Submitted:
13 June 2023
Posted:
14 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Short theoretical background
3. Lanthanide complexes with tripodal ligands
3.1. Complexes with the pyrazolyl bearing tripodal ligand
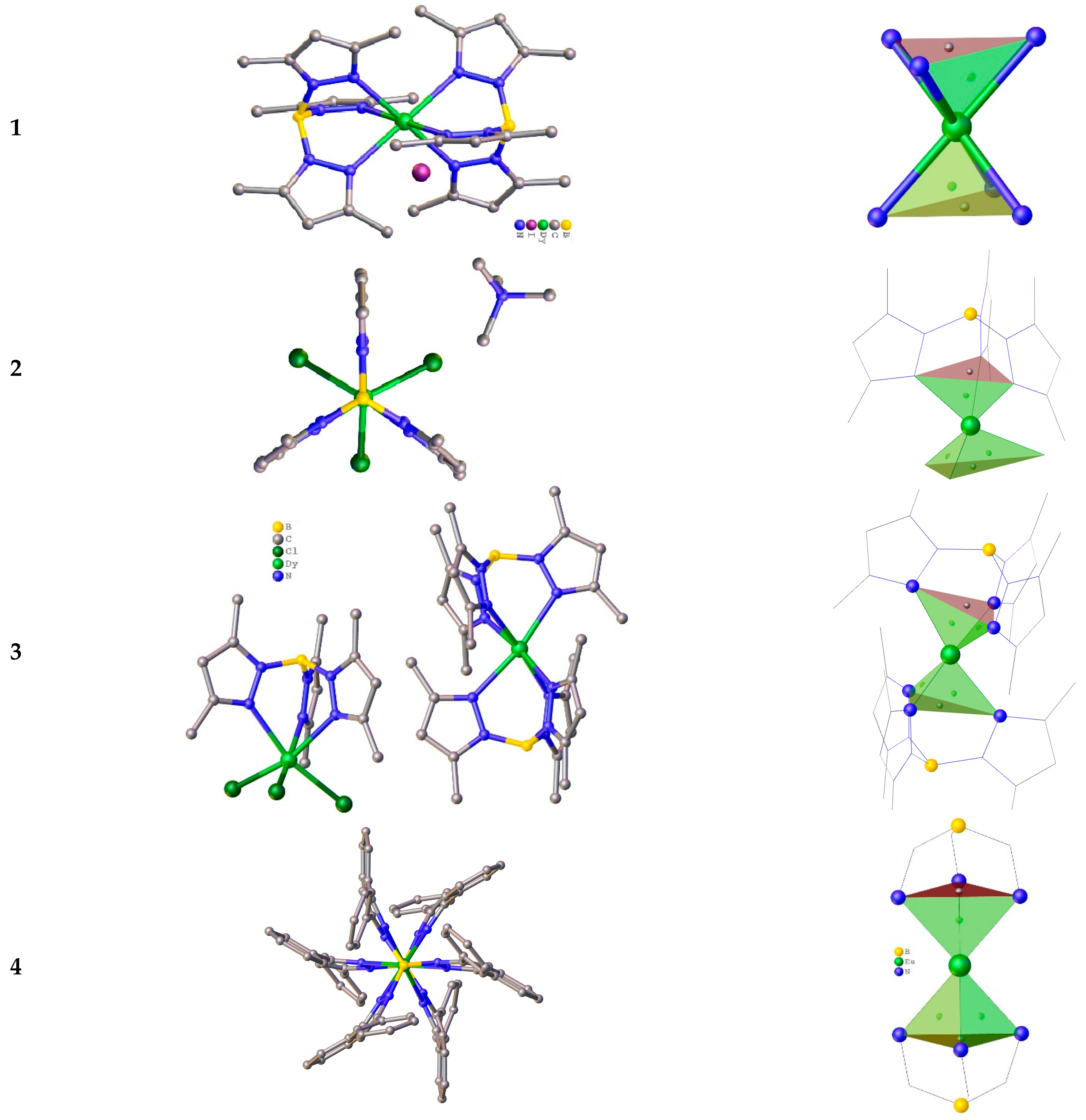
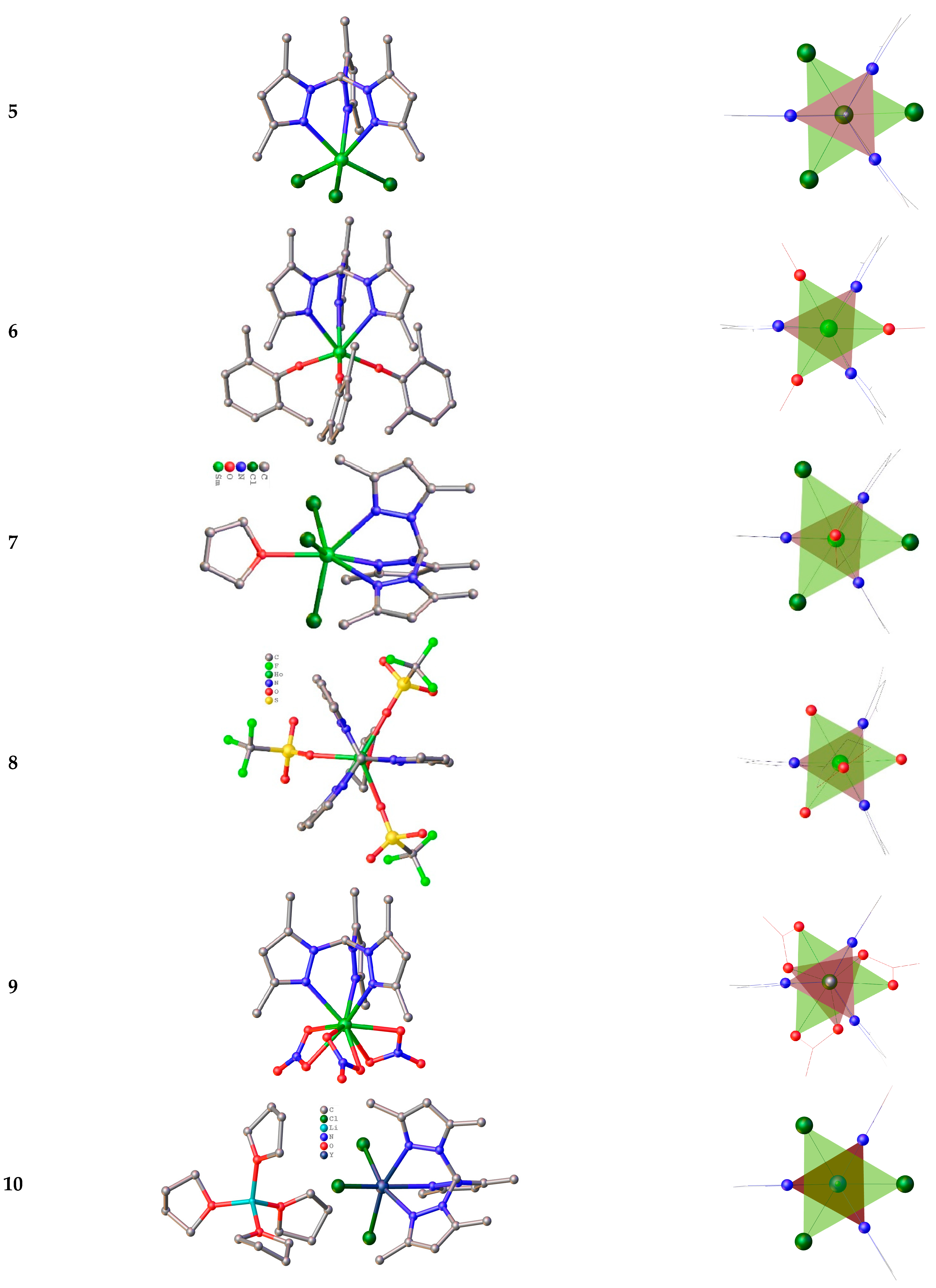
3.1.1. Complexes with tris(pyrazolyl)borates tripodal ligands
3.1.2. Complexes of tris(3,5-dimethylpyrazolyl)methane
3.1.3. Complexes of anionic tripodal ligand − tris(3,5-dimethylpyrazolyl)-methanide
3.2. Complexes with the pyridyl bearing tripodal ligand
3.2.1. Complexes of tris(2-pyridyl)metallates
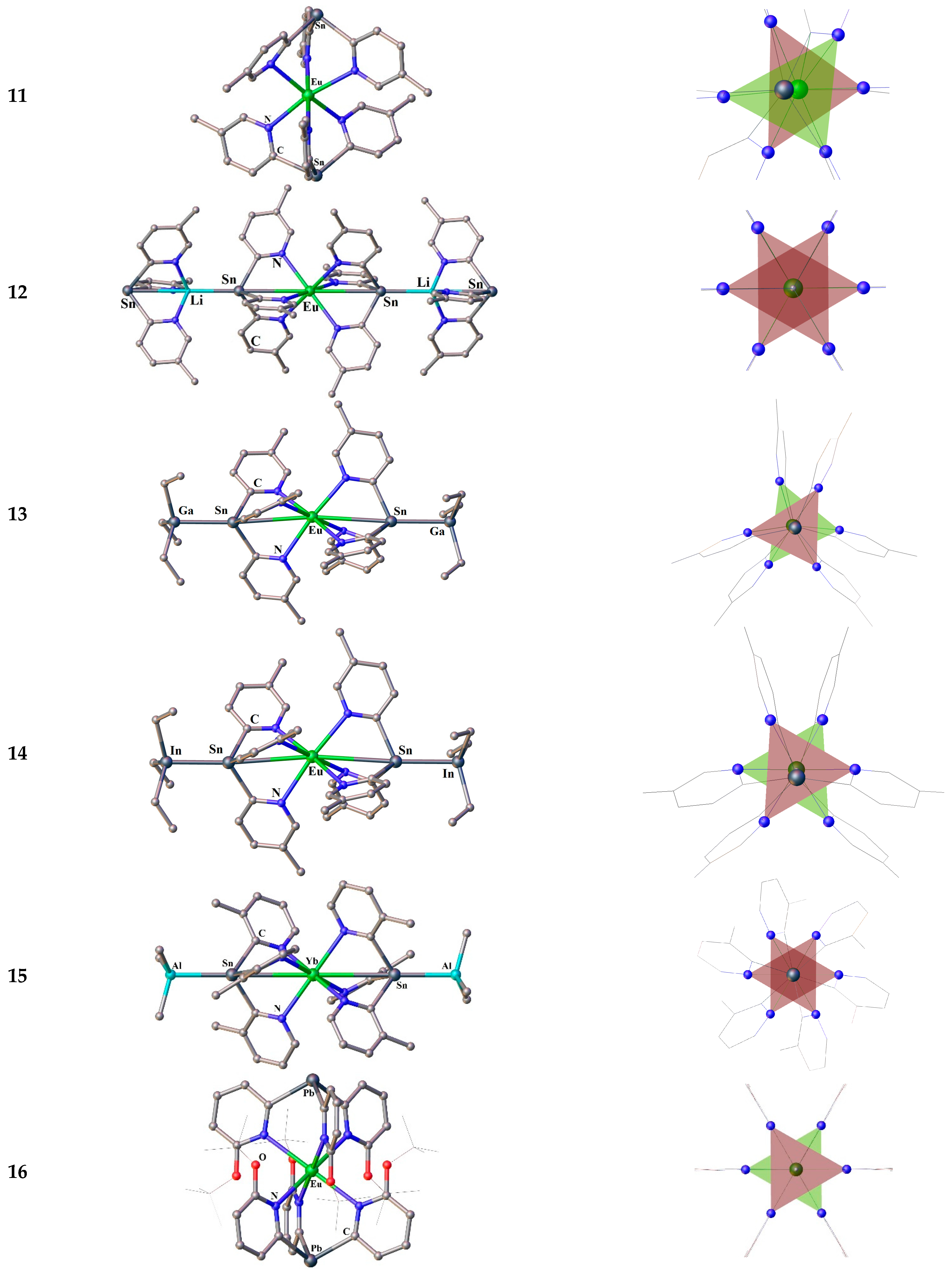
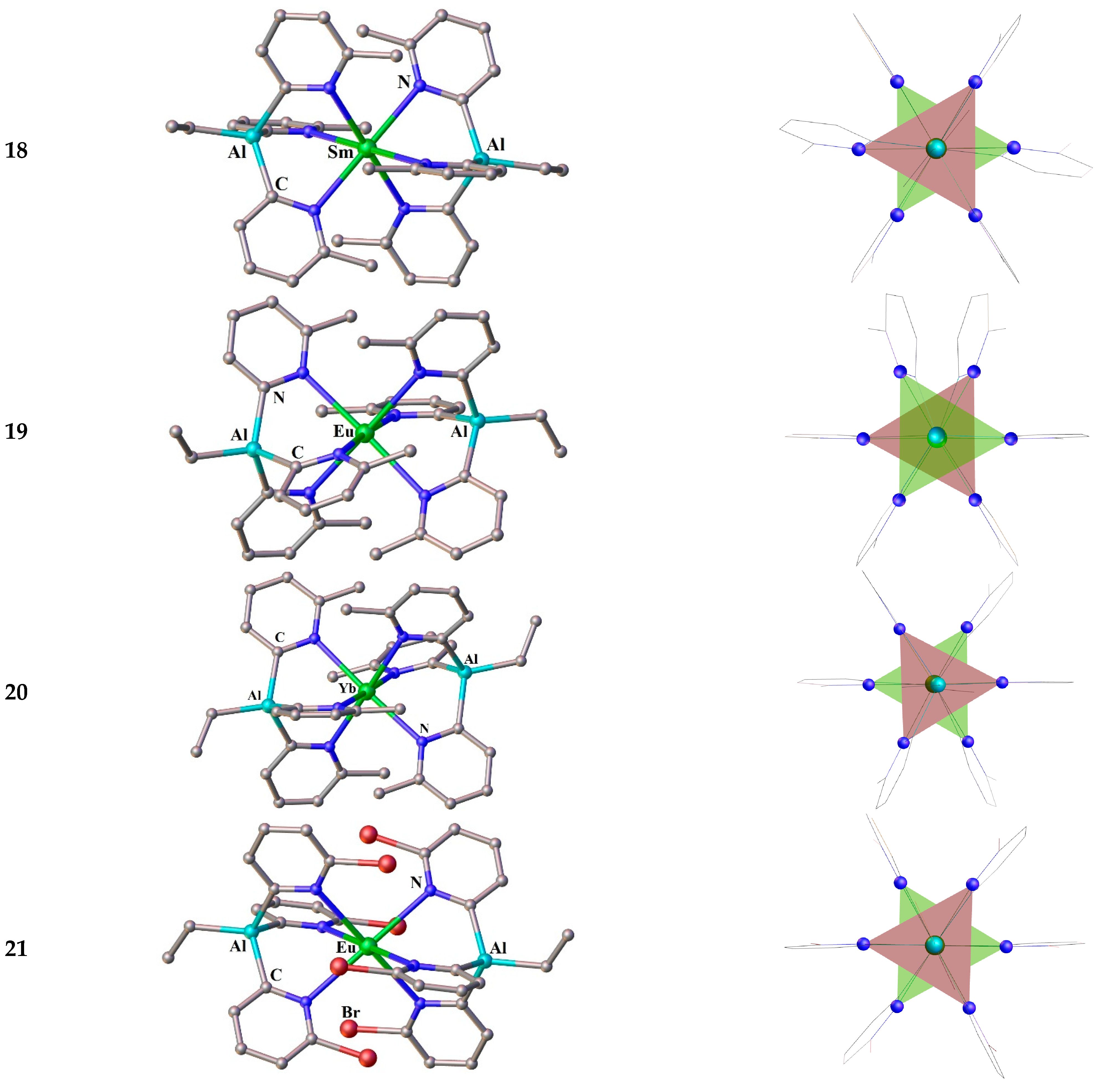
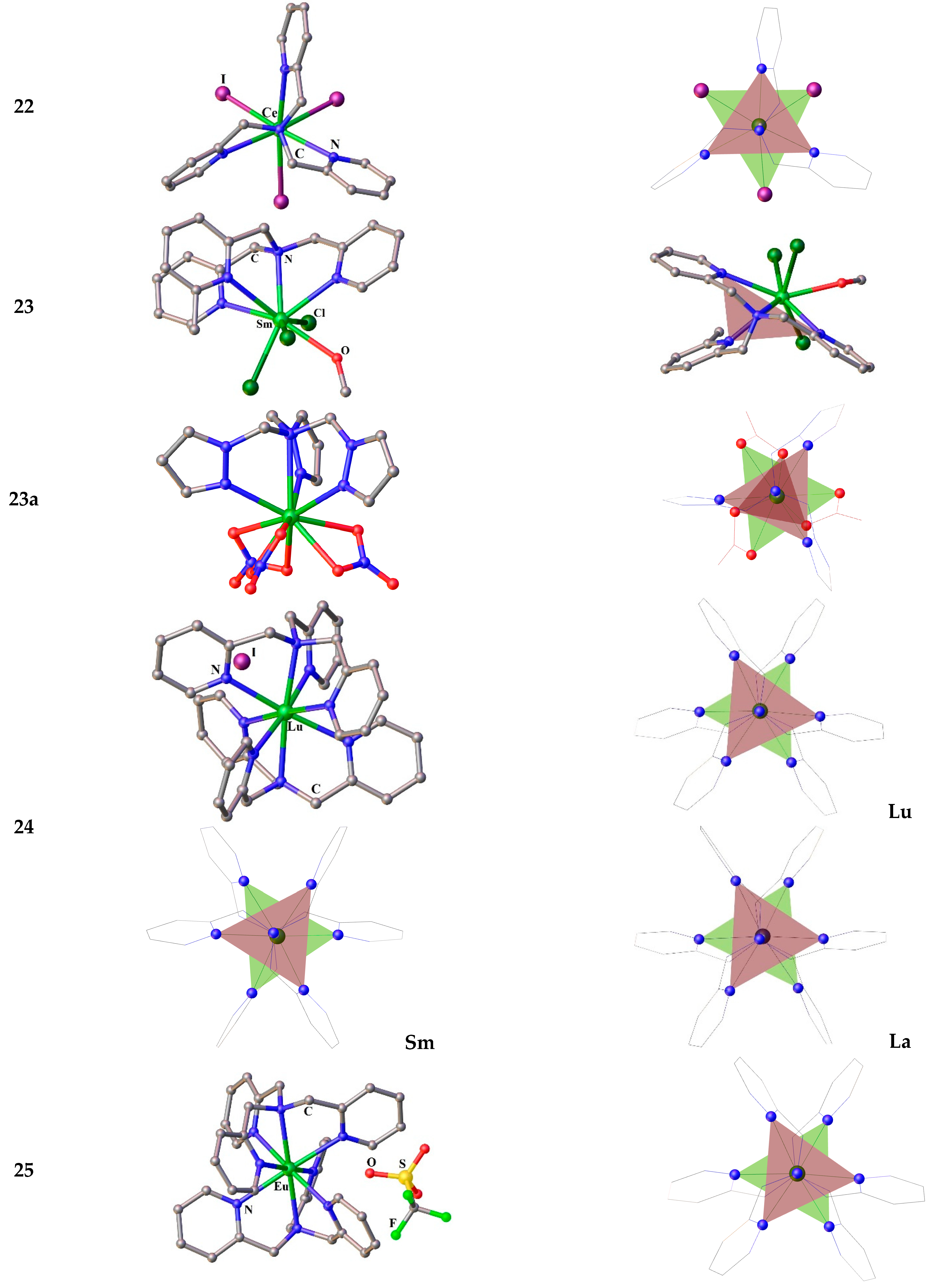
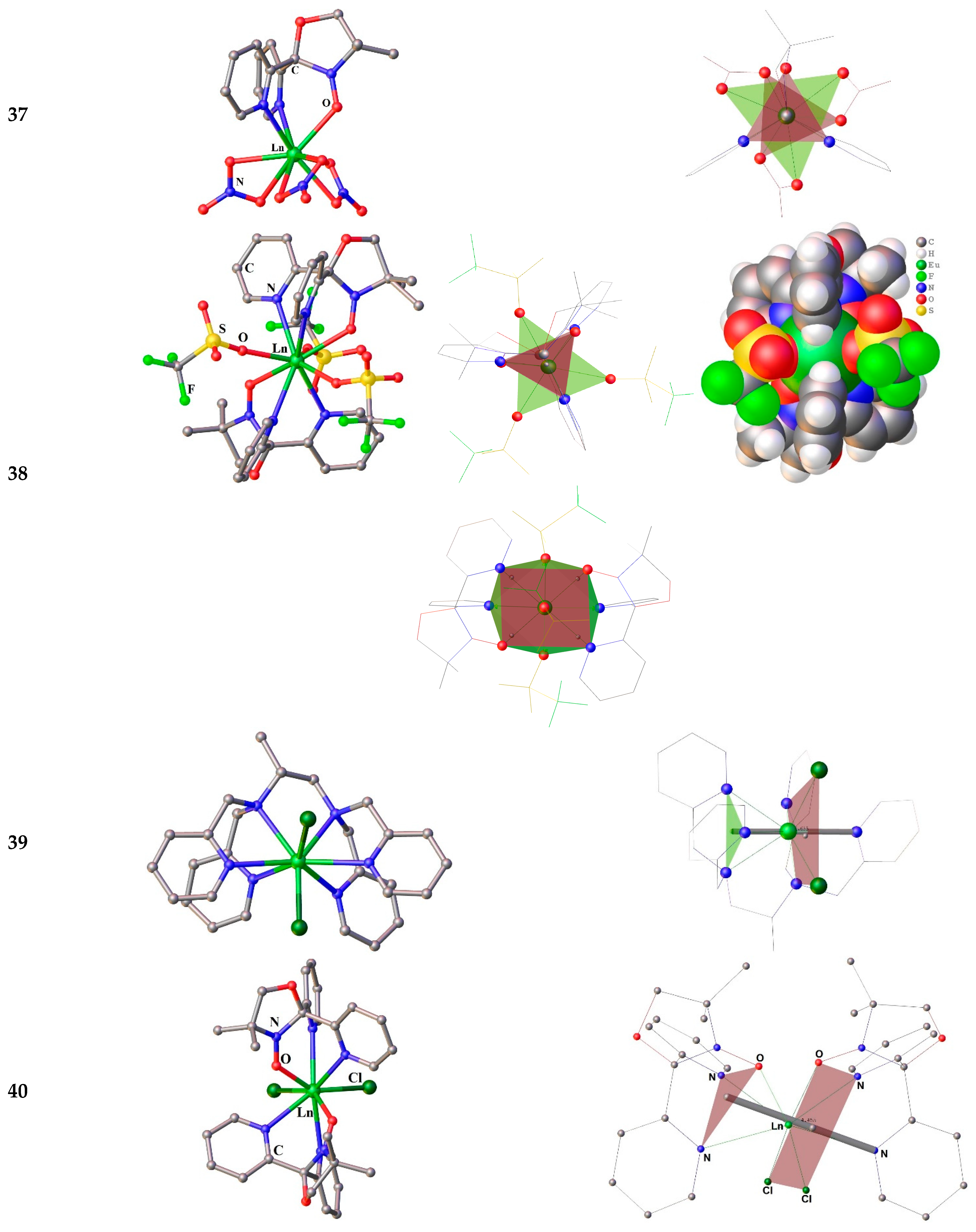
3.2.2. Complexes of tris(2-pyridyl)amines
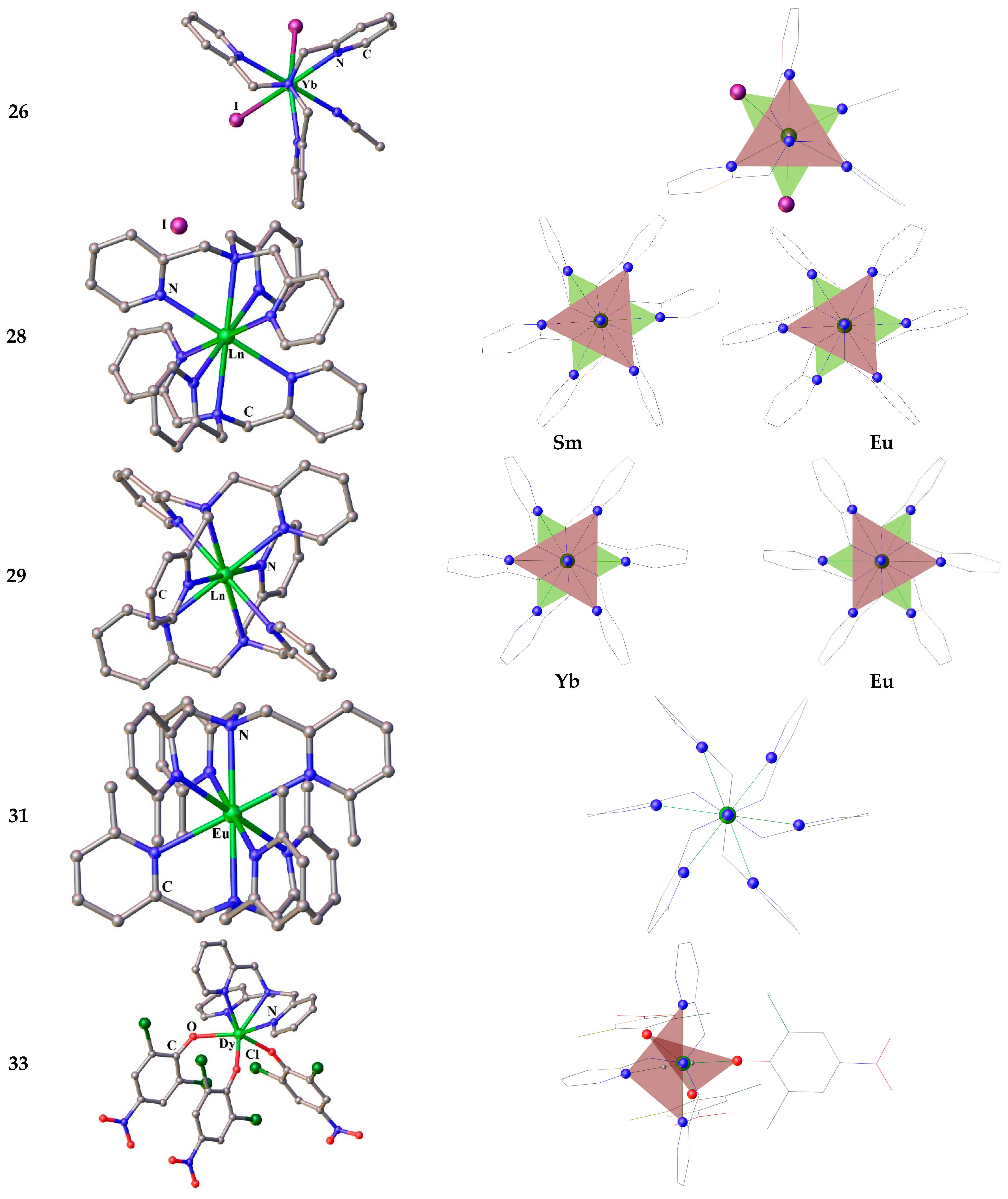
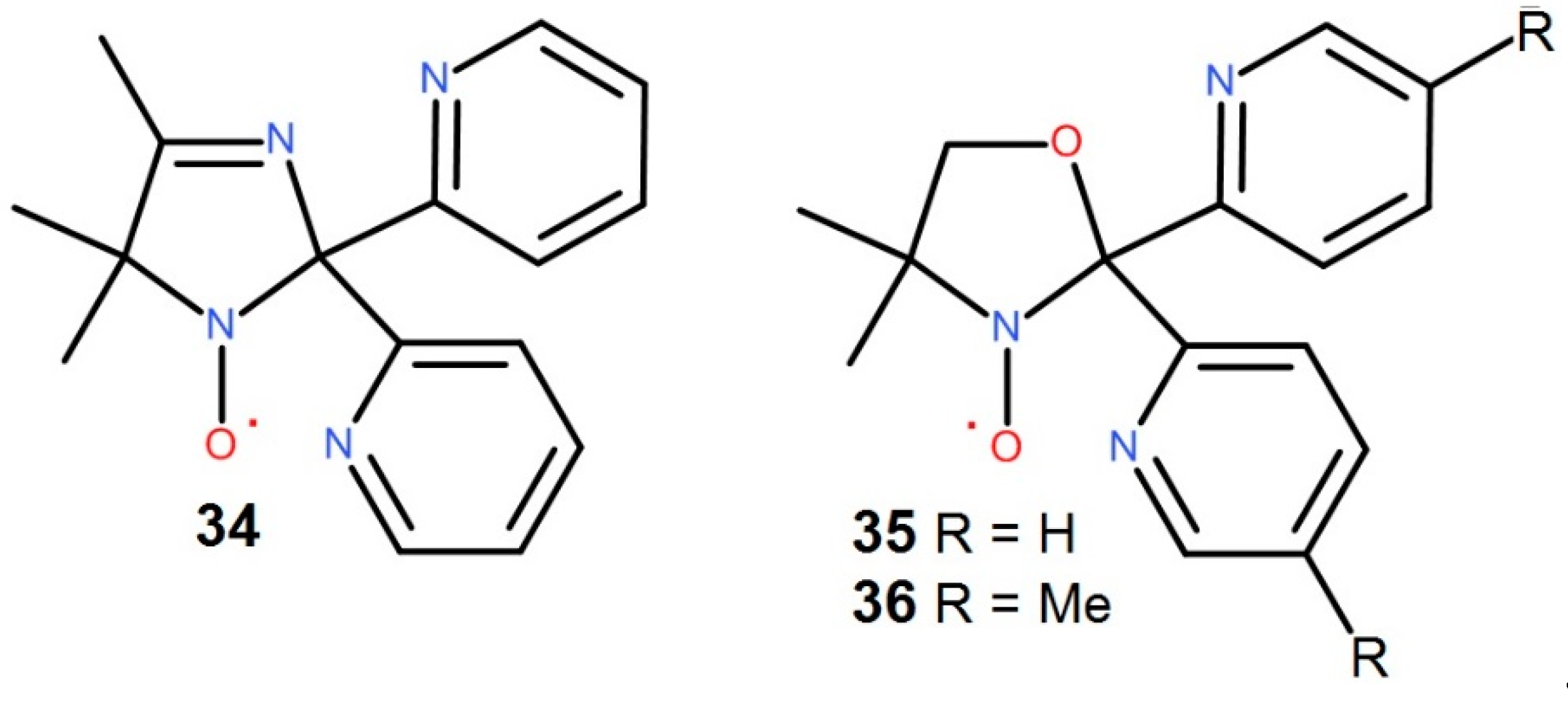
4.1. Complexes of the tripodal nitroxyl radicals
4.1.1. The functionalized by 2-pyridyl groups paramagnetic tripods and their complexes
