1. Introduction
Transseptal puncture (TSP) is currently the gold standard for endovascular procedures that require catheterization of the left atrium (LA). Although its development began almost 100 years ago, recent years brought substantial advancement in the field providing necessary tools for dealing with difficult scenarios as well as increasing the safety and efficacy of the procedure. Even though the TSP is associated with a high success rate and low risk of adverse events, it is of paramount importance for the physician to be aware of these potential complications and take appropriate measures to prevent them. The trend towards performing minimally invasive procedures, the development of new interatrial devices and the need for further simplification of the technique forces rapid progress in the field. In this paper we discuss the evolution of TSP, available techniques, potential complications and current state of the art.
2. Brief History
The first documented cardiac catheterization in a live human was performed almost 100-years ago. In 1929, a 25-year-old surgical trainee Werner Forssmann introduced a 65-cm catheter into his own right atrium through one of his left antecubital veins under fluoroscopy guidance [
1]. The technique was further refined for diagnostic purposes allowing for direct measurement of pressure in right heart chambers. As a result, in 1956, Forssmann together with Cournand and Richards were jointly awarded the Nobel Prize "for their discoveries concerning heart catheterization and pathological changes in the circulatory system”.
It was not until 1947 that Cournand first described transseptal access through a septal defect into the left atrium and pulmonary veins for recording blood pressure [
2]. In 1950 Zimmerman and others [
3] described their method of introducing a catheter through the radial artery and the aorta for left ventricle catheterization. The following years brought further developments in the field. In 1953 Seldinger published a paper presenting his new technique of percutaneous vascular access for angiography [
4], which was later adopted for heart catheterization procedures. The earliest techniques of accessing the left side of the heart also included subxiphoid and apical approach for direct percutaneous puncture of the left ventricle. In order to directly reach the left atrium transbronchial, posterior transthoracic or suprasternal access were used [
5]. The development of the transseptal puncture technique for obtaining a LA access began in the late 1950s. In 1959 Ross and Cope published their papers describing a novel technique for accessing the left atrium through the right atrium with a femoral vein approach by puncturing the interatrial septum with a specially designed needle [
6] [
7]. In 1960, Brockenbrough together with Braunwald published their paper presenting an improved approach using a modified transseptal needle allowing for easier left heart access and manipulation [
8], which was further developed in the following years [
9] [
10]. This breakthrough marked the beginning of a new era in cardiac procedures, as it allowed for safer access to the left side of the heart and paved the way for further advancements in the field.
3. Interatrial Septum Development and Anatomy
The formation of the interatrial septum takes place in the first months of fetal development. It is formed by the fusion of the primary atrial septum, originating from the roof of the atrium, and the secondary septum, created by the infolding of the superior caval vein and right pulmonary veins into the atria. The primary septum in postnatal life becomes the floor of the fossa ovalis (FO), while the secondary septum forms its extensive antero-superior rim [
11]. The importance of these embryology considerations becomes apparent during the transseptal puncture, as the “true” interatrial septum (formed by the FO and its infero-anterior rim) represents only about 20% of the whole interatrial septum area [
12]. Puncturing other parts of the septum, created by the infoldings of the adjacent atrial walls, would lead to injury of the outer wall of the heart and potentially serious complications during the procedure [
13].
Klimek-Piotrowska et al. described four variations of the FO anatomy - “smooth” being the most common (56.3% of cases), persistent foramen ovale (24.4%), right-sided septal pouch (11.9%) and a net-like structure within the FO (7.4%) [
12]. Each of those may present a different challenge during the procedure. The FO might sometimes appear as a floppy, aneurysmal structure, which can elevate the risk of perforating the anterolateral wall of the atrium due to its excessive tenting during the transseptal puncture [
14]. On the contrary, in some cases (such as lipomatous atrial septal hypertrophy or after a previous transseptal puncture) the septum might appear as unusually stiff, requiring applying more force into the puncture and making it difficult to advance the sheath through the septum [
15] [
16]. Accessing the LA through a patent foramen ovale, which is situated in the antero-cepahalad margin of the FO, might be inadvisable since it can lead to problems with catheter and sheath maneuverability [
14]. Similar difficulties might be expected with a right-sided septal pouch, which is formed by a partial fusion of the primary and secondary septum [
17]. The presence of a well-developed net-like structure, similar to a Chiari’s network, could also impede the transseptal puncture and catheter manipulation or potentially even lead to catheter entrapment [
12] [
18].
4. Periprocedural Management
As for most invasive procedures, basic laboratory test results should be obtained and the patient should be fasting for at least 6-8 hours before the procedure. In the case of atrial fibrillation (AF) ablation therapeutic anticoagulation should be initiated at least 3 weeks prior to the procedure. Transesophageal echocardiography (TEE) or computed tomography may be used to exclude left atrial thrombus. If the patient is on oral anticoagulation (OAC), current practice guidelines recommend performing the procedure under uninterrupted anticoagulation, which proved to be a safe approach associated with low rates of stroke/TIA and bleeding complications [
19,
20,
21,
22]. Major bleeding was significantly reduced with uninterrupted direct oral anticoagulants (DOACs) compared to vitamin K antagonists [
23]. After the procedure, the first dose of OAC should be administered according to the previous patient's schedule and the antithrombotic treatment should be continued for at least 2 months, depending on the individual stroke risk profile [
24]. If the OAC was interrupted before the procedure for any reason, administration of DOAC after 3 to 5 hours following the ablation (after ensuring proper hemostasis) should be considered [
25]. Transthoracic echocardiography after the procedure should be performed in order to exclude any significant pericardial effusion following the procedure .
5. Necessary Equipment
In order to perform a transseptal puncture certain equipment must be obtained. A standard kit consists of a long, stainless steel Brockenbrough needle (with a lumen and a stylet inside of it in order to avoid puncturing the sheath while advancing the needle), a J-shaped guidewire and a pre-shaped long transseptal sheath with a dilator compatible with the needle. Depending on the procedure and individual anatomy of the patient different lengths and curvatures are available. Recent years brought substantial development in the available equipment. The use of steerable sheaths and utilization of radiofrequency (RF) energy with transseptal needles and wires allows for a successful TSP in certain challenging scenarios. The development of a dedicated transseptal system made zero-exchange workflow possible, potentially avoiding exchange-related complications and further simplifying the procedure.
