Submitted:
13 June 2023
Posted:
13 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Properties of Chemical Warfare Agents
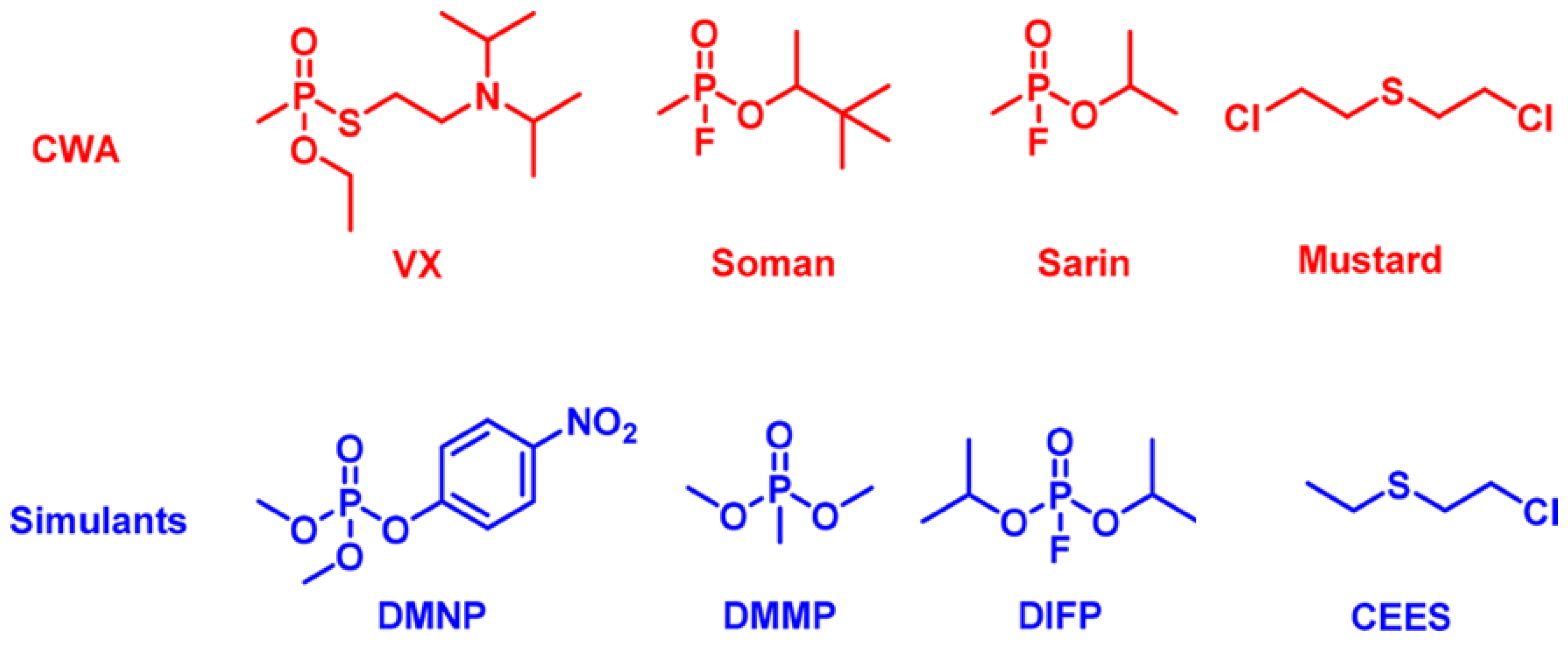
2.1. Nerve Agents, Vesicants, & Their Simulants
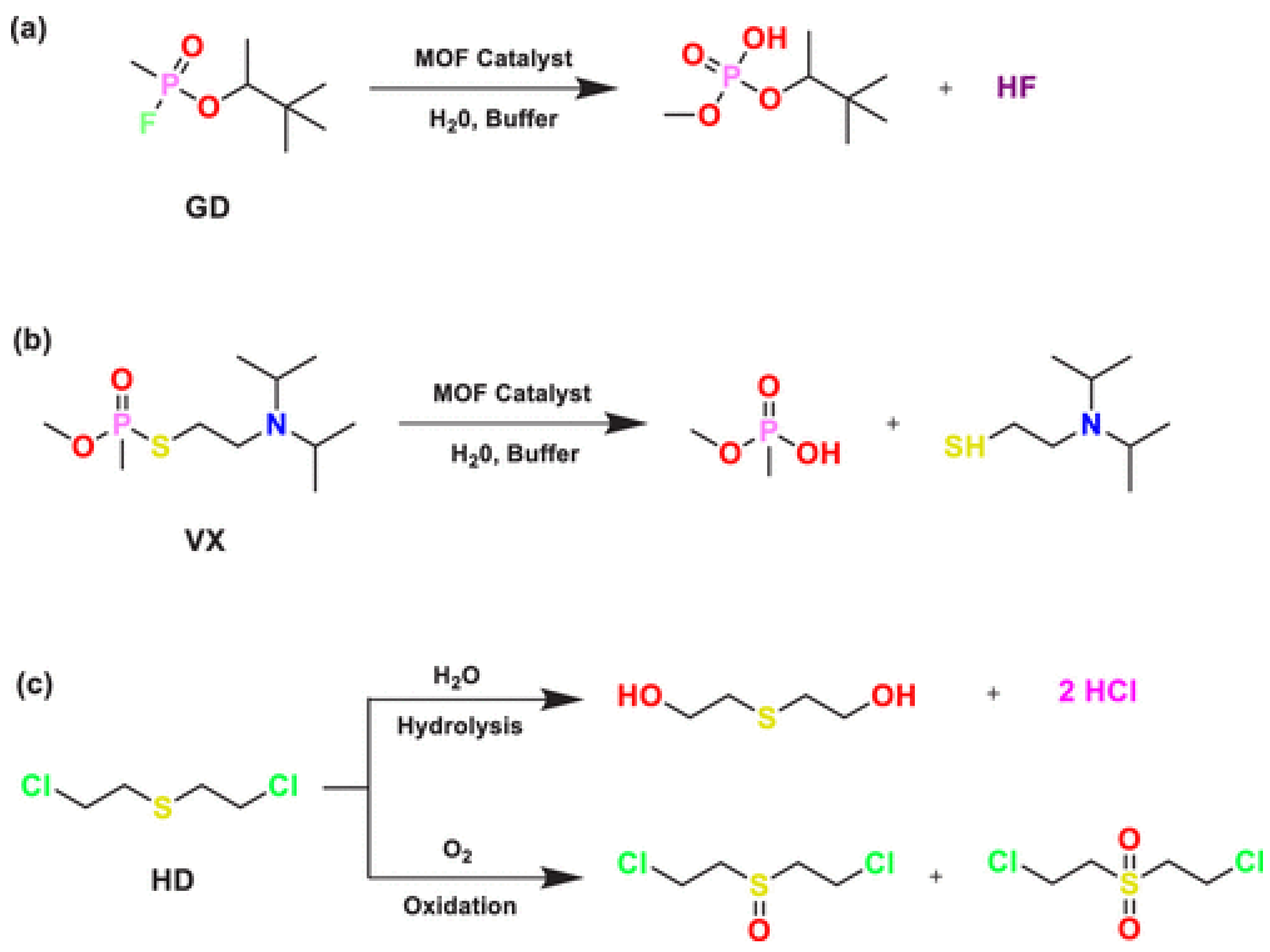
2.2. Degradation Mechanisms
3. CWA Removal by Metal-Organic Frameworks (MOFS)
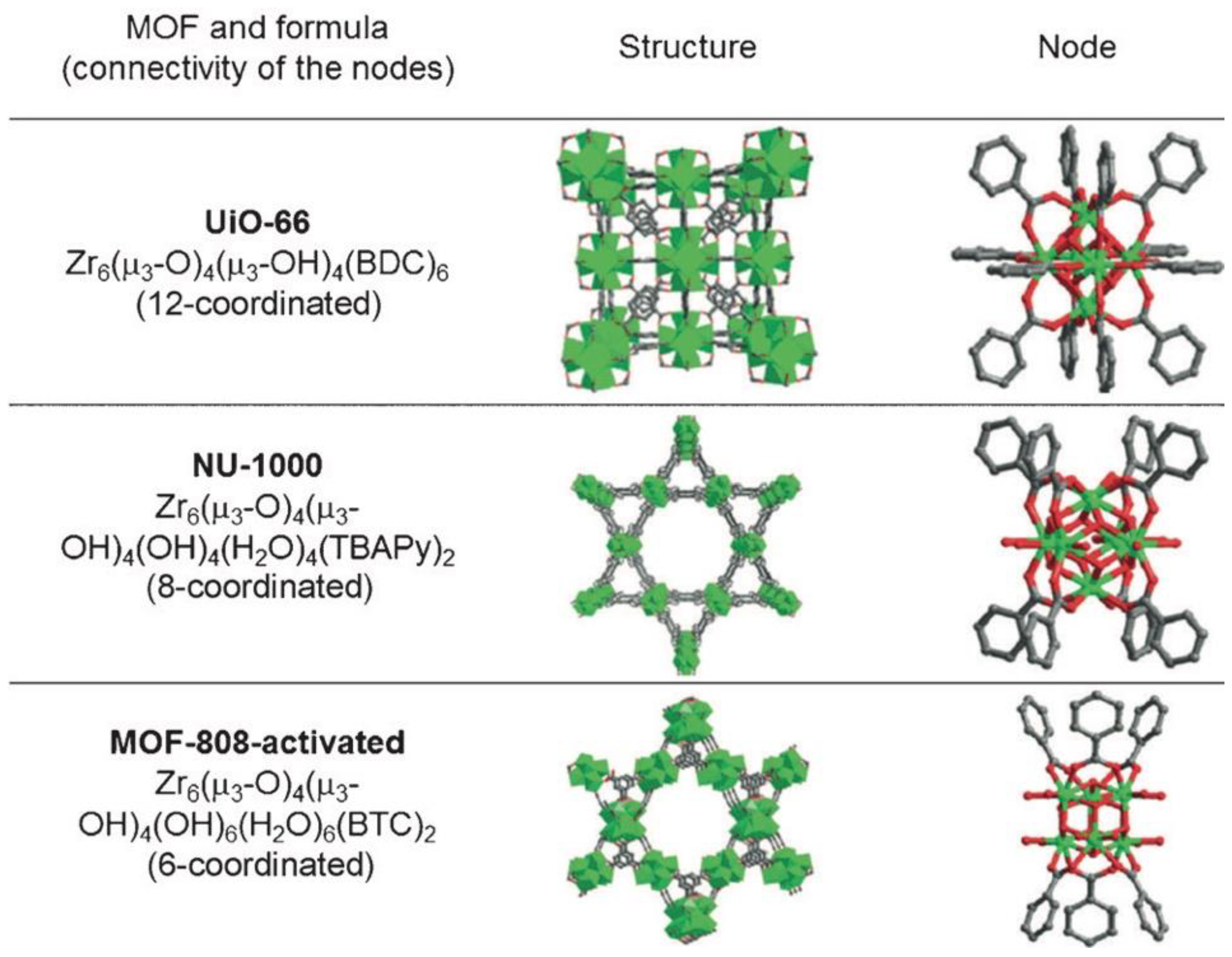
3.1. Structural Features of Promising MOFs
3.1.1. Nodes and Linkers
3.1.2. Pore Sizes and Connectivity
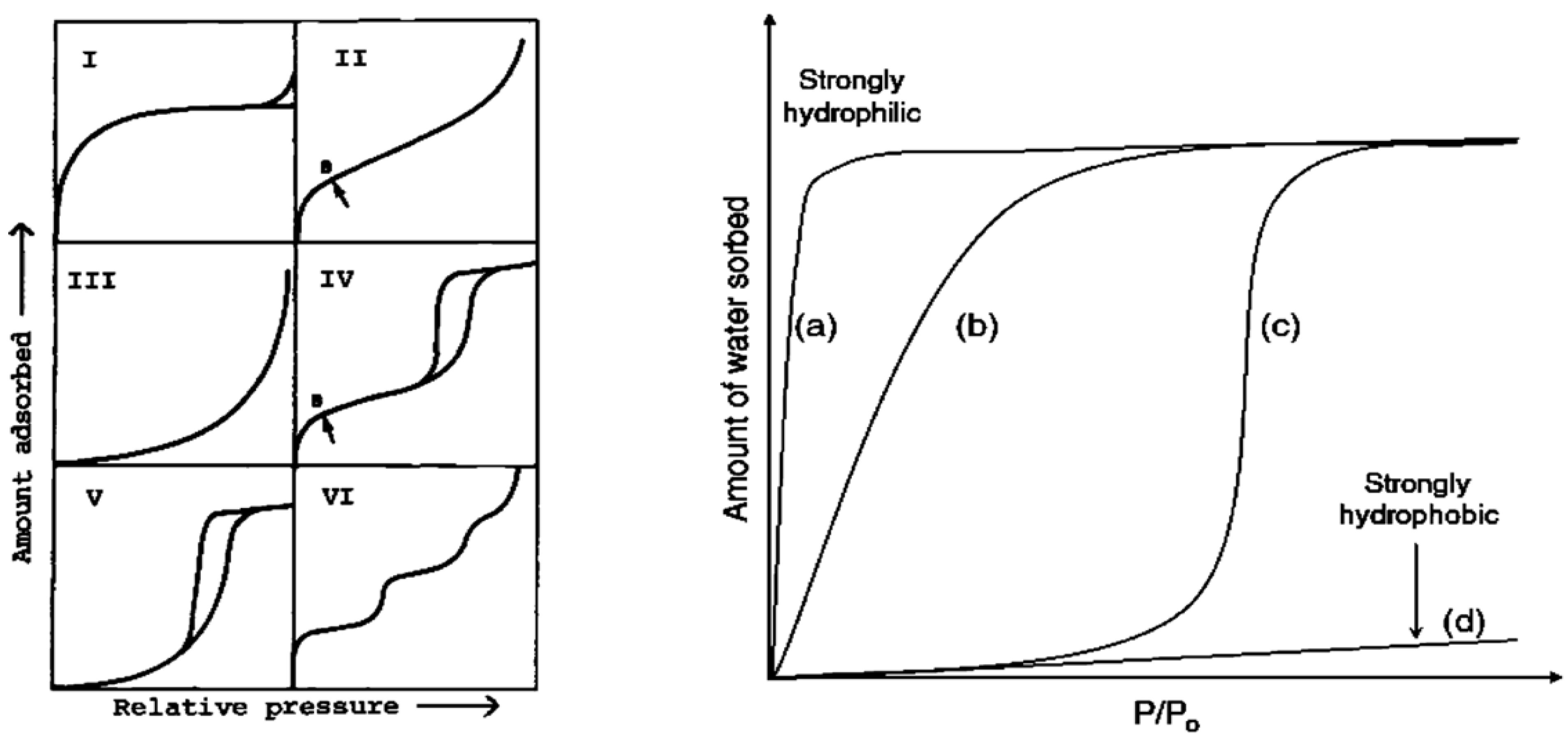
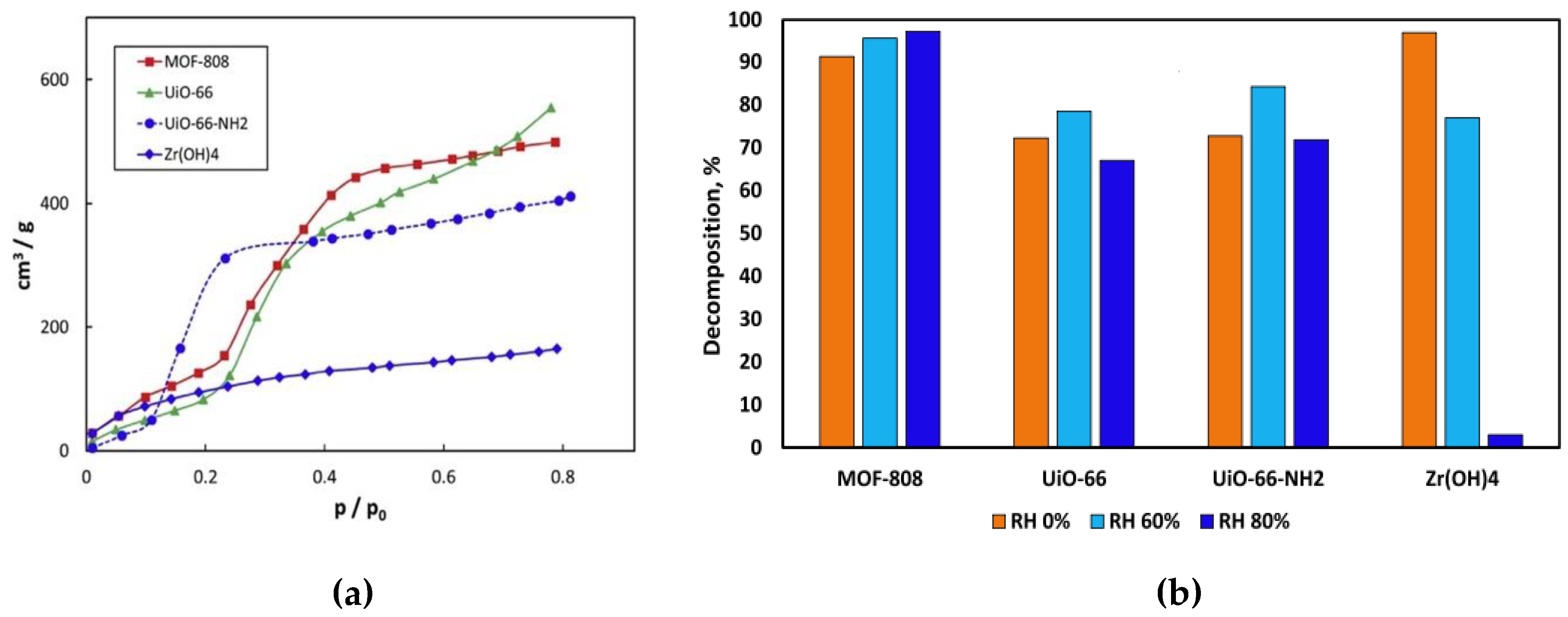
3.1.3. Hydrophilicity and Hydrophobicity
3.2. Nerve Agent Hydrolysis
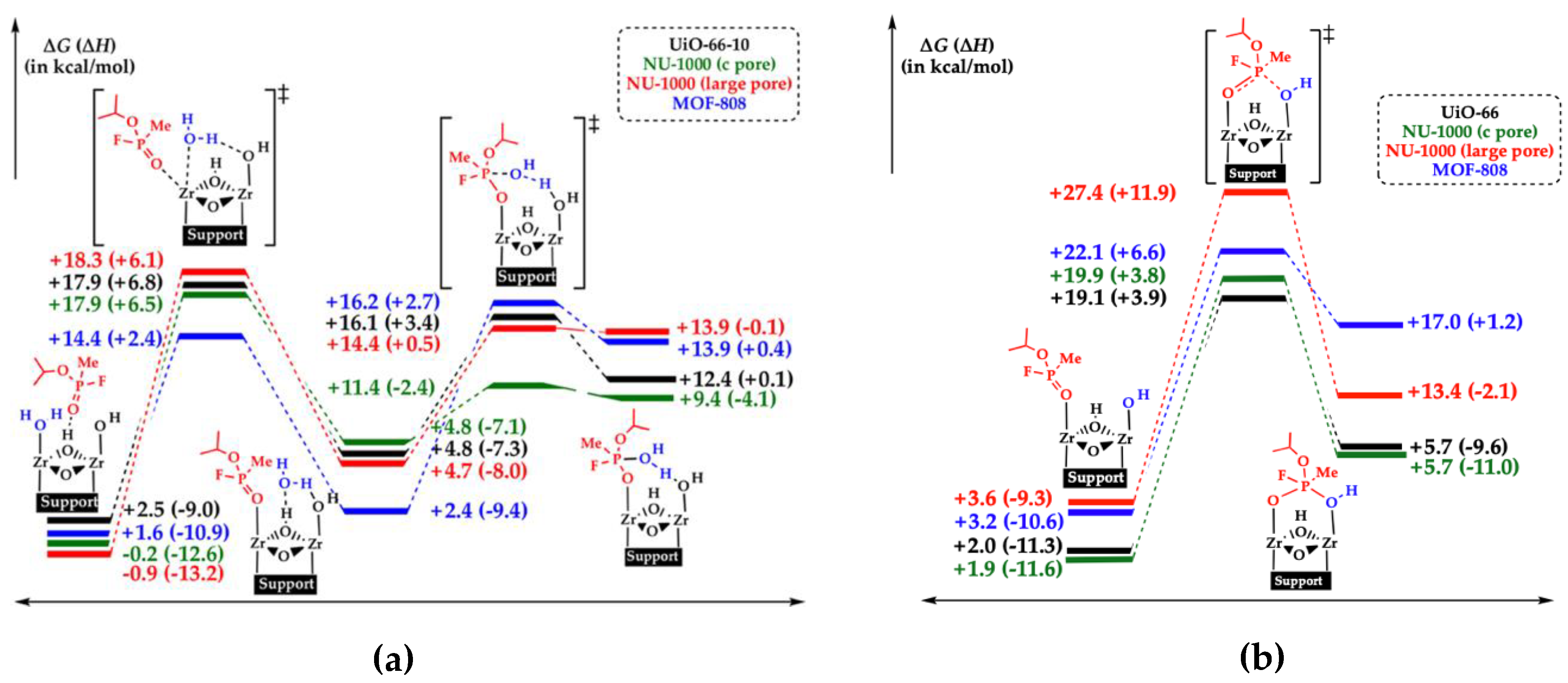
3.2.1. Proposed Hydrolysis Mechanisms in Zr-MOFs
3.2.2. Topology and Reaction Conditions
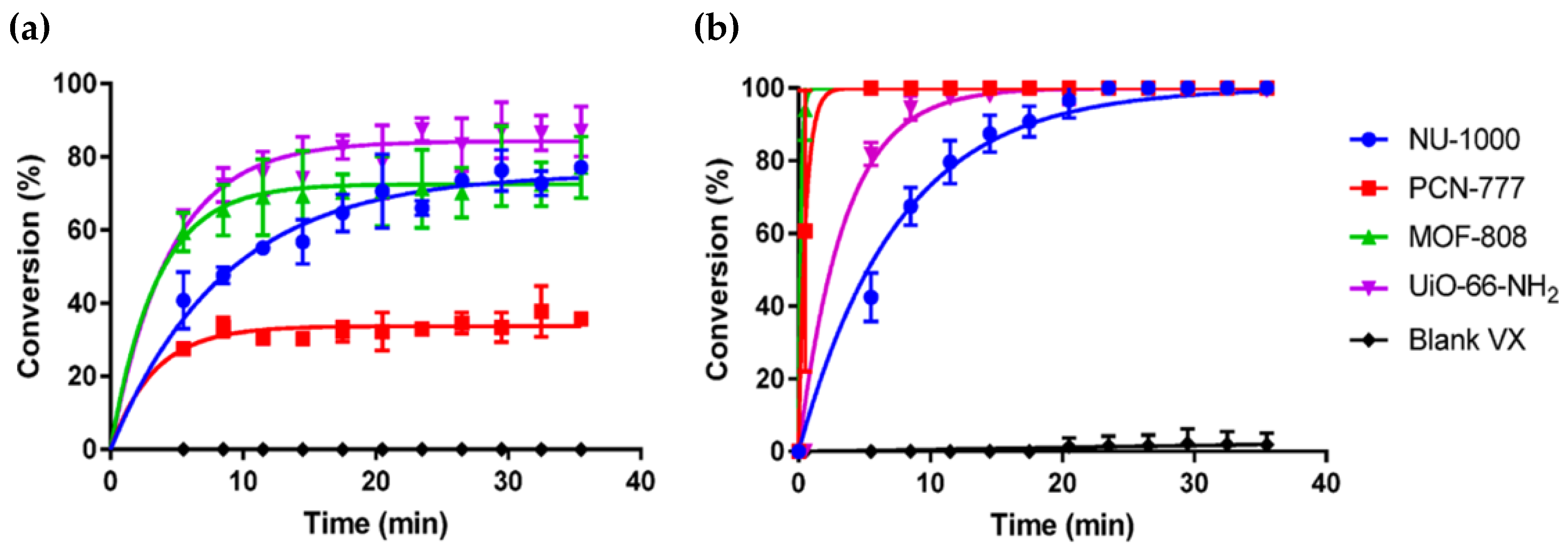
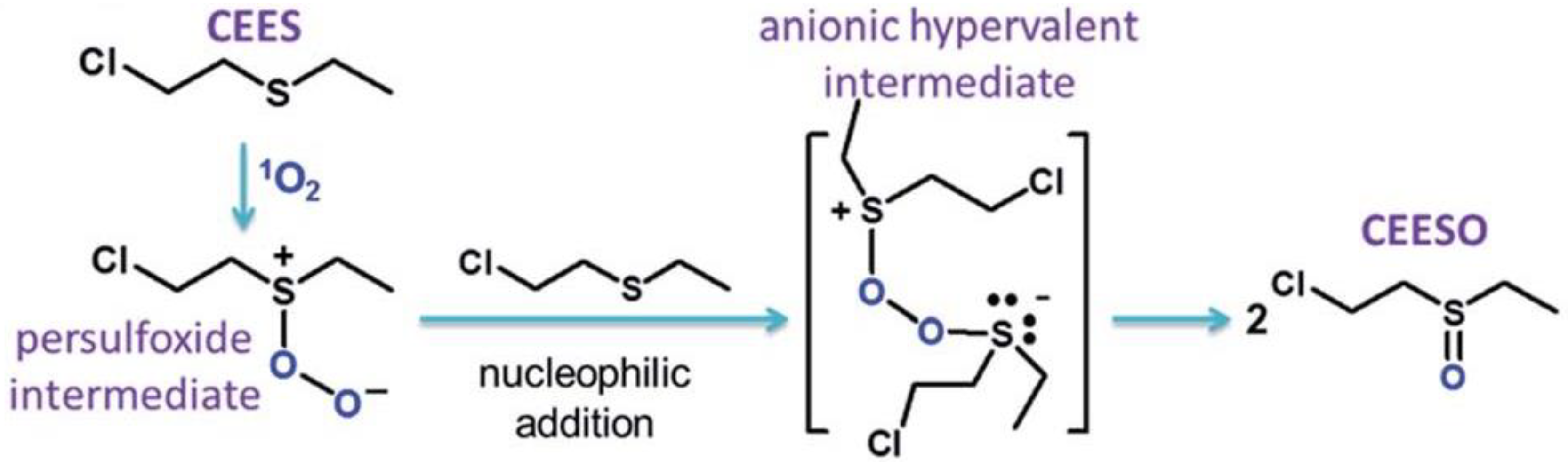
3.3. Sulfur Mustard Oxidation
3.3.1. Photooxidation in Zr-MOFs
3.3.2. Tuning Enhanced Photocatalytic Activity
4. Summary and Outlook
Acknowledgements
Conflicts of Interest
References
- Balasubramanian, S.; Kulandaisamy, A. J.; Babu, K. J.; Das, A.; Balaguru Rayappan, J. B. Metal Organic Framework Functionalized Textiles as Protective Clothing for the Detection and Detoxification of Chemical Warfare Agents—A Review. Ind Eng Chem Res 2021, 60, 4218–4239. [Google Scholar] [CrossRef]