4.1.2. Structural features of the paramagnetic tripod complexes
4.1.3. Magnetic properties of the paramagnetic tripod complexes
4.1.4. Rational design of the paramagnetic tripods and their complexes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Szczepura, L.F.; Witham, L.M.; Takeuchi, K.J. Tris(2-Pyridyl) Tripod Ligands. Coord. Chem. Rev. 1998, 174, 5–32. [CrossRef]
- Bellemin-Laponnaz, S.; Gade, L.H. A Modular Approach to C1 and C3 Chiral N-Tripodal Ligands for Asymmetric Catalysis. Angew. Chemie Int. Ed. 2002, 41, 3473–3475. [CrossRef]
- Natrajan, L.; Pécaut, J.; Mazzanti, M.; LeBrun, C. Controlled Hydrolysis of Lanthanide Complexes of the N-Donor Tripod Tris(2-Pyridylmethyl)Amine versus Bisligand Complex Formation. Inorg. Chem. 2005, 44, 4756–4765. [CrossRef]
- Chen, Y.-J.; Chen, H.-H. 1,1,1-Tris(Hydroxymethyl)Ethane as a New, Efficient, and Versatile Tripod Ligand for Copper-Catalyzed Cross-Coupling Reactions of Aryl Iodides with Amides, Thiols, and Phenols. Org. Lett. 2006, 8, 5609–5612. [CrossRef]
