Submitted:
10 June 2023
Posted:
12 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
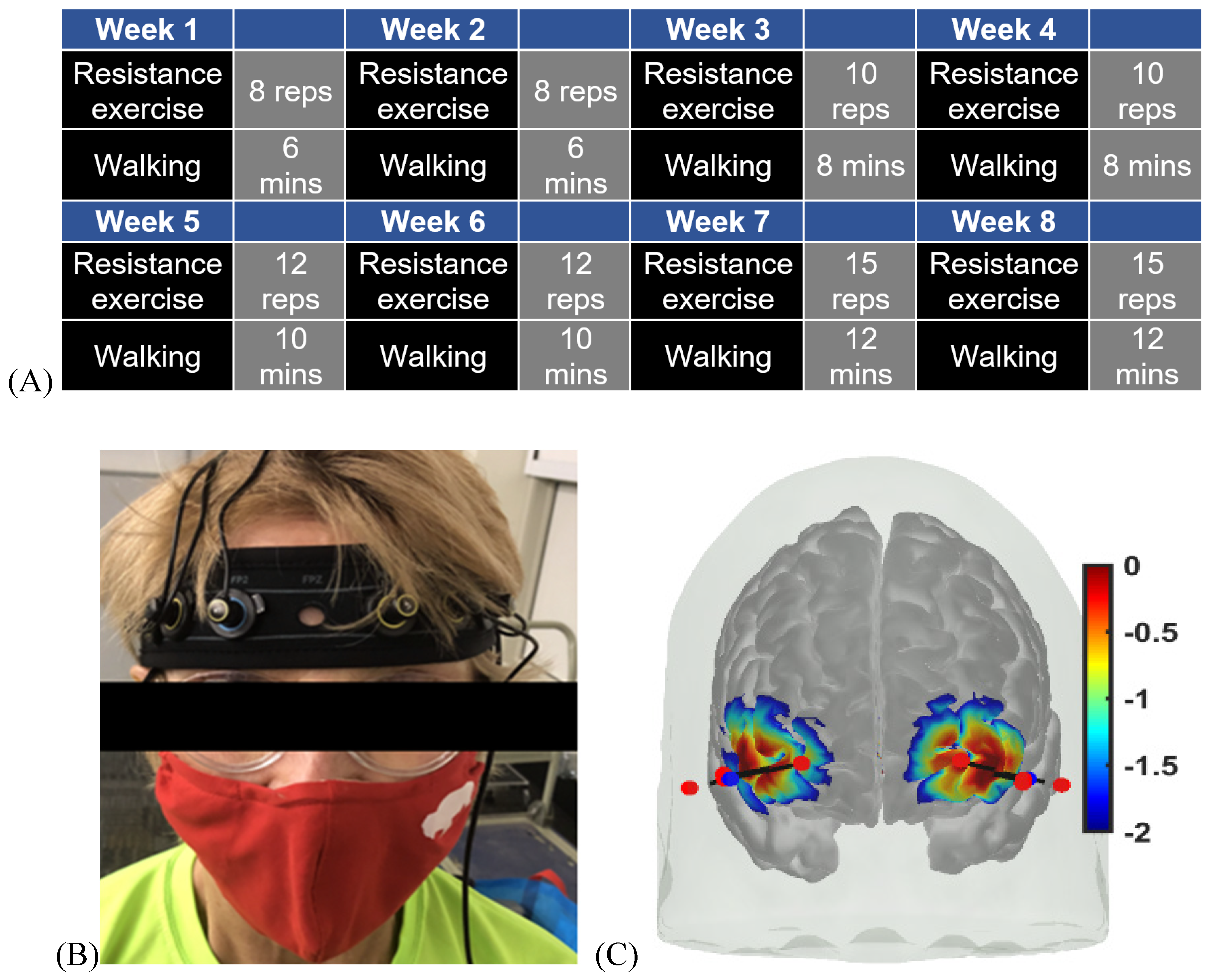
2.1. Study Design
2.2. 2-month Exercise Intervention
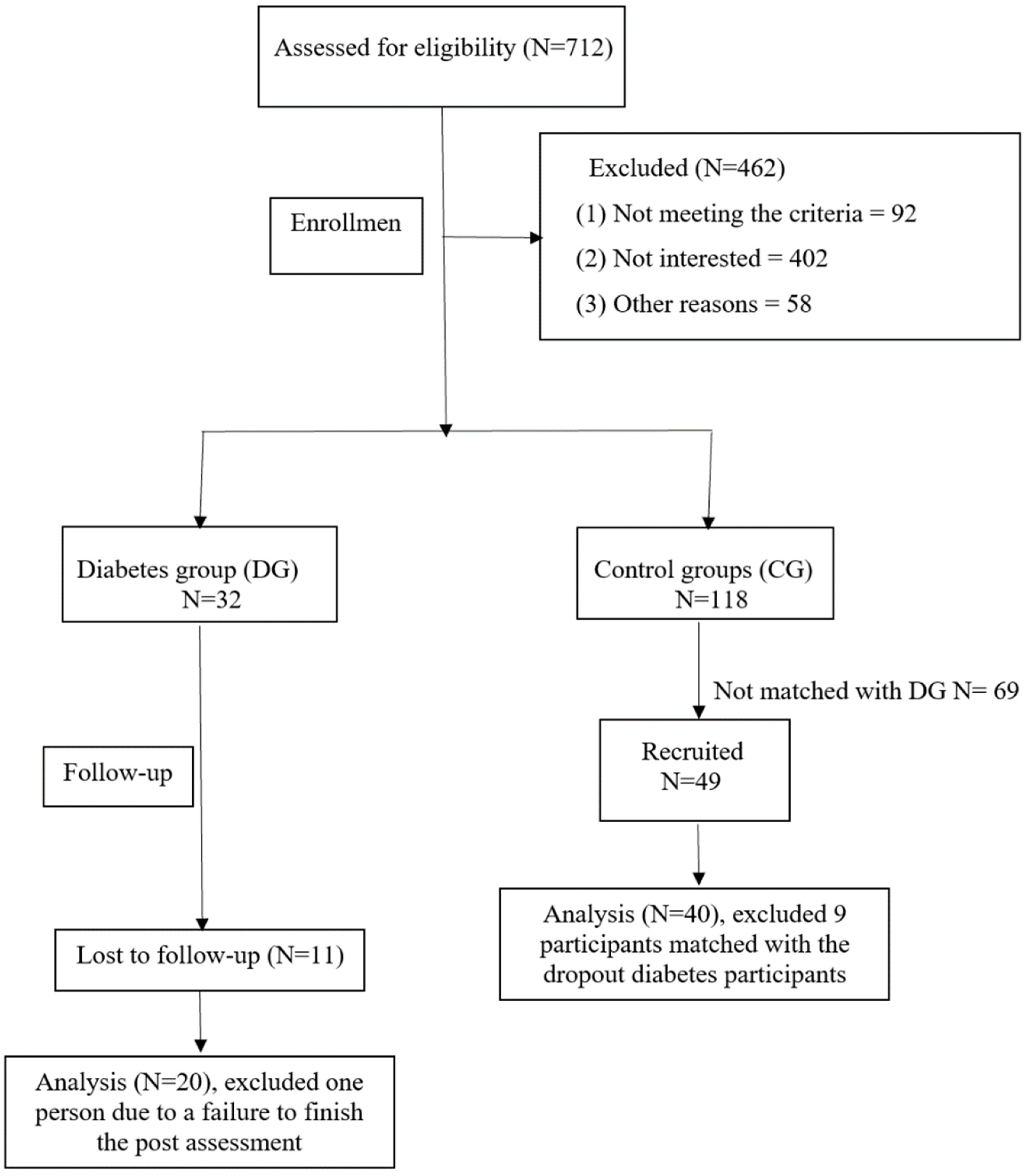
2.3. Sample
| Characteristics | Intervention group at baseline M(SD) |
Sedentary healthy Control group M(SD) |
Active healthy Control group M(SD) |
F or χ2 |
|---|---|---|---|---|
|
Age |
66.1 (4.5) | 66.6 (4.2) |
65.9 (4.2) |
F = .055 (p=.947) |
|
BMI |
34.8 (4.8) |
33.3 (5.3) |
26.1 (4.3)*** |
F = 18.775*** (p<.001) Intervention group at baseline versus Active healthy Control group |
|
Sex Male Female |
50.0% 50.0% |
50.0% 50.0% |
50.0% 50.0% |
χ2 = 1.0 (p=1.000) |
2.3.1. Inclusion and exclusion criteria
2.3.2. Recruitment
| Characteristics | Intervention group at baseline M(SD) |
Control group M(SD) |
Z | Cohen’s d |
|---|---|---|---|---|
| Mini-Cog |
12.79 (2.1) | 14.16 (0.9) | Z=3.273** (p=.0005) |
d = .967 |
| Trail Making Part A (secs) | 39.55 (12.1) | 30.94 (6.8) | Z=2.548** (p=.006) |
d = .972 |
| Trail Making Part B (secs) | 93.45 (26.58) | 69.08 (21.3) | Z=3.293*** (p<.001) |
d = 1.053 |
2.4. Pre- and Post-intervention measurements
2.4.1. Cerebral oxygenation
2.4.2. Muscle oxygenation
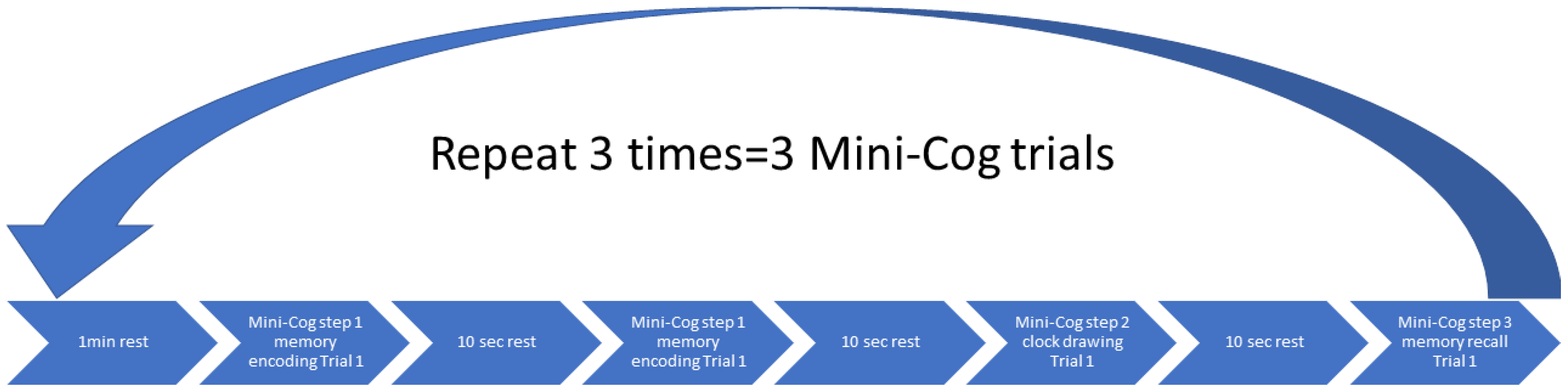
2.4.3. Cognitive and physical function tests
2.5. Protocol
2.6. Statistical analysis
3. Results
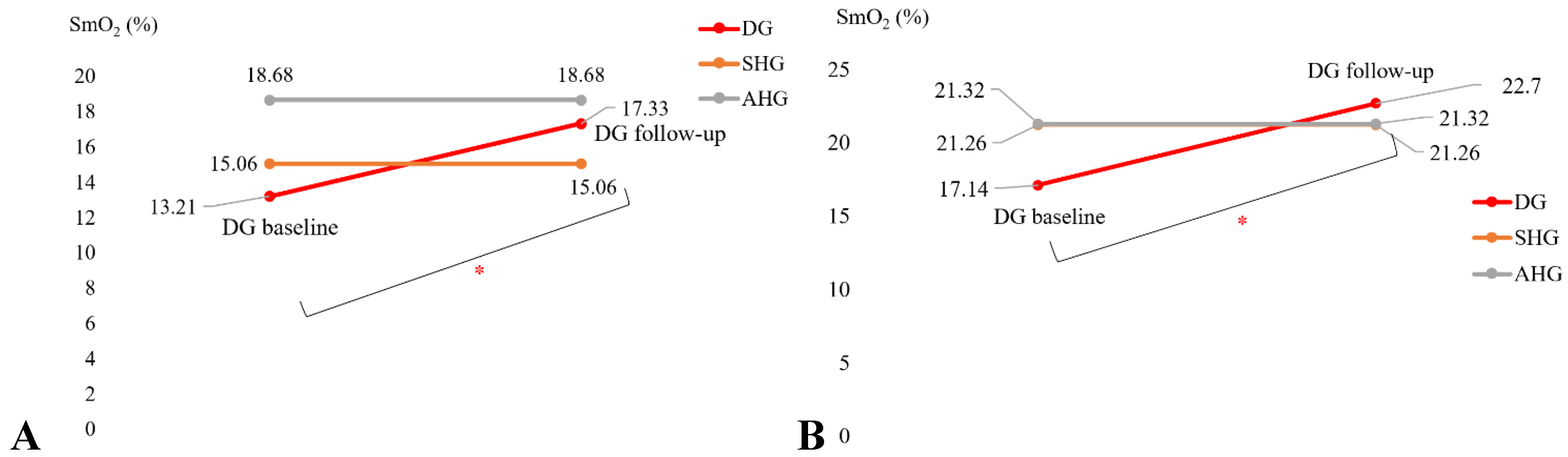
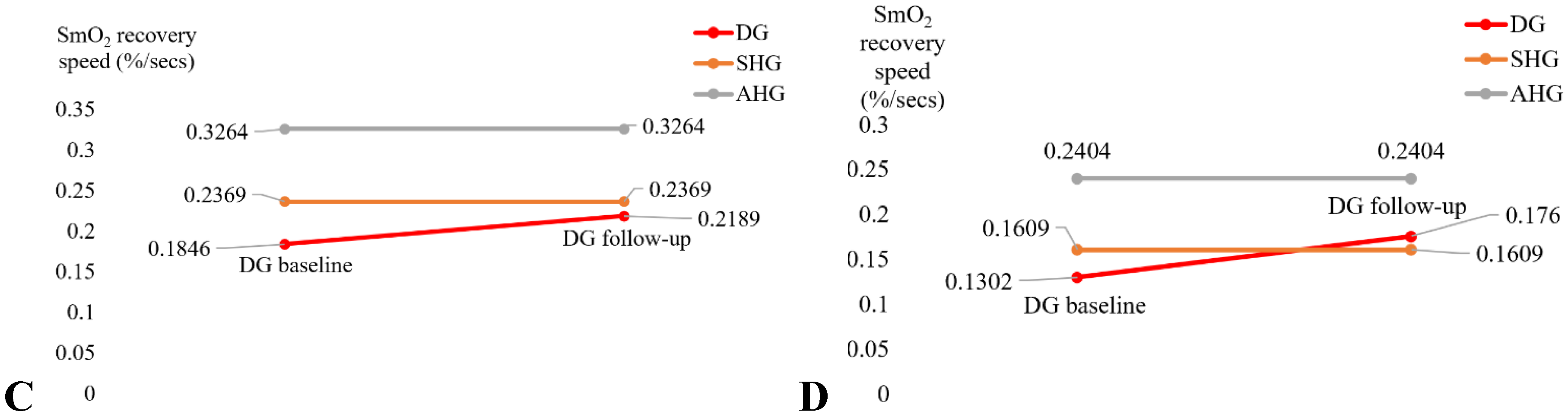
| Characteristics | Baseline M(SD) |
Follow-up M(SD) |
t or Z | Cohen’s d |
| SmO2 drop during BHR test (%) |
13.21 (7.5) | 17.33 (11.6) | t=2.185* (p=.022) |
d = -0.515 |
| SmO2 drop during 6MWT (%) | 17.14 (9.1) | 22.70 (15.4) | t=1.845* (p=.041) |
d = -0.435 |
| BHR SmO2 recovery speed (%/sec) | .1846 (.071) | .2189 (.107) | t=1.714 (p=.052) |
d = -0.404 |
| 6MWT SmO2 recovery speed (%/sec) | .1302(.087) | .1760 (.174) | t=1.094 (p=.145) |
d = -0.258 |
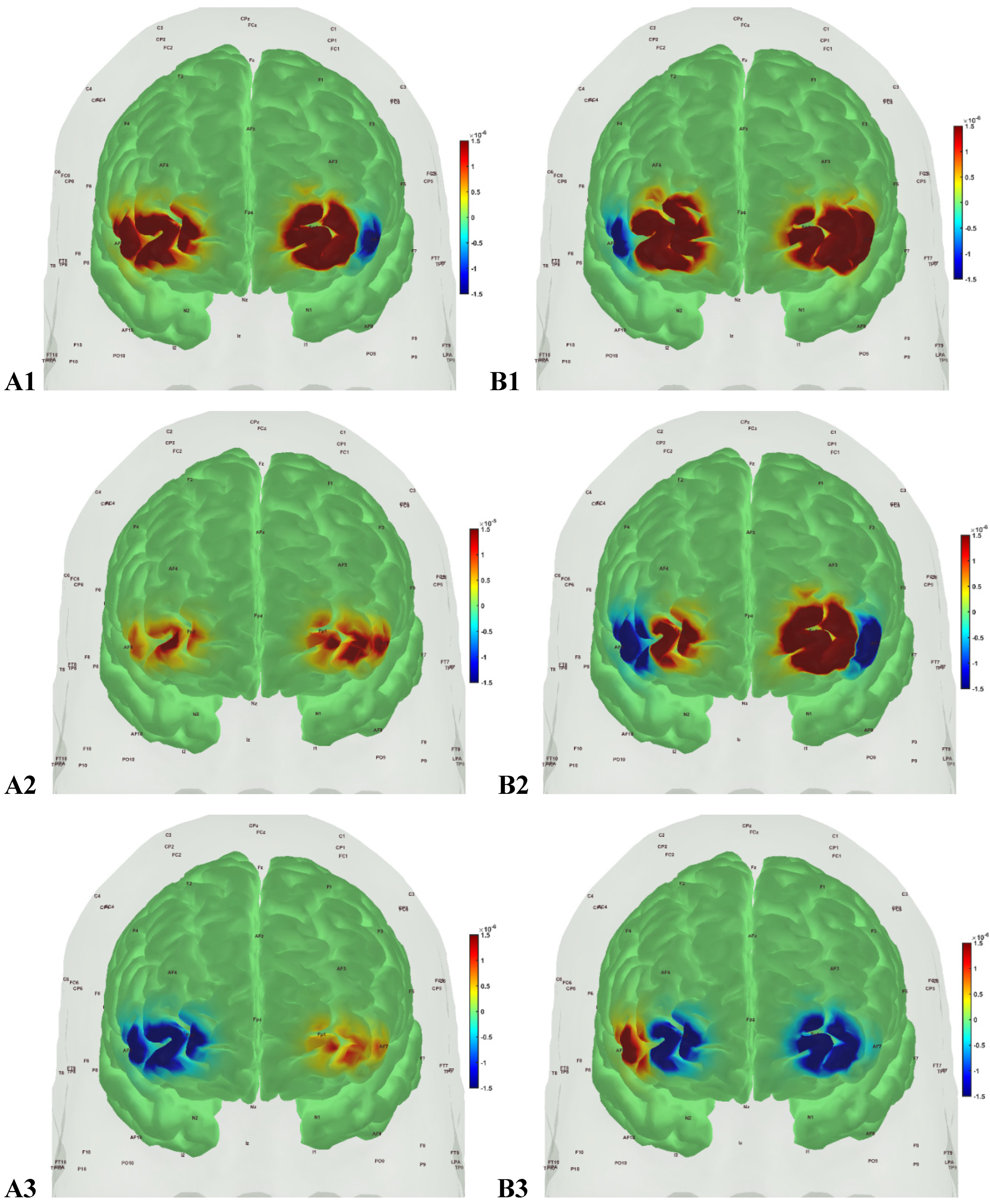
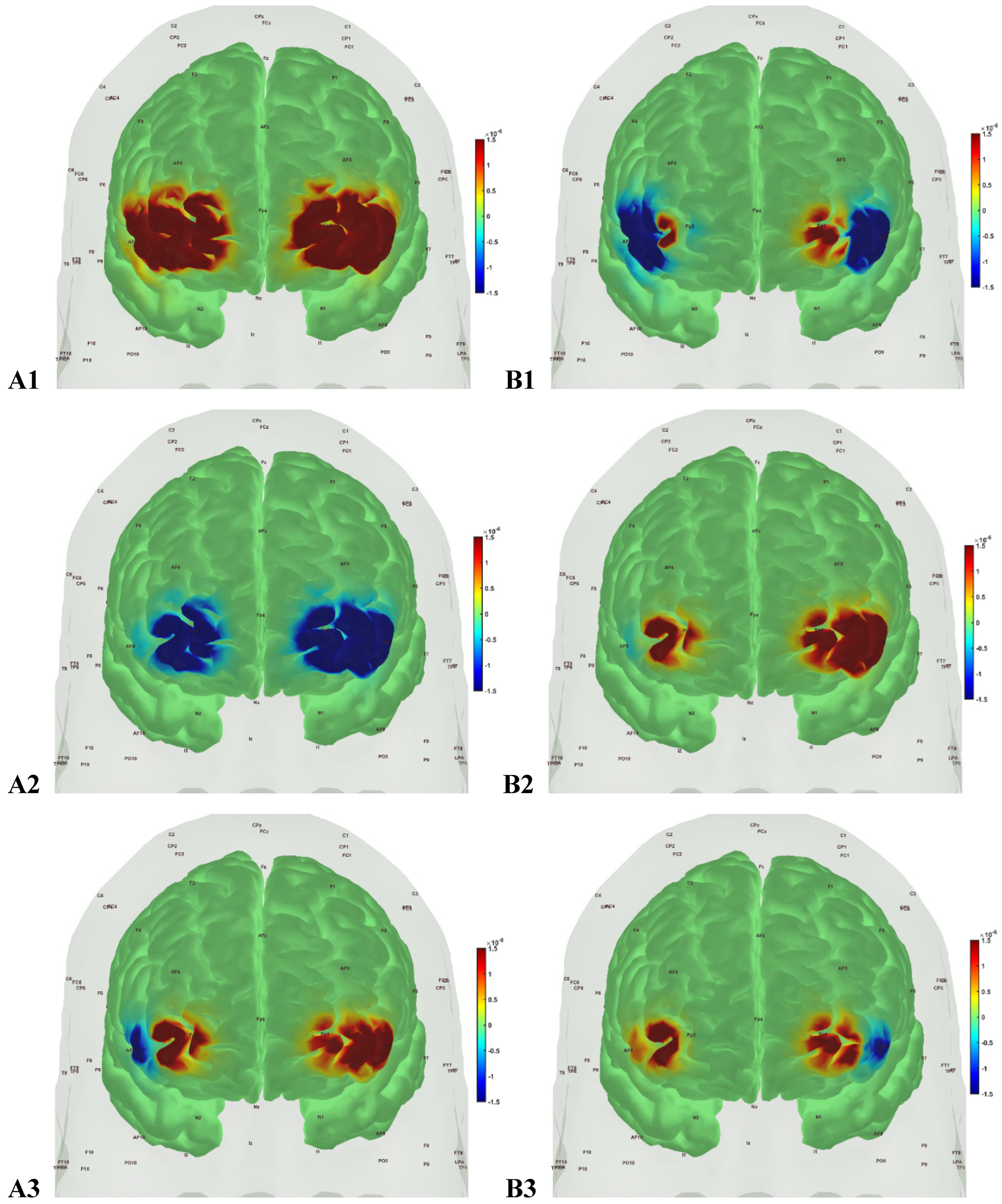
| AAL region | source | detector | type | factor | p | q |
|---|---|---|---|---|---|---|
| Frontal_Inf_Tri_R | 1 | 1 | ’hbo’ | ’cond’ | 0.999999981 | 1 |
| Frontal_Inf_Tri_R | 1 | 1 | ’hbo’ | ’group’ | 0.083678248 | 0.205977227 |
| Frontal_Inf_Tri_R | 1 | 1 | ’hbr’ | ’cond’ | 0.947160368 | 1 |
| Frontal_Inf_Tri_R | 1 | 1 | ’hbr’ | ’group’ | 0.000314435 | 0.001437418 |
| Frontal_Sup_R | 3 | 1 | ’hbo’ | ’cond’ | 0.999987864 | 1 |
| Frontal_Sup_R | 3 | 1 | ’hbo’ | ’group’ | 0.003211619 | 0.012846478 |
| Frontal_Sup_R | 3 | 1 | ’hbr’ | ’cond’ | 1 | 1 |
| Frontal_Sup_R | 3 | 1 | ’hbr’ | ’group’ | 1.97E-10 | 3.15E-09 |
| Frontal_Inf_Tri_L | 5 | 2 | ’hbo’ | ’cond’ | 0.999999039 | 1 |
| Frontal_Inf_Tri_L | 5 | 2 | ’hbo’ | ’group’ | 0.064006828 | 0.170684874 |
| Frontal_Inf_Tri_L | 5 | 2 | ’hbr’ | ’cond’ | 0.994658711 | 1 |
| Frontal_Inf_Tri_L | 5 | 2 | ’hbr’ | ’group’ | 1.82E-05 | 9.70E-05 |
| Frontal_Sup_L | 7 | 2 | ’hbo’ | ’cond’ | 0.375322014 | 0.720721989 |
| Frontal_Sup_L | 7 | 2 | ’hbo’ | ’group’ | 6.15E-07 | 4.92E-06 |
| Frontal_Sup_L | 7 | 2 | ’hbr’ | ’cond’ | 0.999999845 | 1 |
| Frontal_Sup_L | 7 | 2 | ’hbr’ | ’group’ | 3.44E-06 | 2.20E-05 |
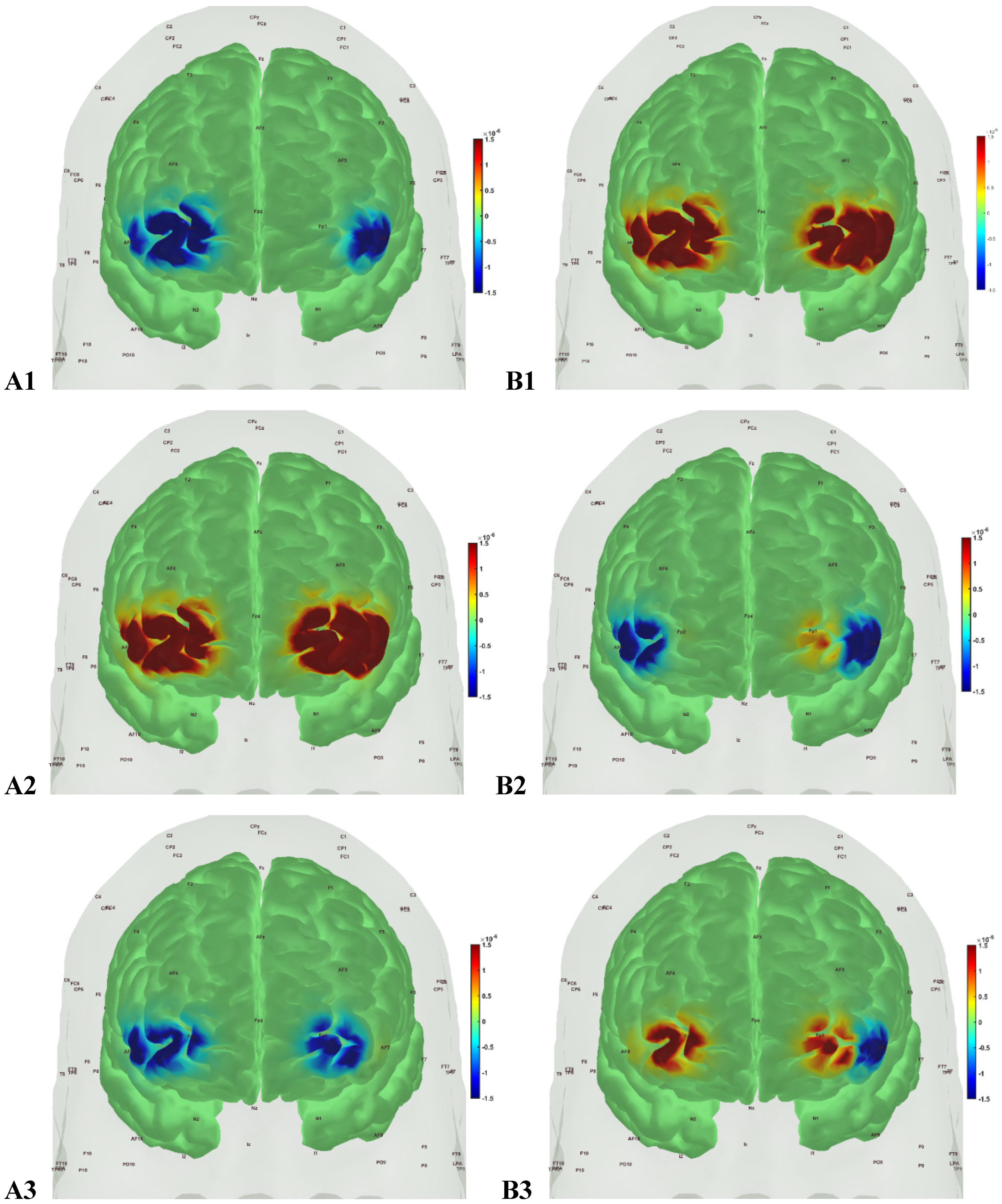
| AAL region | source | detector | type | factor | p | q |
|---|---|---|---|---|---|---|
| Frontal_Inf_Tri_R | 1 | 1 | ’hbo’ | ’cond’ | 0.6481 | 1 |
| Frontal_Inf_Tri_R | 1 | 1 | ’hbo’ | ’group’ | 0.00587 | 0.01879 |
| Frontal_Inf_Tri_R | 1 | 1 | ’hbr’ | ’cond’ | 0.78319 | 1 |
| Frontal_Inf_Tri_R | 1 | 1 | ’hbr’ | ’group’ | 0.62103 | 1 |
| Frontal_Sup_R | 3 | 1 | ’hbo’ | ’cond’ | 0.97924 | 1 |
| Frontal_Sup_R | 3 | 1 | ’hbo’ | ’group’ | 2.39E-06 | 8.50E-06 |
| Frontal_Sup_R | 3 | 1 | ’hbr’ | ’cond’ | 1 | 1 |
| Frontal_Sup_R | 3 | 1 | ’hbr’ | ’group’ | 2.15E-06 | 8.50E-06 |
| Frontal_Inf_Tri_L | 5 | 2 | ’hbo’ | ’cond’ | 1 | 1 |
| Frontal_Inf_Tri_L | 5 | 2 | ’hbo’ | ’group’ | 0.7415 | 1 |
| Frontal_Inf_Tri_L | 5 | 2 | ’hbr’ | ’cond’ | 0.99998 | 1 |
| Frontal_Inf_Tri_L | 5 | 2 | ’hbr’ | ’group’ | 0.56073 | 1 |
| Frontal_Sup_L | 7 | 2 | ’hbo’ | ’cond’ | 1 | 1 |
| Frontal_Sup_L | 7 | 2 | ’hbo’ | ’group’ | 7.59E-08 | 3.47E-07 |
| Frontal_Sup_L | 7 | 2 | ’hbr’ | ’cond’ | 0.99781 | 1 |
| Frontal_Sup_L | 7 | 2 | ’hbr’ | ’group’ | 0.56618 | 1 |
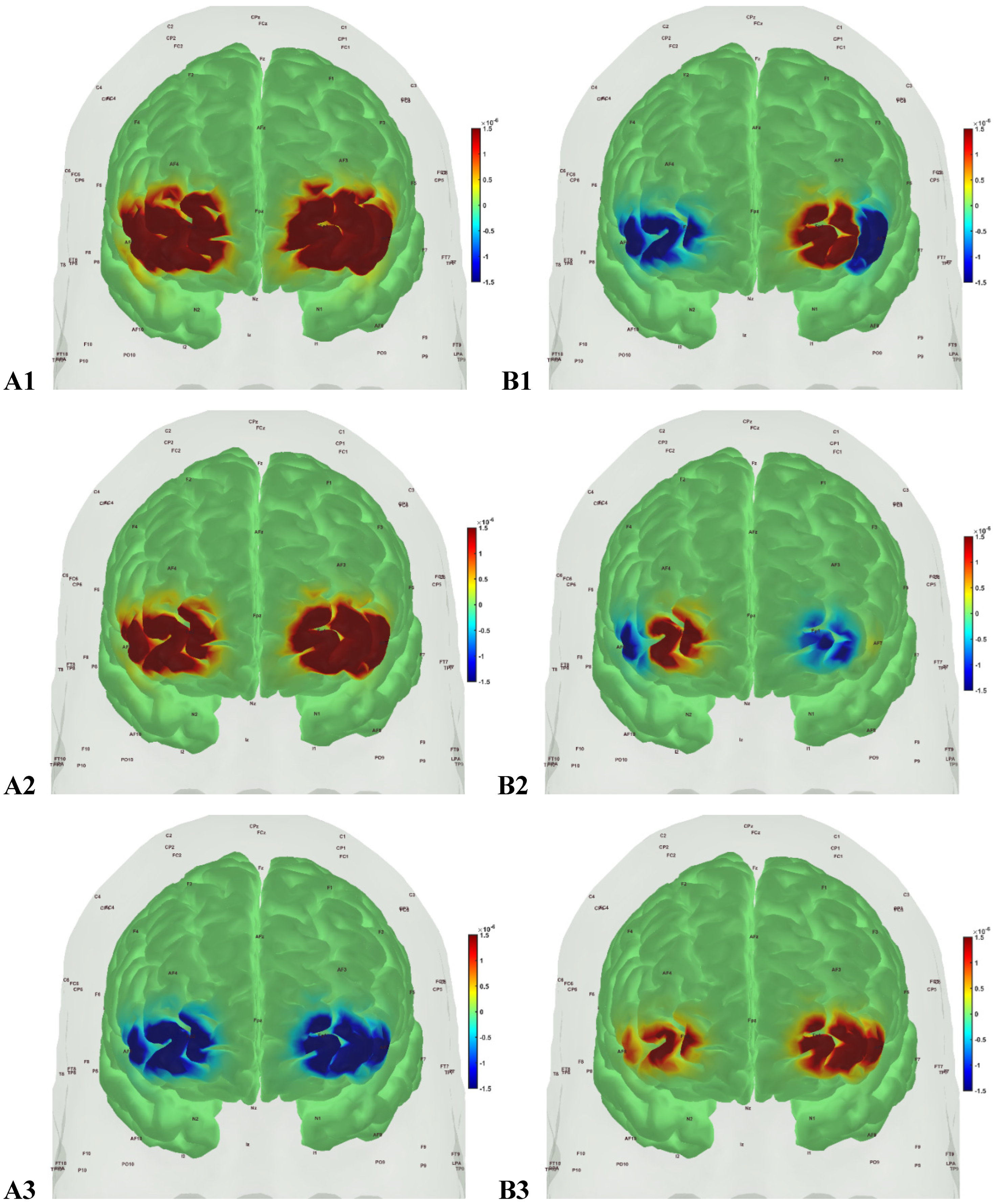
| AAL region | source | detector | type | factor | p | q |
|---|---|---|---|---|---|---|
| Frontal_Inf_Tri_R | 1 | 1 | ’hbo’ | ’cond’ | 0.99999259 | 1 |
| Frontal_Inf_Tri_R | 1 | 1 | ’hbo’ | ’group’ | 0.056004 | 0.17322566 |
| Frontal_Inf_Tri_R | 1 | 1 | ’hbr’ | ’cond’ | 1 | 1 |
| Frontal_Inf_Tri_R | 1 | 1 | ’hbr’ | ’group’ | 0.00543628 | 0.04349027 |
| Frontal_Sup_R | 3 | 1 | ’hbo’ | ’cond’ | 0.99999952 | 1 |
| Frontal_Sup_R | 3 | 1 | ’hbo’ | ’group’ | 0.01244236 | 0.05687937 |
| Frontal_Sup_R | 3 | 1 | ’hbr’ | ’cond’ | 1 | 1 |
| Frontal_Sup_R | 3 | 1 | ’hbr’ | ’group’ | 8.41E-12 | 1.35E-10 |
| Frontal_Inf_Tri_L | 5 | 2 | ’hbo’ | ’cond’ | 0.09426186 | 0.2320292 |
| Frontal_Inf_Tri_L | 5 | 2 | ’hbo’ | ’group’ | 0.06495962 | 0.17322566 |
| Frontal_Inf_Tri_L | 5 | 2 | ’hbr’ | ’cond’ | 0.97629652 | 1 |
| Frontal_Inf_Tri_L | 5 | 2 | ’hbr’ | ’group’ | 0.28616516 | 0.57233033 |
| Frontal_Sup_L | 7 | 2 | ’hbo’ | ’cond’ | 0.01672316 | 0.06689266 |
| Frontal_Sup_L | 7 | 2 | ’hbo’ | ’group’ | 0.96488565 | 1 |
