Submitted:
08 June 2023
Posted:
09 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Ligno-hemicellulolytic enzyme co-production using corn stover
2.3. Enzyme extraction and quantification
2.4. Biological pretreatment reaction system
2.5. Biomass regeneration
2.6. Enzymatic hydrolysis using in-house produced cellulases
2.7. Statistical analysis
3. Results and Discussion
3.1. Microorganism
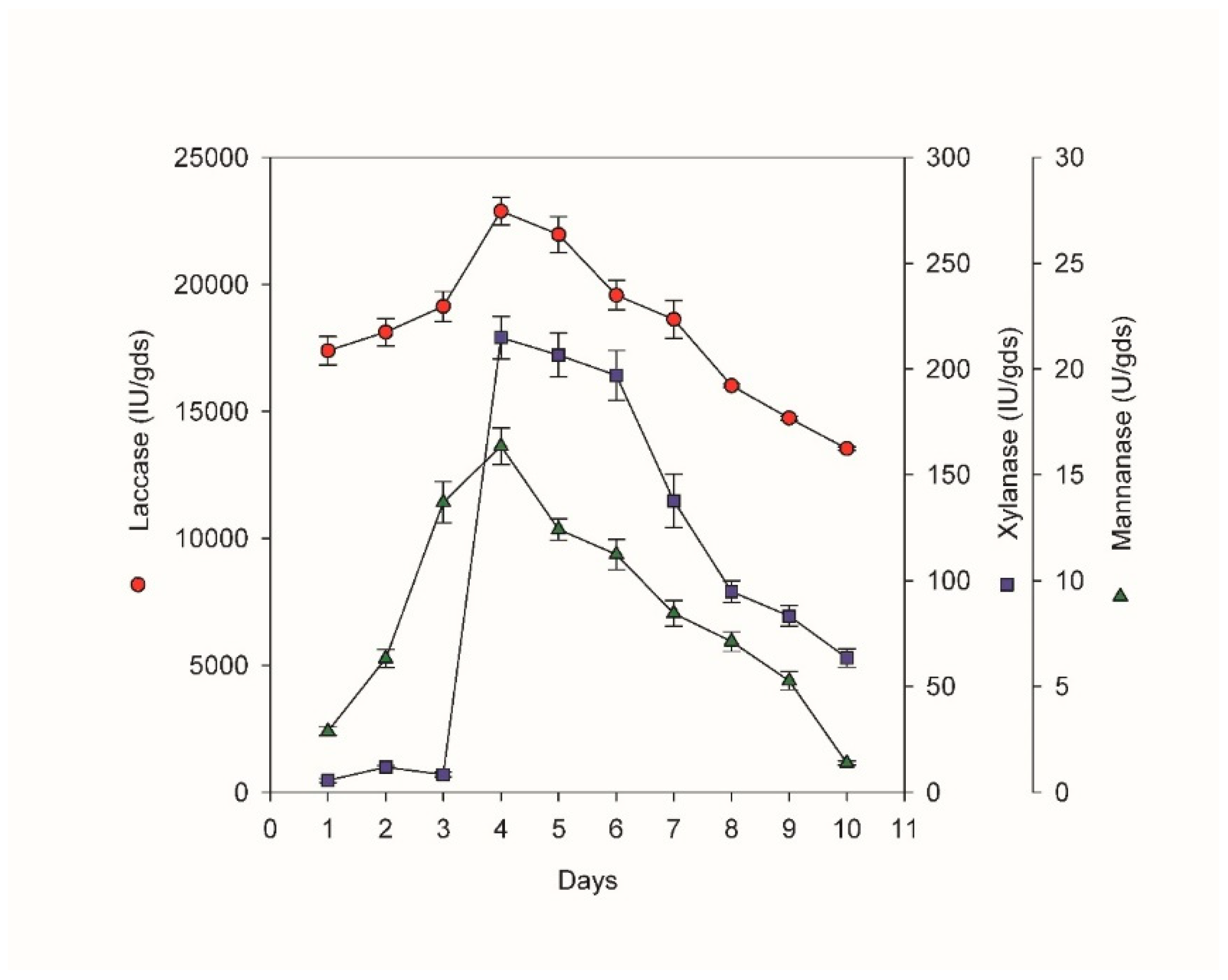
| Substrate | Laccase | Xylanase | Mannanase | Time (days) | Fermentation type | Fungal strain | Reference |
| (U/gds) | |||||||
| Corn stover | 22884.29 | 214.95 | 16.36 | 4 | SSF | P. phaeocomes S-1 | Present study |
| Rice straw | 10859.51 | 22.01 | 10.45 | 8 | SSF | P. phaeocomes S-1 | [19] |
| Corn stover | 6.61 | - | - | 14 | SSF | Myrothecium verrucaria | [31] |
| Corn stover | 1.5 | 4.8 | - | 7 | SSF | Ceriporiopsis subvermispora | [17] |
| Corn stover | 0.8 | 11.22 | - | 7, | SSF | Trametes hirsuta | [32] |
| Soyabean meal | 47.7 | - | 3 | SSF | Aspergillus niger | [33] | |
| Jerusalem artichoke | 2.0 | 106.5 ± 3.3 | - | 5, 20 | SSF | Ceriporiopsis subvermispora | [34] |
| Rice straw | 316.28 | - | - | 6 | SSF | Schizophyllum commune | [35] |
| Wheat straw | 1360 | - | 33.9 | 21 | SSF | Pleurotus ostreatus | [36] |
| Wheat straw | 72.9 | 98.9 | 35.5 | 21 | SSF | Trametes versicolor | [36] |
| Wheat straw | - | 1924.4 | 1.6 | 42 | SSF | Piptoporus betulinus | [36] |
| Rice bark | 2172.28 | - | - | 25 | SSF | Pleorotus ostreatus AMRL 173–6 | [37] |
