Submitted:
10 June 2023
Posted:
12 June 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Material and Methods
- 1.
- Study design
- 2.
- Patients
- 3.
- Ethics statement
- 4.
- Vascular accesses
- 4.1.
- DualCath Insertion
- The first step consists of local anesthesia in the prethoracic area and at the base of the neck region at the top of the Sedillot triangle.
- The second step consists in locating the right internal jugular vein and inserting the two silastic cannulas into the right internal jugular vein based on a percutaneous-based method using a desilet technique relying on an introducer and a vein dilator.
- The third step consists of tunnelling each cannula with a metallic tunneler advanced in the subcutaneous tissue through the cervical hole downward following the anesthetized track and exiting in the prethoracic area. Cannula is firmly attached to the tunneler with a tressed nylon suture which is then passed through tunnel with cannula. The second cannula is tunneled with the same method in a parallel subcutaneous track.
- The fourth step consists in shortening the cannulas to fit chest patient size with use of space band marks, putting on a silicone rubber collar, and then docking the external extension piece ending by luer-lock connector to the cannula. Cannula and tubing extension assembly are then rigidified by plastic stylet and pushed back with a twisting movement through the enlarged skin exit. Cannulas are then rinsed with saline and clamped with a vascular atraumatic clamp. Anchoring the two cannulas together is then performed with a subcutaneous nylon suture through the jugular neck hole. The two threads emerging from the cervical cutaneous orifice are recovered, isolated, and firmly tied together to create a bridge suture between the two cannulas (stays).
- The fifth step consists in closing skin cutaneous orifice with intradermal sutures, rinsing, locking cannulas with antithrombotic solution and capping them with Luer-lock caps.
- 4.2.
- DualCath Looking and Imaging (Figure 1)
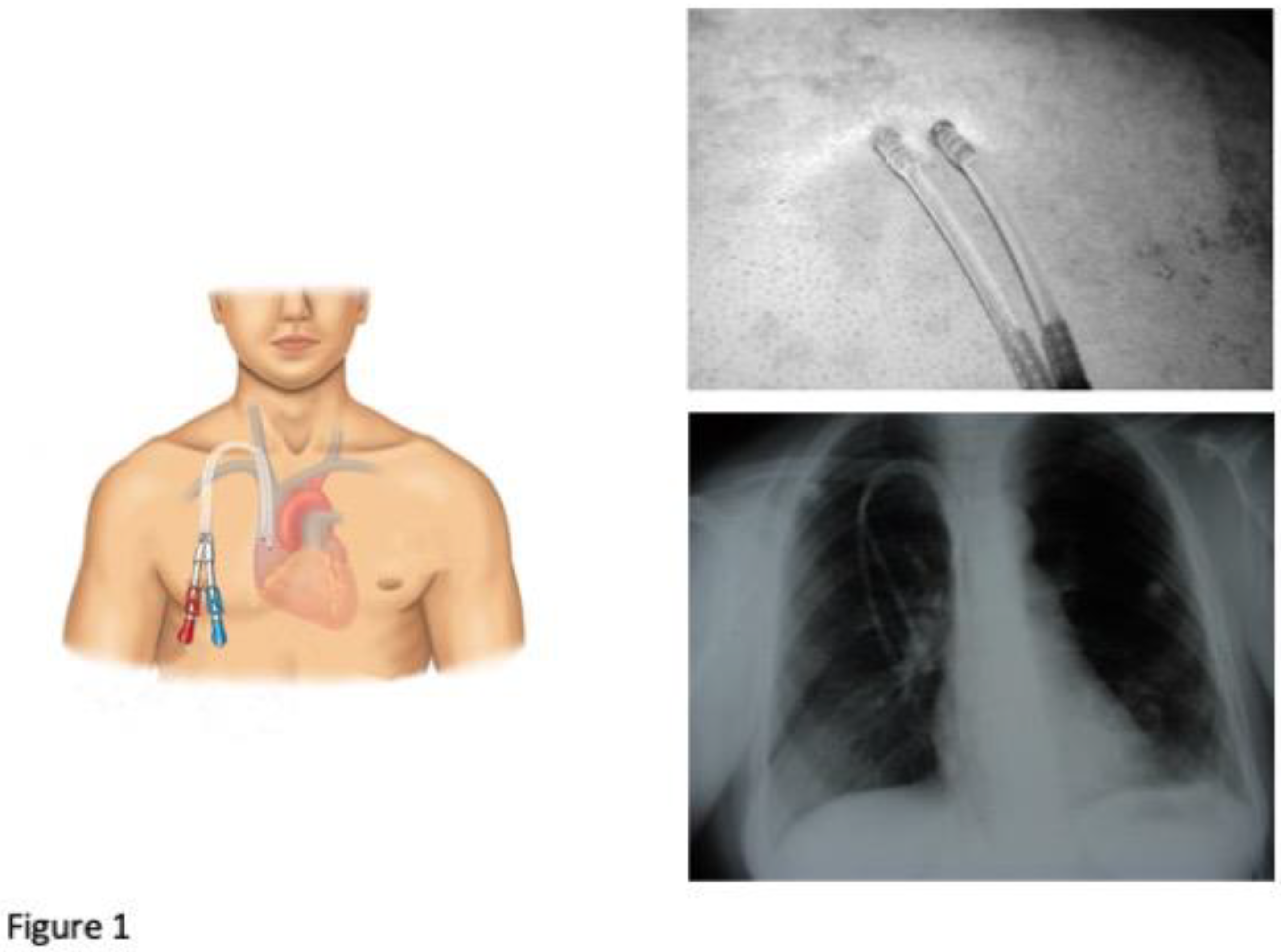
- 4.3.
- DualCath Management and Handling
- 4.4.
- Arteriovenous access management
- 5.
- Clinical performances assessed
- 5.1.
- Blood flow (QB, ml/min)
- 5.2.
- Total blood volume processed (TBVP, L/session)
- 5.3.
- Vascular access recirculation (VA.REC, %)
- Dialysis dose delivery
- 5.4
- Urea Kt/V
- 5.5.
- Ionic dialysance and ocm Kt/V
- 5.6
- Total Kt (TKt, L/session)
- 5.7.
- Total ultrafiltration volume (VUF, L/session)
- 5.8.
- Percent reduction of β2-Microglobulin (PRβ2M)
- 5.9.
- Normalized protein catabolic rate (nPCR)
- 6.