- Eckert, M.; Brethon, A.; Li, Y.-X.; Sheldon, R.A.; Arends, I.W.C.E. Study of the Efficiency of Amino-Functionalized Ruthenium and Ruthenacycle Complexes as Racemization Catalysts in the Dynamic Kinetic Resolution of 1-Phenylethanol. Adv. Synth. Catal. 2007, 349, 2603–2609. [CrossRef]
- Andrez, J.; Bozoklu, G.; Nocton, G.; Pécaut, J.; Scopelliti, R.; Dubois, L.; Mazzanti, M. Lanthanide(II) Complexes Supported by N,O-Donor Tripodal Ligands: Synthesis, Structure, and Ligand-Dependent Redox Behavior. Chem. – A Eur. J. 2015, 21, 15188–15200. [CrossRef]
- Wietzke, R.; Mazzanti, M.; Latour, J.-M.; Pécaut, J.; Cordier, P.-Y.; Madic, C. Lanthanide(III) Complexes of Tripodal N-Donor Ligands: Structural Models for the Species Involved in Solvent Extraction of Actinides(III). Inorg. Chem. 1998, 37, 6690–6697. [CrossRef]
- Kuswandi, B.; N/a, N.; Verboom, W.; Reinhoudt, D.N. Tripodal Receptors for Cation and Anion Sensors. Sensors 2006, 6, 978–1017.
