Submitted:
13 June 2023
Posted:
14 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture Protocol
2.3. Fatty Acid and Neutral Lipid Profile Analysis of NEFA-Treated BeWo Trophoblasts
2.4. Transcriptomic Profiling of NEFA-Treated BeWo Cytotrophoblast Cells
2.5. RT-qPCR Validation of Differentially Expressed Genes Identified by mRNA Microarray
2.6. Cell Collections and Lysis for Untargeted Metabolomic and Lipidomic Profiling of BeWo Cytotrophoblast Cells
2.7. Untargeted Metabolomic Profiling of BeWo Cytotrophoblast Cells
2.8. Integration of BeWo Cytotrophoblast Transcriptomic and Metabolomic Profiles
2.9. Untargeted Lipidomic Profiling of BeWo Cytotrophoblast Cells
3. Results
3.1. NEFA-Treatments Impact Fatty acid Profiles of BeWo Trophoblasts
3.2. The Impact of NEFA Treatments on Desaturation and Elongation Indices in BeWo Trophoblasts
3.3. OA-Treatment Alters Neutral Lipid Profiles of BeWo Trophoblasts
3.4. Transcriptomic Profiles of BeWo Cytotrophoblasts Are Impacted by NEFA Treatment
3.5. RT-qPCR Validation of Differentially Expressed Genes
3.6. Metabolomic Profiles of BeWo Cytotrophoblast in Response to NEFA Treatment
3.7. BeWo Cytotrophoblast Transcriptome and Metabolome Integration
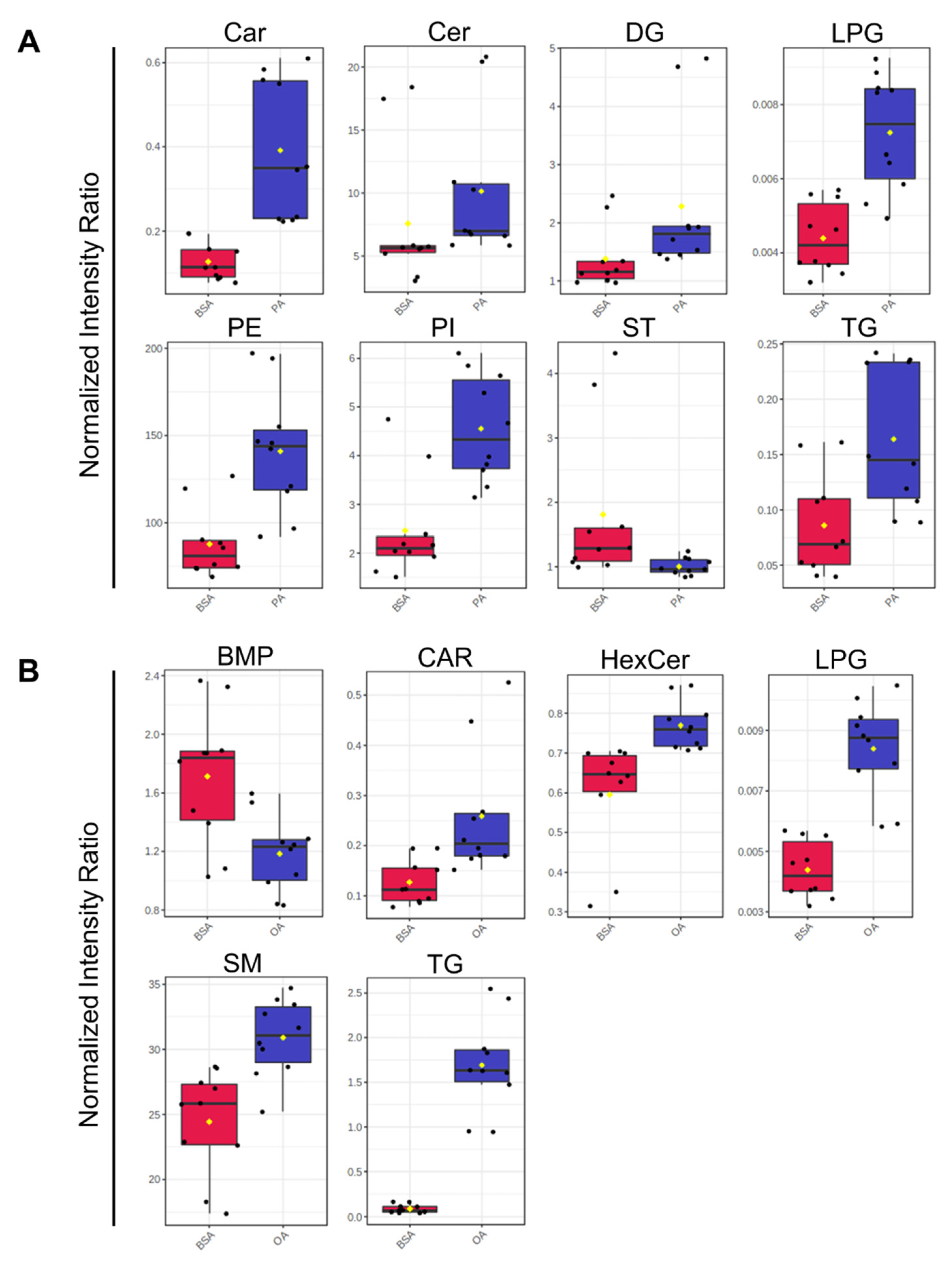
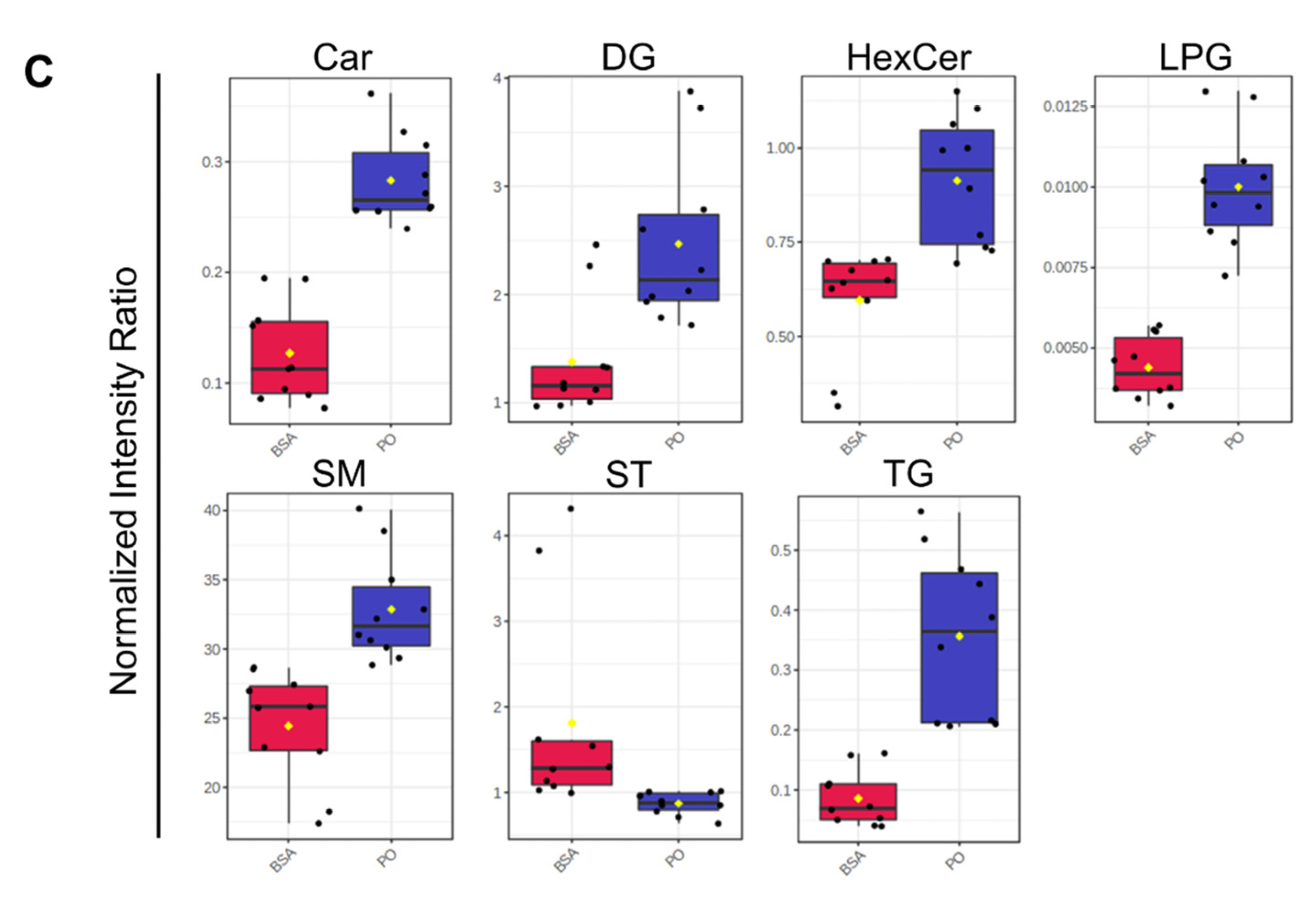
3.8. The Impact of NEFA-Treatment of BeWo Cytotrophoblast Lipidome Profiles
4. Discussion
4.1. Impacts of Dietary NEFA on BeWo FA and Neutral Lipid Profiles
4.2. A multi-Omics Analysis of NEFA-Treated BeWo CT Metabolic Function
4.3. Conclusion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhuang, W.; Lv, J.; Liang, Q.; Chen, W.; Zhang, S.; Sun, X. Adverse Effects of Gestational Diabetes-Related Risk Factors on Pregnancy Outcomes and Intervention Measures. Exp Ther Med 2020. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Phung, H.; Freebairn, L.; Sexton, R.; Raulli, A.; Kelly, P. Contribution of Maternal Overweight and Obesity to the Occurrence of Adverse Pregnancy Outcomes. Australian and New Zealand Journal of Obstetrics and Gynaecology 2019, 59, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Yogev, Y.; Visser, G.H.A. Obesity, Gestational Diabetes and Pregnancy Outcome. Semin Fetal Neonatal Med 2009, 14, 77–84. [Google Scholar] [CrossRef]
- Armitage, J.A.; Poston, L.; Taylor, P.D. Developmental Origins of Obesity and the Metabolic Syndrome: The Role of Maternal Obesity. Front Horm Res 2008, 36, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Manderson, J.; Mullan, B.; Patterson, C.; Hadden, D.; Traub, A.; McCance, D. Cardiovascular and Metabolic Abnormalities in the Offspring of Diabetic Pregnancy. Diabetologia 2002, 45, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Morriseau, T.S.; Kereliuk, S.M.; Doucette, C.A.; Wicklow, B.A.; Dolinsky, V.W. Maternal Obesity, Diabetes during Pregnancy and Epigenetic Mechanisms That Influence the Developmental Origins of Cardiometabolic Disease in the Offspring. Crit Rev Clin Lab Sci 2018, 55, 71–101. [Google Scholar] [CrossRef]
- Catalano, P.M. The Impact of Gestational Diabetes and Maternal Obesity on the Mother and Her Offspring. J Dev Orig Health Dis 2010, 1, 208–215. [Google Scholar] [CrossRef]
- Shrestha, N.; Ezechukwu, H.C.; Holland, O.J.; Hryciw, D.H. Developmental Programming of Peripheral Diseases in Offspring Exposed to Maternal Obesity during Pregnancy. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 2020, 319, R507–R516. [Google Scholar] [CrossRef]
- Whitaker, R.C. Predicting Preschooler Obesity at Birth: The Role of Maternal Obesity in Early Pregnancy. Pediatrics 2004, 114, e29–e36. [Google Scholar] [CrossRef]
- Boney, C.M.; Verma, A.; Tucker, R.; Vohr, B.R. Metabolic Syndrome in Childhood: Association with Birth Weight, Maternal Obesity, and Gestational Diabetes Mellitus. Pediatrics 2005, 115, e290–6. [Google Scholar] [CrossRef]
- Vääräsmäki, M.; Pouta, A.; Elliot, P.; Tapanainen, P.; Sovio, U.; Ruokonen, A.; Hartikainen, A.-L.; McCarthy, M.; Järvelin, M.-R. Adolescent Manifestations of Metabolic Syndrome Among Children Born to Women With Gestational Diabetes in a General-Population Birth Cohort. Am J Epidemiol 2009, 169, 1209–1215. [Google Scholar] [CrossRef]
- Ferrara, A. Increasing Prevalence of Gestational Diabetes Mellitus: A Public Health Perspective. Diabetes Care 2007, 30, S141–S146. [Google Scholar] [CrossRef]
- Kim, S.Y.; Dietz, P.M.; England, L.; Morrow, B.; Callaghan, W.M. Trends in Pre-Pregnancy Obesity in Nine States, 1993–2003*. Obesity 2007, 15, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wei, T.; Ni, W.; Zhang, A.; Zhang, J.; Xing, Y.; Xing, Q. Incidence and Risk Factors of Gestational Diabetes Mellitus: A Prospective Cohort Study in Qingdao, China. Front Endocrinol (Lausanne) 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Hod, M.; Kapur, A.; Sacks, D.A.; Hadar, E.; Agarwal, M.; Di Renzo, G.C.; Roura, L.C.; McIntyre, H.D.; Morris, J.L.; Divakar, H. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on Gestational Diabetes Mellitus: A Pragmatic Guide for Diagnosis, Management, and Care #. International Journal of Gynecology & Obstetrics 2015, 131, S173–S211. [Google Scholar] [CrossRef]
