun 2020, 83, 56–67. [Google Scholar] [CrossRef]
- Miller, A.H.; Haroon, E.; Raison, C.L.; Felger, J.C. Cytokine Targets in the Brain: Impact on Neurotransmitters and Neurocircuits. Depress Anxiety 2013, 30, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Mueller, F.S.; Polesel, M.; Richetto, J.; Meyer, U.; Weber-Stadlbauer, U. Mouse Models of Maternal Immune Activation: Mind Your Caging System! Brain Behav Immun 2018, 73, 643–660. [Google Scholar] [CrossRef]
- Allswede, D.M.; Yolken, R.H.; Buka, S.L.; Cannon, T.D. Cytokine Concentrations throughout Pregnancy and Risk for Psychosis in Adult Offspring: A Longitudinal Case-Control Study. Lancet Psychiatry 2020, 7, 254–261. [Google Scholar] [CrossRef]
- Carbone, E.; Buzzelli, V.; Manduca, A.; Leone, S.; Rava, A.; Trezza, V. Maternal Immune Activation Induced by Prenatal Lipopolysaccharide Exposure Leads to Long-Lasting Autistic-like Social, Cognitive and Immune Alterations in Male Wistar Rats. Int J Mol Sci 2023, 24, 3920. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, L.; Rehavi, M.; Nachman, R.; Weiner, I. Immune Activation during Pregnancy in Rats Leads to a Postpubertal Emergence of Disrupted Latent Inhibition, Dopaminergic Hyperfunction, and Altered Limbic Morphology in the Offspring: A Novel Neurodevelopmental Model of Schizophrenia. Neuropsychopharmacology 2003, 28, 1778–1789. [Google Scholar] [CrossRef]
- Meyer, U. Prenatal Poly(i:C) Exposure and Other Developmental Immune Activation Models in Rodent Systems. Biol Psychiatry 2014, 75, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.R.; Careaga, M.; Van de Water, J.; McAllister, K.; Bauman, M.D.; Ashwood, P. Long-Term Altered Immune Responses Following Fetal Priming in a Non-Human Primate Model of Maternal Immune Activation. Brain Behav Immun 2017, 63, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Luchicchi, A.; Lecca, S.; Melis, M.; De Felice, M.; Cadeddu, F.; Frau, R.; Muntoni, A.L.; Fadda, P.; Devoto, P.; Pistis, M. Maternal Immune Activation Disrupts Dopamine System in the Offspring. Int J Neuropsychopharmacol 2016, 19, pyw007. [Google Scholar] [CrossRef] [PubMed]
- Santoni, M.; Frau, R.; Pistis, M. Transgenerational Sex-Dependent Disruption of Dopamine Function Induced by Maternal Immune Activation. Front Pharmacol 2022, 13, 821498. [Google Scholar] [CrossRef] [PubMed]
- Santoni, M.; Sagheddu, C.; Serra, V.; Mostallino, R.; Castelli, M.P.; Pisano, F.; Scherma, M.; Fadda, P.; Muntoni, A.L.; Zamberletti, E.; et al. Maternal Immune Activation Impairs Endocannabinoid Signaling in the Mesolimbic System of Adolescent Male Offspring. Brain Behav Immun 2023, 109, 271–284. [Google Scholar] [CrossRef] [PubMed]
- De Felice, M.; Melis, M.; Aroni, S.; Muntoni, A.L.; Fanni, S.; Frau, R.; Devoto, P.; Pistis, M. The PPARα Agonist Fenofibrate Attenuates Disruption of Dopamine Function in a Maternal Immune Activation Rat Model of Schizophrenia. CNS Neurosci Ther 2019, 25, 549–561. [Google Scholar] [CrossRef]
- Melis, M.; Carta, S.; Fattore, L.; Tolu, S.; Yasar, S.; Goldberg, S.R.; Fratta, W.; Maskos, U.; Pistis, M. Peroxisome Proliferator-Activated Receptors-Alpha Modulate Dopamine Cell Activity through Nicotinic Receptors. Biol Psychiatry 2010, 68, 256–264. [Google Scholar] [CrossRef]
- Skerrett, R.; Malm, T.; Landreth, G. Nuclear Receptors in Neurodegenerative Diseases. Neurobiol Dis 2014, 72 Pt A, 104–116. [Google Scholar] [CrossRef]