- Dai, Z.; Canary, J.W. Tailoring Tripodal Ligands for Zinc Sensing. New J. Chem. 2007, 31, 1708–1718. [CrossRef]
- Machado, K.; Mukhopadhyay, S.; Videira, R.A.; Mishra, J.; Mobin, S.M.; Mishra, G.S. Polymer Encapsulated Scorpionate Eu3+ Complexes as Novel Hybrid Materials for High Performance Luminescence Applications. RSC Adv. 2015, 5, 35675–35682. [CrossRef]
- Yu, T.; Zhao, Y.; Fan, D.; Hong, Z.; Su, W. Preparation, Photo- and Electro-Luminescent Properties of a Novel Complex of Tb (III) with a Tripod Ligand. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 69, 654–658. [CrossRef]
- Long, J.; Lyubov, D.M.; Mahrova, T. V.; Lyssenko, K.A.; Korlyukov, A.A.; Fedorov, Y. V.; Chernikova, E.Y.; Guari, Y.; Larionova, J.; Trifonov, A.A. Heteroleptic Lanthanide Complexes Coordinated by Tripodal Tetradentate Ligand: Synthesis, Structure, and Magnetic and Photoluminescent Properties. Cryst. Growth Des. 2020, 20, 5184–5192. [CrossRef]
- Zhu, L.; Tang, H.; Harima, Y.; Yamashita, K.; Hirayama, D.; Aso, Y.; Otsubo, T. Electrochemical Properties of Self-Assembled Monolayers of Tripod-Shaped Molecules and Their Applications to Organic Light-Emitting Diodes. Chem. Commun. 2001, 1830–1831. [CrossRef]
- Zheng, X.-L.; Liu, Y.; Pan, M.; Lü, X.-Q.; Zhang, J.-Y.; Zhao, C.-Y.; Tong, Y.-X.; Su, C.-Y. Bright Blue-Emitting Ce3+ Complexes with Encapsulating Polybenzimidazole Tripodal Ligands as Potential Electroluminescent Devices. Angew. Chemie Int. Ed. 2007, 46, 7399–7403. [CrossRef]
- Chen, K.-Y.; Ivashenko, O.; Carroll, G.T.; Robertus, J.; Kistemaker, J.C.M.; London, G.; Browne, W.R.; Rudolf, P.; Feringa, B.L. Control of Surface Wettability Using Tripodal Light-Activated Molecular Motors. J. Am. Chem. Soc. 2014, 136, 3219–3224. [CrossRef]
- Kammerer, C.; Rapenne, G. Scorpionate Hydrotris(Indazolyl)Borate Ligands as Tripodal Platforms for Surface-Mounted Molecular Gears and Motors. Eur. J. Inorg. Chem. 2016, 2016, 2214–2226. [CrossRef]
- Rabinovich, D. Synthetic Bioinorganic Chemistry: Scorpionates Turn 50 BT - 50 Years of Structure and Bonding – The Anniversary Volume. In; Mingos, D.M.P., Ed.; Springer International Publishing: Cham, 2017; pp. 139–157 ISBN 978-3-319-35138-4.
- Eliseeva, S. V.; Bünzli, J.-C.G. Lanthanide Luminescence for Functional Materials and Bio-Sciences. Chem. Soc. Rev. 2010, 39, 189–227. [CrossRef]
- Klitsche, F.; Ramcke, J.; Migenda, J.; Hensel, A.; Vossmeyer, T.; Weller, H.; Gross, S.; Maison, W. Synthesis of Tripodal Catecholates and Their Immobilization on Zinc Oxide Nanoparticles. Beilstein J. Org. Chem. 2015, 11, 678–686. [CrossRef]
- A Fluorescent Sensor-Based Tripodal-Bodipy for Cu (II) Ions: Bio-Imaging on Cells. TURKISH J. Chem. 2021, 45. [CrossRef]
- Brechin, E.K. Using Tripodal Alcohols to Build High-Spin Molecules and Single-Molecule Magnets. Chem. Commun. 2005, 5141–5153. [CrossRef]
- Milios, C.J.; Manoli, M.; Rajaraman, G.; Mishra, A.; Budd, L.E.; White, F.; Parsons, S.; Wernsdorfer, W.; Christou, G.; Brechin, E.K. A Family of [Mn6] Complexes Featuring Tripodal Ligands. Inorg. Chem. 2006, 45, 6782–6793. [CrossRef]
- Domingo, N.; Bellido, E.; Ruiz-Molina, D. Advances on Structuring, Integration and Magnetic Characterization of Molecular Nanomagnets on Surfaces and Devices. Chem. Soc. Rev. 2012, 41, 258–302. [CrossRef]
- Long, J.; Lyubov, D.M.; Mahrova, T. V.; Cherkasov, A. V.; Fukin, G.K.; Guari, Y.; Larionova, J.; Trifonov, A.A. Synthesis, Structure and Magnetic Properties of Tris(Pyrazolyl)Methane Lanthanide Complexes: Effect of the Anion on the Slow Relaxation of Magnetization. Dalt. Trans. 2018, 47, 5153–5156. [CrossRef]
- Ito, A.; Nakano, Y.; Urabe, M.; Tanaka, K.; Shiro, M. Structural and Magnetic Studies of Copper(II) and Zinc(II) Coordination Complexes Containing Nitroxide Radicals as Chelating Ligands. Eur. J. Inorg. Chem. 2006, 2006, 3359–3368. [CrossRef]
- Gass, I.A.; Gartshore, C.J.; Lupton, D.W.; Moubaraki, B.; Nafady, A.; Bond, A.M.; Boas, J.F.; Cashion, J.D.; Milsmann, C.; Wieghardt, K.; et al. Anion Dependent Redox Changes in Iron Bis-Terdentate Nitroxide {NNO} Chelates. Inorg. Chem. 2011, 50, 3052–3064. [CrossRef]
- Gass, I.A.; Tewary, S.; Nafady, A.; Chilton, N.F.; Gartshore, C.J.; Asadi, M.; Lupton, D.W.; Moubaraki, B.; Bond, A.M.; Boas, J.F.; et al. Observation of Ferromagnetic Exchange, Spin Crossover, Reductively Induced Oxidation, and Field-Induced Slow Magnetic Relaxation in Monomeric Cobalt Nitroxides. Inorg. Chem. 2013, 52, 7557–7572. [CrossRef]
- Gass, I.A.; Tewary, S.; Rajaraman, G.; Asadi, M.; Lupton, D.W.; Moubaraki, B.; Chastanet, G.; Létard, J.-F.; Murray, K.S. Solvate-Dependent Spin Crossover and Exchange in Cobalt(II) Oxazolidine Nitroxide Chelates. Inorg. Chem. 2014, 53, 5055–5066. [CrossRef]
- Gass, I.A.; Asadi, M.; Lupton, D.W.; Moubaraki, B.; Bond, A.M.; Guo, S.-X.; Murray, K.S. Manganese(II) Oxazolidine Nitroxide Chelates: Structure, Magnetism, and Redox Properties. Aust. J. Chem. 2014, 67, 1618. [CrossRef]
- Pedersen, A.H.; Geoghegan, B.L.; Nichol, G.S.; Lupton, D.W.; Murray, K.S.; Martínez-Lillo, J.; Gass, I.A.; Brechin, E.K. Hexahalorhenate(IV) Salts of Metal Oxazolidine Nitroxides. Dalt. Trans. 2017, 46, 5250–5259. [CrossRef]
- Gass, I.A.; Lu, J.; Asadi, M.; Lupton, D.W.; Forsyth, C.M.; Geoghegan, B.L.; Moubaraki, B.; Cashion, J.D.; Martin, L.L.; Bond, A.M.; et al. Use of the TCNQF 4 2− Dianion in the Spontaneous Redox Formation of [Fe III (L − ) 2 ][TCNQF 4 ⋅− ]. Chempluschem 2018, 83, 658–668. [CrossRef]
- Gass, I.A.; Lu, J.; Ojha, R.; Asadi, M.; Lupton, D.W.; Geoghegan, B.L.; Moubaraki, B.; Martin, L.L.; Bond, A.M.; Murray, K.S. [FeII(L•)2][TCNQF4•−]2: A Redox-Active Double Radical Salt. Aust. J. Chem. 2019, 72, 769–777. [CrossRef]
- Trofimenko, S. Boron-Pyrazole Chemistry. J. Am. Chem. Soc. 1966, 88, 1842–1844. [CrossRef]
- Oliver, J.D.; Mullica, D.F.; Hutchinson, B.B.; Milligan, W.O. Iron-Nitrogen Bond Lengths in Low-Spin and High-Spin Iron(II) Complexes with Poly(Pyrazolyl)Borate Ligands. Inorg. Chem. 1980, 19, 165–169. [CrossRef]
- Trofimenko, S. Recent Advances in Poly(Pyrazolyl)Borate (Scorpionate) Chemistry. Chem. Rev. 1993, 93, 943–980. [CrossRef]
- Wu, L.P.; Yamagiwa, Y.; Ino, I.; Sugimoto, K.; Kuroda-Sowa, T.; Kamikawa, T.; Munakata, M. Unique Tetranuclear Copper(II) Cluster and Monomeric Iron(II), (III) Complexes with a Tris(Imidazolyl) Chelating Ligand. Polyhedron 1999, 18, 2047–2053. [CrossRef]
- SCORPIONATES II: CHELATING BORATE LIGANDS - Dedicated To Swiatoslaw Trofimenko; Pettinari, C., Ed.; IMPERIAL COLLEGE PRESS and distribiuted by World Scientific: London, 2008; ISBN 978-1-86094-876-3.
- Dougherty, W.G.; Kassel, W.S. Synthesis of a Series of First-Row Tris-Imidazolylphosphine Sandwich Complexes and Their Potential for Formation of Polymetallic Species. Inorganica Chim. Acta 2010, 364, 120–124. [CrossRef]
- Saouma, C.T.; Peters, J.C. ME and ME Complexes of Iron and Cobalt That Emphasize Three-Fold Symmetry (E=O, N, NR). Coord. Chem. Rev. 2011, 255, 920–937. [CrossRef]
- Lavrenova, L.G.; Strekalova, A.D.; Smolentsev, A.I.; Naumov, D.Y.; Bogomyakov, A.S.; Sheludyakova, L.A.; Vasilevskii, S.F. Mono- and Heteroligand Iron(II) Complexes with Tris(3,5-Dimethylpyrazol-1-Yl)Methane. Russ. J. Coord. Chem. 2016, 42, 711–718. [CrossRef]
- Feng, M.; Tong, M.-L. Single Ion Magnets from 3d to 5f: Developments and Strategies. Chem. – A Eur. J. 2018, 24, 7574–7594. [CrossRef]
- Landart-Gereka, A.; Quesada-Moreno, M.M.; Palacios, M.A.; Díaz-Ortega, I.F.; Nojiri, H.; Ozerov, M.; Krzystek, J.; Colacio, E. Pushing up the Easy-Axis Magnetic Anisotropy and Relaxation Times in Trigonal Prismatic CoII Mononuclear SMMs by Molecular Structure Design. Chem. Commun. 2023, 59, 952–955. [CrossRef]
- Apostolidis, C.; Carvalho, A.; Domingos, A.; Kanellakopulos, B.; Maier, R.; Marques, N.; Matos, A.P. de; Rebizant, J. Chloro-Lanthanide, and Plutonium Complexes Containing the Hydrotris (3,5-Dimethylpyrazol-1-Yl)Borate Ligand: The Crystal and Molecular Structures of [PrCl(μ-Cl)TpMe2(3,5-Me2pzH)]2 and YbCl2TpMe2 (THF). Polyhedron 1998, 18, 263–272. [CrossRef]
- Hillier, A.C.; Zhang, X.W.; Maunder, G.H.; Liu, S.Y.; Eberspacher, T.A.; Metz, M. V.; McDonald, R.; Domingos, A.; Marques, N.; Day, V.W.; et al. Synthesis and Structural Comparison of a Series of Divalent Ln(TpR,R)2 (Ln = Sm, Eu, Yb) and Trivalent Sm(TpMe2)2X (X = F, Cl, I, BPh4) Complexes. Inorg. Chem. 2001, 40, 5106–5116. [CrossRef]
- Sella, A.; Brown, S.E.; Steed, J.W.; Tocher, D.A. Synthesis and Solid-State Structures of Pyrazolylmethane Complexes of the Rare Earths. Inorg. Chem. 2007, 46, 1856–1864. [CrossRef]
- Liu, S.-Y.; Maunder, G.H.; Sella, A.; Stevenson, M.; Tocher, D.A. Synthesis and Molecular Structures of Hydrotris(Dimethylpyrazolyl)Borate Complexes of the Lanthanides. Inorg. Chem. 1996, 35, 76–81. [CrossRef]
- Werner, E.J.; Biros, S.M. Supramolecular Ligands for the Extraction of Lanthanide and Actinide Ions. Org. Chem. Front. 2019, 6, 2067–2094. [CrossRef]
- Barraza, R.; Sertage, A.G.; Kajjam, A.B.; Ward, C.L.; Lutter, J.C.; Schlegel, H.B.; Allen, M.J. Properties of Amine-Containing Ligands That Are Necessary for Visible-Light-Promoted Catalysis with Divalent Europium. Inorg. Chem. 2022, 61, 19649–19657. [CrossRef]
- Frost, J.M.; Harriman, K.L.M.; Murugesu, M. The Rise of 3-d Single-Ion Magnets in Molecular Magnetism: Towards Materials from Molecules? Chem. Sci. 2016, 7, 2470–2491. [CrossRef]
- Zhu, Z.; Guo, M.; Li, X.-L.; Tang, J. Molecular Magnetism of Lanthanide: Advances and Perspectives. Coord. Chem. Rev. 2019, 378, 350–364. [CrossRef]
- Zabala-Lekuona, A.; Seco, J.M.; Colacio, E. Single-Molecule Magnets: From Mn12-Ac to Dysprosium Metallocenes, a Travel in Time. Coord. Chem. Rev. 2021, 441, 213984. [CrossRef]
- Gatteschi, D.; Sessoli, R. Quantum Tunneling of Magnetization and Related Phenomena in Molecular Materials. Angew. Chemie Int. Ed. 2003, 42, 268–297. [CrossRef]
- Goodwin, C.A.P.; Ortu, F.; Reta, D. Strangely Attractive: Collaboration and Feedback in the Field of Molecular Magnetism. Int. J. Quantum Chem. 2020, 120, e26248. [CrossRef]
- Tang, J.; Zhang, P. Lanthanide Single Molecule Magnets; Springer Berlin Heidelberg: Berlin, Heidelberg, 2015; ISBN 978-3-662-46998-9.