- Statistics
3. Results
3.1. Patient characteristics
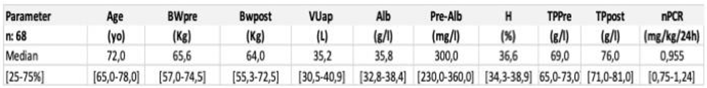
3.2. Renal replacement treatment schedule
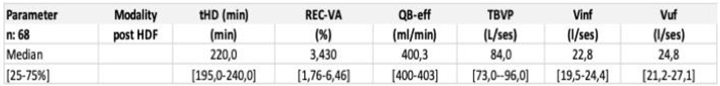
3.3. Baseline clinical performances (3 months)
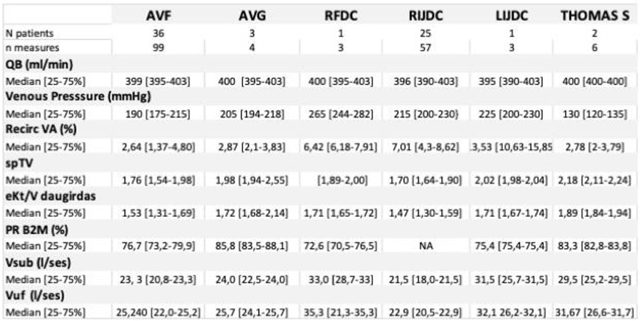
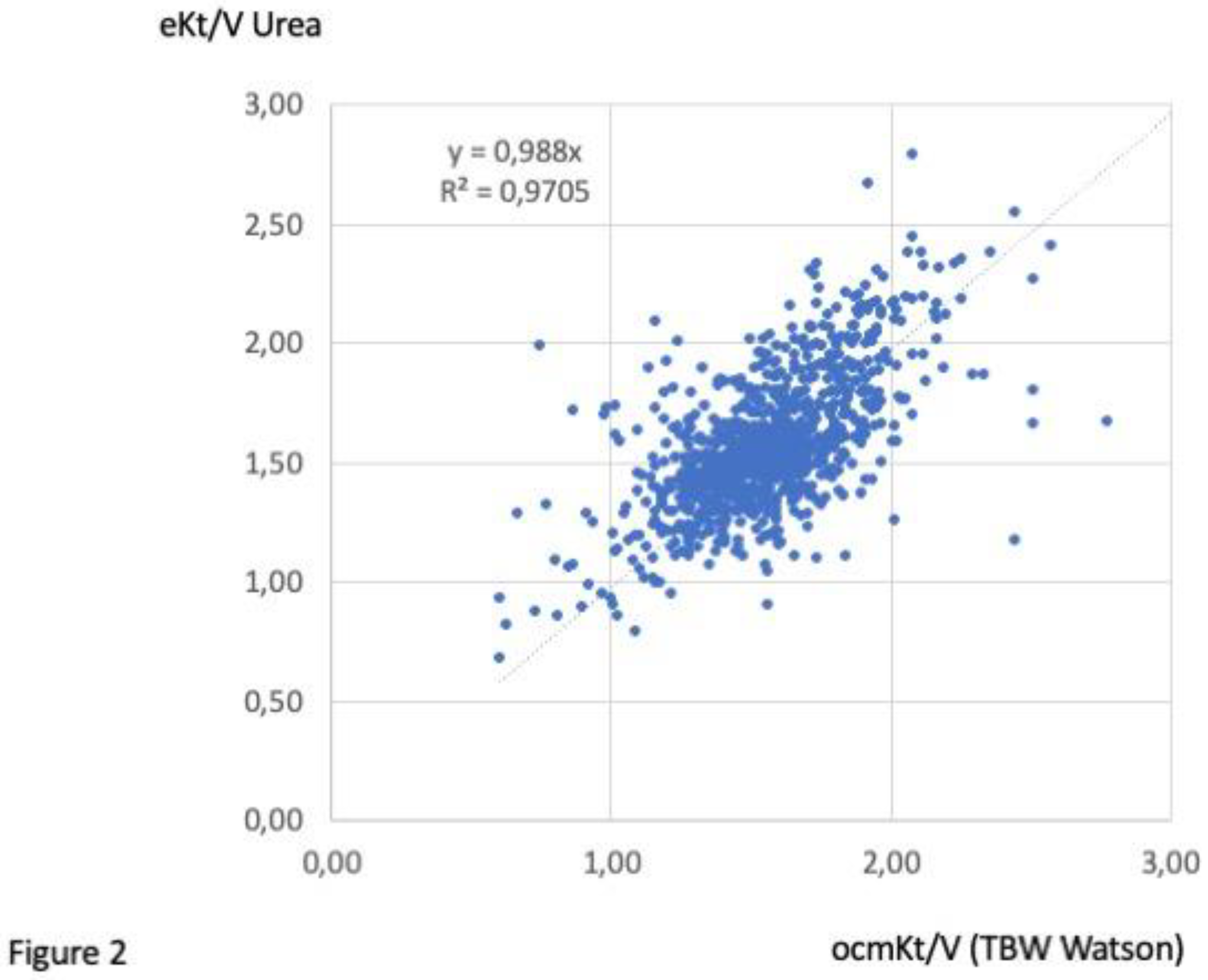
3.4. Clinical performances (over a 30-month follow-up)
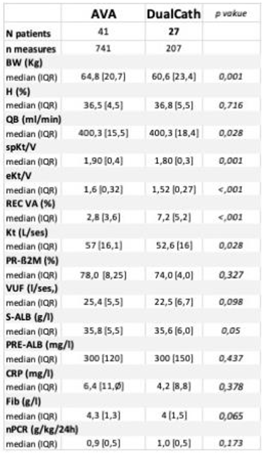
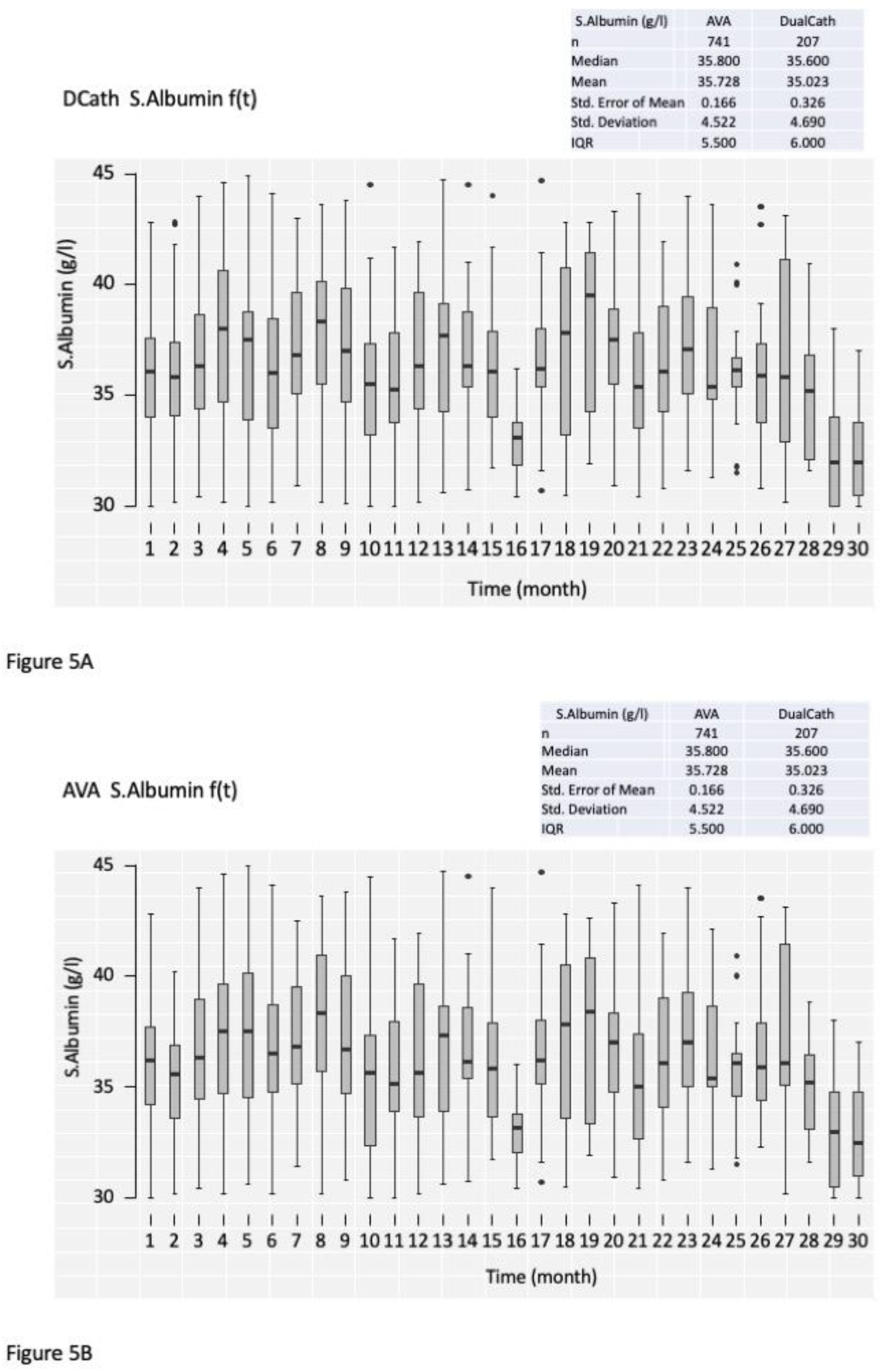
3.4.1. Cumulative clinical performances comparing DualCath (DCath) and grouped Arterio-Venous Accesses (AVA).
