1. Introduction
It is estimated that almost 4% of all NICU (Neonatal Intensive Care Unit) admissions are attributed to
Candida spp. infections [1, 2].
Candida albicans is the leading cause of neonatal candidiasis [1, 3], while
Candida parapsilosis is the most common non-
albicans isolate in VLBW (very low birth weight) infants. Invasive candidiasis in neonates is associated with considerably increased mortality [
1], which is further worsened when the pathogen is isolated from multiple body sites [
4]. Furthemore, long-term neurodevelopmental impairment has been described in clinical cases of neonatal invasive candidiasis [1, 2].
Vaginal delivery and either maternal or healthcare-associated staff skin contact are primarily accused of enabling
Candida colonization in neonates [
1]. Neonatal immune system immaturity, extended antibiotic administration [3, 5, 6] and inadequate integrity of skin and mucosa barriers are among the most recognizable risk factors for the development of invasive fungal infection (IFI) [3, 6]. However, prompt diagnosis of IFI poses a major challenge as signs and symptoms are commonly vague [6, 7]. Also, it is worth mentioning that the clinical management of IFI in children and neonates presents heterogeneity according to the findings of a study conducted in 13 third-level university hospitals in United Kingdom [
8].
Blood cultures [
3] remain the gold standard for the diagnosis of IFI, even though they require time and may exhibit less sensitivity under certain circumstances [4, 9]. That’s the main reason why additional laboratory methods are frequently being utilized in clinical practice for the early diagnosis of IFΙ namely, detection of mannan antigen/mannan antibody in serum against
Candida spp. [
9], β-D-glucan or PCR [9, 10].
The use of mannan antigen (MA) assay in neonates is not well studied. The aim of our study was to evaluate the performance of MA in hospitalized neonates (age <28 days) of a Level II neonatal unit.
2. Materials and Methods
This was a prospective, case-control study of hospitalized neonates in the Special Care (Level II) Neonatal Unit of the 1st Department of Pediatrics of University of Athens, “Aghia Sophia” Children’s Hospital.
Rectal samples for Candida colonization were collected on admission and blood samples were drawn for routine blood tests.
Blood sample for MA detection was collected during the first 3 days of hospitalization. Serum was collected and stored at -20 °C until assayed. Detection of MA was carried out using the commercially available Platelia™ Candida Ag Plus kit (Bio-Rad), according to the manufacturer’s instructions. A positive result, as defined by the manufacturer, was with concentrations less than 62.5 pg/mL, while samples with concentrations between 62.5 and 125 pg/mL were considered to be «intermediate» for MA and those with concentrations that are equal or greater than 125 pg/mL were considered to be «positive» for MA. All samples were run in duplicate. Analysis of the results was carried out blind to the clinical and microbiological data. All neonates were followed up for IFI during the hospitalization period and blood cultures were taken upon clinical indications.
The study protocol was approved by the “Aghia Sophia” Children’s Hospital Ethics Committee (study approval number: 5878) and informed written consent was obtained from infants’ parents.
Statistical analysis was performed using SPSS version 28.0 (SPSS Inc, Chicago, IL). Data were test for normality using the Kolmogorov-Smirnov test and expressed as mean±standard deviation (SD). For comparisons Chi-squared test and Student’s t test were used, as appropriate.