- Driscoll, A.K.; Gregory, E.C.W. Increases in Prepregnancy Obesity: United States, 2016-2019. NCHS Data Brief 2020, 1–8. [Google Scholar]
- Howell, K.R.; Powell, T.L. Effects of Maternal Obesity on Placental Function and Fetal Development. Reproduction 2017, 153, R97–R108. [Google Scholar] [CrossRef]
- Hebert, J.F.; Myatt, L. Placental Mitochondrial Dysfunction with Metabolic Diseases: Therapeutic Approaches. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2021, 1867, 165967. [Google Scholar] [CrossRef]
- Kelly, A.C.; Powell, T.L.; Jansson, T. Placental Function in Maternal Obesity. Clin Sci 2020, 134, 961–984. [Google Scholar] [CrossRef] [PubMed]
- Calabuig-Navarro, V.; Haghiac, M.; Minium, J.; Glazebrook, P.; Ranasinghe, G.C.; Hoppel, C.; Hauguel de-Mouzon, S.; Catalano, P.; O’Tierney-Ginn, P. Effect of Maternal Obesity on Placental Lipid Metabolism. Endocrinology 2017, 158, 2543–2555. [Google Scholar] [CrossRef]
- Nogues, P.; Dos Santos, E.; Couturier-Tarrade, A.; Berveiller, P.; Arnould, L.; Lamy, E.; Grassin-Delyle, S.; Vialard, F.; Dieudonne, M.-N. Maternal Obesity Influences Placental Nutrient Transport, Inflammatory Status, and Morphology in Human Term Placenta. J Clin Endocrinol Metab 2021, 106, 1880–1896. [Google Scholar] [CrossRef] [PubMed]
- Hastie, R.; Lappas, M. The Effect of Pre-Existing Maternal Obesity and Diabetes on Placental Mitochondrial Content and Electron Transport Chain Activity. Placenta 2014, 35, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Mandò, C.; Anelli, G.M.; Novielli, C.; Panina-Bordignon, P.; Massari, M.; Mazzocco, M.I.; Cetin, I. Impact of Obesity and Hyperglycemia on Placental Mitochondria. Oxid Med Cell Longev 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Maloyan, A.; Mele, J.; Muralimanoharan, S.; Myatt, L. Placental Metabolic Flexibility Is Affected by Maternal Obesity. Placenta 2016, 45, 69. [Google Scholar] [CrossRef]
- Mele, J.; Muralimanoharan, S.; Maloyan, A.; Myatt, L. Impaired Mitochondrial Function in Human Placenta with Increased Maternal Adiposity. Am J Physiol Endocrinol Metab 2014, 307, E419–25. [Google Scholar] [CrossRef]
- Valent, A.M.; Choi, H.; Kolahi, K.S.; Thornburg, K.L. Hyperglycemia and Gestational Diabetes Suppress Placental Glycolysis and Mitochondrial Function and Alter Lipid Processing. The FASEB Journal 2021, 35. [Google Scholar] [CrossRef] [PubMed]
- Calabuig-Navarro, V.; Puchowicz, M.; Glazebrook, P.; Haghiac, M.; Minium, J.; Catalano, P.; Hauguel deMouzon, S.; O’Tierney-Ginn, P. Effect of ω-3 Supplementation on Placental Lipid Metabolism in Overweight and Obese Women. Am J Clin Nutr 2016, 103, 1064–1072. [Google Scholar] [CrossRef]
- Hirschmugl, B.; Desoye, G.; Catalano, P.; Klymiuk, I.; Scharnagl, H.; Payr, S.; Kitzinger, E.; Schliefsteiner, C.; Lang, U.; Wadsack, C.; et al. Maternal Obesity Modulates Intracellular Lipid Turnover in the Human Term Placenta. Int J Obes 2017, 41, 317–323. [Google Scholar] [CrossRef]
- Bucher, M.; Montaniel, K.R.C.; Myatt, L.; Weintraub, S.; Tavori, H.; Maloyan, A. Dyslipidemia, Insulin Resistance, and Impairment of Placental Metabolism in the Offspring of Obese Mothers. J Dev Orig Health Dis 2021, 12, 738–747. [Google Scholar] [CrossRef]
- Powell, T.L.; Barner, K.; Madi, L.; Armstrong, M.; Manke, J.; Uhlson, C.; Jansson, T.; Ferchaud-Roucher, V. Sex-Specific Responses in Placental Fatty Acid Oxidation, Esterification and Transfer Capacity to Maternal Obesity. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2021, 1866, 158861. [Google Scholar] [CrossRef]
- Hulme, C.H.; Nicolaou, A.; Murphy, S.A.; Heazell, A.E.P.; Myers, J.E.; Westwood, M. The Effect of High Glucose on Lipid Metabolism in the Human Placenta. Sci Rep 2019, 9, 14114. [Google Scholar] [CrossRef]
- Visiedo, F.; Bugatto, F.; Quintero-Prado, R.; Cózar-Castellano, I.; Bartha, J.L.; Perdomo, G. Glucose and Fatty Acid Metabolism in Placental Explants From Pregnancies Complicated With Gestational Diabetes Mellitus. Reproductive Sciences 2015, 22, 798–801. [Google Scholar] [CrossRef]