- One Hundred Years of Chemical Warfare: Research, Deployment, Consequences; Friedrich, B. , Hoffmann, D., Renn, J., Schmaltz, F., Wolf, M., Eds.; Springer International Publishing: Cham, 2017. [Google Scholar] [CrossRef]
- Mendonca, M. L.; Ray, D.; Cramer, C. J.; Snurr, R. Q. Exploring the Effects of Node Topology, Connectivity, and Metal Identity on the Binding of Nerve Agents and Their Hydrolysis Products in Metal–Organic Frameworks. ACS Appl Mater Interfaces 2020, 12, 35657–35675. [Google Scholar] [CrossRef]
- Wang, H.; Mahle, J. J.; Tovar, T. M.; Peterson, G. W.; Hall, M. G.; DeCoste, J. B.; Buchanan, J. H.; Karwacki, C. J. Solid-Phase Detoxification of Chemical Warfare Agents Using Zirconium-Based Metal Organic Frameworks and the Moisture Effects: Analyze via Digestion. ACS Appl Mater Interfaces 2019, 11, 21109–21116. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Moon, S.-Y.; Hupp, J. T.; Farha, O. K. Dual-Function Metal–Organic Framework as a Versatile Catalyst for Detoxifying Chemical Warfare Agent Simulants. ACS Nano 2015, 9, 12358–12364. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S. S.; Holdt, H.-J. Breaking Down Chemical Weapons by Metal–Organic Frameworks. Angewandte Chemie International Edition 2016, 55, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Mondloch, J. E.; Katz, M. J.; Isley III, W. C.; Ghosh, P.; Liao, P.; Bury, W.; Wagner, G. W.; Hall, M. G.; DeCoste, J. B.; Peterson, G. W.; Snurr, R. Q.; Cramer, C. J.; Hupp, J. T.; Farha, O. K. Destruction of Chemical Warfare