1. Introduction
Salinization does more than just impede the normal growth of plants; it presents a significant challenge to crop yield and global land utilization. Salt stress inflicts three primary forms of damage on plants: ion imbalance, hypertonic stress, and oxidative damage [
1]. It is the root system that first detects the salt signal, reacting swiftly to maintain its function and propagate the signal to other plant organs [
2]. Consequently, plants employ a myriad of complex mechanisms to counter salt stress, including selective ion absorption/rejection, regulation of photosynthesis and energy metabolism, the accumulation of antioxidant enzymes, hormone regulation, and alterations in cell structure [
3]. Therefore, unraveling the salt response mechanism in plant roots to enhance plant salt tolerance represents a primary focus of research in this field.
In recent years, numerous researchers have delved into the molecular mechanisms underlying plant salt tolerance by elucidating the pathways of plant salt signal transduction. The use of plant growth regulators has proven to be an effective strategy for enhancing plant salt tolerance. Abscisic acid (ABA), a critical stress response hormone, holds an indispensable role in seed development, as well as in defense mechanisms against drought and salt stress [
4,
5,
6]. Sucrose non-fermenting 1-associated protein kinases (SnRK2s), activated by ABA, help maintain osmotic homeostasis by regulating the conversion of BAM1- and amy3-dependent starch into sugars and osmolytes [
7,
8]. Moreover, SnRK2.2/2.3/2.6 have the capability to phosphorylate various ABA-responsive element (ABRE)-binding protein/Abre-Binding factor (AREB/ABF) transcription factors, which subsequently regulate stomatal closure in response to osmotic stress in plants [
9]. Gibberellin (GA) is also pivotal in plant growth and development. Some studies have revealed that genes related to GA metabolism, such as
AtGA2ox7 [
10],
OsGA2ox5 [
11], and
OsMYB91 [
12], can enhance plant salt tolerance by decelerating growth. The SLR1 protein, an inhibitor of the GA signaling pathway, can boost plant survival under salt stress by suppressing plant growth [
13]. Salicylic acid (SA) not only has a role in biological stress in plants but is also crucial in the plant response to salt stress. Applying SA can bolster the plant's antioxidant and osmotic systems and can even stimulate photosynthesis under salt stress [
14]. Overexpression of SA receptor
AtNPR1 or
MhNPR1 in tobacco plants has been shown to enhance tobacco's tolerance to oxidative and salt stress by promoting the SA signaling pathway [
15,
16]. Brassinosteroids (BR) have been identified as playing a role in plant responses to various abiotic stresses, including salt stress [
17]. Overexpression of the BR biosynthetic gene SoCYP85A1 in spinach has been shown to enhance salt tolerance [
18]. Furthermore, BR can channel more carbohydrates to the roots and hasten the establishment of bacteria in root nodules under stress, thereby enhancing the plant's salt tolerance [
17].
Basic region/leucine zipper (bZIP) proteins comprise one of the most extensively studied transcription factor families, present across all eukaryotic organisms. TGA transcription factors, belonging to the bZIP protein family, are known to specifically bind to the tgacg central activation sequence 1 (as-1). This binding event regulates the transcription level of target genes, thereby enhancing the plant's capacity to withstand abiotic stress [
19]. Genome-wide studies in
Arabidopsis thaliana have revealed that the TGA gene family, encompassing 10 members, can be further subdivided into five groups based on sequence similarity [
20,
21,
22]. Numerous studies have demonstrated that TGA transcription factors play a significant role in modulating plant stress tolerance, including the enhancement of plant salt tolerance. Jayakannan [
23] discovered that
Arabidopsis NPR1 facilitates the absorption of Na+ into the root system via the SA signaling pathway while inhibiting transport to the above-ground parts, thereby bolstering the plant's salt tolerance. The
MhTGA2 gene in apple trees has been found to enhance the resistance of transgenic tobacco seeds to NaCl during the germination and seedling stages [
24]. Similarly, the
MhNPR1 gene in tobacco augments the resilience of tobacco seeds to NaCl during germination [
16]. Hao's research [
25]showed that
Arabidopsis NPR1 knockout mutants accumulated SA under salt stress, resulting in enhanced salt tolerance. Furthermore, over-accumulating NRP1 in
Arabidopsis double mutants positively influences plant salt tolerance without inducing programmed cell death [
26,
27].
While significant advancements have been made in the study of salt stress genes pertaining to the TGA family, research involving the TGA1 transcription factor remains scant.
Medicago truncatula, a model plant within the legume family [
28], offers considerable importance for studying salt-tolerance response genes. Exploring its salt-tolerance mechanisms could potentially enhance its salt-tolerance capability and improve legume yields. Therefore, this study focuses on the MtTGA1 transcription factor of
Medicago truncatula. Through biotechnological approaches, we investigate the regulatory mechanism of the MtTGA1 transcription factor in response to salt stress. Our findings aim to lay a foundation for expedited breeding of salt-tolerant forage varieties via genetic engineering.
3. Discussion
Salinization is a severe agricultural issue that significantly impacts plant yield and economic benefit. TGA1 transcription factors, belonging to Group I members of the TGA gene family, are primarily involved in plant disease and stress resistance and play a crucial role in mitigating plant salt stress [
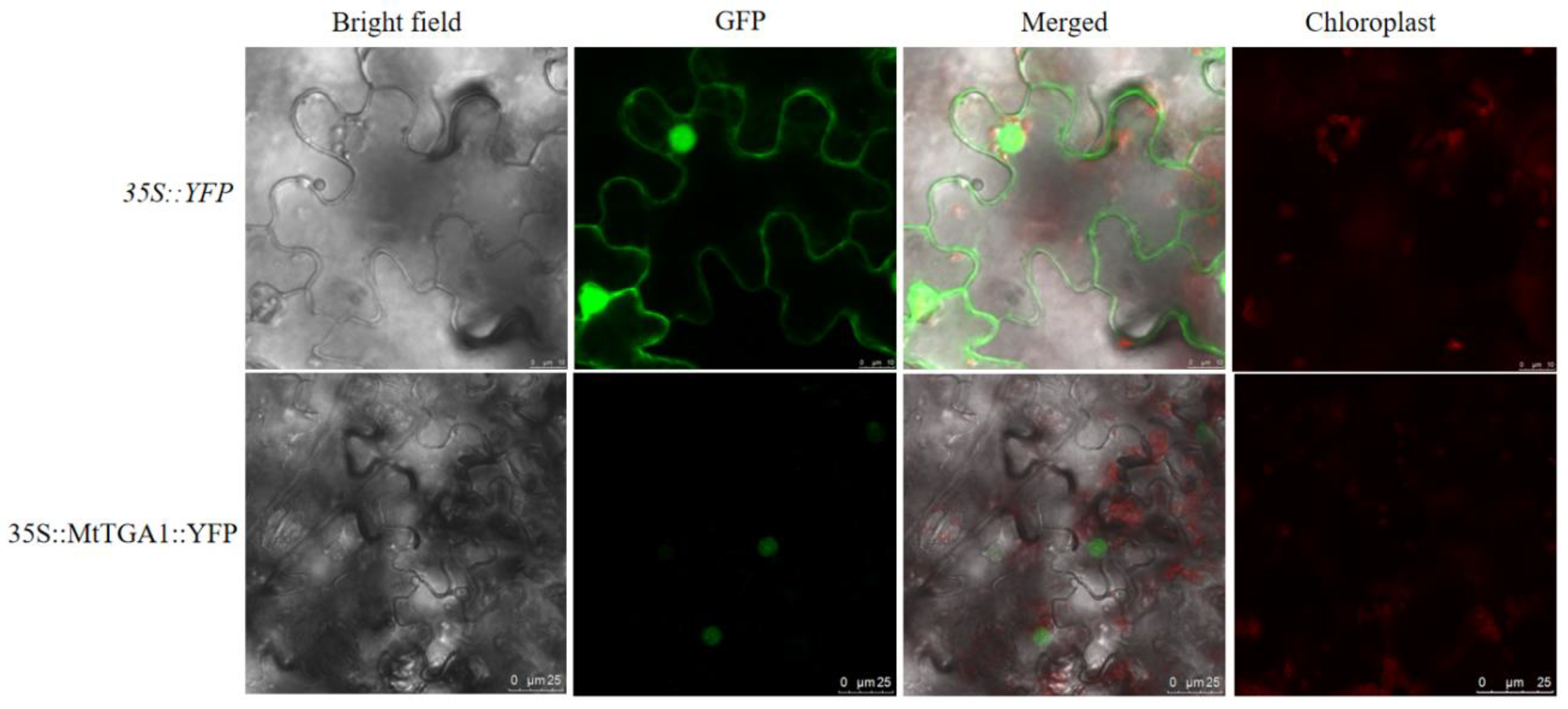
29]. We employed bioinformatics strategies to study the MtTGA1 transcription factor and, through simple analysis, discovered that the MtTGA1 protein contains a polar amino acid residue, a DOG1 structure, and a BZIP domain. It is highly conserved among closely related leguminous homologous proteins, providing a reference for studying other leguminous homologous genes. We fused YFP with MtTGA1 and performed subcellular localization in tobacco. The results showed that MtTGA1 was mainly located in the nucleus (
Figure 3).
