Submitted:
12 June 2023
Posted:
13 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
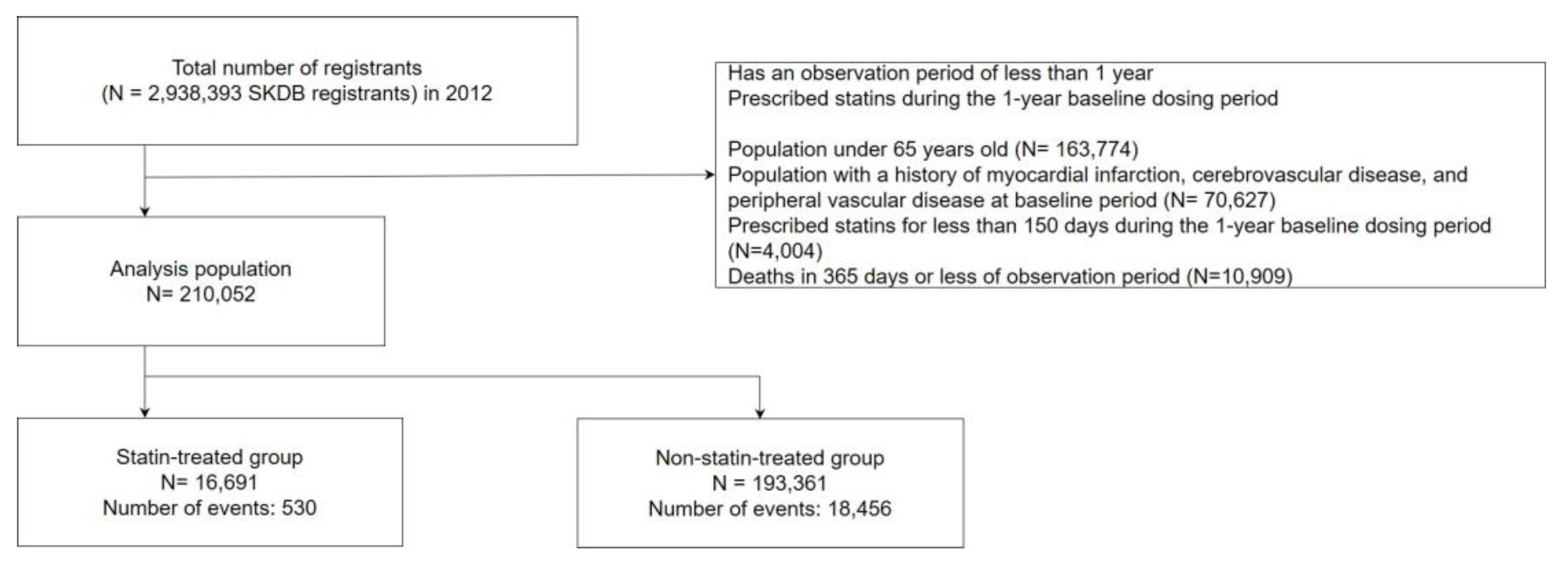
2.1. Data source
2.2. Study design and population
2.3. Outcome and confounder candidates
2.4. Statistical analysis
2.5. Ethics
3. Results
3.1. Demographics of participants
3.2. Effectiveness of statin therapy for survival
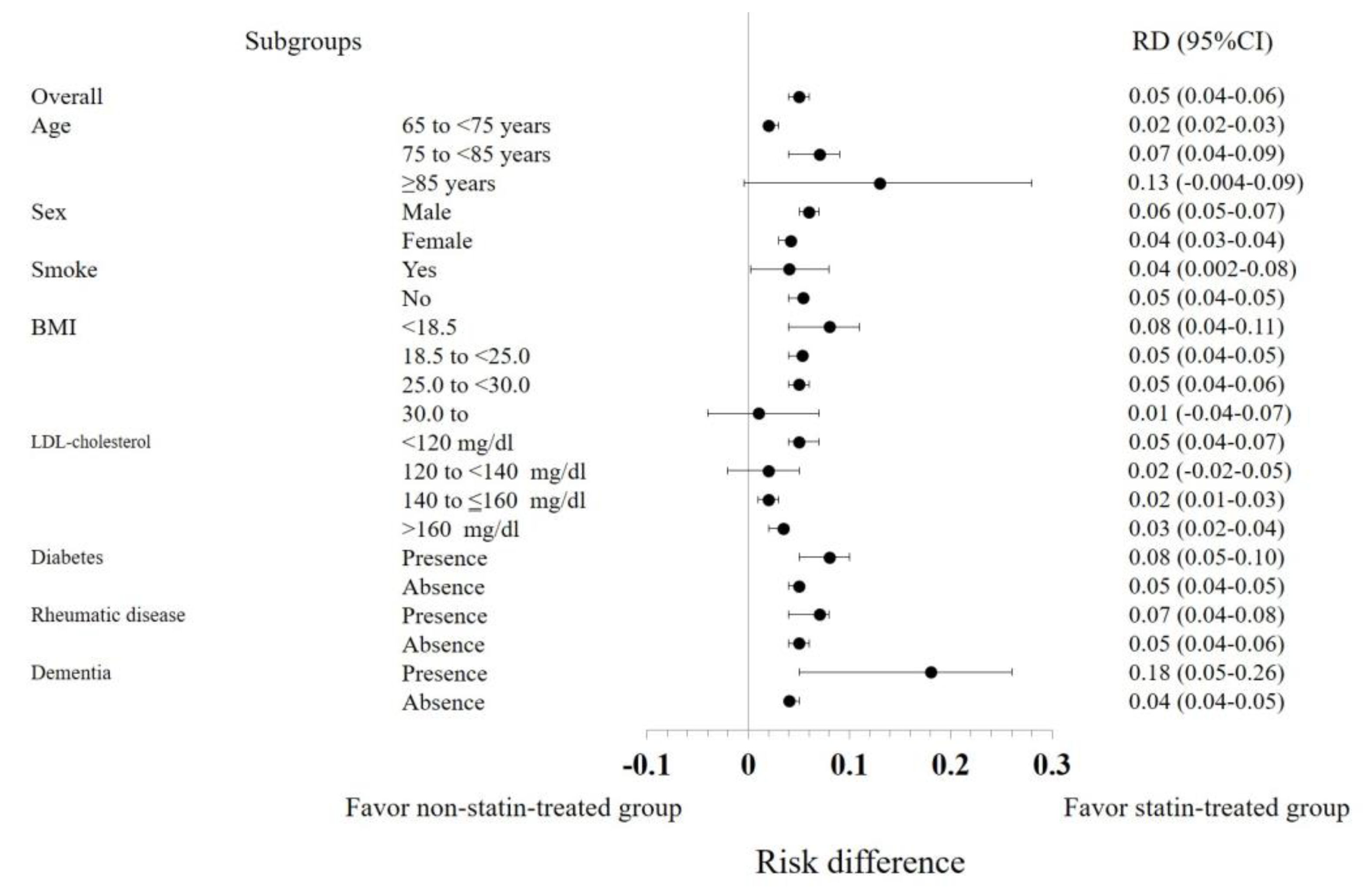
3.3. Subgroup Analyses
4. Discussion
4.2. Strengths
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- White, J.; Swerdlow, D.I.; Preiss, D.; Fairhurst-Hunter, Z.; Keating, B.J.; Asselbergs, F.W.; Sattar, N.; Humphries, S.E.; Hingorani, A.D.; Holmes, M.V. Association of Lipid Fractions With Risks for Coronary Artery Disease and Diabetes. JAMA Cardiol 2016, 1, 692–699. [Google Scholar] [CrossRef]
- Vrecer, M.; Turk, S.; Drinovec, J.; Mrhar, A. Use of Statins in Primary and Secondary Prevention of Coronary Heart Disease and Ischemic Stroke. Meta-Analysis of Randomized Trials. Int. J. Clin. Pharmacol. Ther. 2003, 41, 567–577. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaborators; Mihaylova, B. ; Emberson, J.; Blackwell, L.; Keech, A.; Simes, J.; Barnes, E.H.; Voysey, M.; Gray, A.; Collins, R.; et al. The Effects of Lowering LDL Cholesterol with Statin Therapy in People at Low Risk of Vascular Disease: Meta-Analysis of Individual Data from 27 Randomised Trials. Lancet 2012, 380, 581–590. [Google Scholar]
- Gencer, B.; Marston, N.A.; Im, K.; Cannon, C.P.; Sever, P.; Keech, A.; Braunwald, E.; Giugliano, R.P.; Sabatine, M.S. Efficacy and Safety of Lowering LDL Cholesterol in Older Patients: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Lancet 2020, 396, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Cholesterol Treatment Trialists’ Collaboration Efficacy and Safety of Statin Therapy in Older People: A Meta-Analysis of Individual Participant Data from 28 Randomised Controlled Trials. Lancet 2019, 393, 407–415. [CrossRef] [PubMed]
- Orkaby, A.R.; Driver, J.A.; Ho, Y.-L.; Lu, B.; Costa, L.; Honerlaw, J.; Galloway, A.; Vassy, J.L.; Forman, D.E.; Gaziano, J.M.; et al. Association of Statin Use With All-Cause and Cardiovascular Mortality in US Veterans 75 Years and Older. JAMA 2020, 324, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, E.; Tabara, Y.; Sato, Y.; Tsuchiya, A.; Miyachi, Y. Data Resource Profile of Shizuoka Kokuho Database (SKDB) Using Integrated Health- and Care-Insurance Claims and Health Checkups: The Shizuoka