3. Results
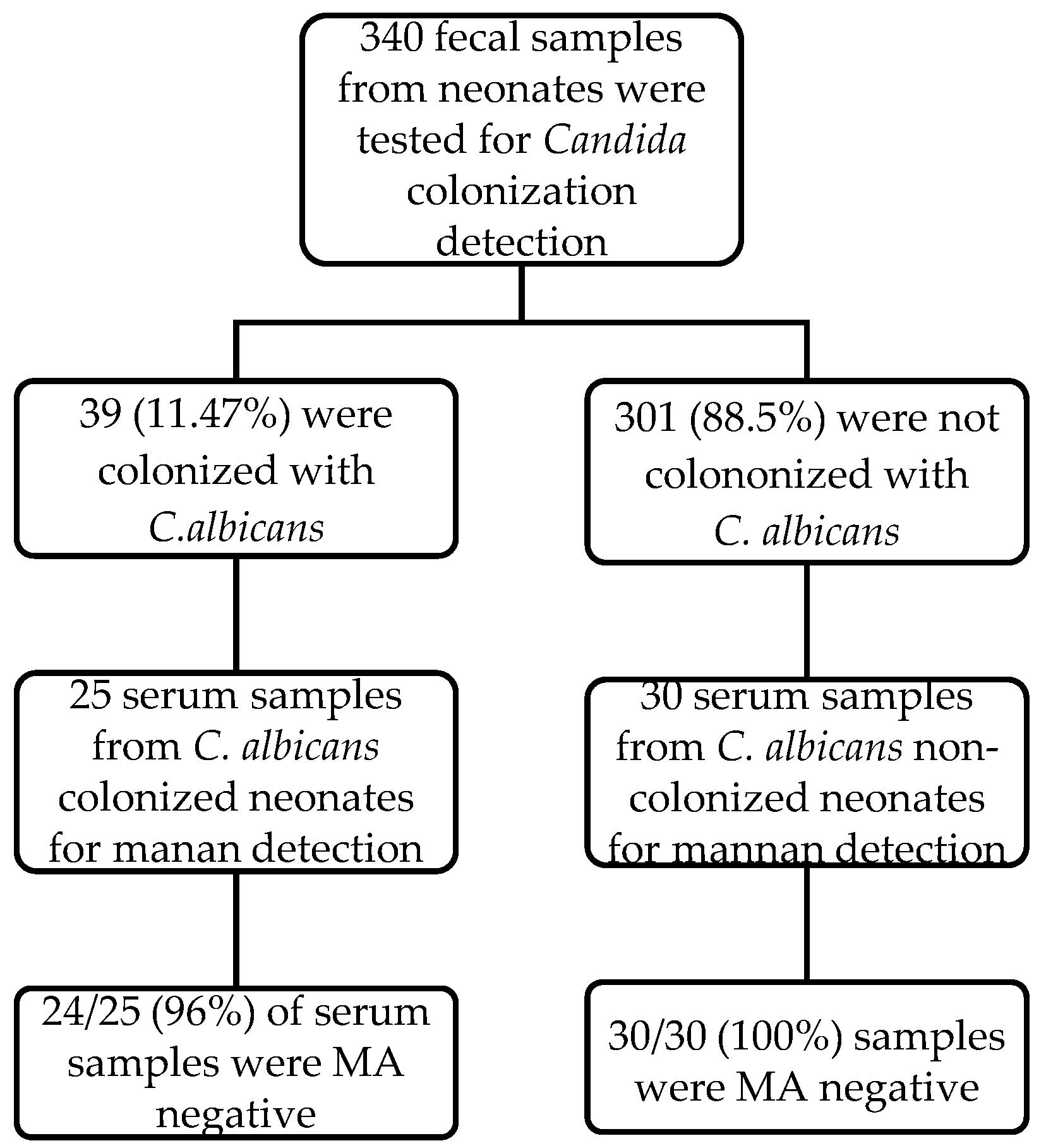
We cultured for rectal Candida colonization 340 neonates and 39 (11.47%) had positive result for Candida rectal colonization. Blood sample was drawn for MA in 25/39 neonates (for 14 neonates there was no written parent consent). 30 neonates, who were hospitalized the same period, with negative result for Candida rectal colonization and similar distribution of age and sex, were included as controls.
The characteristics of the study population are presented in
Table 1.
Quantitive variables are expressed as mean±SD.
The mean±SD gestational age of the study population was 38.2 ± 1.4 weeks, 34 neonates (62%), had vaginal birth, 29 out of 55 neonates were male (52.7%) and at the time of the study were 17.3 ± 7.8 days of age.
The leading causes of admission in both groups were bronchiolitis (36%), jaundice (16.4%), fever (11%), failure to thrive (9%), vomiting (7.3%), irregular breathing or cyanosis (5.5%), diarrhea (3.6%), late prematurity (3.6%), opthalmia neonatorum (3.6%), rash (2%) and heart murmur (2%).
