Submitted:
13 June 2023
Posted:
14 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
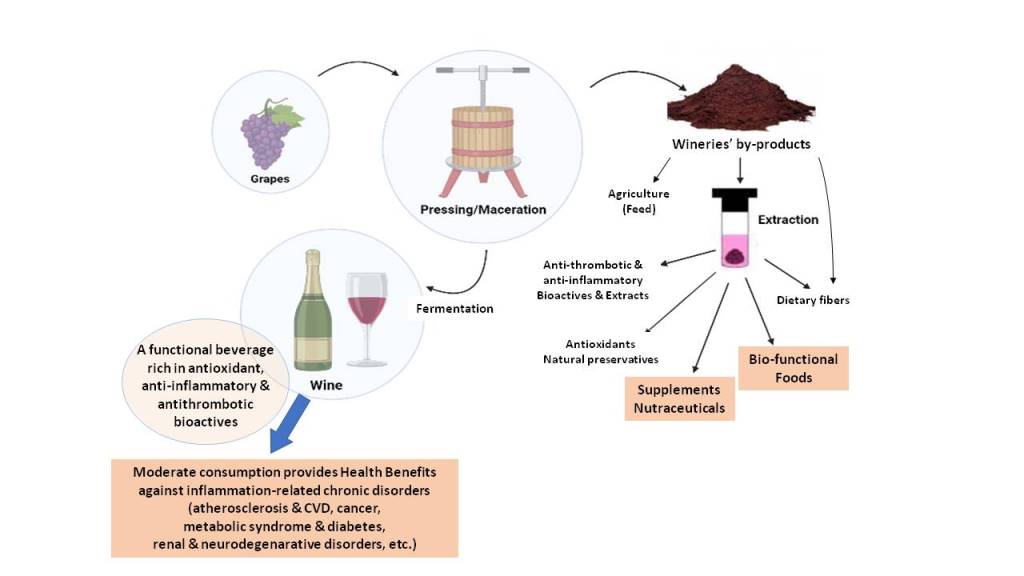
2. Bio-functional compounds and health benefits of the fermented alcoholic beverage, wine and of its by-products
2.1. Composition, nutritional value, bio-functional components, and functional properties
2.1.1. Types of wine; emphasis to red wine production, composition and nutritional value
2.1.2. Wineries by-products - composition and nutritional value
2.2. Bio-functional components and associated health benefits
2.2.1. Wine and its by-products phenolic bioactives with antioxidant, anti-inflammatory and anti-thrombotic beneficial properties
2.2.2. Bioactive lipid compounds of wine and wineries’ by-products
2.2.3. Bio-functional dietary fibres from wineries’ by-products
3. Health benefits of moderate red wine consumption and detrimental effects of alcohol abuse; a coin with two sides
4. Recovery and valorization of bioactive compounds from wineries’ by-products as ingredients for developing health promoting functional foods, supplements and nutraceuticals

4.1. Characteristic extraction methods for the recovery of wineries’ by-products bioactive compounds
4.2. Applications of wineries’ by-products and their bioactives in the food industry, as ingredient(s) for the fortification/production of existing/novel functional food products
4.2.1. Applications of wineries’ by-products and their bioactive ingredients for the fortification/production of functional flour/cereals-based foods
4.2.2. Applications of wineries’ by-products and their bioactive ingredients for the fortification/production of functional dairy-based foods
4.2.3. Applications of wineries’ by-products and their bioactive ingredients for the fortification/production of functional meat-/fish-based foods
4.2.4. Applications of wineries’ by-products and their bioactive ingredients for the fortification/production of other plant-based functional foods and beverages
4.3. Health benefits and applications of wineries’ by-products and their bioactives, as ingredients of bio-functional food products, supplements and nutraceuticals
4.3.1. Anti-oxidant, anti-inflammatory and antithrombotic health promoting effects of grape pomace and of its bioactives, extracts and relevant bio-functional products
4.3.2. Anti-oxidant, anti-inflammatory and antithrombotic health promoting effects of grape seeds and of their bioactives, extracts and relevant bio-functional products
4.3.3. Anticancer protective effects of wineries’ by-products and of their bioactives, extracts and relevant bio-functional products
4.3.4. Antimicrobial protective effects of wineries’ by-products and of their bioactives, extracts and relevant bio-functional products
4.3.5. Bio-delivery systems to improve the bioavailability and bio-functionality of wineries’ by-products and of their bioactives, extracts and relevant bio-functional products
4.4. Limitations in the applications of wineries by-products and of their bioactive ingredients
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basalekou, M.; Kallithraka, S.; Kyraleou, M. Wine bioactive compounds. In Functional Foods and Their Implications for Health Promotion, 1st ed.; Zabetakis, I., Tsoupras, A., Lordan, R., Ramji, D., Eds.; Academic Press, 2023; Volume 13, pp. 341–63. [Google Scholar]