Study. J. Epidemiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Sato, Y.; Nakatani, E.; Kaneda, H.; Yamamoto, S.; Miyachi, Y.; Itoh, H. Statin Exposure and Pancreatic Cancer Incidence: A Japanese Regional Population-Based Cohort Study, the Shizuoka Study. Cancer Prev. Res. 2021, 14, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Yamamoto, H.; Nakatani, E.; Kumamaru, H.; Nishimura, S.; Ichihara, N.; Hirahara, N.; Mori, K.; Kotani, M.; Miyachi, Y.; et al. Real-World Evidence of the Incidence of and Risk Factors for Type 1 Diabetes Mellitus and Hypothyroidism as Immune-Related Adverse Events Associated With Programmed Cell Death-1 Inhibitors. Endocr. Pract. 2021, 27, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Kohsaka, S.; Kumamaru, H.; Nishimura, S.; Shoji, S.; Nakatani, E.; Ichihara, N.; Yamamoto, H.; Miyachi, Y.; Miyata, H. Incidence of Adverse Cardiovascular Events in Type 2 Diabetes Mellitus Patients after Initiation of Glucose-Lowering Agents: A Population-Based Community Study from the Shizuoka Kokuho Database. J. Diabetes Investig. 2021, 12, 1452–1461. [Google Scholar] [CrossRef]
- Ray, W.A. Evaluating Medication Effects Outside of Clinical Trials: New-User Designs. Am. J. Epidemiol. 2003, 158, 915–920. [Google Scholar] [CrossRef]
- Holmes, H.M.; Min, L.C.; Yee, M.; Varadhan, R.; Basran, J.; Dale, W.; Boyd, C.M. Rationalizing Prescribing for Older Patients with Multimorbidity: Considering Time to Benefit. Drugs Aging 2013, 30, 655–666. [Google Scholar] [CrossRef]
- Kutner, J.S.; Blatchford, P.J.; Taylor, D.H., Jr; Ritchie, C.S.; Bull, J.H.; Fairclough, D.L.; Hanson, L.C.; LeBlanc, T.W.; Samsa, G.P.; Wolf, S.; et al. Safety and Benefit of Discontinuing Statin Therapy in the Setting of Advanced, Life-Limiting Illness: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 691–700. [Google Scholar] [CrossRef]
- Rosenbaum, P.R.; Rubin, D.B. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
- Austin, P.C.; Stuart, E.A. Moving towards Best Practice When Using Inverse Probability of Treatment Weighting (IPTW) Using the Propensity Score to Estimate Causal Treatment Effects in Observational Studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef]
- Austin, P.C. Absolute Risk Reductions and Numbers Needed to Treat Can Be Obtained from Adjusted Survival Models for Time-to-Event Outcomes. J. Clin. Epidemiol. 2010, 63, 46–55. [Google Scholar] [CrossRef]
