Submitted:
08 June 2023
Posted:
09 June 2023
You are already at the latest version
Abstract
Keywords:
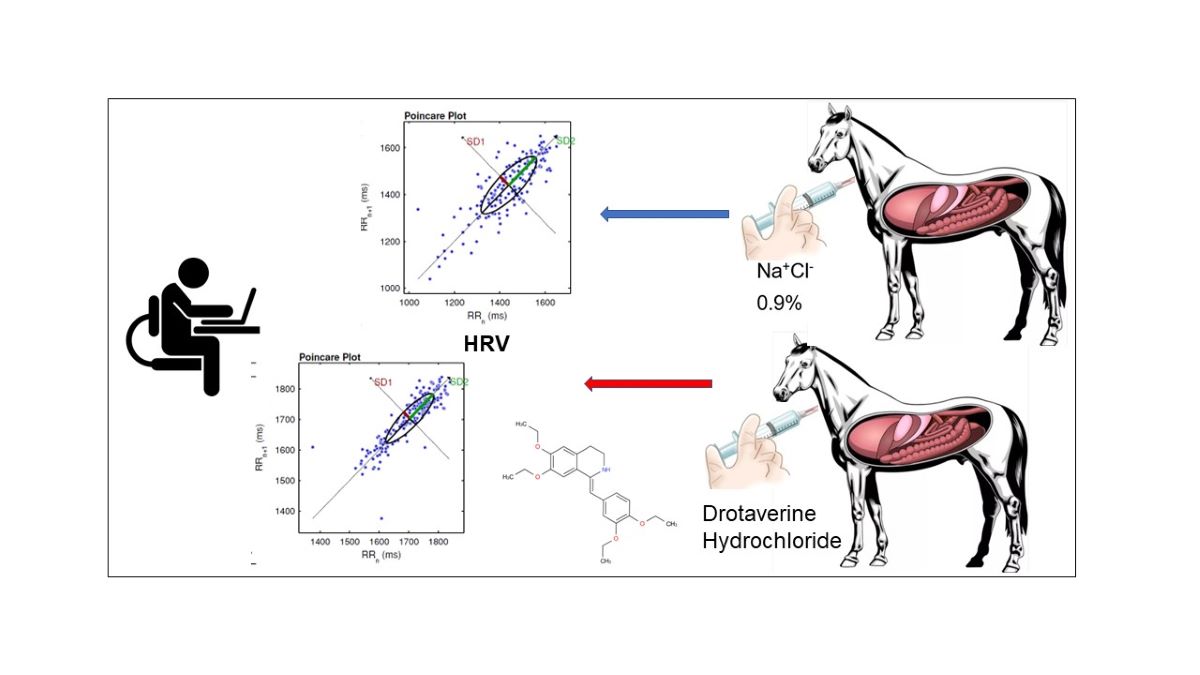
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bolaji, O.O.; Onyeji, E.O.; Ogundaini, A.O.; Olugbade, T.A.; Ogunbona, F.A. Pharmacokinetics and bioavailability of drotaverine in humans. Eur. J. Drug Metab. Pharmacokinet. 1996, 21, 217–221. [Google Scholar] [CrossRef]
- Pap, A.; Topa, L.; Balgha, V.; Kovats-Megyesi, A.; Pozsar, J.; Szikszai, E. Drotaverine antagonizes spasm of Oddi’s sphincter provoked by morphine in man. Gastroenterology 1997, 112, A519. [Google Scholar]
- Hoting, E.; Reiss, J.; Schulz, K.H. Papaverin--wirksam in der Therapie des Pruritus bei Dermatitis atopica? [Papaverin--effective in therapy of pruritus of atopic dermatitis?]. Z Hautkr. 1990, 65, 725–729. [Google Scholar]
- Romics, I.; Molnár, D.L.; Timberg, G.; Mrklic, B.; Jelakovic, B.; Köszegi, G.; Blasko, G. The effect of drotaverine hydrochloride in acute colicky pain caused by renal and ureteric stones. BJU Int. 2003, 92, 92–96. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, M.C. Development and validation of new analytical methods for simultaneous estimation of Drotaverine hydrochloride in combination with Omeprazole in a pharmaceutical dosage form. Arab. J. Chem. 2012, 10, S397–S403. [Google Scholar] [CrossRef]
- Willenbucher, R.F.; Xie, Y.N.; Eysselein, V.E.; Snape, W. JJr. Mechanisms of cAMP-mediated relaxation of distal circular muscle in rabbit colon. Am J Physiol. 1992, 262, G159–64. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Lin, G.; Xin, Z.C.; Lue, T.F. Expression, distribution and regulation of phosphodiesterase 5. Curr. Pharm. 2006, 12, 3439–3457. [Google Scholar] [CrossRef]
- Kusakari, Y.; Hongo, K.; Kawai, M.; Konishi, M.; Kurihara, S. Use of the Ca-shortening curve to estimate the myofilament responsiveness to Ca2+ in tetanized rat ventricular myocytes. J. Physiol. Sci. 2006, 56, 219–226. [Google Scholar] [CrossRef]
- Takashi, O.; Masatoshi, H.; Hiroshi, O. Mechanism of abnormal intestinal motility in inflammatory bowel disease: how smooth muscle contraction is reduced? Smooth Muscle Res. 2007, 43, 43–54. [Google Scholar] [CrossRef]
- Kristev, A.D.; Sirakov, N.V.; Getova, D.P.; Katcarov, V.I.; Sirakov, V.N.; Stefanov, R.S.; Turiiski, V.I.; Velkova, K.G. Comparing hyoscine and drotaverine effects on colon in CT colonography. Cent. Eur. J. Med. 2011, 6, 234–242. [Google Scholar] [CrossRef]
- Eder, P.; Kowalski, P.; Mastalerz-Migas, A.; Skrzydlo-Radomanska, B.; Cichy, W.; Proga, K. Self-Medication with Drotaverine among Patients with Common Abdominal Symptoms and Treatment Efficacy from the Perspectives of Patients and General Practitioners-An Observational, Retrospective, Cross-Sectional Study Using Real-World Data. J. Clin. Med. 2022, 11, 3156. [Google Scholar] [CrossRef]
- Pavel, I.Z.; Heller, L.; Sommerwerk, S.; Loesche, A.; Al-Harrasi, A.; Csuk, R. Drotaverine – a Concealed Cytostatic! Arch Pharm. 2017, 350, 1–e1600289. [Google Scholar] [CrossRef]