6. Current Techniques for TSP
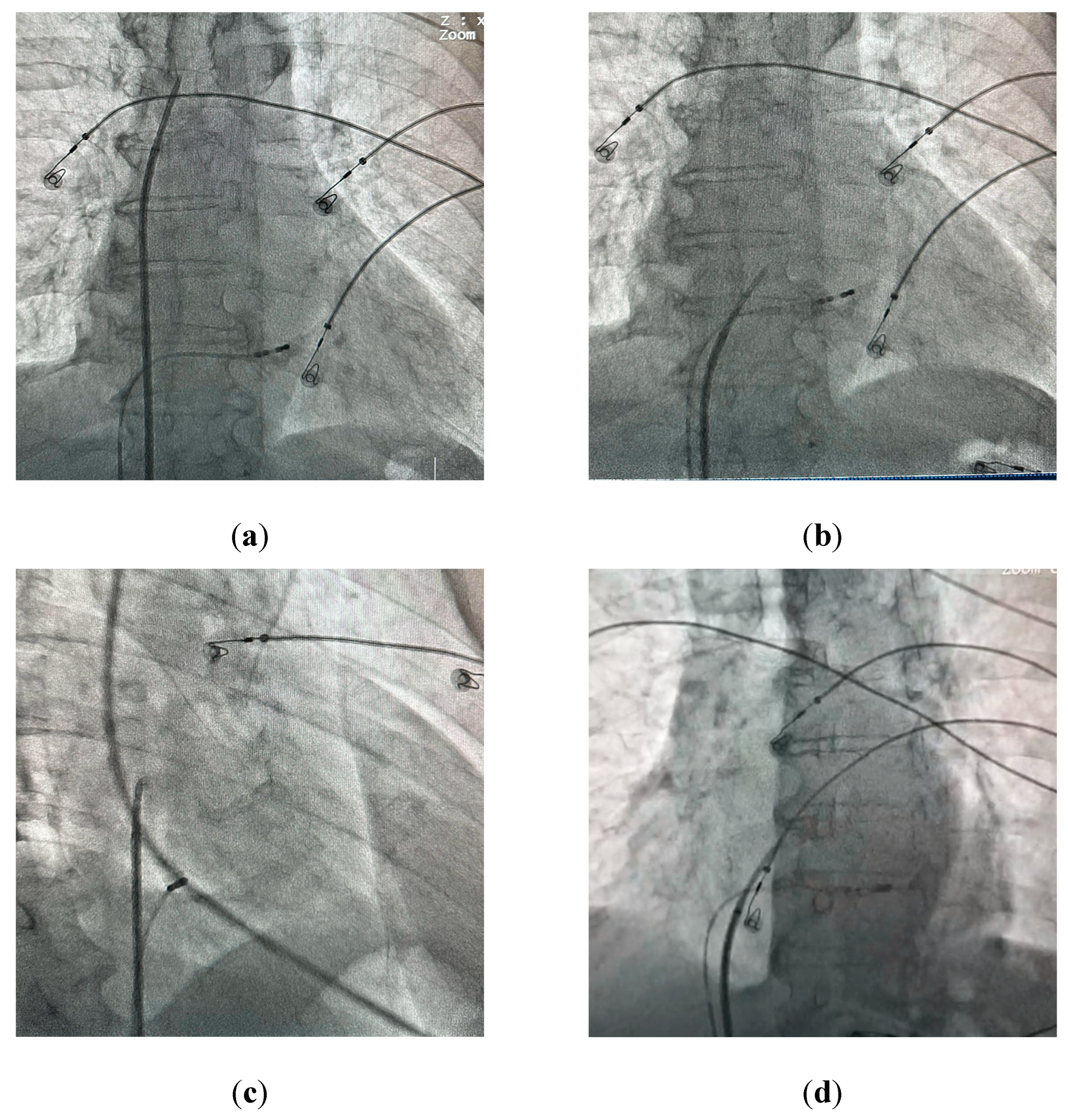
The standard technique for performing a TSP under fluoroscopic guidance involves obtaining venous access through the right femoral vein and placing a J-shaped guidewire into the superior vena cava. After advancing a transseptal sheath with a dilator over-the-wire into the superior vena cava, the guidewire is replaced with a transseptal needle with a stylet inside of it to avoid additional friction. After advancing the needle just below the tip of the dilator the stylet is removed and a syringe with contrast is connected to the needle. The System is then rotated to 4-5 o’clock (
Figure 1a) and pulled down into the right atrium under the anteroposterior (AP) fluoroscopic view. Two “jumps” are encountered on the way down into the desired position on the interatrial septum - the first one after dropping from superior vena cava into the right atrium, and the second one after reaching the fossa ovalis. The assembly should then be carefully advanced into the final position (
Figure 1b). A catheter placed in the coronary sinus is used as a landmark for the puncture; a pigtail catheter placed in the aortic root may also be used for additional orientation. Derejko et al. recently reported that a guidewire positioned between the superior and inferior vena cava can serve as another landmark guiding the TSP, as it should be running in direct vicinity to the FO in mid right anterior oblique (RAO) view [
26]. Two independent fluoroscopic angles are used to confirm the correct position on the interatrial septum (IAS). In a 40° RAO view the assembly should be aligned so that the needle runs parallel to the coronary sinus catheter (
Figure 1c). After verifying the correct position in the RAO view the needle is advanced outside the dilator under a 30° left anterior oblique (LAO) view (
Figure 1d) and contrast is administered in order to confirm reaching the left atrium. Direct pressure monitoring from the transseptal needle may also be used [
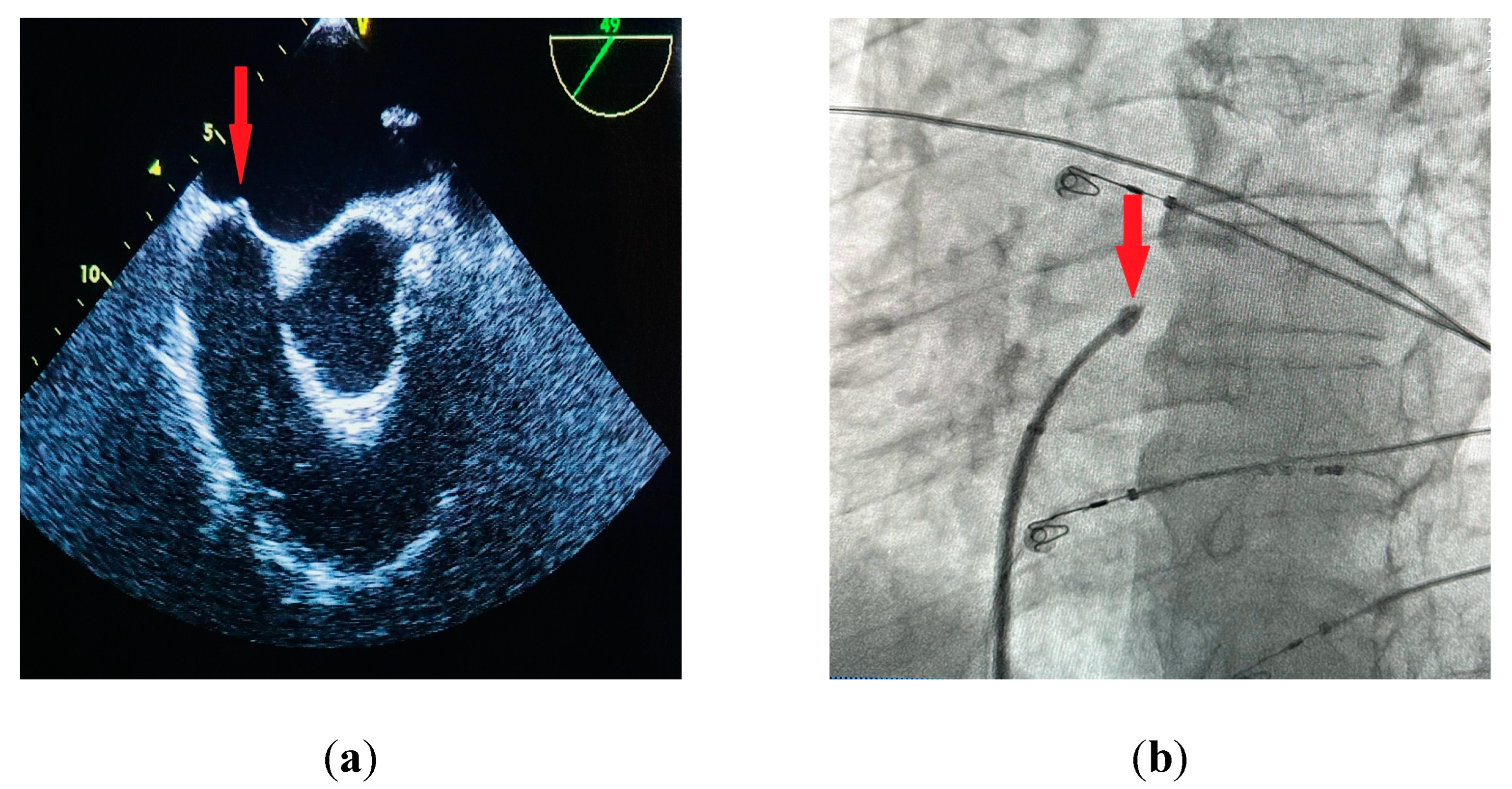
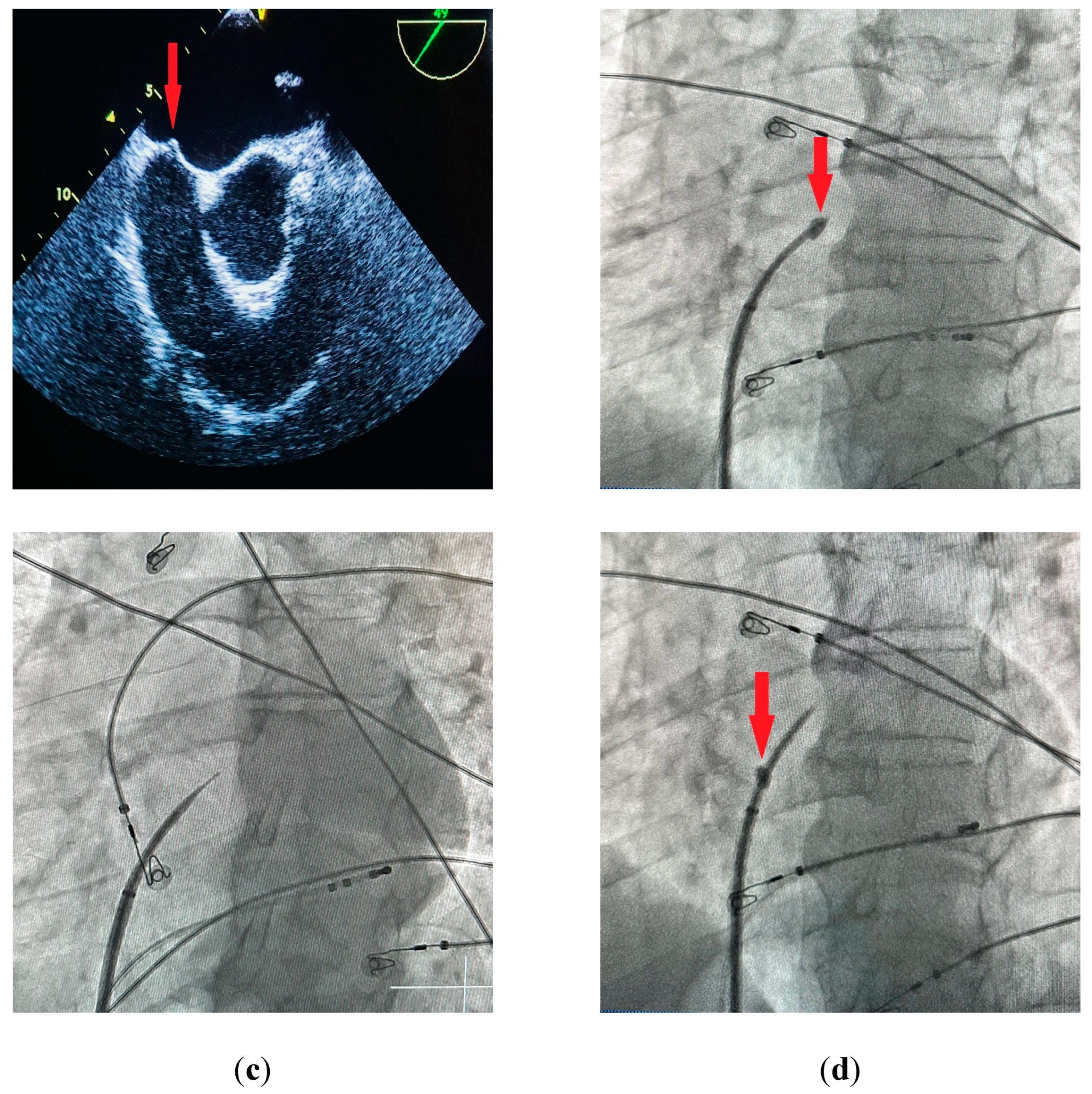
27]. IAS tenting can usually be observed before the needle passes into the left atrium (
Figure 2a,b). The sheath with a dilator is carefully advanced over the needle into the left atrium. The needle is then withdrawn and replaced with a guidewire that is usually placed deep inside the left superior pulmonary vein. At this point the transseptal sheath can be safely exchanged for a dedicated transseptal system depending on the procedure type. Other modalities for TSP include the utilization of transesophageal (TEE) [
27] or intracardiac echocardiography (ICE) [
28] for direct visualization of the needle and its orientation relating to IAS and other heart structures.
7. Alternative Techniques
