1. Introduction
Microbial keratitis is one of the leading causes of corneal blindness in tropical belts of the world, particularly in developing nations of south and east Asia
[1]. It is estimated that in India, approximately two million cases of corneal ulcer occur yearly, most of which are attributed to some form of antecedent injury [
2]. While fungi and bacteria are the commonest etiological agents of keratitis, sophisticated microbiological techniques such as DNA sequencing have revealed the existence of newer pathogens. It is estimated that ten percent of cases of microbial keratitis are associated with these novel organisms [
3].
Pythium insidiosum (PI) is one such new pathogen of the 21
st century. It is an aquatic oomycete which exists in two forms, mycelium and zoospore [
4]. Infection has been reported to be acquired through motile zoospores present in freshwater [
5].
Classical clinical features of PI keratitis are described as reticular dot infiltrates, tentacular projections, and peripheral furrowing , however these hallmark signs may be absent in majority of the cases [
6]. Microbiologically, it mimics fungus due to the presence of branching hyphae on smear examination but unlike fungus, its growth on culture is delayed and it lacks ergosterol in its cell wall [
7].
Since PI keratitis was first reported in 1993, diagnostic techniques and management guidelines for this condition have evolved [
8]. Published reports indicate that it is a fulminant condition and therapeutic keratoplasty is warranted in most of the patients [
9,
10,
11]. Owing to the obstinate nature of the disease , loss of globe and failure of keratoplasty with eventual loss of vision are commonly reported outcomes in PI keratitis [
6,
9]. Herein, we report outcomes of keratoplasty in a cohort of PI keratitis patients from a tertiary eye care institute in India with emphasis on the surgical techniques to eliminate infection.
2. Materials and Methods
This is a retrospective review of outcomes of keratoplasty in culture proven cases of PI keratitis treated between January 2020 to December 2021 at a tertiary eye care institute in India. The study approval was obtained from the institutional review board and the study complied with the tenets of the Declaration of Helsinki.
2.1. Demographic and Disease Parameters
The clinical and microbiological case records of all culture proven cases of PI keratitis who underwent keratoplasty were retrieved from electronic central patient data base of the hospital. Patients with incomplete medical records or those treated for PI keratitis on clinical suspicion alone were excluded.
Preoperative data such as demographic profile, predisposing risk factors, systemic co-morbidity, previous clinical or microbiological diagnosis, visual acuity at presentation, medical therapy received and time to therapeutic penetrating keratoplasty (TPK) from onset of symptoms were recorded.
Intraoperative data recorded included size of graft, special techniques employed during keratoplasty, use of adjuvant intraocular antibiotics, combined posterior segment surgery, if performed, and lens status at the end of the procedure.
Postoperative data were collected to assess outcomes of keratoplasty and complications. Parameters noted were globe integrity, graft survival, best corrected visual acuity (BCVA) and recurrence of infection. Recurrence was further elaborated as type of recurrence, medical or surgical intervention for recurrence and period of disease free follow-up.
2.2. Management Parameters
Patients with microbiological diagnosis of PI keratitis were treated with topical linezolid 0.2% eyedrops hourly, azithromycin 1% eye ointment twice a day, topical cycloplegic (homatropine 2%) eyedrops twice a day and oral azithromycin (500 mg twice a day). Therapeutic penetrating keratoplasty was performed in patients with worsening of disease defined as increase in size and depth of infiltration, involvement of limbus, corneal perforation and corneal thinning.
Postoperatively, eyes were treated with topical linezolid 0.2% eyedrops hourly, topical azithromycin 1% eye ointment twice a day, topical cycloplegic (homatropine 2%) eyedrops twice a day and oral azithromycin (500 mg twice a day). Patients were prescribed steroid prednisolone acetate 1% eyedrops four times a day when the button culture report was found to be negative for fungal growth. Patients were followed up at close interval. Repeat keratoplasty was performed in patients with recurrence not responding to topical and intracameral linezolid treatment.
2.3. Statistical Analysis
Data analysis was performed using the SPSS statistics program (version 29.0, IBM, USA). Continuous data are reported as mean ± SD with range while categorical data are presented as frequencies (n) and percentages (%). BCVA data were converted to logarithm of the minimum angle of resolution (logMAR) values for statistical analysis. Vision categories of counting fingers, hand motion, light perception, and no light perception were assigned logMAR values 2.1, 2.4, 2.7, and 3.0, respectively. We used survival analysis to estimate the average time to repeat penetrating keratoplasty following the first surgery for pythium keratitis.
