Submitted:
12 June 2023
Posted:
13 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants and Recruitment
2.2. Study Assessments and Timeline
2.3. Biometrics and Dietary Recall
2.4. Statistical Analysis
3. Results
3.1. Patient characteristics
3.2. Excess Weight Loss and Responder Status
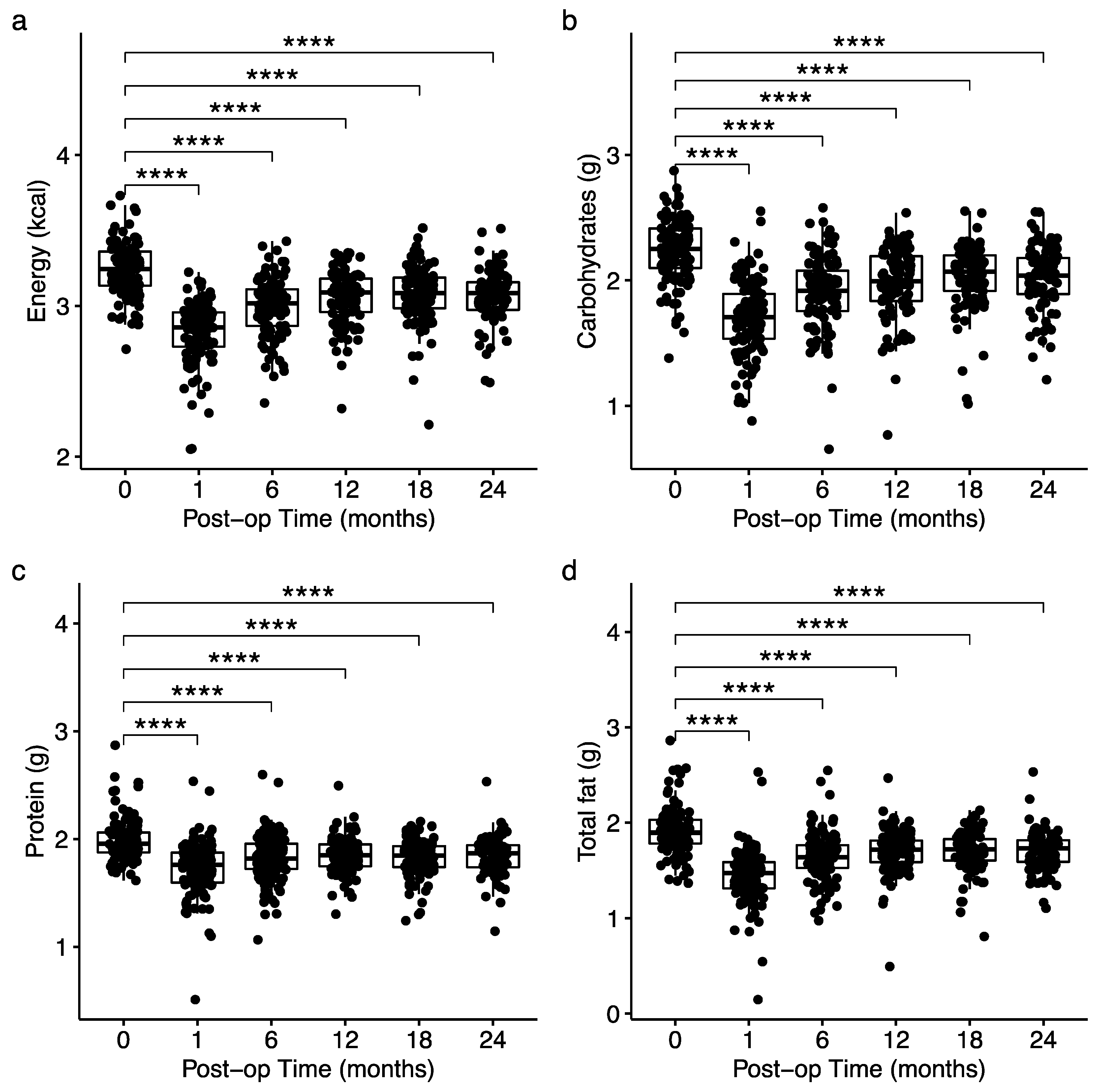
3.3. Changes in Total Energy and Macronutrient Intake
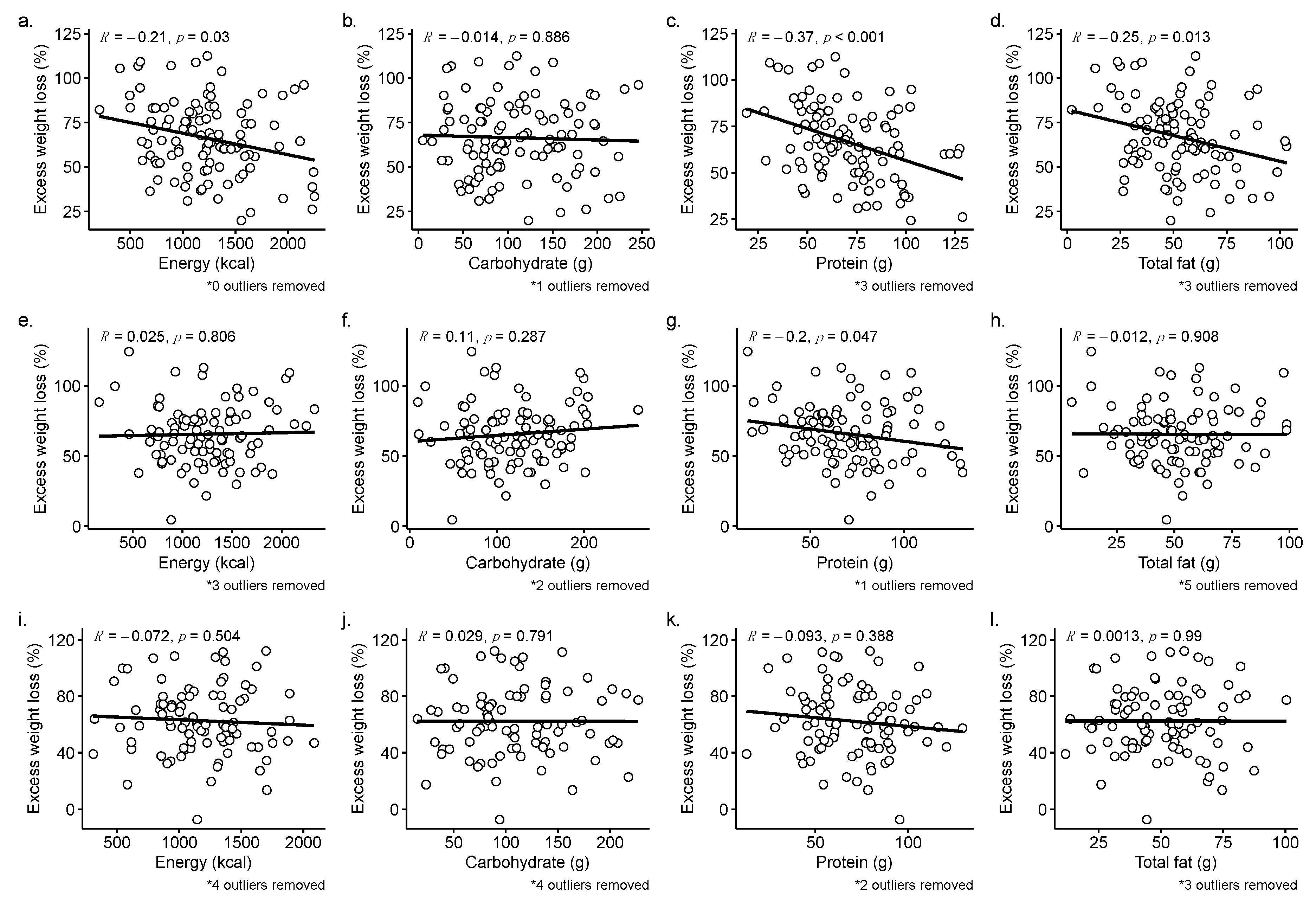
3.4. Direct Associations between Nutrient Intake and %EWL at 12-, 18-, and 24-months
3.5. Changes in Total Energy and Macronutrient Intake over Time by Weight Loss Response
3.5.1. Dietary Intake and Weight Loss Outcomes at 12 months
3.5.2. Dietary Intake and Weight Loss Outcomes at 18 months
3.5.3. Dietary Intake and Weight Loss Outcomes at 24 months
3.6. Differences in Sample Size and Power do not explain Differences in outcome between 12 and 24 months
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- King, W.C.; Hinerman, A.S.; White, G.E.; Courcoulas, A.P.; Saad, M.A.B.; Belle, S.H. Associations Between Physical Activity and Changes in Weight Across 7 Years After Roux-En-Y Gastric Bypass Surgery: A Multicenter Prospective Cohort Study. Annals of Surgery 2022, 275, 718–726. [CrossRef]
- Madsbad, S.; Holst, J.J. Bariatric Surgery—Which Procedure Is the Optimal Choice? The Lancet 2019, 393, 1263–1264. [CrossRef]
- American Society for Metabolic and Bariatric Surgery Estimate of Bariatric Surgery Numbers, 2011-2020 Available online: https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers.