- Scheggi, S.; Melis, M.; De Felice, M.; Aroni, S.; Muntoni, A.L.; Pelliccia, T.; Gambarana, C.; De Montis, M.G.; Pistis, M. PPARα Modulation of Mesolimbic Dopamine Transmission Rescues Depression-Related Behaviors. Neuropharmacology 2016, 110, 251–259. [Google Scholar] [CrossRef]
- Duval, C.; Chinetti, G.; Trottein, F.; Fruchart, J.-C.; Staels, B. The Role of PPARs in Atherosclerosis. Trends Mol Med 2002, 8, 422–430. [Google Scholar] [CrossRef]
- Sagheddu, C.; Torres, L.H.; Marcourakis, T.; Pistis, M. Endocannabinoid-Like Lipid Neuromodulators in the Regulation of Dopamine Signaling: Relevance for Drug Addiction. Front Synaptic Neurosci 2020, 12, 588660. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Vitale, R.M. The Endocannabinoid System and PPARs: Focus on Their Signalling Crosstalk, Action and Transcriptional Regulation. Cells 2021, 10, 586. [Google Scholar] [CrossRef]
- Decara, J.; Rivera, P.; López-Gambero, A.J.; Serrano, A.; Pavón, F.J.; Baixeras, E.; Rodríguez de Fonseca, F.; Suárez, J. Peroxisome Proliferator-Activated Receptors: Experimental Targeting for the Treatment of Inflammatory Bowel Diseases. Front Pharmacol 2020, 11, 730. [Google Scholar] [CrossRef]
- Jiang, Z.; Zamanian-Daryoush, M.; Nie, H.; Silva, A.M.; Williams, B.R.G.; Li, X. Poly(I-C)-Induced Toll-like Receptor 3 (TLR3)-Mediated Activation of NFkappa B and MAP Kinase Is through an Interleukin-1 Receptor-Associated Kinase (IRAK)-Independent Pathway Employing the Signaling Components TLR3-TRAF6-TAK1-TAB2-PKR. J Biol Chem 2003, 278, 16713–16719. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, S.; Khan, D.; Kong, E.; Berger, A.; Pollak, A.; Pollak, D.D. The Poly(I:C)-Induced Maternal Immune Activation Model in Preclinical Neuropsychiatric Drug Discovery. Pharmacol Ther 2015, 149, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Hameete, B.C.; Fernández-Calleja, J.M.S.; de Groot, M.W.G.D.M.; Oppewal, T.R.; Tiemessen, M.M.; Hogenkamp, A.; de Vries, R.B.M.; Groenink, L. The Poly(I:C)-Induced Maternal Immune Activation Model; a Systematic Review and Meta-Analysis of Cytokine Levels in the Offspring. Brain Behav Immun Health 2021, 11, 100192. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; Nyffeler, M.; Engler, A.; Urwyler, A.; Schedlowski, M.; Knuesel, I.; Yee, B.K.; Feldon, J. The Time of Prenatal Immune Challenge Determines the Specificity of Inflammation-Mediated Brain and Behavioral Pathology. J Neurosci 2006, 26, 4752–4762. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, J.H.; Jarskog, L.F.; Vadlamudi, S. Maternal Poly I:C Exposure during Pregnancy Regulates TNFα, BDNF, and NGF Expression in Neonatal Brain and the Maternal–Fetal Unit of the Rat. J Neuroimmunol 2005, 159, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Urakubo, A.; Jarskog, L.F.; Lieberman, J.A.; Gilmore, J.H. Prenatal Exposure to Maternal Infection Alters Cytokine Expression in the Placenta, Amniotic Fluid, and Fetal Brain. Schizophr Res 2001, 47, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Openshaw, R.L.; Kwon, J.; McColl, A.; Penninger, J.M.; Cavanagh, J.; Pratt, J.A.; Morris, B.J. JNK Signalling Mediates Aspects of Maternal Immune Activation: Importance of Maternal Genotype in Relation to Schizophrenia Risk. J Neuroinflammation 2019, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Arrode-Brusés, G.; Brusés, J.L. Maternal Immune Activation by Poly(I:C) Induces Expression of Cytokines IL-1β and IL-13, Chemokine MCP-1 and Colony Stimulating Factor VEGF in Fetal Mouse Brain. J Neuroinflammation 2012, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Ballendine, S.A.; Greba, Q.; Dawicki, W.; Zhang, X.; Gordon, J.R.; Howland, J.G. Behavioral Alterations in Rat Offspring Following Maternal Immune Activation and ELR-CXC Chemokine Receptor Antagonism during Pregnancy: Implications for Neurodevelopmental Psychiatric Disorders. Prog Neuropsychopharmacol Biol Psychiatry 2015, 57, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Cartier, L.; Hartley, O.; Dubois-Dauphin, M.; Krause, K.