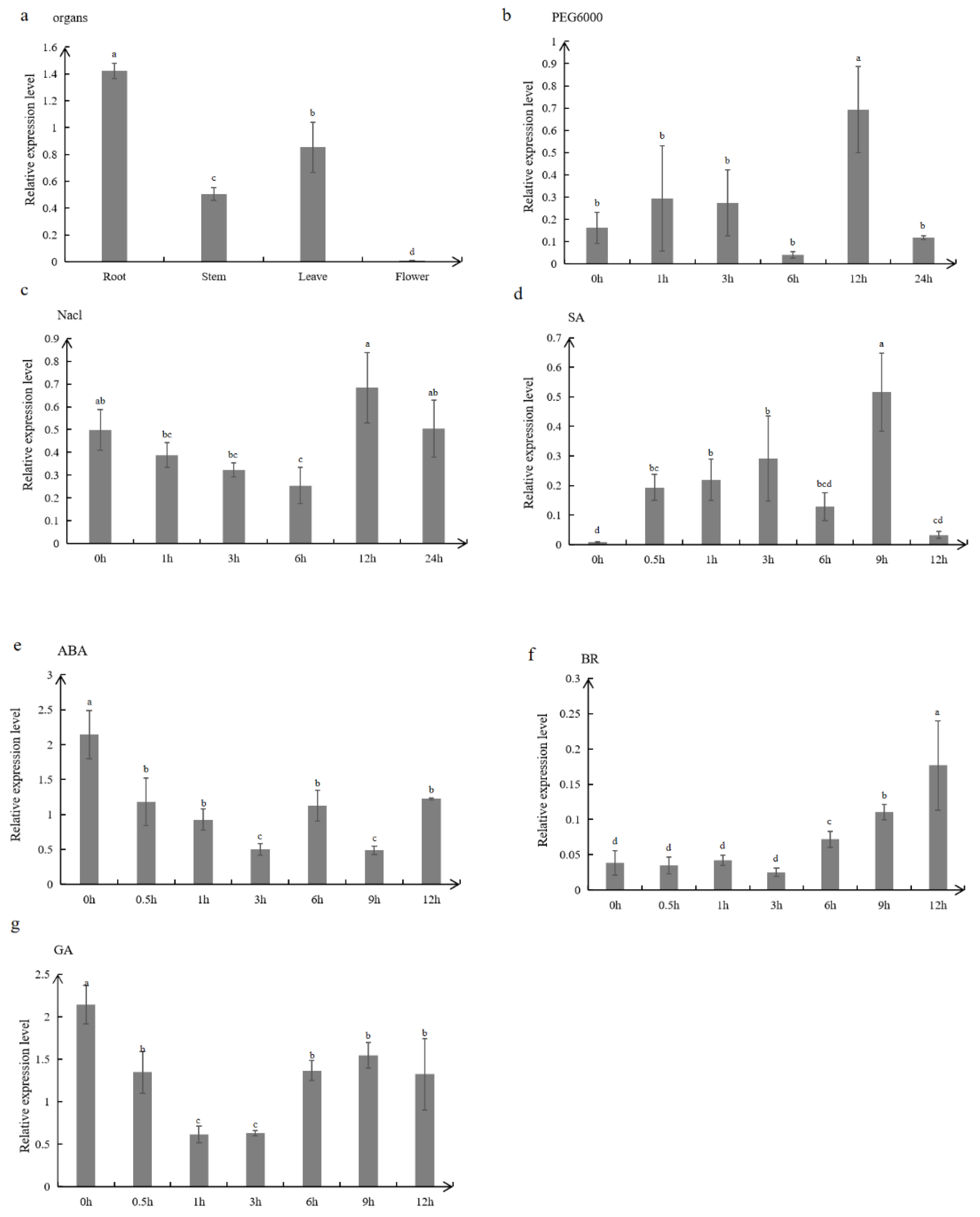
Analysis of the
MtTGA1 gene promoter sequence and hormone-induced expression demonstrated that the
MtTGA1 gene responds to various hormones, including SA, ABA, BR, and GA, which are implicated in plant growth, development, and stress response [
30,
31]. To investigate whether the MtTGA1 salt tolerance mechanism is hormone-related, we examined endogenous hormone contents in transgenic plants. Previous studies have shown that under salinity osmotic stress, endogenous ABA levels rapidly increase, activating SnRK2s proteases, which in turn enhance salt stress [
7]. The dominant inhibitor OsDSK2a protein could degrade the GA inactivating enzyme ELONGATED UPPERMOST INTERNODE (EUI). Under salt stress conditions, OsDSK2a levels decreased, and accumulated EUIs reduced GA levels, slowing down plant growth and indicating that salt stress improved plant stress resistance by decreasing bioactive GA levels [
32].
Arabidopsis BR mutants
det2-1 and
bin2-1 are more sensitive to salt stress, which can be rescued by administration of exogenous BR [
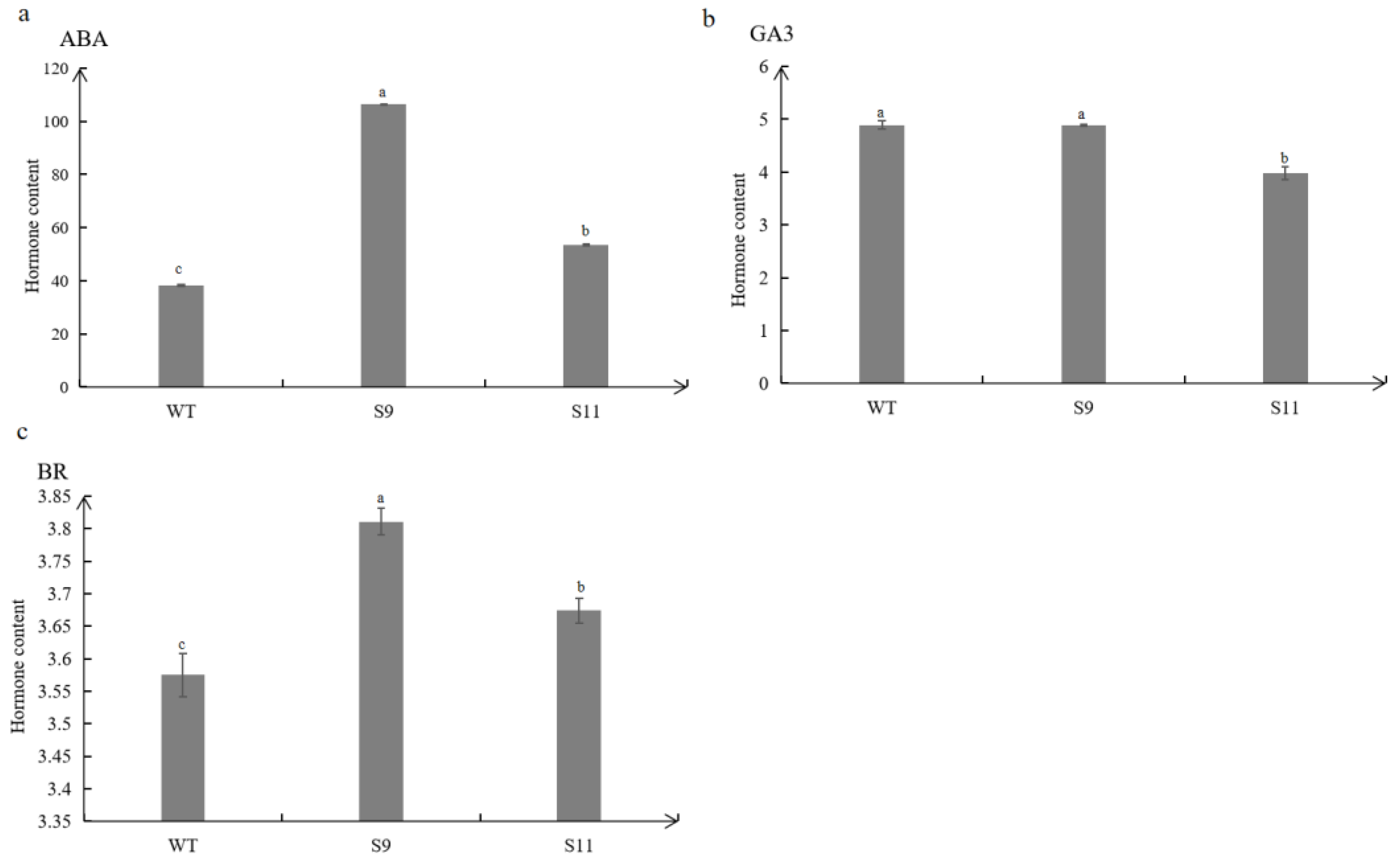
33]. These findings suggest that BR may be a positive regulator of plant salt tolerance. The contents of endogenous hormones ABA and BR in transgenic plants were significantly higher than those of the wild type, and the content of GA3 was significantly lower than that of the wild type (
Figure 6). Therefore, MtTGA1 transgenic plants can enhance salt tolerance by increasing ABA and BR content and reducing GA content.
