Submitted:
07 June 2023
Posted:
08 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
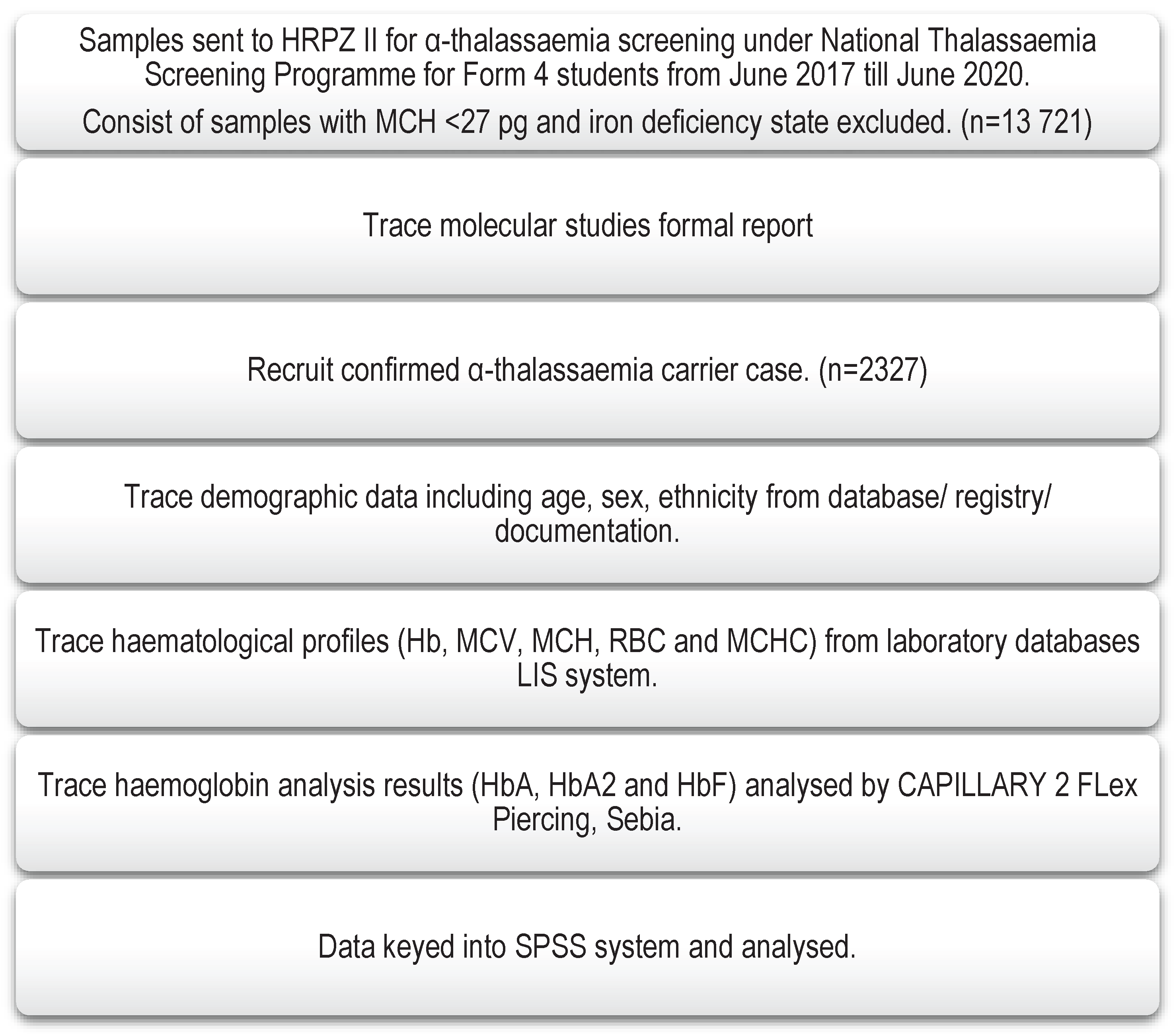
2. Materials and Methods
2.1. Study site
2.2. Study population
2.3. Sampling
3. Results
3.1. Detection of Common deletional and non-deletional α-thalassaemia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bain, B.J. Haemoglobinopathy diagnosis; Blackwell Publising: 2006.