- Visiedo, F.; Bugatto, F.; Sánchez, V.; Cózar-Castellano, I.; Bartha, J.L.; Perdomo, G. High Glucose Levels Reduce Fatty Acid Oxidation and Increase Triglyceride Accumulation in Human Placenta. American Journal of Physiology-Endocrinology and Metabolism 2013, 305, E205–E212. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Saez, M.A.; Sainz, F.; Fraile-Martínez, O.; García-Gallego, S.; Pekarek, L.; Bravo, C.; Coca, S.; Mon, M.Á.-; Buján, J.; et al. Lipidomic Profiling of Chorionic Villi in the Placentas of Women with Chronic Venous Disease. Int J Med Sci 2020, 17, 2790–2798. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Bhattacharjee, J.; Vasanthan, T.; Harris, C.S.; Bainbridge, S.A.; Adamo, K.B. Metabolomics to Understand Placental Biology: Where Are We Now? Tissue Cell 2021, 73, 101663. [Google Scholar] [CrossRef] [PubMed]
- Fattuoni, C.; Mandò, C.; Palmas, F.; Anelli, G.M.; Novielli, C.; Parejo Laudicina, E.; Savasi, V.M.; Barberini, L.; Dessì, A.; Pintus, R.; et al. Preliminary Metabolomics Analysis of Placenta in Maternal Obesity. Placenta 2018, 61, 89–95. [Google Scholar] [CrossRef]
- Segura, M.T.; Demmelmair, H.; Krauss-Etschmann, S.; Nathan, P.; Dehmel, S.; Padilla, M.C.; Rueda, R.; Koletzko, B.; Campoy, C. Maternal BMI and Gestational Diabetes Alter Placental Lipid Transporters and Fatty Acid Composition. Placenta 2017, 57, 144–151. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, Z.; Guo, F.; Wang, H.; Long, W.; Wang, H.; Yu, B. Placental Metabolic Profiling in Gestational Diabetes Mellitus: An Important Role of Fatty Acids. J Clin Lab Anal 2021. [Google Scholar] [CrossRef]
- Furse, S.; Fernandez-Twinn, D.S.; Jenkins, B.; Meek, C.L.; Williams, H.E.L.; Smith, G.C.S.; Charnock-Jones, D.S.; Ozanne, S.E.; Koulman, A. A High-Throughput Platform for Detailed Lipidomic Analysis of a Range of Mouse and Human Tissues. Anal Bioanal Chem 2020, 412, 2851–2862. [Google Scholar] [CrossRef]
- Uhl, O.; Demmelmair, H.; Segura, M.T.; Florido, J.; Rueda, R.; Campoy, C.; Koletzko, B. Effects of Obesity and Gestational Diabetes Mellitus on Placental Phospholipids. Diabetes Res Clin Pract 2015, 109, 364–371. [Google Scholar] [CrossRef]
- Bidne, K.L.; Rister, A.L.; McCain, A.R.; Hitt, B.D.; Dodds, E.D.; Wood, J.R. Maternal Obesity Alters Placental Lysophosphatidylcholines, Lipid Storage, and the Expression of Genes Associated with Lipid Metabolism. Biol Reprod 2021, 104, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Stuart, T.J.; O’Neill, K.; Condon, D.; Sasson, I.; Sen, P.; Xia, Y.; Simmons, R.A. Diet-Induced Obesity Alters the Maternal Metabolome and Early Placenta Transcriptome and Decreases Placenta Vascularity in the Mouse†. Biol Reprod 2018, 98, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.T.; Demmelmair, H.; Krauss-Etschmann, S.; Nathan, P.; Dehmel, S.; Padilla, M.C.; Rueda, R.; Koletzko, B.; Campoy, C. Maternal BMI and Gestational Diabetes Alter Placental Lipid Transporters and Fatty Acid Composition. Placenta 2017, 57, 144–151. [Google Scholar] [CrossRef]
- Delhaes, F.; Giza, S.A.; Koreman, T.; Eastabrook, G.; McKenzie, C.A.; Bedell, S.; Regnault, T.R.H.; de Vrijer, B. Altered Maternal and Placental Lipid Metabolism and Fetal Fat Development in Obesity: Current Knowledge and Advances in Non-Invasive Assessment. Placenta 2018, 69, 118–124. [Google Scholar] [CrossRef]
- Herrera Martínez, A. Hyperlipidemia during Gestational Diabetes and Its Relation with Maternal and Offspring Complications. Nutr Hosp 2018. [Google Scholar] [CrossRef]
- Scifres, C.M.; Catov, J.M.; Simhan, H.N. The Impact of Maternal Obesity and Gestational Weight Gain on Early and Mid-Pregnancy Lipid Profiles. Obesity 2014, 22, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Scholl, T.O.; Leskiw, M.; Savaille, J.; Stein, T.P. Differences in Maternal Circulating Fatty Acid Composition and Dietary Fat Intake in Women with Gestational Diabetes Mellitus or Mild Gestational Hyperglycemia. Diabetes Care 2010, 33, 2049–2054. [Google Scholar] [CrossRef]
- Villa, P.M.; Laivuori, H.; Kajantie, E.; Kaaja, R. Free Fatty Acid Profiles in Preeclampsia. Prostaglandins Leukot Essent Fatty Acids 2009, 81, 17–21. [Google Scholar] [CrossRef]
- Denomme, J.; Stark, K.D.; Holub, B.J. Directly Quantitated Dietary (n-3) Fatty Acid Intakes of Pregnant Canadian Women Are Lower than Current Dietary Recommendations. J Nutr 2005, 135, 206–211. [Google Scholar] [CrossRef]
- Savard, C.; Lemieux, S.; Weisnagel, S.; Fontaine-Bisson, B.; Gagnon, C.; Robitaille, J.; Morisset, A.-S. Trimester-Specific Dietary Intakes in a Sample of French-Canadian Pregnant Women in Comparison with National Nutritional Guidelines. Nutrients 2018, 10, 768. [Google Scholar] [CrossRef]
- Iggman, D.; Risérus, U. Role of Different Dietary Saturated Fatty Acids for Cardiometabolic Risk. Clin Lipidol 2011, 6, 209–223. [Google Scholar] [CrossRef]