| Frontal_Sup_L | 7 | 2 | ’hbr’ | ’cond’ | 1 | 1 |
| Frontal_Sup_L | 7 | 2 | ’hbr’ | ’group’ | 0.04105912 | 0.14598797 |
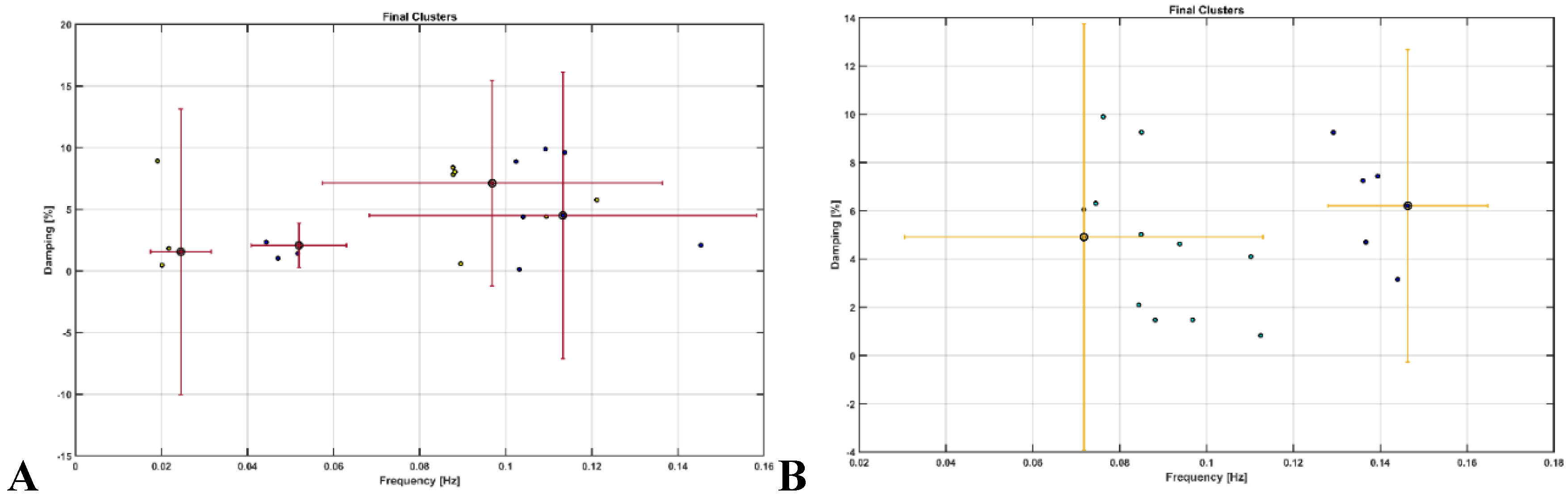
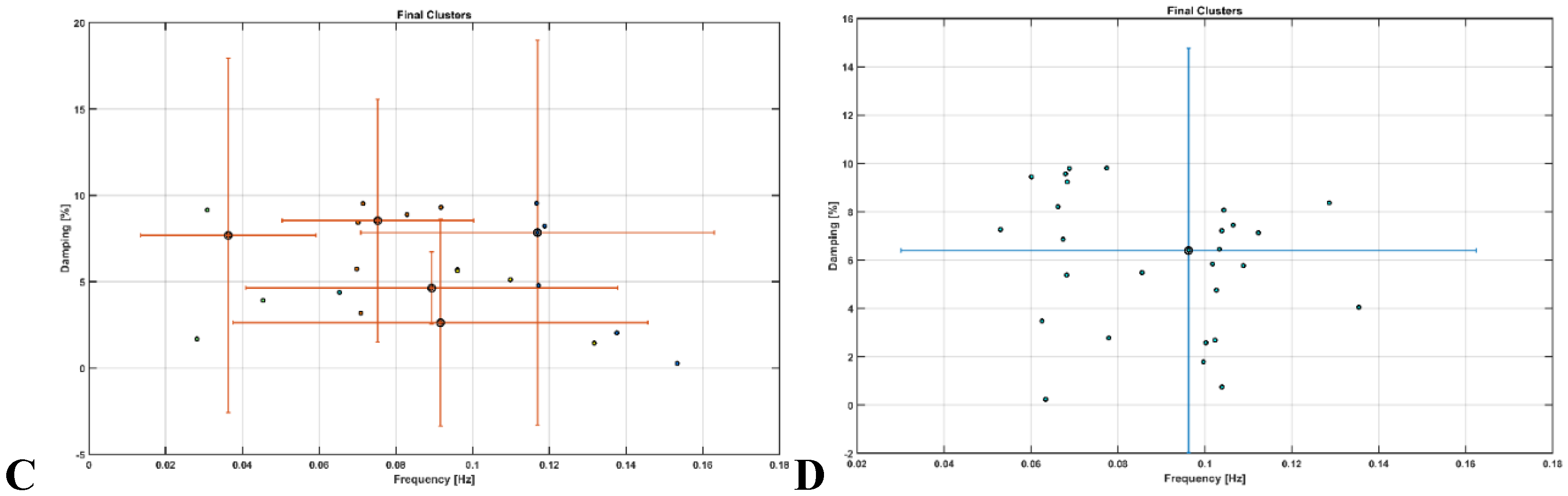
4. Discussion
Supplementary Materials
Funding
Authors’ Contributions
Data Availability Statement
Acknowledgments
Conflict of Interest
References
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. The Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Meisl, G.; Hidari, E.; Allinson, K.; Rittman, T.; DeVos, S.L.; Sanchez, J.S.; Xu, C.K.; Duff, K.E.; Johnson, K.A.; Rowe, J.B.; et al. In Vivo Rate-Determining Steps of Tau Seed Accumulation in Alzheimer’s Disease. Sci Adv 2021, 7, eabh1448. [Google Scholar] [CrossRef]
- Standards of Medical Care in Diabetes—2016 Abridged for Primary Care Providers. Clin Diabetes 2016, 34, 3–21. [CrossRef]
- Zhao, G.; Ford, E.S.; Li, C.; Balluz, L.S. Physical Activity in U.S. Older Adults with Diabetes Mellitus: Prevalence and Correlates of Meeting Physical Activity Recommendations. J Am Geriatr Soc 2011, 59, 132–137. [Google Scholar] [CrossRef]
- Cuff, D.J.; Meneilly, G.S.; Martin, A.; Ignaszewski, A.; Tildesley, H.D.; Frohlich, J.J. Effective Exercise Modality to Reduce Insulin Resistance in Women with Type 2 Diabetes. Diabetes Care 2003, 26, 2977–2982. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, H. Daily Physical Activity and Type 2 Diabetes: A Review. World J Diabetes 2016, 7, 243–251. [Google Scholar] [CrossRef]
- Özdirenç, M.; Biberoğlu, S.; Özcan, A. Evaluation of Physical Fitness in Patients with Type 2 Diabetes Mellitus. Diabetes Research and Clinical Practice 2003, 60, 171–176. [Google Scholar] [CrossRef]
- Advika, T.S.; Idiculla, J.; Kumari, S.J. Exercise in Patients with Type 2 Diabetes: Facilitators and Barriers - A Qualitative Study. J Family Med Prim Care 2017, 6, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Fagour, C.; Gonzalez, C.; Pezzino, S.; Florenty, S.; Rosette-Narece, M.; Gin, H.; Rigalleau, V. Low Physical Activity in Patients with Type 2 Diabetes: The Role of Obesity. Diabetes Metab 2013, 39, 85–87. [Google Scholar] [CrossRef]
- Hamilton, M.T.; Hamilton, D.G.; Zderic, T.W. Sedentary Behavior as a Mediator of Type 2 Diabetes. Med Sport Sci 2014, 60, 11–26. [Google Scholar] [CrossRef]
- Abd El-Kader, S.M.; Al-Jiffri, O.H.; Al-Shreef, F.M. Aerobic Exercises Alleviate Symptoms of Fatigue Related to Inflammatory Cytokines in Obese Patients with Type 2 Diabetes. Afr Health Sci 2015, 15, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Toledo, F.G.S.; Menshikova, E.V.; Azuma, K.; Radiková, Z.; Kelley, C.A.; Ritov, V.B.; Kelley, D.E. Mitochondrial Capacity in Skeletal Muscle Is Not Stimulated by Weight Loss despite Increases in Insulin Action and Decreases in Intramyocellular Lipid Content. Diabetes 2008, 57, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Radaelli, R.; Fleck, S.J.; Leite, T.; Leite, R.D.; Pinto, R.S.; Fernandes, L.; Simão, R. Dose-Response of 1, 3, and 5 Sets of Resistance Exercise on Strength, Local Muscular Endurance, and Hypertrophy. J Strength Cond Res 2015, 29, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Peterson, M.D.; Ogborn, D.; Contreras, B.; Sonmez, G.T. Effects of Low- vs. High-Load Resistance Training on Muscle Strength and Hypertrophy in Well-Trained Men. J Strength Cond Res 2015, 29, 2954–2963. [Google Scholar] [CrossRef]
- Poirier, P.; Garneau, C.; Bogaty, P.; Nadeau, A.; Marois, L.; Brochu, C.; Gingras, C.; Fortin, C.; Jobin, J.; Dumesnil, J.G. Impact of Left Ventricular Diastolic Dysfunction on Maximal Treadmill Performance in Normotensive Subjects with Well-Controlled Type 2 Diabetes Mellitus. Am J Cardiol 2000, 85, 473–477. [Google Scholar] [CrossRef]
- Allen, K.V.; Frier, B.M.; Strachan, M.W.J. The Relationship between Type 2 Diabetes and Cognitive Dysfunction: Longitudinal Studies and Their Methodological Limitations. Eur J Pharmacol 2004, 490, 169–175. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Wilson, R.S.; Bienias, J.L.; Evans, D.A.; Bennett, D.A. Diabetes Mellitus and Risk of Alzheimer Disease and Decline in Cognitive Function. Arch Neurol 2004, 61, 661–666. [Google Scholar] [CrossRef]
- Cukierman, T.; Gerstein, H.C.; Williamson, J.D. Cognitive Decline and Dementia in Diabetes--Systematic Overview of Prospective Observational Studies. Diabetologia 2005, 48, 2460–2469. [Google Scholar] [CrossRef]
- CHOI, S.E.; ROY, B.; FREEBY, M.; MULLUR, R.; WOO, M.A.; KUMAR, R. Prefrontal Cortex Brain Damage and Glycemic Control in Patients with Type 2 Diabetes. J Diabetes 2020, 12, 465–473. [Google Scholar] [CrossRef]
- Reuter-Lorenz, P.A.; Cappell, K.A. Neurocognitive Aging and the Compensation Hypothesis. Curr Dir Psychol Sci 2008, 17, 177–182. [Google Scholar] [CrossRef]
- Wood, A.G.; Chen, J.; Moran, C.; Phan, T.; Beare, R.; Cooper, K.; Litras, S.; Srikanth, V. Brain Activation during Memory Encoding in Type 2 Diabetes Mellitus: A Discordant Twin Pair Study. J Diabetes Res 2016, 2016, 3978428. [Google Scholar] [CrossRef]