- Huang, X.; Xiaokaiti, Y.; Yang, J.; Pan, J.; Li, Z.; Luria, V.; Li, Y.; Song, G.; Zhu, X.; Zhang, HT.; O’Donnell, J.M.; Xu, Y. Inhibition of phosphodiesterase 2 reverses gp91phox oxidase-mediated depression- and anxiety-like behavior. Neuropharmacology 2018, 143, 176–185. [Google Scholar] [CrossRef]
- Debski, R.; Niemiec, T.; Mazurek, M.; Debska, M. Porównanie skuteczności i tolerancji 80 mg drotaweryny i 400 mg ibuprofenu u pacjentek z pierwotnym bolesnym miesiaczkowaniem--badanie DOROTA [Comparative efficacy and tolerability of drotaverine 80 mg and ibuprofen 400 mg in patients with primary dysmenorrhoea--protocol DOROTA]. Ginekol. Pol. 2007, 78, 933–8. [Google Scholar] [PubMed]
- Rzymski, P.; Tomczyk, K.M.; Wilczak, M. The Influence of Oral Drotaverine Administration on Materno–Fetal Circulation during the Second and Third Trimester of Pregnancy. Medicina 2022, 58, 235. [Google Scholar] [CrossRef] [PubMed]
- Dyderski, S.; Grześkowiak, E.; Drobnik, L.; Szałek, E.; Balcerkiewicz, M.; Dubai, V. Bioavailability study of drotaverine from capsule and tablet preparations in healthy volunteers. Arzneimittelforschung 2004, 54, 298–302. [Google Scholar] [CrossRef]
- Tar, A.; Singer, J. A. NO-SPA mellékhatásprofilja [Safety profile of NO-SPA]. Orv Hetil. 2002, 143, 559–62. [Google Scholar] [PubMed]
- Vancea, S.; Gáll, Z.; Donáth-Nagy, G.; Borka-Balás, R. Rapid LC-MS/MS method for determination of drotaverine in a bioequivalence study. J Pharm Biomed Anal. 2014, 98, 417–23. [Google Scholar] [CrossRef] [PubMed]
- Soare, A.-C.; Meltzer, V.; Colbea, C.; Stanculescu, I.; Pincu, E. Compatibility of Drotaverine Hydrochloride with Ibuprofen and Ketoprofen Nonsteroidal Anti-Inflammatory Drugs Mixtures. Materials 2022, 15, 1244. [Google Scholar] [CrossRef] [PubMed]
- Ikeotuonye, A.C.; Umeora, O.J; Nwafor, J.I.; Ojumah, B.O.; Ekwunife, I.C.; Dimejesi, I.B. Drotaverine to shorten the duration of labour in primigravidas: a randomised, double-blind, placebo-controlled trial. Afr Health Sci. 2022, 22, 108–116. [Google Scholar] [CrossRef]
- Dash, A.; Maiti, R.; Akantappa Bandakkanavar, T.K.; Arora, P. Intramuscular drotaverine and diclofenac in acute renal colic: a comparative study of analgesic efficacy and safety. Pain Med. 2012, 13, 466–71. [Google Scholar] [CrossRef]
- Rai, R.R.; Dwivedi, M.; Kumar, N. Efficacy and safety of drotaverine hydrochloride in irritable bowel syndrome: a randomized double-blind placebo-controlled study. Saudi J Gastroenterol. 2014, 20, 378–82. [Google Scholar] [CrossRef]
- Narang, S.; Koli, J. Efficacy and safety of fixed-dose combination of drotaverine hydrochloride (80 mg) and paracetamol (500 mg) in amelioration of abdominal pain in acute infectious gastroenteritis: A randomized controlled trial. J Gastroenterol Hepatol. 2018, 33, 1942–1947. [Google Scholar] [CrossRef] [PubMed]
- Khalif, I.L.; Quigley, E.M.; Makarchuk, P.A.; Golovenko, O.V.; Podmarenkova, L.F.; Dzhanayev, Y.A. Interactions between symptoms and motor and visceral sensory responses of irritable bowel syndrome patients to spasmolytics (antispasmodics). J Gastrointestin Liver Dis. 2009, 18, 17–22. [Google Scholar]