| Leaf of corn cob | 29.31 | - | - | 12 | SSF | Pleurotus eryngii Han 1787 | [38] |
| Pinus tabuliformis | 2.46 | - | - | 14 | SSF | Lentinus edodes Han 1788 | [38] |
| Wheat straw | 25.51 | - | - | 5 | SSF | A. niger | [7] |
| Kraft lignin | 5.68 U/mL | - | - | 10 | SmF | Pleurotus ostreatus | [13] |
3.2. Enzyme quantification and correlation with the biomass cellulose recovery during fungal pretreatment
| Fermentation Duration (days) |
Weight loss (%) |
Cellulose (%) |
APPL (mg/g) |
Total reducing sugars |
Ligno-hemicellulolytic enzymes (IU/gds) |
||
| (mg/g) | Laccase | Xylanase | Mannanase | ||||
| 0 | 7.28 | 17.09 | 80 | 19.26 | 0 | 0 | 0 |
| 4 | 15.80 | 19.21 | 192.8 | 15.78 | 336.3636 | 54.27 | 3.62 |
| 8 | 19.80 | 21.09 | 237.6 | 24.37 | 264.4628 | 46.94 | 3.29 |
| 10 | 21.80 | 22.17 | 255.2 | 28.78 | 227.2727 | 39.61 | 2.94 |
| 15 | 23.20 | 23.36 | 298 | 38.60 | 179.3388 | 37.20 | 2.59 |
| 20 | 25.80 | 25.28 | 333.6 | 42.01 | 183.4711 | 13.71 | 3.01 |
| 30 | 26.13 | 30.12 | 365.6 | 44.21 | 154.5455 | 10.76 | 2.31 |
| 40 | 28.26 | 44.25 | 384.8 | 51.83 | 133.8843 | 14.93 | 1.34 |
| 60 | 30.53 | 43.96 | 224.8 | 49.18 | 53.71901 | 3.98 | 0.44 |
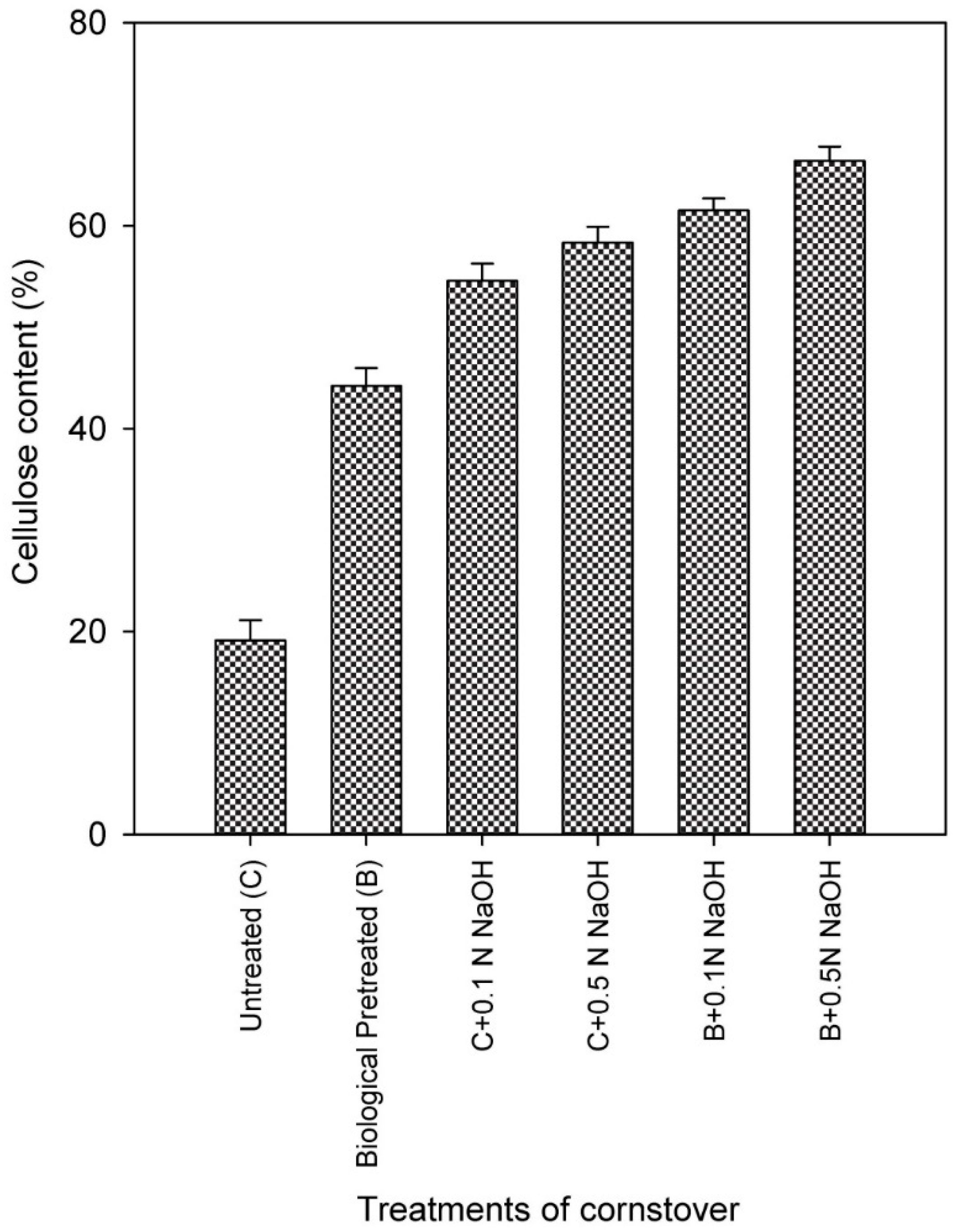
3.3. Enzymatic hydrolysis of pretreated biomass
| Treatment of corn stover | Time (days) | |||||
| 0 | 1 | 4 | 5 | 6 | ||
| S. No. | Total reducing sugars (mg/ml) | |||||
| 1 | Untreated (C) | 0.05 | 1.71 | 2.30 | 3.05 | 3.11 |
| 2 | Biologically pretreated (B) | 1.10 | 3.12 | 6.48 | 12.49 | 16.08 |