A dilatator method for the TSP is an alternative to a standard needle puncture, where the needle is kept within the dilator and the puncture is performed only by forcing the blunt tip of the dilator through the fossa ovalis. In this approach the outer sheath is not used in the assembly. After withdrawing the dilatator with the needle to the right atrium and confirming the correct position with the RAO view (similar to the standard method) the assembly is gently pushed forward in order to catch the lip edge of the FO by the dilatator tip. At this point a gradually increasing force is applied. If the dilator is in the correct position the thin fibrous tissue within the fossa should easily be penetrated without the use of excessive force. Conversely, other parts of the IAS are much thicker and penetrating them would require much more effort, which should prompt the operator to adjust the position of the dilatator. The incidence of severe complications with this alternative approach was lower compared to the needle method, but it required a slightly longer procedure time [
29].
8. Anticoagulation During Procedures
Thromboembolic events are of major concern during procedures requiring left heart catheterization. Current expert consensus on catheter ablation of atrial fibrillation recommends administration of intravenous unfractionated heparin (UFH) directly prior to or immediately after TSP to target activated clotting time (ACT) of over 300 seconds [
25]. The initial dose is dependent on body mass and might vary significantly with different anticoagulation regime. Patients on warfarin and dabigatran require a lower initial dose of UFH in order to reach target ACT compared to the ones receiving rivaroxaban and apixaban - approximately 150 vs 170 IU/kg, respectively [
30]. After the procedure administration of protamine may be considered in order to reverse the effects of heparin.
9. Different Approaches for Different Procedures
Depending on the type of the procedure the TSP localization might be adjusted in order to provide optimal LA access and enable a smooth catheter manipulation. For left atrial appendage occlusion procedures more central puncture is preferred. In mitral valve repair procedures an optimal TS access should be obtained by passing through the superior and posterior-mid part of the fossa ovalis, preferably between 3.5-4 cm above the mitral annulus in order to properly align the clip delivery system [
31]. For ablation procedures the preferred puncture site might vary depending on the arrhythmia - for example posteroinferior access is more desirable during pulmonary vein isolation, although utilization of a persistent foramen ovale might also be considered [
32]. No clear benefit was shown between single or double TSP in atrial fibrillation ablation with similar rates of adverse events [
33].
10. Complications
Overall reported complication rate for TSP is very low (<1%) with a mortality rate of 0.018% [
34]. Available literature provides mostly procedure-related complication rates, without clear division into strictly TSP-related, thus making it often impossible to establish a direct association with TSP. Nevertheless, complications associated with procedures requiring TSP and left atrium catheterization are discussed below.