- Xue, X.C.; Qi, X.X.; Wan, X.Y. Randomized controlled study of efficacy and safety of drotaverine hydrochloride in patients with irritable bowel syndrome. Medicine (Baltimore) 2017, 96, e9235. [Google Scholar] [CrossRef]
- Hasan, T.; Azizunnesa, A.; Parvez, M. A.; Hossain, M. A.; Barman, T. R. Left oblique laparotomy for caesarean section in a cow due to dystocia. Asian J. Med. Biol. Res. 2017, 3, 282–289. [Google Scholar] [CrossRef]
- Hasan, T.; Azizunnesa Parvez, M.A.; Paul, P.; Akter, S.; Faruk, M.O.; Hossain, D. Correction and management of vaginal prolapse in a cow by Buhner’stechnique. Res. J. Vet. Pract. 2017, 5, 1–4. [Google Scholar] [CrossRef]
- Cristina, R.T. Drotaverine (No-Spa) effectiveness in horse colic therapy. Rev. Rom. Med. Vet. 2003, 121–128. [Google Scholar]
- Zájer, J.; Szentmiklósi, P.; Sebestyén, G.; Kökény, G. Effects of drotaverin and depogen on gastric emptying in beagle dogs. Acta Vet Acad Sci Hung. 1981, 29, 173–82. [Google Scholar] [PubMed]
- Nazir, S.; Anwar, F.; Saleem, U.; Ahmad, B.; Raza, Z.; Sanawar, M.; Rehman, A.U.; Ismail, T. Drotaverine Inhibitor of PDE4: Reverses the Streptozotocin Induced Alzheimer’s Disease in Mice. Neurochem Res. 2021, 46, 1814–1829. [Google Scholar] [CrossRef]
- Proudman, C.J. A two-year, prospective survey of equine colic in general practice. Equine Vet. J. 1992, 24, 90–93. [Google Scholar] [CrossRef]
- Abutarbush, S.M.; Carmalt, J.L.; Shoemaker, R.W. Causes of gastrointestinal colic in horses in western Canada: 604 cases (1992 to 2002). Can. Vet. J. 2005, 46, 800–805. [Google Scholar]
- Sundra, T.M.; Harrison, J.L.; Lester, G.D.; Raidal, S.L.; Philips, J.K. The influence of spasmolytic agents on heart rate variability and gastrointestinal motility in normal horses. Res. Vet. Sci. 2012, 93, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Porges, S.W. The polyvagal perspective. Biol Psychol. 2007, 74, 116–43. [Google Scholar] [CrossRef] [PubMed]
- Rietmann, T.; Stuart, A.; Bernasconi, P.; Stauffacher, M. Assessment of mental stress in warmblood horses: heart rate variability in comparison to heart rate and selected behavioural parameters. Appl Anim Behav Sci. 2004, 88, 121–136. [Google Scholar] [CrossRef]
- Rietmann, T.R.; Stau_acher, M.; Bernasconi, P.; Auer, J.A.; Weishaupt, M.A. The association between heart rate, heart rate variability, endocrine and behavioural pain measures in horses suffering from laminitis. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2004, 51, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Von Borell, E.; Langbein, J.; Després, G.; Hansen, S.; Leterrier, C.; Marchant-Forde, J.; Marchant-Forde, R.; Minero, M.; Mohr, E.; Prunier, A.; Valance, D.; Veissier, I. Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals — A review. Physiol. Behav. 2007, 92, 293–316. [Google Scholar] [CrossRef]