Agents Using Metal–Organic Frameworks. Nat Mater 2015, 14, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Grissom, T. G.; Plonka, A. M.; Sharp, C. H.; Ebrahim, A. M.; Tian, Y.; Collins-Wildman, D. L.; Kaledin, A. L.; Siegal, H. J.; Troya, D.; Hill, C. L.; Frenkel, A. I.; Musaev, D. G.; Gordon, W. O.; Karwacki, C. J.; Mitchell, M. B.; Morris, J. R. Metal–Organic Framework- and Polyoxometalate-Based Sorbents for the Uptake and Destruction of Chemical Warfare Agents. ACS Appl Mater Interfaces 2020, 12, 14641–14661. [Google Scholar] [CrossRef] [PubMed]
- Bobbitt, N. S.; Mendonca, M. L.; Howarth, A. J.; Islamoglu, T.; Hupp, J. T.; Farha, O. K.; Snurr, R. Q. Metal–Organic Frameworks for the Removal of Toxic Industrial Chemicals and Chemical Warfare Agents. Chem Soc Rev 2017, 46, 3357–3385. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Farha, O. K.; Roberts, J.; Scheidt, K. A.; Nguyen, S. T.; Hupp, J. T. Metal–Organic Framework Materials as Catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.; Xie, L.-H.; Rutledge, W.; Li, J.-R.; Zhou, H.-C. Zr-Based Metal–Organic Frameworks: Design, Synthesis, Structure, and Applications. Chem Soc Rev 2016, 45, 2327–2367. [Google Scholar] [CrossRef]
- Moon, S.-Y.; Liu, Y.; Hupp, J. T.; Farha, O. K. Instantaneous Hydrolysis of Nerve-Agent Simulants with a Six-Connected Zirconium-Based Metal–Organic Framework. Angewandte Chemie International Edition 2015, 54, 6795–6799. [Google Scholar] [CrossRef]