3. Results
3.1. Patient Characteristics
A summary of the clinical characteristics of the cohort is provided in
Table 1. A total of 16 eyes of 16 patients were included in the study. The mean age at time of presentation was 33.9 ± 13.2 years (range 19-58 years). Out of the 16 patients, ten were males (62.5%) and six (37.5%) were females. History of injury with vegetative matter was found in one patient and one patient reported onset of symptoms following contact lens use. None of the patients had pre-existing ocular comorbidity. One patient had a history of hypertension and coronary artery disease.
Mean duration of symptoms was 23.1 ± 9.9 days (range10-45 days) at the time of presentation to our hospital. All patients had history of previously receiving treatment from elsewhere. Seven (43.75%) patients had been diagnosed as fungal keratitis and were on anti-fungal treatment. Seven (43.75%) patients were on a cocktail therapy of antibacterial and antifungal drugs while two patients were receiving antiviral therapy. None of the patients had been previously diagnosed as cases of PI keratitis. The mean visual acuity at presentation was 2.32 ± 0.36 logMAR (approximately counting fingers close to face). The range of visual acuity at presentation was 1.5-2.7 logMAR (20/600- light perception Snellen).
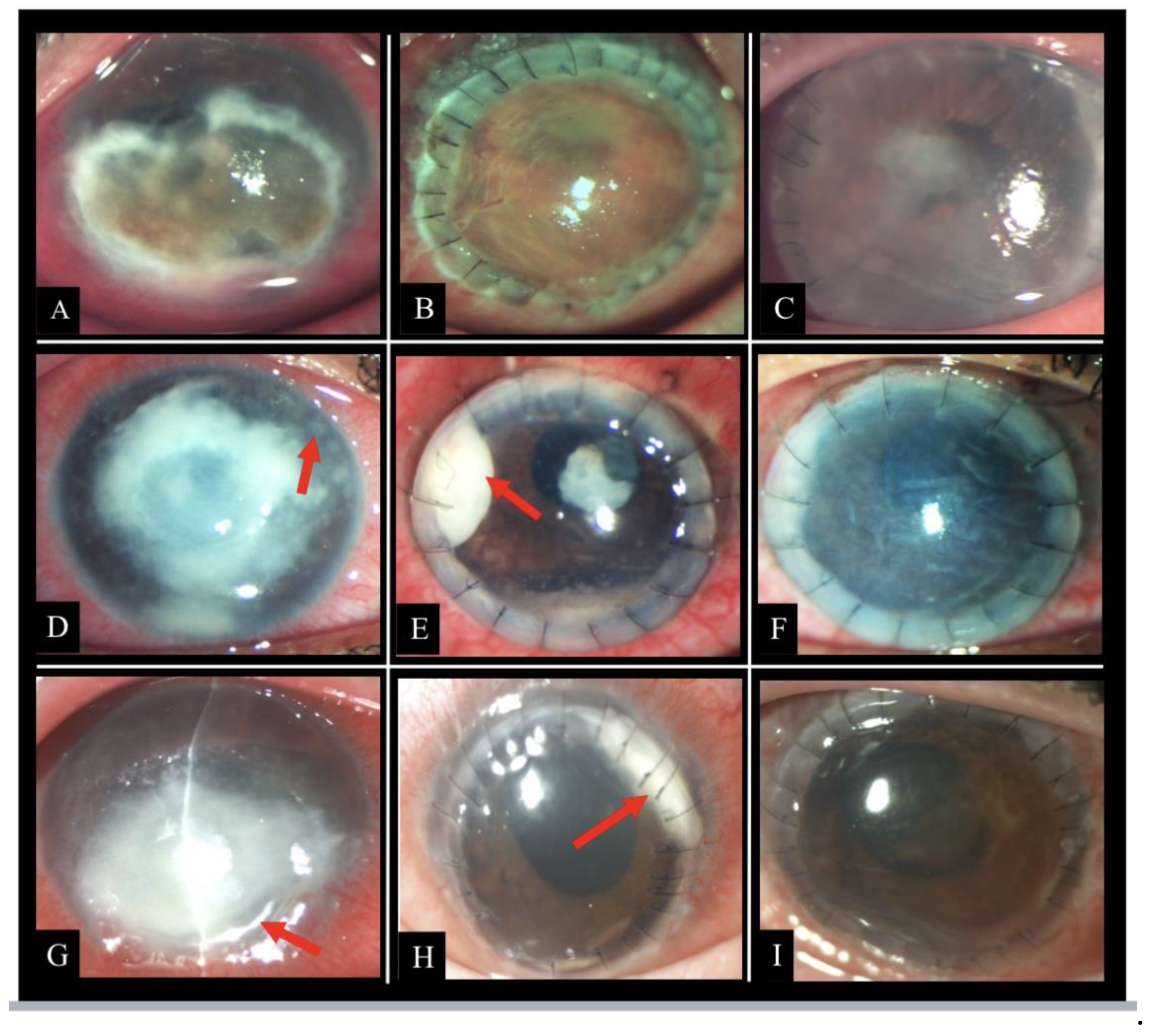
Figure 1 shows representative photographs depicting clinical features and course of disease of patients from the study cohort.