- Bowen, I.M. Ambulatory electrocardiography and heart rate variability. In Cardiology of the Horse; Marr, C.M., Ed.; Saunders Elsevier: Edinburgh, UK, 2010; pp. 127–137. [Google Scholar]
- Ohmura, H.; Hiraga, A.; Aida, H.; Kuwahara, M.; Tsubone, H.; Jones, J.H. Changes in heart rate and heart rate variability in Thoroughbreds during prolonged road transportation. Am. J. Vet. Res. 2006, 67, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Nagy, K.; Bodó, G.; Bárdos, G.; Harnos, A.; Kabai, P. The effect of a feeding stress-test on the behaviour and heart rate variability of control and crib-biting horses (with or without inhibition). Appl. Anim. Behav. Sci. 2009, 121, 140–147. [Google Scholar] [CrossRef]
- Becker-Birck, M.; Schmidt, A.; Lasarzik, J.; Aurich, J.; Mostl, E.; Aurich, C. Cortisol release and heart rate variability in sport horses participating in equestrian competitions. J. Vet. Behav. 2013, 8, 87–94. [Google Scholar] [CrossRef]
- Gehlen, H.; Faust, M.D.; Grzeskowiak, R.M.; Trachsel, D.S. Association Between Disease Severity, Heart Rate Variability (HRV) and Serum Cortisol Concentrations in Horses with Acute Abdominal Pain. Animals 2020, 10, 1563. [Google Scholar] [CrossRef]
- Gehlen, H.; Loschelder, J.; Merle, R.; Walther, M. Evaluation of Stress Response under a Standard Euthanasia Protocol in Horses Using Analysis of Heart Rate Variability. Animals, 2020, 10, 485. [Google Scholar] [CrossRef] [PubMed]
- Perkins, J.D.; Bowen, I.M.; Else, R.W.; Marr, C.M.; Mayhew, I.G. Functional and histopathological evidence of cardiac parasympathetic dysautonomia in equine grass sickness. Vet. Rec. 2000, 146, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Oel, C.; Gerhards, H.; Gehlen, H. Effect of retrobulbar nerve block on heart rate variability during enucleation in horses under general anesthesia. Vet. Ophthalmol. 2014, 17, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology and North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation, 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Stucke, D.; Grosse Ruse, M.; Lebelt, D. Measuring heart rate variability in horses to investigate the autonomic nervous system activity- Pros and cons of different methods. Appl. Anim. Behav. Sci. 2015, 166, 1–10. [Google Scholar] [CrossRef]
- Constable, P.D.; Hinchcliff, K.W.; Done, S.H.; Grünberg, W. Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs, and Goats, 11th ed.; Elsevier: St. Louis, MO, USA, 2017; Volume 1, pp. 657–715. [Google Scholar]
- Demir, E.T.; Erbaş, M. Investigation of proarrhythmic effect of high sugammadex doses: an experimental animal study. J Anesth Analg Crit Care 2022, 2, 53. [Google Scholar] [CrossRef] [PubMed]
- Kenchaiwong, W.; Sangpo, P.; Kusol, A.; Pontaema, T.; Lerdweeraphon, W. The position of ground electrode affects electrocardiographic parameters in horses. Veterinary World 2022, 15, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Tarvainen, M.P. Kubios HRV Version 2.2 User s Guide. Biosignal Analysis and Medical Imaging Group, Department of Applied Physics, University of Eastern Finland, Kuopio, Finland. 2014.