- Calabriso, N.; Scoditti, E.; Massaro, M.; Pellegrino, M.; Storelli, C.; Ingrosso, I.; et al. Multiple anti-inflammatory and anti-atherosclerotic properties of red wine polyphenolic extracts: differential role of hydroxycinnamic acids, flavonols and stilbenes on endothelial inflammatory gene expression. Eur J Nutr 2016, 55, 477–89. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fidalgo, S.; Cárdeno, A.; Villegas, I.; Talero, E.; de la Lastra, C.A. Dietary supplementation of resveratrol attenuates chronic colonic inflammation in mice. Eur J Pharmac. 2010, 633, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; De Freitas, V. Wine Flavonoids in Health and Disease Prevention. Molecules. 2017, 22, 292. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.B.; Fragopoulou, E.; Nomikos, T.; Iatrou, C.; Antonopoulou, S.; Demopoulos, C.A. Characterization of the de novo biosynthetic enzyme of platelet activating factor, DDT-insensitive cholinephosphotransferase, of human mesangial cells. Mediators Inflamm. 2007, 2007, 27683. [Google Scholar] [CrossRef]
- Tsoupras, A.B.; Iatrou, C.; Frangia, C.; Demopoulos, C.A. The implication of platelet activating factor in cancer growth and metastasis: potent beneficial role of PAF-inhibitors and antioxidants. Infect Disord Drug Targets. 2009, 9, 390–9. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, not Cholesterol, Is a Cause of Chronic Disease. Nutrients. 2018, 10, 604. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Nomikos, T.; Tsantila, N.; Mitropoulou, A.; Zabetakis, I.; Demopoulos, C.A. Biological Activity of Total Lipids from Red and White Wine/Must. J Agr Food Chem. 2001, 49, 5186–93. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, E.; Antonopoulou, S.; Demopoulos, C.A. Biologically Active Lipids with Antiatherogenic Properties from White Wine and Must. J Agr Food Chem. 2002, 50, 2684–94. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Antonopoulou, S.; Tsoupras, A.; Tsantila, N.; Grypioti, A.; Gribilas, G.; Gritzapi, H.; Konsta, E.; Skandalou, E.; Papadopoulou, A. Antiatherogenic properties of red/white wine, musts, grape-skins, and yeast. In Proceedings of the 45th International Conference on the Bioscience of Lipids, University of, Ioannina, Ioannina, Greece, 25–29 May 2004; p. 66. [Google Scholar]
- Fragopoulou, E.; Demopoulos, C.A.; Antonopoulou, S. Lipid Minor Constituents in Wines. A Biochemical Approach in the French Paradox. Int. J. Wine Res. 2009, 1, 131–143. [Google Scholar]
- Xanthopoulou, M.N.; Kalathara, K.; Melachroinou, S.; Arampatzi-Menenakou, K.; Antonopoulou, S.; Yannakoulia, M.; Fragopoulou, E. Wine Consumption Reduced Postprandial Platelet Sensitivity against Platelet Activating Factor in Healthy Men. Eur. J. Nutr. 2017, 56, 1485–1492. [Google Scholar] [CrossRef]
- Argyrou, C.; Vlachogianni, I.; Stamatakis, G.; Demopoulos, C.A.; Antonopoulou, S.; Fragopoulou, E. Postprandial Effects of Wine Consumption on Platelet Activating Factor Metabolic Enzymes. Prostaglandins Other Lipid Mediat. 2017, 130, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, E.; Choleva, M.; Antonopoulou, S.; Demopoulos, C.A. Wine and its metabolic effects. A comprehensive review of clinical trials. Metabolism. 2018, 83, 102–19. [Google Scholar] [CrossRef] [PubMed]
- Gavriil, L.; Detopoulou, M.; Petsini, F.; Antonopoulou, S.; Fragopoulou, E. Consumption of plant extract supplement reduces platelet activating factor-induced platelet aggregation and increases platelet activating factor catabolism: a randomised, double-blind and placebo-controlled trial. Br J Nutr 2019, 121, 982–991. [Google Scholar] [CrossRef]
- Choleva, M.; Boulougouri, V.; Panara, A.; Panagopoulou, E.; Chiou, A.; Thomaidis, N.S.; Antonopoulou, S.; Fragopoulou, E. Evaluation of Anti-Platelet Activity of Grape Pomace Extracts. Food Funct. 2019, 10, 8069–8080. [Google Scholar] [CrossRef]
- Choleva, M.; Tsota, M.; Boulougouri, V.; Panara, A.; Thomaidis, N.; Antonopoulou, S.; & Fragopoulou, E. Anti-platelet and anti-inflammatory properties of an ethanol-water red grape pomace extract. Proceedings of the Nutrition Society, 2020, 79(OCE2), E370.