- Reinhold, R.B. Critical Analysis of Long Term Weight Loss Following Gastric Bypass. Surg Gynecol Obstet 1982, 155, 385–394.
- van de Laar, A.; de Caluwé, L.; Dillemans, B. Relative Outcome Measures for Bariatric Surgery. Evidence Against Excess Weight Loss and Excess Body Mass Index Loss from a Series of Laparoscopic Roux-En-Y Gastric Bypass Patients. OBES SURG 2011, 21, 763–767. [CrossRef]
- Hall, K.D.; Kahan, S. Maintenance of Lost Weight and Long-Term Management of Obesity. Med Clin North Am 2018, 102, 183–197. [CrossRef]
- Ruiz-Lozano, T.; Vidal, J.; de Hollanda, A.; Scheer, F.A.J.L.; Garaulet, M.; Izquierdo-Pulido, M. Timing of Food Intake Is Associated with Weight Loss Evolution in Severe Obese Patients after Bariatric Surgery. Clinical Nutrition 2016, 35, 1308–1314. [CrossRef]
- Handzlik-Orlik, G.; Holecki, M.; Orlik, B.; Wyleżoł, M.; Duława, J. Nutrition Management of the Post–Bariatric Surgery Patient. Nutrition in Clinical Practice 2015, 30, 383–392. [CrossRef]
- Sherf Dagan, S.; Goldenshluger, A.; Globus, I.; Schweiger, C.; Kessler, Y.; Kowen Sandbank, G.; Ben-Porat, T.; Sinai, T. Nutritional Recommendations for Adult Bariatric Surgery Patients: Clinical Practice. Advances in Nutrition 2017, 8, 382–394. [CrossRef]
- Miller, G.D.; Norris, A.; Fernandez, A. Changes in Nutrients and Food Groups Intake Following Laparoscopic Roux-En-Y Gastric Bypass (RYGB). OBES SURG 2014, 24, 1926–1932. [CrossRef]
- Raatz, S.K.; Johnson, L.K.; Caliquary, A.; King, W.C.; Kalarchian, M.A.; Devlin, M.J.; Marcus, M.D.; Mitchell, J.E. Reported Nutrient Intake over 7 Years after Roux-En-Y Gastric Bypass in the Longitudinal Assessment of Bariatric Surgery-3 (LABS-3) Psychosocial Study. Surg Obes Relat Dis 2020, 16, 1022–1029. [CrossRef]
- Kanerva, N.; Larsson, I.; Peltonen, M.; Lindroos, A.-K.; Carlsson, L.M. Changes in Total Energy Intake and Macronutrient Composition after Bariatric Surgery Predict Long-Term Weight Outcome: Findings from the Swedish Obese Subjects (SOS) Study. The American Journal of Clinical Nutrition 2017, 106, 136–145. [CrossRef]
- Karmali, S.; Brar, B.; Shi, X.; Sharma, A.M.; de Gara, C.; Birch, D.W. Weight Recidivism Post-Bariatric Surgery: A Systematic Review. OBES SURG 2013, 23, 1922–1933. [CrossRef]
- Courcoulas, A.P.; Christian, N.J.; Belle, S.H.; Berk, P.D.; Flum, D.R.; Garcia, L.; Horlick, M.; Kalarchian, M.A.; King, W.C.; Mitchell, J.E.; et al. Weight Change and Health Outcomes at 3 Years After Bariatric Surgery Among Individuals With Severe Obesity. JAMA 2013, 310, 2416–2425. [CrossRef]
- Lim, H.-S.; Kim, Y.J.; Lee, J.; Yoon, S.-J.; Lee, B. Establishment of Adequate Nutrient Intake Criteria to Achieve Target Weight Loss in Patients Undergoing Bariatric Surgery. Nutrients 2020, 12, 1774. [CrossRef]
- Heinberg, L.J.; Bond, D.S.; Carroll, I.; Crosby, R.; Fodor, A.; Fouladi, F.; Gunstad, J.; Mitchell, J.; Peat, C.; Steffen, K. Identifying Mechanisms That Predict Weight Trajectory after Bariatric Surgery: Rationale and Design of the BioBehavioral Trial. Surg Obes Relat Dis 2020, 16, 1816–1826. [CrossRef]
- National Institutes of Health Automated Self-Administered 24-Hour (ASA24®) Dietary Assessment Tool Available online: https://epi.grants.cancer.gov/asa24/.