- VanderWeele, T.J.; Ding, P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann. Intern. Med. 2017, 167, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Mathur, M.B.; Ding, P.; Riddell, C.A.; VanderWeele, T.J. Web Site and R Package for Computing E-Values. Epidemiology 2018, 29, e45–e47. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.; Blauw, G.J.; Murphy, M.B.; Bollen, E.L.E.M.; Buckley, B.M.; Cobbe, S.M.; Ford, I.; Gaw, A.; Hyland, M.; Jukema, J.W.; et al. Pravastatin in Elderly Individuals at Risk of Vascular Disease (PROSPER): A Randomised Controlled Trial. Lancet 2002, 360, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Everett, B.M.; Glynn, R.J.; MacFadyen, J.G.; Ridker, P.M. Rosuvastatin in the Prevention of Stroke among Men and Women with Elevated Levels of C-Reactive Protein: Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER). Circulation 2010, 121, 143–150. [Google Scholar] [CrossRef]
- Downs, J.R.; Clearfield, M.; Weis, S.; Whitney, E.; Shapiro, D.R.; Beere, P.A.; Langendorfer, A.; Stein, E.A.; Kruyer, W.; Gotto, A.M. , Jr Primary Prevention of Acute Coronary Events with Lovastatin in Men and Women with Average Cholesterol Levels: Results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998, 279, 1615–1622. [Google Scholar] [CrossRef]
- Nakamura, H.; Arakawa, K.; Itakura, H.; Kitabatake, A.; Goto, Y.; Toyota, T.; Nakaya, N.; Nishimoto, S.; Muranaka, M.; Yamamoto, A.; et al. Primary Prevention of Cardiovascular Disease with Pravastatin in Japan (MEGA Study): A Prospective Randomised Controlled Trial. Lancet 2006, 368, 1155–1163. [Google Scholar] [CrossRef]
- Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of Cholesterol Lowering with Simvastatin in 20,536 High-Risk Individuals: A Randomised Placebo-Controlled Trial. Lancet 2002, 360, 7–22. [CrossRef] [PubMed]
- Roberts, C.G.P.; Guallar, E.; Rodriguez, A. Efficacy and Safety of Statin Monotherapy in Older Adults: A Meta-Analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 879–887. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaboration; Fulcher, J. ; O’Connell, R.; Voysey, M.; Emberson, J.; Blackwell, L.; Mihaylova, B.; Simes, J.; Collins, R.; Kirby, A.; et al. Efficacy and Safety of LDL-Lowering Therapy among Men and Women: Meta-Analysis of Individual Data from 174,000 Participants in 27 Randomised Trials. Lancet 2015, 385, 1397–1405. [Google Scholar]