- Oliver, M. C.; Wang, S.; Huang, L.; Kasule, M.; Wu, Y. Vapor-Like Water in the NU-1000 Zr-MOF: A Molecular Level Understanding of Balanced Hydrophobicity in Humid Conditions. The Journal of Physical Chemistry C 2023, 127, 6503–6514. [Google Scholar] [CrossRef]
- Islamoglu, T.; Chen, Z.; Wasson, M. C.; Buru, C. T.; Kirlikovali, K. O.; Afrin, U.; Mian, M. R.; Farha, O. K. Metal–Organic Frameworks against Toxic Chemicals. Chem Rev 2020, 120, 8130–8160. [Google Scholar] [CrossRef]
- Liu, Y.; Howarth, A. J.; Vermeulen, N. A.; Moon, S.-Y.; Hupp, J. T.; Farha, O. K. Catalytic Degradation of Chemical Warfare Agents and Their Simulants by Metal-Organic Frameworks. Coord Chem Rev 2017, 346, 101–111. [Google Scholar] [CrossRef]
- Sferopoulos, R. A Review of Chemical Warfare Agent (CWA) Detector Technologies and Commercial-Off-The-Shelf Items; 2009.
- Liao, Y.; Sheridan, T.; Liu, J.; Farha, O.; Hupp, J. Product Inhibition and the Catalytic Destruction of a Nerve Agent Simulant by Zirconium-Based Metal–Organic Frameworks. ACS Appl Mater Interfaces 2021, 13, 30565–30575. [Google Scholar] [CrossRef] [PubMed]
- <i>Decontamination of Warfare Agents: Enzymatic Methods for the Removal of B/C, Weapons</i>; Richardt, A. 18. Decontamination of Warfare Agents: Enzymatic Methods for the Removal of B/C Weapons, 2008. [Google Scholar]
- Hoenig, S. L. Compendium of Chemical Warfare Agents; 2007.