- Kien, C.L.; Bunn, J.Y.; Stevens, R.; Bain, J.; Ikayeva, O.; Crain, K.; Koves, T.R.; Muoio, D.M. Dietary Intake of Palmitate and Oleate Has Broad Impact on Systemic and Tissue Lipid Profiles in Humans. Am J Clin Nutr 2014, 99, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, F.L.; Calabuig-Navarro, V.; Haghiac, M.; Puchowicz, M.; Tsai, P.-J.S.; O’Tierney-Ginn, P. Maternal Obesity Is Not Associated with Placental Lipid Accumulation in Women with High Omega-3 Fatty Acid Levels. Placenta 2018, 69, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Kolahi, K.; Valent, A.; Thornburg, K.L. Cytotrophoblast, Not Syncytiotrophoblast, Dominates Glycolysis and Oxidative Phosphorylation in Human Term Placenta. Sci Rep 2017, 1–12. [Google Scholar] [CrossRef]
- Kolahi, K.; Louey, S.; Varlamov, O.; Thornburg, K. Real-Time Tracking of BODIPY-C12 Long-Chain Fatty Acid in Human Term Placenta Reveals Unique Lipid Dynamics in Cytotrophoblast Cells. PLoS One 2016, 11, 1–23. [Google Scholar] [CrossRef]
- Easton, Z.J.W.; Delhaes, F.; Mathers, K.; Zhao, L.; Vanderboor, C.M.G.; Regnault, T.R.H. Syncytialization and Prolonged Exposure to Palmitate Impacts BeWo Respiration. Reproduction 2021, 161, 73–88. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A SIMPLE METHOD FOR THE ISOLATION AND PURIFICATION OF TOTAL LIPIDES FROM ANIMAL TISSUES. Journal of Biological Chemistry 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Klaiman, J.M.; Price, E.R.; Guglielmo, C.G. Fatty Acid Composition of Pectoralis Muscle Membrane, Intramuscular Fat Stores and Adipose Tissue of Migrant and Wintering White-Throated Sparrows ( Zonotrichia Albicollis ). Journal of Experimental Biology 2009, 212, 3865–3872. [Google Scholar] [CrossRef]
- Sarr, O.; Mathers, K.E.; Zhao, L.; Dunlop, K.; Chiu, J.; Guglielmo, C.G.; Bureau, Y.; Cheung, A.; Raha, S.; Lee, T.-Y.; et al. Western Diet Consumption through Early Life Induces Microvesicular Hepatic Steatosis in Association with an Altered Metabolome in Low Birth Weight Guinea Pigs. J Nutr Biochem 2019, 67, 219–233. [Google Scholar] [CrossRef]
- Merino, D.M.; Johnston, H.; Clarke, S.; Roke, K.; Nielsen, D.; Badawi, A.; El-Sohemy, A.; Ma, D.W.L.; Mutch, D.M. Polymorphisms in FADS1 and FADS2 Alter Desaturase Activity in Young Caucasian and Asian Adults. Mol Genet Metab 2011, 103, 171–178. [Google Scholar] [CrossRef]
- Chajès, V.; Joulin, V.; Clavel-Chapelon, F. The Fatty Acid Desaturation Index of Blood Lipids, as a Biomarker of Hepatic Stearoyl-CoA Desaturase Expression, Is a Predictive Factor of Breast Cancer Risk. Curr Opin Lipidol 2011, 22, 6–10. [Google Scholar] [CrossRef]
- Jeyakumar, S.M.; Lopamudra, P.; Padmini, S.; Balakrishna, N.; Giridharan, N. V; Vajreswari, A. Fatty Acid Desaturation Index Correlates with Body Mass and Adiposity Indices of Obesity in Wistar NIN Obese Mutant Rat Strains WNIN/Ob and WNIN/GR-Ob. Nutr Metab (Lond) 2009, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Massih, Y.N.; Hall, A.G.; Suh, J.; King, J.C. Zinc Supplements Taken with Food Increase Essential Fatty Acid Desaturation Indices in Adult Men Compared with Zinc Taken in the Fasted State. J Nutr 2021, 151, 2583–2589. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Vilarrubla, A.; Mas-Parés, B.; Díaz, M.; Xargay-Torrent, S.; Carreras-Badosa, G.; Jové, M.; Martin-Gari, M.; Bonmatí-Santané, A.; de Zegher, F.; Ibañez, L.; et al. Fatty Acids in the Placenta of Appropiate- versus Small-for-Gestational-Age Infants at Term Birth. Placenta 2021, 109, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, K.P. Skeletal Muscle Lipid Metabolism and Markers of Insulin Resistance in Young Male Low Birth Weight Offspring in Combination With a Postnatal Western Diet. Electronic Thesis and Dissertation Respository 2015, 2946. [Google Scholar]
- Easton, Z.J.W.; Luo, X.; Li, L.; Regnault, T.R.H. The Impact of Hyperglycemia upon BeWo Trophoblast Cell Metabolic Function: A Multi-OMICS and Functional Metabolic Analysis. PLoS One 2023, 18. [Google Scholar] [CrossRef]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.-F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1α-Responsive Genes Involved in Oxidative Phosphorylation Are Coordinately Downregulated in Human Diabetes. Nat Genet 2003, 34, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proceedings of the National Academy of Sciences 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, J.; Jaehnig, E.J.; Shi, Z.; Zhang, B. WebGestalt 2019: Gene Set Analysis Toolkit with Revamped UIs and APIs. Nucleic Acids Res 2019, 47, W199–W205. [Google Scholar] [CrossRef]
- Khan, M.I.; Dębski, K.J.; Dabrowski, M.; Czarnecka, A.M.; Szczylik, C. Gene Set Enrichment Analysis and Ingenuity Pathway Analysis of Metastatic Clear Cell Renal Cell Carcinoma Cell Line. American Journal of Physiology-Renal Physiology 2016, 311, F424–F436. [Google Scholar] [CrossRef]
- Guo, K.; Li, L. Differential 12 C-/ 13 C-Isotope Dansylation Labeling and Fast Liquid Chromatography/Mass Spectrometry for Absolute and Relative Quantification of the Metabolome. Anal Chem 2009, 81, 3919–3932. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, L. Chemical Derivatization in LC-MS-Based Metabolomics Study. TrAC Trends in Analytical Chemistry 2020, 131, 115988. [Google Scholar] [CrossRef]