| 3 | C +0.1 N NaOH treated | 0.78 | 2.59 | 5.26 | 10.02 | 14.83 |
| 4 | C +0.5 N NaOH treated | 0.93 | 3.21 | 6.10 | 11.58 | 16.32 |
| 5 | B + 0.1N NaOH treated | 1.11 | 4.10 | 12.46 | 19.65 | 23.86 |
| 6 | B + 0.5N NaOH treated | 1.16 | 4.69 | 12.94 | 20.31 | 26.52 |
| Total reducing sugars (mg/g) | ||||||
| 1 | Untreated (C) | 0.78 | 25.79 | 34.54 | 45.75 | 46.67 |
| 2 | Biologically pretreated (B) | 16.61 | 46.85 | 97.32 | 187.38 | 251.22 |
| 3 | C +0.1 N NaOH treated | 11.83 | 38.98 | 79.04 | 150.34 | 222.47 |
| 4 | C +0.5 N NaOH treated | 14.07 | 48.15 | 91.51 | 173.77 | 244.83 |
| 5 | B + 0.1N NaOH treated | 16.67 | 61.53 | 186.91 | 294.83 | 358.02 |
| 6 | B + 0.5N NaOH treated | 17.52 | 70.46 | 194.18 | 304.65 | 397.84 |
| Substrate |
Fungi and duration |
FPU |
Hydrolysis efficiency (%)/ reducing sugars (mg/g) |
Reference |
| Corn stover alkali extracted |
P. phaeocomes S-1 40 days |
5 FPU/g | 53.97% 397.84 23.86g/L |
Present study |
| Corn stover | Bacillus sp. | 20 FPU/ g | 56% 55.50±0.74 |
[30] |
|
Corn stover |
P. sajor-caju 25 days |
20 FPU Sigma Cellic® CTec2 |
71.24% 13.65 g/L |
[10] |
| Corn stover | Fomes sp. EUM1 | 0.5 FPU/ml | 34.1% 147.4 |
[28] |
| Corn stover | Ceriporiopsis subvermispora | 5 FPU/g 20 xylanase |
21.02% glucose yield | [50] |
| Corn stover |
Cyathus stercoreus NRRL-6573 30 days |
3 commercial enzyme (cellulase, β-glucosidase, hemicellulase) | 394 ± 13 g/L | [46] |
| Corn stover | Pycnoporus sanguineus FP-10356-Sp | 3 commercial enzyme (cellulase, β-glucosidase, hemicellulase) | 393 ± 17 g/L | [46] |