It is important to note that hormones do not act individually within the plant body but work together with other hormones to regulate plant growth and development. Microarray data revealed that many genes were regulated by BR and ABA [
34]. Under our experimental conditions, spraying BR and ABA hormones altered MtTGA1 expression (
Figure 4e-f), we speculate that MtTGA1 transcription factors can be jointly regulated by BR and ABA. Previous studies have shown that the redox cofactor NPR1 has a positive role in BR-mediated salt stress tolerance and that NPR1 monomers can interact with TGA and activate the expression of defense genes [
35]. This coincides with our results, where the MtTGA1 transcription factor is activated under 150 mM NaCl treatment to promote plant root growth by participating in the hormonal regulatory network, enhancing plant salt tolerance (
Figure 5a-b). Brassinosteroid signaling kinase 5 (BSK5) is a crucial enzyme in the brassin synthesis pathway, activated by BR and ABA, as well as salt stress [
36]. A previous study showed that BSK5 overexpression inhibited the developmental defects of
bril mutants, suggesting that BSK5 has a positive effect on BR signaling [
37]. BR signals are well known to improve plant stress resistance through interaction between redox signals [
38]. Lv et al. demonstrated that the crosstalk nodes between BR and reactive oxygen species (ROS) pathways were related to plant root growth and development [
39]. Thus, BR may directly act on the MtTGA1 transcription factor. When plant receive salt stress signals, they activate BSK5, promoting BR hormone synthesis, leading to an increase in MtTGA1 expression, and subsequently enhancing plant salt stress capacity by increasing antioxidant enzyme activity. Conversely, while ABA inhibits BR signal output, BR can enhance the response of ABA by increasing the level of ABA under certain conditions, thereby improving plant stress tolerance [
35,
40]. BR also modulates plant adaptation to salt by directly inducing ROS clearance (
Figure 10) [
41]. The interaction between BR and ABA hormones and MtTGA1 transcription factors can provide a new perspective to explore the regulatory mechanism of