- Bartelt-Hunt, S. L.; Knappe, D. R. U.; Barlaz, M. A. A Review of Chemical Warfare Agent Simulants for the Study of Environmental Behavior. Crit Rev Environ Sci Technol 2008, 38, 112–136. [Google Scholar] [CrossRef]
- Vishnyakov, A.; Gor, G. Yu.; Lee, M.-T.; Neimark, A. V. Molecular Modeling of Organophosphorous Agents and Their Aqueous Solutions. J Phys Chem A 2011, 115, 5201–5209. [Google Scholar] [CrossRef]
- Lee, M.-T.; Vishnyakov, A.; Gor, G. Yu.; Neimark, A. V. Interactions of Phosphororganic Agents with Water and Components of Polyelectrolyte Membranes. J Phys Chem B 2011, 115, 13617–13623. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Sava Gallis, D. F.; Greathouse, J. A.; Sholl, D. S. How Useful Are Common Simulants of Chemical Warfare Agents at Predicting Adsorption Behavior? The Journal of Physical Chemistry C 2018, 122, 26061–26069. [Google Scholar] [CrossRef]
- Emelianova, A.; Reed, A.; Basharova, E. A.; Kolesnikov, A. L.; Gor, G. Y. Closer Look at Adsorption of Sarin and Simulants on Metal–Organic Frameworks. ACS Appl Mater Interfaces 2023, 15, 18559–18567. [Google Scholar] [CrossRef]
- Mendonca, M. L.; Snurr, R. Q. Screening for Improved Nerve Agent Simulants and Insights into Organophosphate Hydrolysis Reactions from DFT and QSAR Modeling. Chemistry – A European Journal 2019, 25, 9217–9229. [Google Scholar] [CrossRef] [PubMed]
- Bartelt-Hunt, S. L.; Knappe, D. R. U.; Barlaz, M. A. A Review of Chemical Warfare Agent Simulants for the Study of Environmental Behavior. Crit Rev Environ Sci Technol 2008, 38, 112–136. [Google Scholar] [CrossRef]
- Butrow, A. B.; Buchanan, J. H.; Tevault, D. E. Vapor Pressure of Organophosphorus Nerve Agent Simulant Compounds. J Chem Eng Data 2009, 54, 1876–1883. [Google Scholar] [CrossRef]
- Piao, H.; Marx, R. B.; Schneider, S.; Irvine, D. A.; Staton, J. Analysis of VX Nerve Agent Hydrolysis Products in Wastewater Effluents by Ion Chromatography with Amperometric and Conductivity Detection. J Chromatogr A 2005, 1089, (1–2). [Google Scholar] [CrossRef] [PubMed]
- Che Sulaiman, I. S.; Chieng, B. W.; Pojol, F. E.; Ong, K. K.; Abdul Rashid, J. I.; Wan Yunus, W. M. Z.; Mohd Kasim, N. A.; Abdul Halim, N.; Mohd Noor, S. A.; Knight, V. F. A Review on Analysis Methods for Nerve Agent Hydrolysis Products. Forensic Toxicol 2020, 38, 297–313. [Google Scholar] [CrossRef]
- Moon, S.; Proussaloglou, E.; Peterson, G. W.; DeCoste, J. B.; Hall, M. G.; Howarth, A. J.; Hupp, J. T.; Farha, O. K. Detoxification of Chemical Warfare Agents Using a Zr 6 -Based Metal–Organic Framework/Polymer Mixture. Chemistry – A European Journal 2016, 22, 14864–14868. [Google Scholar] [CrossRef]
- Couzon, N.; Dhainaut, J.; Campagne, C.; Royer, S.; Loiseau, T.; Volkringer, C. Porous Textile Composites (PTCs) for the Removal and the Decomposition of Chemical Warfare Agents (CWAs) – A Review. Coord Chem Rev 2022, 467, 214598. [Google Scholar] [CrossRef]
- Vellingiri, K.; Philip, L.; Kim, K.-H. Metal–Organic Frameworks as Media for the Catalytic Degradation of Chemical Warfare Agents. Coord Chem Rev 2017, 353, 159–179. [Google Scholar] [CrossRef]
- Oheix, E.; Gravel, E.; Doris, E. Catalytic Processes for the Neutralization of Sulfur Mustard. Chemistry – A European Journal 2021, 27, 54–68. [Google Scholar] [CrossRef]
- Wang, H.; Wagner, G. W.; Lu, A. X.; Nguyen, D. L.; Buchanan, J. H.; McNutt, P. M.; Karwacki, C. J. Photocatalytic Oxidation of Sulfur Mustard and Its Simulant on BODIPY-Incorporated Polymer Coatings and Fabrics. ACS Appl Mater Interfaces 2018, 10, 18771–18777. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Buru, C. T.; Howarth, A. J.; Mahle, J. J.; Buchanan, J. H.; DeCoste, J. B.; Hupp, J. T.; Farha, O. K. Efficient and Selective Oxidation of Sulfur Mustard Using Singlet Oxygen Generated by a Pyrene-Based Metal–Organic Framework. J Mater Chem A Mater 2016, 4, 13809–13813. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Kapteijn, F. Water and Metal–Organic Frameworks: From Interaction toward Utilization. Chem Rev 2020, 120, 8303–8377. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Idrees, K. B.; Chen, Z.; Knapp, J.; Chen, Y.; Wang, X.; Cao, R.; Zhang, X.; Xing, H.; Islamoglu, T.; Farha, O. K. Nanoporous Water-Stable Zr-Based Metal–Organic Frameworks for Water Adsorption. ACS Appl Nano Mater 2021, 4, 4346–4350. [Google Scholar] [CrossRef]
- Low, J. J.; Benin, A. I.; Jakubczak, P.; Abrahamian, J. F.; Faheem, S. A.; Willis, R. R. Virtual High Throughput Screening Confirmed Experimentally: Porous Coordination Polymer Hydration. J Am Chem Soc 2009, 131, 15834–15842. [Google Scholar] [CrossRef]
- Mouchaham, G.; Wang, S.; Serre, C. The Stability of Metal-Organic Frameworks. In Metal-Organic Frameworks; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018. [Google Scholar] [CrossRef]
- Pearson, R. G. Hard and Soft Acids and Bases. J Am Chem Soc 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; Wang, Q.; Zou, L.; Zhang, Y.; Zhang, L.; Fang, Y.; Li, J.; Zhou, H.-C. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Advanced Materials 2018, 30. [Google Scholar] [CrossRef]
- Wang, B.; Lv, X.-L.; Feng, D.; Xie, L.-H.; Zhang, J.; Li, M.; Xie, Y.; Li, J.-R.; Zhou, H.-C. Highly Stable Zr(IV)-Based Metal–Organic Frameworks for the Detection and Removal of Antibiotics and Organic Explosives in Water. J Am Chem Soc 2016, 138, 6204–6216. [Google Scholar] [CrossRef]
- Cavka, J. H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K. P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J Am Chem Soc 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Mondloch, J. E.; Katz, M. J.; Planas, N.; Semrouni, D.; Gagliardi, L.; Hupp, J. T.; Farha, O. K. Are Zr6-Based MOFs Water Stable? Linker Hydrolysis vs. Capillary-Force-Driven Channel Collapse. Chemical Communications 2014, 50, 8944–8946. [Google Scholar] [CrossRef]