- Hoffbrand, A.V.; Keeling, D.M.; Mehta, A.B.; Higgs, D.R. Haemoglobin and The Inherited Disorders of Globin Synthesis. 2016; pp. 72-97.
- Anders Enevold; Michael Alifrangis; Juan J. Sanchez; Ilona Carneiro; Cally Roper; Claus Børsting; John Lusingu; Lasse S. Vestergaard; Martha M. Lemnge; Niels Morling; et al. Associations between α+-Thalassemia and Plasmodium falciparum Malarial Infection in Northeastern Tanzania The Journal of Infectious Diseases 2007, 196, 451-459. [CrossRef]
- Mockenhaupt, F.P.; Ehrhardt, S.; Gellert, S.; Otchwemah, R.N.; Dietz, E.; Anemana, S.D.; Bienzle, U. α+-thalassemia protects African children from severe malaria. Blood 2004, 104, 2003-2006.
- Ghartey-Kwansah, G.; Boampong, J.N.; Aboagye, B.; Afoakwah, R.; Ameyaw, E.O.; Quashie, N.B. The prevalence of α-thalassemia and its relation to plasmodium falciparum infection in patients presenting to clinics in two distinct ecological zones in Ghana. Hemoglobin 2016, 40, 32-37.
- Opoku-Okrah, C.; Gordge, M.; Kweku Nakua, E.; Abgenyega, T.; Parry, M.; Robertson, C.; Smith, C.L. An investigation of the protective effect of alpha+-thalassaemia against severe P lasmodium falciparum amongst children in K umasi, G hana. International journal of laboratory hematology 2014, 36, 62-70.
- Helena Lamptey; Michael Fokuo Ofori; Bright Adu; Kwadwo Asamoah Kusi; Emmanuel Kakra Dickson; Isabella Quakyi; Alifrangis, M. Association between alpha-thalassaemia trait, Plasmodium falciparum asexual parasites and gametocyte carriage in a malaria endemic area in Southern Ghana. BMC Res Notes 2019, 12, 134. [CrossRef]
- Rosnah, B.; Rosline, H.; Zaidah, A.W.; Noor Haslina, M.N.; Marini, R.; Shafini, M.Y. Detection of Common Deletional Alpha-Thalassemia Spectrum by Molecular Technique in Kelantan, Northeastern Malaysia. International Scholarly Research Network Hematology 2012, 2012, 3-3. [CrossRef]
- Rosline, H.; Ahmed, S.A.; Al-Joudi, F.S.; Rapiaah, M.; Naing, N.N.; Nor Atifah, M.A. Thalassemia among blood donors at the Hospital Universiti Sains Malaysia. Southeast Asian J Trop Med Public Health 2006, 37, 549-552.
- Jameela, S.; Sabirah, S.O.S.; Phan, C.L.; Visalachy, P.; Chang, K.M.; Salwana, M.A.; Zuraidah, A.; Subramanian, Y.; Rahimah, A. Thalassaemia screening among students in a secondary school in Ampang, Malaysia. Med J Malaysia 2011, 66, 522-524.
- Azma, R.Z.; Ainoon, O.; Hafiza, A.; Azlin, I.; Noor Farisah, A.R.; Nor Hidayati, S.; Noor Hamidah, H. Molecular characteristic of alpha thalassaemia among patients diagnosed in UKM Medical Centre. The Malaysian Journal of Pathology 2014, 36, 27-32.
- Rahimah, A.; Saleem, M.; Aloysious, N.S.; Yelumalai, P.; Mohamed, N.; Hassan, S. Distribution of Alpha Thalassaemia Gene variants in Diverse Ethnic Populations in Malaysia: Data from the Institute For Medical Research. International Journal of Molecular Sciences 2013, 14, 18599-18614. [CrossRef]
- Cornelis L Harteveld; Higgs, D.R. α-Thalassaemia. Orphanet Journal of Rare Diseases 2010, 5, 13.