- Schmidt, A.; Aurich, J.; Möstl, E.; Müller, J.; Aurich, C. Changes in cortisol release and heart rate and heart rate variability during the initial training of 3-year-old sport horses. Horm. Behav. 2010, 58, 628–636. [Google Scholar] [CrossRef]
- Tarvainen, M.P.; Ranta-Aho, P.O.; Karjalainen, P.A. An advanced detrending method with application to HRV analysis. IEEE Trans Biomed Eng. 2002, 49, 172–5. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Hahn, A.W.; Sollers, J.J.; van Doornen, L.; Johnson, P.J. Heart rate variability in the horse by ambulatory monitoring. Biomed Sci Instrum. 1997, 33, 482–485. [Google Scholar] [PubMed]
- Gehrke, E.K.; Baldwin, A.; Schiltz, P.M. Heart Rate Variability in Horses Engaged in Equine-Assisted Activities. J. Equine Vet. Sci. 2011, 31, 78–84. [Google Scholar] [CrossRef]
- Szabó, C.; Vizesi, Z.; Vincze, A. Heart Rate and Heart Rate Variability of Amateur Show Jumping Horses Competing on Different Levels. Animals 2021, 11, 693. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Murata, A.; Lee, I.; Yamada, H. Evaluation of equine cecal motility by ausculation, ultrasonography and electrointestinography after jejunocecostomy. Res. Vet. Sci. 2008, 84, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Visser, E.K; van Reenen, C.; van der Werf, J.; Schilder, M.; Knaap, J.; Barnveld, A.; Blokhuis, H.J. Heart rate and heart rate variability during a novel object test and a handling test in young horses. Physiol Behav. 2002, 76, 289–96. [Google Scholar] [CrossRef] [PubMed]
- Nagel, C.; Aurich, J.; Aurich, C. Heart rate and heart rate variability in the pregnant mare and its foetus. Reprod Domest Anim. 2011, 46, 990–3. [Google Scholar] [CrossRef] [PubMed]
- Jonckheer-Sheehy, V.S.M.; Vinke, C.M.; Ortolani, A. Validation of a Polar human heart rate monitor for measuring heart rate and heart rate variability in adult dogs under stationary conditions. J. Vet. Behav.: Clin. Appl. Res. 2012, 7, 205–212. [Google Scholar] [CrossRef]
- Parker, M.; Goodwin, D.; Eager, R.A.; Redhead, E.S.; Marlin, D.J. Comparison of Polar heart rate interval data with simultaneously recorded ECG signals in horses. Comp. Exerc. Physiol. 2010, 6, 137e142. [Google Scholar] [CrossRef]
- Ille, N.; von Lewinski, M.; Erber, R.; Wulf, M.; Aurich, J.; Möstl, E.; Aurich, C. Effects of the level of experience of horses and their riders on cortisol release, heart rate and heart rate variability during a jumping course. Anim. Welf. 2013, 22, 457e465. [Google Scholar] [CrossRef]
- Kleiger, R.E.; Stein, P.K.; Bosner, M.S.; Rottman, J.N. Time-domain measurements of heart rate variability. In Heart rate variability; Malik, M., Camm, A.J., Eds.; Futura Publishing: Armonk, New York, 1995. [Google Scholar]
- Fei, L.; Copie, X.; Malik, M.; Camm, A.J. Short and long-term assessment of heart rate variability for risk stratification after acute myocardial infarction. Am. J. Cardiol. 1996, 77, 681–4. [Google Scholar] [CrossRef] [PubMed]
- Von Lewinski, M.; Biau, S.; Erber, R.; Ille, N.; Aurich, J.; Faure, J.M.; Möstl, E.; Aurich, C. Cortisol release, heart rate and heart rate variability in the horse and its rider: Different responses to training and performance. Vet. J. 2013, 197, 229e232. [Google Scholar] [CrossRef] [PubMed]
- Nagel, C.; Aurich, J.; Aurich, C. Determination of heart rate and heart rate variability in the equine fetus by fetomaternal electrocardiography. Theriogenology 2010, 73, 973e983. [Google Scholar] [CrossRef] [PubMed]