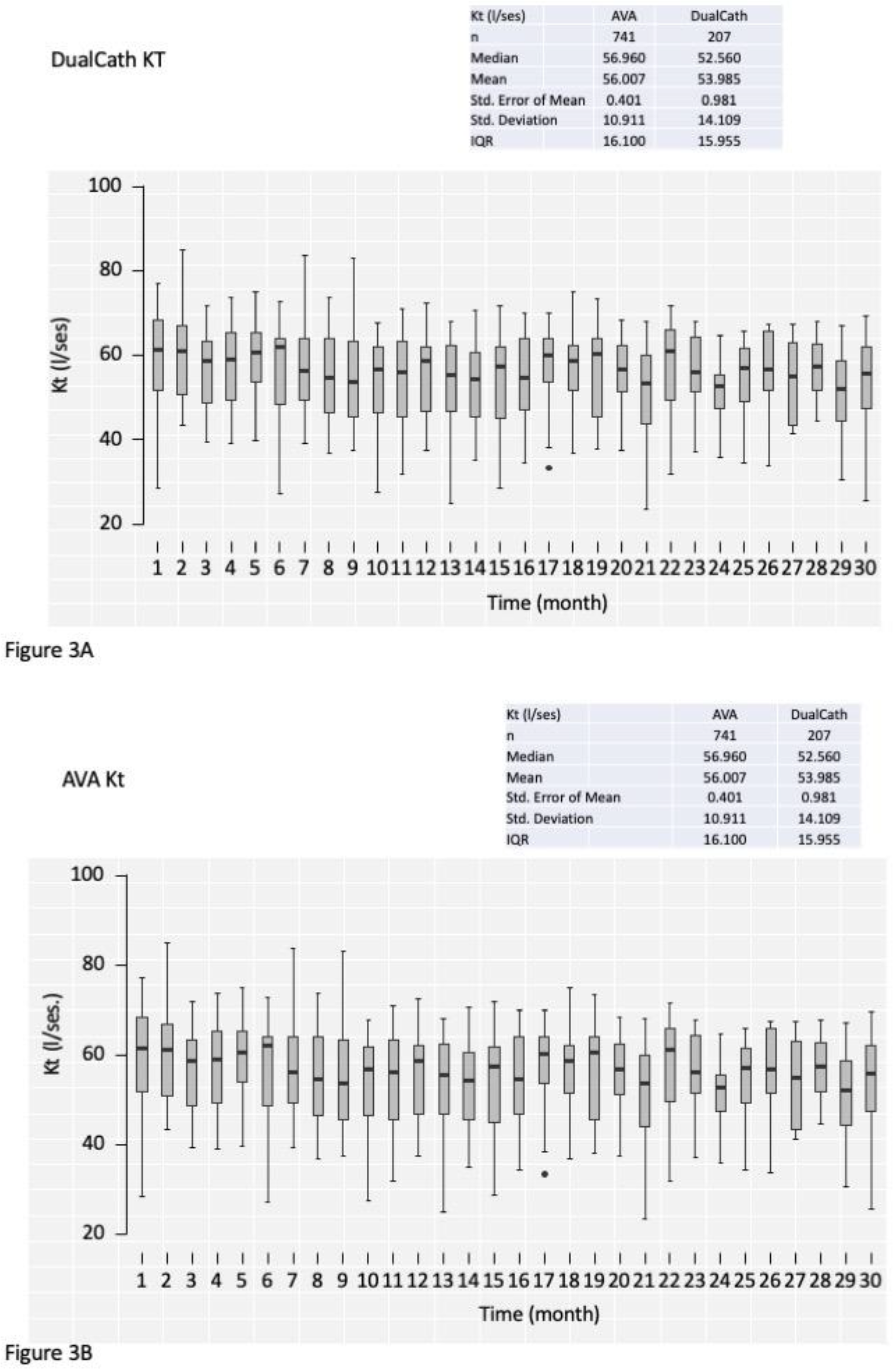
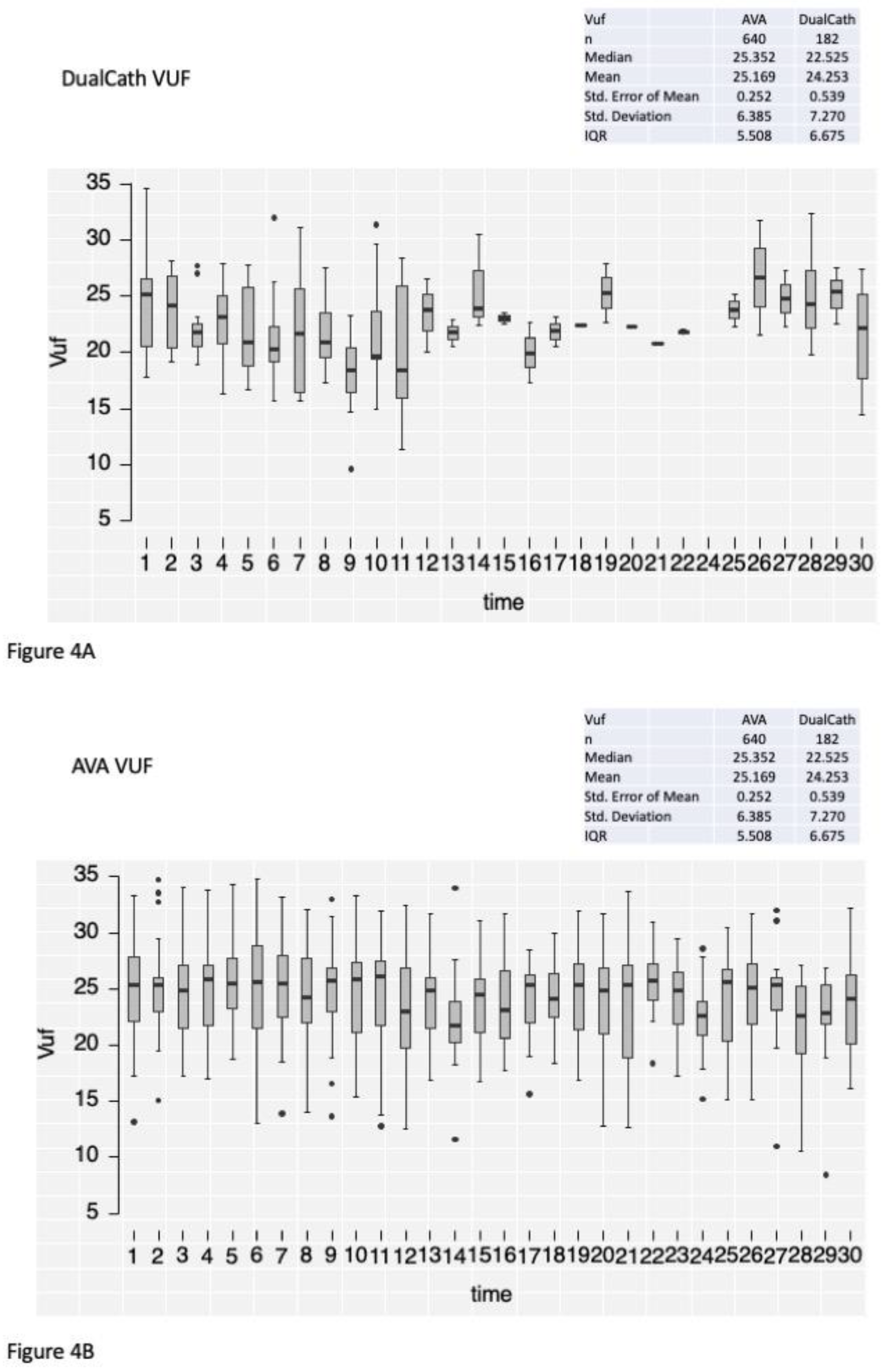
3.4.2. Longitudinal follow-up
4. Discussion
- Main findings of our study:
- Literature comparison:
- Strength and weakness of our study:
- Implications for clinical practices:
Conclusion
References
- Schmidli J, Widmer MK, Basile C, et al. Editor’s Choice - Vascular Access: 2018 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2018;55:757-818. [CrossRef]
- Lok CE, Huber TS, Lee T, et al. KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update. Am J Kidney Dis 2020;75:S1-s164. [CrossRef]
- Tordoir J, Canaud B, Haage P, et al. EBPG on Vascular Access. Nephrol Dial Transplant 2007;22 Suppl 2:ii88-117.
- Ravani P, Palmer SC, Oliver MJ, et al. Associations between hemodialysis access type and clinical outcomes: a systematic review. J Am Soc Nephrol 2013;24:465-73.
- Fissell RB, Fuller DS, Morgenstern H, et al. Hemodialysis patient preference for type of vascular access: variation and predictors across countries in the DOPPS. J Vasc Access 2013;14:264-72. [CrossRef]
- Pisoni RL. Vascular access use and outcomes: results from the DOPPS. Contrib Nephrol 2002:13-9.
- Pisoni RL, Young EW, Dykstra DM, et al. Vascular access use in Europe and the United States: results from the DOPPS. Kidney Int 2002;61:305-16. [CrossRef]
- Rayner HC, Besarab A, Brown WW, Disney A, Saito A, Pisoni RL. Vascular access results from the Dialysis Outcomes and Practice Patterns Study (DOPPS): performance against Kidney Disease Outcomes Quality Initiative (K/DOQI) Clinical Practice Guidelines. Am J Kidney Dis 2004;44:22-6.