- He, X.-S.; Wang, Z.-X.; Zhu, Y.-Z.; Wang, N.; Hu, X.; Zhang, D.-R.; Zhu, D.-F.; Zhou, J.-N. Hyperactivation of Working Memory-Related Brain Circuits in Newly Diagnosed Middle-Aged Type 2 Diabetics. Acta Diabetol 2015, 52, 133–142. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, S.; Liu, C.; Zhang, H.; Zhou, X.; Ni, C.; Qin, W.; Zhang, Q. Altered Brain Activation and Functional Connectivity in Working Memory Related Networks in Patients with Type 2 Diabetes: An ICA-Based Analysis. Sci Rep 2016, 6, 23767. [Google Scholar] [CrossRef]
- Barloese, M.C.J.; Bauer, C.; Petersen, E.T.; Hansen, C.S.; Madsbad, S.; Siebner, H.R. Neurovascular Coupling in Type 2 Diabetes With Cognitive Decline. A Narrative Review of Neuroimaging Findings and Their Pathophysiological Implications. Front Endocrinol (Lausanne) 2022, 13, 874007. [Google Scholar] [CrossRef]
- Sorond, F.A.; Schnyer, D.M.; Serrador, J.M.; Milberg, W.P.; Lipsitz, L.A. Cerebral Blood Flow Regulation during Cognitive Tasks: Effects of Healthy Aging. Cortex 2008, 44, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Beishon, L.C.; Hosford, P.; Gurung, D.; Brassard, P.; Minhas, J.S.; Robinson, T.G.; Haunton, V.; Panerai, R.B. The Role of the Autonomic Nervous System in Cerebral Blood Flow Regulation in Dementia: A Review. Auton Neurosci 2022, 240, 102985. [Google Scholar] [CrossRef] [PubMed]
- Kisler, K.; Nelson, A.R.; Montagne, A.; Zlokovic, B.V. Cerebral Blood Flow Regulation and Neurovascular Dysfunction in Alzheimer Disease. Nat Rev Neurosci 2017, 18, 419–434. [Google Scholar] [CrossRef]
- Binder, J.R.; Rao, S.M.; Hammeke, T.A.; Frost, J.A.; Bandettini, P.A.; Hyde, J.S. Effects of Stimulus Rate on Signal Response during Functional Magnetic Resonance Imaging of Auditory Cortex. Cognitive Brain Research 1994, 2, 31–38. [Google Scholar] [CrossRef]
- Jiang, D.; Lu, H. Cerebral Oxygen Extraction Fraction MRI: Techniques and Applications. Magnetic Resonance in Medicine 2022, 88, 575–600. [Google Scholar] [CrossRef]
- Buxton, R.B.; Griffeth, V.E.M.; Simon, A.B.; Moradi, F. Variability of the Coupling of Blood Flow and Oxygen Metabolism Responses in the Brain: A Problem for Interpreting BOLD Studies but Potentially a New Window on the Underlying Neural Activity. Front Neurosci 2014, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Buxton, R.B. Interpreting Oxygenation-Based Neuroimaging Signals: The Importance and the Challenge of Understanding Brain Oxygen Metabolism. Front Neuroenergetics 2010, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Buxton, R.B.; Frank, L.R. A Model for the Coupling between Cerebral Blood Flow and Oxygen Metabolism during Neural Stimulation. J Cereb Blood Flow Metab 1997, 17, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Van Ryckeghem, L.; Keytsman, C.; Verboven, K.; Verbaanderd, E.; Frederix, I.; Bakelants, E.; Petit, T.; Jogani, S.; Stroobants, S.; Dendale, P.; et al. Exercise Capacity Is Related to Attenuated Responses in Oxygen Extraction and Left Ventricular Longitudinal Strain in Asymptomatic Type 2 Diabetes Patients. European Journal of Preventive Cardiology 2021, 28, 1756–1766. [Google Scholar] [CrossRef] [PubMed]
- Van Ryckeghem, L.; Keytsman, C.; De Brandt, J.; Verboven, K.; Verbaanderd, E.; Marinus, N.; Franssen, W.M.A.; Frederix, I.; Bakelants, E.; Petit, T.; et al. Impact of Continuous vs. Interval Training on Oxygen Extraction and Cardiac Function during Exercise in Type 2 Diabetes Mellitus. Eur J Appl Physiol 2022, 122, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Kim, M.; Ahn, Y.-B.; Lim, H.-K.; Kang, S.-G.; Cho, J.; Park, S.-J.; Song, S.-W. Effect of Dance Exercise on Cognitive Function in Elderly Patients with Metabolic Syndrome: A Pilot Study. J Sports Sci Med 2011, 10, 671–678. [Google Scholar] [PubMed]
- Holwerda, S.W.; Restaino, R.M.; Manrique, C.; Lastra, G.; Fisher, J.P.; Fadel, P.J. Augmented Pressor and Sympathetic Responses to Skeletal Muscle Metaboreflex Activation in Type 2 Diabetes Patients. Am J Physiol Heart Circ Physiol 2016, 310, H300–H309. [Google Scholar] [CrossRef] [PubMed]
- Pinna, V.; Doneddu, A.; Roberto, S.; Magnani, S.; Ghiani, G.; Mulliri, G.; Sanna, I.; Serra, S.; Hosseini Kakhak, S.A.; Milia, R.; et al. Combined Mental Task and Metaboreflex Impair Cerebral Oxygenation in Patients with Type 2 Diabetes Mellitus. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 2021, 320, R488–R499. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.; Tarantini, S.; Csiszar, A.; Ungvari, Z. Functional Vascular Contributions to Cognitive Impairment and Dementia: Mechanisms and Consequences of Cerebral Autoregulatory Dysfunction, Endothelial Impairment, and Neurovascular Uncoupling in Aging. Am J Physiol Heart Circ Physiol 2017, 312, H1–H20. [Google Scholar] [CrossRef]
- Bherer, L.; Erickson, K.I.; Liu-Ambrose, T. A Review of the Effects of Physical Activity and Exercise on Cognitive and Brain Functions in Older Adults. J Aging Res 2013, 2013, 657508. [Google Scholar] [CrossRef]
- Smith, J.C.; Nielson, K.A.; Antuono, P.; Lyons, J.-A.; Hanson, R.J.; Butts, A.M.; Hantke, N.C.; Verber, M.D. Semantic Memory Functional MRI and Cognitive Function after Exercise Intervention in Mild Cognitive Impairment. Journal of Alzheimer’s Disease 2013, 37, 197–215. [Google Scholar] [CrossRef]
- Liao, Y.-Y.; Chen, I.-H.; Hsu, W.-C.; Tseng, H.-Y.; Wang, R.-Y. Effect of Exergaming versus Combined Exercise on Cognitive Function and Brain Activation in Frail Older Adults: A Randomised Controlled Trial. Ann Phys Rehabil Med 2021, 64, 101492. [Google Scholar] [CrossRef]