- Widaman, A.M.; Keim, N.L.; Burnett, D.J.; Miller, B.; Witbracht, M.G.; Widaman, K.F.; Laugero, K.D. A Potential Tool for Clinicians; Evaluating a Computer-Led Dietary Assessment Method in Overweight and Obese Women during Weight Loss. Nutrients 2017, 9, 218. [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing 2018.
- Courcoulas, A.; Coley, R.Y.; Clark, J.M.; McBride, C.L.; Cirelli, E.; McTigue, K.; Arterburn, D.; Coleman, K.J.; Wellman, R.; Anau, J.; et al. Interventions and Operations 5 Years After Bariatric Surgery in a Cohort From the US National Patient-Centered Clinical Research Network Bariatric Study. JAMA Surgery 2020, 155, 194–204. [CrossRef]
- Gutiérrez-Repiso, C.; Molina-Vega, M.; Bernal-López, M.R.; Garrido-Sánchez, L.; García-Almeida, J.M.; Sajoux, I.; Moreno-Indias, I.; Tinahones, F.J. Different Weight Loss Intervention Approaches Reveal a Lack of a Common Pattern of Gut Microbiota Changes. Journal of Personalized Medicine 2021, 11, 109. [CrossRef]
- Lanyon, R.I.; Maxwell, B.M.; Kraft, A.J. Prediction of Long-Term Outcome after Gastric Bypass Surgery. OBES SURG 2009, 19, 439–445. [CrossRef]
- Pinto-Bastos, A.; de Lourdes, M.; Brandão, I.; Machado, P.P.P.; Conceição, E.M. Weight Loss Trajectories and Psychobehavioral Predictors of Outcome of Primary and Reoperative Bariatric Surgery: A 2-Year Longitudinal Study. Surgery for Obesity and Related Diseases 2019, 15, 1104–1112. [CrossRef]
- Colles, S.L.; Dixon, J.B.; O’Brien, P.E. Grazing and Loss of Control Related to Eating: Two High-Risk Factors Following Bariatric Surgery. Obesity 2008, 16, 615–622. [CrossRef]
- Fouladi, F.; Carroll, I.M.; Sharpton, T.J.; Bulik-Sullivan, E.; Heinberg, L.; Steffen, K.; Fodor, A.A. A Microbial Signature Following Bariatric Surgery Is Robustly Consistent Across Multiple Cohorts. medRxiv 2020, 2020.11.12.20230581. [CrossRef]
- Fouladi, F.; Brooks, A.E.; Fodor, A.A.; Carroll, I.M.; Bulik-Sullivan, E.C.; Tsilimigras, M.C.B.; Sioda, M.; Steffen, K.J. The Role of the Gut Microbiota in Sustained Weight Loss Following Roux-En-Y Gastric Bypass Surgery. OBES SURG 2019, 29, 1259–1267. [CrossRef]