- Farashi, S.; Harteveld, C.L. Molecular basis of alpha-thalassemia. Blood Cells Mol Dis 2018, 70, 43-53. [CrossRef]
- Hockham, C.; Ekwattanakit, S.; Bhatt, S.; Penman, B.S.; Gupta, S.; Viprakasit, V.; Piel, F.B. Estimating the burden of α-thalassaemia in Thailand using a comprehensive prevalence database for Southeast Asia. Elife 2019, e40580. [CrossRef]
- Alibakhshi, R.; Mehrabi, M.; Omidniakan, L.; Shafieenia, S. The spectrum of α-thalassemia mutations in Kermanshah Province, West Iran. Hemoglobin 2015, 39, 403-406. [CrossRef]
- Singh, S.A.; Sarangi, S.; Appiah-Kubi, A.; Hsu, P.; Smith, W.B.; Gallagher, P.G.; Glader, B.; Chui, D.H.K. Hb Adana (HBA2 or HBA1: c.179G > A) and alpha thalassemia: genotype-phenotype correlation. Pediatr Blood Cancer 2018, 65, e27220. [CrossRef]
- Lam, J.C.M.; Soh, S.Y.; Law, H.Y. Clinical and haematological features of non-deletional alpha thalassaemia mutations in Singapore. Pathology 2014, 46, S94. [CrossRef]
- Fucharoen, S.; Viprakasit, V. Hb H disease: clinical course and disease modifiers. Hematology 2009, 2009, 26-34. [CrossRef]
- Galanello, R.; Cao, A. Gene test review. Alpha-thalassemia. Genet Med 2011, 13, 83-88. [CrossRef]
- Viprakasit, V. Alpha-thalassemia syndromes: from clinical and molecular diagnosis to bedside management. Hematology Education: the education program for the annual congress of the European Hematology Association 2013, 7, 329-338.
- Carinna, H.; Samit, B.; Bridget S., P.; Sunetra, G.; Vip, V.; Frederic B., P.; Supachai, E. Estimating the burden of alpha thalassaemia in Thailand using cmprehensive prevalence databae for Southeast Asia. eLife Research Communication 2019, 8. [CrossRef]
- Hua-bing, Z.; De-Pei, L.; Chih-Chuan, L. The Control of Expression of the α-Globin Gene Cluster. International Journal of Hematology 2002, 76, 420-426. [CrossRef]
- Bozdogan, S.T.; Yuregir, O.O.; Buyukkurt, N.; Aslan, H.; Ozdemir, Z.C.; Gambin, T. Alpha-thalassemia mutations in Adana Province, Southern Turkey: genotype-phenotype correlation. Indian J Hematol Blood Transfus 2015, 31, 223-228. [CrossRef]
- Aksu, T.; Yaralı, N.; Bayram, C.; Fettah, A.; Avcı, Z.; Tunç, B. Homozygosity for HBA1: c.179G > A: Hb Adana in an Infant. Hemoglobin 2014, 38, 449-450. [CrossRef]
- George, E.; Jama, T.; Azian, A.S.; Rahimah, A.; Zubaidah, Z. A rare case of alpha-thalassaemia intermedia in a Malay patient double heterozygous for alpha(+)-thalassaemia and a mutation in alpha1 globin gene CD59 (GGC --> GAC). Med J Malaysia 2009, 64, 321-322.
- Alauddin, H.; Jaapar, N.A.; Azma, R.Z.; Ithnin, A.; Razak, N.F.; Loh, C.K.; Alias, H.; Abdul-Latiff, Z.; Othman, A. A case series of α-thalassemia intermedia due to compound heterozygosity for Hb Adana [HBA2: c179G>A (or HBA1); p.Gly60Asp] with other α-thalassemias in Malay families. Hemoglobin 2014, 38, 277-281. [CrossRef]
- Nainggolan, I.M.; Harahap, A.; Setianingsih, I. Hydrops fetalis associated with homozygosity for Hb Adana [alpha59(E8)Gly-->Asp (alpha2)]. Hemoglobin 2010, 34, 394-401. [CrossRef]
- Nainggolan, I.M.; Harahap, A.; Ambarwati, D.D.; Liliani, R.V.; Megawati, D.; Swastika, M.; Setianingsih, I. Interaction of Hb Adana (HBA2: c.179G>A) with deletional and nondeletional α(+)-thalassemia mutations: diverse hematological and clinical features. Hemoglobin 2013, 37, 297-305. [CrossRef]
- Lee, T.Y.; Lai, M.I.; Ismail, P.; Ramachandran, V.; Tan, J.A.; Teh, L.K.; Othman, R.; Hussein, N.H.; George, E. Analysis of α1 and α2 globin genes among patients with hemoglobin Adana in Malaysia. Genet Mol Res 2016, 15. [CrossRef]
- Al-Allawi N.A.;Badi A.I.;Imanian H. et al. Molecular characterization of alpha-thalassemia in the Dohuk region of Iraq. Hemoglobin. 2009,33, pp. 37-44.