Medicago truncatula salt stress response.
Plant roots are primarily responsible for sensing salinity signals. Under salt stress conditions, Na
+ first enters the roots; most Na
+ is expelled from the root cells via the root plasma membrane (PM) Na
+/H
+ antiporters (NHXs), while the rest is isolated into vacuoles or transported above ground [
42,
43,
44]. In response to increased Na
+ concentrations, plant root growth typically slows down rapidly and can gradually recover under moderate salt stress [
45]. In our study, the length of S9 and S11 roots was significantly longer than the wild type under 150 mM NaCl stress, indicating that S9 and S11 are less sensitive to salt stress compared to controls (
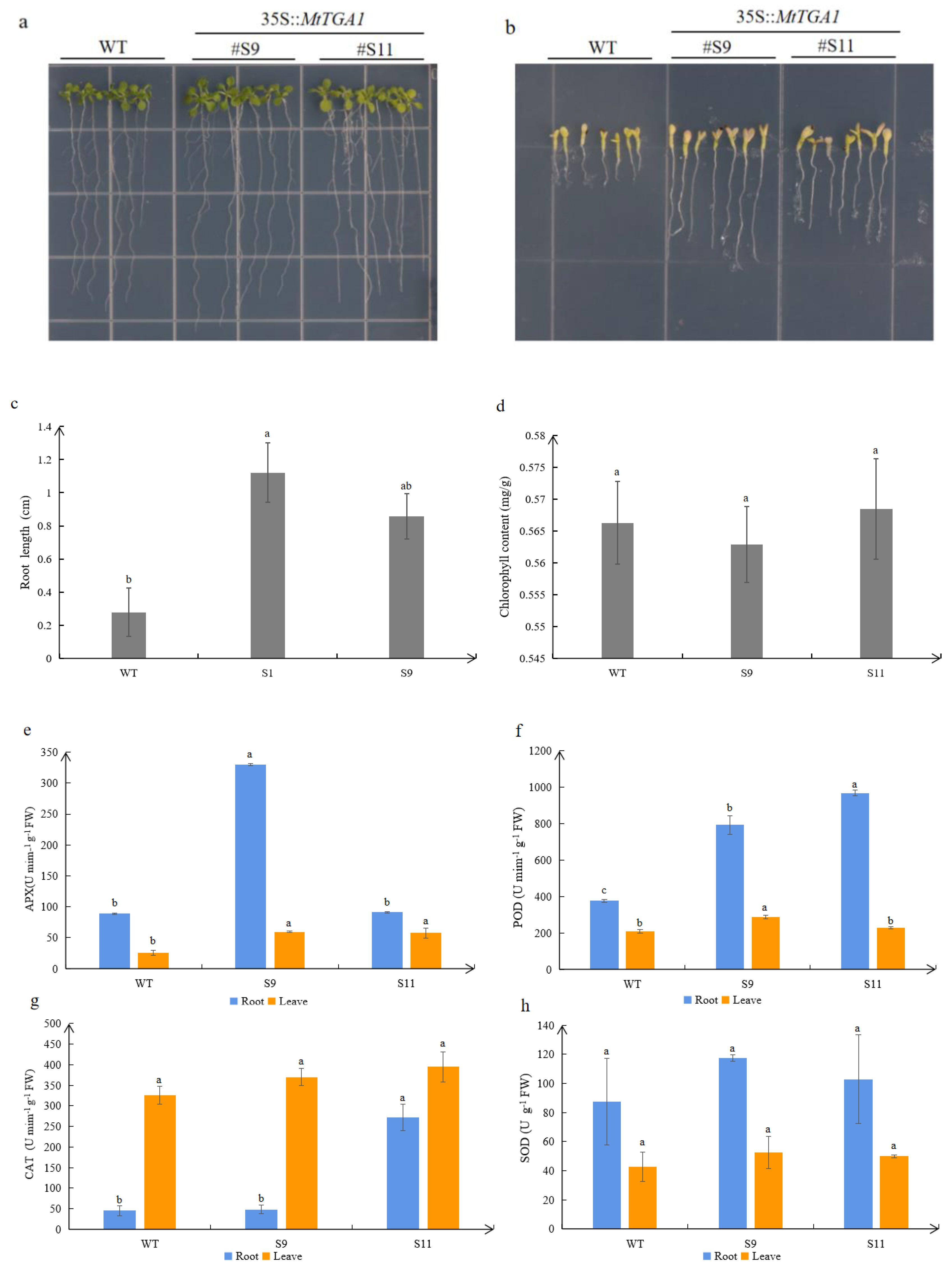
Figure 5a-c). We also observed that the chlorophyll content of S9 was slightly lower than that of the wild type (
Figure 5d). On the one hand, it may be due to mechanical damage during sampling, which affects the normal transport of Na
+ to the ground, resulting in an increase in the concentration of Na
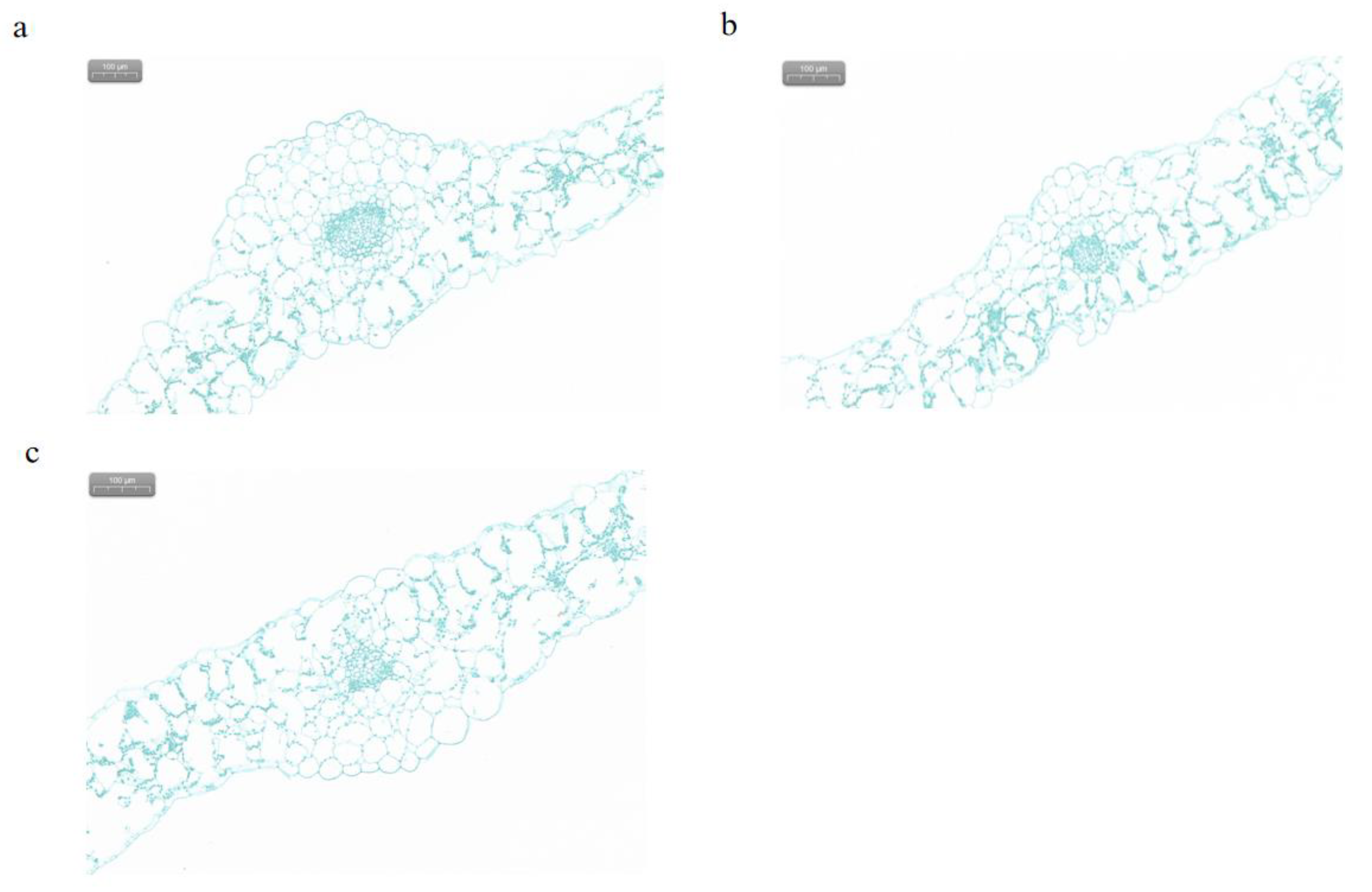
+ transport into the leaf, which hinders the synthesis of chlorophyll. On the other hand, the decrease in cortical tissue thickness of transgenic plants may lead to a decrease in the number of chloroplasts in the leaves of transgenic plants and a slowdown in chlorophyll synthesis (
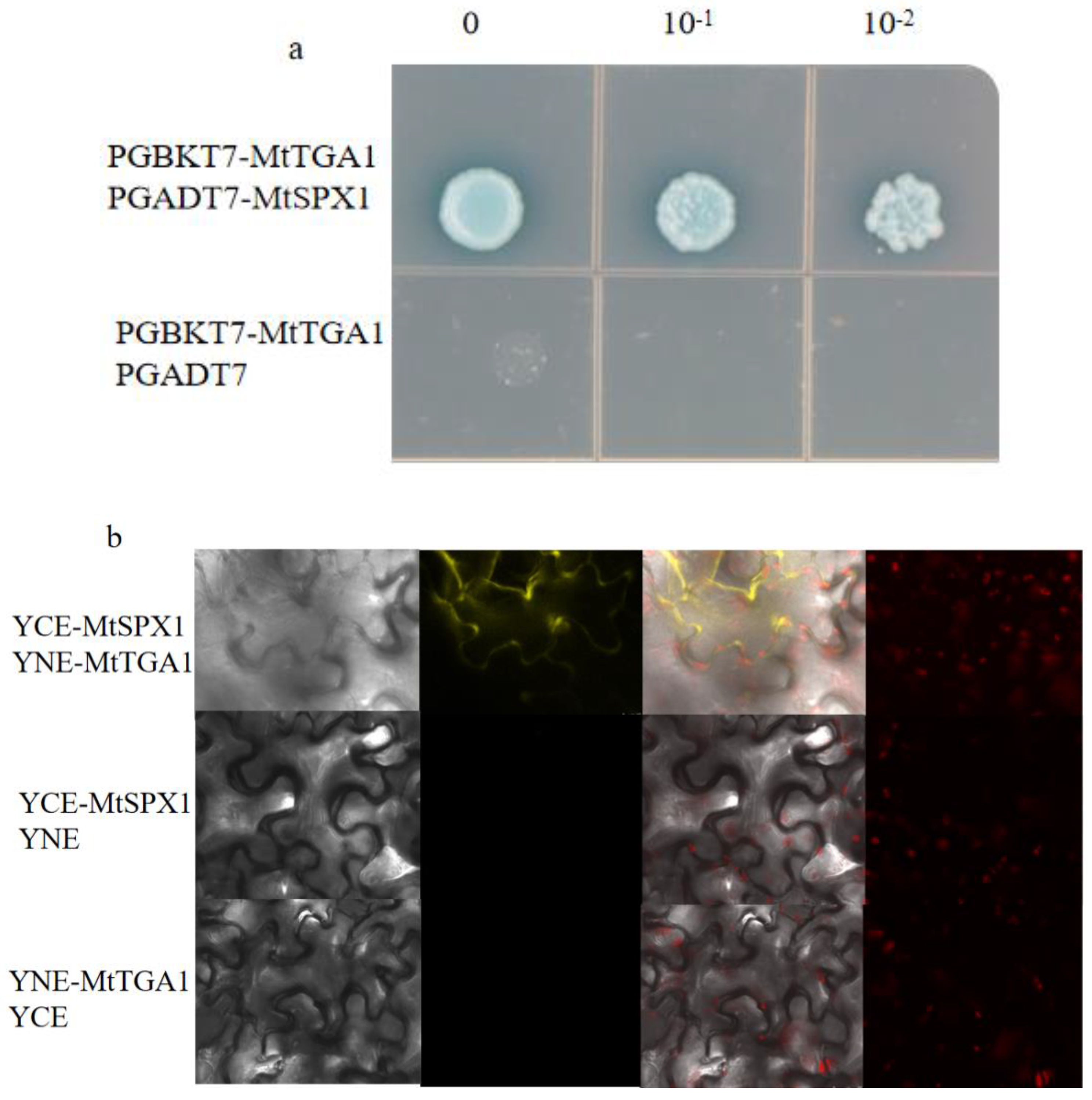
Figure 7). Additionally, we found that the MtTGA1 transcription factor interacts with MtSPX1 (
Figure 8a-b). Proteins containing the SPX1 domain are key to regulating Pi homeostasis and signaling [
46]. Studies have shown that the rice OsSOX4 protein can interact with the nitrate transporter OsNRT1.1B to regulate the balance of nitrate and Pi in rice [
47]. Interestingly, TGA1 is a crucial regulator of the nitrate response of
Arabidopsis thaliana roots and can regulate the expression of
NRT1.1 homologous gene
NRT2.1, thereby promoting root development [
48]. Thus, MtTGA1 and MtSPX1 might jointly regulate the balance of nitrate and Pi in root, and possibly regulate the root length of plant under salt stress through this pathway (
Figure 10).