The laboratory results are presented in
Table 2. One neonate colonized with
C. albicans had a positive blood culture for coagulase-negative
Staphylococci. During the study period no invasive fungal infection was detected. Urinary tract infection was detected in 3/25 (10,7%)
C. albicans colonized and in 3/30 (10%) non-colonized neonates. All neonates were negative for central nervous system infection.
The description of the MA case-control study is presented in
Figure 1. MA was negative in all uncolonized neonates (30/30, 100% specificity) and in 24 out of 25 colonized neonates (96% specificity). The one candida colonized neonate, that was detected positive with the MA assay, was admitted at 13 days of postnatal age for fever and has started oral topical miconazole treatment for oral
Candida mucositis. The neonate had no signs of invasive candidiasis, cultures were negative and blood culture for IFI was also negative.
4. Discussion
The present study aimed to evaluate the use of MA assay in a Level II neonatal unit and compared between C. albicans colonized and non-colonized infants. According to our results, none of the non-colonized infants had positive MA, whereas only one from the colonized group had positive result, within the colonized group. However, this neonate had oral Candida mucositis that could possibly facilitate the presence of the antigen in the blood. During the study period there was not any IFI.
Prolonged antibiotic administration, use of steroids or histamine-2 receptor antagonists and lipids emulsions are among the most well-established risk factors for
Candida colonization in neonates and subsequent development of invasive candidiasis [
3]. Unlike bacterial infections, invasive candidiasis could present with a pattern of non-specific signs and symptoms, especially after the 3rd week of life in preterm neonates [
2]. Apnea, respiratory distress syndrome or feeding intolerance may be the most prominent signs of IC [2, 3].
In the present study laboratory results did not differ between colonized and non-colonized neonates. Furthermore, full blood count, including platelets, and CRP did not differ between positive and negative carriers. The role of laboratory findings and/or biomarkers in the accurate diagnosis of IC has been evaluated in a clinical study of Guo et al [
6], in which white blood cell count, platelet count, hs-CRP levels, PCT and β-D-glucan (BDG) were compared between 30 neonates with IC, 25 neonates with bacterial infection and 25 neonates considered as controls. The study reported that, WBCs and PCT levels did not differ between neonates with IC compared to controls. On the contrary, CRP levels were statistically significant higher and platelet count was statistically significant lower in neonates with IC compared to controls. BDG was the only biomarker that was found statistically higher in neonates with IC compared to neonates with bacterial infection or controls [
6]. BDG, also may aid to the diagnosis of IC [
6], however, it should be noticed that is not specific and can also be detected in patients with bacteremia either from gram-positive or gram-negative pathogens [
9].
The performance of MA has been previously studied in adults and neonates. The third European Conference on Infections in Leukemia (ECIL-3) meeting conducted a systematic literature review [
11], including 14 studies of adult patients with hemato-oncology diseases and invasive candidiasis, and tried to evaluate the sensitivity and specificity of MA and mannan antibody. According to their results there is significant heterogeneity among studies, however, the combined test of MA and mannan antibody performed better with 83% sensitivity and 86% specificity.
Regarding neonates, according to the study by Oliveri et al. [
12] MA was considered positive at two least samples with levels above 0.5 ng/mL. The MA was negative only in one patient out of 12, who was diagnosed with invasive candidiasis by
C. parapsilosis. It is remarkable that in 8/12 neonates the antigen was positive before the blood culture. However, 3 neonates out of 58 without candidiasis had false positive MA. According to the above results, the sensitivity of the assay was 94.4% and the specificity 94.2%. Furthermore, in a multicentre survey in NICU in Southern Italy in 2010, MA was positive in 5 out of 7 neonates with IFI [
13]. However, there is no recent study evaluating possible MA presence in the serum of neonates with rectal colonization.
Limitations of the present study include that in the population, there were not many early preterm or VLBW neonates, which may explain that we did not detect any IFI episode, even in the C. albicans colonized neonates. For this reason we could not estimate the sensitivity of the assay for the diagnosis of IFI. However, the importance of the study is that we found a very high specificity in both colonized and non-colonized babies.