- Marceau, P.; Hould, F.-S.; Simard, S.; Lebel, S.; Bourque, R.-A.; Potvin, M.; Biron, S. Biliopancreatic Diversion with Duodenal Switch. World J. Surg. 1998, 22, 947–954. [CrossRef]
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A.; et al. The US Department of Agriculture Automated Multiple-Pass Method Reduces Bias in the Collection of Energy Intakes. The American Journal of Clinical Nutrition 2008, 88, 324–332. [CrossRef]
- Yuan, C.; Spiegelman, D.; Rimm, E.B.; Rosner, B.A.; Stampfer, M.J.; Barnett, J.B.; Chavarro, J.E.; Rood, J.C.; Harnack, L.J.; Sampson, L.K.; et al. Relative Validity of Nutrient Intakes Assessed by Questionnaire, 24-Hour Recalls, and Diet Records as Compared With Urinary Recovery and Plasma Concentration Biomarkers: Findings for Women. American Journal of Epidemiology 2018, 187, 1051–1063. [CrossRef]
- Foster, E.; Lee, C.; Imamura, F.; Hollidge, S.E.; Westgate, K.L.; Venables, M.C.; Poliakov, I.; Rowland, M.K.; Osadchiy, T.; Bradley, J.C.; et al. Validity and Reliability of an Online Self-Report 24-h Dietary Recall Method (Intake24): A Doubly Labelled Water Study and Repeated-Measures Analysis. J Nutr Sci 2019, 8, e29. [CrossRef]
- Grivetti, L.E. Culture, Diet, and Nutrition: Selected Themes and Topics. BioScience 1978, 28, 171–177. [CrossRef]
- Inocian, E.P.; Nolfi, D.A.; Felicilda-Reynaldo, R.F.D.; Bodrick, M.M.; Aldohayan, A.; Kalarchian, M.A. Bariatric Surgery in the Middle East and North Africa: Narrative Review with Focus on Culture-Specific Considerations. Surgery for Obesity and Related Diseases 2021, 17, 1933–1941. [CrossRef]
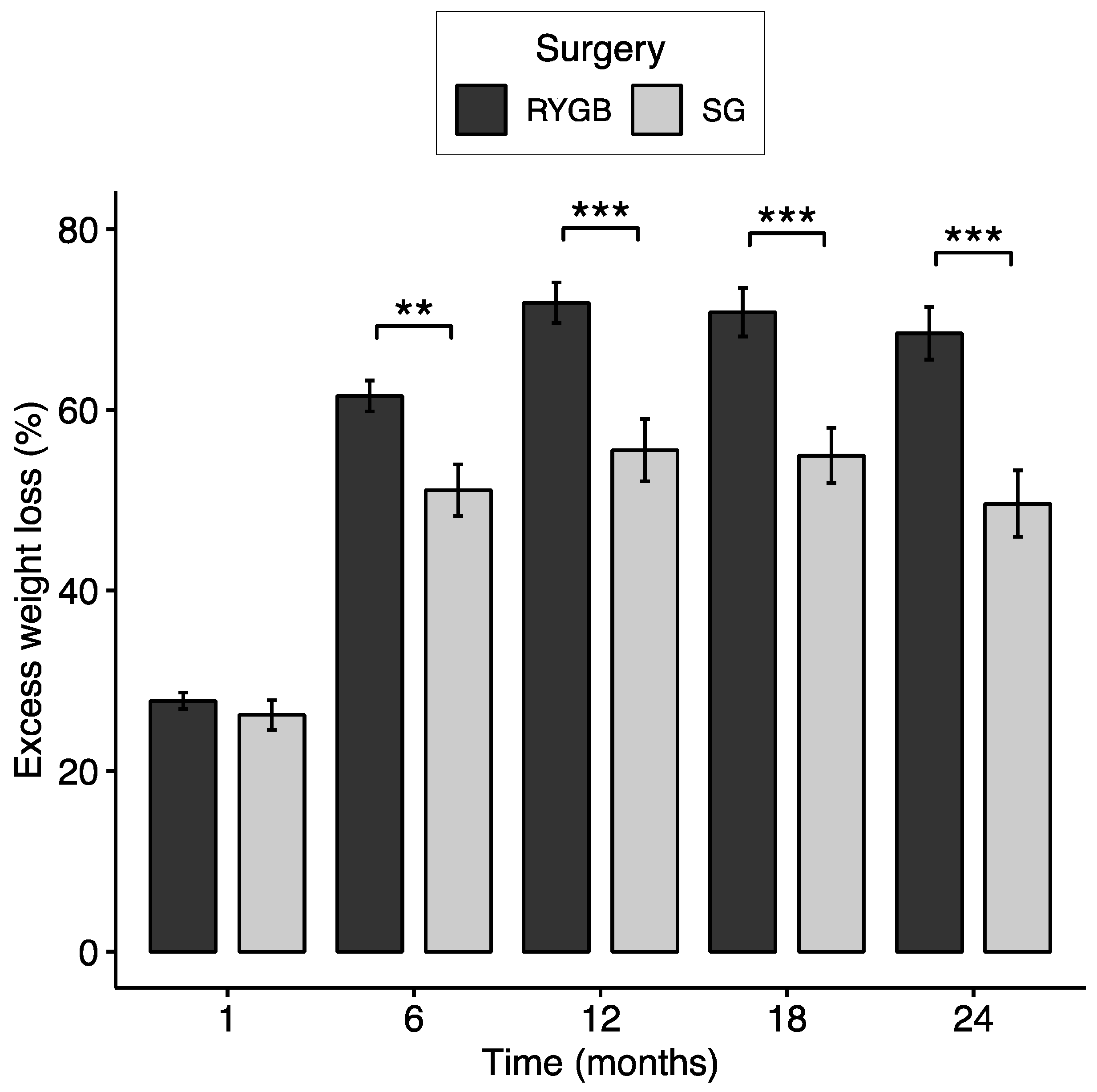
| Timepoint | n | Weight (kg) | BMI (kg/m2) | Excess weight (kg) | Percent loss (%) | EWL (%) | ||
|---|---|---|---|---|---|---|---|---|
| Baseline | 124 | 131.64 ± 25.21 | 45.93 ± 6.79 | 60.1 ± 21.17 | - | - | ||
| RYGB – 87 | 129.51 ± 21.58 | 45.28 ± 5.66 | 58.05 ± 17.56 | - | - | |||
| SG – 37 | 136.66 ± 31.97 | 47.46 ± 8.82 | 64.93 ± 27.58 | - | - | |||
| p = 0.219 | p = 0.348 | p = 0.263 | ||||||