- Sikma, R. E.; Katyal, N.; Lee, S.-K.; Fryer, J. W.; Romero, C. G.; Emslie, S. K.; Taylor, E. L.; Lynch, V. M.; Chang, J.-S.; Henkelman, G.; Humphrey, S. M. Low-Valent Metal Ions as MOF Pillars: A New Route Toward Stable and Multifunctional MOFs. J Am Chem Soc 2021, 143, 13710–13720. [Google Scholar] [CrossRef]
- Kang, I. J.; Khan, N. A.; Haque, E.; Jhung, S. H. Chemical and Thermal Stability of Isotypic Metal–Organic Frameworks: Effect of Metal Ions. Chemistry – A European Journal 2011, 17, 6437–6442. [Google Scholar] [CrossRef] [PubMed]
- Towsif Abtab, S. M.; Alezi, D.; Bhatt, P. M.; Shkurenko, A.; Belmabkhout, Y.; Aggarwal, H.; Weseliński, Ł. J.; Alsadun, N.; Samin, U.; Hedhili, M. N.; Eddaoudi, M. Reticular Chemistry in Action: A Hydrolytically Stable MOF Capturing Twice Its Weight in Adsorbed Water. Chem 2018, 4, 94–105. [Google Scholar] [CrossRef]
- Lian, X.; Feng, D.; Chen, Y.-P.; Liu, T.-F.; Wang, X.; Zhou, H.-C. The Preparation of an Ultrastable Mesoporous Cr( <scp>iii</Scp> )-MOF via Reductive Labilization. Chem Sci 2015, 6, 7044–7048. [Google Scholar] [CrossRef] [PubMed]
- Rieth, A. J.; Dincă, M. Tricking Inert Metals into Water-Absorbing MOFs. Joule 2018, 2, 18–20. [Google Scholar] [CrossRef]
- Burtch, N. C.; Jasuja, H.; Walton, K. S. Water Stability and Adsorption in Metal–Organic Frameworks. Chem Rev 2014, 114, 10575–10612. [Google Scholar] [CrossRef] [PubMed]
- Emerson, A. J.; Hawes, C. S.; Marshall, M.; Knowles, G. P.; Chaffee, A. L.; Batten, S. R.; Turner, D. R. High-Connectivity Approach to a Hydrolytically Stable Metal–Organic Framework for CO 2 Capture from Flue Gas. Chemistry of Materials 2018, 30, 6614–6618. [Google Scholar] [CrossRef]
- Ling, S.; Slater, B. Dynamic Acidity in Defective UiO-66. Chem Sci 2016, 7, 4706–4712. [Google Scholar] [CrossRef]
- Wang, S.; Oliver, M. C.; An, Y.; Chen, E.; Su, Z.; Kleinhammes, A.; Wu, Y.; Huang, L. A Computational Study of Isopropyl Alcohol Adsorption and Diffusion in UiO-66 Metal–Organic Framework: The Role of Missing Linker Defect. J Phys Chem B 2021, 125, 3690–3699. [Google Scholar] [CrossRef]
- Ghosh, P.; Colón, Y. J.; Snurr, R. Q. Water Adsorption in UiO-66: The Importance of Defects. Chem. Commun. 2014, 50, 11329–11331. [Google Scholar] [CrossRef]
- Wang, G.; Sharp, C.; Plonka, A. M.; Wang, Q.; Frenkel, A. I.; Guo, W.; Hill, C.; Smith, C.; Kollar, J.; Troya, D.; Morris, J. R. Mechanism and Kinetics for Reaction of the Chemical Warfare Agent Simulant, DMMP( g ), with Zirconium(IV) MOFs: An Ultrahigh-Vacuum and DFT Study. The Journal of Physical Chemistry C 2017, 121, 11261–11272. [Google Scholar] [CrossRef]
- Grant Glover, T.; Peterson, G. W.; Schindler, B. J.; Britt, D.; Yaghi, O. MOF-74 Building Unit Has a Direct Impact on Toxic Gas Adsorption. Chem Eng Sci 2011, 66, 163–170. [Google Scholar] [CrossRef]
- Ng, E.-P.; Mintova, S. Nanoporous Materials with Enhanced Hydrophilicity and High Water Sorption Capacity. Microporous and Mesoporous Materials 2008, 114, (1–3). [Google Scholar] [CrossRef]
- Islamoglu, T.; Goswami, S.; Li, Z.; Howarth, A. J.; Farha, O. K.; Hupp, J. T. Postsynthetic Tuning of Metal–Organic Frameworks for Targeted Applications. Acc Chem Res 2017, 50, 805–813. [Google Scholar] [CrossRef]
- Matito-Martos, I.; Moghadam, P. Z.; Li, A.; Colombo, V.; Navarro, J. A. R.; Calero, S.; Fairen-Jimenez, D. Discovery of an Optimal Porous Crystalline Material for the Capture of Chemical Warfare Agents. Chemistry of Materials 2018, 30, 4571–4579. [Google Scholar] [CrossRef]
- Son, F. A.; Wasson, M. C.; Islamoglu, T.; Chen, Z.; Gong, X.; Hanna, S. L.; Lyu, J.; Wang, X.; Idrees, K. B.; Mahle, J. J.; Peterson, G. W.; Farha, O. K. Uncovering the Role of Metal–Organic Framework Topology on the Capture and Reactivity of Chemical Warfare Agents. Chemistry of Materials 2020, 32, 4609–4617. [Google Scholar] [CrossRef]
- Canivet, J.; Fateeva, A.; Guo, Y.; Coasne, B.; Farrusseng, D. Water Adsorption in MOFs: Fundamentals and Applications. Chem. Soc. Rev. 2014, 43, 5594–5617. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, A. M.; Levasseur, B.; Bandosz, T. J. Interactions of NO2 with Zr-Based MOF: Effects of the Size of Organic Linkers on NO2 Adsorption at Ambient Conditions. Langmuir 2013, 29, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S. G.; Kim, M.-K.; Park, M.; Jang, S. O.; Kim, S. H.; Jung, H. Availability of Zr-Based MOFs for the Degradation of Nerve Agents in All Humidity Conditions. Microporous and Mesoporous Materials 2019, 274, 9–16. [Google Scholar] [CrossRef]
- Yao, A.; Jiao, X.; Chen, D.; Li, C. Bio-Inspired Polydopamine-Mediated Zr-MOF Fabrics for Solar Photothermal-Driven Instantaneous Detoxification of Chemical Warfare Agent Simulants. ACS Appl Mater Interfaces 2020, 12, 18437–18445. [Google Scholar] [CrossRef]
- Kirlikovali, K. O.; Chen, Z.; Islamoglu, T.; Hupp, J. T.; Farha, O. K. Zirconium-Based Metal–Organic Frameworks for the Catalytic Hydrolysis of Organophosphorus Nerve Agents. ACS Appl Mater Interfaces 2020, 12, 14702–14720. [Google Scholar] [CrossRef] [PubMed]
- Momeni, M. R.; Cramer, C. J. Dual Role of Water in Heterogeneous Catalytic Hydrolysis of Sarin by Zirconium-Based Metal–Organic Frameworks. ACS Appl Mater Interfaces 2018, 10, 18435–18439. [Google Scholar] [CrossRef] [PubMed]
- Devulapalli, V. S. D.; Richard, M.; Luo, T.-Y.; De Souza, M. L.; Rosi, N. L.; Borguet, E. Tuning the Lewis Acidity of Metal–Organic Frameworks for Enhanced Catalysis. Dalton Transactions 2021, 50, 3116–3120. [Google Scholar] [CrossRef] [PubMed]