| Corn stover | Phlebia brevispora NRRL-13108). | 3 commercial enzyme (cellulase, β-glucosidase, hemicellulase) | 383 ± 13 g/L | [46] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes : An overview. Renew. Energy. 2012, 37, 19–27. [CrossRef]
- Soni, S. K.; Parkash, O.; Manhas, R.; Tewari, R.; Soni, R. Value added products from lignocellulosic agricultural residues: an overview. Int. j. food ferment. technol. 2019, 9(2), 101-115.
- Yadav, M.; Balan, V.; Varjani, S. Multidisciplinary Pretreatment Approaches to Improve the Bio-methane Production from Lignocellulosic Biomass. Bioenerg. Res. 2023, 16, 228–247. [CrossRef]
- Tian, L.; Branford-White, C.; Wang, W.; Nie, H.; Zhu, L. Laccase-mediated system pretreatment to enhance the effect of hydrogen peroxide bleaching of cotton fabric. Int. J. Biol. Macromol. 2012, 50, 782–787. [CrossRef]
- Kaur, M.; Gill, M. K.; Sharma, S.; Kocher, G. S.; Sodhi, H. S. Biological Pretreatment Strategies for Second-Generation Lignocellulosic Biomass to Enhance Ethanol Production. In Agroindustrial Waste for Green Fuel Application; Singapore: Springer Nature Singapore. 2023, pp. 169-203.
- Sobti, R. C.; Sharma, A.; Soni, S. K. Applications of Biotechnological Techniques in Mitigating Environmental Concerns.; CRC Press: Boca Raton, FL, USA, 2022; pp. 249–312.
- Hasan, S.; Anwar, Z. Khalid, W. Afzal, F. Zafar, M. Ali, U. Aljobair, M. O. Laccase production from local biomass using solid state fermentation. Fermentation, 2023, 9(2), 179.