| Postop 1 month | 122 | 115.89 ± 22.41 | 40.52 ± 6.37 | 44.43 ± 19.01 | 11.81 ± 3.44 | 27.32 ± 8.94 | ||
| RYGB – 86 | 114.07 ± 18.46 | 39.9 ± 5.02 | 42.59 ± 14.86 | 12.02 ± 3.4 | 27.77 ± 8.49 | |||
| SG – 36 | 120.23 ± 29.68 | 41.99 ± 8.72 | 48.82 ± 26.19 | 11.31 ± 3.55 | 26.23 ± 9.96 | |||
| p = 0.295 | p = 0.485 | p = 0.386 | p = 0.117 | p = 0.341 | ||||
| Postop 6 months | 114 | 97.85 ± 20.93 | 34.37 ± 6.09 | 26.77 ± 17.87 | 25.3 ± 5.95 | 58.32 ± 16.36 | ||
| RYGB – 79 | 94.29 ± 15.76 | 33.26 ± 4.58 | 23.39 ± 12.91 | 26.69 ± 5.66 | 61.53 ± 15.16 | |||
| SG – 35 | 105.88 ± 28.12 | 36.88 ± 8.1 | 34.4 ± 24.35 | 22.15 ± 5.43 | 51.09 ± 16.88 | |||
| p = 0.032 | p = 0.024 | p = 0.020 | p < 0.001 | p = 0.002 | ||||
| Postop 12 months | 110 | 93.13 ± 21.66 | 32.69 ± 6.34 | 22.05 ± 18.66 | 28.79 ± 7.99 | 66.51 ± 21.19 | ||
| RYGB – 74 | 88.32 ± 16.32 | 31.12 ± 4.68 | 17.43 ± 13.38 | 31 ± 7.29 | 71.85 ± 19.44 | |||
| SG – 36 | 103.02 ± 27.5 | 35.91 ± 7.99 | 31.55 ± 23.93 | 24.23 ± 7.52 | 55.53 ± 20.64 | |||
| p = 0.004 | p = 0.002 | p = 0.002 | p < 0.001 | p < 0.001 | ||||
| Postop 18 months | 101 | 93.31 ± 21.76 | 32.73 ± 6.16 | 22.28 ± 18.37 | 28.25 ± 8.48 | 65.46 ± 21.93 | ||
| RYGB – 67 | 88.61 ± 17.32 | 31.21 ± 4.86 | 17.78 ± 14.21 | 30.46 ± 8.57 | 70.81 ± 21.98 | |||
| SG – 34 | 102.58 ± 26.5 | 35.73 ± 7.32 | 31.14 ± 22.29 | 23.89 ± 6.45 | 54.93 ± 17.86 | |||
| p = 0.009 | p = 0.003 | p = 0.003 | p < 0.001 | p < 0.001 | ||||
| Postop 24 months | 93 | 94.87 ± 21.64 | 33.36 ± 6.41 | 23.91 ± 18.89 | 27.25 ± 9.26 | 62.59 ± 23.8 | ||
| RYGB – 64 | 89.3 ± 17.45 | 31.62 ± 5.03 | 18.83 ± 14.76 | 29.45 ± 9.01 | 68.47 ± 23.24 | |||
| SG – 29 | 107.18 ± 25 | 37.19 ± 7.5 | 35.12 ± 22.2 | 22.42 ± 7.99 | 49.62 ± 19.82 | |||
| p = 0.001 | p = 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | ||||
| Responder | Suboptimal responder | |||||
|---|---|---|---|---|---|---|
| (n = 85) | (n = 25) | R2 | p-value | |||
| Baseline | Weight (kg) | 126.01 ± 21.13 | 146.67 ± 31.57 | 0.118 | 0.002 | |
| Energy (kcal) | 1865.58 ± 842.97 | 1973.37 ± 756.42 | 0.003 | 0.392 | ||