3.2. Microbiological Diagnosis
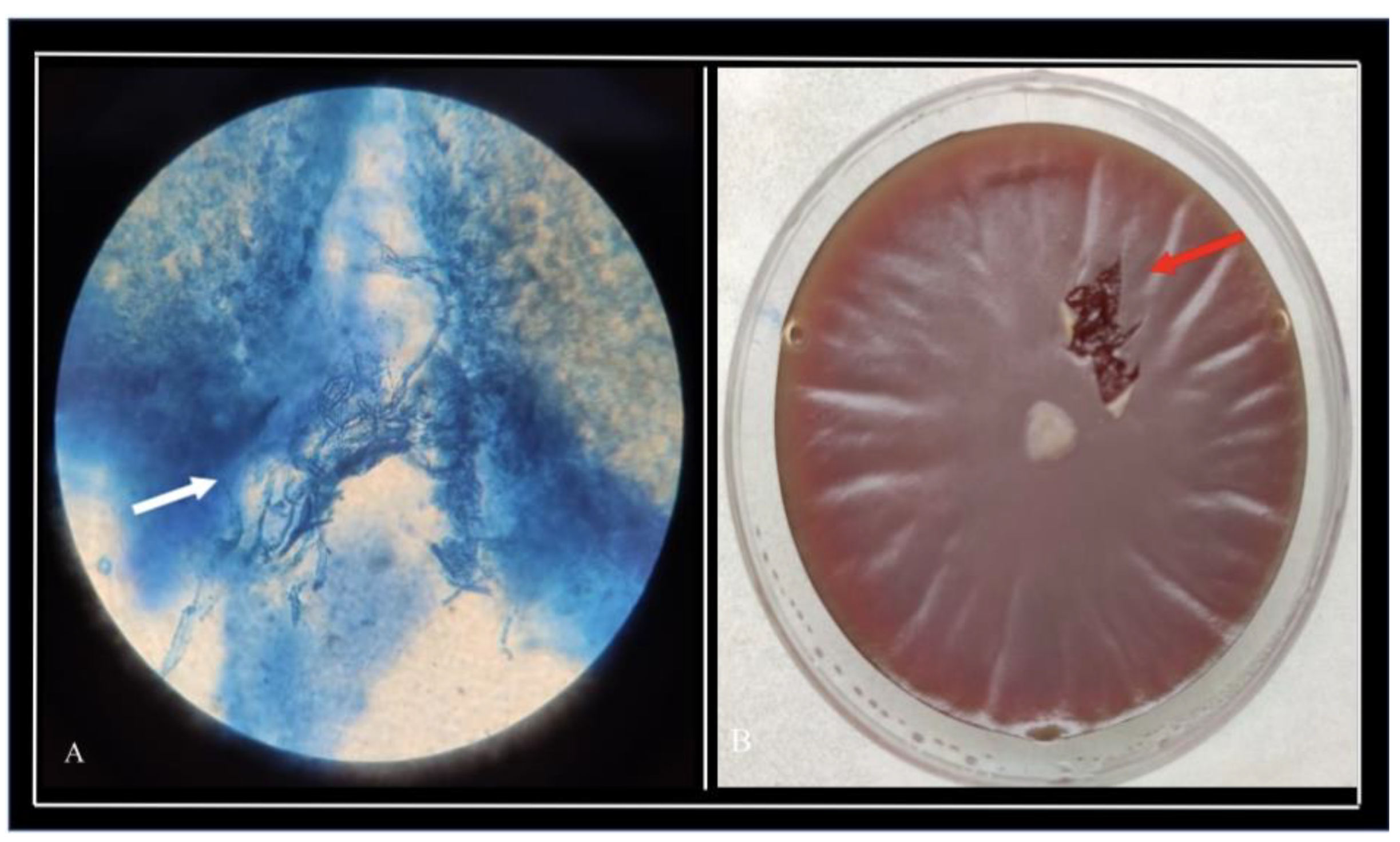
Corneal scraping and microbiological examination were performed for all patients. Long, sparsely septate hyaline hyphae with numerous vesicles suggestive of
Pythium insiodiosum species were observed on Potassium hydroxide(KOH) with Calcofluorowhite (CFW)smears in 11 patients (
Figure 2a). Additionally, Gram staining of the smear showed thick cell wall, few septae, and mass of vesicles within the hyphae. Flat, feathery-edged, partially submerged, colourless, or light brown glabrous colonies with filiform margins on blood agar were grown from corneal scrapings from all patients in the cohort (
Figure 2b).