- Saha, B.C.; Kennedy, G.J.; Qureshi, N.; Cotta, M.A. Biological pretreatment of corn stover with Phlebia brevispora NRRL-13108 for enhanced enzymatic hydrolysis and efficient ethanol production. Biotechnol. Prog. 2017, 33, 365–374. [CrossRef]
- Waghmare, P.R.; Khandare, R. V.; Jeon, B.H.; Govindwar, S.P. Enzymatic hydrolysis of biologically pretreated sorghum husk for bioethanol production. Biofuel Res. J. 2018, 5, 846–853. [CrossRef]
- Ding, C.; Wang, X.; Li, M. Evaluation of six white-rot fungal pretreatments on corn stover for the production of cellulolytic and ligninolytic enzymes, reducing sugars, and ethanol. Appl. Microbiol. Biotechnol. 2019, 103, 5641–5652. [CrossRef]
- Qin, X.; Su, X.; Luo, H.; Ma, R.; Yao, B.; Ma, F. Deciphering lignocellulose deconstruction by the white rot fungus Irpex lacteus based on genomic and transcriptomic analyses. Biotechnol. Biofuels, 2018, 11, 1–14. [CrossRef]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49-58.
- Franco, P. C.; Shiraishi, I. S.; Dekker, R. F.; Barbosa-Dekker, A. M.; Borsato, D.; Angilelli, K. B.; Daniel, J. F. Optimization of laccase production by Pleurotus ostreatus Florida and evaluation of metabolites generated during Kraft lignin biotransformation. Waste Biomass Valori. 2023, 1-9. [CrossRef]
- Bak, J.S.; Kim, M.D.; Choi, I.G.; Kim, K.H. Biological pretreatment of rice straw by fermenting with Dichomitus squalens. N. Biotechnol. 2010, 27, 424–434. [CrossRef]
- Taniguchi, M.; Suzuki, H.; Watanabe, D.; Sakai, K.; Hoshino, K.; Tanaka, T. Evaluation of pretreatment with Pleurotus ostreatus for enzymatic hydrolysis of rice straw. J. Biosci. Bioeng. 2005, 100, 637–643. [CrossRef]
- Tiwari, R.; Rana, S.; Singh, S.; Arora, A.; Kaushik, R.; Agrawal, V.V.; Saxena, A.K.; Nain, L. Biological delignification of paddy straw and Parthenium sp. using a novel micromycete Myrothecium roridum LG7 for enhanced saccharification. Bioresour. Technol. 2013, 135, 7–11. [CrossRef]
- Wan, C.; Li, Y. Microbial delignification of corn stover by Ceriporiopsis subvermispora for improving cellulose digestibility. Enzyme Microb. Technol. 2010, 47, 31–36. [CrossRef]
- Mohanram, S.; Rajan, K.; Carrier, D. J.; Nain, L.; Arora, A. Insights into biological delignification of rice straw by Trametes hirsuta and Myrothecium roridum and comparison of saccharification yields with dilute acid pretreatment. Biomass Bioenerg. 2015, 76, 54-60. [CrossRef]
- Rastogi, S.; Soni, R.; Kaur, J.; Soni, S.K. Unravelling the capability of Pyrenophora phaeocomes S-1 for the production of ligno-hemicellulolytic enzyme cocktail and simultaneous bio-delignification of rice straw for enhanced enzymatic saccharification. Bioresour. Technol. 2016, 222, 458–469. [CrossRef]
- Jhadav, A.; Vamsi, K.K.; Khairnar, Y.; Boraste, A.; Gupta, N.; Trivedi, S.; Patil, P.; Gupta, G.; Gupta, M. Optimization of production and partial purification of laccase by Phanerochaete chrysosporium using submerged fermenation. Int. J. Microbiol. Res. 2009, 1, 9–12. [CrossRef]
- Bailey, M.J.; Biely, P.; Poutanen, K. Interlaboratory testing of methods for assay of xylanase activity. J. Biotechnol. 1992, 23, 257–270. [CrossRef]
- Markovič, O.; Slezárik, A.; Labudová, I. Purification and characterization of pectinesterase and polygalacturonase from Trichoderma reesei. FEMS microbiol. Lett. 1985, 27(3), 267-271.