- Nagel, C.; Erber, R.; Bergmaier, C.; Wulf, M.; Aurich, J. , Möstl, E.; Aurich, C. Cortisol and progestin release, heart rate and heart rate variability in the pregnant and postpartum mare, fetus and newborn foal. Theriogenology 2012, 78, 759e767. [Google Scholar] [CrossRef]
- Erber, R.; Wulf, M.; Rose-Meierhöfer, S.; Becker-Birck, M.; Möstl, E.; Aurich, J.; Hoffmann, G.; Aurich, C. . Behavioral and physiological responses of young horses to different weaning protocols: A pilot study. Stress, 2012, 15, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Erber, R.; Wulf, M.; Becker-Birck, M.; Kaps, S.; Aurich, J.; Möstl, E.; Aurich, C. . Physiological and behavioural responses of young horses to hot iron branding and microchip implantation. Vet. J. 2012, 191, 171–175. [Google Scholar] [CrossRef]
- Schmidt, A.; Biau, S.; Möstl, E.; Becker-Birck, M.; Morillon, B.; Aurich, J.; Faure, J.M.; Aurich, C. Changes in cortisol release and heart rate variability in sport horses during a long-distance road transport. Domest. Anim. Endocrinol. 2010, 38, 179–189. [Google Scholar] [CrossRef]
- Schmidt, A.; Hödl, E.; Möstl, E.; Aurich, J.; Müller, J.; Aurich, C. Cortisol release, heart rate, and heart rate variability in transport-naive horses during repeated road transport. Domest. Anim. Endocrinol. 2010, 39, 205–213. [Google Scholar] [CrossRef]
- Schmidt, A.; Möstl, E.; Wehnert, C.; Aurich, J.; Müller, J.; Aurich, C. Cortisol release and heart rate variability in horse during road transport. Horm. Behav. 2010, 57, 209–215. [Google Scholar] [CrossRef]
- Munsters, C.C.; de Gooijer, J.W.; van den Broek, J.; van Oldruitenborgh-Oosterbaan, M.M. Heart rate, heart rate variability and behaviour of horses during air transport. Vet Rec. 2013, 172, 15. [Google Scholar] [CrossRef]
- Erber, R.; Wulf, M.; Aurich, J.; Rose-Meierhöfer, S.; Hoffmann, G.; Becker-Birck, M.; Möstl, E.; Aurich, C. Stress response of three-year-old horse mares to changes in husbandry system during initial equestrian training. J. Equine Vet.Sci. 2013, 33, 1088–1094. [Google Scholar] [CrossRef]
- Pollard, J.C.; Littlejohn, R.P. Effects of social isolation and restraint on heart rate and behaviour of alpacas. Appl Anim Behav Sci. 1995, 45, 165–74. [Google Scholar] [CrossRef]
- Weisenberger, M.E.; Krausman, P.R.; Wallace, M.C.; Deyoung, D.W. Maughan, O.E.Z. Effects of simulated jet aircraft noise on heart rate and behavior of desert ungulates. J Wildl Manage. 1996, 60, 52–61. [Google Scholar] [CrossRef]
- Kuwahara, M.; Hashimoto, S.; Ishii, K.; Yagi, Y.; Hada, T.; Hiraga, A.; Kai, M.; Kubo, K.; Oki, H.; Tsubone, H.; Sugano, S. Assessment of autonomic nervous function by power spectral analysis of heart rate variability in the horse. J. Auton. Nerv. Syst. 1996, 60, 43–48. [Google Scholar] [CrossRef]
- Malliani, A. Association of heart rate variability components with physiological regulatory mechanisms. In Heart rate variability; Malik, M., Camm, A.J., Eds.; Futura Publishing: Armonk, New York, 1995. [Google Scholar]
- Houle, M.S.; Billman, G.E. Low-frequency component of the heart rate spectrum: a poor marker of sympathetic activity. Am. J. Physiol. Heart Circ. Physiol. 1999, 276, 215–23. [Google Scholar] [CrossRef]
- Kuwahara, M.; Suzuki, A.; Tsutsumi, H.; Tanigawa, M.; Tsubone, H.; Sugano, S. Power spectral analysis of heart rate variability for assessment of diurnal variation of autonomic nervous activity in miniature swine. Lab. Anim. Sci. 1999, 49, 202–8. [Google Scholar] [PubMed]
- Kanters, J.L.; Hojgaard, M.V.; Agner, E.; Holsteinrathlou, N.H. Short- and long-term variations in non-linear dynamics of heart rate variability. Cardiovasc. Res. 1996, 31, 400–9. [Google Scholar] [CrossRef]