- Fragopoulou, E.; Antonopoulou, S. The French paradox three decades later: Role of inflammation and thrombosis. Clin Chim Acta. 2020, 510, 160–9. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Argyrou, C.; Detopoulou, M.; Tsitsou, S.; Seremeti, S.; Yannakoulia, M.; Antonopoulou, S.; Kolovou, G.; Kalogeropoulos, P. The Effect of Moderate Wine Consumption on Cytokine Secretion by Peripheral Blood Mononuclear Cells: A Randomized Clinical Study in Coronary Heart Disease Patients. Cytokine 2021, 146, 155629. [Google Scholar] [CrossRef]
- Choleva, M.; Argyrou, C.; Detopoulou, M.; Donta, M.-E.; Gerogianni, A.; Moustou, E.; Papaemmanouil, A.; Skitsa, C.; Kolovou, G.; Kalogeropoulos, P.; et al. Effect of Moderate Wine Consumption on Oxidative Stress Markers in Coronary Heart Disease Patients. Nutrients 2022, 14, 1377. [Google Scholar] [CrossRef]
- Choleva, M.; Matalliotaki, E.; Antoniou, S.; Asimomyti, E.; Drouka, A.; Stefani, M.; Yannakoulia, M.; Fragopoulou, E. Postprandial Metabolic and Oxidative Stress Responses to Grape Pomace Extract in Healthy Normal and Overweight/Obese Women: A Randomized, Double-Blind, Placebo-Controlled Crossover Study. Nutrients 2023, 15, 156. [Google Scholar] [CrossRef]
- Karantonis, H.C.; Tsoupras, A.; Moran, D.; Zabetakis, I.; Nasopoulou, C. Olive, apple, and grape pomaces with antioxidant and anti-inflammatory bioactivities for functional foods. In Functional Foods and Their Implications for Health Promotion, 1st ed.; Zabetakis, I., Tsoupras, A., Lordan, R., Ramji, D., Eds.; Academic Press; 2023; Volume 5, pp. 131–159.
- Sabra, A.; Netticadan, T.; Wijekoon, C. Grape bioactive molecules, and the potential health benefits in reducing the risk of heart diseases. Food Chem X. 2021, 12, 100149. [Google Scholar] [CrossRef]
- Tsoupras, A; Lordan, R.; Zabetakis, I. Inflammation and Cardiovascular Diseases. In The Impact of Nutrition and Statins on Cardiovascular Diseases, 1st ed. Zabetakis, I., Lordan, R., Tsoupras, A., Eds. Academic Press; 2019. Volume 3, pp. 53–117.
- van Bussel, B.C.T.; Henry, R.M.A.; Schalkwijk, C.G.; Dekker, J.M.; Nijpels, G.; Feskens, E.J.M.; et al. Alcohol and red wine consumption, but not fruit, vegetables, fish or dairy products, are associated with less endothelial dysfunction and less low-grade inflammation: the Hoorn Study. Eur J Nutr. 2018, 57, 1409–19. [Google Scholar] [CrossRef] [PubMed]
- Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; et al. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am J Clin Nutr. 2012, 95, 1323–34. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Arvaniti, F.; Stefanadis, C. Adherence to the Mediterranean Food Pattern Predicts the Prevalence of Hypertension, Hypercholesterolemia, Diabetes and Obesity, among Healthy Adults; the Accuracy of the MedDietScore. Prev. Med. 2007, 44, 335–340. [Google Scholar] [CrossRef]
- Esposito, K.; Maiorino, M.I.; Bellastella, G.; Panagiotakos, D.B.; Giugliano, D. Mediterranean diet for type 2 diabetes: cardiometabolic benefits. Endocrine. 2017, 56, 27–32. [Google Scholar] [CrossRef]
- Piano, M.R. Alcohol's Effects on the Cardiovascular System. Alcohol Res. 2017, 38, 219–41. [Google Scholar]
- Markoski, M.M.; Garavaglia, J.; Oliveira, A.; Olivaes, J.; Marcadenti, A. Molecular Properties of Red Wine Compounds and Cardiometabolic Benefits. Nutr Metabol Ins. 2016, 9, 51–7. [Google Scholar] [CrossRef]
- Poklar Ulrih, N.; Opara, R.; Skrt, M.; Košmerl, T.; Wondra, M.; Abram, V. Part I. Polyphenols composition and antioxidant potential during 'Blaufränkisch' grape maceration and red wine maturation, and the effects of trans-resveratrol addition. Food Chem Toxicol. 2020, 137, 111122. [Google Scholar] [CrossRef]
- Xiang, L.; Xiao, L.; Wang, Y.; Li, H.; Huang, Z.; He, X. Health benefits of wine: Don’t expect resveratrol too much. Food Chem. 2014, 156, 258–63. [Google Scholar] [CrossRef] [PubMed]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Arboleda Mejia, J.A.; Ricci, A.; Figueiredo, A.S.; Versari, A.; Cassano, A.; Parpinello, G.P.; De Pinho, M.N. Recovery of Phenolic Compounds from Red Grape Pomace Extract through Nanofiltration Membranes. Foods 2020, 9, 1649. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, M.S.; Torres, J.S.; Nuñez, M.J. ;. Extraction of polyphenols from white distilled grape pomace: Optimization and modelling. Biores Tech 2008, 99, 1311–1318. [Google Scholar] [CrossRef]