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [CrossRef]
- Updegraff, D.M. Semimicro determination of cellulose inbiological materials. Anal. Biochem. 1969, 32, 420–424. [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Laboratory Analytical Procedure: Determination of Structural carbohydrates and Lignin in Biomass. Golden, colorado: National Renewable Energy Laboratory, 2011, pp. 1-15.
- Bansal, N.; Janveja, C.; Tewari, R.; Soni, R.; Soni, S.K. Highly thermostable and pH-stable cellulases from Aspergillus niger NS-2: properties and application for cellulose hydrolysis. Appl. Biochem. Biotechnol. 2014, 172, 141–56.
- Gao, W.; Lei, Z.; Tabil, L.G.; Zhao, R. Biological Pretreatment by Solid-State Fermentation of Oat Straw to Enhance Physical Quality of Pellets. Hindawi J. Chem. 2020, 3060475, 1-13. [CrossRef]
- Méndez-Hernández, J.E.; Loera, O.; Méndez-Hernández, E.M.; Herrera, E.; Arce-Cervantes, O.; Soto-Cruz, N.Ó. Fungal Pretreatment of Corn Stover by Fomes sp. EUM1: Simultaneous Production of Readily Hydrolysable Biomass and Useful Biocatalysts. Waste Biomass Valori. 2019, 10, 2637–2650. [CrossRef]
- Ma, K.; Ruan, Z. Production of a lignocellulolytic enzyme system for simultaneous bio-delignification and saccharification of corn stover employing co-culture of fungi. Bioresour. Technol. 2015, 175, 586–593. [CrossRef]
- Wu, Y.; Guo, H.; Rahman, S.; Chen, X.; Zhang, J.; Liu, Y. Biological pretreatment of corn stover for enhancing enzymatic hydrolysis using Bacillus sp. P3. Bioresour. Bioprocess. 2021, 8, 1-10. [CrossRef]
- Su, Y.; Yu, X.; Sun, Y.; Wang, G.; Chen, H.; Chen, G. Evaluation of Screened Lignin-degrading Fungi for the Biological Pretreatment of Corn Stover. Sci. Rep. 2018, 8, 1–11. [CrossRef]
- Sun, F. H.; Li, J.; Yuan, Y. X.; Yan, Z. Y. Liu, X. F. Effect of biological pretreatment with Trametes hirsuta yj9 on enzymatic hydrolysis of corn stover. Int. Biodeterior. Biodegradation, 2011, 65(7), 931-938. [CrossRef]
- Vitcosque, G. L.; Fonseca, R. F.; Rodríguez-Zúñiga, U. F.; Bertucci Neto, V.; Couri, S.; Farinas, C. S. Production of biomass-degrading multienzyme complexes under solid-state fermentation of soybean meal using a bioreactor. Enzyme Res. 2012. 248983, 1-9. [CrossRef]
- Zhu, N.; Liu, J.; Yang, J.; Lin, Y.; Yang, Y.; Ji, L.; Yuan, H. Comparative analysis of the secretomes of Schizophyllum commune and other wood-decay basidiomycetes during solid-state fermentation reveals its unique lignocellulose-degrading enzyme system. Biotechnol. biofuels, 2016, 9, 1-22. [CrossRef]
- Asgher, M.; Wahab, A.; Bilal, M.; Iqbal, H. M. N. Lignocellulose degradation and production of lignin modifying enzymes by Schizophyllum commune IBL-06 in solid-state fermentation. Biocatal. Agric. Biotechnol. 2016, 6, 195-201. [CrossRef]
- Valášková, V.; Baldrian, P. Estimation of bound and free fractions of lignocellulose-degrading enzymes of wood-rotting fungi Pleurotus ostreatus, Trametes versicolor and Piptoporus betulinus. Res. microbial. 2006, 157(2), 119-124. [CrossRef]
- Melanouri, E. M.; Dedousi, M,M; Diamantopoulou, P. Cultivating Pleurotus ostreatus and Pleurotus eryngii mushroom strains on agro-industrial residues in solid-state fermentation. Part I: Screening for growth, endoglucanase, laccase and biomass production in the colonization phase. Carbon Resour. Convers. 2022, 5, 61-70. [CrossRef]
- An, Q.; Li, C.S.; Yuan, Y.N.; Dou, X.Y.; Wang, Y.H.; Guo, S.; Chen, Z.; Ping, A.Q.; Zhang, T.X.; Yang, Q.Y.; Han, M.L. Utilization of Agroindustrial Wastes for the Production of Laccase by Pleurotus eryngii Han 1787 and Lentinus edodes Han1788. BioResources, 2023, 18(1), 570-583. [CrossRef]
- Pinheiro, V.E.; Preto, R.; Preto, R. Screening and cocktail optimization using experimental mixture design : enzymatic saccharification as a biological pretreatment strategy. Biofuels, Bioprod. Biorefining, 2021, 15(5), 1–14.