- Malonzo, C. D.; Shaker, S. M.; Ren, L.; Prinslow, S. D.; Platero-Prats, A. E.; Gallington, L. C.; Borycz, J.; Thompson, A. B.; Wang, T. C.; Farha, O. K.; Hupp, J. T.; Lu, C. C.; Chapman, K. W.; Myers, J. C.; Penn, R. L.; Gagliardi, L.; Tsapatsis, M.; Stein, A. Thermal Stabilization of Metal–Organic Framework-Derived Single-Site Catalytic Clusters through Nanocasting. J Am Chem Soc 2016, 138, 2739–2748. [Google Scholar] [CrossRef] [PubMed]
- Troya, D. Reaction Mechanism of Nerve-Agent Decomposition with Zr-Based Metal Organic Frameworks. The Journal of Physical Chemistry C 2016, 120, 29312–29323. [Google Scholar] [CrossRef]
- Chen, H.; Liao, P.; Mendonca, M. L.; Snurr, R. Q. Insights into Catalytic Hydrolysis of Organophosphate Warfare Agents by Metal–Organic Framework NU-1000. The Journal of Physical Chemistry C 2018, 122, 12362–12368. [Google Scholar] [CrossRef]
- de Koning, M. C.; Vieira Soares, C.; van Grol, M.; Bross, R. P. T.; Maurin, G. Effective Degradation of Novichok Nerve Agents by the Zirconium Metal–Organic Framework MOF-808. ACS Appl Mater Interfaces 2022, 14, 9222–9230. [Google Scholar] [CrossRef]
- Vermoortele, F.; Bueken, B.; Le Bars, G.; Van de Voorde, B.; Vandichel, M.; Houthoofd, K.; Vimont, A.; Daturi, M.; Waroquier, M.; Van Speybroeck, V.; Kirschhock, C.; De Vos, D. E. Synthesis Modulation as a Tool To Increase the Catalytic Activity of Metal–Organic Frameworks: The Unique Case of UiO-66(Zr). J Am Chem Soc 2013, 135, 11465–11468. [Google Scholar] [CrossRef]
- Bůžek, D.; Adamec, S.; Lang, K.; Demel, J. Metal–Organic Frameworks vs. Buffers: Case Study of UiO-66 Stability. Inorg Chem Front 2021, 8, 720–734. [Google Scholar] [CrossRef]
- de Koning, M. C.; van Grol, M.; Breijaert, T. Degradation of Paraoxon and the Chemical Warfare Agents VX, Tabun, and Soman by the Metal–Organic Frameworks UiO-66-NH 2, MOF-808, NU-1000, and PCN-777. Inorg Chem 2017, 56, 11804–11809. [Google Scholar] [CrossRef]
- Peterson, G. W.; Moon, S.-Y.; Wagner, G. W.; Hall, M. G.; DeCoste, J. B.; Hupp, J. T.; Farha, O. K. Tailoring the Pore Size and Functionality of UiO-Type Metal–Organic Frameworks for Optimal Nerve Agent Destruction. Inorg Chem 2015, 54, 9684–9686. [Google Scholar] [CrossRef]
- Caratelli, C.; Hajek, J.; Cirujano, F. G.; Waroquier, M.; Llabrés i Xamena, F. X.; Van Speybroeck, V. Nature of Active Sites on UiO-66 and Beneficial Influence of Water in the Catalysis of Fischer Esterification. J Catal 2017, 352, 401–414. [Google Scholar] [CrossRef]
- Moon, S.-Y.; Wagner, G. W.; Mondloch, J. E.; Peterson, G. W.; DeCoste, J. B.; Hupp, J. T.; Farha, O. K. Effective, Facile, and Selective Hydrolysis of the Chemical Warfare Agent VX Using Zr 6 -Based Metal–Organic Frameworks. Inorg Chem 2015, 54, 10829–10833. [Google Scholar] [CrossRef]
- Katz, M. J.; Moon, S.-Y.; Mondloch, J. E.; Beyzavi, M. H.; Stephenson, C. J.; Hupp, J. T.; Farha, O. K. Exploiting Parameter Space in MOFs: A 20-Fold Enhancement of Phosphate-Ester Hydrolysis with UiO-66-NH 2. Chem Sci 2015, 6, 2286–2291. [Google Scholar] [CrossRef]
- Islamoglu, T.; Ortuño, M. A.; Proussaloglou, E.; Howarth, A. J.; Vermeulen, N. A.; Atilgan, A.; Asiri, A. M.; Cramer, C. J.; Farha, O. K. Presence versus Proximity: The Role of Pendant Amines in the Catalytic Hydrolysis of a Nerve Agent Simulant. Angewandte Chemie International Edition 2018, 57, 1949–1953. [Google Scholar] [CrossRef]
- Ma, K.; Wasson, M. C.; Wang, X.; Zhang, X.; Idrees, K. B.; Chen, Z.; Wu, Y.; Lee, S.-J.; Cao, R.; Chen, Y.; Yang, L.; Son, F. A.; Islamoglu, T.; Peterson, G. W.; Mahle, J. J.; Farha, O. K. Near-Instantaneous Catalytic Hydrolysis of Organophosphorus Nerve Agents with Zirconium-Based MOF/Hydrogel Composites. Chem Catalysis 2021, 1, 721–733. [Google Scholar] [CrossRef]
- Luo, H.-B.; Castro, A. J.; Wasson, M. C.; Flores, W.; Farha, O. K.; Liu, Y. Rapid, Biomimetic Degradation of a Nerve Agent Simulant by Incorporating Imidazole Bases into a Metal–Organic Framework. ACS Catal 2021, 11, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Howarth, A. J.; Hupp, J. T.; Farha, O. K. Selective Photooxidation of a Mustard-Gas Simulant Catalyzed by a Porphyrinic Metal-Organic Framework. Angewandte Chemie International Edition 2015, 54, 9001–9005. [Google Scholar] [CrossRef] [PubMed]
- Atilgan, A.; Islamoglu, T.; Howarth, A. J.; Hupp, J. T.; Farha, O. K. Detoxification of a Sulfur Mustard Simulant Using a BODIPY-Functionalized Zirconium-Based Metal–Organic Framework. ACS Appl Mater Interfaces 2017, 9, 24555–24560. [Google Scholar] [CrossRef] [PubMed]
- Tanielian, C.; Wolff, C.; Esch, M. Singlet Oxygen Production in Water: Aggregation and Charge-Transfer Effects. J Phys Chem 1996, 100, 6555–6560. [Google Scholar] [CrossRef]
- Hao, Y.; Papazyan, E. K.; Ba, Y.; Liu, Y. Mechanism-Guided Design of Metal–Organic Framework Composites for Selective Photooxidation of a Mustard Gas Simulant under Solvent-Free Conditions. ACS Catal 2022, 12, 363–371. [Google Scholar] [CrossRef]
- Buru, C. T.; Majewski, M. B.; Howarth, A. J.; Lavroff, R. H.; Kung, C.-W.; Peters, A. W.; Goswami, S.; Farha, O. K. Improving the Efficiency of Mustard Gas Simulant Detoxification by Tuning the Singlet Oxygen Quantum Yield in Metal–Organic Frameworks and Their Corresponding Thin Films. ACS Appl Mater Interfaces 2018, 10, 23802–23806. [Google Scholar] [CrossRef]
- Howarth, A. J.; Buru, C. T.; Liu, Y.; Ploskonka, A. M.; Hartlieb, K. J.; McEntee, M.; Mahle, J. J.; Buchanan, J. H.; Durke, E. M.; Al-Juaid, S. S.; Stoddart, J. F.; DeCoste, J. B.; Hupp, J. T.; Farha, O. K. Postsynthetic Incorporation of a Singlet Oxygen Photosensitizer in a Metal-Organic Framework for Fast and Selective Oxidative Detoxification of Sulfur Mustard. Chemistry - A European Journal 2017, 23, 214–218. [Google Scholar] [CrossRef]