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M.V. From grape to wine: Changes in phenolic composition and its influence on antioxidant activity. Food Chem 2016, 208, 228–38. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Göksel, Z.; Erdoğan, S.S.; Öztürk, A.; Atak, A.; Özer, C. Antioxidant Activity and Phenolic Content of Seed, Skin and Pulp Parts of 22 Grape (Vitis vinifera L.) Cultivars (4 Common and 18 Registered or Candidate for Registration). J Food Proc Preserv. 2015, 39, 1682–91. [Google Scholar] [CrossRef]
- Ferri, M.; Bin, S.; Vallini, V.; Fava, F.; Michelini, E.; Roda, A.; Minnucci, G.; Bucchi, G.; Tassoni, A. Recovery of polyphenols from red grape pomace and assessment of their antioxidant and anti-cholesterol activities. New Biotechnol. 2016, 33, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Iora, S.R.F.; Maciel, G.M.; Zielinski, A.A.F.; da Silva, M.V.; Pontes, P.V. de A.; Haminiuk, C.W.I.; Granato, D. Evaluation of the bioactive compounds and the antioxidant capacity of grape pomace. Int J Food Sci Tech 2015, 50, 62–69. [Google Scholar] [CrossRef]
- Negro, C.; Tommasi, L.; Miceli, A. Phenolic compounds and antioxidant activity from red grape marc extracts. Biores Tech 2003, 87, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Szabó, É.; Marosvölgyi, T.; Szilágyi, G.; Kőrösi, L.; Schmidt, J.; Csepregi, K.; Márk, L.; Bóna, Á. Correlations between Total Antioxidant Capacity, Polyphenol and Fatty Acid Content of Native Grape Seed and Pomace of Four Different Grape Varieties in Hungary. Antioxidants 2021, 10, 1101. [Google Scholar] [CrossRef]
- Chedea, V.S.; Macovei, Ș.O.; Bocșan, I.C.; Măgureanu, D.C.; Levai, A.M.; Buzoianu, A.D.; Pop, R.M. Grape Pomace Polyphenols as a Source of Compounds for Management of Oxidative Stress and Inflammation—A Possible Alternative for Non-Steroidal Anti-Inflammatory Drugs? Molecules 2022, 27, 6826. [Google Scholar] [CrossRef]
- Visioli, F.; Panaite, S.A.; Tomé-Carneiro, J. Wine’s Phenolic Compounds and Health: A Pythagorean View. Molecules 2020, 25, 4105. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Saucier, C.; Glories, Y. Grape and Wine Phenolics: History and Perspective. Am J Enol Vitic. 2006, 57, 239–248. [Google Scholar] [CrossRef]
- Lago-Vanzela, E.; Alves Baffi, M.; Castilhos, M.; Ribeiro-Pinto, M.; Del Bianchi, V.; Ramos, A.; Stringheta, P.; Hermosín-Gutiérrez, I.; Da Silva, R. Phenolic compounds in grapes and wines: Chemical and biochemical characteristics and technological quality. In Grapes: production, phenolic composition and potential biomedical effects, 1st ed.; Câmara J.S., Eds.; Nova Science Publishers Inc.; 2014; Volume 3, pp. 1–18.
- Zhou, D.-D.; Li, J.; Xiong, R.-G.; Saimaiti, A.; Huang, S.-Y.; Wu, S.-X.; Yang, Z.-J.; Shang, A.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Bioactive Compounds, Health Benefits and Food Applications of Grape. Foods 2022, 11, 2755. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds From Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef]
- Cordova, A.C.; Sumpio, B.E. Polyphenols are medicine: Is it time to prescribe red wine for our patients? Int J Angiol 2009, 18, 111–117. [Google Scholar] [CrossRef]
- Snopek, L.; Mlcek, J.; Sochorova, L.; Baron, M.; Hlavacova, I.; Jurikova, T.; Kizek, R.; Sedlackova, E.; Sochor, J. Contribution of Red Wine Consumption to Human Health Protection. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Hrelia, S.; Di Renzo, L.; Bavaresco, L.; Bernardi, E.; Malaguti, M.; Giacosa, A. Moderate Wine Consumption and Health: A Narrative Review. Nutrients 2023, 15, 175. [Google Scholar] [CrossRef]
- Aviram, M.; Fuhrman, B. Wine Flavonoids Protect against LDL Oxidation and Atherosclerosis. Ann N Y Acad Sci 2002, 957, 146–161. [Google Scholar] [CrossRef]