- Zhao, S.; Li, H.; Han, W.; Chan, W.; Li, L. Metabolomic Coverage of Chemical-Group-Submetabolome Analysis: Group Classification and Four-Channel Chemical Isotope Labeling LC-MS. Anal Chem 2019, 91, 12108–12115. [Google Scholar] [CrossRef] [PubMed]
- Zardini Buzatto, A.; Abdel Jabar, M.; Nizami, I.; Dasouki, M.; Li, L.; Abdel Rahman, A.M. Lipidome Alterations Induced by Cystic Fibrosis, CFTR Mutation, and Lung Function. J Proteome Res 2021, 20, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Zardini Buzatto, A.; Tatlay, J.; Bajwa, B.; Mung, D.; Camicioli, R.; Dixon, R.A.; Li, L. Comprehensive Serum Lipidomics for Detecting Incipient Dementia in Parkinson’s Disease. J Proteome Res 2021, 20, 4053–4067. [Google Scholar] [CrossRef]
- Kind, T.; Liu, K.-H.; Lee, D.Y.; DeFelice, B.; Meissen, J.K.; Fiehn, O. LipidBlast in Silico Tandem Mass Spectrometry Database for Lipid Identification. Nat Methods 2013, 10, 755–758. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Aoyagi, R.; Ikeda, K.; Isobe, Y.; Arita, M. Comprehensive Analyses of Oxidized Phospholipids Using a Measured MS/MS Spectra Library. J Lipid Res 2017, 58, 2229–2237. [Google Scholar] [CrossRef]
- Ma, Y.; Kind, T.; Vaniya, A.; Gennity, I.; Fahrmann, J.F.; Fiehn, O. An in Silico MS/MS Library for Automatic Annotation of Novel FAHFA Lipids. J Cheminform 2015, 7, 53. [Google Scholar] [CrossRef]
- Fattuoni, C.; Mandò, C.; Palmas, F.; Anelli, G.M.; Novielli, C.; Parejo Laudicina, E.; Savasi, V.M.; Barberini, L.; Dessì, A.; Pintus, R.; et al. Preliminary Metabolomics Analysis of Placenta in Maternal Obesity. Placenta 2018, 61, 89–95. [Google Scholar] [CrossRef]
- Brown, S.H.J.; Eather, S.R.; Freeman, D.J.; Meyer, B.J.; Mitchell, T.W. A Lipidomic Analysis of Placenta in Preeclampsia: Evidence for Lipid Storage. PLoS One 2016, 11, e0163972. [Google Scholar] [CrossRef]
- Amusquivar, E.; Herrera, E. Influence of Changes in Dietary Fatty Acids during Pregnancy on Placental and Fetal Fatty Acid Profile in the Rat. Neonatology 2003, 83, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Ferchaud-Roucher, V.; Barner, K.; Jansson, T.; Powell, T.L. Maternal Obesity Results in Decreased Syncytiotrophoblast Synthesis of Palmitoleic Acid, a Fatty Acid with Anti-inflammatory and Insulin-sensitizing Properties. The FASEB Journal 2019, 33, 6643–6654. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.M.; Simão, J.J.; Sá, R.D.C.C. de; Farias, T.S.M.; Silva, V.S. da; Abdala, F.; Antraco, V.J.; Armelin-Correa, L.; Alonso-Vale, M.I.C. Palmitoleic Acid Decreases Non-Alcoholic Hepatic Steatosis and Increases Lipogenesis and Fatty Acid Oxidation in Adipose Tissue From Obese Mice. Front Endocrinol (Lausanne) 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.M.; Lopes, A.B.; Crisma, A.R.; de Sá, R.C.C.; Kuwabara, W.M.T.; Curi, R.; de Andrade, P.B.M.; Alonso-Vale, M.I.C. Palmitoleic Acid (16:1n7) Increases Oxygen Consumption, Fatty Acid Oxidation and ATP Content in White Adipocytes. Lipids Health Dis 2018, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- Bláhová; Harvey; Pšenička; Mráz Assessment of Fatty Acid Desaturase (Fads2) Structure-Function Properties in Fish in the Context of Environmental Adaptations and as a Target for Genetic Engineering. Biomolecules 2020, 10, 206. [CrossRef] [PubMed]
- Dave, K.; Kaur, L.; Sundrani, D.; Sharma, P.; Bayyana, S.; Mehendale, S.; Randhir, K.; Chandak, G.R.; Joshi, S. Association of Placental Fatty Acid Desaturase 2 (FADS2) Methylation with Maternal Fatty Acid Levels in Women with Preeclampsia. Prostaglandins Leukot Essent Fatty Acids 2022, 184, 102472. [Google Scholar] [CrossRef]
- Muralidharan, J.; Papandreou, C.; Sala-Vila, A.; Rosique-Esteban, N.; Fitó, M.; Estruch, R.; Angel Martínez-González, M.; Corella, D.; Ros, E.; Razquín, C.; et al. Fatty Acids Composition of Blood Cell Membranes and Peripheral Inflammation in the PREDIMED Study: A Cross-Sectional Analysis. Nutrients 2019, 11, 576. [Google Scholar] [CrossRef]
- Kapoor, R.; Huang, Y.-S. Gamma Linolenic Acid: An Antiinflammatory Omega-6 Fatty Acid. Curr Pharm Biotechnol 2006, 7, 531–534. [Google Scholar] [CrossRef]
- Colvin, B.N.; Longtine, M.S.; Chen, B.; Costa, M.L.; Nelson, D.M. Oleate Attenuates Palmitate-Induced Endoplasmic Reticulum Stress and Apoptosis in Placental Trophoblasts. Reproduction 2017, 153, 369–380. [Google Scholar] [CrossRef]
- Natarajan, S.K.; Bruett, T.; Muthuraj, P.G.; Sahoo, P.K.; Power, J.; Mott, J.L.; Hanson, C.; Anderson-Berry, A. Saturated Free Fatty Acids Induce Placental Trophoblast Lipoapoptosis. PLoS One 2021, 16, e0249907. [Google Scholar] [CrossRef] [PubMed]
