Submitted:
07 June 2023
Posted:
08 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reagents
2.3. Nitrogen replacement in the culture vessel
2.4. Co-culture of S. epidermidis and cosmetics
2.5. Extraction of exogenous metabolites (removal of foreign substances)
2.6. Short-chain fatty acid (BTB method) analysis by high-performance liquid chromatography (HPLC)
2.7. Peak identification
2.8. Statistical Analysis
3. Results
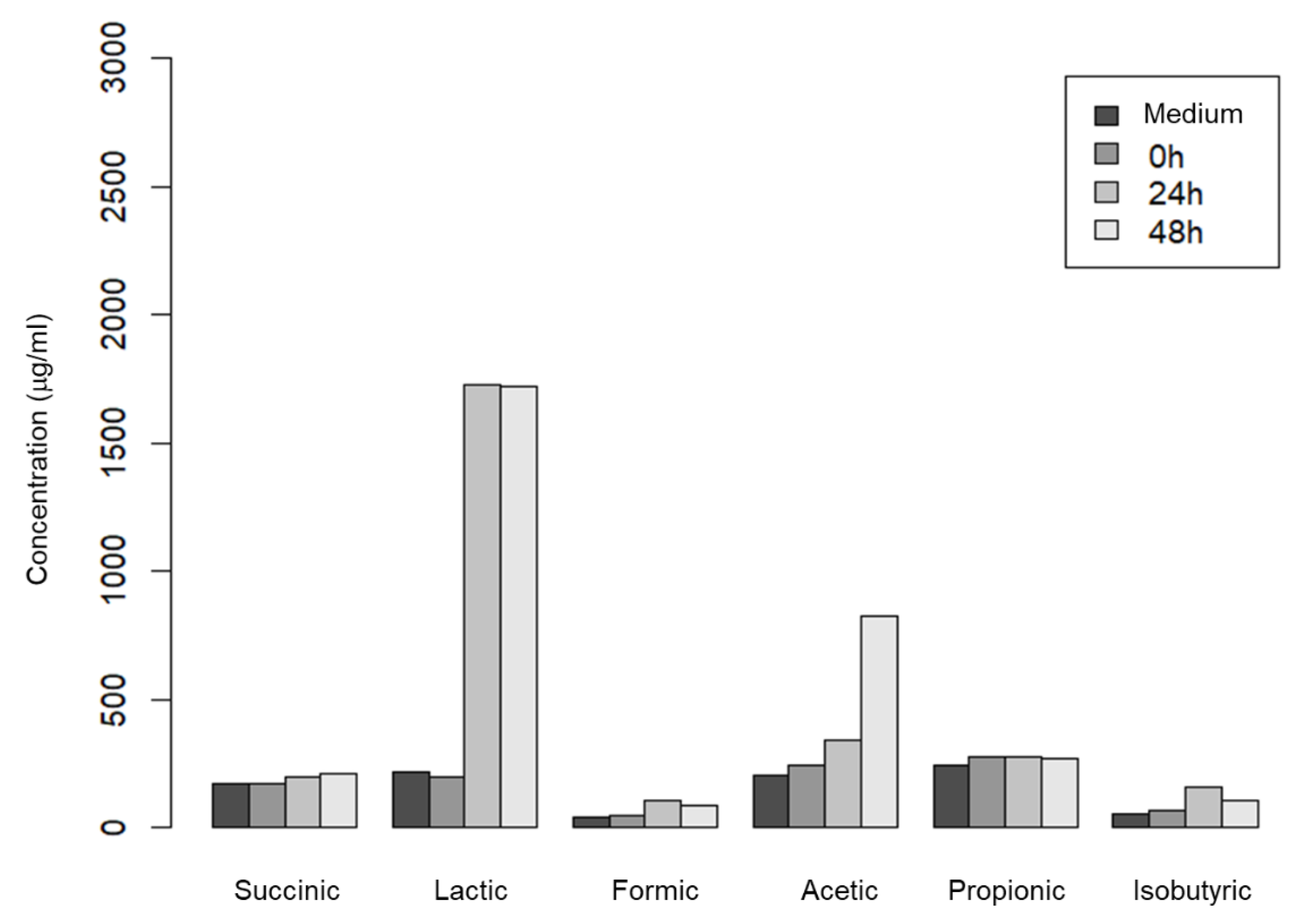
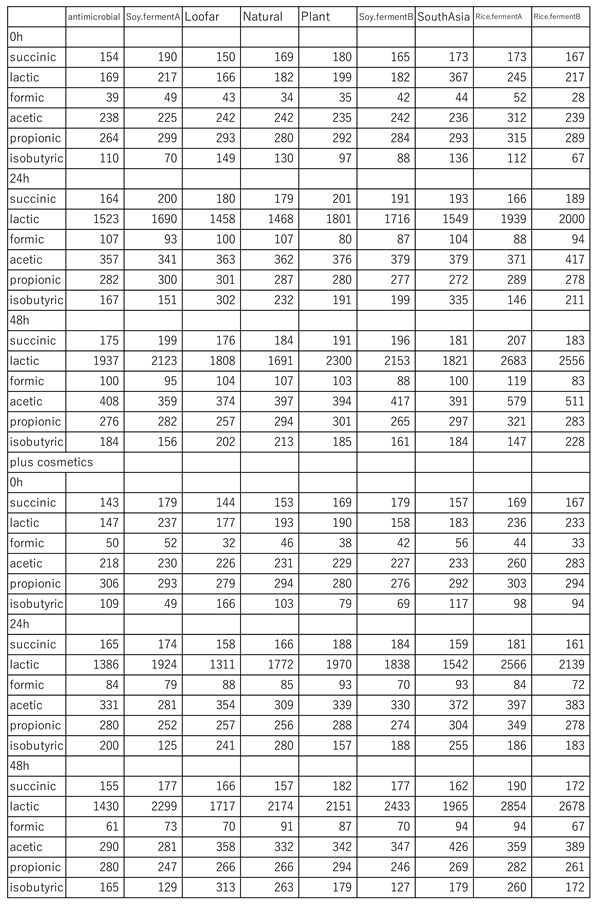
3.1. Change in short-chain fatty acid production by S. epidermidis upon addition of cosmetics
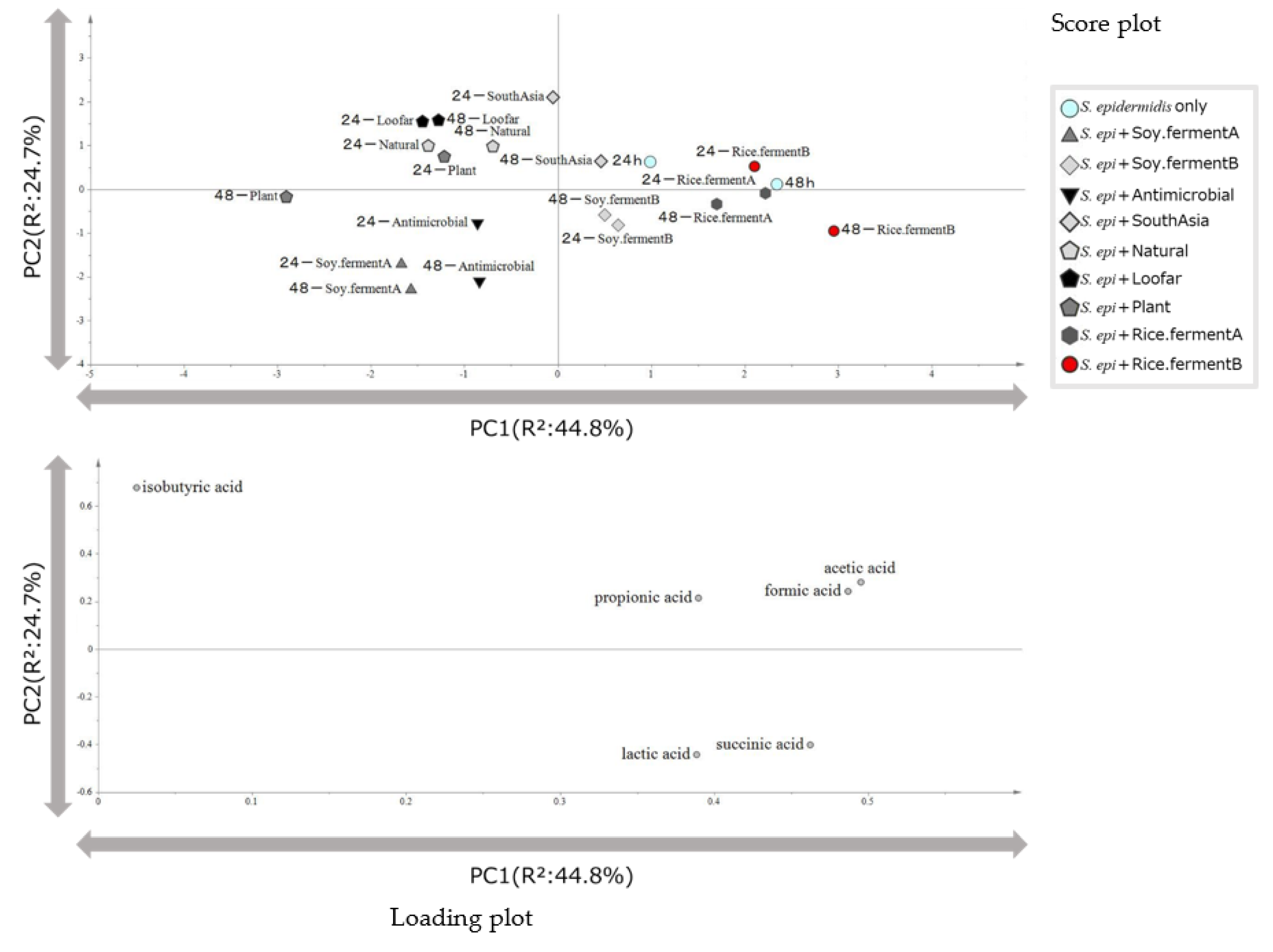
3.2. Classification of cosmetics based on principal component analysis of the amount of short-chain fatty acids produced by indigenous skin bacteria
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamamura, H., History of Japanese cosmetics, Yoshikawa Kobunkan, 2016, 19-30.