- Nigdikar, S.V.; Williams, N.R.; Griffin, B.A.; Howard, A.N. Consumption of red wine polyphenols reduces the susceptibility of low-density lipoproteins to oxidation in vivo. Am J Clin Nutr 1998, 68, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Apostolidou, C.; Adamopoulos, K.; Lymperaki, E.; Iliadis, S.; Papapreponis, P.; Kourtidou-Papadeli, C. Cardiovascular risk and benefits from antioxidant dietary intervention with red wine in asymptomatic hypercholesterolemics. Clin Nutr ESPEN. 2015, 10, e224–e33. [Google Scholar] [CrossRef] [PubMed]
- Stranieri, C.; Guzzo, F.; Gambini, S.; Cominacini, L.; Fratta Pasini, A.M. Intracellular Polyphenol Wine Metabolites Oppose Oxidative Stress and Upregulate Nrf2/ARE Pathway. Antioxidants 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Schrieks, I.C.; van den Berg, R.; Sierksma, A.; Beulens, J.W.; Vaes, W.H.; Hendriks, H.F. Effect of red wine consumption on biomarkers of oxidative stress. Alcohol Alcohol. 2013, 48, 153–9. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Arranz, S.; Lamuela-Raventos, R.M.; Estruch, R. Effects of wine, alcohol and polyphenols on cardiovascular disease risk factors: Evidences from human studies. Alcohol Alcohol. 2013, 48, 270–7. [Google Scholar] [CrossRef] [PubMed]
- Mangge, H.; Becker, K.; Fuchs, D.; Gostner, J.M. Antioxidants, inflammation and cardiovascular disease. World J Cardiol 2014, 6, 462–477. [Google Scholar] [CrossRef] [PubMed]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; et al. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int J Mol Sci. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Habauzit, V.; Morand, C. Evidence for a protective effect of polyphenols-containing foods on cardiovascular health: an update for clinicians. Ther Adv Chronic Dis. 2012, 3, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L,; Marsella, L. T.; Carraro, A.; Valente, R.; Gualtieri, P.; Gratteri, S.; et al. Changes in LDL Oxidative Status and Oxidative and Inflammatory Gene Expression after Red Wine Intake in Healthy People: A Randomized Trial. Mediators Inflamm. 2015, 2015. [Google Scholar]
- Torres, A.; Cachofeiro, V.; Millán, J.; Lahera, V.; Nieto, M.L.; Martín, R.; Bello, E.; Alvarez-Sala, L.A. Red wine intake but not other alcoholic beverages increases total antioxidant capacity and improves pro-inflammatory profile after an oral fat diet in healthy volunteers. Rev Clin Esp (Barc). 2015, 215, 486–94. [Google Scholar] [CrossRef]
- Weseler, A.R.; Ruijters, E.J.B.; Drittij-Reijnders, M-J. ; Reesink, K.D.; Haenen, G.R.M.M.; Bast, A. Pleiotropic Benefit of Monomeric and Oligomeric Flavanols on Vascular Health - A Randomized Controlled Clinical Pilot Study. Plos One. 2011, 6, e28460. [Google Scholar] [CrossRef]
- Yang, H.; Xiao, L.; Yuan, Y.; Luo, X.; Jiang, M.; Ni, J.; et al. Procyanidin B2 inhibits NLRP3 inflammasome activation in human vascular endothelial cells. Biochem Pharmacol. 2014, 92, 599–606. [Google Scholar] [CrossRef]
- de Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999, 99, 779–85. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, J.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health benefits and molecular mechanisms of resveratrol: A narrative review. Foods 2020, 9, 340. [Google Scholar] [CrossRef]
- Khattar, S.; Khan, S.A.; Zaidi, S.A.A.; Darvishikolour, M.; Farooq, U.; Naseef, P.P.; Kurunian, M.S.; Khan, M.Z.; Shamim, A.; Khan, M.M.U.; Iqbal, Z.; Mirza, M.A. Resveratrol from Dietary Supplement to a Drug Candidate: An Assessment of Potential. Pharmaceuticals 2022, 15, 957. [Google Scholar] [CrossRef]
- Zhang, L.X.; Li, C.X.; Kakar, M.U.; Khan, M.S.; Wu, P.F.; Amir, R.M.; Dai, D.F.; Naveed, M.; Li, Q.Y.; Saeed, M.; Shen, J.Q.; Rajput, S.A.; Li, J.H. Resveratrol (RV): A pharmacological review and call for further research. Biomed Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Zam, W.; Kumar, M.; Cardoso, S.M.; Pereira, O.R.; Ademiluyi, A.O.; Adeleke, O.; Moreira, A.C.; Živković, J. Phenolic bioactives as antiplatelet aggregation factors: the pivotal ingredients in maintaining cardiovascular health. Oxid. Med. Cell. Longev., 2021, 2021, 2195902. [Google Scholar] [CrossRef]