- How Accurate Is the Mini-Cog Test When Used to Assess Dementia in General Practice? Available online: https://www.cochrane.org/CD011415/DEMENTIA_how-accurate-mini-cog-test-when-used-assess-dementia-general-practice (accessed on 30 May 2023).
- Pinti, P.; Tachtsidis, I.; Hamilton, A.; Hirsch, J.; Aichelburg, C.; Gilbert, S.; Burgess, P.W. The Present and Future Use of Functional Near-infrared Spectroscopy (FNIRS) for Cognitive Neuroscience. Annals of the New York Academy of Sciences 2020, 1464, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Belardinelli, R.; Georgiou, D.; Scocco, V.; Barstow, T.J.; Purcaro, A. Low Intensity Exercise Training in Patients with Chronic Heart Failure. J Am Coll Cardiol 1995, 26, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Grassi, B.; Quaresima, V.; Marconi, C.; Ferrari, M.; Cerretelli, P. Blood Lactate Accumulation and Muscle Deoxygenation during Incremental Exercise. J Appl Physiol (1985) 1999, 87, 348–355. [Google Scholar] [CrossRef] [PubMed]
- van der Zwaard, S.; de Ruiter, C.J.; Noordhof, D.A.; Sterrenburg, R.; Bloemers, F.W.; de Koning, J.J.; Jaspers, R.T.; van der Laarse, W.J. Maximal Oxygen Uptake Is Proportional to Muscle Fiber Oxidative Capacity, from Chronic Heart Failure Patients to Professional Cyclists. Journal of Applied Physiology 2016, 121, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.; Zierath, J.R. Exercise Metabolism and the Molecular Regulation of Skeletal Muscle Adaptation. Cell Metabolism 2013, 17, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Barstow, T.J. Understanding near Infrared Spectroscopy and Its Application to Skeletal Muscle Research. J Appl Physiol (1985) 2019, 126, 1360–1376. [Google Scholar] [CrossRef] [PubMed]
- Farzam, P.; Starkweather, Z.; Franceschini, M.A. Validation of a Novel Wearable, Wireless Technology to Estimate Oxygen Levels and Lactate Threshold Power in the Exercising Muscle. Physiological Reports 2018, 6, e13664. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Chiesa, S.T.; Chaturvedi, N.; Hughes, A.D. Recent Developments in Near-Infrared Spectroscopy (NIRS) for the Assessment of Local Skeletal Muscle Microvascular Function and Capacity to Utilise Oxygen. Artery Research 2016, 16, 25–33. [Google Scholar] [CrossRef]
- Lagerwaard, B.; Nieuwenhuizen, A.G.; de Boer, V.C.J.; Keijer, J. In Vivo Assessment of Mitochondrial Capacity Using NIRS in Locomotor Muscles of Young and Elderly Males with Similar Physical Activity Levels. GeroScience 2019, 42, 299–310. [Google Scholar] [CrossRef]
- Boone, J.; Celie, B.; Dumortier, J.; Barstow, T.J.; De Bleecker, J.; Smet, J.; Van Lander, A.; Van Coster, R.; Bourgois, J. Forearm Muscle Oxygenation Responses during and Following Arterial Occlusion in Patients with Mitochondrial Myopathy. Respiratory Physiology & Neurobiology 2014, 190, 70–75. [Google Scholar] [CrossRef]
- Malagoni, A.M.; Felisatti, M.; Mandini, S.; Mascoli, F.; Manfredini, R.; Basaglia, N.; Zamboni, P.; Manfredini, F. Resting Muscle Oxygen Consumption by Near-Infrared Spectroscopy in Peripheral Arterial Disease: A Parameter to Be Considered in a Clinical Setting? Angiology 2010, 61, 530–536. [Google Scholar] [CrossRef]
- Vardi, M.; Nini, A. Near-Infrared Spectroscopy for Evaluation of Peripheral Vascular Disease. A Systematic Review of Literature. European Journal of Vascular and Endovascular Surgery 2008, 35, 68–74. [Google Scholar] [CrossRef]
- Malagoni, A.M.; Felisatti, M.; Lamberti, N.; Basaglia, N.; Manfredini, R.; Salvi, F.; Zamboni, P.; Manfredini, F. Muscle Oxygen Consumption by NIRS and Mobility in Multiple Sclerosis Patients. BMC Neurol 2013, 13, 52. [Google Scholar] [CrossRef]
- Fu, T.-C.; Wang, C.-H.; Lin, P.-S.; Hsu, C.-C.; Cherng, W.-J.; Huang, S.-C.; Liu, M.-H.; Chiang, C.-L.; Wang, J.-S. Aerobic Interval Training Improves Oxygen Uptake Efficiency by Enhancing Cerebral and Muscular Hemodynamics in Patients with Heart Failure. Int J Cardiol 2013, 167, 41–50. [Google Scholar] [CrossRef]
- Southern, W.M.; Ryan, T.E.; Kepple, K.; Murrow, J.R.; Nilsson, K.R.; McCully, K.K. Reduced Skeletal Muscle Oxidative Capacity and Impaired Training Adaptations in Heart Failure. Physiol Rep 2015, 3, e12353. [Google Scholar] [CrossRef]
- Belardinelli, R.; Barstow, T.J.; Porszasz, J.; Wasserman, K. Changes in Skeletal Muscle Oxygenation during Incremental Exercise Measured with near Infrared Spectroscopy. Eur J Appl Physiol Occup Physiol 1995, 70, 487–492. [Google Scholar] [CrossRef]
- Zwaard, S. van der; Jaspers, R.T.; Blokland, I.J.; Achterberg, C.; Visser, J.M.; Uil, A.R. den; Hofmijster, M.J.; Levels, K.; Noordhof, D.A.; Haan, A. de; et al. Oxygenation Threshold Derived from Near-Infrared Spectroscopy: Reliability and Its Relationship with the First Ventilatory Threshold. PLOS ONE 2016, 11, e0162914. [Google Scholar] [CrossRef]
- Wang, L.; Yoshikawa, T.; Hara, T.; Nakao, H.; Suzuki, T.; Fujimoto, S. Which Common NIRS Variable Reflects Muscle Estimated Lactate Threshold Most Closely? Appl Physiol Nutr Metab 2006, 31, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Cheung, M.; Dutta, A.; Fisher, N.; Tomita, M. Exercises to Determine Older Adults’ Muscle Oxygenation Change Rate by Various Physical Performance Levels. Archives of Physical Medicine and Rehabilitation 2019, 100, e178–e179. [Google Scholar] [CrossRef]