-H. Chemokine Receptors in the Central Nervous System: Role in Brain Inflammation and Neurodegenerative Diseases. Brain Res Brain Res Rev 2005, 48, 16–42. [Google Scholar] [CrossRef] [PubMed]
- Charo, I.F.; Ransohoff, R.M. The Many Roles of Chemokines and Chemokine Receptors in Inflammation. N Engl J Med 2006, 354, 610–621. [Google Scholar] [CrossRef]
- Deverman, B.E.; Patterson, P.H. Cytokines and CNS Development. Neuron 2009, 64, 61–78. [Google Scholar] [CrossRef]
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.N.; Van De Water, J. Associations of Impaired Behaviors with Elevated Plasma Chemokines in Autism Spectrum Disorders. J Neuroimmunol 2011, 232, 196–199. [Google Scholar] [CrossRef]
- Reale, M.; Patruno, A.; De Lutiis, M.A.; Pesce, M.; Felaco, M.; Di Giannantonio, M.; Di Nicola, M.; Grilli, A. Dysregulation of Chemo-Cytokine Production in Schizophrenic Patients versus Healthy Controls. BMC Neurosci 2011, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.J.; Baune, B.T. Chemokines and Chemokine Receptors in Mood Disorders, Schizophrenia, and Cognitive Impairment: A Systematic Review of Biomarker Studies. Neurosci Biobehav Rev 2014, 42, 93–115. [Google Scholar] [CrossRef]
- Cieślik, M.; Gąssowska-Dobrowolska, M.; Jęśko, H.; Czapski, G.A.; Wilkaniec, A.; Zawadzka, A.; Dominiak, A.; Polowy, R.; Filipkowski, R.K.; Boguszewski, P.M.; et al. Maternal Immune Activation Induces Neuroinflammation and Cortical Synaptic Deficits in the Adolescent Rat Offspring. Int J Mol Sci 2020, 21, 4097. [Google Scholar] [CrossRef]
- Ding, S.; Hu, Y.; Luo, B.; Cai, Y.; Hao, K.; Yang, Y.; Zhang, Y.; Wang, X.; Ding, M.; Zhang, H.; et al. Age-Related Changes in Neuroinflammation and Prepulse Inhibition in Offspring of Rats Treated with Poly I:C in Early Gestation. Behav Brain Funct 2019, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Kamerman, P.; Skosana, M.; Loram, L.; Mitchell, B.; Weber, J. Fever and Inflammatory Cytokine Response in Rats Injected Subcutaneously with Viral Double-Stranded RNA Analog, Polyinosinic:Polycytidylic Acid (Poly-I:C). J Therm Biol 2011, 36, 397–402. [Google Scholar] [CrossRef]
- Koga, K.; Cardenas, I.; Aldo, P.; Abrahams, V.M.; Peng, B.; Fill, S.; Romero, R.; Mor, G. Activation of TLR3 in the Trophoblast Is Associated with Preterm Delivery. Am J Reprod Immunol 2009, 61, 196–212. [Google Scholar] [CrossRef] [PubMed]
- McColl, E.R.; Piquette-Miller, M. Poly(I:C) Alters Placental and Fetal Brain Amino Acid Transport in a Rat Model of Maternal Immune Activation. Am J Reprod Immunol 2019, 81, e13115. [Google Scholar] [CrossRef] [PubMed]