- Watkins, O.C.; Islam, M.O.; Selvam, P.; Pillai, R.A.; Cazenave-Gassiot, A.; Bendt, A.K.; Karnani, N.; Godfrey, K.M.; Lewis, R.M.; Wenk, M.R.; et al. Metabolism of 13C-Labeled Fatty Acids in Term Human Placental Explants by Liquid Chromatography–Mass Spectrometry. Endocrinology 2019, 160, 1394–1408. [Google Scholar] [CrossRef]
- Colvin, B.N.; Longtine, M.S.; Chen, B.; Costa, M.L.; Nelson, D.M. Oleate Attenuates Palmitate-Induced Endoplasmic Reticulum Stress and Apoptosis in Placental Trophoblasts. Reproduction 2017, 153, 369–380. [Google Scholar] [CrossRef]
- Campbell, F.M.; Clohessy, A.M.; Gordon, M.J.; Page, K.R.; Dutta-Roy, A.K. Uptake of Long Chain Fatty Acids by Human Placental Choriocarcinoma (BeWo) Cells: Role of Plasma Membrane Fatty Acid-Binding Protein. J Lipid Res 1997, 38, 2558–2568. [Google Scholar] [CrossRef]
- Tobin, K.A.R.; Johnsen, G.M.; Staff, A.C.; Duttaroy, A.K. Long-Chain Polyunsaturated Fatty Acid Transport across Human Placental Choriocarcinoma (BeWo) Cells. Placenta 2009, 30, 41–47. [Google Scholar] [CrossRef]
- Ferchaud-Roucher, V.; Rudolph, M.C.; Jansson, T.; Powell, T.L. Fatty Acid and Lipid Profiles in Primary Human Trophoblast over 90 h in Culture. Prostaglandins Leukot Essent Fatty Acids 2017, 121, 14–20. [Google Scholar] [CrossRef]
- Mashek, D.G.; McKenzie, M.A.; Van Horn, C.G.; Coleman, R.A. Rat Long Chain Acyl-CoA Synthetase 5 Increases Fatty Acid Uptake and Partitioning to Cellular Triacylglycerol in McArdle-RH7777 Cells. Journal of Biological Chemistry 2006, 281, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Dutta-Roy, A.K. Cellular Uptake of Long-Chain Fatty Acids: Role of Membrane-Associated Fatty-Acid-Binding/Transport Proteins. Cellular and Molecular Life Sciences 2000, 57, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, G.M.; Weedon-Fekjær, M.S.; Tobin, K.A.R.; Staff, A.C.; Duttaroy, A.K. Long-Chain Polyunsaturated Fatty Acids Stimulate Cellular Fatty Acid Uptake in Human Placental Choriocarcinoma (BeWo) Cells. Placenta 2009, 30, 1037–1044. [Google Scholar] [CrossRef]
- Bildirici, I.; Schaiff, W.T.; Chen, B.; Morizane, M.; Oh, S.-Y.; O’Brien, M.; Sonnenberg-Hirche, C.; Chu, T.; Barak, Y.; Nelson, D.M.; et al. PLIN2 Is Essential for Trophoblastic Lipid Droplet Accumulation and Cell Survival During Hypoxia. Endocrinology 2018, 159, 3937–3949. [Google Scholar] [CrossRef]
- Stirm, L.; Kovářová, M.; Perschbacher, S.; Michlmaier, R.; Fritsche, L.; Siegel-Axel, D.; Schleicher, E.; Peter, A.; Pauluschke-Fröhlich, J.; Brucker, S.; et al. BMI-Independent Effects of Gestational Diabetes on Human Placenta. J Clin Endocrinol Metab 2018, 103, 3299–3309. [Google Scholar] [CrossRef] [PubMed]
- Adamo, K.B.; Dent, R.; Langefeld, C.D.; Cox, M.; Williams, K.; Carrick, K.M.; Stuart, J.S.; Sundseth, S.S.; Harper, M.-E.; McPherson, R.; et al. Peroxisome Proliferator-Activated Receptor γ 2 and Acyl-CoA Synthetase 5 Polymorphisms Influence Diet Response*. Obesity 2007, 15, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Becher, S.; Wallert, M.; Maeß, M.B.; Abhari, M.; Rennert, K.; Mosig, A.S.; Große, S.; Heller, R.; Grün, M.; et al. The Peroxisome Proliferator–Activated Receptor (PPAR)- γ Antagonist 2-Chloro-5-Nitro-N-Phenylbenzamide (GW9662) Triggers Perilipin 2 Expression via PPAR δ and Induces Lipogenesis and Triglyceride Accumulation in Human THP-1 Macrophages. Mol Pharmacol 2020, 97, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, J.; Ichiki, T.; Takahara, Y.; Kojima, H.; Sankoda, C.; Kitamoto, S.; Tokunou, T.; Sunagawa, K. PPARγ Agonists Attenuate Palmitate-Induced ER Stress through Up-Regulation of SCD-1 in Macrophages. PLoS One 2015, 10, e0128546. [Google Scholar] [CrossRef]
- Yao-Borengasser, A.; Rassouli, N.; Varma, V.; Bodles, A.M.; Rasouli, N.; Unal, R.; Phanavanh, B.; Ranganathan, G.; McGehee, R.E.; Kern, P.A. Stearoyl-Coenzyme A Desaturase 1 Gene Expression Increases after Pioglitazone Treatment and Is Associated with Peroxisomal Proliferator-Activated Receptor-γ Responsiveness. J Clin Endocrinol Metab 2008, 93, 4431–4439. [Google Scholar] [CrossRef]
- Brunmeir, R.; Xu, F. Functional Regulation of PPARs through Post-Translational Modifications. Int J Mol Sci 2018, 19, 1738. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Satoh, A.; Tezuka, H.; Han, S.; Takei, K.; Iwasaki, H.; Yatoh, S.; Yahagi, N.; Suzuki, H.; Iwasaki, Y.; et al. CREB3L3 Controls Fatty Acid Oxidation and Ketogenesis in Synergy with PPARα. Sci Rep 2016, 6, 39182. [Google Scholar] [CrossRef]
- Wade, H.; Pan, K.; Su, Q. CREBH: A Complex Array of Regulatory Mechanisms in Nutritional Signaling, Metabolic Inflammation, and Metabolic Disease. Mol Nutr Food Res 2021, 65, 2000771. [Google Scholar] [CrossRef]
- Sampieri, L.; Di Giusto, P.; Alvarez, C. CREB3 Transcription Factors: ER-Golgi Stress Transducers as Hubs for Cellular Homeostasis. Front Cell Dev Biol 2019, 7. [Google Scholar] [CrossRef]