4. Materials and Methods
4.1. Plant Materials, Growth Conditions and Stress Treatments
Seeds of Medicago truncatula (R108), Arabidopsis thaliana and Nicotiana benthamiana, plant expression vectors 3302Y and 3302-strep, and the Agrobacterium tumefaciens strain EHA105, used in this study, were stored and cultivated at Beijing Forestry University. Following a 4-day vernalization at 4℃, Medicago truncatula and Nicotiana benthamiana, seeds were grown under conditions of 16 hours of light at 26℃ and 8 hours of darkness at 24℃ until root development. Seedlings were transferred to a nutrient medium containing peat, vermiculite, and perlite, and growth continued under the same conditions. Arabidopsis thaliana seeds were sown on plastic Petri plates containing half-strength Murashige-Skoog (MS) medium (PhytoTech, America), with or without 150mM NaCl. The seeds underwent cold stratification at 4℃ for 3 days and were then transferred to an incubator set at 23℃ light for 16 h and 21℃ dark for 8 h.
4.2. Bioinformation Analysis
4.3. Expression Pattern
Mature
Medicago truncatula leaves (two months old) were selected and sprayed with hormones including 50 μmol/L ABA, 0.5mmol/L SA,10μmol/L GA3, 0.1μmol/L BR, 150mmol/L NaCl, and 25% PEG6000. Plant tissues were collected at 0h, 0.5h, 1h, 3h, 6h, 9h, 12h and 24h for temporal expression analysis. Rhizomes and leaf flowers in the same state were quickly frozen with liquid nitrogen and stored at -80℃ for further spatial differential expression analysis. Total RNA from different treatment samples was extracted using a Plant RNA Kit (Omega Bio-tek, Inc., USA), and cDNA was synthesized using the PrimeScript™ RT reagent Kit (TaKaRa, Japan) for real-time quantitative RT-PCR (qRT-PCR) with the primer pair TGA1-RT-F/R (
Supplementary Table S1). The expression of the MtTGA1 gene was calculated using the 2-ΔΔCT method, with three biological replicates [
53].
4.4. Subcellular Localization
The MtTGA1-pMD19-T vector and MtTGA1-YFP-F/R primers (
Supplementary Table S1) were used to construct the subcellular localization vector 35S::MtTGA1-YFP, which was transformed into EHA105
A. tumefaciens. The aforementioned bacterial solution was injected into tobacco leaves and cultured in darkness for 48 h. The YFP signal's location within the cells was then observed under a confocal microscope (Leica TCS SP8).
4.5. Transformation of Plants
The TGA1-F/R primers (
Supplementary Table S1) were used to clone the coding domain sequence (CDS) from
Medicago truncatula. The TGA1-strep-F/R primer was employed to construct the expression vector 35S::TGA1. This expression vector was transformed into EHA105
A. tumefaciens and subsequently into
Arabidopsis thaliana using the Agrobacterium-mediated floral dip method [
54]. Three different lines (S1, S9, and S11) demonstrating high expression levels were selected for further experiments.
4.6. Hormone Measurement and Microscopy Analysis
Leaves from 40-day-old transgenic and wild-type plants at a similar growth stage were collected. The metabolites were extracted, concentrated, and the endogenous hormones were detected by enzyme-linked immunoassay (ELISA) [
55]. Leaf morphology of transgenic and wild-type plants at 40 days of leaf age was analyzed. The leaves were preserved using a 50% FAA fixative, followed by saffrine solid green staining and paraffin sectionalization and analysis. The samples were observed under an optical microscope and images were captured [
56]. These processes were conducted at Servicebio Biotechnology Co., Ltd. (Servicebio, Wuhan, China).
4.7. Next Generation Sequencing and Analysis
RNA-seq technology was employed to analyze the transgenic and wild-type groups under identical conditions. Differentially expressed genes (DEGs) were identified using the DESeq R package (version 1.18.0) [
57] with parameters set at an adjusted p-value <0.05 and |log
2FC|≥1. Gene Ontology (GO) analysis of DEGs between samples was performed using the GOseq R package (version: Release 2.12) [
58]. The KOBAS software (version: 2.0) was used for functional analysis of DEGs in the KEGG pathway [
59].
4.8. Yeast Two-Hybrid and BiFC
Primers PGBKT7-TGA1-F/R and PGADT7-SPX1-F/R (
Supplementary Table S1) were used to construct yeast two-hybrid vectors. The recombinant plasmids PGBKT7-TGA1 and PGADT7-SPX1 were transformed into yeast strains Y2H and Y187, respectively, using the Yeastmaker™ Yeast Transformation System (Clontech Laboratories, Mountain View, CA, USA). The two yeast strains were then mixed in an SD solid medium lacking Leu, Trp, His, and Ade, supplemented with 40 μg·ml
−1 X-α-gal and 200 ng·ml
−1 Aureobasidin A, and cultured at 29.5℃ for 3-5 days. As a control, PGBKT7-TGA1 was paired with yeast strains containing empty PGADT7 vectors.
Bimolecular Fluorescence Complementation (BiFC) expression vectors were constructed using the N-terminal vector primer TGA1-NE2-F/R and the C-terminal vector primer SPX-CE4-F/R, which contained the YFP protein. These were then separately transformed into the EH105 strain. The strains harboring TGA1-NE2 and SPX-CE4 were combined to form the treatment group, while those carrying NE2 or CE4 empty vectors were mixed with the aforementioned strains to serve as control groups. After a 2-day incubation in the dark, the YFP fluorescence in the tobacco cells was observed using a LEICA SP-8 confocal microscope.
4.9. Antioxidant Enzyme Activity and Chlorophyll Contents
The activity of ascorbate peroxidase (APX) was determined using ascorbic acid colorimetry. A spectrophotometer cuvette (1cm path length) was filled with 50mM phosphate buffer (PBS, pH 7.0), 30μl of 50mM ascorbic acid, 30μl of 10mM EDTA, 100μl of enzyme extract, and 30μl of 10mM H2O2. The mixture was stirred and the optical density (OD) was measured at 290nm within 1 minute at 5-second intervals, using pH 7.0 PBS in place of the enzyme solution for reference. Peroxidase (POD) activity was determined using the guaiacol method. A spectrophotometer cuvette (1cm path length) was filled with 1ml of 50mM PBS (pH 7.0), 1ml of 0.3% H2O2, 0.95ml of 0.2% guaiacol, and 50μl of enzyme solution. The OD values were measured at 470nm for 1 minute with 5-second intervals. Catalase (CAT) activity was determined using an ultraviolet spectrophotometer. A quartz cuvette was filled with 2.84ml of 50mM PBS (pH 7.0), 30μl of 1.5M H2O2, and 100μl of enzyme solution. The OD values were measured at 240nm within 1 minute at 5-second intervals, using pH 7.0 PBS as the blank control. Superoxide dismutase (SOD) activity was determined using the nitro blue tetrazolium (NBT) photoreduction method. Each 100μl of enzyme solution was mixed with 2.97ml of reaction solution and 0.03ml of 130 μmol·L-1 riboflavin solution. The mixture was kept in the dark as a control at 25℃, exposed to a light intensity of 60μmol·m-2·s-1 for 20-30 minutes, and the OD values were measured at 560nm.