The diagnosis was further confirmed on histopathological analysis of the growth. Periodic Acid Schiff (PAS) staining was negative for fungal hyphae and showed only unstained fragments of sparsely septate hyphae which were broad and unfolded. Grocott-Gomori's methenamine silver (GMS) stain showed distinctly stained oomycetes on Potassium Iodide–Sulfuric acid (IKI-H2SO4) treatment suggestive of Pythium species. Following TPK, the excised corneal button was cultured and processed for species identification for all cases. Corneal button cultures showed Pythium growth after first surgery in all cases. Three out of seven (42.9%) corneal button cultures from repeat keratoplasty patients also showed Pythium growth.
3.3. Intraoperative Assessment and Surgical Technique
Eleven patients underwent therapeutic penetrating keratoplasty (TPK) under local anesthesia with facial block by a trained cornea surgeon. General anesthesia was administered in five patients. Mean time to the first or primary keratoplasty from onset of symptoms was 31.3 ± 8.6 days (range 16-49 days). Mean primary graft size was 10.34 ± 0.87 mm (range 9.5-12 mm). Infection was controlled after primary keratoplasty in ten patients (62.50%) while six patients (37.5%) required repeat keratoplasty. One patient required three keratoplasties for elimination of infection.
Trephination of the host bed was performed with the diameter of trephination enough to include more than 1mm of the surrounding normal cornea. Careful dissection of corneal tissue was done while separating adherent tissue from all sides. Peripheral iridectomy was performed in all cases. 1mm difference between donor and host trephination size was ensured. Sixteen to 24 well-spaced, interrupted sutures were placed depending on the donor graft size. In one patient, who had scleral infiltration, an incision was made at the site of infiltration to partially deroof the sclera and drain the infiltration (Supplemental video). The deroofed area was left uncovered to allow better penetration of antibiotics postoperatively. Intracameral linezolid (200 micrograms in 0.1ml) was administered to all patients at the end of surgery. Double freeze thaw cryotherapy at the site of scleral infiltration was done in addition to TPK in one patient.
Lens was preserved in 14 out of 16 patients lens after first TPK. Three out of 6 patients who underwent repeat TPK had spontaneous expulsion of lens during the procedure. Anterior vitrectomy was performed in all cases of lens expulsion.
3.4. Postoperative Course and Complications
All patients were kept under close follow up post-surgery. Nine patients (56.25 %) had recurrence of infection. Mean time to presentation with recurrence was 8.9 days (range 2-34 days). Recurrence of the disease manifested as graft infiltration (4 patients), endo-exudates (2 patients), graft infiltrate along with endo-exudates (1 patient), combination of graft host junction infiltration and endo-exudates (1 patient), and graft melt (1 patient).
Recurrence of infection was managed by repeat penetrating keratoplasty in six patients, application of cryotherapy to the graft host junction in one patient and intracameral linezolid (200 micrograms in 0.1 ml, thrice over 4 days) in two patients. Mean graft size for the first TPK in patients who presented with recurrence was 10.06 ± 1.15 mm (range 8.5-10 mm) while mean graft size of the repeat TPK was 11.7 ± 1.56 mm (range 10-14 mm). In two of the six patients, infection could not be controlled even after 4 doses of intracameral linezolid. These 2 patients subsequently underwent repeat keratoplasty for resolution of infection. Topical steroid (prednisolone acetate 1%) eyedrops were prescribed two-hourly to all patients after repeat keratoplasty in addition to antibiotics.
Mean postoperative follow up period in the study was 8.9 ± 9.8 months. Graft outcome was assessed at six months from last surgery. Seven patients who did not have recurrence of infection after the first keratoplasty had varied graft outcome. One patient had a clear compact graft, edematous failed graft in 5 patients and one patient progressed to phthisis bulbi. Mean time to graft failure was 12.16 ± 4.54 weeks (range 2-32 weeks). Two of the five patients with graft failure underwent optical keratoplasty subsequently.
Six patients in whom repeat keratoplasty was performed had graft outcomes in form of clear compact graft in 1 patient, edematous failed graft in 4 patients and 1 patient progressed to phthisis bulbi.