- Desmeules S, Canaud B. Venous access for chronic hemodialysis: “undesirable yet unavoidable”. Artif Organs 2004;28:611-6. [CrossRef]
- Di Iorio B. Central Venous Catheters in hemodialysis: an actual conundrum without solutions. J Vasc Access 2002;3:174-6. [CrossRef]
- Sohail MA, Vachharajani TJ, Anvari E. Central Venous Catheters for Hemodialysis-the Myth and the Evidence. Kidney Int Rep 2021;6:2958-68. [CrossRef]
- Ash SR. The evolution and function of central venous catheters for dialysis. Semin Dial 2001;14:416-24.
- Ash SR. Advances in tunneled central venous catheters for dialysis: design and performance. Semin Dial 2008;21:504-15.
- Silverstein DM, Trerotola SO, Clark T, et al. Clinical and Regulatory Considerations for Central Venous Catheters for Hemodialysis. Clin J Am Soc Nephrol 2018;13:1924-32. [CrossRef]
- Tal MG, Peixoto AJ, Crowley ST, Denbow N, Eliseo D, Pollak J. Comparison of side hole versus non side hole high flow hemodialysis catheters. Hemodial Int 2006;10:63-7. [CrossRef]
- Leblanc M, Bosc JY, Paganini EP, Canaud B. Central venous dialysis catheter dysfunction. Adv Ren Replace Ther 1997;4:377-89. [CrossRef]
- McIntyre CW, Hulme LJ, Taal M, Fluck RJ. Locking of tunneled hemodialysis catheters with gentamicin and heparin. Kidney Int 2004;66:801-5. [CrossRef]
- Saxena AK, Panhotra BR. Locking hemodialysis catheters with cefotaxime instead of gentamicin to avoid potential ototoxicity. Kidney Int 2005;67:2505-6. [CrossRef]
- Stas KJ, Vanwalleghem J, De Moor B, Keuleers H. Trisodium citrate 30% vs. heparin 5% as catheter lock in the interdialytic period in twin- or double-lumen dialysis catheters for intermittent haemodialysis. Nephrol Dial Transplant 2001;16:1521-2.
- Polderman KH, Girbes AJ. Central venous catheter use. Part 1: mechanical complications. Intensive Care Med 2002;28:1-17.
- Trerotola SO, Shah H, Johnson M, et al. Randomized comparison of high-flow versus conventional hemodialysis catheters. J Vasc Interv Radiol 1999;10:1032-8. [CrossRef]
- Van Der Meersch H, De Bacquer D, Vandecasteele SJ, et al. Hemodialysis catheter design and catheter performance: a randomized controlled trial. Am J Kidney Dis 2014;64:902-8. [CrossRef]
- Canaud B, Beraud JJ, Joyeux H, Mion C. Internal jugular vein cannulation using 2 silastic catheters. A new, simple and safe long-term vascular access for extracorporeal treatment. Nephron 1986;43:133-8.
- Canaud B, Leray-Moragues H, Garred LJ, Turc-Barron C, Mion C. What is the Role of Permanent Central Vein Access in Hemodialysis Patients. Semin Dial 1996;9:397-400.
- Canaud B, Leray-Moragues H, Garrigues V, Mion C. Permanent twin catheter: a vascular access option of choice for haemodialysis in elderly patients. Nephrol Dial Transplant 1998;13 Suppl 7:82-8.
- Canaud B, Leray-Moragues H, Kerkeni N, Bosc JY, Martin K. Effective flow performances and dialysis doses delivered with permanent catheters: a 24-month comparative study of permanent catheters versus arterio-venous vascular accesses. Nephrol Dial Transplant 2002;17:1286-92.
- FA G. Hemodialysis: Technique and kinetic considerations. in The Kidney, edited by Brenner BM, Rector FC Jr, Philadelphia, WB Saunders Company, 1976 1976;Chapter 41:1673.
- Thomas M, Argiles A, Kerr PG, Canaud B, Flavier JL, Mion CM. Measurement of vascular access recirculation without contralateral venous puncture. Nephron 1992;62:224-5. [CrossRef]
- Leblanc M, Bosc JY, Vaussenat F, Maurice F, Leray-Moragues H, Canaud B. Effective blood flow and recirculation rates in internal jugular vein twin catheters: measurement by ultrasound velocity dilution. Am J Kidney Dis 1998;31:87-92. [CrossRef]
- Aslam S, Saggi SJ, Salifu M, Kossmann RJ. Online measurement of hemodialysis adequacy using effective ionic dialysance of sodium-a review of its principles, applications, benefits, and risks. Hemodial Int 2018;22:425-34.