- Ramos, R.; Comas-Cufí, M.; Martí-Lluch, R.; Balló, E.; Ponjoan, A.; Alves-Cabratosa, L.; Blanch, J.; Marrugat, J.; Elosua, R.; Grau, M.; et al. Statins for Primary Prevention of Cardiovascular Events and Mortality in Old and Very Old Adults with and without Type 2 Diabetes: Retrospective Cohort Study. BMJ 2018, 362, k3359. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, C.J.; Shim, C.-Y.; Kim, J.-S.; Kim, B.-K.; Park, S.; Chang, H.-J.; Hong, G.-R.; Ko, Y.-G.; Kang, S.-M.; et al. Statin and Clinical Outcomes of Primary Prevention in Individuals Aged >75 Years: The SCOPE-75 Study. Atherosclerosis 2019, 284, 31–36. [Google Scholar] [CrossRef]
- Jun, J.E.; Cho, I.-J.; Han, K.; Jeong, I.-K.; Ahn, K.J.; Chung, H.Y.; Hwang, Y.-C. Statins for Primary Prevention in Adults Aged 75 Years and Older: A Nationwide Population-Based Case-Control Study. Atherosclerosis 2019, 283, 28–34. [Google Scholar] [CrossRef]
- Stone, N.J.; Greenland, P.; Grundy, S.M. Statin Usage in Primary Prevention-Comparing the USPSTF Recommendations With the AHA/ACC/Multisociety Guidelines. JAMA Cardiol 2022. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.K.; Laufs, U. Pleiotropic Effects of Statins. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 89–118. [Google Scholar] [CrossRef]
- Kataoka, Y.; St John, J.; Wolski, K.; Uno, K.; Puri, R.; Tuzcu, E.M.; Nissen, S.E.; Nicholls, S.J. Atheroma Progression in Hyporesponders to Statin Therapy. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Sandfort, V.; Lai, S.; Ahlman, M.A.; Mallek, M.; Liu, S.; Sibley, C.T.; Turkbey, E.B.; Lima, J.A.C.; Bluemke, D.A. Obesity Is Associated With Progression of Atherosclerosis During Statin Treatment. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Won, K.-B.; Hur, S.-H.; Nam, C.-W.; Ann, S.H.; Park, G.-M.; Lee, S.-G.; Kim, H.-E.; Cho, Y.-K.; Yoon, H.-J.; Park, H.-S.; et al. Evaluation of the Impact of Statin Therapy on the Obesity Paradox in Patients with Acute Myocardial Infarction: A Propensity Score Matching Analysis from the Korea Acute Myocardial Infarction Registry. Medicine 2017, 96, e7180. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.E.; MacInnis, R.J.; Wattanapenpaiboon, N.; Nowson, C.A. BMI and All-Cause Mortality in Older Adults: A Meta-Analysis. Am. J. Clin. Nutr. 2014, 99, 875–890. [Google Scholar] [CrossRef] [PubMed]