| Carbohydrates (g) | 198.29 ± 119.14 (43.7%) | 209.78 ± 101.33 (46.5%) | 0.002 | 0.448 | ||
| Protein (g) | 109.6 ± 86.56 (27.7%) | 117.28 ± 70.75 (30.2%) | 0.002 | 0.267 | ||
| Total fat (g) | 98.8 ± 94.24 (52.4%) | 111.46 ± 70.2 (62.8%) | 0.004 | 0.053 | ||
| Postop | Weight (kg) | 110.27 ± 18.12 | 130.76 ± 29.13 | 0.143 | 0.001 | |
| 1 month | Energy (kcal) | 668.69 ± 253.94 | 930.85 ± 295.61 | 0.148 | <0.001 | |
| Carbohydrates (g) | 58.41 ± 50.72 (35.8%) | 79.96 ± 47.85 (33.5%) | 0.031 | 0.013 | ||
| Protein (g) | 59.97 ± 45.52 (36.8%) | 64.17 ± 22.47 (28.9%) | 0.002 | 0.105Δ | ||
| Total fat (g) | 33.71 ± 44.01 (46.7%) | 39.33 ± 17.15 (37.5%) | 0.003 | 0.007 | ||
| Postop | Weight (kg) | 91.35 ± 15.01 | 119.82 ± 25.38 | 0.311 | <0.001 | |
| 6 months | Energy (kcal) | 1021.7 ± 427.11 | 1285.95 ± 458.12 | 0.06 | 0.009Δ | |
| Carbohydrates (g) | 91.51 ± 63.07 (35.1%) | 110.9 ± 54.65 (34.4%) | 0.017 | 0.038Δ | ||
| Protein (g) | 74.4 ± 53.24 (30.9%) | 76.09 ± 28.62 (24.0%) | <0.001 | 0.247 | ||
| Total fat (g) | 50.88 ± 48.29 (45.0%) | 59.21 ± 25.22 (41.2%) | 0.006 | 0.017Δ | ||
| Postop | Weight (kg) | 85.72 ± 14.63 | 118.35 ± 22.9 | 0.402 | <0.001 | |
| 12 months | Energy (kcal) | 1156.08 ± 416.19 | 1436.55 ± 466.82 | 0.072 | 0.018Δ | |
| Carbohydrates (g) | 110.25 ± 61.81 (36.8%) | 116.61 ± 58.31 (31.1%) | 0.002 | 0.685Δ | ||
| Protein (g) | 71.27 ± 36.12 (26.0%) | 85.48 ± 24.49 (25.1%) | 0.031 | 0.002Δ | ||
| Total fat (g) | 52.42 ± 33.14 (40.6%) | 66.43 ± 25.31 (41.5%) | 0.034 | 0.005Δ |
| Responder | Suboptimal responder | ||||
|---|---|---|---|---|---|
| (n = 64) | (n = 29) | R2 | p-value | ||
| Baseline | Weight (kg) | 126.81 ± 19.42 | 138.62 ± 32.7 | 0.049 | 0.121 |
| Energy (kcal) | 1807.96 ± 681.19 | 1820.37 ± 762.13 | <0.001 | 0.879 | |
| Carbohydrates (g) | 179.03 ± 81.77 (38.7%) | 222.75 ± 161.77 (53%) | 0.032 | 0.673 | |
| Protein (g) | 91.19 ± 28.57 (21.8%) | 141.41 ± 141 (38.3%) | 0.077 | 0.167 | |
| Total fat (g) | 76.24 ± 31.16 (37.7%) | 133.3 ± 142.57 (79.2%) | 0.094 | 0.048 | |