- Sorace, L.; Gatteschi, D. Electronic Structure and Magnetic Properties of Lanthanide Molecular Complexes. In Lanthanides and Actinides in Molecular Magnetism; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 1–26.
- Goodwin, C.A.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular Magnetic Hysteresis at 60 Kelvin in Dysprosocenium. Nature 2017, 548, 439–442. [CrossRef]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. A Dysprosium Metallocene Single-Molecule Magnet Functioning at the Axial Limit. Angew. Chemie Int. Ed. 2017, 56, 11445–11449. [CrossRef]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic Hysteresis up to 80 Kelvin in a Dysprosium Metallocene Single-Molecule Magnet. Science (80-. ). 2018, 362, 1400–1403. [CrossRef]
- Vincent, A.H.; Whyatt, Y.L.; Chilton, N.F.; Long, J.R. Strong Axiality in a Dysprosium(III) Bis(Borolide) Complex Leads to Magnetic Blocking at 65 K. J. Am. Chem. Soc. 2023, 145, 1572–1579. [CrossRef]
- Rinehart, J.D.; Long, J.R. Exploiting Single-Ion Anisotropy in the Design of f-Element Single-Molecule Magnets. Chem. Sci. 2011, 2, 2078–2085. [CrossRef]
- Chilton, N.F. Design Criteria for High-Temperature Single-Molecule Magnets. Inorg. Chem. 2015, 54, 2097–2099. [CrossRef]
- Harriman, K.L.M.; Errulat, D.; Murugesu, M. Magnetic Axiality: Design Principles from Molecules to Materials. Trends Chem. 2019, 1, 425–439. [CrossRef]
- Guo, F.-S.; Bar, A.K.; Layfield, R.A. Main Group Chemistry at the Interface with Molecular Magnetism. Chem. Rev. 2019, 119, 8479–8505. [CrossRef]
- Chiesa, A.; Cugini, F.; Hussain, R.; Macaluso, E.; Allodi, G.; Garlatti, E.; Giansiracusa, M.; Goodwin, C.A.P.; Ortu, F.; Reta, D.; et al. Understanding Magnetic Relaxation in Single-Ion Magnets with High Blocking Temperature. Phys. Rev. B 2020, 101, 174402. [CrossRef]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [CrossRef]
- Jiang, S.-D.; Wang, B.-W.; Gao, S. Advances in Lanthanide Single-Ion Magnets BT - Molecular Nanomagnets and Related Phenomena. In; Gao, S., Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2015; pp. 111–141 ISBN 978-3-662-45723-8.
- Gupta, S.K.; Murugavel, R. Enriching Lanthanide Single-Ion Magnetism through Symmetry and Axiality. Chem. Commun. 2018, 54, 3685–3696. [CrossRef]
- Vogel, R.; Müntener, T.; Häussinger, D. Intrinsic Anisotropy Parameters of a Series of Lanthanoid Complexes Deliver New Insights into the Structure-Magnetism Relationship. Chem 2021, 7, 3144–3156. [CrossRef]
- Jiang, S.-D.; Qin, S.-X. Prediction of the Quantized Axis of Rare-Earth Ions: The Electrostatic Model with Displaced Point Charges. Inorg. Chem. Front. 2015, 2, 613–619. [CrossRef]
- Liu, J.-L.; Chen, Y.-C.; Tong, M.-L. Symmetry Strategies for High Performance Lanthanide-Based Single-Molecule Magnets. Chem. Soc. Rev. 2018, 47, 2431–2453. [CrossRef]
- Chen, Y.-C.; Tong, M.-L. Single-Molecule Magnets beyond a Single Lanthanide Ion: The Art of Coupling. Chem. Sci. 2022. [CrossRef]
- Molecular Nanomagnets and Related Phenomena —Structure and Bonding; Gao, S., Ed.; Springer Berlin: Heidelberg, 2015; ISBN 978-3-662-45722-1.
- Kahn, O. Molecular Magnetism; VCH: New York, NY, USA, 1993; ISBN 978-1-56081-566-2.
- Series Page. In Theoretical Foundations of Molecular Magnetism; Boča, R.B.T.-C.M. in I.C., Ed.; Elsevier, 1999; Vol. 1, p. ii ISBN 1873-0418.
- Chen, Y.-C.; Liu, J.-L.; Wernsdorfer, W.; Liu, D.; Chibotaru, L.F.; Chen, X.-M.; Tong, M.-L. Hyperfine-Interaction-Driven Suppression of Quantum Tunneling at Zero Field in a Holmium(III) Single-Ion Magnet. Angew. Chemie Int. Ed. 2017, 56, 4996–5000. [CrossRef]
- Skomski, R.; Sellmyer, D.J. Anisotropy of Rare-Earth Magnets. J. Rare Earths 2009, 27, 675–679. [CrossRef]
- Zhu, Z.; Tang, J. Lanthanide Single-Molecule Magnets with High Anisotropy Barrier: Where to from Here? Natl. Sci. Rev. 2022, 9, nwac194. [CrossRef]
- Wang, J.; Sun, C.; Zheng, Q.; Wang, D.; Chen, Y.; Ju, J.; Sun, T.; Cui, Y.; Ding, Y.; Tang, Y. Lanthanide Single-molecule Magnets: Synthetic Strategy, Structures, Properties and Recent Advances. Chem. – An Asian J. 2023, 18. [CrossRef]
- Trofimenko, S. The Coordination Chemistry of Polypyrazolylborate Ligand; IMPERIAL COLLEGE PRESS, 1999; ISBN 978-1-86094-172-6.
- Cheng, J.; Takats, J.; Ferguson, M.J.; McDonald, R. Heteroleptic Tm(II) Complexes: One More Success for Trofimenko’s Scorpionates. J. Am. Chem. Soc. 2008, 130, 1544–1545. [CrossRef]
- Dei, A.; Gatteschi, D.; Pécaut, J.; Poussereau, S.; Sorace, L.; Vostrikova, K. Crystal Field and Exchange Effects in Rare Earth Semiquinone Complexes. Comptes Rendus l’Academie des Sci. - Ser. IIC Chem. 2001, 4. [CrossRef]
- Zhang, P.; Perfetti, M.; Kern, M.; Hallmen, P.P.; Ungur, L.; Lenz, S.; Ringenberg, M.R.; Frey, W.; Stoll, H.; Rauhut, G.; et al. Exchange Coupling and Single Molecule Magnetism in Redox-Active Tetraoxolene-Bridged Dilanthanide Complexes. Chem. Sci. 2018, 9, 1221–1230. [CrossRef]
- Xu, G.-F.; Gamez, P.; Tang, J.; Clérac, R.; Guo, Y.-N.; Guo, Y. MIIIDyIII3 (M = FeIII, CoIII) Complexes: Three-Blade Propellers Exhibiting Slow Relaxation of Magnetization. Inorg. Chem. 2012, 51, 5693–5698. [CrossRef]
- Xu, G.-F.; Wang, Q.-L.; Gamez, P.; Ma, Y.; Clérac, R.; Tang, J.; Yan, S.-P.; Cheng, P.; Liao, D.-Z. A Promising New Route towards Single-Molecule Magnets Based on the Oxalate Ligand. Chem. Commun. 2010, 46, 1506–1508. [CrossRef]
- Mikhalyova, E.A.; Zeller, M.; Jasinski, J.P.; Butcher, R.J.; Carrella, L.M.; Sedykh, A.E.; Gavrilenko, K.S.; Smola, S.S.; Frasso, M.; Cazorla, S.C.; et al. Combination of Single-Molecule Magnet Behaviour and Luminescence Properties in a New Series of Lanthanide Complexes with Tris(Pyrazolyl)Borate and Oligo(β-Diketonate) Ligands. Dalt. Trans. 2020, 49, 7774–7789. [CrossRef]
- Kandel, A. V.; Mikhalyova, E.A.; Zeller, M.; Addison, A.W.; Pavlishchuk, V. V. Influence of the Structure of 3-Arylacetylacetonate Ligands on the Luminescence Properties of Eu3+ and Tb3+ Complexes. Theor. Exp. Chem. 2017, 53, 180–186. [CrossRef]
- Depperman, E.C.; Bodnar, S.H.; Vostrikova, K.E.; Shultz, D.A.; Kirk, M.L. Spin Robustness of a New Hybrid Inorganic−Organic High-Spin Molecule. J. Am. Chem. Soc. 2001, 123, 3133–3134. [CrossRef]
- Wang, S.; Zuo, J.-L.; Zhou, H.-C.; Choi, H.J.; Ke, Y.; Long, J.R.; You, X.-Z. [(Tp)8(H2O)6CuII6FeIII8(CN)24]4+: A Cyanide-Bridged Face-Centered-Cubic Cluster with Single-Molecule-Magnet Behavior. Angew. Chemie Int. Ed. 2004, 43, 5940–5943. [CrossRef]
- Wang, S.; Zuo, J.-L.; Gao, S.; Song, Y.; Zhou, H.-C.; Zhang, Y.-Z.; You, X.-Z. The Observation of Superparamagnetic Behavior in Molecular Nanowires. J. Am. Chem. Soc. 2004, 126, 8900–8901. [CrossRef]
- Alexandropoulos, D.I.; Vignesh, K.R.; Xie, H.; Dunbar, K.R. Switching on Single-Molecule Magnet Properties of Homoleptic Sandwich Tris(Pyrazolyl)Borate Dysprosium(III) Cations via Intermolecular Dipolar Coupling. Dalt. Trans. 2019, 48, 10610–10618. [CrossRef]
- Kühling, M.; Wickleder, C.; Ferguson, M.J.; Hrib, C.G.; McDonald, R.; Suta, M.; Hilfert, L.; Takats, J.; Edelmann, F.T. Investigation of the “Bent Sandwich-like” Divalent Lanthanide Hydro-Tris(Pyrazolyl)Borates Ln(TpIPr2)2 (Ln = Sm, Eu, Tm, Yb). New J. Chem. 2015, 39, 7617–7625. [CrossRef]
- Qi, H.; Zhao, Z.; Zhan, G.; Sun, B.; Yan, W.; Wang, C.; Wang, L.; Liu, Z.; Bian, Z.; Huang, C. Air Stable and Efficient Rare Earth Eu(II) Hydro-Tris(Pyrazolyl)Borate Complexes with Tunable Emission Colors. Inorg. Chem. Front. 2020, 7, 4593–4599. [CrossRef]
- Takats, J.; Zhang, X.W.; Day, V.W.; Eberspacher, T.A. Synthesis and Structure of Bis[Hydrotris(3,5-Dimethylpyrazolyl)Borato]Samarium(II), Sm[HB(3,5-Me2pz)3]2, and the Product of Its Reaction with Azobenzene. Organometallics 1993, 12, 4286–4288. [CrossRef]
- Maunder, G.H.; Sella, A.; Tocher, D.A. Synthesis and Molecular Structures of a Redox-Related Pair of Lanthanide Complexes. J. Chem. Soc. Chem. Commun. 1994, 885. [CrossRef]
- Momin, A.; Carter, L.; Yang, Y.; McDonald, R.; Essafi (née Labouille), S.; Nief, F.; Del Rosal, I.; Sella, A.; Maron, L.; Takats, J. To Bend or Not To Bend: Experimental and Computational Studies of Structural Preference in Ln(TpiPr2)2 (Ln = Sm, Tm). Inorg. Chem. 2014, 53, 12066–12075. [CrossRef]