- Kuhlmann U, Goldau R, Samadi N, et al. Accuracy and safety of online clearance monitoring based on conductivity variation. Nephrol Dial Transplant 2001;16:1053-8. [CrossRef]
- Lowrie EG, Li Z, Ofsthun N, Lazarus JM. The online measurement of hemodialysis dose (Kt): clinical outcome as a function of body surface area. Kidney Int 2005;68:1344-54. [CrossRef]
- Maduell F, Ramos R, Varas J, et al. Hemodialysis patients receiving a greater Kt dose than recommended have reduced mortality and hospitalization risk. Kidney Int 2016;90:1332-41. [CrossRef]
- Tattersall JE, Ward RA. Online haemodiafiltration: definition, dose quantification and safety revisited. Nephrol Dial Transplant 2013;28:542-50. [CrossRef]
- Daugirdas JT. Simplified equations for monitoring Kt/V, PCRn, eKt/V, and ePCRn. Adv Ren Replace Ther 1995;2:295-304. [CrossRef]
- Daugirdas JT, Schneditz D. Overestimation of hemodialysis dose depends on dialysis efficiency by regional blood flow but not by conventional two pool urea kinetic analysis. Asaio j 1995;41:M719-24. [CrossRef]
- Bergström J, Wehle B. No change in corrected beta 2-microglobulin concentration after cuprophane haemodialysis. Lancet 1987;1:628-9.
- Garred LJ, Canaud B, Argiles A, Flavier JL, Mion C. Protein catabolic rate determination from a single measurement of dialyzed urea. Asaio j 1995;41:M804-9. [CrossRef]
- Trerotola SO. Catheters: A Necessary Evil? Journal of Vascular and Interventional Radiology 2004;15:P206-P9.
- Trerotola SO, Kraus M, Shah H, et al. Randomized comparison of split tip versus step tip high-flow hemodialysis catheters. Kidney Int 2002;62:282-9. [CrossRef]
- Lok CE, Huber TS, Lee T, et al. KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update. Am J Kidney Dis 2020;75:S1-S164. [CrossRef]
- Power A, Hill P, Singh SK, Ashby D, Taube D, Duncan N. Comparison of Tesio and LifeCath twin permanent hemodialysis catheters: the VyTes randomized trial. J Vasc Access 2014;15:108-15. [CrossRef]
- Vesely TM. Central venous catheter tip position: a continuing controversy. J Vasc Interv Radiol 2003;14:527-34. [CrossRef]
- El Khudari H, Ozen M, Kowalczyk B, Bassuner J, Almehmi A. Hemodialysis Catheters: Update on Types, Outcomes, Designs and Complications. Semin Intervent Radiol 2022;39:90-102. [CrossRef]
- Lucas TC, Tessarolo F, Jakitsch V, et al. Blood flow in hemodialysis catheters: a numerical simulation and microscopic analysis of in vivo-formed fibrin. Artif Organs 2014;38:556-65. [CrossRef]
- Vanholder R, Canaud B, Fluck R, et al. Diagnosis, prevention and treatment of haemodialysis catheter-related bloodstream infections (CRBSI): a position statement of European Renal Best Practice (ERBP). NDT Plus 2010;3:234-46. [CrossRef]
- Jaffer Y, Selby NM, Taal MW, Fluck RJ, McIntyre CW. A meta-analysis of hemodialysis catheter locking solutions in the prevention of catheter-related infection. Am J Kidney Dis 2008;51:233-41. [CrossRef]
- Weijmer MC, Debets-Ossenkopp YJ, Van De Vondervoort FJ, ter Wee PM. Superior antimicrobial activity of trisodium citrate over heparin for catheter locking. Nephrol Dial Transplant 2002;17:2189-95.
- Weijmer MC, Kars SM, ter Wee PM. A scanning electron microscopy analysis of a spontaneous hemodialysis catheter fracture. Am J Kidney Dis 2001;38:858-61. [CrossRef]
- Weijmer MC, van den Dorpel MA, Van de Ven PJ, et al. Randomized, clinical trial comparison of trisodium citrate 30% and heparin as catheter-locking solution in hemodialysis patients. J Am Soc Nephrol 2005;16:2769-77. [CrossRef]
- Marcelli D, Scholz C, Ponce P, et al. High-volume postdilution hemodiafiltration is a feasible option in routine clinical practice. Artif Organs 2015;39:142-9. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