- Parsamanesh, N.; Asghari, A.; Sardari, S.; Tasbandi, A.; Jamialahmadi, T.; Xu, S.; Sahebkar, A. Resveratrol and endothelial function: a literature review. Pharmacol. Res., 2021, 170, 105725. [Google Scholar] [CrossRef]
- Muñoz-Bernal, Ó.A.; Coria-Oliveros, A.J.; de la Rosa, L.A.; Rodrigo-García, J.; Del Rocío Martínez-Ruiz, N.; Sayago-Ayerdi, S.G.; Alvarez-Parrilla, E. Cardioprotective effect of red wine and grape pomace. Food Res Int. 2021, 140, 110069. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Socha, M.; Walczak, M.; Wódkiewicz, E.; Malinowski, B.; Rewerski, S.; Górski, K.; Pawlak-Osińska, K. Beneficial Effects of Resveratrol Administration-Focus on Potential Biochemical Mechanisms in Cardiovascular Conditions. Nutrients. 2018, 10, 1813. [Google Scholar] [CrossRef]
- Kuršvietienė, L.; Stanevičienė, I.; Mongirdienė, A.; Bernatonienė, J. Multiplicity of effects and health benefits of resveratrol. Medicina (Kaunas). 2016, 52, 148–55. [Google Scholar] [CrossRef]
- Riccioni, G.; Gammone, M.A.; Tettamanti, G.; Bergante, S.; Pluchinotta, F.R.; D'Orazio, N. Resveratrol and anti-atherogenic effects. Int J Food Sci Nutr. 2015, 66, 603–10. [Google Scholar] [CrossRef] [PubMed]
- Tamer, F.; Tullemans, B.M.E.; Kuijpers, M.J.E.; Claushuis, T.A.M.; Heemskerk, J.W.M. Nutrition Phytochemicals Affecting Platelet Signaling and Responsiveness: Implications for Thrombosis and Hemostasis. Thromb Haemost. 2022, 122, 879–894. [Google Scholar] [CrossRef] [PubMed]
- Shahcheraghi, S.H.; Salemi, F.; Small, S.; Syed, S.; Salari, F.; Alam, W.; et al. Resveratrol regulates inflammation and improves oxidative stress via Nrf2 signaling pathway: Therapeutic and biotechnological prospects. Phytother Res. 2023. [Google Scholar] [CrossRef]
- Rius, C.; Abu-Taha, M.; Hermenegildo, C.; et al. , “Trans- but Not Cis-Resveratrol impairs angiotensin-II-mediated vascular inflammation through inhibition of NF-κB activation and peroxisome proliferator-activated Receptor-γ upregulation,” J Immunol 2010, 185, 3718–3727.
- Csiszar, A.; Smith, K.; Labinskyy, N.; Orosz, Z.; Rivera, A.; Ungvari, Z. Resveratrol attenuates TNF-alpha-induced activation of coronary arterial endothelial cells: role of NF-kappaB inhibition. Am J Physiol Heart Circ Physiol. 2006, 291, H1694–9. [Google Scholar] [CrossRef]
- Toaldo, I.M.; Van Camp, J.; Gonzales, G.B.; Kamiloglu, S.; Bordignon-Luiz, M.T.; Smagghe, G.; et al. Resveratrol improves TNF-α-induced endothelial dysfunction in a coculture model of a Caco-2 with an endothelial cell line. J Nutr Biochem. 2016, 36, 21–30. [Google Scholar] [CrossRef]
- Chalons, P.; Amor, S.; Courtaut, F.; Cantos-Villar, E.; Richard, T.; Auger, C.; et al. Study of potential anti-inflammatory effects of red wine extract and resveratrol through a modulation of interleukin-1-beta in macrophages. Nutrients. 2018, 10. [Google Scholar] [CrossRef]
- Fukuda, M.; Ogasawara, Y.; Hayashi, H.; Inoue, K.; Sakashita, H. Resveratrol Inhibits Proliferation and Induces Autophagy by Blocking SREBP1 Expression in Oral Cancer Cells. Molecules. 2022, 27. [Google Scholar] [CrossRef]
- Zucchi, A.; Claps, F.; Pastore, A.L.; Perotti, A.; Biagini, A.; Sallicandro, L.; Gentile, R.; Caglioti, C.; Palazzetti, F.; Fioretti, B. Focus on the Use of Resveratrol in Bladder Cancer. Int J Mol Sci. 2023, 24, 4562. [Google Scholar] [CrossRef]
- Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Beneficial Effects of Red Wine Polyphenols on Human Health: Comprehensive Review. Curr Issues Mol Biol. 2023, 45, 782–798. [Google Scholar] [CrossRef]
- Chimento, A.; D'Amico, M.; De Luca, A.; Conforti, F.L.; Pezzi, V.; De Amicis, F. Resveratrol, Epigallocatechin Gallate and Curcumin for Cancer Therapy: Challenges from Their Pro-Apoptotic Properties. Life 2023, 13, 261. [Google Scholar] [CrossRef]