Ischemic stroke, transient ischemic events and silent cerebral ischemic events
Thromboembolic events during left heart procedure are of major concern and extra care should be taken to provide a proper anticoagulation. Thrombus formation immediately after the TSP has been reported [
35] which could be avoided with administration of heparin before the puncture [
36]. The reported rate of clinically significant arterial thromboembolism (mostly cerebral transient ischemic attack) associated with TSP was extremely low (0.07%) with symptoms resolving spontaneously within 12 hours [
34]. In patients undergoing transcatheter ablation for AF, transcatheter edge-to-edge repair (TEER) of the mitral valve or placement of the left atrial appendage occlusion (LAAO) devices the total rates of periprocedural stroke was 0.23-0.4% [
37] [
38], 1% [
39] and 1.1% [
40] respectively. On the contrary, reported rates of silent cerebral ischemic events (SCI) during procedures requiring catheterization of the left atrium are high, ranging from 4.2% - 26.5% in cryoballoon ablation [
41,
42] and 14% in RF ablation for pulmonary vein isolation [
43], 4.8 - 54.8% in LAAO procedures [
44,
45] up to 86% during transcatheter edge-to-edge repair of the mitral valve [
46]. Factors that have been associated with SCI are nonparoxysmal AF, noncompliance to the anticoagulation protocol [
47], subtherapeutic levels of ACT, and cardioversion to sinus rhythm during the procedure [
43], procedure duration and lower left ventricle ejection fraction [
41]. The significance of those findings is still uncertain, although some reports suggest a possible association with dementia and cognitive decline [
48,
49]. Rigorous adherence to an anticoagulation protocol with strict ACT monitoring during the procedure should be maintained in order to minimize the risk of SCI.
Cardiac tamponade and inadvertent puncture of other heart structures
Another potentially serious complication of TSP is inadvertent puncture of the aortic root and atrial wall (with reported rates of 0.05% [
50] and 0.24% respectively), which can lead to cardiac tamponade in approximately 0.09% of cases, as reported by De Ponti [
34]. Reported rates of cardiac tamponade during different procedures requiring transseptal catheterization are significantly higher (approximately 1.2-1.3% [
37,
51]), although the origin of the perforation is not always clear (i.e TSP-related vs. RF/catheter manipulation-related). After accidental needle-only puncture of other heart structures, without further advancement of the dilator/sheath, the needle can usually be safely withdrawn without further complications. It is crucial to confirm an accurate TSP with contrast injection under fluoroscopy or continuous pressure recording before advancing the sheath further, which could potentially have catastrophic consequences in case of an improper puncture. Utilization of TEE or ICE with direct needle visualization may also help to avoid inadvertent puncture of other heart structures, especially in challenging scenarios.
Iatrogenic atrial septal defect
Persistent iatrogenic atrial septal defect (iASD) after TSP is common with rates up to 87% directly after the procedure [
52], decreasing over time to 37% after a median follow-up of 2.9 years [
53] and generally should not be considered a complication, but rather an expected occurrence. The prevalence increases with more catheter manipulation across the IAS during the procedure as well as with larger sheath diameter [
54]. Although reports of patients requiring the closure of iASD exist [
55] and some studies have shown a potential benefit of concomitant closure of the defect at the end of the procedure [
56], they are generally well tolerated and clinically insignificant. In a randomized trial involving patients after transcatheter mitral valve repair closure of iASD did not improve functional or clinical midterm outcomes [
57]. Furthermore, reports are available describing positive hemodynamic effect of the newly created iASD in patients undergoing TEER of the mitral valve [
58] although recent trials describing the placement of dedicated interatrial shunt devices in patients with heart failure with preserved ejection fraction did not show any clinical benefit [
59]. No differences in adverse events associated with persistent iASD after catheter ablation for pulmonary vein isolation, implantation of LAAO devices or TEER of the mitral valve were described [
53,
60,
61,
62].
Mortality
In a multicenter survey spanning 12 years with 5520 procedures only 1 death directly associated with TSP was reported [
34]. Mortality rates for procedures requiring left atrium catheterization are 0.15 % for catheter ablation of atrial fibrillation [
37], 0.53% for placement of LAAO devices [
63] up to 1.1-4.3% for TEER of the mitral valve [
64].
11. Managing Difficult Anatomies, Recent Developments in the Field
The standard transseptal needle for use in adults usually comes in three usable lengths of 71, 89 and 98 cm with different curvatures, which allows for a better fit depending on the patient's anatomy and desired TSP localization. Although the TSP with a standard needle yields a high success rate of over 98% with a low incidence of complications [
65] certain scenarios might require a different approach. The NRG® transseptal needle (Baylis Medical) allows for a delivery of radiofrequency energy through a rounded atraumatic tip which might be helpful especially in a case of fibrotic or aneurysmal septum [
66]. In a recently published randomized trial comparing conventional vs RF transseptal needle with pigtail wire the use of the latter was associated with shorter time to left atrial access and reduced fluoroscopy time [
67]. Another option for overcoming a challenging septum is utilization of a 0.014-inch diameter sharp nitinol guidewire (SafeSept® Pressure Products Inc.), which assumes an atraumatic “J” shape after achieving a successful puncture, providing additional safety in patients at higher risk for complications [
68]. It can also be used as a bailout strategy in case of an unsuccessful TSP with conventional and RF needles [
69]. Similarly, the VersaCross® wire (Baylis Medical) allows for TSP using RF energy and simultaneously serves as an exchange rail for delivering therapy sheaths, further shortening the procedure time [
70,
71,
72]. One of the biggest pitfalls of the techniques mentioned above is the need for a sheath exchange, which is associated with prolonged procedure time and brings serious concerns about potential air aspiration during the exchange and subsequent air embolism. Recently, new techniques have emerged that allow for a zero-exchange workflow during the procedure [
73]. The VersaCross Connect™ LAAC Access Solution (Baylis Medical) dedicated for WATCHMAN FLX™ (Boston Scientific) implantation provides an exchangeless workflow during LAAO device placement [
74]. The AcQCross
TM (Medtronic) transseptal access system is compatible with most of the available therapy sheaths and allows for obtaining direct TS access (mechanical or RF-based) without the need for a sheath exchange, leading to improved procedural efficiency [
75]. A recent study has proved the feasibility and safety of a zero exchange workflow during pulse field ablation procedures by using a direct over-the-needle transseptal access with the Faradrive sheath [
76]. Similar reports are available describing a direct TSP through the FlexCath sheath with a standard transseptal needle [
77,
78] and a SafeSept wire [
79].
Despite the development of new modalities for TSP, certain scenarios can still prove to be challenging. In patients with extra stiff septum, after a successful puncture of the IAS, problems with advancing the sheath into the LA may be encountered. Exchanging for a stiff guidewire or repositioning the wire into the right superior pulmonary vein can provide a different angle of approach, leading to successful sheath insertion. If the above maneuvers prove to be unsuccessful, additional dilation of the IAS before advancing the sheath might be necessary. One of the available techniques for dilating the septum is the “shoehorn” maneuver, utilizing an electrophysiological catheter already being used for the procedure. After performing the TSP and placing a guidewire in the left superior pulmonary vein, the transseptal sheath is withdrawn and the catheter is advanced through the puncture site into the LA by following the guidewire under fluoroscopy guidance. The sheath is then advanced and pressure is applied on the IAS while simultaneously withdrawing the catheter into the right atrium, which enables the sheath to pass through the septum [
80]. Another possible technique is an atrial balloon septoplasty using a noncompliant balloon advanced over a stiff wire through the septum [
81] or a sequential dilation of the IAS with progressively larger bore dilators. The former though requires additional sheath exchange which, if possible, should be avoided during LA catheterization.
Patients who have undergone implantation of atrial septal defect (ASD) closure devices and require transseptal left atrial catheterization form a relatively small subset. Although challenging, the TSP in such cases is still a feasible approach. In the case of smaller devices a puncture posteroinferior to the occluder is preferred. If the device is >26mm a direct TSP through the device might be required [
82], preferably away from the attachment hub in the case of a double-disc occluder [
83] .