Further investigation is needed in high-risk neonates, that have higher propability of IFI, to estimate the sensitivity of the MA assay. In these groups it is very important to have bioassays with good performance characteristics to guide proper antifungal treatment [2, 14].
5. Conclusions
MA found to have high specificity in candida colonized and non-colonized neonates. That is important for further evaluation of the assay for the detection of invasive fungal infection in neonatal units and the timely administration of antifungals.
Author Contributions
Conceptualization, G.V., A.M., T.S.; methodology, A.M., V.B., T.S., G.V., investigation, V.B., K.T., T.S., G.V., A.M.; resources G.V.; data curation V.B., T.S., A.M.; writing—review and editing V.B., A.T., A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved the “Aghia Sophia” Children’s Hospital Ethics Committee (No 5878).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Botero-Calderon L, Benjamin DK, Cohen-Wolkowiez M. Advances in the treatment of invasive neonatal candidiasis. Expert Opin Pharmacother. 2015, 16, 1035–1048. [CrossRef] [PubMed]
- Hornik CD, Bondi DS, Greene NM, Cober MP, John B. Review of fluconazole treatment and prophylaxis for invasive candidiasis in neonates. J. Pediatr. Pharmacol. Ther. 2021, 26, 115–122. [CrossRef]
- Weimer KED, Smith PB, Puia-Dumitrescu M, Aleem S. Invasive fungal infections in neonates: A review. Pediatr Res. 2022, 91, 404–412. [CrossRef] [PubMed]
- Walsh TJ, Katragkou A, Chen T, Salvatore CM, Roilides E. Invasive candidiasis in infants and children: Recent advances in epidemiology, diagnosis, and treatment. J. Fungi 2019, 5. [CrossRef]
- Manzoni P, Mostert M, Castagnola E. Update on the management of Candida infections in preterm neonates. Arch Dis Child Fetal Neonatal Ed. 2015, 100, F454–F459. [CrossRef]
- Guo J, Wu Y, Lai W, Lu W, Mu X. The diagnostic value of (1,3)-β-D-glucan alone or combined with traditional inflammatory markers in neonatal invasive candidiasis. BMC Infect Dis. 2019, 19. [CrossRef]
- Warris A; European Paediatric Mycology Network (EPMyN)*.The European Paediatric Mycology Network (EPMyN): Towards a Better Understanding and Management of Fungal Infections in Children. Curr Fungal Infect Rep. 2016, 10, 7–9. [CrossRef] [PubMed]
- Ferreras-Antolín L, Sharland M, Warris A. Management of Invasive Fungal Disease in Neonates and Children. Pediatr. Infect. Dis. J. 2019, 38, S2–S6. [CrossRef] [PubMed]
- Clancy CJ, Nguyen MH. Finding the missing 50% of invasive candidiasis: How nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin. Infect. Dis. 2013, 56, 1284–1292. [CrossRef] [PubMed]
- Clancy CJ, Nguyen MH. Rapid diagnosis of invasive candidiasis: Ready for prime-time? Curr Opin Infect Dis. 2019, 32, 546–552. [CrossRef] [PubMed]
- Marchetti O, Lamoth F, Mikulska M; et al. ECIL recommendations for the use of biological markers for the diagnosis of invasive fungal diseases in leukemic patients and hematopoietic SCT recipients. Bone Marrow Transplant. 2012, 47, 846–854. [CrossRef]
- Oliveri S, Trovato L, Betta P, Romeo MG, Nicoletti G. Experience with the Platelia Candida ELISA for the diagnosis of invasive candidosis in neonatal patients. Clin Microbiol Infect. 2008, 14, 391–393. [CrossRef]
- Montagna MT, Lovero G, De Giglio O; et al. Invasive fungal infections in neonatal intensive care units of Southern Italy: A multicentre regional active surveillance (AURORA project). J Prev Med Hyg. 2010, 51, 125–130.
- Scott BL, Hornik CD, Zimmerman K. Pharmacokinetic, efficacy, and safety considerations for the use of antifungal drugs in the neonatal population. Expert Opin Drug Metab Toxicol. 2020, 16, 605–616. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).