- Wahl, M.P.; Scalzo, R.L.; Regensteiner, J.G.; Reusch, J.E.B. Mechanisms of Aerobic Exercise Impairment in Diabetes: A Narrative Review. Front Endocrinol (Lausanne) 2018, 9, 181. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Seifert, T.; Brassard, P.; Rasmussen, P.; Vaag, A.; Nielsen, H.B.; Secher, N.H.; van Lieshout, J.J. Impaired Cerebral Blood Flow and Oxygenation during Exercise in Type 2 Diabetic Patients. Physiol Rep 2015, 3, e12430. [Google Scholar] [CrossRef]
- Fowler, M.J. Microvascular and Macrovascular Complications of Diabetes. Clinical Diabetes 2008, 26, 77–82. [Google Scholar] [CrossRef]
- Rask-Madsen, C.; King, G.L. Vascular Complications of Diabetes: Mechanisms of Injury and Protective Factors. Cell Metab 2013, 17, 20–33. [Google Scholar] [CrossRef] [PubMed]
- McClatchey, P.M.; Schafer, M.; Hunter, K.S.; Reusch, J.E.B. The Endothelial Glycocalyx Promotes Homogenous Blood Flow Distribution within the Microvasculature. Am J Physiol Heart Circ Physiol 2016, 311, H168–H176. [Google Scholar] [CrossRef] [PubMed]
- Dogné, S.; Flamion, B.; Caron, N. Endothelial Glycocalyx as a Shield Against Diabetic Vascular Complications. Arterioscler Thromb Vasc Biol 2018, 38, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Kröpfl, J.M.; Beltrami, F.G.; Rehm, M.; Gruber, H.-J.; Stelzer, I.; Spengler, C.M. Acute Exercise-Induced Glycocalyx Shedding Does Not Differ between Exercise Modalities, but Is Associated with Total Antioxidative Capacity. Journal of Science and Medicine in Sport 2021, 24, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, B.; Niehues, H.; Lenders, M.; Thorwesten, L.; Klose, A.; Krüger, M.; Brand, E.; Brand, S.-M. Effects of High-Intensity Interval Training on Microvascular Glycocalyx and Associated MicroRNAs. American Journal of Physiology-Heart and Circulatory Physiology 2019, 316, H1538–H1551. [Google Scholar] [CrossRef] [PubMed]
- Machin, D.R.; Phuong, T.T.T.; Donato, A.J. The Role of the Endothelial Glycocalyx in Advanced Age and Cardiovascular Disease. Curr Opin Pharmacol 2019, 45, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.G.; Patel, V.; Dull, R.O. Human Glycocalyx Shedding: Systematic Review and Critical Appraisal. Acta Anaesthesiologica Scandinavica 2021, 65, 590–606. [Google Scholar] [CrossRef]
- Targosz-Korecka, M.; Jaglarz, M.; Malek-Zietek, K.E.; Gregorius, A.; Zakrzewska, A.; Sitek, B.; Rajfur, Z.; Chlopicki, S.; Szymonski, M. AFM-Based Detection of Glycocalyx Degradation and Endothelial Stiffening in the Db/Db Mouse Model of Diabetes. Sci Rep 2017, 7, 15951. [Google Scholar] [CrossRef] [PubMed]
- Sandoo, A.; van Zanten, J.J.C.S.V.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The Endothelium and Its Role in Regulating Vascular Tone. Open Cardiovasc Med J 2010, 4, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.G. Endothelial Control of Vasomotion and Nitric Oxide Production: A Potential Target for Risk Factor Management. Cardiol Clin 1996, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Haselden, W.D.; Kedarasetti, R.T.; Drew, P.J. Spatial and Temporal Patterns of Nitric Oxide Diffusion and Degradation Drive Emergent Cerebrovascular Dynamics. PLOS Computational Biology 2020, 16, e1008069. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.G.; Widder, J.; Grumbach, I.; Chen, W.; Weber, M.; Searles, C. Endothelial Mechanotransduction, Nitric Oxide and Vascular Inflammation. Journal of Internal Medicine 2006, 259, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.; Sahay, R. Diabetes Fatigue Syndrome. Diabetes Ther 2018, 9, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry, 5th ed.; W.H. Freeman; NCBI: New York, [Bethesda, MD], 2002. [Google Scholar]
- Kemp, G.J.; Hands, L.J.; Ramaswami, G.; Taylor, D.J.; Nicolaides, A.; Amato, A.; Radda, G.K. Calf Muscle Mitochondrial and Glycogenolytic Atp Synthesis in Patients with Claudication Due to Peripheral Vascular Disease Analysed Using 31P Magnetic Resonance Spectroscopy. Clinical Science 1995, 89, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.A.; Reusch, J.E.B.; Levi, M.; Regensteiner, J.G. Skeletal Muscle Deoxygenation After the Onset of Moderate Exercise Suggests Slowed Microvascular Blood Flow Kinetics in Type 2 Diabetes. Diabetes Care 2007, 30, 2880–2885. [Google Scholar] [CrossRef]
- Zhao, F.; Tomita, M.R.; Dutta, A. Functional Near-Infrared Spectroscopy of Prefrontal Cortex during Memory Encoding and Recall in Elderly with Type 2 Diabetes Mellitus. Annu Int Conf IEEE Eng Med Biol Soc 2022, 2022, 3323–3326. [Google Scholar] [CrossRef]
- Das, A.; Murphy, K.; Drew, P.J. Rude Mechanicals in Brain Haemodynamics: Non-Neural Actors That Influence Blood Flow. Philosophical Transactions of the Royal Society B: Biological Sciences 2020, 376, 20190635. [Google Scholar] [CrossRef]
- Aalkjær, C.; Nilsson, H. Vasomotion: Cellular Background for the Oscillator and for the Synchronization of Smooth Muscle Cells. Br J Pharmacol 2005, 144, 605–616. [Google Scholar] [CrossRef]