- Kentner, A.C.; Bilbo, S.D.; Brown, A.S.; Hsiao, E.Y.; McAllister, A.K.; Meyer, U.; Pearce, B.D.; Pletnikov, M.V.; Yolken, R.H.; Bauman, M.D. Maternal Immune Activation: Reporting Guidelines to Improve the Rigor, Reproducibility, and Transparency of the Model. Neuropsychopharmacology 2019, 44, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Valtanen, P.; Diermen, B.A.; Lakhan, N.; Lousberg, E.L.; Robertson, S.A.; Hayball, J.D.; Diener, K.R. Maternal Host Responses to Poly(I:C) during Pregnancy Leads to Both Dysfunctional Immune Profiles and Altered Behaviour in the Offspring. Am J Reprod Immunol 2020, 84. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.; Sharma, R.; Smith, K.B.; Mar, K.D.; Barve, R.; Lukasik, M.; Pirwani, A.F.; Malette-Guyon, E.; Lamba, S.; Thomas, B.J.; et al. Probiotic Consumption during Puberty Mitigates LPS-Induced Immune Responses and Protects against Stress-Induced Depression- and Anxiety-like Behaviors in Adulthood in a Sex-Specific Manner. Brain Behav Immun 2019, 81, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Ashdown, H.; Dumont, Y.; Ng, M.; Poole, S.; Boksa, P.; Luheshi, G.N. The Role of Cytokines in Mediating Effects of Prenatal Infection on the Fetus: Implications for Schizophrenia. Mol Psychiatry 2006, 11, 47–55. [Google Scholar] [CrossRef]
- Carpentier, P.A.; Dingman, A.L.; Palmer, T.D. Placental TNF-α Signaling in Illness-Induced Complications of Pregnancy. Am J Pathol 2011, 178, 2802–2810. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, E.; Pakan, J.M.P.; Yilmazer-Hanke, D.; McDermott, K.W. Acute in Utero Exposure to Lipopolysaccharide Induces Inflammation in the Pre- and Postnatal Brain and Alters the Glial Cytoarchitecture in the Developing Amygdala. J Neuroinflammation 2017, 14, 212. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research. Drug Approvals and Databases Tricor (Fenofibrate) Tablets https://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/021656s000_TricorTOC.cfm. (accessed on 30 May 2023).
- European Medicines Agency Science Medicines Health European Commission final decision Fibrates https://www.ema.europa.eu/en/medicines/human/referrals/fibrates. (accessed on 30 May 2023).
- Jin, L.; Hua, H.; Ji, Y.; Jia, Z.; Peng, M.; Huang, S. Anti-Inflammatory Role of Fenofibrate in Treating Diseases. Biomol Biomed 2023, 23, 376–391. [Google Scholar] [CrossRef]
- Sagheddu, C.; Melis, M.; Muntoni, A.L.; Pistis, M. Repurposing Peroxisome Proliferator-Activated Receptor Agonists in Neurological and Psychiatric Disorders. Pharmaceuticals (Basel) 2021, 14, 1025. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Ren, X.; Han, T.; Chen, Y.; Qiu, H.; Liu, W.; Hu, Y. Fenofibrate Attenuates Fatty Acid-Induced Islet β-Cell Dysfunction and Apoptosis via Inhibiting the NF-ΚB/MIF Dependent Inflammatory Pathway. Metabolism 2017, 77, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, A.; Hattori, Y.; Inoue, T.; Hattori, S.; Kasai, K. Fenofibrate Suppresses Microvascular Inflammation and Apoptosis through Adenosine Monophosphate-Activated Protein Kinase Activation. Metabolism 2011, 60, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Jiang, T.; Bao, Y.-Y.; Chen, G.-J.; Chen, L.; Zhang, Q.; Lu, Y.-X. Fenofibrate Improves High-Fat Diet-Induced and Palmitate-Induced Endoplasmic Reticulum Stress and Inflammation in Skeletal Muscle. Life Sci 2016, 157, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, R.M.; Baugé, E.; Staels, B.; Gervois, P. Systemic and Distal Repercussions of Liver-Specific Peroxisome Proliferator-Activated Receptor-Alpha Control of the Acute-Phase Response. Endocrinology 2008, 149, 3215–3223. [Google Scholar] [CrossRef]
- Guo, Y.-Q.; Zheng, L.-N.; Wei, J.-F.; Hou, X.-L.; Yu, S.-Z.; Zhang, W.-W.; Jing, J.-M. Expression of CCL2 and CCR2 in the Hippocampus and the Interventional Roles of Propofol in Rat Cerebral Ischemia/Reperfusion. Exp Ther Med 2014, 8, 657–661. [Google Scholar] [CrossRef]