12. Conclusions and Future Directions
Although its development began in the early previous century, the TSP technique has undergone remarkable progress in recent years, following the rapid evolution of minimally-invasive techniques for left heart procedures, while new techniques are still being researched. Currently there are ongoing clinical trials investigating the safety and efficacy of transcatheter microguidewire drilling (TMD) for transseptal left atrial access in patients with abnormal atrial septal morphology [
84] and evaluating the usefulness of 3D printing of both atria for optimum TSP site planning before the procedure [
85].
The availability of new modalities provides operators with a wider range of options for addressing challenging scenarios which has further increased the safety and efficacy of the procedure. Novel techniques that allow for direct needle and heart structures visualization during the procedure (such as electroanatomical mapping and the utilization of ICE) made fluoroless TSP possible, removing one of its pitfalls [
86,
87,
88,
89] and helping with more site-specific puncture [
90,
91]. Recently, the first attempts at implementing augmented reality (AR) for transseptal puncture during cardiac procedures have been described, reporting a low misalignment error rate with an AR guidance system [
92,
93,
94]. James et al. in their proof of concept feasibility study suggest that AR guidance could potentially accelerate the learning curve and provide better accuracy for the TSP in the future [
95]. The ongoing implementation of robotics in medicine provides another potential direction for cardiac procedures. There are documented reports describing the safety and feasibility of conducting TSP through a robotic catheter control system in a remote manner during ablation procedures [
96]. The use of an autonomous robotic ICE controller for a zero-fluoroscopy transseptal puncture has recently been described, providing additional assistance and visualization of cardiac structures without engaging the operator in the process [
97]. One of the main benefits of using robotics in medicine is increased precision and accuracy, which can be especially beneficial in delicate cardiac procedures potentially leading to improved patient outcomes. However, issues related to cost, access, and the need for specialized training remain the limiting factors for its wider use. Artificial intelligence (AI) is another rapidly growing field with the potential to revolutionize many areas of medicine, as it uses machine learning techniques to analyze complex data sets and identify patterns that might be missed by human analysis. Using AI for automated multislice computed tomography analysis for preoperative planning of the transseptal puncture site during transcatheter procedures has recently been described [
98], significantly accelerating image analysis and providing potential assistance with handling rapidly increasing volumes of patients.
As the demand for minimally invasive procedures requiring transseptal catheterization increases, simpler techniques with a steeper learning curve may become preferable in order to make the TSP more accessible to a broader range of physicians. The zero exchange solutions for LA catheterization seem to be a promising perspective for a more streamlined workflow, allowing for further simplification of the procedure and avoiding potentially serious exchange-related complications. The rapidly evolving fields of robotics, AR and AI in medicine provide an exciting perspective for the future.
Author Contributions
Conceptualization, P.D., M.S., A.G.; Methodology, A.G., M.S., ; Validation M.S., P.B., A.G. and J.P-K.; Investigation, P.D., A.D., M.S., ; Resources, P.D, A.D., J.P-K.; Writing—Original Draft Preparation P.D, A.D. M.S. and A.G.; Writing—Review & Editing, J.P-K., A.G.; Visualization M.S., J.P-K. .; Supervision, M.S., P.B., A.G.; Project Administration, A.G, P.D.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Forssmann, W. Die Sondierung des Rechten Herzens. Klin Wochenschr. 1929, 8, 2085–2087. [Google Scholar] [CrossRef]
- Cournand, A.; Motley, H.L. Recording of blood pressure from the left auricle and the pulmonary veins in human subjects with interauricular septal defect. Am J Physiol. 1947, 150, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, H.A.; Scott, R.W.; Becker, N.O. Catheterization of the left side of the heart in man. Circulation. 1950, 1, 357–359. [Google Scholar] [CrossRef]
- Seldinger, S.I. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta radiol. 1953, 39, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Morrow, A.G.; Braunwald, E.; Ross, J., Jr. Left heart catheterization: an appraisal of techniques and their applications in cardiovascular diagnosis. Arch Intern Med. 1960, 105, 645–655. [Google Scholar] [CrossRef]
- Ross, J., Jr. Transeptal left heart catheterization: a new method of left atrial puncture. Ann Surg. 1959, 149, 395–401. [Google Scholar] [CrossRef]
- Cope, C. Technique for transseptal catheterization of the left atrium; preliminary report. J Thorac Surg. 1959, 37, 482–486. [Google Scholar] [CrossRef]
- Brockenbrough, E.G.; Braunwald, E. A new technic for left ventricular angiocardiography and transseptal left heart catheterization. Am J Cardiol. 1960, 6, 1062–1064. [Google Scholar] [CrossRef]
- Brockenbrough, E.C.; Braunwald, E.; Ross, J. Transseptal left heart catheterization. A review of 450 studies and description of an improved technic. Circulation. 1962, 25, 15–21. [Google Scholar] [CrossRef]
- Ross, J., Jr. Considerations regarding the technique for transseptal left heart catheterization. Circulation. 1966, 34, 391–399. [Google Scholar] [CrossRef]
- Anderson, R.H.; Brown, N.A.; Webb, S. Development and structure of the atrial septum. Heart. 2002, 88, 104–110. [Google Scholar] [CrossRef]
- Klimek-Piotrowska, W.; Hołda, M.K.; Koziej, M.; Piątek, K.; Hołda, J. Anatomy of the true interatrial septum for transseptal access to the left atrium. Ann Anat. 2016, 205, 60–64. [Google Scholar] [CrossRef]
- Anderson, R.H.; Webb, S.; Brown, N.A. Clinical anatomy of the atrial septum with reference to its developmental components. Clin Anat. 1999, 12, 362–374. [Google Scholar] [CrossRef]
- O’Brien, B.; Zafar, H.; De Freitas, S.; Sharif, F. Transseptal puncture - Review of anatomy, techniques, complications and challenges. Int J Cardiol. 2017, 233, 12–22. [Google Scholar] [CrossRef]
- Laura, D.M.; Donnino, R.; Kim, E.E.; Benenstein, R.; Freedberg, R.S.; Saric, M. Lipomatous Atrial Septal Hypertrophy: A Review of Its Anatomy, Pathophysiology, Multimodality Imaging, and Relevance to Percutaneous Interventions. J Am Soc Echocardiogr. 2016, 29, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, D.R.; Sabharwal, N.; Bashir, Y.; Betts, T.R. Interatrial septum thickness and difficulty with transseptal puncture during redo catheter ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2008, 31, 1606–1611. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.; Jasinska, K.A.; Walocha, J.A. The morphology, clinical significance and imaging methods of the atrial septal pouch: A critical review. Translational Research in Anatomy. 2018, 13, 7–11. [Google Scholar] [CrossRef]