- Saliu, K.O.; Takats, J.; Ferguson, M.J. Bis[Tris(3-Tert-Butyl-5-Methylpyrazol-1-Yl)Hydridoborato]Ytterbium(II) Toluene Solvate. Acta Crystallogr. Sect. E Struct. Reports Online 2009, 65, m643–m644. [CrossRef]
- Suta, M.; Kühling, M.; Liebing, P.; Edelmann, F.T.; Wickleder, C. Photoluminescence Properties of the “Bent Sandwich-like” Compounds [Eu(TpIPr2)2] and [Yb(TpIPr2)2]– Intermediates between Nitride-Based Phosphors and Metallocenes. J. Lumin. 2017, 187, 62–68. [CrossRef]
- Arikawa, Y.; Inada, K.; Onishi, M. Side-on Coordination Mode of a Pyrazolyl Group in the Structure of a Divalent [Sm{B(3-Mepz)4}2] Complex (3-Mepz Is 3-Methylpyrazol-1-Yl). Acta Crystallogr. Sect. C Struct. Chem. 2016, 72, 838–841. [CrossRef]
- Li, Z.-H.; Zhai, Y.-Q.; Chen, W.-P.; Ding, Y.-S.; Zheng, Y.-Z. Air-Stable Hexagonal Bipyramidal Dysprosium(III) Single-Ion Magnets with Nearly Perfect D6h Local Symmetry. Chem. – A Eur. J. 2019, 25, 16219–16224. [CrossRef]
- Canaj, A.B.; Dey, S.; Martí, E.R.; Wilson, C.; Rajaraman, G.; Murrie, M. Insight into D 6 h Symmetry: Targeting Strong Axiality in Stable Dysprosium(III) Hexagonal Bipyramidal Single-Ion Magnets. Angew. Chemie Int. Ed. 2019, 58, 14146–14151. [CrossRef]
- Li, Q.-W.; Wan, R.-C.; Chen, Y.-C.; Liu, J.-L.; Wang, L.-F.; Jia, J.-H.; Chilton, N.F.; Tong, M.-L. Unprecedented Hexagonal Bipyramidal Single-Ion Magnets Based on Metallacrowns. Chem. Commun. 2016, 52, 13365–13368. [CrossRef]
- Gil, Y.; Castro-Alvarez, A.; Fuentealba, P.; Spodine, E.; Aravena, D. Lanthanide SMMs Based on Belt Macrocycles: Recent Advances and General Trends. Chem. – A Eur. J. 2022, 28, e202200336. [CrossRef]
- Meyer, G. All the Lanthanides Do It and Even Uranium Does Oxidation State +2. Angew. Chemie Int. Ed. 2014, 53, 3550–3551. [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE, Version 2.1, Program for the Stereochemical Analysis of Molecular Fragments by Means of Continuous Shape Measures and Associated Tools. Univ. Barcelona, Barcelona, Spain 2013, 2103.
- Li, T.; Zhang, G.; Guo, J.; Wang, S.; Leng, X.; Chen, Y. Tris(Pyrazolyl)Methanide Complexes of Trivalent Rare-Earth Metals. Organometallics 2016, 35, 1565–1572. [CrossRef]
- Keene, F.R.; Snow, M.R.; Stephenson, P.J.; Tiekink, E.R.T. Ruthenium(II) Complexes of the C3v Ligands Tris(2-Pyridyl)Amine, Tris(2-Pyridyl)Methane, and Tris(2-Pyridyl)Phosphine. 1. Synthesis and x-Ray Structural Studies of the Bis(Ligand) Complexes. Inorg. Chem. 1988, 27, 2040–2045. [CrossRef]
- García, F.; Hopkins, A.D.; Humphrey, S.M.; McPartlin, M.; Rogers, M.C.; Wright, D.S. The First Example of a Si-Bridged Tris(Pyridyl) Ligand; Synthesis and Structure of [MeSi(2-C5H4N)3LiX] (X = 0.2Br, 0.8Cl). Dalt. Trans. 2004, 361–362. [CrossRef]
- MANN, F.G.; WATSON, J. Conditions of Salt Formation in Polyamines And Kindred Compounds. Salt Formation in the Tertiary 2-Pyridylamines, Phosphines and Arsines. J. Org. Chem. 1948, 13, 502–531. [CrossRef]
- García, F.; Hopkins, A.D.; Kowenicki, R.A.; McPartlin, M.; Rogers, M.C.; Silvia, J.S.; Wright, D.S. Syntheses and Structure of Heterometallic Complexes Containing Tripodal Group 13 Ligands [RE(2-Py)3]- (E = Al, In). Organometallics 2006, 25, 2561–2568. [CrossRef]
- Beswick, M.A.; Belle, C.J.; Davies, M.K.; Halcrow, M.A.; Raithby, P.R.; Steiner, A.; Wright, D.S. One-Pot Synthesis of a Novel Tridentate Tin(IV) Ligand; Syntheses and Structures of [BunSn(NC5H4-C,N)3MBr](M = Li, Cu). Chem. Commun. 1996, 2619. [CrossRef]
- Zeckert, K.; Zahn, S.; Kirchner, B. Tin–Lanthanoid Donor–Acceptor Bonds. Chem. Commun. 2010, 46, 2638. [CrossRef]
- Beswick, M.A.; Davies, M.K.; Raithby, P.R.; Steiner, A.; Wright, D.S. Synthesis and Structure of [Pb(2-Py)3Li·THF], Containing a Low-Valent Group 14 Tris(Pyridyl) Ligand (2-Py = 2-Pyridyl). Organometallics 1997, 16, 1109–1110. [CrossRef]
- Goura, J.; McQuade, J.; Shimoyama, D.; Lalancette, R.A.; Sheridan, J.B.; Jäkle, F. Electrophilic and Nucleophilic Displacement Reactions at the Bridgehead Borons of Tris(Pyridyl)Borate Scorpionate Complexes. Chem. Commun. 2022, 58, 977–980. [CrossRef]
- Reichart, F.; Kischel, M.; Zeckert, K. Lanthanide(II) Complexes of a Dual Functional Tris(2-Pyridyl)Stannate Derivative. Chem. – A Eur. J. 2009, 15, 10018–10020. [CrossRef]
- Zeckert, K. Syntheses and Structures of Lanthanoid(Ii) Complexes Featuring Sn–M (M = Al, Ga, In) Bonds. Dalt. Trans. 2012, 41, 14101–14106. [CrossRef]
- Zeckert, K. Pyridyl Compounds of Heavier Group 13 and 14 Elements as Ligands for Lanthanide Metals. Organometallics 2013, 32, 1387–1393. [CrossRef]
- Zeckert, K.; Griebel, J.; Kirmse, R.; Weiß, M.; Denecke, R. Versatile Reactivity of a Lithium Tris(Aryl)Plumbate(II) Towards Organolanthanoid Compounds: Stable Lead-Lanthanoid-Metal Bonds or Redox Processes. Chem. - A Eur. J. 2013, 19, 7718–7722. [CrossRef]
- Hellmann, K.W.; Gade, L.H.; Gevert, O.; Steinert, P.; Lauher, J.W. Tripodal Triamidostannates and -Plumbates. Inorg. Chem. 1995, 34, 4069–4078. [CrossRef]
- García-Rodríguez, R.; Simmonds, H.R.; Wright, D.S. Formation of a Heterometallic AlIII/SmIII Complex Involving a Novel [EtAl(2-Py)2O]2– Ligand (2-Py = 2-Pyridyl). Organometallics 2014, 33, 7113–7117. [CrossRef]
- García-Rodríguez, R.; Kopf, S.; Wright, D.S. Modifying the Donor Properties of Tris(Pyridyl)Aluminates in Lanthanide(Ii) Sandwich Compounds. Dalt. Trans. 2018, 47, 2232–2239. [CrossRef]
- Hajiashrafi, T.; Nemati Kharat, A.; Love, J.A.; Patrick, B.O. Synthesis, Characterization and Crystal Structure of Three New Lanthanide (III) Complexes with the [(6-Methyl-2-Pyridyl)Methyl]Bis(2-Pyridylmethyl)Amine (MeTPA) Ligand; New Precursors for Lanthanide (III) Oxide Nano-Particles. Polyhedron 2013, 60, 30–38. [CrossRef]
- Hay, M.A.; Gable, R.W.; Boskovic, C. Modulating the Electronic Properties of Divalent Lanthanoid Complexes with Subtle Ligand Tuning. Dalt. Trans. 2023, 52, 3315–3324. [CrossRef]
- Zhang, C.; Cheng, Z.; Tan, P.; Lv, W.; Cui, H.; Chen, L.; Cai, X.; Zhao, Y.; Yuan, A. Tuning the Ligand Field in Seven-Coordinate Dy(III) Complexes to Perturb Single-Ion Magnet Behavior. New J. Chem. 2021, 45, 8591–8596. [CrossRef]
- Demir, S.; Jeon, I.-R.; Long, J.R.; Harris, T.D. Radical Ligand-Containing Single-Molecule Magnets. Coord. Chem. Rev. 2015, 289–290, 149–176. [CrossRef]
- Ishikawa, N.; Sugita, M.; Tanaka, N.; Ishikawa, T.; Koshihara, S.; Kaizu, Y. Upward Temperature Shift of the Intrinsic Phase Lag of the Magnetization of Bis(Phthalocyaninato)Terbium by Ligand Oxidation Creating an S = 1 / 2 Spin. Inorg. Chem. 2004, 43, 5498–5500. [CrossRef]
- Pederson, R.; Wysocki, A.L.; Mayhall, N.; Park, K. Multireference Ab Initio Studies of Magnetic Properties of Terbium-Based Single-Molecule Magnets. J. Phys. Chem. A 2019, 123, 6996–7006. [CrossRef]
- Rinehart, J.D.; Fang, M.; Evans, W.J.; Long, J.R. A N 2 3– Radical-Bridged Terbium Complex Exhibiting Magnetic Hysteresis at 14 K. J. Am. Chem. Soc. 2011, 133, 14236–14239. [CrossRef]
- Rinehart, J.D.; Fang, M.; Evans, W.J.; Long, J.R. Strong Exchange and Magnetic Blocking in N23−-Radical-Bridged Lanthanide Complexes. Nat. Chem. 2011, 3, 538–542. [CrossRef]
- Vieru, V.; Iwahara, N.; Ungur, L.; Chibotaru, L.F. Giant Exchange Interaction in Mixed Lanthanides. Sci. Rep. 2016, 6, 24046. [CrossRef]
- Eugene V Tretyakov; Victor I Ovcharenko The Chemistry of Nitroxide Radicals in the Molecular Design of Magnets. Russ. Chem. Rev. 2009, 78, 971. [CrossRef]
- Vostrikova, K.E. High-Spin Molecules Based on Metal Complexes of Organic Free Radicals. Coord. Chem. Rev. 2008, 252, 1409–1419. [CrossRef]
- Ovcharenko, V.I.; Vostrikova, K.E.; Romanenko, G. V; Ikorski, V.N.; Podberezskaya, N. V; Larionov, S. V Synthesis, Crystal Structure and Magnetic Properties of Di(Methanol) and Di(Ethanol)-Bis 2,2,5,5-Tetramethyl-1-Oxyl-3-Imidazoline-4-(3’,3’,3’-Trifluoromethyl-1-Propenyl-2’-Oxyato Nickel(II) - a New Type of Low Temperature Ferromagnetics. Dokl. Akad. Nauk SSSR 1989, 306, 115–118, doi:WOS:A1989U689100027.