- Lalani, A.R.; Fakhari, F.; Radgoudarzi, S.; Rastegar-Pouyani, N.; Moloudi, K.; Khodamoradi, E.; Taeb, S.; Najafi, M. Immunoregulation by resveratrol; implications for normal tissue protection and tumour suppression. Clin Exp Pharmacol Physiol. 2023, 50, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Angellotti, G.; Di Prima, G.; Belfiore, E.; Campisi, G.; De Caro, V. Chemopreventive and Anticancer Role of Resveratrol against Oral Squamous Cell Carcinoma. Pharmaceutics. 2023, 15, 275. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.S.; Gadi, V.; Kaur, G.; Chintamaneni, M.; Tuli, H.; Ramniwas, S.; Sethi, G. Resveratrol and Its Role in the Management of B-Cell Malignancies-A Recent Update. Biomedicines. 2023, 11, 221. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Fan, F.; Hou, X.; Gao, C.; Meng, L.; Meng, S.; Huang, S.; Wu, H. Resveratrol suppresses pulmonary tumor metastasis by inhibiting platelet-mediated angiogenic responses. J Surg Res. 2017, 217, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Bae, J.U.; Kim, I.S.; Chang, C.L.; Oh, S.O.; Kim, C.D. SIRT1 prevents pulmonary thrombus formation induced by arachidonic acid via downregulation of PAF receptor expression in platelets. Platelets. 2016, 27, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Michno, A.; Grużewska, K.; Ronowska, A.; Gul-Hinc, S.; Zyśk, M.; Jankowska-Kulawy, A. Resveratrol Inhibits Metabolism and Affects Blood Platelet Function in Type 2 Diabetes. Nutrients. 2022, 14, 1633. [Google Scholar] [CrossRef] [PubMed]
- Crescente, M.; Jessen, G.; Momi, S.; et al. , “Interactions of gallic acid, resveratrol, quercetin and aspirin at the platelet cyclooxygenase-1 level. Functional and modelling studies. Thromb Haem, 2009, 102, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Marumo, M.; Ekawa, K.; Wakabayashi, I. Resveratrol inhibits Ca2+ signals and aggregation of platelets. Environ Health Prev Med. 2020, 25, 70. [Google Scholar] [CrossRef] [PubMed]
- Vlachogianni, I.C.; Fragopoulou, E.; Stamatakis, G.M.; Kostakis, I.K.; Antonopoulou, S. Platelet Activating Factor (PAF) biosynthesis is inhibited by phenolic compounds in U-937 cells under inflammatory conditions. Prostaglandins Other Lipid Mediat. 2015, 121, 176–83. [Google Scholar] [CrossRef] [PubMed]
- Eräsalo, H.; Hämäläinen, M.; Leppänen, T.; Mäki-Opas, I.; Laavola, M.; Haavikko, R.; Yli-Kauhaluoma, J.; Moilanen, E. Natural Stilbenoids Have Anti-Inflammatory Properties in Vivo and Down-Regulate the Production of Inflammatory Mediators NO, IL6, and MCP1 Possibly in a PI3K/Akt-Dependent Manner. J Nat Prod. 2018, 81, 1131–1142. [Google Scholar] [CrossRef]
- Dutra, L.A.; Guanaes, J.F.O.; Johmann, N.; Lopes Pires, M.E.; Chin, C.M.; Marcondes, S.; Dos Santos, J.L. Synthesis, antiplatelet and antithrombotic activities of resveratrol derivatives with NO-donor properties. Bioorg. Med. Chem. Lett. 2017, 27, 2450–2453. [Google Scholar] [CrossRef]
- Deng, Y.-H.; Alex, D.; Huang, H.-Q.; et al. Inhibition of TNF-α-mediated endothelial cell-monocyte cell adhesion and adhesion molecules expression by the resveratrol derivative, trans-3,5,4'-trimethoxystilbene. Phytotherapy Research. 2011, 25, 451–457. [Google Scholar] [CrossRef]
- Nash, V.; Ranadheera, C.S.; Georgousopoulou, E.N.; Mellor, D.D.; Panagiotakos, D.B.; McKune, A.J.; et al. The effects of grape and red wine polyphenols on gut microbiota – A systematic review. Food Res Internat. 2018, 113, 277–87. [Google Scholar] [CrossRef]
- Dueñas, M.; Cueva, C.; Muñoz-González, I.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; et al. Studies on Modulation of Gut Microbiota by Wine Polyphenols: From Isolated Cultures to Omic Approaches. Antioxidants. 2015, 4, 1–21. [Google Scholar] [CrossRef]
- O'Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef]
- Haas, E.A.; Saad, M.J.A.; Santos, A.; Vitulo, N.; Lemos, W.J.F.; Martins, A.M.A.; et al. A red wine intervention does not modify plasma trimethylamine N-oxide but is associated with broad shifts in the plasma metabolome and gut microbiota composition. Am J Clin Nutr. 2022, 116, 1515–29. [Google Scholar] [CrossRef]