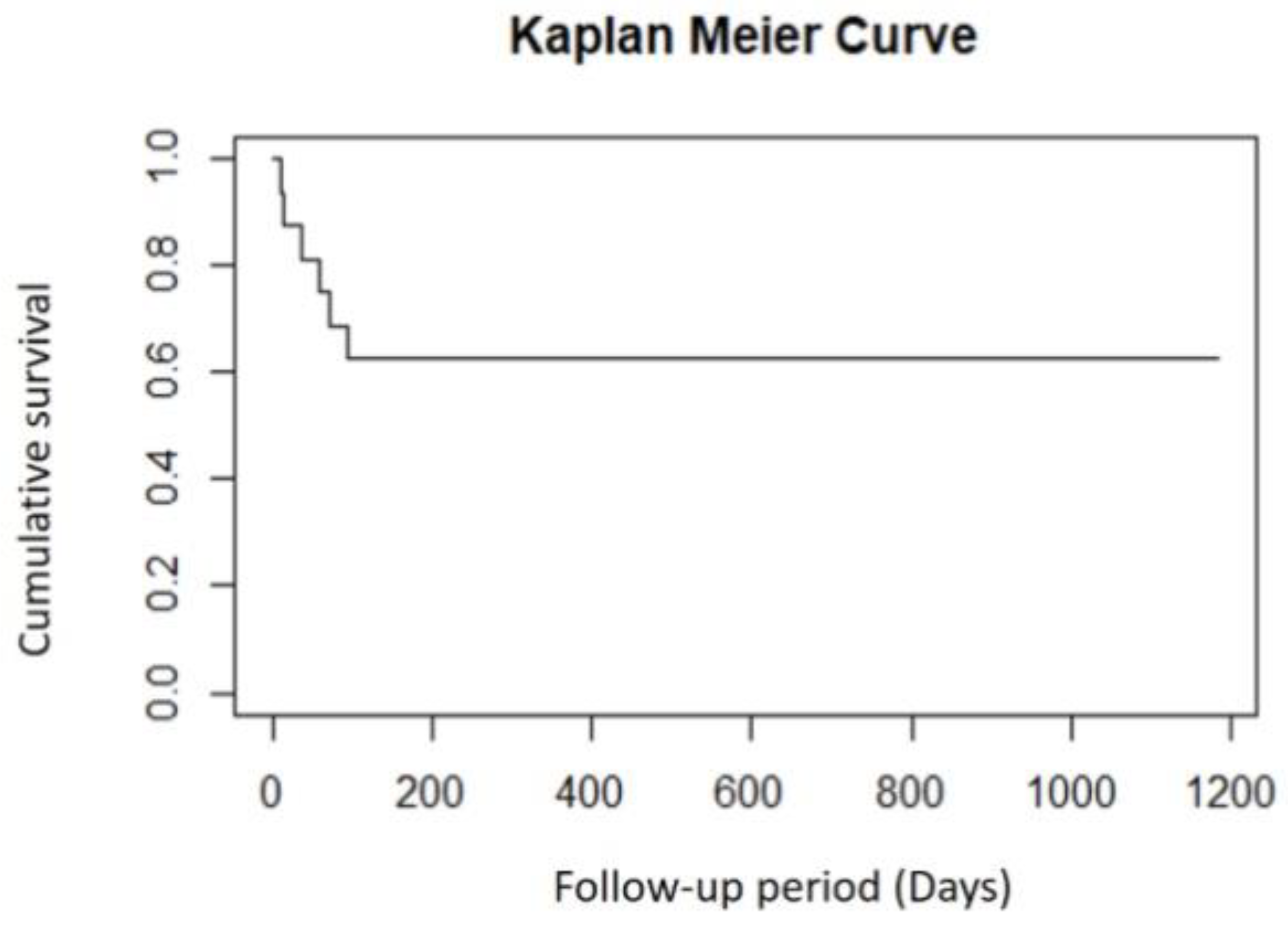
Figure 3 depicts graft survival following repeat penetrating keratoplasty. Out of the 4 patients with graft failure, 1 underwent optical keratoplasty and another patient underwent DSAEK for visual rehabilitation. Secondary glaucoma (1 patient) and retinal detachment (1 patient) were additional complications that were observed along with graft failure in this group. Retinal detachment was managed by vitrectomy and endolaser application along with a repeat keratoplasty.
The mean uncorrected visual acuity at six month follow up was 2.04±0.77 logMAR (20/2000 Snellen) in the study.
Table 2 summarizes the intraoperative and post-operative details of patients.
4. Discussion
Our study describes demographic details, clinical course and detailed outcomes of therapeutic penetrating keratoplasty in a cohort of patients with culture proven PI keratitis. PI keratitis is observed to afflict patients in young and middle age groups and is found to be more common in males compared to females as has been reported in other studies[
4,
12]. Swimming in water bodies, contact lens wear and trauma have been commonly reported as risk factors for developing pythium keratitis in literature[
13,
14,
15]. While most patients in our study did not report a specific predisposing event, history of corneal injury with vegetative matter and contact lens use were found respectively in two of the patients. Mean time for patients to present to our hospital was 23 days from onset of symptoms and none of the patients had been diagnosed as cases of PI keratitis. Time to presentation to hospital in patients with PI keratitis has been reported to range between 2 to 60 days in various studies[
4,
16]. Delay in presentation and accurate diagnosis subsequently adversely affect clinical outcomes in this condition.