- Zhang, K.; Shen, X.; Wu, J.; Sakaki, K.; Saunders, T.; Rutkowski, D.T.; Back, S.H.; Kaufman, R.J. Endoplasmic Reticulum Stress Activates Cleavage of CREBH to Induce a Systemic Inflammatory Response. Cell 2006, 124, 587–599. [Google Scholar] [CrossRef]
- Saben, J.; Zhong, Y.; Gomez-Acevedo, H.; Thakali, K.M.; Borengasser, S.J.; Andres, A.; Shankar, K. Early Growth Response Protein-1 Mediates Lipotoxicity-Associated Placental Inflammation: Role in Maternal Obesity. American Journal of Physiology-Endocrinology and Metabolism 2013, 305, E1–E14. [Google Scholar] [CrossRef]
- Lien, Y.-C.; Zhang, Z.; Barila, G.; Green-Brown, A.; Elovitz, M.A.; Simmons, R.A. Intrauterine Inflammation Alters the Transcriptome and Metabolome in Placenta. Front Physiol 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.C.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.; Krüger, A.; Tauqeer Alam, M.; et al. The Return of Metabolism: Biochemistry and Physiology of the Pentose Phosphate Pathway. Biological Reviews 2015, 90, 927–963. [Google Scholar] [CrossRef]
- Moe, A.J.; Farmer, D.R.; Nelson, D.M.; Smith, C.H. Pentose Phosphate Pathway in Cellular Trophoblasts from Full-Term Human Placentas. American Journal of Physiology-Cell Physiology 1991, 261, C1042–C1047. [Google Scholar] [CrossRef]
- Beaconsfield, P.; Ginsburg, J.; Jeacock, M.K. Glucose Metabolism via the Pentose Phosphate Pathway Relative to Nucleic Acid and Protein Synthesis in the Human Placenta. Dev Med Child Neurol 2008, 6, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Riganti, C.; Gazzano, E.; Polimeni, M.; Aldieri, E.; Ghigo, D. The Pentose Phosphate Pathway: An Antioxidant Defense and a Crossroad in Tumor Cell Fate. Free Radic Biol Med 2012, 53, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.M. Metabolic Reconfiguration Is a Regulated Response to Oxidative Stress. J Biol 2008, 7, 1. [Google Scholar] [CrossRef]
- Duan, Y.; Li, F.; Li, Y.; Tang, Y.; Kong, X.; Feng, Z.; Anthony, T.G.; Watford, M.; Hou, Y.; Wu, G.; et al. The Role of Leucine and Its Metabolites in Protein and Energy Metabolism. Amino Acids 2016, 48, 41–51. [Google Scholar] [CrossRef]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine Transport and Fatty Acid Oxidation. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2016, 1863, 2422–2435. [Google Scholar] [CrossRef] [PubMed]
- Lambertucci, R.H.; Hirabara, S.M.; Silveira, L. dos R.; Levada-Pires, A.C.; Curi, R.; Pithon-Curi, T.C. Palmitate Increases Superoxide Production through Mitochondrial Electron Transport Chain and NADPH Oxidase Activity in Skeletal Muscle Cells. J Cell Physiol 2008, 216, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Rosca, M.G.; Vazquez, E.J.; Chen, Q.; Kerner, J.; Kern, T.S.; Hoppel, C.L. Oxidation of Fatty Acids Is the Source of Increased Mitochondrial Reactive Oxygen Species Production in Kidney Cortical Tubules in Early Diabetes. Diabetes 2012, 61, 2074–2083. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Mitochondrial ROS Promote Mitochondrial Dysfunction and Inflammation in Ischemic Acute Kidney Injury by Disrupting TFAM-Mediated MtDNA Maintenance. Theranostics 2021, 11, 1845–1863. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, H.; Pal, S.; Sabnam, S.; Pal, A. High Glucose Augments ROS Generation Regulates Mitochondrial Dysfunction and Apoptosis via Stress Signalling Cascades in Keratinocytes. Life Sci 2020, 241, 117148. [Google Scholar] [CrossRef]
- Honda, A.; Yamashita, K.; Ikegami, T.; Hara, T.; Miyazaki, T.; Hirayama, T.; Numazawa, M.; Matsuzaki, Y. Highly Sensitive Quantification of Serum Malonate, a Possible Marker for de Novo Lipogenesis, by LC-ESI-MS/MS. J Lipid Res 2009, 50, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Rutkowsky, J.M.; Knotts, T.A.; Ono-Moore, K.D.; McCoin, C.S.; Huang, S.; Schneider, D.; Singh, S.; Adams, S.H.; Hwang, D.H. Acylcarnitines Activate Proinflammatory Signaling Pathways. Am J Physiol Endocrinol Metab 2014, 306, E1378–87. [Google Scholar] [CrossRef] [PubMed]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.B.; Newgard, C.B.; et al. Mitochondrial Overload and Incomplete Fatty Acid Oxidation Contribute to Skeletal Muscle Insulin Resistance. Cell Metab 2008, 7, 45–56. [Google Scholar] [CrossRef]
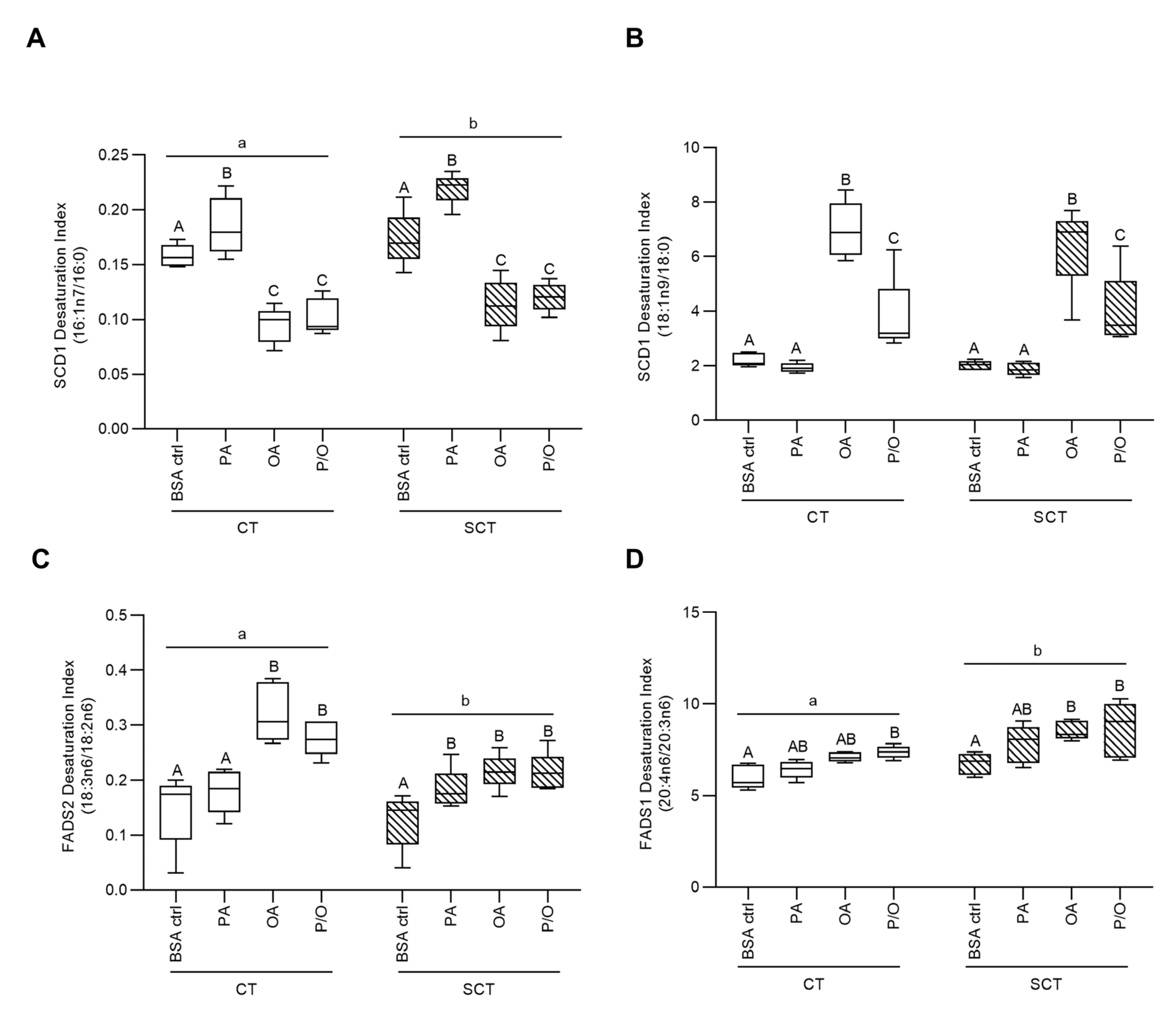
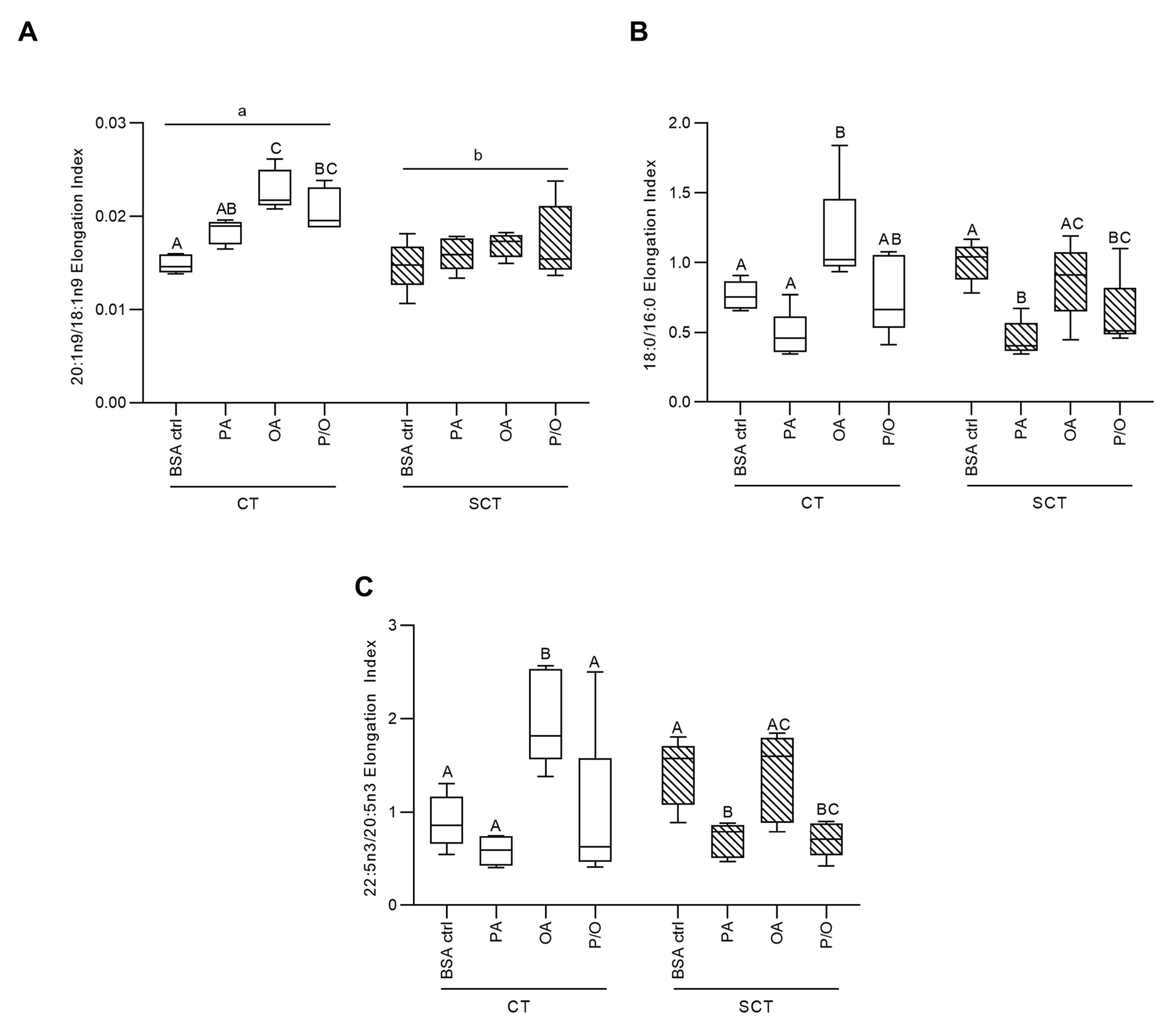
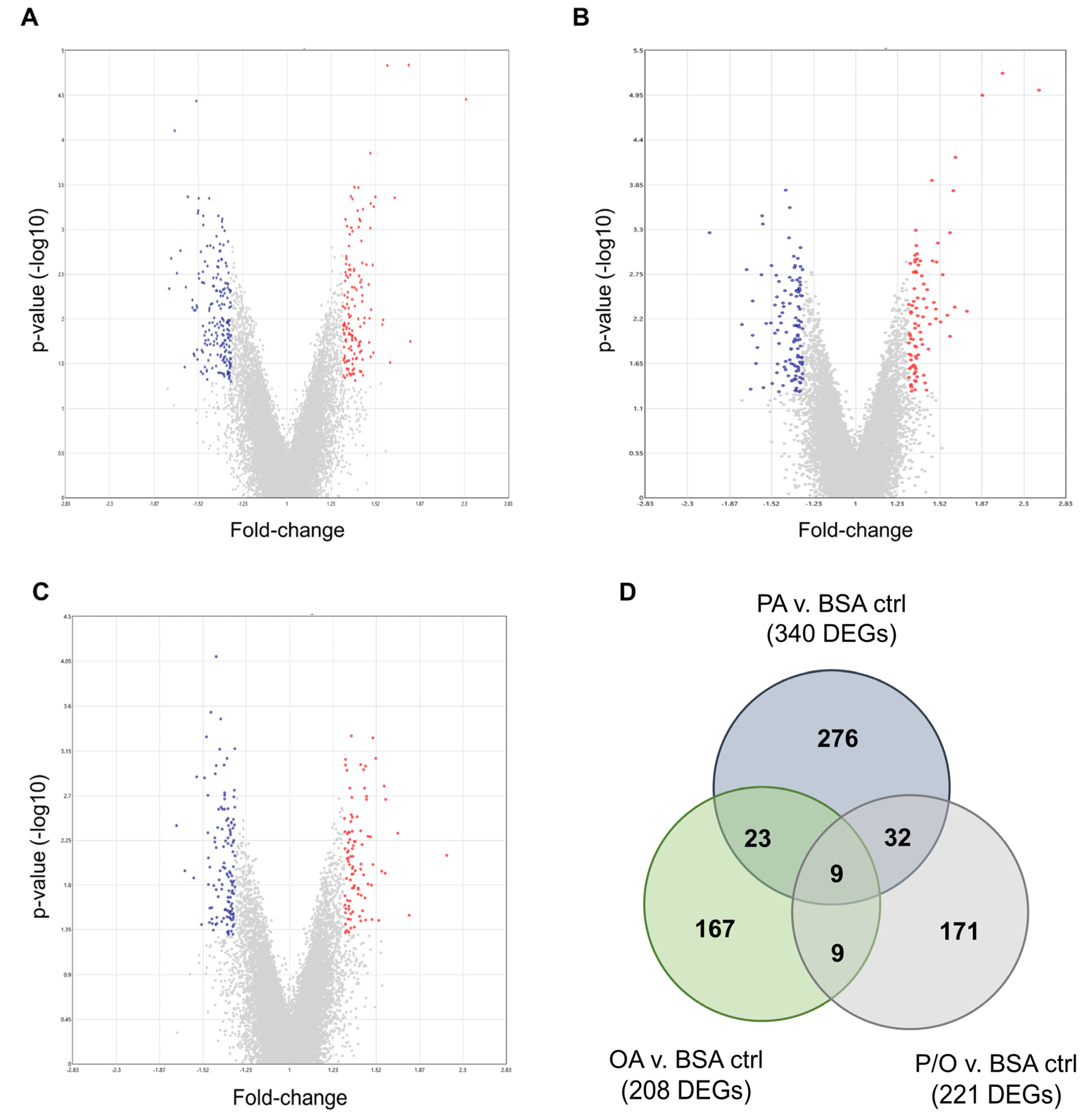
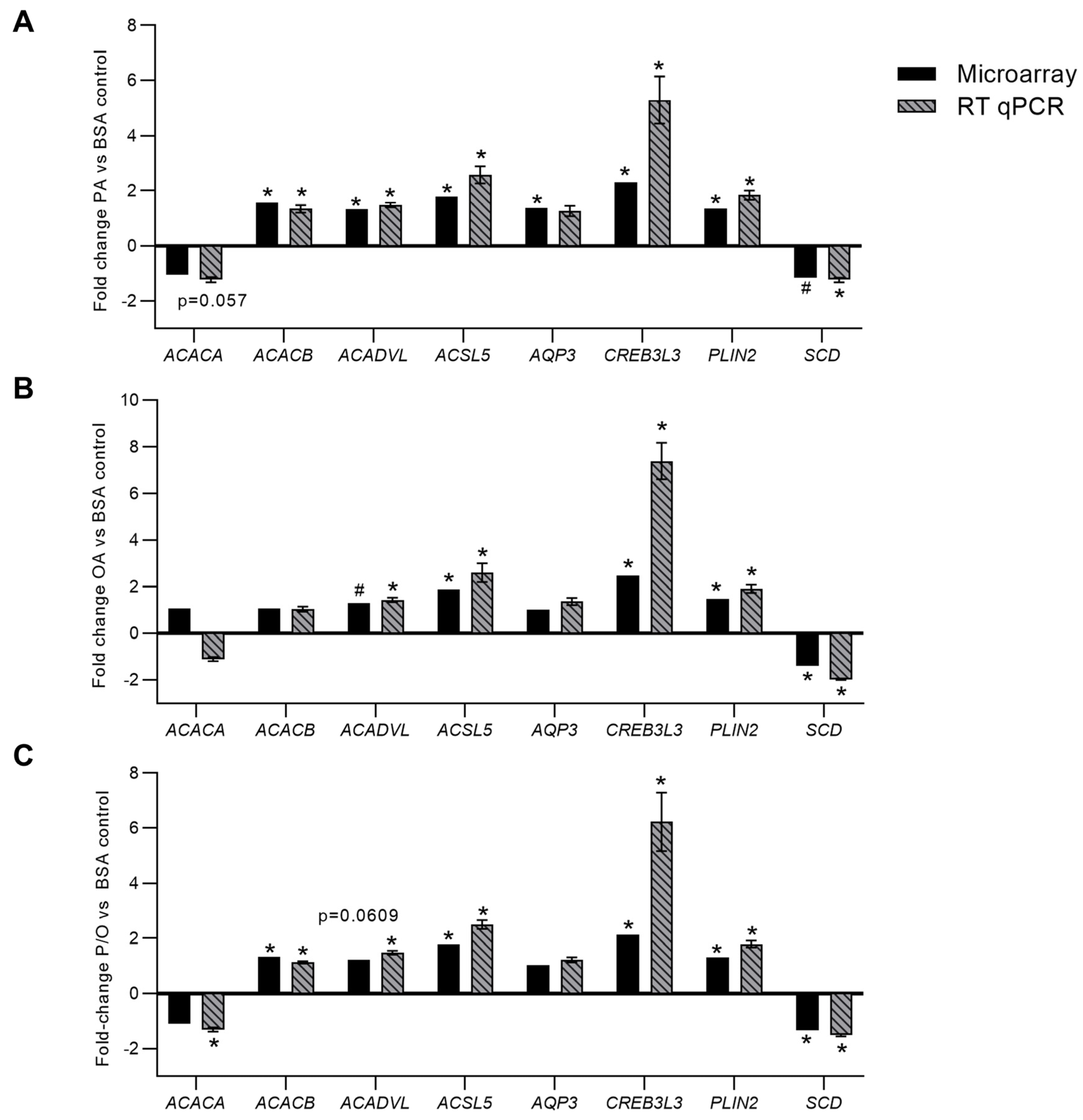
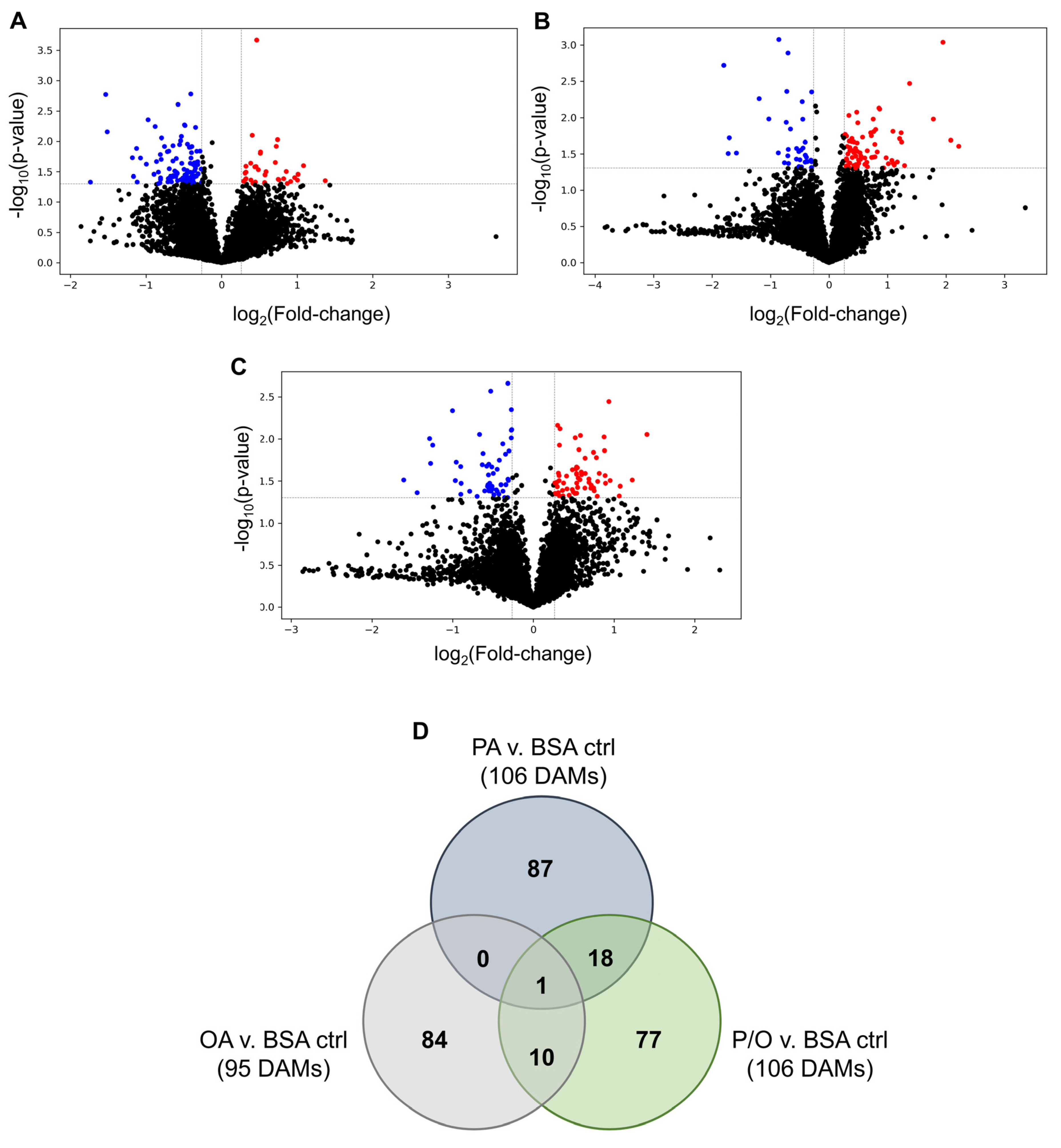
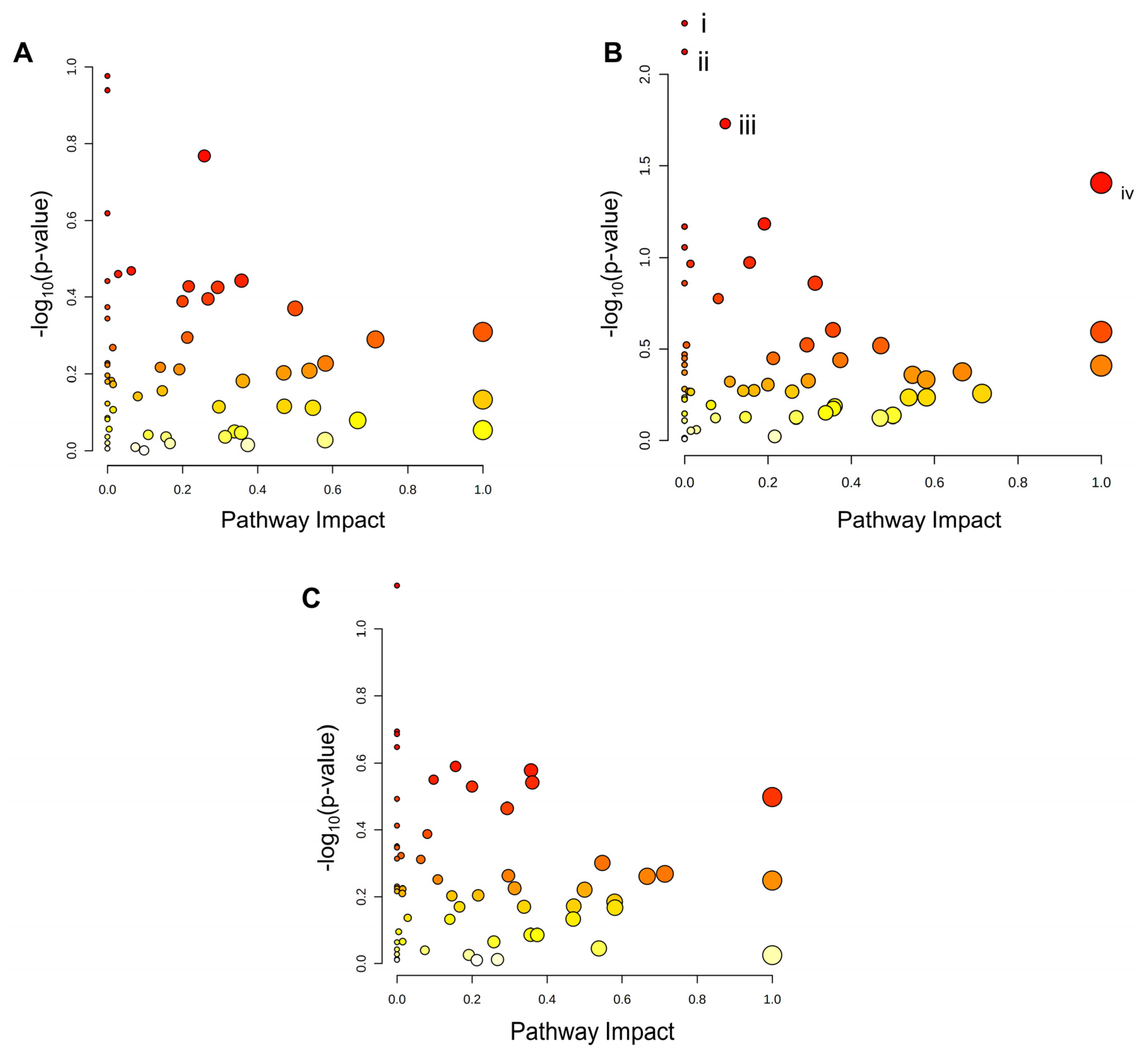
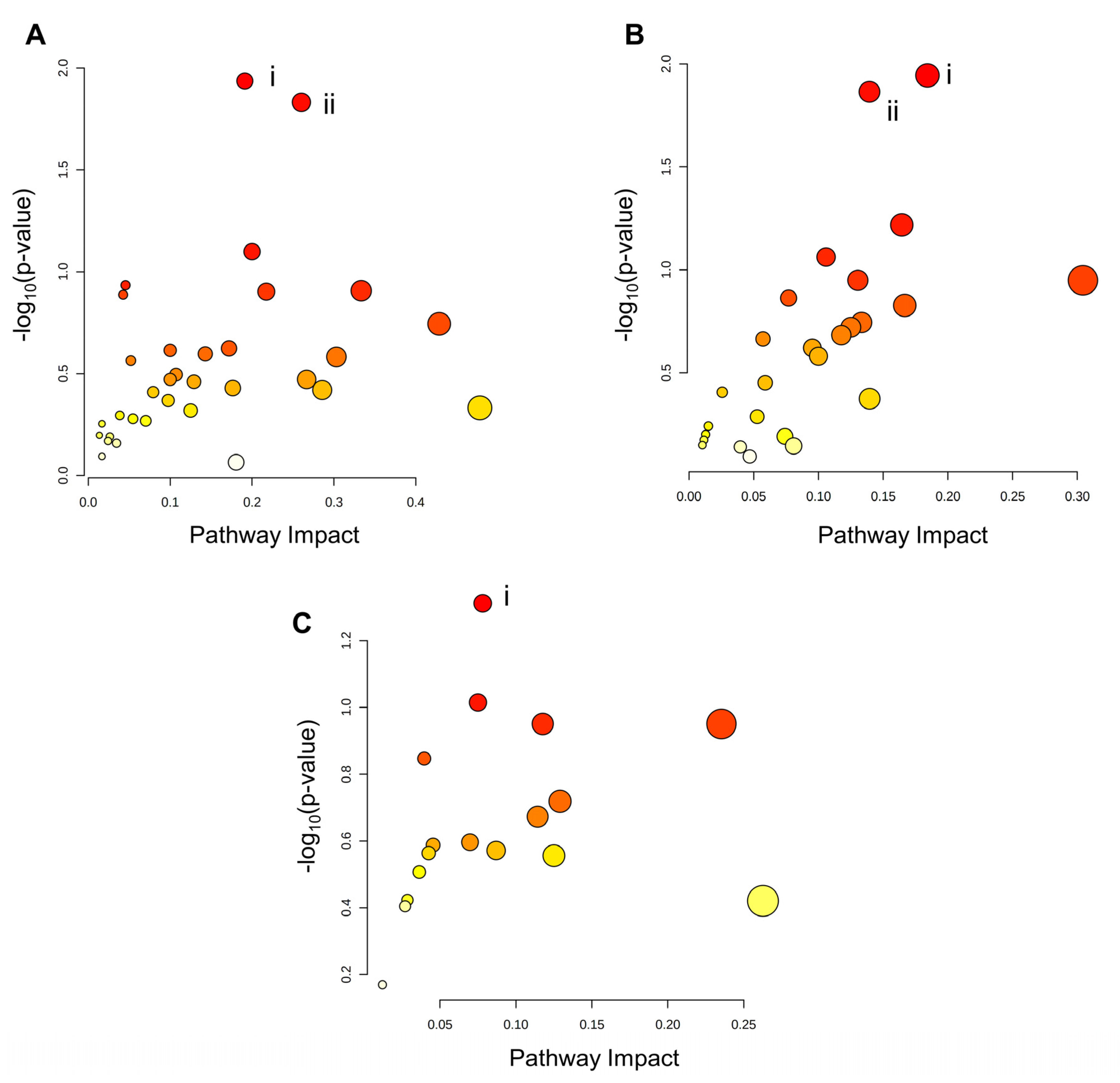
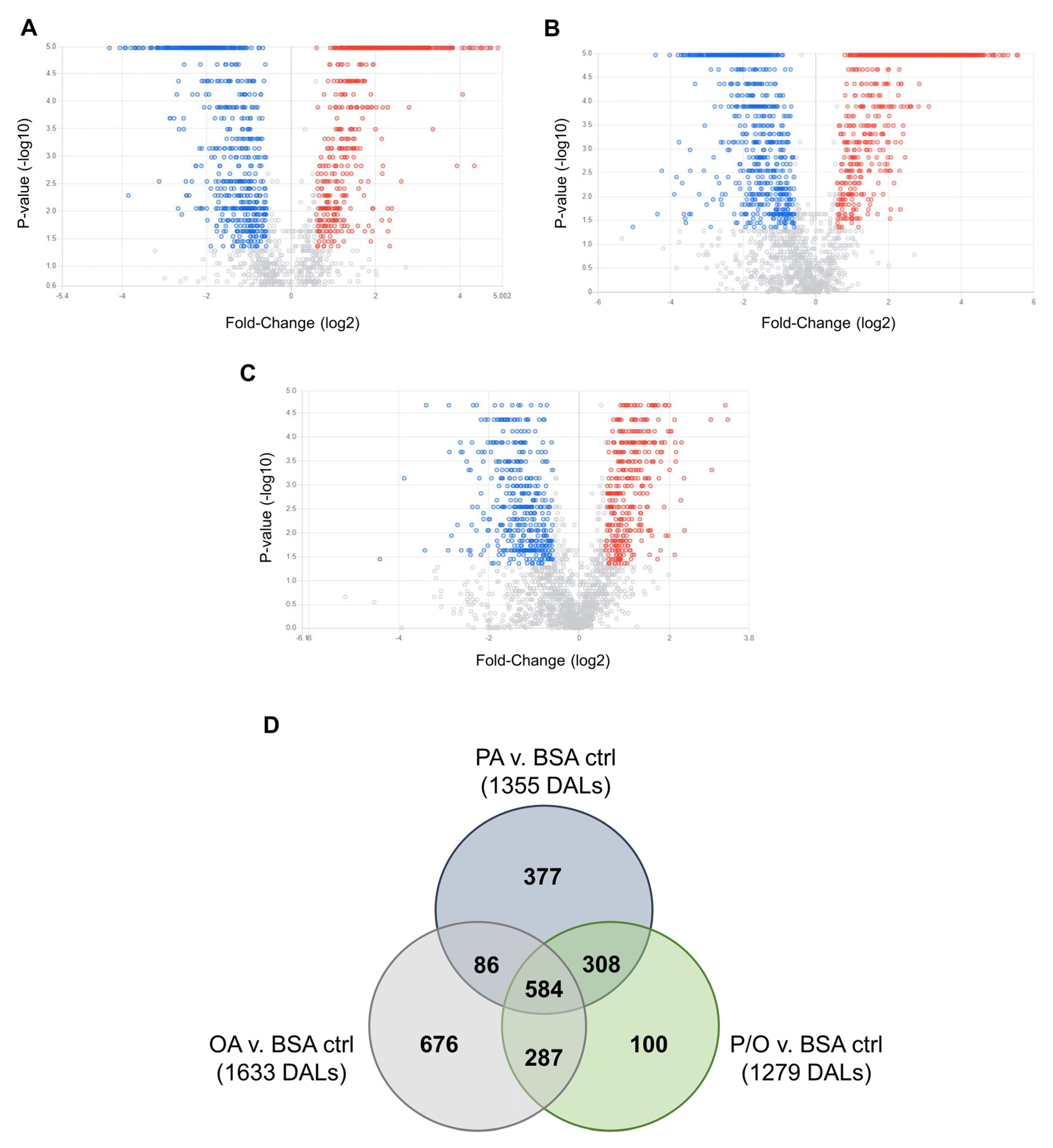
| Fatty Acid Species | CT | SCT | Differentiation State Difference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BSA Ctrl | PA | OA | P/O | BSA Ctrl | PA | OA | P/O | ||