Chlorophyll content was determined using 95% ethanol extraction, and the total chlorophyll content, including Chl a, Chl b, and carotenoid content, were calculated based on absorbance [
60]. Each sample was tested in triplicate.
5. Conclusion
In summary, the MtTGA1 transcription factor acts as a salt stress regulator, modulating the adaptation of Arabidopsis thaliana to salinity through a hormonal regulatory network and antioxidant enzyme activity. Additionally, MtTGA1 transcription factors can be induced by SA, ABA, BR, and GA, and genes from multiple stress-related families were identified through next-generation sequencing analysis. Under salt stress conditions, BR and ABA hormones accumulated abundantly, and the activities of APX, POD, CAT, and SOD increased, thereby improving the salt tolerance of plants. This study provides a new understanding of the regulation of plant salt tolerance mechanisms mediated by BR and ABA, offering a molecular theoretical basis for enhancing plant salt tolerance.
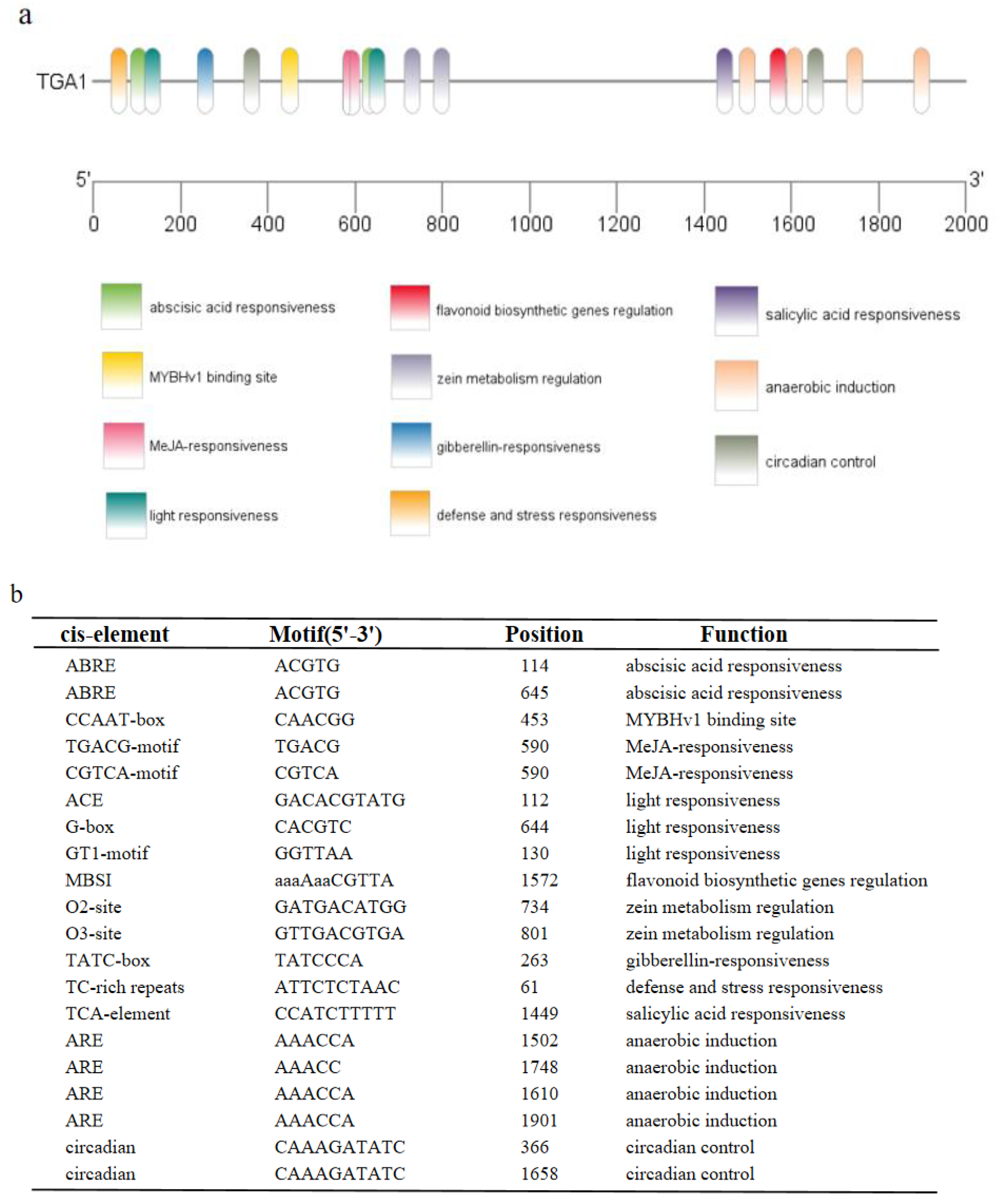
Figure 1.
Analysis of upstream sequence of MtTGA1 transcription factor. (a) Cis-acting elements upstream of MtTGA1transcription factor. (b) List of predicted binding sites for the transcription factors upstream of MtTGA1 transcription factor.
Figure 1.
Analysis of upstream sequence of MtTGA1 transcription factor. (a) Cis-acting elements upstream of MtTGA1transcription factor. (b) List of predicted binding sites for the transcription factors upstream of MtTGA1 transcription factor.
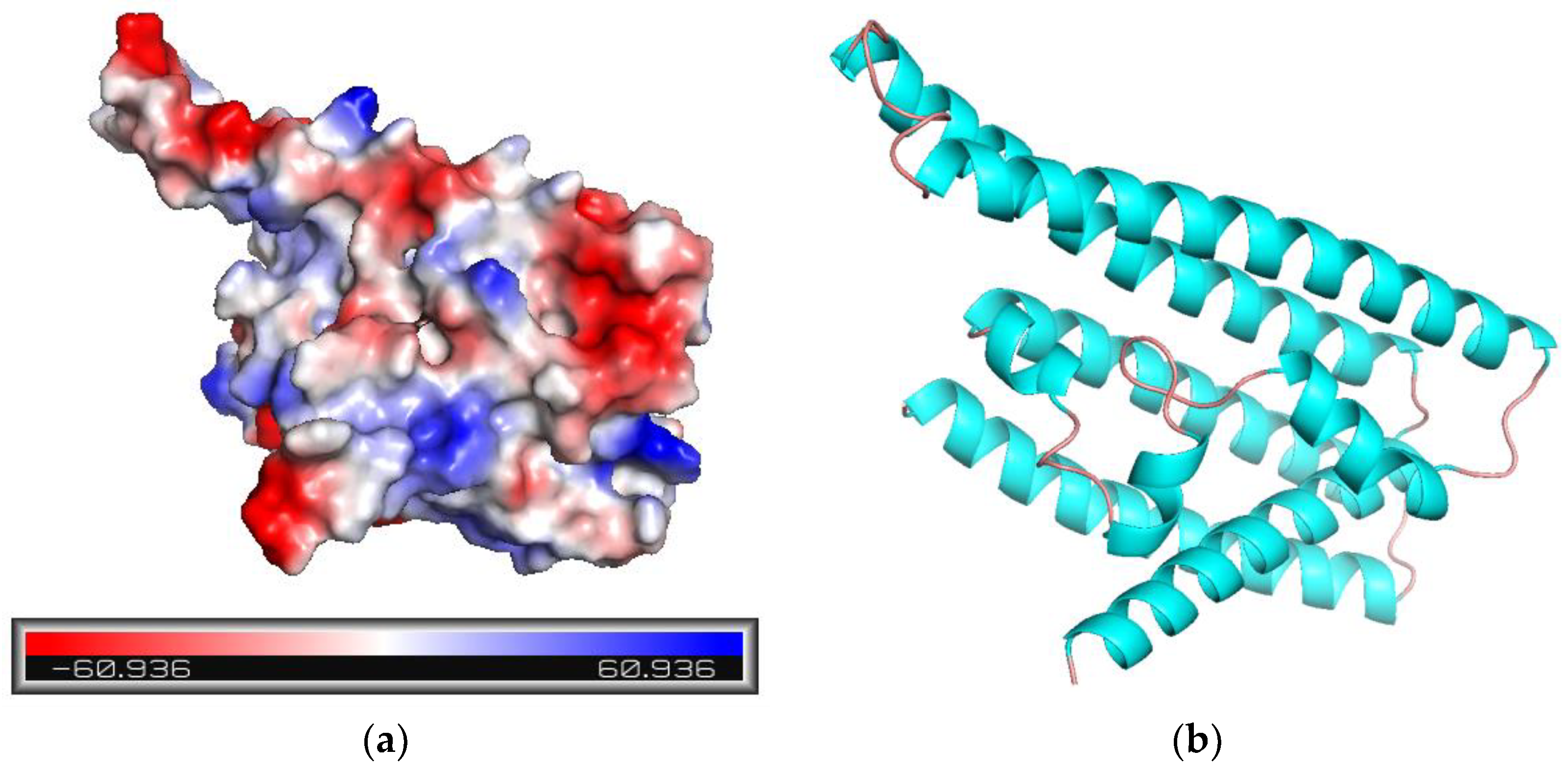
Figure 2.