- Sankaralingam, R.; Sengottuvelan, B.; Venkat, P.; Selvaraj, M. Arunachalam, V. Natarajan, J. Experimental investigation on varying flame characteristics of benzoic resin solid fuel pellets. Renew. Energy, 2020, 147, 1500–1510. [CrossRef]
- Moiceanu, G.; Bianca, S.; Ferdes, M. Microorganisms and Enzymes Used in the Biological Pretreatment of the Substrate to Enhance Biogas Production : A Review. Sustainability, 2020, 12(17), 7205. [CrossRef]
- Saritha, M.; Arora, A.; Nain, L. Pretreatment of paddy straw with Trametes hirsuta for improved enzymatic saccharification. Bioresour. Technol. 2012, 104, 459-465. [CrossRef]
- Savy, D.; Piccolo, A. Physical–chemical characteristics of lignins separated from biomasses for second-generation ethanol. Biomass bioenergy. 2014, 62, 58-67. [CrossRef]
- Arora, A.; Priya, S.; Sharma, P.; Sharma, S.; Nain, L. Evaluating biological pretreatment as a feasible methodology for ethanol production from paddy straw. Biocatal. Agric. Biotechnol. 2016, 8, 66-72. [CrossRef]
- Ghorbani, F.; Karimi, M.; Biria, D.; Kariminia, H. R.; Jeihanipour, A. Enhancement of fungal delignification of rice straw by Trichoderma viride sp. to improve its saccharification. Biochem. Eng. J. 2015, 101, 77-84. [CrossRef]
- Saha, B. C.; Qureshi, N.; Kennedy, G. J.; Cotta, M. A. Biological pretreatment of corn stover with white-rot fungus for improved enzymatic hydrolysis. Int Biodeterior Biodegradation, 2016, 109, 29-35. [CrossRef]
- Baramee, S.; Siriatcharanon, A.; Ketbot, P.; Teeravivattanakit, T.; Waeonukul, R.; Pason, P.; Tachaapaikoon, C.; Ratanakhanokchai, K.; Phitsuwan, P. Biological pretreatment of rice straw with cellulase-free xylanolytic enzyme-producing Bacillus firmus K-1: Structural modification and biomass digestibility. Renew. Energy. 2020, 160, 555-563. [CrossRef]
- Martínez-Patiño, J.C.; Lu-Chau, T.A.; Gullón, B.; Ruiz, E.; Romero, I.; Castro, E.; Lema, J.M. Application of a combined fungal and diluted acid pretreatment on olive tree biomass. Ind. Crops Prod. 2018, 121, 10–17. [CrossRef]
- Dai, Y.; Si, M.; Chen, Y.; Zhang, N.; Zhou, M.; Liao, Q.; Liu, Y. Combination of biological pretreatment with NaOH/Urea pretreatment at cold temperature to enhance enzymatic hydrolysis of rice straw. Bioresour. Technol. 2015, 198, 725-731. [CrossRef]
- Huang, W.; Wachemo, A. C.; Yuan, H.; Li, X. Modification of corn stover for improving biodegradability and anaerobic digestion performance by Ceriporiopsis subvermispora. Bioresour. Technol. 2019, 283, 76-85. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