- Li B.;Han X.;Ma J. et al. Mutation spectrum and erythrocyte indices characterisation of α-thalassaemia and β-thalassaemia in Sichuan women in China: a thalassaemia screening survey of 42 155 women. J Clin Pathol. 2021,74, pp. 182-186.
- Chen F.E.;Ooi C.;Ha S.Y. et al. Genetic and clinical features of Hemoglobin H disease in Chinese patients. N Engl J Med. 2000,343, pp. 544-50.
- Akhtar M.S.;Qaw F.;Borgio J.F. et al. Spectrum of α-thalassemia mutations in transfusion-dependent β-thalassemia patients from the Eastern Province of Saudi Arabia. Hemoglobin. 2013,37, pp. 65-73.
- Azma, R.Z.; Ainoon, O.; Hafiza, A.; Azlin, I.; Noor Farisah, A.R.; Nor Hidayati, S.; Noor Hamidah, H. Molecular characteristic of alpha thalassaemia among patients diagnosed in UKM Medical Centre. Malay J Pathol 2014, 36, 27-32.
- Ahmad, R.; Saleem, M.; Aloysious, N.S.; Yelumalai, P.; Mohamed, N.; Hassan, S. Distribution of alpha thalassaemia gene variants in diverse ethnic populations in malaysia: data from the institute for medical research. Int J Mol Sci 2013, 14, 18599-18614. [CrossRef]
- Rahimah, A.N.; Nisha, S.; Safiah, B.; Roshida, H.; Punithawathy, Y.; Nurul, H.; Syahzuwan, H.; Zubaidah, Z. Distribution of alpha thalassaemia in 16 year old Malaysian Students in Penang, Melaka and Sabah. Med J Malaysia 2012, 67, 565-570.
- Nur Zaireena, Z.; Alauddin, H.; Ahmad, S.; Hamidah Hussin, N.; Hamidah Hussin, N. Thalassemia with Haemoglobin Adana mutation: prenatal diagnosis Malay J Pathol 2014, 36, 207-211.
- Tan, J.; Kho, S.L.; Ngim, C.F.; Chua, K.H.; Goh, A.S.; Yeoh, S.L.; George, E. DNA studies are necessary for accurate patient diagnosis in compound heterozygosity for Hb Adana (HBA2:c.179>A) with deletional or nondeletional alpha-thalassaemia. Sci Rep 2016, 6, 26994. [CrossRef]
- Chong, S.S.; Boehm, C.D.; Higgs, D.R.; Cutting, G.R. Single-tube multiplex-PCR screen for common deletional determinants of α-thalassemia. Blood 2000, 95, 360-362. [CrossRef]
- Eng, B.; Patterson, M.; Walker, L.; Chui, D.H.K.; Waye, J.S. Detection of Severe Nondeletional α-Thalassemia Mutations Using a Single-Tube Multiplex ARMS Assay. Genetic Testing 2001, 5, 327-329. [CrossRef]
- Department of Statistics, M. Population estimates based on the adjusted population and housing census of Malaysia 2010. 2021. Availabe online: https://www.dosm.gov.my/v1/index.php?r=column/cone&menu_id=RU84WGQxYkVPeVpodUZtTkpPdnBmZz09 (accessed on 18 Oct 2021).
- Kelantan State, Malaysia. Population, Charts, Maps. 2010. Availabe online: https://www.citypopulation.de/en/malaysia/admin/03__kelantan/ (accessed on 5 Jan).