- Donini, L.M.; Savina, C.; Gennaro, E.; De Felice, M.R.; Rosano, A.; Pandolfo, M.M.; Del Balzo, V.; Cannella, C.; Ritz, P.; Chumlea, W.C. A Systematic Review of the Literature Concerning the Relationship between Obesity and Mortality in the Elderly. J. Nutr. Health Aging 2012, 16, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, K.R.; McCubrey, R.; Mehta, T.; Pajewski, N.M.; Keith, S.W.; Bangalore, S.S.; Crespo, C.J.; Allison, D.B. Body Mass Index and Mortality Rate among Hispanic Adults: A Pooled Analysis of Multiple Epidemiologic Data Sets. Int. J. Obes. 2012, 36, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Kit, B.K.; Orpana, H.; Graubard, B.I. Association of All-Cause Mortality with Overweight and Obesity Using Standard Body Mass Index Categories: A Systematic Review and Meta-Analysis. JAMA 2013, 309, 71–82. [Google Scholar] [CrossRef]
- Yamazaki, K.; Suzuki, E.; Yorifuji, T.; Tsuda, T.; Ohta, T.; Ishikawa-Takata, K.; Doi, H. Is There an Obesity Paradox in the Japanese Elderly Population? A Community-Based Cohort Study of 13 280 Men and Women. Geriatr. Gerontol. Int. 2017, 17, 1257–1264. [Google Scholar] [CrossRef]
- Ozen, G.; Dell’Aniello, S.; Pedro, S.; Michaud, K.; Suissa, S. Reduction of Cardiovascular Disease and Mortality versus Risk of New Onset Diabetes with Statin Use in Patients with Rheumatoid Arthritis. Arthritis Care Res. 2022. [Google Scholar] [CrossRef]
- Chhibber, A.; Hansen, S.; Biskupiak, J. Statin Use and Mortality in Rheumatoid Arthritis: An Incident User Cohort Study. J Manag Care Spec Pharm 2021, 27, 296–305. [Google Scholar] [CrossRef]
- Schoenfeld, S.R.; Lu, L.; Rai, S.K.; Seeger, J.D.; Zhang, Y.; Choi, H.K. Statin Use and Mortality in Rheumatoid Arthritis: A General Population-Based Cohort Study. Ann. Rheum. Dis. 2016, 75, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Petek, B.; Xu, H.; Villa-Lopez, M.; Winblad, B.; Kramberger, M.G.; Eriksdotter, M.; Garcia-Ptacek, S. Statins, Risk of Death and Ischemic Stroke in Patients with Dementia: A Registry-Based Observational Cohort Study. Curr. Alzheimer Res. 2020, 17, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, A.; Oristrell, J.; Pla, X.; Ruggiero, C.; Ferretti, R.; Diestre, G.; Clarfield, A.M.; Crome, P.; Hertogh, C.; Lesauskaite, V.; et al. The Persistent Exclusion of Older Patients from Ongoing Clinical Trials Regarding Heart Failure. Arch. Intern. Med. 2011, 171, 550–556. [Google Scholar] [CrossRef] [PubMed]
| Variable | Category | Before adjustment | After adjustment | ||||
|---|---|---|---|---|---|---|---|
| statin-exposure | statin-non-exposure | SMD | statin-exposure | statin-non-exposure | SMD | ||
| (n=16,691) | (n=193,361) | (n=2,753) | (n=31,466) | ||||
| Age | 65-<75 | 12637 (75.7) | 121013 (62.6) | 0.33 | 1826.95 (66.4) | 20965.04 (66.6) | 0.04 |