- Miki, M. Types and ways of using cosmetics-skin care cosmetics-. J. Jpn. Cosmet. Sci. Ind. 2018, 42, 109–124. [Google Scholar]
- Ryuen, H. Cosmetic industry and consumers. J. Jpn. Res. Assoc. Text. End-Uses 2012, 53, 1032–1037. [Google Scholar]
- Miyagawa, M.; Fujikawa, A.; Nagadome, M.; Kohama, K.; Ogami, T.; Kitamura, S.; Kitagaki, H. Glycosylceramides Purified from the Japanese Traditional Non-Pathogenic Fungus Aspergillus and Koji Increase the Expression of Genes Involved in Tight Junctions and Ceramide Delivery in Normal Human Epidermal Keratinocytes. Fermentation 2019, 5, 43. [Google Scholar] [CrossRef]
- Otsuka, A.; Moriguchi, C.; Shigematsu, Y.; Tanabe, K.; Haraguchi, N.; Iwashita, S.; Tokudome, Y.; Kitagaki, H. Fermented Cosmetics and Metabolites of Skin Microbiota—A New Approach to Skin Health. Fermentation 2022, 8, 703. [Google Scholar] [CrossRef]
- Matt Venus, et al. Basic physiology of the skin. Surgery (Oxford) 2010, 28, 469–472. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Joachim W, et al. Glycerol Regulates Stratum Corneum Hydration in Sebaceous Gland Deficient (Asebia) Mice. Investigate Dermatory. 2003, 120, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Nodake, Y. , et al. Pilot study on novel skin care method by augmentation with Staphylococcus epidermidis, an autologous skin microbe – A blinded randomized clinical trial. Science Direct. 2015, 79, 119–126. [Google Scholar]
- Mizukoshi, K. Effects of lactic acid on the flexibility of the stratum corneum. Ski. Res. Technol. 2020, 26, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; et al. Compartmentalized Control of Skin Immunity by Resident Commensals. Science 2012, 337, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Prince, A. Staphylococcus aureus metabolites promote IL-10. Nat. Microbiol. 2020, 5, 1183–1184. [Google Scholar] [CrossRef]
- Y Wang, et al. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: implications of probiotics in acne vulgaris”. Microb. Cell Physiol. 2014, 98, 411‒424.