MtTGA1 protein structure analysis. (a) Electrostatic distribution of proteins. The electrostatic point value is positively correlated with the color depth, with blue representing positive potential and red representing negative potential. (b) MtTGA1 protein structure model. Blue represents Helix and red represents Loop.
Figure 2.
MtTGA1 protein structure analysis. (a) Electrostatic distribution of proteins. The electrostatic point value is positively correlated with the color depth, with blue representing positive potential and red representing negative potential. (b) MtTGA1 protein structure model. Blue represents Helix and red represents Loop.
Figure 3.
Subcellular localization of MtTGA1. The MtTGA1 is confirmed to be located in the nucleus by detecting of YFP signal. Scale bars= 10 μm/25μm.
Figure 3.
Subcellular localization of MtTGA1. The MtTGA1 is confirmed to be located in the nucleus by detecting of YFP signal. Scale bars= 10 μm/25μm.
Figure 4.
Expression analysis of MtTGA1 qRT-PCR. (a) Relative expression level of MtTGA1 in the root, stem, leaf and flower. (b-c) Relative expression level of MtTGA1 after 25% PEG6000 and 150mM Nacl. (d-g) Relative expression level of MtTGA1 under different hormones, including 0.5 mM SA, 50 μM ABA , 0.1μM BR or 10 μM GA3 treatments. The values are means ± SD (n=3). Lowercase letters above columns indicate differences in means (p<0.05).
Figure 4.
Expression analysis of MtTGA1 qRT-PCR. (a) Relative expression level of MtTGA1 in the root, stem, leaf and flower. (b-c) Relative expression level of MtTGA1 after 25% PEG6000 and 150mM Nacl. (d-g) Relative expression level of MtTGA1 under different hormones, including 0.5 mM SA, 50 μM ABA , 0.1μM BR or 10 μM GA3 treatments. The values are means ± SD (n=3). Lowercase letters above columns indicate differences in means (p<0.05).
Figure 5.
Responses of WT and transgenic plants to NaCl treatment. (a,b) Transgenic and WT plants were planted on 1/2 MS medium with or without 150 mM NaCl for 15 days. (c) The root lengths of transgenic and WT plants were measured 15 days after 150 mM NaCl treatment. (d) Chlorophyll content of transgenic and wild-type plants treated with 150 mM NaCl. APX enzyme (e), POD enzyme (f), CAT enzyme (g) and SOD (h) enzyme in roots and leaves of transgenic and WT plants treated with 150 mM NaCl. The values are means ± SD (n=3). Lowercase letters above columns indicate differences in means (p<0.05).
Figure 5.
Responses of WT and transgenic plants to NaCl treatment. (a,b) Transgenic and WT plants were planted on 1/2 MS medium with or without 150 mM NaCl for 15 days. (c) The root lengths of transgenic and WT plants were measured 15 days after 150 mM NaCl treatment. (d) Chlorophyll content of transgenic and wild-type plants treated with 150 mM NaCl. APX enzyme (e), POD enzyme (f), CAT enzyme (g) and SOD (h) enzyme in roots and leaves of transgenic and WT plants treated with 150 mM NaCl. The values are means ± SD (n=3). Lowercase letters above columns indicate differences in means (p<0.05).
Figure 6.
Determination of endogenous hormones ABA(a), GA3(b), BR(c). The values are means ± SD (n=3). Lowercase letters above columns indicate differences in means (p<0.05).
Figure 6.
Determination of endogenous hormones ABA(a), GA3(b), BR(c). The values are means ± SD (n=3). Lowercase letters above columns indicate differences in means (p<0.05).
Figure 7.
Observation of leaf morphology in transverse section. (a) WT blade transverse section. (b) S9 blade transverse section. (c) S11blade transverse section. 200×, scale bar = 100 μm.
Figure 7.
Observation of leaf morphology in transverse section. (a) WT blade transverse section. (b) S9 blade transverse section. (c) S11blade transverse section. 200×, scale bar = 100 μm.
Figure 8.
MtTGA1 Interacts with MtSPX1. (a) Interaction between MtTGA1and MtSPX1. The number indicates dilution times of transformed yeast cells.The control was PGBKT7-MtTGA1 and PGADT7. The control was not long and remained blue.(b) BIFC shows the interaction of MtTGA1and MtSPX1 in living cells. MtTGA1 interacts with MtSPX1in the cytoplasm by detecting the YFP signal. Negative control was YCE-MtSPX1 co-expression with YNE or YNE-MtTGA1 co-expression with YCE. Scale bars =25 μm.
Figure 8.
MtTGA1 Interacts with MtSPX1. (a) Interaction between MtTGA1and MtSPX1. The number indicates dilution times of transformed yeast cells.The control was PGBKT7-MtTGA1 and PGADT7. The control was not long and remained blue.(b) BIFC shows the interaction of MtTGA1and MtSPX1 in living cells. MtTGA1 interacts with MtSPX1in the cytoplasm by detecting the YFP signal. Negative control was YCE-MtSPX1 co-expression with YNE or YNE-MtTGA1 co-expression with YCE. Scale bars =25 μm.