All patients in our cohort presented with visual acuity worse than counting fingers at 2 meters. Interestingly, all 16 patients had received non-specific medical treatment for microbial keratitis before presentation to our center. Administration of empirical antibiotic therapy without confirmatory microbiological diagnosis continues to be widely practiced at primary care level[
17]. Unfamiliarity with clinical features and common misdiagnosis as fungal keratitis due to overlapping features are some of the other challenges in managing these patients. Clinically, rapid progression of disease is the hallmark of PI keratitis[
18,
19]. Large size of infiltration, involvement of limbus, dense vascularization, endothelium and anterior chamber infiltration at presentation were the high-risk factors for rapid deterioration of keratitis in our study. Favorable response in PI keratitis on treatment with antibiotic combination of linezolid eyedrops and azithromycin eye ointment have been reported in literature[
19,
20,
21]. However, keratoplasty has been stated as the mainstay in management of severe, refractory and rapidly worsening cases of PI keratitis[
6,
12].
Even though the primary aim of TPK in these patients is eradication of infective foci and restoration of globe integrity, achieving a good visual outcome with optically clear graft is desirable. The long-term success of keratoplasty additionally depends on the development of various post-operative sequelae, causative agent, preoperative medications, timing of surgery, quality of donor tissue, and size of the graft[
22]. In our study, mean size of the graft in primary keratoplasty and repeat keratoplasty were 10.4 mm and 11.7 mm respectively indicating large extent of infiltration in our cohort of patients. Meticulous clearing of infiltration with a disease-free margin of at least 1-2 mm was ensured in all cases to prevent recurrence. Scleral spread has been frequently reported in this condition as was seen in two patients in our study[
23]. A wide peritomy and thorough exploration of the sclera beyond limbus was carried out in and on evidence of scleral infection, partial deroofing of the sclera and drainage of infection was performed in one patient. Cryotherapy to the infected sclera was another technique employed to treat scleral infiltration in one patient as has been described by Agarwal et al[
24]. Additionally, intracameral linezolid was administered at the end of surgery in all cases for maximal benefit.
Post-operative period management can be challenging in cases of PI keratitis. More than half of the patients in our study presented with recurrence of disease in varied forms such as graft infiltration, endo-exudates or graft deshiscence. Recurrence of disease after keratoplasty has been reported to be more common in cases of PI keratitis as compared to other causes of microbial keratitis[
4,
24,
25]. Agarwal et al reported post TPK recurrence in seven out of ten patients two underwent evisceration eventually[
26]. Keratoplasty with a larger graft size and meticulous clean-up of infiltration was performed in six patients in our study for recurrence of disease. Additionally, topical steroids were prescribed two- hourly in the post-operative period in view of increased inflammation observed in the post-operative period in these patients.
Enucleation and evisceration for endophthalmitis in PI keratitis are devastating outcomes and have been commonly reported before[
12,
27,
28,
29]. In recent times, Vishwakarma et al have reported anatomical success of TPK in 72.2 % patients of PI keratitis in their study with three patients requiring evisceration for infection control[
12]. Globe was successfully salvaged (eyes which did not need evisceration or progressed to phthisis bulbi) in 14 out of 16 patients (87.5 %) in our study. None of the eyes developed endophthalmitis or required evisceration for infection control. This can be attributed to better understanding of disease over the years, taking adequate surgical precautions, administration of adjuvant antibiotic therapy and early control of post-operative inflammation with vigorous administration of topical steroids. While successful salvage of the globe and control of infection are a step forward in managing these patients, the rate of graft failure continues to be high in this cohort as was observed in our study[
14,
30]. For visual rehabilitation, optical keratoplasty, (penetrating or DSAEK) was performed in failed grafts. Mean uncorrected visual acuity improved marginally from 2.32 to 2.04 logMAR after surgery which is comparable with reports in literature[
6,
12].
5. Conclusions
PI keratitis is a challenging condition which often presents with severe infiltration that is refractory to medical treatment, necessitating management with therapeutic penetrating keratoplasty. Recurrence of infection after surgery is the main concern in managing these patients. Per-operative appropriately large sized grafts and meticulous clean-up of infiltration is advised. Furthermore, surgeons must be prepared for repeat surgeries and a prolonged and arduous postoperative course.