- Paniagua, O.A.; Bryant, M.B.; Panza, J.A. Role of Endothelial Nitric Oxide in Shear Stress–Induced Vasodilation of Human Microvasculature. Circulation 2001, 103, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.Z.; Goligorsky, M.S. Biomechanical Properties of Endothelial Glycocalyx: An Imperfect Pendulum. Matrix Biol Plus 2021, 12, 100087. [Google Scholar] [CrossRef] [PubMed]
- Farina, A.; Rosso, F.; Fasano, A. A Continuum Mechanics Model for the Fåhræus-Lindqvist Effect. J Biol Phys 2021, 47, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Forouzan, O.; Yang, X.; Sosa, J.M.; Burns, J.M.; Shevkoplyas, S.S. Spontaneous Oscillations of Capillary Blood Flow in Artificial Microvascular Networks. Microvasc Res 2012, 84, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Au, S.-K.; Brownjohn, J.M.W.; Li, B.; Raby, A. Understanding and Managing Identification Uncertainty of Close Modes in Operational Modal Analysis. Mechanical Systems and Signal Processing 2021, 147, 107018. [Google Scholar] [CrossRef]
- Akazawa, N.; Tanahashi, K.; Kosaki, K.; Ra, S.; Matsubara, T.; Choi, Y.; Zempo-Miyaki, A.; Maeda, S. Aerobic Exercise Training Enhances Cerebrovascular Pulsatility Response to Acute Aerobic Exercise in Older Adults. Physiol Rep 2018, 6, e13681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, J.; He, X.; Cao, W.; Peng, X.; Li, G. Pulsatility Protects the Endothelial Glycocalyx during Extracorporeal Membrane Oxygenation. Microcirculation 2021, 28, e12722. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.L.; Palta, P.; Tanaka, H.; Deal, J.A.; Wright, J.; Knopman, D.S.; Griswold, M.E.; Mosley, T.H.; Heiss, G. Association of Central Arterial Stiffness and Pressure Pulsatility with Mild Cognitive Impairment and Dementia: The Atherosclerosis Risk in Communities Study-Neurocognitive Study (ARIC-NCS). J. Alzheimers Dis. 2017, 57, 195–204. [Google Scholar] [CrossRef]
- Nieuwdorp, M.; van Haeften, T.W.; Gouverneur, M.C.L.G.; Mooij, H.L.; van Lieshout, M.H.P.; Levi, M.; Meijers, J.C.M.; Holleman, F.; Hoekstra, J.B.L.; Vink, H.; et al. Loss of Endothelial Glycocalyx During Acute Hyperglycemia Coincides With Endothelial Dysfunction and Coagulation Activation In Vivo. Diabetes 2006, 55, 480–486. [Google Scholar] [CrossRef]
- Jahani, S.; Fantana, A.L.; Harper, D.; Ellison, J.M.; Boas, D.A.; Forester, B.P.; Yücel, M.A. FNIRS Can Robustly Measure Brain Activity during Memory Encoding and Retrieval in Healthy Subjects. Scientific Reports 2017, 7, 9533. [Google Scholar] [CrossRef]
- Sun, S.; Yang, B.; Zhang, Q.; Wüchner, R.; Pan, L.; Zhu, H. Fast Online Implementation of Covariance-Driven Stochastic Subspace Identification. Mechanical Systems and Signal Processing 2023, 197, 110326. [Google Scholar] [CrossRef]
- Zhao, F. Cerebral and Muscular Oxygenation Changes after Moderate-Intensity Exercise in Sedentary Older Adults with Type 2 Diabetes. Ph.D., State University of New York at Buffalo: United States -- New York, 2022.
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B.; American College of Sports Medicine; et al. Exercise and Type 2 Diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint Position Statement. Diabetes Care 2010, 33, e147–167. [Google Scholar] [CrossRef] [PubMed]
- Irvine, C.; Taylor, N.F. Progressive Resistance Exercise Improves Glycaemic Control in People with Type 2 Diabetes Mellitus: A Systematic Review. Aust J Physiother 2009, 55, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.A.; Winters-Stone, K.; Nail, L.M.; Scherer, J. Definitions of Sedentary in Physical-Activity-Intervention Trials: A Summary of the Literature. J Aging Phys Act 2006, 14, 456–477. [Google Scholar] [CrossRef] [PubMed]
- Murkin, J.M.; Arango, M. Near-Infrared Spectroscopy as an Index of Brain and Tissue Oxygenation. British Journal of Anaesthesia 2009, 103, i3–i13. [Google Scholar] [CrossRef] [PubMed]
- Huppert, T.J.; Hoge, R.D.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. A Temporal Comparison of BOLD, ASL, and NIRS Hemodynamic Responses to Motor Stimuli in Adult Humans. Neuroimage 2006, 29, 368–382. [Google Scholar] [CrossRef] [PubMed]
- Mehagnoul-Schipper, D.J.; van der Kallen, B.F.W.; Colier, W.N.J.M.; van der Sluijs, M.C.; van Erning, L.J.T.O.; Thijssen, H.O.M.; Oeseburg, B.; Hoefnagels, W.H.L.; Jansen, R.W.M.M. Simultaneous Measurements of Cerebral Oxygenation Changes during Brain Activation by Near-Infrared Spectroscopy and Functional Magnetic Resonance Imaging in Healthy Young and Elderly Subjects. Hum Brain Mapp 2002, 16, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Huppert, T.J.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. HomER: A Review of Time-Series Analysis Methods for near-Infrared Spectroscopy of the Brain. Appl Opt 2009, 48, D280–D298. [Google Scholar] [CrossRef]
- Arora, Y.; Dutta, A. Perspective: Disentangling the Effects of TES on Neurovascular Unit. Frontiers in Neurology 2023, 13. [Google Scholar] [CrossRef]
- Santosa, H.; Zhai, X.; Fishburn, F.; Huppert, T. The NIRS Brain AnalyzIR Toolbox. Algorithms 2018, 11, 73. [Google Scholar] [CrossRef]
- Barker, J.W.; Aarabi, A.; Huppert, T.J. Autoregressive Model Based Algorithm for Correcting Motion and Serially Correlated Errors in FNIRS. Biomed Opt Express 2013, 4, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Brincker, R.; Andersen, P.; Jacobsen, N.-J. Automated Frequency Domain Decomposition for Operational Modal Analysis.; 2007.