- Meng, X.; Shi, W.; Cheng, P. Magnetism in One-Dimensional Metal–Nitronyl Nitroxide Radical System. Coord. Chem. Rev. 2019, 378, 134–150. [CrossRef]
- Fegy, K.; Vostrikova, K.E.; Luneau, D.; Rey, P. New Nitroxide Based Molecular Magnetic Materials. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A. Mol. Cryst. Liq. Cryst. 1997, 305, 69–80. [CrossRef]
- Ovcharenko, V.I.; Vostrikova, K.E.; Ikorskii, V.N.; Larionov, S. V; Sagdeev, R.Z. The Low Temperature Ferromagnet Dimethanol-Bis-[2,2,5,5-Tetramethyl-1-Oxyl-3-Imidazoline-4-(3’,3’,3’-Tri Fluoromethyl-1’-Propenyl-2’-Oxyato)] Cobalt(II), CoL2(CH3OH)2. Dokl. Akad. Nauk SSSR 1989, 306, 660–662, doi:WOS:A1989AC74400036.
- Hintermaier, F.; Volodarsky, L.B.; Polborn, K.; Beck, W. New 2,5-Dihydroimidazole-1-Oxyls with Functional Side Groups (N, O, S Donors). Liebigs Ann. 1995, 1995, 2189–2194. [CrossRef]
- Hintermaier, F.; Sünkel, K.; Volodarsky, L.B.; Beck, W. Synthesis, Structure, and Magnetic Properties of Transition Metal Complexes of the Nitroxide 2,5-Dihydro-4,5,5-Trimethyl-2,2-Bis(2-Pyridyl)Imidazole-1-Oxyl. Inorg. Chem. 1996, 35, 5500–5503. [CrossRef]
- Perfetti, M.; Caneschi, A.; Sukhikh, T.S.; Vostrikova, K.E. Lanthanide Complexes with a Tripodal Nitroxyl Radical Showing Strong Magnetic Coupling. Inorg. Chem. 2020, 59, 16591–16598. [CrossRef]
- Rey, P.; Smolentsev, A.I.; Vostrikova, K.E. Oxazolidine Nitroxide Transformation in a Coordination Sphere of the Ln3+ Ions. Molecules 2022, 27, 1626. [CrossRef]
- Rey, P.; Caneschi, A.; Sukhikh, T.S.; Vostrikova, K.E. Tripodal Oxazolidine-N-Oxyl Diradical Complexes of Dy3+ and Eu3+. Inorganics 2021, 9, 91. [CrossRef]
- Köhn, R.D.; Pan, Z.; Kociok-Köhn, G.; Mahon, M.F. New Sandwich Complexes of Praseodymium(Iii) Containing Triazacyclohexane Ligands. J. Chem. Soc. Dalt. Trans. 2002, 2344–2347. [CrossRef]
- Wedal, J.C.; Ziller, J.W.; Evans, W.J. Trimethyltriazacyclohexane Coordination Chemistry of Simple Rare-Earth Metal Salts. Dalt. Trans. 2023, 52, 4787–4795. [CrossRef]
- Hazama, R.; Umakoshi, K.; Kabuto, C.; Kabuto, K.; Sasaki, Y. A Europium(III)-N,N,N′,N′-Tetrakis(2-Pyridylmethyl)-(R)-Propylenediamine Complex as a New Chiral Lanthanide NMR Shift Reagent for Aqueous Neutral Solution. Chem. Commun. 1996, 15–16. [CrossRef]
- Kira E. Vostrikova, Denis G. Samsonenko, CCDC 2268033: Experimental Crystal Structure Determination, DOI: 10.5517/ccdc.csd.cc2g42c2.
- Ishida, T.; Murakami, R.; Kanetomo, T.; Nojiri, H. Magnetic Study on Radical-Gadolinium(III) Complexes. Relationship between the Exchange Coupling and Coordination Structure. Polyhedron 2013, 66, 183–187. [CrossRef]
- Kanetomo, T.; Ishida, T. Strongest Exchange Coupling in Gadolinium(III) and Nitroxide Coordination Compounds. Inorg. Chem. 2014, 53, 10794–10796. [CrossRef]
- Kanetomo, T.; Yoshitake, T.; Ishida, T. Strongest Ferromagnetic Coupling in Designed Gadolinium(III)–Nitroxide Coordination Compounds. Inorg. Chem. 2016, 55, 8140–8146. [CrossRef]
- Hu, P.; Zhu, M.; Mei, X.; Tian, H.; Ma, Y.; Li, L.; Liao, D. Single-Molecule Magnets Based on Rare Earth Complexes with Chelating Benzimidazole-Substituted Nitronyl Nitroxide Radicals. Dalt. Trans. 2012, 41, 14651. [CrossRef]