Author Contributions
Conceptualisation and hypothesis: MA; methodology: ICH VN; data Collector: VN; Data Analysis and Statistics: AS, VN; investigations: AG; manuscript writing: MA, AS; supervision: MA. All authors have read and agreed to the published version of manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to acknowledge Mr. Sajy Thomas for microbiology, Dr. Atanu Majumdar for statistical analysis and Mr. Mukesh Kumar for image acquisition.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ung, L.; Bispo, P.J.M.; Shanbhag, S.S.; Gilmore, M.S.; Chodosh, J. The Persistent Dilemma of Microbial Keratitis: Global Burden, Diagnosis, and Antimicrobial Resistance. Surv Ophthalmol 2019, 64, 255–271. [Google Scholar] [CrossRef] [PubMed]
- WHITCHER, J.; SRINIVASAN, M. Corneal Ulceration in the Developing World—a Silent Epidemic. Br J Ophthalmol 1997, 81, 622–623. [Google Scholar] [CrossRef]
- Van Gelder, R.N. Ocular Pathogens for the Twenty-First Century. Am J Ophthalmol 2010, 150, 595–597. [Google Scholar] [CrossRef]
- Hasika, R.; Lalitha, P.; Radhakrishnan, N.; Rameshkumar, G.; Prajna, N.V.; Srinivasan, M. Pythium Keratitis in South India: Incidence, Clinical Profile, Management, and Treatment Recommendation. Indian J Ophthalmol 2019, 67, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Gaastra, W.; Lipman, L.J.A.; De Cock, A.W.A.M.; Exel, T.K.; Pegge, R.B.G.; Scheurwater, J.; Vilela, R.; Mendoza, L. Pythium Insidiosum: An Overview. Vet Microbiol 2010, 146, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gurnani, B.; Christy, J.; Narayana, S.; Rajkumar, P.; Kaur, K.; Gubert, J. Retrospective Multifactorial Analysis of Pythium Keratitis and Review of Literature. Indian J Ophthalmol 2021, 69, 1095–1101. [Google Scholar] [CrossRef]
- Yolanda, H.; Krajaejun, T. Review of Methods and Antimicrobial Agents for Susceptibility Testing against Pythium Insidiosum. Heliyon 2020, 6, e03737. [Google Scholar] [CrossRef]
- Virgile, R.; Perry, H.D.; Pardanani, B.; Szabo, K.; Rahn, E.K.; Stone, J.; Salkin, I.; Dixon, D.M. Human Infectious Corneal Ulcer Caused by Pythium Insidiosum. Cornea 1993, 12, 81–83. [Google Scholar] [CrossRef]
- Puangsricharern, V.; Chotikkakamthorn, P.; Tulvatana, W.; Kittipibul, T.; Chantaren, P.; Reinprayoon, U.; Kasetsuwan, N.; Satitpitakul, V.; Worasilchai, N.; Chindamporn, A. Clinical Characteristics, Histopathology, and Treatment Outcomes of Pythium Keratitis: A Retrospective Cohort Study. Clin Ophthalmol 2021, 15, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Thanathanee, O.; Bhoomibunchoo, C.; Anutarapongpan, O.; Suwan-Apichon, O.; Charoensuk, K.; Chindamporn, A. Role of Immunotherapy in Pythium Insidiosum Keratitis. Am J Trop Med Hyg 2022, 107, 110–112. [Google Scholar] [CrossRef]
- Agarwal, S.; Iyer, G.; Srinivasan, B.; Benurwar, S.; Agarwal, M.; Narayanan, N.; Lakshmipathy, M.; Radhika, N.; Rajagopal, R.; Krishnakumar, S.; et al. Clinical Profile, Risk Factors and Outcome of Medical, Surgical and Adjunct Interventions in Patients with Pythiuminsidiosum Keratitis. Br J Ophthalmol 2019, 103, 296–300. [Google Scholar] [CrossRef]
- Vishwakarma, P.; Mohanty, A.; Kaur, A.; Das, S.; Priyadarshini, S.R.; Mitra, S.; Mittal, R.; Sahu, S.K. Pythium Keratitis: Clinical Profile, Laboratory Diagnosis, Treatment, and Histopathology Features Post-Treatment at a Tertiary Eye Care Center in Eastern India. Indian J Ophthalmol 2021, 69, 1544–1552. [Google Scholar] [CrossRef]
- Lelievre, L.; Borderie, V.; Garcia-Hermoso, D.; Brignier, A.C.; Sterkers, M.; Chaumeil, C.; Lortholary, O.; Lanternier, F. Imported Pythium Insidiosum Keratitis After a Swim in Thailand by a Contact Lens-Wearing Traveler. Am J Trop Med Hyg 2015, 92, 270–273. [Google Scholar] [CrossRef]
- Hasika, R.; Lalitha, P.; Radhakrishnan, N.; Rameshkumar, G.; Prajna, N.V.; Srinivasan, M. Pythium Keratitis in South India: Incidence, Clinical Profile, Management, and Treatment Recommendation. Indian J Ophthalmol 2019, 67, 42–47. [Google Scholar] [CrossRef]