- Signorini, M.G.; Cerutti, S.; Guzzetti, S.; Parola, R. Non-linear dynamics of cardiovascular variability signals. Methods Inf Med. 1994, 33, 81–4. [Google Scholar]
- Yamamoto, Y.; Hughson, R.L. On the fractal nature of heart rate variability in humans—effects of data length and beta-adrenergic blockade. Am J Physiol: Regul, Integr Comp Physiol. 1994, 266, R40–9. [Google Scholar] [CrossRef]
- Mohr, E.; Langbein, J.; Nürnberg, G. Heart rate variability—a noninvasive approach to measure stress in calves and cows. Physiol Behav. 2002, 75, 251–9. [Google Scholar] [CrossRef]
- Hagen, K.; Langbein, J.; Schmied, C.; Lexer, D.; Waiblinger, S. Heart rate variability in dairy cows — influences of breed and milking system. Physiol Behav. 2005, 85, 195–204. [Google Scholar] [CrossRef]
- Ciccone, A.B.; Siedlik, J.A.; Wecht, J.M.; Deckert, J.A.; Nguyen, N.D.; Weir, J.P. Reminder: RMSSD and SD1 are identical heart rate variability metrics. Muscle Nerve 2017, 56, 674–678. [Google Scholar] [CrossRef]
- Brennan, M.; Palaniswami, M.; Kamen, P. Poincare plot interpretation using a physiological model of HRV based on a network of oscillators. Am. J. Physiol. -Heart Circ. Physiol. 2002, 283, H1873–H1886. [Google Scholar] [CrossRef]
- Charlet, A.; Rodeau, J.L.; Poisbeau, P. Poincaré plot descriptors of heart rate variability as markers of persistent pain expression in freely moving rats. Physiol Behav. 2011, 104, 694–701. [Google Scholar] [CrossRef]
- Gomaa, N.; Uhlig, A.; Schusser, G.F. Effect of Buscopan compositum on the motility of the duodenum, cecum and left ventral colon in healthy conscious horses. Berliner und Münchener tierärztliche Wochenschrift 2011, 124, 168–174. [Google Scholar]
- Roelvink, M.E.; Goossens, L.; Kalsbeek, H.C.; Wensing, T.H. Analgesic and spasmolytic effects of dipyrone, hyosine-n-butylbromide and a combination of the two in ponies. Vet. Rec. 1991, 129, 378–380. [Google Scholar] [CrossRef] [PubMed]
- De Ferrari, G.M.; Mantica, M.; Vanoli, E.; Hull, S.S., Jr.; Schwartz, P.J. Scopolamine increases vagal tone and vagal reflexes in patients after myocardial infarction. J. Am. Coll. Cardiol. 1993, 22, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Casadei, B.; Pipilis, A.; Sessa, F.; Conway, J.; Sleight, P. Low doses of scopolamine increase cardiac vagal tone in the acute phase of myocardial infarction. Circulation 1993, 88, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Vybiral, T.; Bryg, R.J.; Maddens, M.E.; Bhasin, S.S.; Cronin, S.; Boden, W.E.; Lehmann, M.H. Effects of transdermal scopolamine on heart rate variability in normal subjects. Am. J. Cardiol. 1990, 65, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Amitabh, D.; Rituparna, M.; Bandakkanavar, T.K.A.; Puneet, A. Intramuscular Drotaverine and Diclofenac in Acute Renal Colic: A Comparative Study of Analgesic Efficacy and Safety. Medicine 2012, 13, 466–471. [Google Scholar] [CrossRef]
- Williams, D.C.; Aleman, M.; Holliday, T.A.; Fletcher, D.J.; Tharp, B.; Kass, P.H.; Steffey, E.P.; LeCouteur, R.A. Qualitative and quantitative characteristics of the electroencephalogram in normal horses during spontaneous drowsiness and sleep. J Vet Intern Med. 2008, 22, 630–8. [Google Scholar] [CrossRef]