- Khor, G.L.; Shariff, Z.M. Do not neglect the indigenous peoples when reporting health and nutrition issues of the socio-economically disadvantaged populations in Malaysia. BMC Public Health 2019, 19, 1685. [CrossRef]
- Vengadesan, M. Bullied, separated-national education failing Orang Asli children. 2019. Availabe online: https://www.malaysiakini.com/news/472558 (accessed on 3 March).
- UNESCO. 2011. Malaysia: the millennium development goals at 2010. Availabe online: (accessed on 20 Oct 2021).
- Azimi, A.; Tahmasebi, S.; Moradi, K.; Nejati, P.; Alibakhshi, R. Severe α-Thalassemia Due to Compound Heterozygosity for Hb Adana (α59 Gly>Asp) (HBA1: c.179G > A) and Codon 127 (A > T) (HBA2: c.382A > T) in an Iranian Family. Hemoglobin 2020, 44, 139-142. [CrossRef]
- Achour, A.; de Grouw, E.; van Erp, F.; Arkesteijn, S.; Schaap, R.; Huurne, J.T.; Bisoen, S.; Verschuren, M.; Harteveld, C.L. The first report of Hemoglobin E in combination with the highly unstable alpha-globin variant Hb Adana: the importance of molecular confirmation. Int J Lab Hematol 2019, 41, e76-e78. [CrossRef]
- Chin-yuet M.;Ezalia E.;Aliza M.Y. et al. Screening for hemoglobinopathies among patients in a government hospital and health clinics in Perlis, Malaysia. J Med Sci Technol. 2014,3, pp. 82-86.
- Nuinoon M.;Kruachan K.;Sengking W. et al. Thalassemia and Hemoglobin E in Southern Thai blood donors. Adv Hematol. 2014,2014, pp. 932306.
- Fucharoen, S.; Weatherall, D.J. The Hemoglobin E thalassemias. Cold Spring Harb Perspect Med 2012, 2, a011734-a011734. [CrossRef]
- Thein, S.L.; Rees, D. Haemoglobin and the inherited disorders of globin synthesis. In Postgraduate Haematology, Hoffbrand, A.V., Catovsky, D., Tuddenham, E.G., Green, A.R., Eds. Blackwell Publishing Ltd: 2016; pp. 72-97.
- Pornprasert, S.; Tookjai, M.; Punyamung, M.; Pongpunyayuen, P. HbE level and red cell parameters in heterozygous HbE with and without α0-thalassemia trait. Indian J Hematol Blood Transfus 2018, 34, 662-665. [CrossRef]
- Vichinsky, E. Hemoglobin E syndromes. Hematology Am Soc Hematol Educ Program 2007, 79-83. [CrossRef]
- Fucharoen, G.; Srivorakun, H.; Singsanan, S.; Fucharoen, S. Presumptive diagnosis of common haemoglobinopathies in Southeast Asia using a capillary electrophoresis system. Int J Lab Hematol 2011, 33, 424-433. [CrossRef]
- Jomoui, W.; Fucharoen, G.; Sanchaisuriya, K.; Nguyen, V.H.; Fucharoen, S. Hemoglobin Constant Spring among Southeast Asian Populations: Haplotypic Heterogeneities and Phylogenetic Analysis. PLoS One 2015, 10, e0145230. [CrossRef]
| DNA Analysis Results | Frequency (n) | Percentage (%) |
|---|---|---|
| Non deletional α-thalassaemia αCD59 α / αα αQZα / αα αCSα / αα |
31 24 1234 |
1.33% 1.03% 53.03% |
| Deletional α-thalassaemia -α3.7/αα -α4.2/αα -α3.7/-α3.7 -α3.7/ -α4.2 --SEA/αα --THAI/αα |
659 43 55 11 260 9 |