| 75-<85 | 3667 (22.0) | 57563 (29.8) | 0.33 | 740.26 (26.9) | 8484.53 (27.0) | 0.04 | |
| ≥85 | 387 (2.3) | 14785 (7.6) | 0.33 | 185.83 (6.8) | 2016.64 (6.4) | 0.04 | |
| Sex | Men | 5884 (35.3) | 94368 (48.8) | -0.28 | 1176.54 (42.7) | 13756.10 (43.7) | -0.02 |
| Current smoker | Yes | 1441 (8.6) | 19694 (10.2) | -0.05 | 223.79 (8.1) | 2912.78 (9.3) | -0.04 |
| BMI | <18.5 | 1114 (6.7) | 21748 (11.3) | 0.15 | 216.59 (7.9) | 2602.25 (8.3) | 0.06 |
| 18.5 to <25.0 | 12044 (72.3) | 136537 (70.8) | 0.15 | 1902.15 (69.1) | 21933.86 (69.7) | 0.06 | |
| 25.0 to <30.0 | 3216 (19.3) | 31488 (16.3) | 0.15 | 560.53 (20.4) | 6182.11 (19.6) | 0.06 | |
| 30.0 to | 292 (1.8) | 3137 (1.6) | 0.15 | 73.77 (2.7) | 747.99 (2.4) | 0.06 | |
| Missing number | 25 | 451 | 0.15 | 0.00 | 0.00 | 0.00 | |
| Alcohol intake | <40 g/day | 13794 (96.0) | 151595 (93.5) | -0.11 | 2588.07 (94.0) | 29500.87 (93.8) | -0.01 |
| ≥40 g/day | 569 (4.0) | 10543 (6.5) | -0.11 | 164.98 (6.0) | 1965.34 (6.2) | -0.01 | |
| Missing number | 2328 | 31223 | -0.11 | 0.00 | 0.00 | 0.00 | |
| With exercise habits* | Yes | 6577 (47.2) | 72654 (46.4) | 0.02 | 1305.5 (47.4) | 14517.14 (46.1) | 0.02 |
| Missing number | 2747 | 36699 | 0.02 | 0.00 | 0.00 | 0.00 | |
| Health conscience† | Low | 7816 (56.8) | 95792 (62.1) | 0.11 | 1571.89 (57.1) | 18653.94 (59.3) | 0.07 |
| Intermediate | 1926 (14.0) | 16976 (11.0) | 0.11 | 339.46 (12.3) | 4004.75 (12.7) | 0.07 | |
| High | 4018 (29.2) | 41529 (26.9) | 0.11 | 841.70 (30.6) | 8807.52 (28.0) | 0.07 | |
| Missing number | 2931 | 39064 | 0.11 | 0.00 | 0.00 | 0.00 | |
| eGFR | >60 | 11369 (71.0) | 130944 (71.3) | 0.14 | 1855.67 (67.4) | 21362.33 (67.9) | 0.04 |
| >45,<=60 | 3962 (24.7) | 43519 (23.7) | 0.14 | 718.25 (26.1) | 8117.45 (25.8) | 0.04 | |
| >30,<=45 | 605 (3.8) | 7821 (4.3) | 0.14 | 151.58 (5.5) | 1691.87 (5.4) | 0.04 | |
| <=30 | 74 (0.5) | 1319 (0.7) | 0.14 | 27.55 (1.0) | 294.56 (0.9) | 0.04 | |
| Missing number | 681 | 9758 | 0.14 | 0.00 | 0.00 | 0.00 | |
| HbA1c | <6.5 | 14891 (90.5) | 164986 (87.4) | 0.14 | 2308.38 (83.8) | 26261.23 (83.5) | 0.08 |
| <7,6.5≥ | 771 (4.7) | 13923 (7.4) | 0.14 | 257.19 (9.3) | 2876.13 (9.1) | 0.08 | |
| <7.5,7≥ | 324 (2.0) | 4960 (2.6) | 0.14 | 98.04 (3.6) | 1180.08 (3.8) | 0.08 | |
| <8,7.5≥ | 163 (1.0) | 1931 (1.0) | 0.14 | 40.51 (1.5) | 498.82 (1.6) | 0.08 | |
| ≥8 | 302 (1.8) | 2970 (1.6) | 0.14 | 48.91 (1.8) | 649.93 (2.1) | 0.08 | |
| Missing number | 240 | 4591 | 0.14 | 0.00 | 0.00 | 0.00 | |