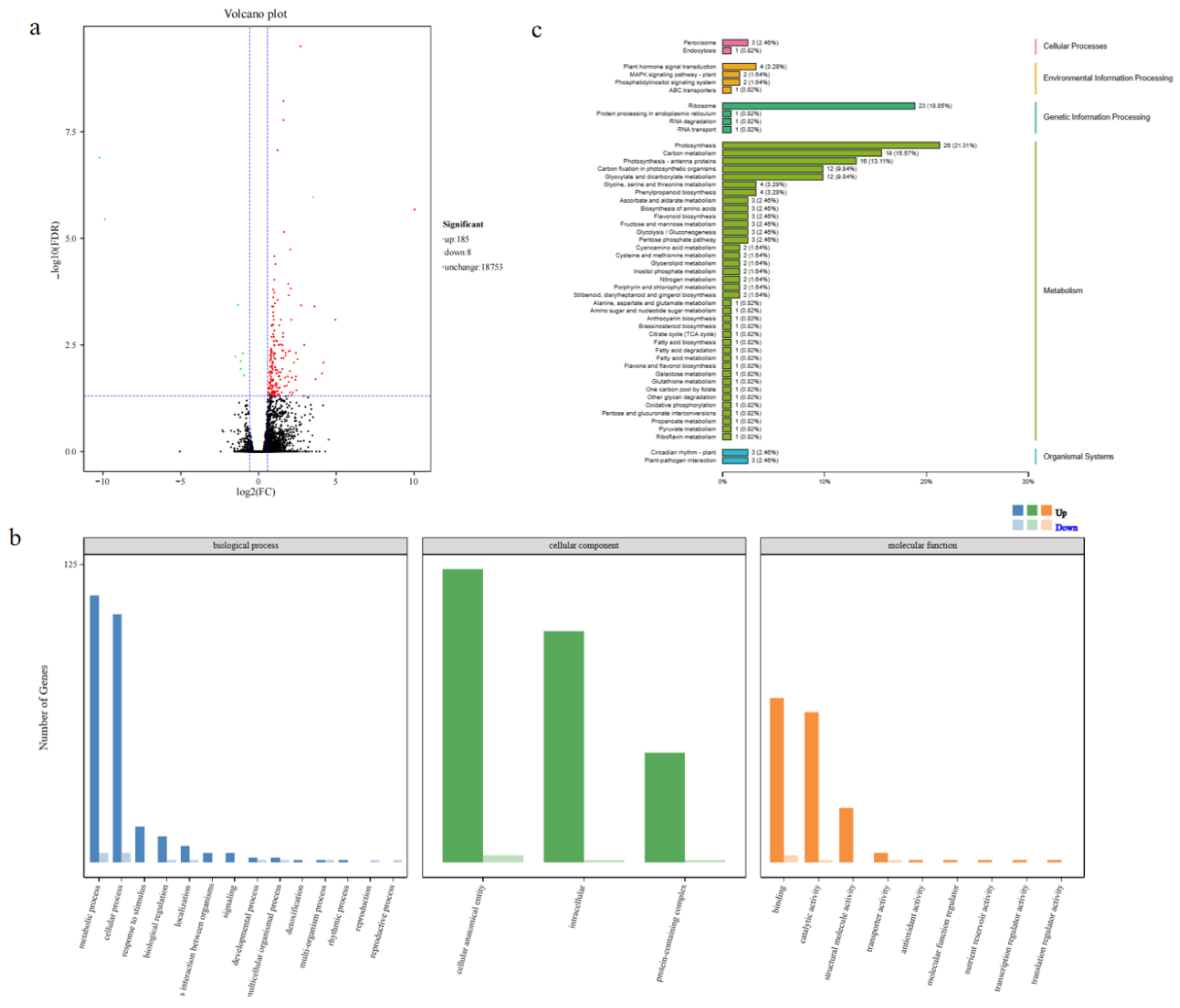
Figure 9.
Transcriptome analysis of transgenic and wild-type Arabidopsis thaliana. Volcano plot of DEGs (a). In graphs, each plot represents one gene with three colors, including red (up-regulated), green (down-regulated) and blank (unchanged). The X-axis represents the value of log2 (Fold Change) in the two samples, and the Y-axis indicates the negative value of log10 (FDR). GO classification enrichment analysis of DEGs(b). In graphs, the X-axis represents the GO classification and the Y-axis represents the number of genes. KEGG pathway types of DEGs (c). In graphs, the X-axis represents the KEGG metabolic pathway name, and the Y-axis represents the ratio of the number of genes annotated to the pathway to the total number of annotated genes.
Figure 9.
Transcriptome analysis of transgenic and wild-type Arabidopsis thaliana. Volcano plot of DEGs (a). In graphs, each plot represents one gene with three colors, including red (up-regulated), green (down-regulated) and blank (unchanged). The X-axis represents the value of log2 (Fold Change) in the two samples, and the Y-axis indicates the negative value of log10 (FDR). GO classification enrichment analysis of DEGs(b). In graphs, the X-axis represents the GO classification and the Y-axis represents the number of genes. KEGG pathway types of DEGs (c). In graphs, the X-axis represents the KEGG metabolic pathway name, and the Y-axis represents the ratio of the number of genes annotated to the pathway to the total number of annotated genes.
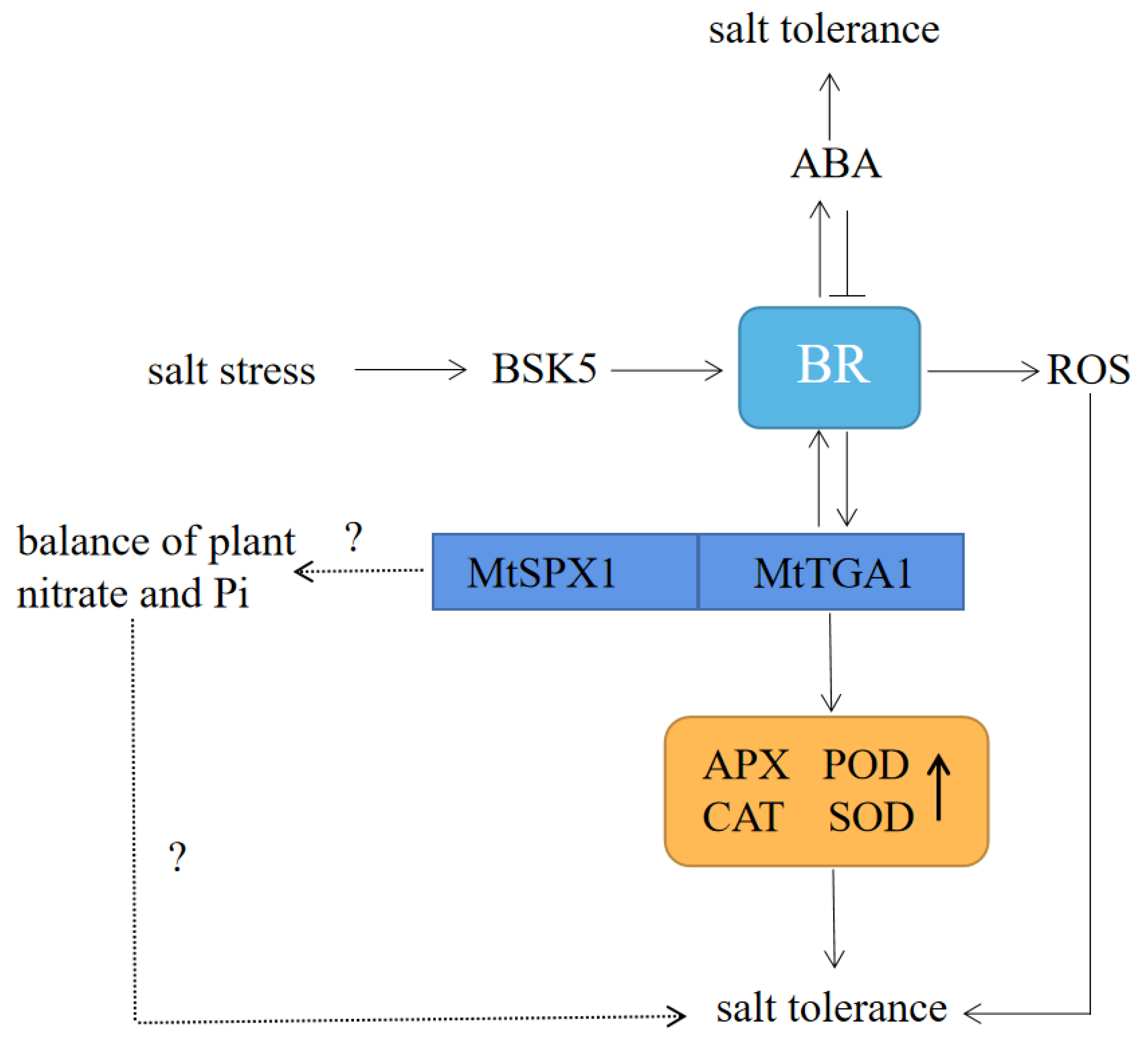
Figure 10.
Role model of MtTGA1 regulatory chain in Arabidopsis thaliana salt stress response. BSK5 promotes the synthesis of BR, induces the expression of MtTGA1 transcription factor, enhances the activity of antioxidant enzymes, and enhances salt stress in plants. BR increases ABA levels, and ABA inhibits BR signal output. BR regulates plant adaptation to salt by regulating ROS. MtTGA1 and MtSPX1 work together to regulate the balance of nitrate and Pi and improve salt tolerance through this pathway.
Figure 10.
Role model of MtTGA1 regulatory chain in Arabidopsis thaliana salt stress response. BSK5 promotes the synthesis of BR, induces the expression of MtTGA1 transcription factor, enhances the activity of antioxidant enzymes, and enhances salt stress in plants. BR increases ABA levels, and ABA inhibits BR signal output. BR regulates plant adaptation to salt by regulating ROS. MtTGA1 and MtSPX1 work together to regulate the balance of nitrate and Pi and improve salt tolerance through this pathway.