- Quininir, L.; Luk, P.P.; McGuire, M.A. Catheter entrapment in the Chiari network during catheter ablation. HeartRhythm Case Rep. 2020, 6, 896–898. [Google Scholar] [CrossRef] [PubMed]
- Calkins, H.; Willems, S.; Gerstenfeld, E.P.; Verma, A.; Schilling, R.; Hohnloser, S.H.; et al. Uninterrupted Dabigatran versus Warfarin for Ablation in Atrial Fibrillation. N Engl J Med. 2017, 376, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Cappato, R.; Marchlinski, F.E.; Hohnloser, S.H.; Naccarelli, G.V.; Xiang, J.; Wilber, D.J.; et al. Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur Heart J. 2015, 36, 1805–1811. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Haeusler, K.G.; Blank, B.; De Bono, J.; Callans, D.; Elvan, A.; et al. Apixaban in patients at risk of stroke undergoing atrial fibrillation ablation. Eur Heart J. 2018, 39, 2942–2955. [Google Scholar] [CrossRef] [PubMed]
- Hohnloser, S.H.; Camm, J.; Cappato, R.; Diener, H.-C.; Heidbüchel, H.; Mont, L.; et al. Uninterrupted edoxaban vs. vitamin K antagonists for ablation of atrial fibrillation: the ELIMINATE-AF trial. Eur Heart J. 2019, 40, 3013–3021. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.; Knijnik, L.; Bhonsale, A.; Miller, J.; Nasi, G.; Rivera, M.; et al. An updated meta-analysis of novel oral anticoagulants versus vitamin K antagonists for uninterrupted anticoagulation in atrial fibrillation catheter ablation. Heart Rhythm. 2018, 15, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021, 42, 373–498. [Google Scholar]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.-H.; Saad, E.B.; Aguinaga, L.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. Europace. 2018, 20, 157–208. [Google Scholar] [CrossRef] [PubMed]
- Derejko, P.; Hasiec, A.; Bardyszewski, A.; Kuśnierz, J.; Dzwonkowska, D.; Szumowski, Ł.; et al. Distances between transseptal puncture site and anatomical landmarks. J Cardiovasc Electrophysiol. 2019, 30, 2841–2848. [Google Scholar] [CrossRef]
- Earley, M.J. How to perform a transseptal puncture. Heart. 2009, 95, 85–92. [Google Scholar] [CrossRef]
- Ruisi, C.P.; Brysiewicz, N.; Asnes, J.D.; Sugeng, L.; Marieb, M.; Clancy, J.; et al. Use of intracardiac echocardiography during atrial fibrillation ablation. Pacing Clin Electrophysiol. 2013, 36, 781–788. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, Y.M.; Mohanty, P.; Natale, A.; Li, L.; Wu, W.F.; et al. Dilator method and needle method for atrial transseptal puncture: a retrospective study from a cohort of 4443 patients. Europace. 2012, 14, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Zeljkovic, I.; Brusich, S.; Scherr, D.; Velagic, V.; Traykov, V.; Pernat, A.; et al. Differences in activated clotting time and total unfractionated heparin dose during pulmonary vein isolation in patients on different anticoagulation therapy. Clin Cardiol. 2021, 44, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Radinovic, A.; Mazzone, P.; Landoni, G.; Agricola, E.; Regazzoli, D.; Della-Bella, P. Different transseptal puncture for different procedures: Optimization of left atrial catheterization guided by transesophageal echocardiography. Ann Card Anaesth. 2016, 19, 589–593. [Google Scholar]
- Sweda, R.; Haeberlin, A.; Seiler, J.; Servatius, H.; Noti, F.; Lam, A.; et al. How to Reach the Left Atrium in Atrial Fibrillation Ablation? Circ Arrhythm Electrophysiol. 2019, 12, e006744. [Google Scholar] [CrossRef]
- Stauber, A.; Kornej, J.; Sepehri Shamloo, A.; Dinov, B.; Bacevicius, J.; Dagres, N.; et al. Impact of single versus double transseptal puncture on outcome and complications in pulmonary vein isolation procedures. Cardiol J. 2021, 28, 671–677. [Google Scholar] [CrossRef]
- De Ponti, R.; Cappato, R.; Curnis, A.; Della Bella, P.; Padeletti, L.; Raviele, A.; et al. Trans-septal catheterization in the electrophysiology laboratory: data from a multicenter survey spanning 12 years. J Am Coll Cardiol. 2006, 47, 1037–1042. [Google Scholar] [CrossRef]
- Pręgowski, J.; Kłapyta, A.; Chmielak, Z.; Skowroński, J.; Szymański, P.; Mintz, G.S.; et al. Incidence, clinical correlates, timing, and consequences of acute thrombus formation in patients undergoing the MitraClip procedure. Kardiol Pol. 2020, 78, 45–50. [Google Scholar] [CrossRef]
- Asbach, S.; Biermann, J.; Bode, C.; Faber, T.S. Early Heparin Administration Reduces Risk for Left Atrial Thrombus Formation during Atrial Fibrillation Ablation Procedures. Cardiol Res Pract. 2011, 2011, 615087. [Google Scholar] [CrossRef]
- Cappato, R.; Calkins, H.; Chen, S.-A.; Davies, W.; Iesaka, Y.; Kalman, J.; et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010, 3, 32–38. [Google Scholar] [CrossRef]
- Noseworthy, P.A.; Kapa, S.; Madhavan, M.; Van Houten, H.; Haas, L.; McLeod, C.; et al. Abstract 16200: Risk of Stroke After Catheter Ablation or Cardioversion for Atrial Fibrillation: Results From a Large Administrative Database, 2008-2012. Circulation.
- Abdelfattah, O.M.; Saad, A.M.; Hisung, I.; Abushouk, A.I.; Gad, M.M.; Okasha, O.; et al. Temporal Trends of Transcatheter Edge-to-Edge Repair of the Mitral Valve Short-Term Outcomes in the United States: Nationwide Representative Study. Structural Heart. 2021, 5, 279–286. [Google Scholar] [CrossRef]
- Holmes, D.R.; Reddy, V.Y.; Turi, Z.G.; Doshi, S.K.; Sievert, H.; Buchbinder, M.; et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009, 374, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Glowniak, A.; Tarkowski, A.; Janczarek, M.; Wysokinski, A. Silent cerebral infarcts following pulmonary vein isolation with different atrial fibrillation ablation techniques - incidence and risk factors. Arch Med Sci. 2022, 18, 632–638. [Google Scholar] [CrossRef]
- Miyazaki, S.; Kajiyama, T.; Yamao, K.; Hada, M.; Yamaguchi, M.; Nakamura, H.; et al. Silent cerebral events/lesions after second-generation cryoballoon ablation: How can we reduce the risk of silent strokes? Heart Rhythm. 2019, 16, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Gaita, F.; Caponi, D.; Pianelli, M.; Scaglione, M.; Toso, E.; Cesarani, F.; et al. Radiofrequency catheter ablation of atrial fibrillation: a cause of silent thromboembolism? Magnetic resonance imaging assessment of cerebral thromboembolism in patients undergoing ablation of atrial fibrillation. Circulation. 2010, 122, 1667–1673. [Google Scholar] [CrossRef]
- Laible, M.; Möhlenbruch, M.; Horstmann, S.; Pfaff, J.; Geis, N.A.; Pleger, S.; et al. Peri-procedural silent cerebral infarcts after left atrial appendage occlusion. Eur J Neurol. 2017, 24, 53–57. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, K.; Lu, S.; Zhang, L.; Li, M.; Ju, W.; et al. Surgical and Percutaneous Left Atrial Appendage Intervention: Silent Cerebral Embolism Considerations. Eur J Cardiothorac Surg. 2023. [CrossRef] [PubMed]