- Barequet, I.S.; Lavinsky, F.; Rosner, M. Long-Term Follow-up after Successful Treatment of Pythium Insidiosum Keratitis in Israel. Semin Ophthalmol 2013, 28, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Gurnani, B.; Christy, J.; Narayana, S.; Rajkumar, P.; Kaur, K.; Gubert, J. Retrospective Multifactorial Analysis of Pythium Keratitis and Review of Literature. Indian J Ophthalmol 2021, 69, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Egrilmez, S.; Yildirim-Theveny, Ş. Treatment-Resistant Bacterial Keratitis: Challenges and Solutions. Clin Ophthalmol 2020, 14, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Kunavisarut, S.; Nimvorapan, T.; Methasiri, S. Pythium Corneal Ulcer in Ramathibodi Hospital. J Med Assoc Thai 2003, 86, 338–342. [Google Scholar]
- Gurnani, B.; Narayana, S.; Christy, J.; Rajkumar, P.; Kaur, K.; Gubert, J. Successful Management of Pediatric Pythium Insidiosum Keratitis with Cyanoacrylate Glue, Linezolid, and Azithromycin: Rare Case Report. Eur J Ophthalmol 2022, 32, NP87–NP91. [Google Scholar] [CrossRef]
- Bagga, B.; Sharma, S.; Madhuri Guda, S.J.; Nagpal, R.; Joseph, J.; Manjulatha, K.; Mohamed, A.; Garg, P. Leap Forward in the Treatment of Pythium Insidiosum Keratitis. Br J Ophthalmol 2018, 102, 1629–1633. [Google Scholar] [CrossRef]
- Ramappa, M.; Nagpal, R.; Sharma, S.; Chaurasia, S. Successful Medical Management of Presumptive Pythium Insidiosum Keratitis. Cornea 2017, 36, 511–514. [Google Scholar] [CrossRef]
- Sharma, N.; Jain, M.; Sehra, S.V.; Maharana, P.; Agarwal, T.; Satpathy, G.; Vajpayee, R.B. Outcomes of Therapeutic Penetrating Keratoplasty from a Tertiary Eye Care Centre in Northern India. Cornea 2014, 33, 114–118. [Google Scholar] [CrossRef]
- Neufeld, A.; Seamone, C.; Maleki, B.; Heathcote, J.G. Pythium Insidiosum Keratitis: A Pictorial Essay of Natural History. Can J Ophthalmol 2018, 53, e48–e50. [Google Scholar] [CrossRef]
- Agarwal, S.; Iyer, G.; Srinivasan, B.; Agarwal, M.; Panchalam Sampath Kumar, S.; Therese, L.K. Clinical Profile of Pythium Keratitis: Perioperative Measures to Reduce Risk of Recurrence. Br J Ophthalmol 2018, 102, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Anitha, V.; Vanathi, M. Commentary: A Retrospective Multifactorial Analysis of Pythium Keratitis and Review of the Literature. Indian J Ophthalmol 2021, 69, 1101–1102. [Google Scholar] [CrossRef]
- Agarwal, S.; Iyer, G.; Srinivasan, B.; Agarwal, M.; Panchalam Sampath Kumar, S.; Therese, L.K. Clinical Profile of Pythium Keratitis: Perioperative Measures to Reduce Risk of Recurrence. Br J Ophthalmol 2018, 102, 153–157. [Google Scholar] [CrossRef]
- Permpalung, N.; Worasilchai, N.; Manothummetha, K.; Torvorapanit, P.; Ratanawongphaibul, K.; Chuleerarux, N.; Plongla, R.; Chindamporn, A. Clinical Outcomes in Ocular Pythiosis Patients Treated with a Combination Therapy Protocol in Thailand: A Prospective Study. Med Mycol 2019, 57, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Lekhanont, K.; Chuckpaiwong, V.; Chongtrakool, P.; Aroonroch, R.; Vongthongsri, A. Pythium Insidiosum Keratitis in Contact Lens Wear: A Case Report. Cornea 2009, 28, 1173–1177. [Google Scholar] [CrossRef]
- Agarwal, S.; Iyer, G.; Srinivasan, B.; Benurwar, S.; Agarwal, M.; Narayanan, N.; Lakshmipathy, M.; Radhika, N.; Rajagopal, R.; Krishnakumar, S.; et al. Clinical Profile, Risk Factors and Outcome of Medical, Surgical and Adjunct Interventions in Patients with Pythiuminsidiosum Keratitis. Br J Ophthalmol 2019, 103, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Sane, S.S.; Madduri, B.; Mohan, N.; Mittal, R.; Raghava, J.V.; Fernandes, M. Improved Outcome of Pythium Keratitis With a Combined Triple Drug Regimen of Linezolid and Azithromycin. Cornea 2021, 40, 888–893. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).