| LDL | <120 | 1548 (9.3) | 86789 (44.9) | 1.18 | 940.52 (34.2) | 10587.78 (33.6) | 0.04 |
| 120<-139 | 2953 (17.8) | 54297 (28.1) | 1.18 | 842.02 (30.6) | 9612.41 (30.5) | 0.04 | |
| 140<-160 | 5015 (30.3) | 34876 (18.1) | 1.18 | 689.34 (25.0) | 7489.79 (23.8) | 0.04 | |
| >160 | 7061 (42.6) | 17238 (8.9) | 1.18 | 281.18 (10.2) | 3776.23 (12.0) | 0.04 | |
| Missing number | 114 | 161 | 1.18 | 0.00 | 0.00 | 0.00 | |
| Any malignancy‡ | Presence | 1422 (8.5) | 17222 (8.9) | -0.01 | 316.73 (11.5) | 3110.49 (9.9) | 0.05 |
| Liver disease | Presence | 2187 (13.1) | 23427 (12.1) | 0.03 | 616.01 (22.4) | 6971.22 (22.2) | 0.01 |
| Hypertension | Presence | 9712 (58.2) | 83181 (43.0) | 0.31 | 1770.22 (64.3) | 19118.98 (60.8) | 0.07 |
| Diabetes | Presence | 807 (4.8) | 6651 (3.4) | 0.07 | 180.37 (6.6) | 1978.72 (6.3) | 0.01 |
| Rheumatic disease | Presence | 466 (2.8) | 5330 (2.8) | 0.00 | 110.94 (4.0) | 1152.58 (3.7) | 0.02 |
| Dementia | Presence | 323 (1.9) | 4527 (2.3) | -0.03 | 74.60 (2.7) | 758.89 (2.4) | 0.02 |
| Renal disease | Presence | 537 (3.2) | 3529 (1.7) | 0.10 | 95.31 (3.5) | 940.35 (3.0) | 0.03 |
| Peptic ulcer disease | Presence | 2328 (13.9) | 26015 (13.5) | 0.01 | 513.61 (18.7) | 5605.58 (17.8) | 0.02 |
| Alcohol abuse | Presence | 58 (0.3) | 908 (0.5) | -0.02 | 17.88 (0.6) | 253.18 (0.8) | -0.19 |
| Anemia | Presence | 739 (4.4) | 9310 (4.8) | -0.02 | 186.85 (6.8) | 1944.47 (6.2) | 0.02 |
| Depression | Presence | 729 (4.4) | 6991 (3.6) | 0.04 | 146.84 (5.3) | 1592.70 (5.1) | 0.01 |
| Hemiplegia or paraplegia | Presence | 21 (0.1) | 241 (0.1) | 0.00 | 3.25 (0.1) | 49.10 (0.2) | -0.01 |
| Fatigue | Presence | 128 (0.8) | 1351 (0.7) | 0.01 | 35.59 (1.3) | 312.13 (1.0) | 0.03 |
| Gait Abnormality or difficulty walking | Presence | 45 (0.3) | 643 (0.3) | -0.01 | 13.47 (0.5) | 113.23 (0.4) | 0.02 |
| Hyperlipidemia | Presence | 16189 (97.0) | 38352 (19.8) | 2.52 | 2542.36 (92.3) | 28498.23 (90.6) | 0.06 |
| Sleep Apnea | Presence | 104 (0.6) | 977 (0.5) | 0.02 | 18.98 (0.7) | 248.64 (0.8) | -0.01 |
| COPD | Presence | 889 (5.3) | 11165 (5.8) | -0.02 | 204.44 (7.4) | 2311.47 (7.3) | 0.00 |
| ACE inhibitor | Presence | 517 (3.1) | 4618 (2.4) | 0.04 | 108.80 (4.0) | 1099.75 (3.5) | 0.02 |
| α-Blocker | Presence | 1157 (6.9) | 15573 (8.1) | -0.04 | 261.47 (9.5) | 2869.89 (9.1) | 0.01 |
| Angiotensin receptor blocker | Presence | 5045 (30.2) | 44749 (23.1) | 0.16 | 926.70 (33.7) | 10306.97 (32.8) | 0.02 |