- Schnitzler, K.; Hell, M.; Geyer, M.; Kreidel, F.; Münzel, T.; von Bardeleben, R.S. Complications Following MitraClip Implantation. Curr Cardiol Rep. 2021, 23, 131. [Google Scholar] [CrossRef] [PubMed]
- Di Biase, L.; Gaita, F.; Toso, E.; Santangeli, P.; Mohanty, P.; Rutledge, N.; et al. Does periprocedural anticoagulation management of atrial fibrillation affect the prevalence of silent thromboembolic lesion detected by diffusion cerebral magnetic resonance imaging in patients undergoing radiofrequency atrial fibrillation ablation with open irrigated catheters? Results from a prospective multicenter study. Heart Rhythm. 2014, 11, 791–798. [Google Scholar]
- Vermeer, S.E.; Prins, N.D.; den Heijer, T.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M.B. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003, 348, 1215–1222. [Google Scholar] [CrossRef]
- De Groot, J.C.; De Leeuw, F.-E.; Oudkerk, M.; Van Gijn, J.; Hofman, A.; Jolles, J.; et al. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol. 2002, 52, 335–341. [Google Scholar] [CrossRef]
- Wasmer, K.; Zellerhoff, S.; Köbe, J.; Mönnig, G.; Pott, C.; Dechering, D.G.; et al. Incidence and management of inadvertent puncture and sheath placement in the aorta during attempted transseptal puncture. Europace. 2017, 19, 447–457. [Google Scholar] [CrossRef]
- Katritsis, G.D.; Siontis, G.C.M.; Giazitzoglou, E.; Fragakis, N.; Katritsis, D.G. Complications of transseptal catheterization for different cardiac procedures. Int J Cardiol. 2013, 168, 5352–5354. [Google Scholar] [CrossRef] [PubMed]
- Rillig, A.; Meyerfeldt, U.; Birkemeyer, R.; Treusch, F.; Kunze, M.; Jung, W. Persistent iatrogenic atrial septal defect after pulmonary vein isolation : incidence and clinical implications. J Interv Card Electrophysiol. 2008, 22, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Linhart, M.; Werner, J.T.; Stöckigt, F.; Kohlmann, A.T.; Lodde, P.C.; Linneborn, L.P.T.; et al. High rate of persistent iatrogenic atrial septal defect after single transseptal puncture for cryoballoon pulmonary vein isolation. J Interv Card Electrophysiol. 2018, 52, 141–148. [Google Scholar] [CrossRef]
- Mcginty, P.M.; Smith, T.W.; Rogers, J.H. Transseptal Left Heart Catheterization and the Incidence of Persistent Iatrogenic Atrial Septal Defects. J Interv Cardiol. 2011, 24, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, J.M.; Sukiennik, A.; Woźnicki, M.; Kubica, J. Percutaneous closure of the iatrogenic atrial septal defect following the transcatheter edge-to-edge mitral valve repair with MitraClip system led to instant improvement of hypoxemia — case report. Med Res J. 2022, 7, 350–354. [Google Scholar] [CrossRef]
- Streb, W.; Fiszer, R.; Podolecki, T.; Mitręga, K.; Kalarus, Z. Uzasadnienie zamknięcia jatrogennego ubytku przegrody międzyprzedsionkowej podczas zabiegu MitraClip. Folia Cardiol. 2022, 17, 157–162. [Google Scholar] [CrossRef]
- Lurz, P.; Unterhuber, M.; Rommel, K.-P.; Kresoja, K.-P.; Kister, T.; Besler, C.; et al. Closure of Iatrogenic Atrial Septal Defect After Transcatheter Mitral Valve Repair: The Randomized MITHRAS Trial. Circulation. 2021, 143, 292–294. [Google Scholar] [CrossRef]
- Hoffmann, R.; Altiok, E.; Reith, S.; Brehmer, K.; Almalla, M. Functional effect of new atrial septal defect after percutaneous mitral valve repair using the MitraClip device. Am J Cardiol. 2014, 113, 1228–1233. [Google Scholar] [CrossRef]
- Shah, S.J.; Borlaug, B.A.; Chung, E.S.; Cutlip, D.E.; Debonnaire, P.; Fail, P.S.; et al. Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP-HF II): a randomised, multicentre, blinded, sham-controlled trial. Lancet. 2022, 399, 1130–1140. [Google Scholar] [CrossRef]
- Watanabe, T.; Miyazaki, S.; Kajiyama, T.; Ichijo, S.; Takagi, T.; Igarashi, M.; et al. Persistence of an iatrogenic atrial septal defect after a second-generation cryoballoon ablation of atrial fibrillation. Heart Vessels. 2018, 33, 1060–1067. [Google Scholar] [CrossRef]
- Alachkar, M.N.; Alnaimi, A.; Reith, S.; Altiok, E.; Schröder, J.; Marx, N.; et al. Incidence and clinical relevance of persistent iatrogenic atrial septal defect after percutaneous mitral valve repair. Sci Rep. 2021, 11, 12700. [Google Scholar] [CrossRef]
- Nelles, D.; Vij, V.; Al-Kassou, B.; Weber, M.; Vogelhuber, J.; Beiert, T.; et al. Incidence, persistence, and clinical relevance of iatrogenic atrial septal defects after percutaneous left atrial appendage occlusion. Echocardiography. 2022, 39, 65–73. [Google Scholar] [CrossRef]
- Kogan, E.V.; Sciria, C.T.; Liu, C.F.; Wong, S.C.; Bergman, G.; Ip, J.E.; et al. Early Stroke and Mortality After Percutaneous Left Atrial Appendage Occlusion in Patients With Atrial Fibrillation. Stroke. 2023, 54, 947–954. [Google Scholar] [CrossRef]
- Wiebe, J.; Franke, J.; Lubos, E.; Boekstegers, P.; Schillinger, W.; Ouarrak, T.; et al. Percutaneous mitral valve repair with the MitraClip system according to the predicted risk by the logistic EuroSCORE: preliminary results from the German Transcatheter Mitral Valve Interventions (TRAMI) Registry. Catheter Cardiovasc Interv. 2014, 84, 591–598. [Google Scholar] [CrossRef]
- Winkle, R.A.; Mead, R.H.; Engel, G.; Patrawala, R.A. The use of a radiofrequency needle improves the safety and efficacy of transseptal puncture for atrial fibrillation ablation. Heart Rhythm. 2011, 8, 1411–1415. [Google Scholar] [CrossRef]
- Jauvert, G.; Grimard, C.; Lazarus, A.; Alonso, C. Comparison of a radiofrequency powered flexible needle with a classic rigid Brockenbrough needle for transseptal punctures in terms of safety and efficacy. Heart Lung Circ. 2015, 24, 173–178. [Google Scholar] [CrossRef]
- Andrade, J.G.; Macle, L.; Bennett, M.T.; Hawkins, N.M.; Essebag, V.; Champagne, J.; et al. Randomized trial of conventional versus radiofrequency needle transseptal puncture for cryoballoon ablation: the CRYO-LATS trial. J Interv Card Electrophysiol. 2022, 65, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, M.; Hoeltgen, R.; Akin, E.; Salili, A.R. Use of a novel needle wire in patients undergoing transseptal puncture associated with severe septal tenting. J Interv Card Electrophysiol. 2010, 27, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Zucchetti, M.; Casella, M.; DelloRusso, A.; Fassini, G.; Carbucicchio, C.; Russo, E.; et al. Difficult case of a trans-septal puncture: Use of a “SafeSept” guidewire. World J Cardiol. 2015, 7, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Janardhan, A.H.; Berggren, K.; Lampert, T. Improved Left Atrial Catheterization Efficiency and Consistency using a novel steerable transseptal puncture sheath. Authorea Preprints. 2022 [cited 12 Apr 2023]. [CrossRef]
- Dewland, T.A.; Gerstenfeld, E.P.; Moss, J.D.; Lee, A.C.; Vedantham, V.; Lee, R.J.; et al. Randomized Comparison of a Radiofrequency Wire Versus a Radiofrequency Needle System for Transseptal Puncture. JACC: Clinical Electrophysiology. 2022. [Google Scholar] [CrossRef] [PubMed]