- Hunter, L.B.; Haskell, M.J.; Langford, F.M.; O’Connor, C.; Webster, J.R.; Stafford, K.J. Heart Rate and Heart Rate Variability Change with Sleep Stage in Dairy Cows. Animals 2021, 11, 2095. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.R.; Rahman, K.; Lloyd, A.; Vollmer-Conna, U. Reduced heart rate variability predicts poor sleep quality in a case–control study of chronic fatigue syndrome. Exp Brain Res. 2010, 204, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Sternberg, E. Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci. 2006, 1088, 361–72. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Yamamoto, S.S.; Brosschot, J.F. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010, 141, 122–31. [Google Scholar] [CrossRef] [PubMed]
- Korte, S.M. Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci. Biobehav. Rev. 2001, 25, 117–142. [Google Scholar] [CrossRef]
- Janczarek, I.; Kedzierski, W.; Wilk, I.; Wnuk–Pawlak, E.; Rakowskac, A. Comparison of daily heart rate variability in old and young horses: A preliminary study. J. Vet. Behav. 2020, 38, 1–7. [Google Scholar] [CrossRef]
- Clement, F.; Barrey, E. Heart rate fluctuations in the horse at rest: (2) Biological variation factors related to behavioural profile. C R Acad. Sci. III 1995, 318, 867–972. [Google Scholar]
- Nagel, C.; Aurich, J.; Palm, F.; Aurich, C. Heart rate and heart rate variability in pregnant warmblood and Shetland mares as well as their fetuses. Anim. Reprod. Sci. 2011, 127, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Konig von Borstel, U.; Euent, S.; Graf, P.; Konig, S.; Gauly, M. Equine behaviour and heart rate in temperament tests with or without rider or handler. Physiol. Behav. 2011, 104, 454–463. [Google Scholar] [CrossRef]
| HRVparameter | Group | Recording Time | |||||
|---|---|---|---|---|---|---|---|
| T0 | T30 | T60 | T90 | T120 | T180 | ||
| RR interval (ms) | S | 1576.05±149.58 | 1606.7±149.52 | 1557.25±121.54 | 1634.25±130.96 | 1514.15±74.32 | 1553.85±125.26 |
| D | 1626.9±163.33 | 1771.55±74.76 | 1824.25±75.31** | 1545.5±37.90 | 1696.1±175.49 | 1594.35±286.95 | |
| SDNN (ms) | S | 152.05±30.72 | 169.55±3.23 | 194.25±11.22 | 166.35±18.78 | 155.25±31.49 | 164.25±44.09 |
| D | 163±34.94 | 113.85±35.43* | 94.75±2.79*** | 179.3±73.72 | 183.45±20.21 | 160.35±5.09 | |
| Heart rate(beats/min) | S | 38.76±3.56 | 38.12±3.57 | 39.425±3.06 | 37.35±2.92 | 40.21±2.2 | 38.35±4.02 |
| D | 37.61±3.65 | 34.08±1.34* | 33.035±1.36** | 39.215±0.60 | 36.23±3.91 | 39.15±7.10 | |
| RMSSD (ms) | S | 64.8±14.67 | 147.95±48.36 | 56.56±12.42 | 68.35±1.25 | 83.86±45.61 | 71.05±3.45 |
| D | 74.9±6.35 | 63.55±24.48* | 45.75±5.31* | 80.55±7.50** | 104.05±26.67* | 131.3±56.52* | |
| LF (ms2) | S | 2574±1191.84 | 5391.5±594.27 | 5477.5±4567.46 | 1363±915.79 | 6734±3191.03 | 2485±1289.34 |
| D | 4729±369.16* | 2811±2751.75* | 342.5±0.54* | 3964.5±1446.53** | 8157±1146.93* | 5339±1940.03* | |
| HF (ms2) | S | 773±281.52 | 5901±3456.13 | 2265±1680.41 | 698.5±164.86 | 5300.5±1644.81 | 880.5±163.76 |
| D | 4161.5±2378.75* | 522±422.84* | 448±283.72* | 1078.5±560.32* | 3758±3040.96* | 1939±1048.34* | |
| LF/HF | S | 3.21±0.37 | 1.34±0.89 | 2.16±0.41 | 3.74±0.56 | 1.55±1.08 | 2.67±0.96 |
| D | 5.10±3.17 | 4.03±2.00** | 1.14±0.72* | 3.99±0.73* | 4.32±3.19* | 4.23±3.29 | |
| SD1 (ms) | S | 44.9±8.43 | 105.05±34.34 | 66.3±19.82 | 48.55±0.82 | 86.75±2.57 | 50.4±2.40 |
| D | 53.2±4.38 | 45.15±17.47* | 36.15±3.88* | 57.15±5.42* | 73.9±18.84* | 93.25±40.03* | |
| SD2 (ms) | S | 209.55±46.39 | 213.35±11.44 | 265.8±21.68 | 229.8±28.59 | 200.3±50.06 | 226.4±64.85 |
| D | 209.55±46.39 | 213.35±11.44 | 265.8±21.68 | 229.8±28.59 | 200.3±50.06 | 226.4±64.85 | |
| GITmotility | S | Normal | Normal | Normal | Normal | Normal | Normal |
| D | Normal | Reduced | Reduced | Reduced | Reduced | Normal | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