| β-Blocker | Presence | 1670 (10.0) | 12253 (6.3) | 0.13 | 274.17 (10.0) | 2801.00 (8.9) | 0.04 |
| Calcium channel blocker | Presence | 6814 (40.8) | 59300 (30.7) | 0.21 | 1216.87 (44.2) | 13327.32 (42.4) | 0.04 |
| Diuretics | Presence | 1257 (7.5) | 12965 (6.7) | 0.03 | 245.17 (8.9) | 2676.15 (8.5) | 0.01 |
| Non-statin lipid-lowering drug | Presence | 2181 (13.1) | 11370 (5.9) | 0.25 | 606.60 (22.0) | 6655.94 (21.2) | 0.02 |
| Variable | Category | Number of cases after adjustment | Number of statin group | Cox regression analysis in adjusted population | RR | RD | RD 95% CI | NNT | NNT 95% CI | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | ||||||||||||
| Overall population | - | 34,219 | 2,753 | 0.40 | 0.33 | 0.48 | <.0001 | 0.31 | 0.05 | 0.04 | 0.06 | 21.20 | 18.10 | 24.70 |
| Age | 65 to <75 years | 21,479 | 2,062 | 0.30 | 0.20 | 0.44 | <.0001 | 0.22 | 0.02 | 0.02 | 0.03 | 44.64 | 39.18 | 54.57 |
| 75 to <85 years | 10,284 | 613 | 0.42 | 0.31 | 0.57 | <.0001 | 0.37 | 0.07 | 0.04 | 0.09 | 14.78 | 11.75 | 22.39 | |
| ≥85 years | 3,011 | 75 | 0.59 | 0.37 | 0.93 | 0.02 | 0.55 | 0.13 | -0.004 | 0.28 | 7.63 | 3.63 | -244.3 | |
| Sex | Men | 17,643 | 1,027 | 0.35 | 0.26 | 0.48 | <.0001 | 0.25 | 0.06 | 0.05 | 0.07 | 15.68 | 13.45 | 19.21 |
| Current smoker | No | 30,474 | 2,535 | 0.38 | 0.31 | 0.47 | <.0001 | 0.29 | 0.05 | 0.04 | 0.05 | 20.95 | 18.34 | 24.42 |
| BMI | <18.5 | 2,847 | 133 | 0.23 | 0.10 | 0.55 | 0.0008 | 0.27 | 0.08 | 0.04 | 0.11 | 12.02 | 8.97 | 25.33 |
| ≥30.0 | 798 | 71 | 0.64 | 0.22 | 1.83 | 0.41 | 0.77 | 0.01 | -0.04 | 0.07 | 73.34 | 13.55 | -22.85 | |
| LDL cholesterol | <120 mg/dl | 27,956 | 436 | 0.57 | 0.37 | 0.86 | 0.008 | 0.20 | 0.05 | 0.04 | 0.07 | 20.34 | 14.62 | 23.46 |
| 140 to ≤160 mg/dl | 8,781 | 1,132 | 0.47 | 0.34 | 0.66 | <.0001 | 0.53 | 0.02 | 0.01 | 0.03 | 45.42 | 31.91 | 75.48 | |
| >160 mg/dl | 7,223 | 2,134 | 0.51 | 0.39 | 0.67 | <.0001 | 0.36 | 0.03 | 0.02 | 0.04 | 34.19 | 25.29 | 51.32 | |
| Diabetes | Yes | 2,223 | 239 | 0.31 | 0.16 | 0.58 | 0.0002 | 0.25 | 0.08 | 0.05 | 0.10 | 12.78 | 9.66 | 20.96 |
| No | 31,225 | 2,516 | 0.41 | 0.34 | 0.50 | <.0001 | 0.32 | 0.05 | 0.04 | 0.05 | 22.14 | 18.85 | 26.45 | |
| Rheumatic disease | Yes | 1,350 | 118 | 0.40 | 0.17 | 0.97 | 0.04 | 0.11 | 0.07 | 0.04 | 0.08 | 14.75 | 12.11 | 24.07 |
| Dementia | Yes | 861 | 60 | 0.35 | 0.19 | 0.67 | 0.0015 | 0.42 | 0.18 | 0.05 | 0.26 | 5.69 | 3.83 | 18.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