- Wang, X.; Zhu, M.; Wang, J.; Li, L. Unusual Gd–Nitronyl Nitroxide Antiferromagnetic Coupling and Slow Magnetic Relaxation in the Corresponding Tb Analogue. Dalt. Trans. 2015, 44, 13890–13896. [CrossRef]
- Wang, J.; Miao, H.; Xiao, Z.-X.; Zhou, Y.; Deng, L.-D.; Zhang, Y.-Q.; Wang, X.-Y. Syntheses, Structures and Magnetic Properties of the Lanthanide Complexes of the Pyrimidyl-Substituted Nitronyl Nitroxide Radical. Dalt. Trans. 2017, 46, 10452–10461. [CrossRef]
- Benelli, C.; Caneschi, A.; Gatteschi, D.; Pardi, L. Gadolinium(III) Complexes with Pyridine-Substituted Nitronyl Nitroxide Radicals. Inorg. Chem. 1992, 31, 741–746. [CrossRef]
- Hu, P.; Sun, Z.; Wang, X.; Li, L.; Liao, D.; Luneau, D. Magnetic Relaxation in Mononuclear Tb Complex Involving a Nitronyl Nitroxide Ligand. New J. Chem. 2014, 38, 4716–4721. [CrossRef]
- Chen, P.Y.; Wu, M.Z.; Shi, X.J.; Tian, L. A Family of Multi-Spin Rare-Earth Complexes Based on a Triazole Nitronyl Nitroxide Radical: Synthesis, Structure and Magnetic Properties. RSC Adv. 2018, 8, 15480–15486. [CrossRef]
- Nakamura, T.; Ishida, T. Magnetic Exchange Interaction in Gadolinium(III) Complex Having Aliphatic Nitroxide Radical TEMPO.; 2016; p. 020016.
- Caneschi, A.; Dei, A.; Gatteschi, D.; Sorace, L.; Vostrikova, K. Antiferromagnetic Coupling in a Gadolinium(III) Semiquinonato Complex. Angew. Chemie - Int. Ed. 2000, 39. [CrossRef]
- Zheludev, A.; Barone, V.; Bonnet, M.; Delley, B.; Grand, A.; Ressouche, E.; Rey, P.; Subra, R.; Schweizer, J. Spin Density in a Nitronyl Nitroxide Free Radical. Polarized Neutron Diffraction Investigation and Ab Initio Calculations. J. Am. Chem. Soc. 1994, 116, 2019–2027. [CrossRef]
- Hamada, D.; Fujinami, T.; Yamauchi, S.; Matsumoto, N.; Mochida, N.; Ishida, T.; Sunatsuki, Y.; Tsuchimoto, M.; Coletti, C.; Re, N. Luminescent DyIII Single Ion Magnets with Same N6O3 Donor Atoms but Different Donor Atom Arrangements, ‘Fac’-[DyIII(HLDL-Ala)3]·8H2O and ‘Mer’-[DyIII(HLDL-Phe)3]·7H2O. Polyhedron 2016, 109, 120–128. [CrossRef]
- Murakami, R.; Nakamura, T.; Ishida, T. Doubly TEMPO-Coordinated Gadolinium(III), Lanthanum(III), and Yttrium(III) Complexes. Strong Superexchange Coupling across Rare Earth Ions. Dalt. Trans. 2014, 43, 5893–5898. [CrossRef]
- Tkachenko, I.A.; Petrochenkova, N. V.; Mirochnik, A.G.; Karasev, V.E.; Kavun, V.Y. Carboxylato-Bis-Dibenzoylmethanates of Europium(III): Luminescence and Magnetic Properties. Russ. J. Phys. Chem. A 2012, 86, 681–684. [CrossRef]
- Kahn, M.L.; Sutter, J.-P.; Golhen, S.; Guionneau, P.; Ouahab, L.; Kahn, O.; Chasseau, D. Systematic Investigation of the Nature of the Coupling between a Ln(III) Ion (Ln = Ce(III) to Dy(III)) and Its Aminoxyl Radical Ligands. Structural and Magnetic Characteristics of a Series of {Ln(Radical)2} Compounds and the Related {Ln(Nitrone)2} Derivat. J. Am. Chem. Soc. 2000, 122, 3413–3421. [CrossRef]
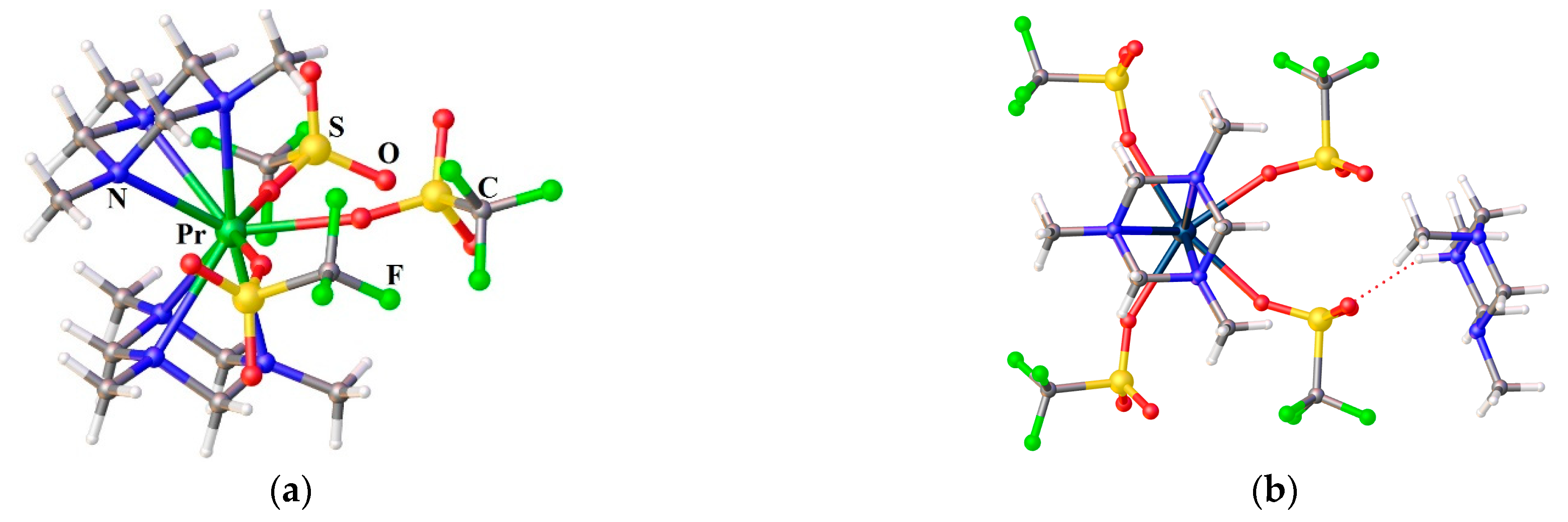
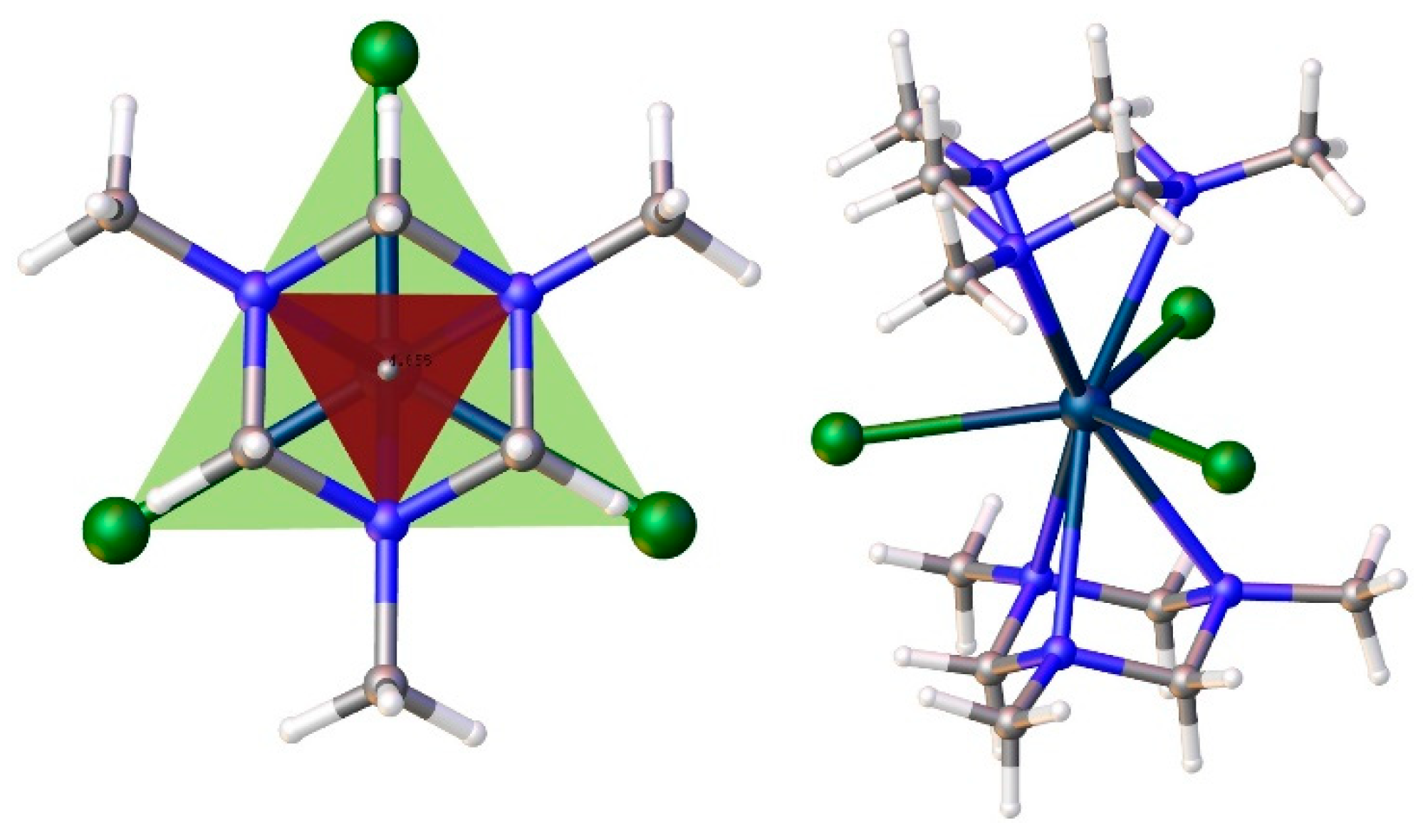
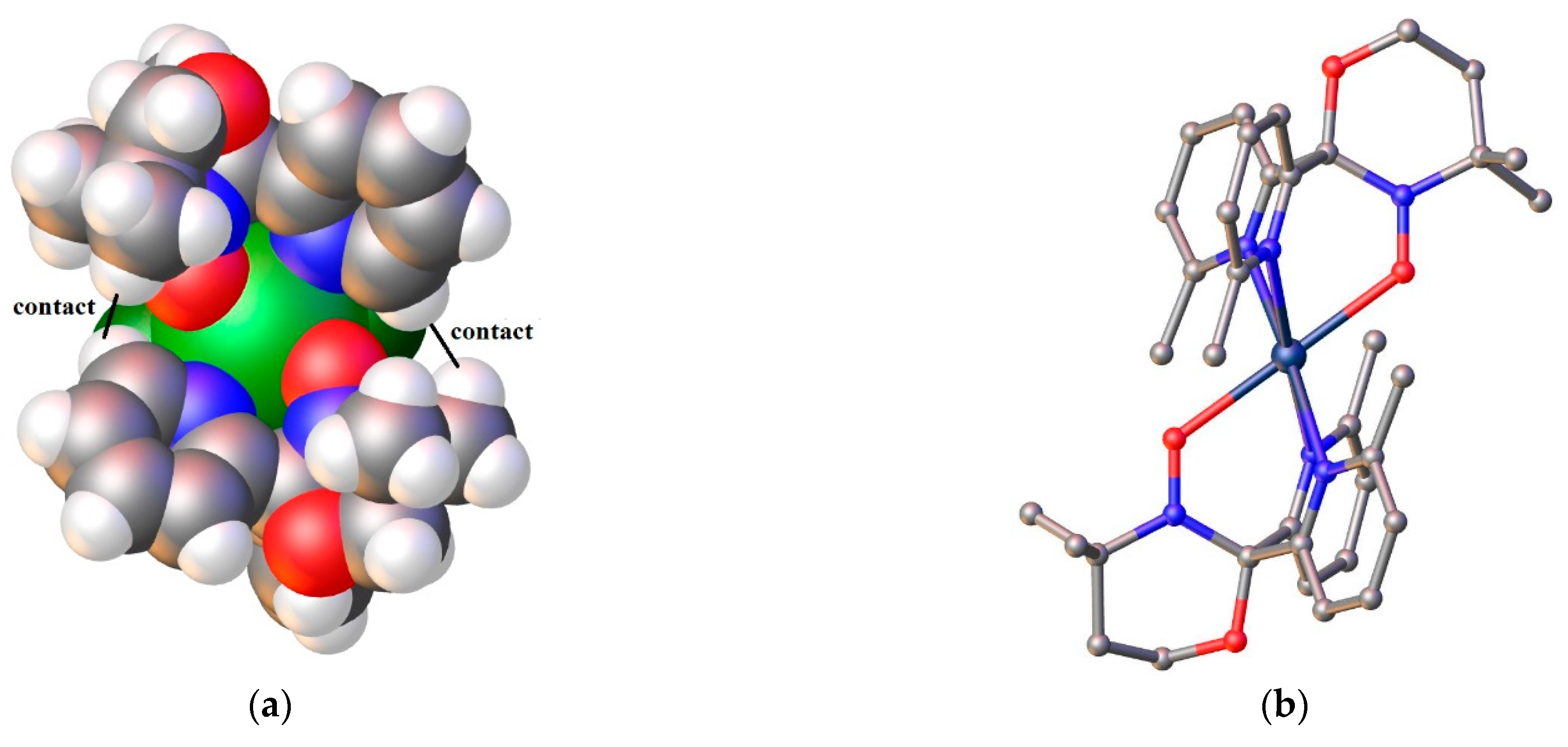
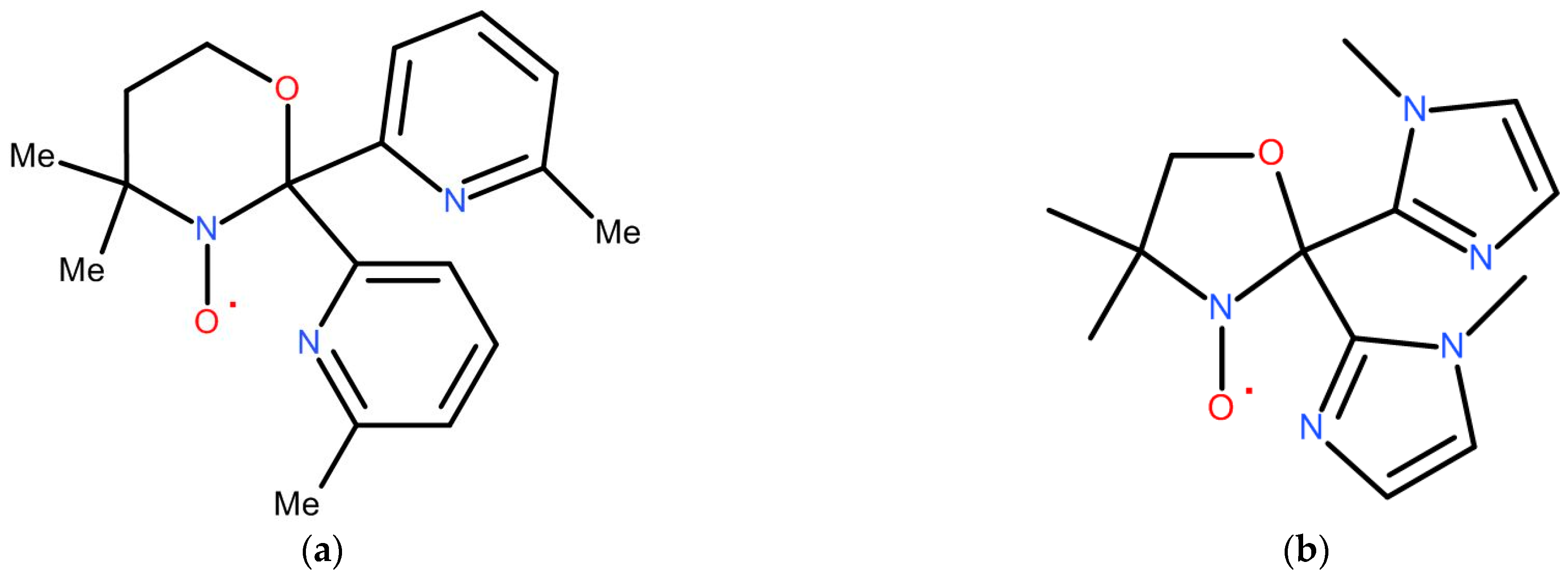
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