- Y Wang, et al. Propionic acid and its esterified derivative suppress the growth of methicillin-resistant Staphylococcus aureus USA300. Benef. Microbes 2014, 5, 161–168. [Google Scholar] [CrossRef]
- Sigurdsson, J. , Björnsson, A., Gudmundsson, S. T. Formic acid burn--local and systemic effects. Report of a case. Burns Incl Therm Inj. 1983, 9, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J. H. , Lee, S., Lee, H. G., Choi, D., Lim, K. M. Evaluation of Skin Irritation of Acids Commonly Used in Cleaners in 3D-Reconstructed Human Epidermis Model, KeraSkinTM. Toxics. 2022, 10, 558. [Google Scholar] [CrossRef]
- Becker, L. C. , Bergfeld, W. F., Belsito, D. V., Hill, R. A., Klaassen, C. D., Liebler, D. C., Marks, J. G., Jr, Shank, R. C., Slaga, T. J., Snyder, P. W., Gill, L. J., & Heldreth, B. Safety Assessment of Glycerin as Used in Cosmetics. Int. J. Toxicol. 2019, 38, 6S–22S. [Google Scholar] [CrossRef]
- Dubal, Z. B., Paturkar, A. M., Waskar, V. S., Zende, R. J., Latha, C., Rawool, D. B., & Kadam, M. M. Effect of food grade organic acids on inoculated S. aureus, L. monocytogenes, E. coli and S. Typhimurium in sheep/goat meat stored at refrigeration temperature. Meat science 2004, 66, 817–821. [CrossRef]
- Lowy F., D. Staphylococcus aureus infections. The New England journal of medicine 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Iwase, T., Uehara, Y., Shinji, H., Tajima, A., Seo, H., Takada, K., Agata, T., & Mizunoe, Y. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 2010, 465, 346–349. [CrossRef]
- Al-Mahrous, M. , Sandiford, S. K., Tagg, J. R., & Upton, M. Purification and characterization of a novel delta-lysin variant that inhibits Staphylococcus aureus and has limited hemolytic activity. Peptides 2010, 31, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Cogen, A. L. , Yamasaki, K., Sanchez, K. M., Dorschner, R. A., Lai, Y., MacLeod, D. T., Torpey, J. W., Otto, M., Nizet, V., Kim, J. E., & Gallo, R. L. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. The Journal of investigative dermatology 2010, 130, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.J.M. , Scholz, C.F.P., Enghild, J. et al. Antagonism between Staphylococcus epidermidis and Propionibacterium acnes and its genomic basis. BMC Genomics 2016, 17, 152. [Google Scholar] [CrossRef] [PubMed]
- Grice, E. A. , & Segre, J. A. The skin microbiome. Nature reviews. Microbiology 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, J. , & Vilcinskas, A. Antimicrobial peptides: the ancient arm of the human immune system. Virulence 2010, 1, 440–464. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G. J. , & Brüggemann, H. Bacterial skin commensals and their role as host guardians. Beneficial microbes 2014, 5, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y. , Di Nardo, A., Nakatsuji, T., Leichtle, A., Yang, Y., Cogen, A. L., Wu, Z. R., Hooper, L. V., Schmidt, R. R., von Aulock, S., Radek, K. A., Huang, C. M., Ryan, A. F., & Gallo, R. L. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat. Med. 2009, 15, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- van der Krieken, D. A. , Ederveen, T. H., van Hijum, S. A., Jansen, P. A., Melchers, W. J., Scheepers, P. T., Schalkwijk, J., & Zeeuwen, P. L. An In vitro Model for Bacterial Growth on Human Stratum Corneum. Acta dermato-venereologica 2016, 96, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Niehues, H. , Bouwstra, J. A., El Ghalbzouri, A., Brandner, J. M., Zeeuwen, P. L. J. M., & van den Bogaard, E. H. 3D skin models for 3R research: The potential of 3D reconstructed skin models to study skin barrier function. Experimental dermatology 2018, 27, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Sfriso, R. , Egert, M., Gempeler, M., Voegeli, R., & Campiche, R. Revealing the secret life of skin - with the microbiome you never walk alone. International journal of cosmetic science 2020, 42, 116–126. [Google Scholar] [CrossRef]
- Holland, K. T. , & Bojar, R. A. Cosmetics: what is their influence on the skin microflora? American journal of clinical dermatology 2002, 3, 445–449. [Google Scholar] [CrossRef]
- Kong, H. H., & Segre, J. A. Skin microbiome: looking back to move forward. The Journal of investigative dermatology 2012, 132(3 Pt 2), 933–939. [CrossRef]
- Harris-Tryon, T. A., & Grice, E. A. (2022). Microbiota and maintenance of skin barrier function. Science (New York, N.Y.), 376(6596), 940–945. [CrossRef]
- Aykut, B., Pushalkar, S., Chen, R., Li, Q., Abengozar, R., Kim, J. I., Shadaloey, S. A., Wu, D., Preiss, P., Verma, N., Guo, Y., Saxena, A., Vardhan, M., Diskin, B., Wang, W., Leinwand, J., Kurz, E., Kochen Rossi, J. A., Hundeyin, M., Zambrinis, C., … Miller, G. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature, 2019, 574(7777), 264–267. [CrossRef]
- David Boothe, W., Tarbox, J.A., Tarbox, M.B. (2017). Atopic Dermatitis: Pathophysiology. In: Fortson, E., Feldman, S., Strowd, L. (eds) Management of Atopic Dermatitis. Advances in Experimental Medicine and Biology, vol 1027. Springer, Cham. [CrossRef]
- Clausen M, Agner T, Lilje B, Edslev SM, Johannesen TB, Andersen PS. Association of Disease Severity With Skin Microbiome and Filaggrin Gene Mutations in Adult Atopic Dermatitis. JAMA Dermatol. 2018;154(3):293–300. [CrossRef]
- https://www.precedenceresearch.com/cosmetics-market accessed 2023.6.3.
- R. Jugé and others, Shift in skin microbiota of Western European women across aging, Journal of Applied Microbiology, Volume 125, Issue 3, 1 September 2018, Pages 907–916. 1 September; 3. [CrossRef]
- Gannesen, A. V., Borrel, V., Lefeuvre, L., Netrusov, A. I., Plakunov, V. K., & Feuilloley, M. G. J. (2019). Effect of two cosmetic compounds on the growth, biofilm formation activity, and surface properties of acneic strains of Cutibacterium acnes and Staphylococcus aureus. MicrobiologyOpen, 8(3), e00659. 3. [CrossRef]
- Filaire, E.; Vialleix, C.; Cadoret, J.-P.; Guénard, S.; Muller, C.; Dreux-Zigha, A.; Berthon, J.-Y. Characterization of Reactive and Sensitive Skin Microbiota: Effect of Halymenia durvillei (HD) Extract Treatment. Cosmetics 2019, 6, 69. [Google Scholar] [CrossRef]
- Saising, J., & Voravuthikunchai, S. P. (2012). Anti Propionibacterium acnes activity of rhodomyrtone, an effective compound from Rhodomyrtus tomentosa (Aiton) Hassk. leaves. Anaerobe, 18(4), 400–404. [CrossRef]
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