- Brincker, R.; Zhang, L.; Andersen, P. Modal Identification of Output-Only Systems Using Frequency Domain Decomposition. Smart Mater. Struct. 2001, 10, 441. [Google Scholar] [CrossRef]
- Arora, Y.; Dutta, A. Human-In-The-Loop Optimization of Transcranial Electrical Stimulation Effects: A Computational Perspective Based on Systems Analysis 2022.
- Arora, Y.; Walia, P.; Hayashibe, M.; Muthalib, M.; Chowdhury, S.R.; Perrey, S.; Dutta, A. Grey-Box Modeling and Hypothesis Testing of Functional near-Infrared Spectroscopy-Based Cerebrovascular Reactivity to Anodal High-Definition TDCS in Healthy Humans. PLOS Computational Biology 2021, 17, e1009386. [Google Scholar] [CrossRef]
- Bicciato, G.; Keller, E.; Wolf, M.; Brandi, G.; Schulthess, S.; Friedl, S.G.; Willms, J.F.; Narula, G. Increase in Low-Frequency Oscillations in FNIRS as Cerebral Response to Auditory Stimulation with Familiar Music. Brain Sci 2021, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Neu, E.; Janser, F.; Khatibi, A.A.; Orifici, A.C. Fully Automated Operational Modal Analysis Using Multi-Stage Clustering. Mechanical Systems and Signal Processing 2017, 84, 308–323. [Google Scholar] [CrossRef]
- Crum, E.M.; O’Connor, W.J.; Van Loo, L.; Valckx, M.; Stannard, S.R. Validity and Reliability of the Moxy Oxygen Monitor during Incremental Cycling Exercise. Eur J Sport Sci 2017, 17, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Borson, S.; Scanlan, J.; Brush, M.; Vitaliano, P.; Dokmak, A. The Mini-Cog: A Cognitive “vital Signs” Measure for Dementia Screening in Multi-Lingual Elderly. Int J Geriatr Psychiatry 2000, 15, 1021–1027. [Google Scholar] [CrossRef]
- Tsoi, K.K.F.; Chan, J.Y.C.; Hirai, H.W.; Wong, S.Y.S.; Kwok, T.C.Y. Cognitive Tests to Detect Dementia: A Systematic Review and Meta-Analysis. JAMA Intern Med 2015, 175, 1450–1458. [Google Scholar] [CrossRef]
- Balke, B. A SIMPLE FIELD TEST FOR THE ASSESSMENT OF PHYSICAL FITNESS. REP 63-6. Rep Civ Aeromed Res Inst US 1963, 1–8. [Google Scholar]
- Chan, W.L.; Chan, H.L.; Chen, K.M.; Fan, H.L.; Lai, W.C.; Yu, S.W. Reliability and Validity of Walk Tests for Older Adults with Dementia: A Systematic Review. Alzheimer’s & Dementia 2021, 17, e050371. [Google Scholar] [CrossRef]
- Lunsford, B.R.; Perry, J. The Standing Heel-Rise Test for Ankle Plantar Flexion: Criterion for Normal. Physical Therapy 1995, 75, 694–698. [Google Scholar] [CrossRef]
- Fairclough, S.H.; Burns, C.; Kreplin, U. FNIRS Activity in the Prefrontal Cortex and Motivational Intensity: Impact of Working Memory Load, Financial Reward, and Correlation-Based Signal Improvement. Neurophotonics 2018, 5, 035001. [Google Scholar] [CrossRef]
- du Boisgueheneuc, F.; Levy, R.; Volle, E.; Seassau, M.; Duffau, H.; Kinkingnehun, S.; Samson, Y.; Zhang, S.; Dubois, B. Functions of the Left Superior Frontal Gyrus in Humans: A Lesion Study. Brain 2006, 129, 3315–3328. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T.; Huang, C.-C.; Lin, C.-P.; Feng, J.; Joliot, M. Automated Anatomical Labelling Atlas 3. NeuroImage 2020, 206, 116189. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ide, J.S.; Zhang, S.; Li, C.R. The Right Superior Frontal Gyrus and Individual Variation in Proactive Control of Impulsive Response. J. Neurosci. 2016, 36, 12688–12696. [Google Scholar] [CrossRef] [PubMed]
- Cerebral Correlates of Autonomic Cardiovascular Arousal: A Functional Neuroimaging Investigation in Humans.
- ter Laan, M.; van Dijk, J.M.C.; Elting, J.W.J.; Staal, M.J.; Absalom, A.R. Sympathetic Regulation of Cerebral Blood Flow in Humans: A Review. British Journal of Anaesthesia 2013, 111, 361–367. [Google Scholar] [CrossRef]
- Stanford, K.I.; Goodyear, L.J. Muscle-Adipose Tissue Cross Talk. Cold Spring Harb Perspect Med 2018, 8, a029801. [Google Scholar] [CrossRef] [PubMed]
- Rosano, C.; Newman, A.; Santanasto, A.; Zhu, X.; Goodpaster, B.; Miljkovic, I. Increase in Skeletal Muscular Adiposity and Cognitive Decline in a Biracial Cohort of Older Men and Women. J Am Geriatr Soc 2023. [Google Scholar] [CrossRef] [PubMed]
- Silveira-Rodrigues, J.G.; Pires, W.; Gomes, P.F.; Ogando, P.H.M.; Melo, B.P.; Aleixo, I.M.S.; Soares, D.D. Combined Exercise Training Improves Specific Domains of Cognitive Functions and Metabolic Markers in Middle-Aged and Older Adults with Type 2 Diabetes Mellitus. Diabetes Res Clin Pract 2021, 173, 108700. [Google Scholar] [CrossRef] [PubMed]
- Leischik, R.; Schwarz, K.; Bank, P.; Brzek, A.; Dworrak, B.; Strauss, M.; Litwitz, H.; Gerlach, C.E. Exercise Improves Cognitive Function-A Randomized Trial on the Effects of Physical Activity on Cognition in Type 2 Diabetes Patients. J Pers Med 2021, 11, 530. [Google Scholar] [CrossRef] [PubMed]
- Berchicci, M.; Lucci, G.; Di Russo, F. Benefits of Physical Exercise on the Aging Brain: The Role of the Prefrontal Cortex. J Gerontol A Biol Sci Med Sci 2013, 68, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-Y.; Chen, I.-H.; Wang, R.-Y. Effects of Kinect-Based Exergaming on Frailty Status and Physical Performance in Prefrail and Frail Elderly: A Randomized Controlled Trial. Sci Rep 2019, 9, 9353. [Google Scholar] [CrossRef] [PubMed]
- Bertram, S.; Brixius, K.; Brinkmann, C. Exercise for the Diabetic Brain: How Physical Training May Help Prevent Dementia and Alzheimer’s Disease in T2DM Patients. Endocrine 2016, 53, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Lustig, C.; Shah, P.; Seidler, R.; Reuter-Lorenz, P.A. Aging, Training, and the Brain: A Review and Future Directions. Neuropsychol Rev 2009, 19, 504–522. [Google Scholar] [CrossRef]
- Dickerson, B.C.; Salat, D.H.; Greve, D.N.; Chua, E.F.; Rand-Giovannetti, E.; Rentz, D.M.; Bertram, L.; Mullin, K.; Tanzi, R.E.; Blacker, D.; et al. Increased Hippocampal Activation in Mild Cognitive Impairment Compared to Normal Aging and AD. Neurology 2005, 65, 404–411. [Google Scholar] [CrossRef]
- Reuter-Lorenz, P.A.; Lustig, C. Brain Aging: Reorganizing Discoveries about the Aging Mind. Curr Opin Neurobiol 2005, 15, 245–251. [Google Scholar] [CrossRef]
- Bruckmaier, M.; Tachtsidis, I.; Phan, P.; Lavie, N. Attention and Capacity Limits in Perception: A Cellular Metabolism Account. J Neurosci 2020, 40, 6801–6811. [Google Scholar] [CrossRef]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.-F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1α-Responsive Genes Involved in Oxidative Phosphorylation Are Coordinately Downregulated in Human Diabetes. Nat Genet 2003, 34, 267–273. [Google Scholar] [CrossRef]
- Patti, M.E.; Butte, A.J.; Crunkhorn, S.; Cusi, K.; Berria, R.; Kashyap, S.; Miyazaki, Y.; Kohane, I.; Costello, M.; Saccone, R.; et al. Coordinated Reduction of Genes of Oxidative Metabolism in Humans with Insulin Resistance and Diabetes: Potential Role of PGC1 and NRF1. Proc Natl Acad Sci U S A 2003, 100, 8466–8471. [Google Scholar] [CrossRef]
- Petersen, K.F.; Befroy, D.; Dufour, S.; Dziura, J.; Ariyan, C.; Rothman, D.L.; DiPietro, L.; Cline, G.W.; Shulman, G.I. Mitochondrial Dysfunction in the Elderly: Possible Role in Insulin Resistance. Science 2003, 300, 1140–1142. [Google Scholar] [CrossRef]
- Schrauwen-Hinderling, V.B.; Kooi, M.E.; Hesselink, M.K.C.; Jeneson, J. a. L.; Backes, W.H.; van Echteld, C.J.A.; van Engelshoven, J.M.A.; Mensink, M.; Schrauwen, P. Impaired in Vivo Mitochondrial Function but Similar Intramyocellular Lipid Content in Patients with Type 2 Diabetes Mellitus and BMI-Matched Control Subjects. Diabetologia 2007, 50, 113–120. [Google Scholar] [CrossRef]
- Szendroedi, J.; Schmid, A.I.; Chmelik, M.; Toth, C.; Brehm, A.; Krssak, M.; Nowotny, P.; Wolzt, M.; Waldhausl, W.; Roden, M. Muscle Mitochondrial ATP Synthesis and Glucose Transport/Phosphorylation in Type 2 Diabetes. PLoS Med 2007, 4, e154. [Google Scholar] [CrossRef]
- Zilberter, Y.; Zilberter, M. The Vicious Circle of Hypometabolism in Neurodegenerative Diseases: Ways and Mechanisms of Metabolic Correction. Journal of Neuroscience Research 2017, 95, 2217–2235. [Google Scholar] [CrossRef]
- Mullins, R.; Reiter, D.; Kapogiannis, D. Magnetic Resonance Spectroscopy Reveals Abnormalities of Glucose Metabolism in the Alzheimer’s Brain. Ann Clin Transl Neurol 2018, 5, 262–272. [Google Scholar] [CrossRef]
- Attwell, D.; Gibb, A. Neuroenergetics and the Kinetic Design of Excitatory Synapses. Nat Rev Neurosci 2005, 6, 841–849. [Google Scholar] [CrossRef]
- Barbiellini Amidei, C.; Fayosse, A.; Dumurgier, J.; Machado-Fragua, M.D.; Tabak, A.G.; van Sloten, T.; Kivimäki, M.; Dugravot, A.; Sabia, S.; Singh-Manoux, A. Association Between Age at Diabetes Onset and Subsequent Risk of Dementia. JAMA 2021, 325, 1640–1649. [Google Scholar] [CrossRef]
- Kandimalla, R.; Thirumala, V.; Reddy, P.H. Is Alzheimer’s Disease a Type 3 Diabetes? A Critical Appraisal. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2017, 1863, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Wei, Y.; Sowers, J.R. Role of Mitochondrial Dysfunction in Insulin Resistance. Circulation Research 2008, 102, 401–414. [Google Scholar] [CrossRef] [PubMed]
- M. d, L.H.; Mann, J.J. Test–Retest Reliability of Brain Mitochondrial Cytochrome-c-Oxidase Assessed by Functional near-Infrared Spectroscopy. JBO 2018, 23, 056006. [Google Scholar] [CrossRef]
- Dutta, A. Bidirectional Interactions between Neuronal and Hemodynamic Responses to Transcranial Direct Current Stimulation (TDCS): Challenges for Brain-State Dependent TDCS. Frontiers in Systems Neuroscience 2015, 107. [Google Scholar] [CrossRef]