- Song, H.; Zhang, X.; Chen, R.; Miao, J.; Wang, L.; Cui, L.; Ji, H.; Liu, Y. Cortical Neuron-Derived Exosomal MicroRNA-181c-3p Inhibits Neuroinflammation by Downregulating CXCL1 in Astrocytes of a Rat Model with Ischemic Brain Injury. Neuroimmunomodulation 2019, 26, 217–233. [Google Scholar] [CrossRef]
- Suzuki, K.; Matsuzaki, H.; Iwata, K.; Kameno, Y.; Shimmura, C.; Kawai, S.; Yoshihara, Y.; Wakuda, T.; Takebayashi, K.; Takagai, S.; et al. Plasma Cytokine Profiles in Subjects with High-Functioning Autism Spectrum Disorders. PLoS One 2011, 6, e20470. [Google Scholar] [CrossRef]
- Shen, Y.; Ou, Ji.; Liu, M.; Shi, L.; Li, Y.; Xiao, L.; Dong, H.; Zhang, F.; Xia, K.; Zhao, J. Altered Plasma Levels of Chemokines in Autism and Their Association with Social Behaviors. Psychiatry Res 2016, 244, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.; Breen, E.J.; Alvares, G.A.; Glozier, N.; Hickie, I.B.; Hunt, A.; Hui, J.; Beilby, J.; Ravine, D.; Wray, J.; et al. Cytokine Levels and Associations with Symptom Severity in Male and Female Children with Autism Spectrum Disorder. Mol Autism 2017, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Heuer, L.S.; Croen, L.A.; Jones, K.L.; Yoshida, C.K.; Hansen, R.L.; Yolken, R.; Zerbo, O.; DeLorenze, G.; Kharrazi, M.; Ashwood, P.; et al. An Exploratory Examination of Neonatal Cytokines and Chemokines as Predictors of Autism Risk: The Early Markers for Autism Study. Biol Psychiatry 2019, 86, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Chitu, V.; Stanley, E.R. Colony-Stimulating Factor-1 in Immunity and Inflammation. Curr Opin Immunol 2006, 18, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.A. Colony-Stimulating Factors in Inflammation and Autoimmunity. Nat Rev Immunol 2008, 8, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Becher, B.; Tugues, S.; Greter, M. GM-CSF: From Growth Factor to Central Mediator of Tissue Inflammation. Immunity 2016, 45, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.A.; Raposo, H.F.; Wanschel, A.C.B.A.; Nardelli, T.R.; Oliveira, H.C.F. Apolipoprotein CIII Overexpression-Induced Hypertriglyceridemia Increases Nonalcoholic Fatty Liver Disease in Association with Inflammation and Cell Death. Oxid Med Cell Longev 2017, 2017, 1838679. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, C.K.; Ferguson, J.F.; Tabita-Martinez, J.; Kong, S.; Shah, R.Y.; Patel, P.N.; Master, S.R.; Usman, M.H.U.; Propert, K.J.; Shah, R.; et al. Peroxisome Proliferator–Activated Receptor-α Agonism With Fenofibrate Does Not Suppress Inflammatory Responses to Evoked Endotoxemia. J Am Heart Assoc 2012, 1, e002923. [Google Scholar] [CrossRef]
- Gu, X.; Song, Y.; Chai, Y.; Lu, F.; Gonzalez, F.J.; Fan, G.; Qi, Y. GC-MS Metabolomics on PPARα-Dependent Exacerbation of Colitis. Mol Biosyst 2015, 11, 1329–1337. [Google Scholar] [CrossRef]
- Škop, V.; Trnovská, J.; Oliyarnyk, O.; Marková, I.; Malínská, H.; Kazdová, L.; Zídek, V.; Landa, V.; Mlejnek, P.; Šimáková, M.; et al. Hepatotoxic Effects of Fenofibrate in Spontaneously Hypertensive Rats Expressing Human C-Reactive Protein. Physiol Res 2016, 65, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Margiani, G.; Castelli, M.P.; Pintori, N.; Frau, R.; Ennas, M.G.; Zottola, A.C.P.; Orrù, V.; Serra, V.; Fiorillo, E.; Fadda, P.; et al. Correction to: Adolescent Self-administration of the Synthetic Cannabinoid Receptor Agonist JWH-018 Induces Neurobiological and Behavioral Alterations in Adult Male Mice. Psychopharmacology (Berl) 2023, 240, 1199–1199. [Google Scholar] [CrossRef] [PubMed]

 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