| Postop | Weight (kg) | 110.99 ± 16.18 | 124.25 ± 30.1 | 0.077 | 0.042 |
| 1 month | Energy (kcal) | 676.96 ± 263.61 | 833.98 ± 291.5 | 0.068 | 0.011 |
| Carbohydrates (g) | 58.46 ± 49.11 (34.8%) | 69.71 ± 41.96 (32.8%) | 0.012 | 0.094 | |
| Protein (g) | 58.87 ± 42.55 (36.9%) | 62.09 ± 24.22 (30.4%) | 0.002 | 0.229Δ | |
| Total fat (g) | 32.14 ± 41.13 (45%) | 34.11 ± 15.19 (36.6%) | 0.001 | 0.091 | |
| Postop | Weight (kg) | 92 ± 14.54 | 110.37 ± 27.77 | 0.162 | <0.001 |
| 6 months | Energy (kcal) | 1016.07 ± 434.96 | 1206.94 ± 488.45 | 0.037 | 0.053Δ |
| Carbohydrates (g) | 90.74 ± 67.43 (35.1%) | 99.74 ± 53.14 (33%) | 0.004 | 0.205Δ | |
| Protein (g) | 77.92 ± 60.31 (32.5%) | 75.54 ± 31.86 (25.5%) | <0.001 | 0.39 | |
| Total fat (g) | 53.3 ± 55.04 (47.7%) | 54.91 ± 26.24 (40.2%) | <0.001 | 0.086Δ | |
| Postop | Weight (kg) | 85.46 ± 14.82 | 109.01 ± 26.16 | 0.252 | <0.001 |
| 12 months | Energy (kcal) | 1143.68 ± 426.09 | 1338 ± 476.48 | 0.04 | 0.141Δ |
| Carbohydrates (g) | 108.7 ± 66.45 (36.3%) | 109.96 ± 57.76 (31.6%) | <0.001 | 0.905Δ | |
| Protein (g) | 72.11 ± 38.97 (26.9%) | 84.65 ± 25.65 (26.9%) | 0.026 | 0.005Δ | |
| Total fat (g) | 52.15 ± 36.69 (40.8%) | 61.4 ± 27.24 (40.8%) | 0.016 | 0.109Δ | |
| Postop | Weight (kg) | 85.99 ± 14.88 | 110.35 ± 24.31 | 0.28 | <0.001 |
| 18 months | Energy (kcal) | 1261.91 ± 498.21 | 1364.14 ± 530.58 | 0.009 | 0.467 |
| Carbohydrates (g) | 121.91 ± 61.32 (37.4%) | 121.67 ± 60.63 (35.3%) | <0.001 | 0.754 | |
| Protein (g) | 65.56 ± 21.83 (22.7%) | 84.3 ± 28.94 (25.2%) | 0.115 | 0.003 | |
| Total fat (g) | 53.67 ± 23.26 (38.3%) | 59.44 ± 25.43 (39.1%) | 0.012 | 0.402 | |
| Postop | Weight (kg) | 86.36 ± 14.77 | 113.67 ± 22.7 | 0.346 | <0.001 |
| 24 months | Energy (kcal) | 1218.42 ± 472.41 | 1296.49 ± 542.18 | 0.005 | 0.451 |
| Carbohydrates (g) | 116.14 ± 62.55 (39.9%) | 121.33 ± 71.11 (36.3%) | 0.001 | 0.808 | |
| Protein (g) | 75.65 ± 41.86 (28.6%) | 71.23 ± 22.48 (23.9%) | 0.003 | 0.976 | |
| Total fat (g) | 56.64 ± 43.52 (47.6%) | 53.28 ± 21.87 (38%) | 0.002 | 0.724 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