| ΣSFA | 39.64 ± 1.1 a | 44.91 ± 1.63 b | 20.73 ± 1.04 c | 33.28 ± 2.99 d | 37.20 ± 0.92 a | 44.67 ± 0.85 b | 24.32 ± 3.18 c | 34.25 ± 3.29 a | NS |
| 8:0 | 0.63 ± 0.23 | 0.32 ± 0.11 | 0.40 ± 0.16 | 0.54 ± 0.10 | 0.49 ± 0.14 | 0.34 ± 0.12 | 0.45 ± 0.11 | 0.45 ± 0.06 | NS |
| 10:0 | 0.40 ± 0.14 | 0.31 ± 0.09 | 0.37 ± 0.11 | 0.37 ± 0.11 | 0.21 ± 0.07 | 0.24 ± 0.05 | 0.30 ± 0.07 | 0.21 ± 0.05 | NS |
| 14:0 | 1.43 ± 0.35 a | 0.85 ± 0.31 ab | 0.52 ± 0.12 b | 0.74 ± 0.25 b | 1.03 ± 0.33 | 0.68 ± 0.24 | 0.40 ± 0.08 | 0.58 ± 0.18 | NS |
| 15:0 | 0.54 ± 0.16 | 0.51 ± 0.19 | 0.19 ± 0.03 | 0.34 ± 0.14 | 0.45 ± 0.10 | 0.59 ± 0.19 | 0.28 ± 0.02 | 0.48 ± 0.14 | NS |
| 16:0 | 19.73 ± 0.81 a | 27.84 ± 2.12 b | 8.27 ± 0.81 c | 17.52 ± 2.87 a | 16.32 ± 0.78 ab | 28.07 ± 1.37 c | 12.17 ± 3.01 a | 19.74 ± 2.80 b | NS |
| 18:0 | 14.98 ± 0.48 a | 12.79 ± 0.76 b | 9.23 ± 0.41 c | 12.04 ± 0.79 b | 16.20 ± 0.36 a | 12.46 ± 0.89 b | 9.26 ± 0.41 c | 10.98 ± 0.43 bc | NS |
| 20:0 | 0.78 ± 0.06 | 0.94 ± 0.09 | 0.79 ± 0.16 | 0.74 ± 0.07 | 1.21 ± 0.17 | 1.10 ± 0.24 | 0.73 ± 0.10 | 0.92 ± 0.14 | NS |
| 22:0 | 0.68 ± 0.11 | 0.80 ± 0.16 | 0.47 ± 0.10 | 0.53 ± 0.06 | 0.76 ± 0.11 ab | 0.91 ± 0.08 a | 0.47 ± 0.07 b | 0.74 ± 0.08 ab | NS |
| 23:0 | 0.49 ± 0.14 | 0.58 ± 0.11 | 0.52 ± 0.02 | 0.64 ± 0.05 | 0.23 ± 0.03 | 0.44 ± 0.12 | 0.27 ± 0.03 | 0.41 ± 0.09 | NS |
| 24:0 | 1.02 ± 0.05 a | 1.08 ± 0.07 a | 0.70 ± 0.06 b | 0.79 ± 0.07 b | 0.98 ± 0.05 a | 0.89 ± 0.05 a | 0.54 ± 0.02 b | 0.63 ± 0.05 b | NS |
| ΣMUFA | 42.82 ± 1.14 a | 36.29 ± 1.21 b | 68.11 ± 1.26 c | 49.99 ± 4.19 d | 42.91 ± 1.15 a | 34.91 ± 0.43 b | 63.40 ± 4.28 c | 49.35 ± 4.35 d | NS |
| 16:1n7 | 3.12 ± 0.18 a | 5.23 ± 0.67 b | 0.80 ± 0.12 c | 1.87 ± 0.42 c | 2.86 ± 0.33 a | 6.20 ± 0.46 b | 1.43 ± 0.41 c | 2.42 ± 0.39 ab | NS |
| 18:1n9c | 33.09 ± 1.32 a | 24.76 ± 1.59 b | 63.73 ± 1.47 c | 43.84 ± 4.64 d | 32.65 ± 1.29 a | 23.08 ± 0.54 b | 62.64 ± 0.68 c | 38.52 ± 0.83 ab | NS |
| 18:1n9t | 0.23 ± 0.02 | 0.34 ± 0.08 | 0.38 ± 0.07 | 0.23 ± 0.04 | 0.28 ± 0.03 | 0.46 ± 0.23 | 0.33 ± 0.07 | 0.41 ± 0.19 | NS |
| 18:1n7 | 5.99 ± 0.26 a | 5.69 ± 0.23 a | 2.91 ± 0.17 b | 3.78 ± 0.31 c | 6.86 ± 0.23 a | 4.89 ± 0.20 b | 3.03 ± 0.16 c | 3.39 ± 0.18 c | NS |
| 20:1n9 | 0.49 ± 0.02 a | 0.45 ± 0.02 a | 1.46 ± 0.08 b | 0.92 ± 0.14 c | 0.48 ± 0.04 a | 0.37 ± 0.02 a | 0.99 ± 0.10 b | 0.77 ± 0.17 c | * |
| 22:1n9 | 0.29 ± 0.13 | 0.21 ± 0.08 | 0.19 ± 0.01 | 0.20 ± 0.03 | 0.16 ± 0.03 | 0.24 ± 0.09 | 0.18 ± 0.02 | 0.20 ± 0.06 | NS |
| ΣPUFA | 16.30 ± 0.94 ab | 17.47 ± 0.82 a | 9.18 ± 0.44 c | 15.02 ± 1.79 b | 18.89 ± 0.78 a | 18.96 ± 0.42 a | 10.68 ± 1.24 b | 14.84 ± 1.27 b | NS |
| 16:3n4 | 0.34 ± 0.04 ab | 0.23 ± 0.02 ab | 0.16 ± 0.02 b | 0.22 ± 0.03 b | 0.37 ± 0.04 a | 0.30 ± 0.05 a | 0.19 ± 0.02 b | 0.28 ± 0.03 ab | NS |
| 18:2n6 | 2.52 ± 1.21 ab | 1.36 ± 0.24 a | 0.61 ± 0.04 b | 0.82 ± 0.08 b | 3.24 ± 0.98 a | 1.45 ± 0.04 b | 0.94 ± 0.04 c | 1.11 ± 0.07 c | * |
| 18:3n6 | 0.230 ± 0.005 | 0.234 ± 0.029 | 0.194 ± 0.006 | 0.226 ± 0.022 | 0.324 ± 0.016 a | 0.265 ± 0.024 ab | 0.201 ± 0.009 c | 0.235 ± 0.011 bc | NS |
| 20:2 | 1.48 ± 0.12 ab | 3.03 ± 0.24 b | 1.69 ± 0.11 a | 3.23 ± 0.48 b | 0.97 ± 0.15 a | 2.00 ± 0.18 b | 1.23 ± 0.27 a | 1.83 ± 0.23 b | * |
| 20:3n6 | 1.45 ± 0.07 ab | 1.43 ± 0.08 a | 0.72 ± 0.05 b | 1.09 ± 0.12 c | 1.66 ± 0.06 a | 1.46 ± 0.05 a | 0.76 ± 0.08 b | 1.03 ± 0.08 c | NS |
| 20:4n6 | 8.68 ± 0.39 ab | 9.18 ± 0.50 a | 5.08 ± 0.25 b | 8.07 ± 0.94 a | 11.12 ± 0.28 a | 11.34 ± 0.38 a | 6.52 ± 0.78 b | 8.82 ± 0.85 c | * |
| 20:4n3 | 0.15 ± 0.06 | 0.19 ± 0.10 | 0.07 ± 0.01 | 0.15 ± 0.04 | 0.14 ± 0.03 | 0.28 ± 0.11 | 0.08 ± 0.02 | 0.21 ± 0.09 | NS |
| 20:5n3 | 0.54 ± 0.14 ac | 0.95 ± 0.14 b | 0.16 ± 0.02 a | 0.64 ± 0.15 bc | 0.39 ± 0.05 a | 0.92 ± 0.13 b | 0.27 ± 0.06 a | 0.63 ± 0.11 ab | NS |
| 22:5n3 | 0.43 ± 0.05 a | 0.52 ± 0.03 b | 0.31 ± 0.01 c | 0.39 ± 0.04 ac | 0.53 ± 0.01 a | 0.61 ± 0.02 a | 0.33 ± 0.02 b | 0.41 ± 0.04 b | NS |
| Neutral Lipid Species | CT | SCT | ||||||
|---|---|---|---|---|---|---|---|---|
| BSA Ctrl | PA | OA | P/O | BSA Ctrl | PA | OA | P/O | |
| Cholesterol Esters | 10.07 ± 2.66 | 25.46 ± 9.52 | 11.29 ± 2.28 | 15.44 ± 4.39 | 9.03 ± 1.69 | 17.56 ± 6.19 | 12.19 ± 4.33 | 19.18 ± 7.54 |
| Free Fatty Acids | 10.46 ± 3.67 | 11.91 ± 5.12 | 8.90 ± 3.36 | 10.05 ± 4.04 | 11.33 ± 3.69 | 9.93 ± 2.81 | 7.81 ± 2.89 | 8.72 ± 2.55 |
| Triglycerides | 15.99 ± 5.14 a | 17.12 ± 2.63 a | 39.47 ± 2.59 b | 18.35 ± 2.88 a | 14.34 ± 1.97 a | 14.74 ± 1.51 a | 30.86 ± 4.95 b | 17.96 ± 1.19 a |
| Free Cholesterol | 61.80 ± 6.21 a | 43.6 ± 5.91 bc | 37.84 ± 1.32 b | 53.79 ± 3.88 ac | 62.88 ± 3.19 a | 56.39 ± 5.57 ab | 46.40 ± 4.62 b | 52.24 ± 6.66 ab |
| Diacylglycerols | 1.68 ± 0.32 | 1.91 ± 0.77 | 2.51 ± 0.35 | 2.37 ± 0.42 | 2.43 ± 0.89 | 1.38 ± 0.24 | 2.75 ± 0.07 | 1.90 ± 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