- Zhang, W.-Q.; Cheng, K.; Zhang, H.; Li, Q.-Y.; Ma, Z.; Wang, Z.; Sheng, J.; Li, Y.; Zhao, X.; Wang, X.-J. Highly Efficient and Selective Photooxidation of Sulfur Mustard Simulant by a Triazolobenzothiadiazole-Moiety-Functionalized Metal–Organic Framework in Air. Inorg Chem 2018, 57, 4230–4233. [Google Scholar] [CrossRef]
- Goswami, S.; Miller, C. E.; Logsdon, J. L.; Buru, C. T.; Wu, Y.-L.; Bowman, D. N.; Islamoglu, T.; Asiri, A. M.; Cramer, C. J.; Wasielewski, M. R.; Hupp, J. T.; Farha, O. K. Atomistic Approach toward Selective Photocatalytic Oxidation of a Mustard-Gas Simulant: A Case Study with Heavy-Chalcogen-Containing PCN-57 Analogues. ACS Appl Mater Interfaces 2017, 9, 19535–19540. [Google Scholar] [CrossRef] [PubMed]
- Stasheuski, A. S.; Galievsky, V. A.; Stupak, A. P.; Dzhagarov, B. M.; Choi, M. J.; Chung, B. H.; Jeong, J. Y. Photophysical Properties and Singlet Oxygen Generation Efficiencies of Water-Soluble Fullerene Nanoparticles. Photochem Photobiol 2014, 90, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Hynek, J.; Chahal, M. K.; Payne, D. T.; Labuta, J.; Hill, J. P. Porous Framework Materials for Singlet Oxygen Generation. Coord Chem Rev 2020, 425, 213541. [Google Scholar] [CrossRef]
- Wu, S.; Wang, L.; Zhu, H.; Liang, J.; Ge, L.; Li, C.; Miao, T.; Li, J.; Cheng, Z. Catalytic Degradation of CWAs with MOF-808 and PCN-222: Toward Practical Application. J Chem Res 2022, 46. [Google Scholar] [CrossRef]
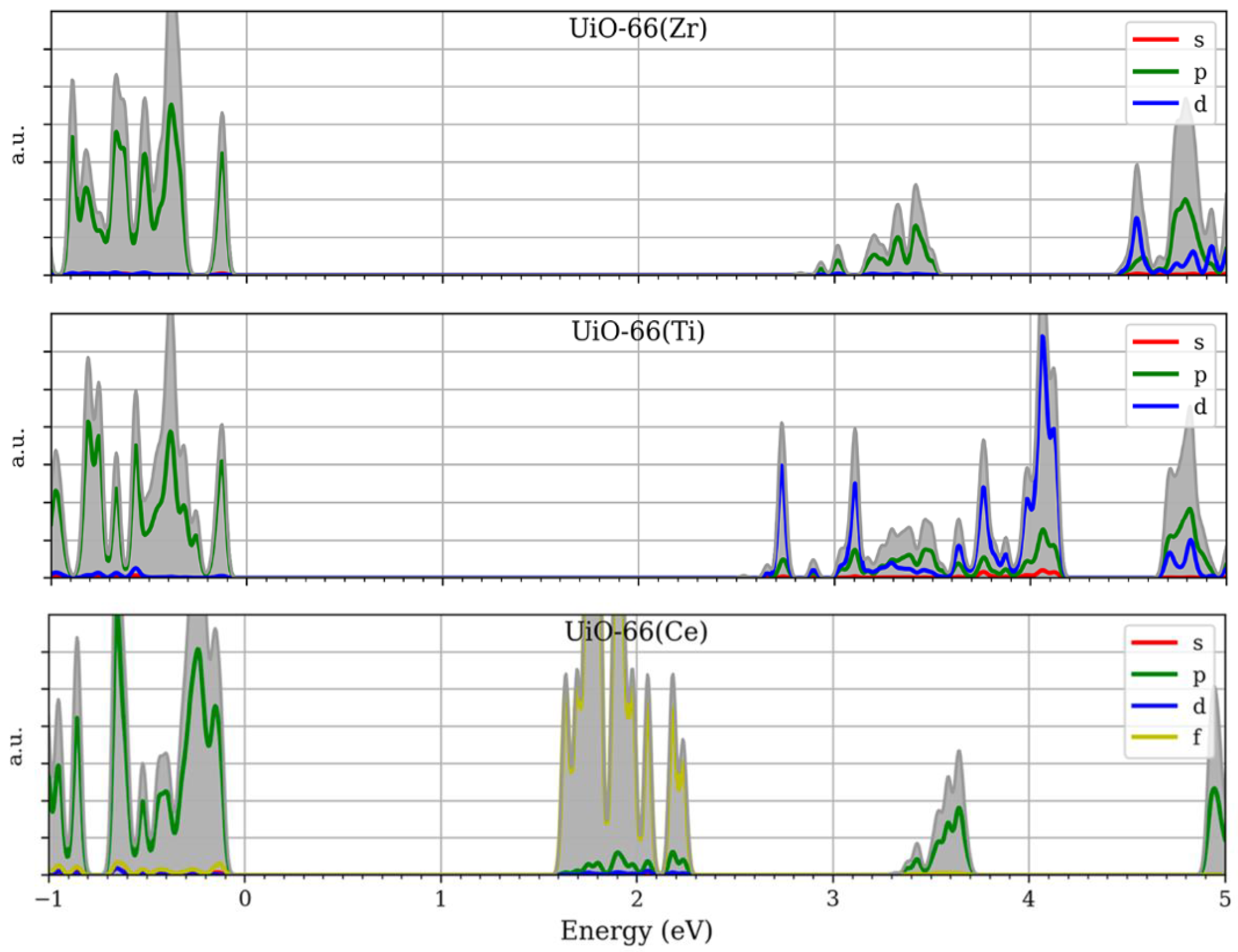
- Hendrickx, K.; Joos, J. J.; De Vos, A.; Poelman, D.; Smet, P. F.; Van Speybroeck, V.; Van Der Voort, P.; Lejaeghere, K. Exploring Lanthanide Doping in UiO-66: A Combined Experimental and Computational Study of the Electronic Structure. Inorg Chem 2018, 57, 5463–5474. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-P.; Gagliardi, L.; Truhlar, D. G. Cerium Metal–Organic Framework for Photocatalysis. J Am Chem Soc 2018, 140, 7904–7912. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Choudhuri, I.; Truhlar, D. G. Computational Studies of Photocatalysis with Metal–Organic Frameworks. ENERGY & ENVIRONMENTAL MATERIALS 2019, 2, 251–263. [Google Scholar] [CrossRef]
- Gomes Silva, C.; Luz, I.; Llabrés i Xamena, F. X.; Corma, A.; García, H. Water Stable Zr-Benzenedicarboxylate Metal-Organic Frameworks as Photocatalysts for Hydrogen Generation. Chemistry - A European Journal 2010, 16, 11133–11138. [Google Scholar] [CrossRef]
- Hendrickx, K.; Vanpoucke, D. E. P.; Leus, K.; Lejaeghere, K.; Van Yperen-De Deyne, A.; Van Speybroeck, V.; Van Der Voort, P.; Hemelsoet, K. Understanding Intrinsic Light Absorption Properties of UiO-66 Frameworks: A Combined Theoretical and Experimental Study. Inorg Chem 2015, 54, 10701–10710. [Google Scholar] [CrossRef]
- Yang, J.; He, X.; Dai, J.; Tian, R.; Yuan, D. Photo-Assisted Enhancement Performance for Rapid Detoxification of Chemical Warfare Agent Simulants over Versatile ZnIn2S4/UiO-66-NH2 Nanocomposite Catalysts. J Hazard Mater 2021, 417, 126056. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y. H.; Ma, K.; van Leeuwen, H. C.; Wasson, M. C.; Wang, X.; Idrees, K. B.; Gong, W.; Cao, R.; Mahle, J. J.; Islamoglu, T.; Peterson, G. W.; de Koning, M. C.; Xin, J. H.; Farha, O. K. Immobilized Regenerable Active Chlorine within a Zirconium-Based MOF Textile Composite to Eliminate Biological and Chemical Threats. J Am Chem Soc 2021, 143, 16777–16785. [Google Scholar] [CrossRef]
- Jiao, L.; Wan, G.; Zhang, R.; Zhou, H.; Yu, S.; Jiang, H. From Metal–Organic Frameworks to Single-Atom Fe Implanted N-doped Porous Carbons: Efficient Oxygen Reduction in Both Alkaline and Acidic Media. Angewandte Chemie International Edition 2018, 57, 8525–8529. [Google Scholar] [CrossRef]
- Lee, B.-H.; Park, S.; Kim, M.; Sinha, A. K.; Lee, S. C.; Jung, E.; Chang, W. J.; Lee, K.-S.; Kim, J. H.; Cho, S.-P.; Kim, H.; Nam, K. T.; Hyeon, T. Reversible and Cooperative Photoactivation of Single-Atom Cu/TiO2 Photocatalysts. Nat Mater 2019, 18, 620–626. [Google Scholar] [CrossRef]
- Abdel-Mageed, A. M.; Rungtaweevoranit, B.; Impeng, S.; Bansmann, J.; Rabeah, J.; Chen, S.; Häring, T.; Namuangrak, S.; Faungnawakij, K.; Brückner, A.; Behm, R. J. Unveiling the CO Oxidation Mechanism over a Molecularly Defined Copper Single-Atom Catalyst Supported on a Metal-Organic Framework. Angewandte Chemie International Edition 2023. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Qiu, F.; Xu, R.; Zhao, Q.; Guo, L.; Chen, D.; Li, C.; Jiao, X. Dual-Function Detoxifying Nanofabrics against Nerve Agent and Blistering Agent Simulants. ACS Appl Mater Interfaces 2023, 15, 1265–1275. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, Q.; Zhang, L.; Zhou, Y.; Zhong, Y.; Yu, J.; Liu, J.; Huang, C.; Wang, Y. Combining Two into One: A Dual-Function H 5 PV 2 Mo 10 O 40 @MOF-808 Composite as a Versatile Decontaminant for Sulfur Mustard and Soman. Inorg Chem 2020, 59, 11595–11605. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-Y.; Sun, Z.-B.; Zhang, M.; Zhao, S.-N.; Luo, P.; Gong, C.-H.; Liu, W.-X.; Zang, S.-Q. Cooperative Catalysis between Dual Copper Centers in a Metal–Organic Framework for Efficient Detoxification of Chemical Warfare Agent Simulants. J Am Chem Soc 2022, 144, 21046–21055. [Google Scholar] [CrossRef]


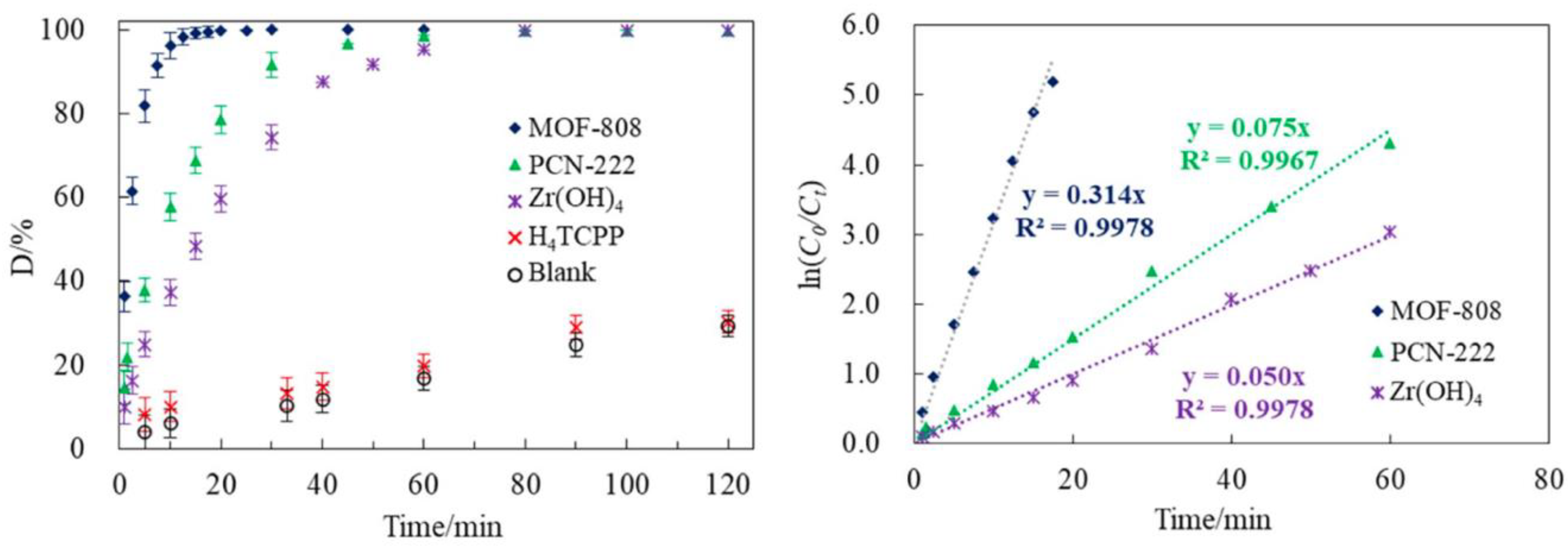
| MOF | Time to Reach 80% Conversion of GD | ||
| pH 10 Buffer Solution | Ryu et al.63 | Wang et al.4 | |
| UiO-66 | ~ 10 min75 | 5-10 min | ~ 1 day |
| UiO-66-NH2 | ~ 4 min75 | 5-10 min | ~ 1 day |
| MOF-808 | < 1 min74 | < 5 min | - |
| NU-1000 | < 1 min74 | - | > 5 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