28.32% 1.85% 2.36% 0.47% 11.17% 0.39% |
| Compound heterozygous αCD59α / -α3.7 |
1 |
0.04% |
| Total | 2327 | 100.00% |
| DNA Analysis Results | Ethnic, n(%) | |||
|---|---|---|---|---|
| Malay | Chinese | Orang Asli | Siamese | |
| Non deletional α-thalassaemia | ||||
| αCD59 α / αα | 27 (1.19) | 0 | 4 (44.44) | 0 |
| αQZα / αα | 23 (1.01) | 1 (3.45) | 0 (0.00) | 0 (0.00) |
| αCSα / αα | 1223 (53.69) | 6 (20.69) | 5 (55.56) | 0 (0.00) |
| Deletional α-thalassaemia | ||||
| -α3.7/αα | 645 (28.31) | 7 (24.14) | 0 (0.00) | 7 (63.64) |
| -α4.2/αα | 43 (1.89) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| -α3.7/-α3.7 | 53 (2.33) | 1 (3.45) | 0 (0.00) | 1 (9.09) |
| -α3.7/ -α4.2 | 10 (0.44) | 1 (3.45) | 0 (0.00) | 0 (0.00) |
| --SEA/αα | 244 (10.71) | 13 (44.83) | 0 (0.00) | 3 (27.27) |
| --THAI/αα | 9 (0.39) | 0 | 0 | 0 |
| Compound heterozygous | ||||
| αCD59α / -α3.7 | 1 (0.04) | 0 | 0 | 0 |
| Total | 2278 | 29 | 9 | 11 |
| DNA Analysis Results | Gender, n (%) | |
|---|---|---|
| Male | Female | |
| Non deletional α-thalassaemia | ||
| αCD59 α / αα | 15 (1.52) | 16 (1.19) |
| αQZα / αα | 11 (1.11) | 13 (0..97) |
| αCSα / αα | 583 (59.01) | 651 (48.62) |
| Deletional α-thalassaemia | ||
| -α3.7/αα | 204 (20.65) | 455 (33.98) |
| -α4.2/αα | 20 (2.02) | 23 (1.72) |
| -α3.7/-α3.7 | 22 (2.23) | 33 (2.46) |
| -α3.7/ -α4.2 | 6 (0.61) | 5 (0.37) |
| --SEA/αα | 123 (12.45) | 137 (10.23) |
| --THAI/αα | 4 (0.40) | 5 (0.37) |
| Compound heterozygous | ||
| αCD59α / -α3.7 | 0 (0.00) | 1 (0.07) |
| Total | 988 | 1339 |
| Parameters | Heterozygous Hb Adana αCD59 α / αα (n=31) Range |
Mean (SD) | Compound heterozygous αCD59α / -α3.7 (n=1) |
|---|---|---|---|
| Hb (g/l) | 95.00-153.00 | 133.61 (±12.31) | 124.0 |
| RBC (x109/L) | 4.82-6.50 | 5.71 (±0.42) | 5.78 |
| MCV (fL) | 58.90-79.60 | 72.93 (±3.57) | 71.1 |
| MCH (pg) | 17.50-24.90 | 23.39 (±1.36) | 21.5 |
| MCHC (g/dl) | 29.80- 34.20 | 32.06 (±0.99) | 30.2 |
| Hb A (%) | 96.70- 98.10 | 97.46 (±0.25) | 97.5 |
| Hb A2 (%) | 1.90-3.00 | 2.51 (±0.20) | 2.1 |
| Hb F (%) | 0.00-0.50 | 0.03 (±0.10) | 0.4 |
| Double heterozygous Hb E/ Hb Adana (n = 7) | |||||||
|---|---|---|---|---|---|---|---|
| Parameters | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Hb (g/l) | 122.0 | 124.0 | 131.0 | 132.0 | 133.0 | 136.0 | 141 |
| RBC (x109/L) | 4.94 | 5.12 | 5.45 | 5.42 | 6.31 | 5.91 | 5.83 |
| MCV (fL) | 73.50 | 70.30 | 72.70 | 72.90 | 64.8 | 69.5 | 71.0 |
| MCH (pg) | 24.70 | 24.20 | 24.0 | 24.40 | 21.1 | 23.0 | 24.2 |
| MCHC (g/dl) | 33.60 | 34.50 | 33.10 | 33.40 | 32.5 | 33.10 | 34.1 |
| Hb A (%) | 74.4 | 72.60 | 75.6 | 75.6 | 73.50 | 74.9 | 74.4 |
| Hb A2 (%) | 3.5 | 3.6 | 3.4 | 3.6 | 4.1 | 3.7 | 3.9 |
| Hb E (%) | 21.9 | 22.4 | 21.0 | 20.8 | 22.4 | 21.4 | 21.7 |
| Hb F (%) | 0.2 | 1.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