- Shaw, K.; Bell, L.; Boyd, K.; Grijseels, D.M.; Clarke, D.; Bonnar, O.; Crombag, H.S.; Hall, C.N. Neurovascular Coupling and Oxygenation Are Decreased in Hippocampus Compared to Neocortex Because of Microvascular Differences. Nat Commun 2021, 12, 3190. [Google Scholar] [CrossRef]
- Arora, Y.; Chowdhury, S.R.; Dutta, A. Physiological Neurovascular Modeling of Cerebrovascular Effects of Transcranial Electrical Current Stimulation. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation 2021, 14, 1597–1598. [Google Scholar] [CrossRef]
- Fujii, H.; Ito, H.; Aihara, K.; Ichinose, N.; Tsukada, M. Dynamical Cell Assembly Hypothesis — Theoretical Possibility of Spatio-Temporal Coding in the Cortex. Neural Networks 1996, 9, 1303–1350. [Google Scholar] [CrossRef]
- Stefanovska, A.; Bracic, M.; Kvernmo, H.D. Wavelet Analysis of Oscillations in the Peripheral Blood Circulation Measured by Laser Doppler Technique. IEEE Trans Biomed Eng 1999, 46, 1230–1239. [Google Scholar] [CrossRef]
- Gibbons, C.H.; Freeman, R. Treatment-Induced Neuropathy of Diabetes: An Acute, Iatrogenic Complication of Diabetes. Brain 2015, 138, 43–52. [Google Scholar] [CrossRef]
- Geddes, J.B.; Carr, R.T.; Wu, F.; Lao, Y.; Maher, M. Blood Flow in Microvascular Networks: A Study in Nonlinear Biology. Chaos 2010, 20, 045123. [Google Scholar] [CrossRef]
- Irace, C.; Carallo, C.; Scavelli, F.; De Franceschi, M.S.; Esposito, T.; Gnasso, A. Blood Viscosity in Subjects with Normoglycemia and Prediabetes. Diabetes Care 2014, 37, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Kistenmacher, A.; Manneck, S.; Wardzinski, E.K.; Martens, J.C.; Gohla, G.; Melchert, U.H.; Jauch-Chara, K.; Oltmanns, K.M. Persistent Blood Glucose Reduction upon Repeated Transcranial Electric Stimulation in Men. Brain Stimul 2017, 10, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Scholey, A.B.; Harper, S.; Kennedy, D.O. Cognitive Demand and Blood Glucose. Physiol Behav 2001, 73, 585–592. [Google Scholar] [CrossRef]
- Chan, C.C.; Fage, B.A.; Burton, J.K.; Smailagic, N.; Gill, S.S.; Herrmann, N.; Nikolaou, V.; Quinn, T.J.; Noel-Storr, A.H.; Seitz, D.P. Mini-Cog for the Diagnosis of Alzheimer’s Disease Dementia and Other Dementias within a Secondary Care Setting. Cochrane Database Syst Rev 2019, 9, CD011414. [Google Scholar] [CrossRef]
- Zhao, F.; Tomita, M.; Dutta, A. Functional Near-Infrared Spectroscopy Of Prefrontal Cortex During Memory Encoding And Recall In Elderly With Type 2 Diabetes Mellitus. 2022. [Google Scholar] [CrossRef]
- Wong, A.D.; Ye, M.; Levy, A.F.; Rothstein, J.D.; Bergles, D.E.; Searson, P.C. The Blood-Brain Barrier: An Engineering Perspective. Front Neuroeng 2013, 6, 7. [Google Scholar] [CrossRef]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.-Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E.; et al. Brain Insulin Resistance in Type 2 Diabetes and Alzheimer Disease: Concepts and Conundrums. Nat Rev Neurol 2018, 14, 168–181. [Google Scholar] [CrossRef]
- Demarest, T.G.; Varma, V.R.; Estrada, D.; Babbar, M.; Basu, S.; Mahajan, U.V.; Moaddel, R.; Croteau, D.L.; Thambisetty, M.; Mattson, M.P.; et al. Biological Sex and DNA Repair Deficiency Drive Alzheimer’s Disease via Systemic Metabolic Remodeling and Brain Mitochondrial Dysfunction. Acta Neuropathol 2020, 140, 25–47. [Google Scholar] [CrossRef]
- Karanth, S.S.; Mujumdar, R.; Sahoo, J.P.; Das, A.; Stachowiak, M.K.; Dutta, A. Human Brain Organoid Platform for Neuroengineering Optical Theranostics in Neonatal Sepsis. In Proceedings of the Converging Clinical and Engineering Research on Neurorehabilitation IV; Torricelli, D., Akay, M., Pons, J.L., Eds.; Springer International Publishing: Cham, 2022; pp. 753–757. [Google Scholar]
- Lombardi, F.; Herrmann, H.J.; de Arcangelis, L. Balance of Excitation and Inhibition Determines 1/f Power Spectrum in Neuronal Networks. Chaos 2017, 27, 047402. [Google Scholar] [CrossRef]
- Dutta, A.; Karanth, S.S.; Bhattacharya, M.; Liput, M.; Augustyniak, J.; Cheung, M.; Stachowiak, E.K.; Stachowiak, M.K. A Proof of Concept ‘Phase Zero’ Study of Neurodevelopment Using Brain Organoid Models with Vis/near-Infrared Spectroscopy and Electrophysiology. Scientific Reports 2020, 10, 20987. [Google Scholar] [CrossRef]
- Zhang, L.; Su, F.; Buizer, S.; Lu, H.; Gao, W.; Tian, Y.; Meldrum, D. A Dual Sensor for Real-Time Monitoring of Glucose and Oxygen. Biomaterials 2013, 34, 10–1016. [Google Scholar] [CrossRef] [PubMed]
- Arora, Y.; Dutta, A. Human-in-the-Loop Optimization of Transcranial Electrical Stimulation at the Point of Care: A Computational Perspective. Brain Sciences 2022, 12, 1294. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xu, Z.; Xiao, L.; Shi, T.; Xiao, H.; Wang, Y.; Li, Y.; Xue, F.; Zeng, W. Review on the Vascularization of Organoids and Organoids-on-a-Chip. Frontiers in Bioengineering and Biotechnology 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Arora, Y.; Walia, P.; Hayashibe, M.; Muthalib, M.; Chowdhury, S.R.; Perrey, S.; Dutta, A. Grey-Box Modeling and Hypothesis Testing of Functional near-Infrared Spectroscopy-Based Cerebrovascular Reactivity to Anodal High-Definition TDCS in Healthy Humans 2021.
- Petrou, M.; Pop-Busui, R.; Foerster, B.R.; Edden, R.A.; Callaghan, B.C.; Harte, S.E.; Harris, R.E.; Clauw, D.J.; Feldman, E.L. Altered Excitation-Inhibition Balance in the Brain of Patients with Diabetic Neuropathy. Acad Radiol 2012, 19, 607–612. [Google Scholar] [CrossRef]
- Sood, M.; Besson, P.; Muthalib, M.; Jindal, U.; Perrey, S.; Dutta, A.; Hayashibe, M. NIRS-EEG Joint Imaging during Transcranial Direct Current Stimulation: Online Parameter Estimation with an Autoregressive Model. J. Neurosci. Methods 2016, 274, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Excitability Changes Induced in the Human Motor Cortex by Weak Transcranial Direct Current Stimulation. J. Physiol. (Lond.) 2000, 527 Pt 3, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Jamil, A.; Batsikadze, G.; Kuo, H.-I.; Meesen, R.L.J.; Dechent, P.; Paulus, W.; Nitsche, M.A. Current Intensity- and Polarity-Specific Online and Aftereffects of Transcranial Direct Current Stimulation: An FMRI Study. Human Brain Mapping 2020, 41, 1644–1666. [Google Scholar] [CrossRef] [PubMed]
- Bahr-Hosseini, M.; Bikson, M. Neurovascular-Modulation: A Review of Primary Vascular Responses to Transcranial Electrical Stimulation as a Mechanism of Action. Brain Stimulation 2021, 14, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Jacob, A.; Chowdhury, S.R.; Das, A.; Nitsche, M.A. EEG-NIRS Based Assessment of Neurovascular Coupling during Anodal Transcranial Direct Current Stimulation--a Stroke Case Series. J Med Syst 2015, 39, 205. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A. Simultaneous Functional Near-Infrared Spectroscopy (FNIRS) and Electroencephalogram (EEG) to Elucidate Neurovascular Modulation by Transcranial Electrical Stimulation (TES). Brain Stimul 2021, 14, 1093–1094. [Google Scholar] [CrossRef] [PubMed]
- Grubb, S.; Cai, C.; Hald, B.O.; Khennouf, L.; Murmu, R.P.; Jensen, A.G.K.; Fordsmann, J.; Zambach, S.; Lauritzen, M. Precapillary Sphincters Maintain Perfusion in the Cerebral Cortex. Nat Commun 2020, 11, 395. [Google Scholar] [CrossRef]
- Zambach, S.A.; Cai, C.; Helms, H.C.C.; Hald, B.O.; Dong, Y.; Fordsmann, J.C.; Nielsen, R.M.; Hu, J.; Lønstrup, M.; Brodin, B.; et al. Precapillary Sphincters and Pericytes at First-Order Capillaries as Key Regulators for Brain Capillary Perfusion. Proc Natl Acad Sci U S A 2021, 118, e2023749118. [Google Scholar] [CrossRef]
- Lundgaard, I.; Li, B.; Xie, L.; Kang, H.; Sanggaard, S.; Haswell, J.D.R.; Sun, W.; Goldman, S.; Blekot, S.; Nielsen, M.; et al. Direct Neuronal Glucose Uptake Heralds Activity-Dependent Increases in Cerebral Metabolism. Nat Commun 2015, 6, 6807. [Google Scholar] [CrossRef]
- Drew, P.J.; Mateo, C.; Turner, K.L.; Yu, X.; Kleinfeld, D. Ultra-Slow Oscillations in FMRI and Resting-State Connectivity: Neuronal and Vascular Contributions and Technical Confounds. Neuron 2020, 107, 782–804. [Google Scholar] [CrossRef] [PubMed]
- van Veluw, S.J.; Hou, S.S.; Calvo-Rodriguez, M.; Arbel-Ornath, M.; Snyder, A.C.; Frosch, M.P.; Greenberg, S.M.; Bacskai, B.J. Vasomotion as a Driving Force for Paravascular Clearance in the Awake Mouse Brain. Neuron 2020, 105, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Dagar, S.; Chowdhury, S.R.; Bapi, R.S.; Dutta, A.; Roy, D. Near-Infrared Spectroscopy – Electroencephalography-Based Brain-State-Dependent Electrotherapy: A Computational Approach Based on Excitation–Inhibition Balance Hypothesis. Front Neurol 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- A ‘Phase Zero’ Human Brain Organoid Platform for Neuroengineering Optical Theranostics | NYC Neuromodulation Online 2020. Available online: https://neuromodec.com/nyc-neuromodulation-online-2020 (accessed on 19 April 2021).
- Cai, C.; Zambach, S.A.; Grubb, S.; Thomsen, K.J.; Lind, B.L.; Hald, B.O.; Lønstrup, M.; Nielsen, R.M.; Lauritzen, M.J. Impaired Dynamics of Brain Precapillary Sphincters and Pericytes at First Order Capillaries Explains Reduced Neurovascular Functions in Aging 2021, 2021.08.05.455300.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