- Inohara, T.; Gilhofer, T.; Luong, C.; Tsang, M.; Saw, J. VersaCross radiofrequency system reduces time to left atrial access versus conventional mechanical needle. J Interv Card Electrophysiol. 2022, 63, 9–12. [Google Scholar] [CrossRef]
- Rizzi, S.; Pannone, L.; Monaco, C.; Bisignani, A.; Miraglia, V.; Gauthey, A.; et al. First experience with a transseptal puncture using a novel transseptal crossing device with integrated dilator and needle. J Interv Card Electrophysiol. 2022, 65, 731–737. [Google Scholar] [CrossRef]
- Perrin, N.; McAlister, C.; Tsang, M.; Mondésert, B.; Ibrahim, R.; Saw, J. Procedural simplification of left atrial appendage occlusion using the VersaCross connect system: First in-human experience. Catheter Cardiovasc Interv. 2023, 101, 227–230. [Google Scholar] [CrossRef]
- Yap, S.-C.; Bhagwandien, R.E.; Szili-Torok, T. Use of a novel integrated dilator-needle system in cryoballoon procedures: a zero-exchange approach. J Interv Card Electrophysiol. 2022, 65, 527–534. [Google Scholar] [CrossRef]
- Kueffer, T.; Madaffari, A.; Thalmann, G.; Mühl, A.; Galuszka, O.; Baldinger, S.; et al. Eliminating transseptal sheath exchange for pulsed field ablation procedures using a direct over-the-needle transseptal access with the Faradrive sheath. Europace. 2023. [CrossRef] [PubMed]
- Denysiuk, P.; Szczasny, M.; Głowniak, A.; Stadnik, M.; Błaszczak, P. Zero-exchange approach using a steerable FlexCath Advance sheath for direct transseptal access in cryoballoon ablation for pulmonary vein isolation – case study. In Good Rythm. 2022, 2, 26–29. [Google Scholar] [CrossRef]
- Ströker, E.; De Greef, Y.; Schwagten, B.; Kupics, K.; Coutiño, H.E.; Takarada, K.; et al. Over-the-needle trans-septal access using the cryoballoon delivery sheath and dilator in atrial fibrillation ablation. Pacing Clin Electrophysiol. 2019, 42, 868–873. [Google Scholar] [CrossRef]
- Tomaiko, E.; Ahmad, Z.; Su, W. ELIMINATING TRANSSEPTAL CATHETER EXCHANGE DURING CRYOBALLOON ABLATION WITH THE SAFESEPT TRANSSEPTAL GUIDEWIRE IN FLEXCATH SHEATH. J Am Coll Cardiol. 2019, 73, 429. [Google Scholar] [CrossRef]
- Kaplan, R.M.; Wasserlauf, J.; Knight, B.P. Transseptal access: A review of contemporary tools. J Cardiovasc Electrophysiol. 2022, 33, 1927–1931. [Google Scholar] [CrossRef]
- Liang, J.J.; Mohanty, S.; Fahed, J.; Muser, D.; Briceno, D.F.; Burkhardt, J.D.; et al. Bailout Atrial Balloon Septoplasty to Overcome Challenging Left Atrial Transseptal Access for Catheter Ablation of Atrial Fibrillation. JACC Clin Electrophysiol. 2018, 4, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wissner, E.; Kamioka, M.; Makimoto, H.; Rausch, P.; Metzner, A.; et al. Safety and feasibility of transseptal puncture for atrial fibrillation ablation in patients with atrial septal defect closure devices. Heart Rhythm. 2014, 11, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, N.; Keaney, J.; Keelan, E.; Walsh, K.P.; Széplaki, G. Picking the locked door: Experiences and techniques in transseptal puncture post-atrial septal defect occlusion. JACC Case Rep. 2023, 14, 101827. [Google Scholar] [CrossRef] [PubMed]
- Transcatheter Microguidewire Drilling for Transseptal Left Atrial Access - Full Text View - ClinicalTrials.Gov. [cited 30 Apr 2023]. Available: https://clinicaltrials.gov/ct2/show/NCT04561908?cond=transseptal&draw=3&rank=2.
- The Usefulness of Biatrial 3D Printing to Plan Transseptal Puncture for the Left Atrial Appendage Closure. [cited 30 Apr 2023]. Available: https://clinicaltrials.gov/ct2/show/NCT05743322?cond=transseptal+puncture&draw=2&rank=1.
- Chokesuwattanaskul, R.; Ananwattanasuk, T.; Hughey, A.B.; Stuart, E.A.; Shah, M.M.; Atreya, A.R.; et al. Three-dimensional-guided and ICE-guided transseptal puncture for cardiac ablations: A propensity score match study. J Cardiovasc Electrophysiol. 2023, 34, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Žižek, D.; Antolič, B.; Prolič Kalinšek, T.; Štublar, J.; Kajdič, N.; Jelenc, M.; et al. Intracardiac echocardiography-guided transseptal puncture for fluoroless catheter ablation of left-sided tachycardias. J Interv Card Electrophysiol. 2021, 61, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Guarguagli, S.; Cazzoli, I.; Kempny, A.; Gatzoulis, M.A.; Ernst, S. Initial Experience Using the Radiofrequency Needle Visualization on the Electroanatomical Mapping System for Transseptal Puncture. Cardiol Res Pract. 2020, 2020, 5420909. [Google Scholar] [CrossRef]
- Yu, R.; Liu, N.; Lu, J.; Zhao, X.; Hu, Y.; Zhang, J.; et al. 3-Dimensional Transseptal Puncture Based on Electrographic Characteristics of Fossa Ovalis: A Fluoroscopy-Free and Echocardiography-Free Method. JACC Cardiovasc Interv. 2020, 13, 1223–1232. [Google Scholar] [CrossRef]
- Li, D.; Ze, F.; Yuan, C.-Z.; Zhou, X.; Wang, L.; Duan, J.-B.; et al. The safety and efficiency of fluoroless site-specific transseptal puncture guided by three-dimensional intracardiac echocardiography. J Interv Card Electrophysiol. 2022, 65, 643–649. [Google Scholar] [CrossRef]
- Site-specific Transseptal Cardiac Catheterization Guided by Intracardiac Echocardiography for emerging electrophysiology applications. [cited 30 Apr 2023]. Available: https://www.innovationsincrm.com/cardiac-rhythm-management/2013/october/512-transseptal-cardiaccatheterization.
- Liu, J.; Al’Aref, S.J.; Singh, G.; Caprio, A.; Moghadam, A.A.A.; Jang, S.-J.; et al. An augmented reality system for image guidance of transcatheter procedures for structural heart disease. PLoS One. 2019, 14, e0219174. [Google Scholar] [CrossRef]
- Jang, S.-J.; Liu, J.; Singh, G.; Al’Aref, S.J.; Caprio, A.; Amiri Moghadam, A.A.; et al. Abstract 11714: Augmented Reality Guidance for Transcatheter Septal Puncture Procedure in Structural Heart Interventions. Circulation.
- Jung, C.; Wolff, G.; Wernly, B.; Bruno, R.R.; Franz, M.; Schulze, P.C.; et al. Virtual and augmented reality in cardiovascular care: State-of-the-art and future perspectives. JACC Cardiovasc Imaging. 2022, 15, 519–532. [Google Scholar] [CrossRef] [PubMed]
- James, R.C.; Monsky, W.L.; Jorgensen, N.W.; Seslar, S.P. Virtual-Reality Guided Versus Fluoroscopy-Guided Transseptal Puncture in a Cardiac Phantom. J Invasive Cardiol. 2020, 32, 76–81. [Google Scholar] [PubMed]
- Saliba, W.; Cummings, J.E.; Oh, S.; Zhang, Y.; Mazgalev, T.N.; Schweikert, R.A.; et al. Novel robotic catheter remote control system: feasibility and safety of transseptal puncture and endocardial catheter navigation. J Cardiovasc Electrophysiol. 2006, 17, 1102–1105. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Collins, J.; Kim, Y.-H.; Chinnadurai, P.; Mansi, T.; Lin, C.H. ZERO-FLUOROSCOPY TRANSSEPTAL PUNCTURE GUIDED BY INTELLIGENT INTRACARDIAC ECHOCARDIOGRAPHY ROBOTICS. J Am Coll Cardiol. 2021, 77, 970. [Google Scholar] [CrossRef]
- Michiels, K.; Heffinck, E.; Astudillo, P.; Wong, I.; Mortier, P.; Bavo, A.M. Automated MSCT Analysis for Planning Left Atrial Appendage Occlusion Using Artificial Intelligence. J Interv Cardiol. 2022, 2022, 5797431. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).