- Suo, H.; Shishir, M.R.I.; Xiao, J.; Wang, M.; Chen, F.; Cheng, K-W. Red Wine High-Molecular-Weight Polyphenolic Complex: An Emerging Modulator of Human Metabolic Disease Risk and Gut Microbiota. J Agr Food Chem. 2021, 69, 10907–19. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Yin, N.; Wele, P.; Li, F.; Dave, S.; et al. Resveratrol in disease prevention and health promotion: A role of the gut microbiome. Crit Rev Food Sci Nutr. 2023, 1–18. [Google Scholar] [CrossRef]
- Tsoupras, A.; Brummell, C.; Kealy, C.; Vitkaitis, K.; Redfern, S.; Zabetakis, I. Cardio-Protective Properties and Health Benefits of Fish Lipid Bioactives; The Effects of Thermal Processing. Mar Drugs. 2022, 20, 187. [Google Scholar] [CrossRef]
- Nunez, D.; Randon, J.; Gandhi, C.; Siafaka-Kapadai, A.; Olson, M.S.; Hanahan, D.J. The inibition of platelet-activating factor-induced platelet activation by oleic acid is associated with a decrease in polyphosphoinositide metabolism. J. Biol. Chem 1990, 265, 18330–18338. [Google Scholar] [CrossRef]
- Perdomo, L.; Beneit, N.; Otero, Y.F.; Escribano, O.; Diaz-Castroverde, S.; Gómez-Hernández, A.; Benito, M. Protective role of oleic acid against cardiovascular insulin resistance and in the early and late cellular atherosclerotic process. Cardiovasc. Diabetol. 2015, 14, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Delgado, G.E.; Krämer, B.K.; Lorkowski, S.; März, W.; Von Schacky, C.; Kleber, M.E. Individual omega-9 monounsaturated fatty acids and mortality—The Ludwigshafen Risk and Cardiovascular Health Study. J. Clin. Lipidol. 2017, 11, 126–135. [Google Scholar] [CrossRef]
- Holy, E.W.; Forestier, M.; Richter, E.K.; Akhmedov, A.; Leiber, F.; Camici, G.C.; Mocharla, P.; Lüscher, T.F.; Beer, J.H.; Tanner, F.C. Dietary α-linolenic acid inhibits arterial thrombus formation, tissue factor expression, and platelet activation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Bazán-Salinas, I.L.; Matías-Pérez, D.; Pérez-Campos, E.; Pérez-Campos Mayoral, L.; García-Montalvo, I.A. Reduction of platelet aggregation from ingestion of oleic and linoleic acids found in Vitis vinifera and Arachis hypogaea Oils. Am. J. Ther. 2016, 23, e1315–e1319. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Rombaut, N.; Savoire, R.; Thomasset, B.; Castello, J.; Van Hecke, E.; Lanoisellé, J.-L. Optimization of oil yield and oil total phenolic content during grape seed cold screw pressing. Industr Crops Prod 2015, 63, 26–33. [Google Scholar] [CrossRef]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadenti, A. ;. Grape Seed Oil Compounds: Biological and Chemical Actions for Health. Nutr Metab Insights 2016, 9, NMI–S32910. [Google Scholar] [CrossRef]
- Matthäus, B. . Virgin grape seed oil: Is it really a nutritional highlight? Europ J Lipid Sci Techn 2008, 110, 645–650. [Google Scholar] [CrossRef]
- Moran, D.; Fleming, M.; Daly, E.; Gaughan, N.; Zabetakis, I.; Traas, C.; Tsoupras, A. Anti-Platelet Properties of Apple Must/Skin Yeasts and of Their Fermented Apple Cider Products. Beverages, 2021, 7, 54. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Harrington, J.; Pienaar, R.; Devaney, K.; Heaney, S.; Koidis, A.; Zabetakis, I. The Effects of Oxidation on the Antithrombotic Properties of Tea Lipids against PAF, Thrombin, Collagen, and ADP. Foods 2020, 9, 385. [Google Scholar] [CrossRef]
- Janssen, I.; Landay, A.L; Ruppert, K.; Powell, L.H. Moderate wine consumption is associated with lower hemostatic and inflammatory risk factors over 8 years: The study of women's health across the nation (SWAN). Nutr Aging. 2014, 2, 91–9. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Kouli, G-M. ; Magriplis, E.; Kyrou, I.; Georgousopoulou, E.N.; Chrysohoou, C.; et al. Beer, wine consumption, and 10-year CVD incidence: the ATTICA study. Eur J Clin Nutr. 2019, 73, 1015–23. [Google Scholar] [CrossRef] [PubMed]
- Taborsky, M.; Ostadal, P.; Adam, T.; Moravec, O.; Gloger, V.; Schee, A.; et al. Red or white wine consumption effect on atherosclerosis in healthy individuals (In Vino Veritas study). Bratisl Lek Listy. 2017, 118, 292–8. [Google Scholar] [CrossRef] [PubMed]
- Salazar, H.M.; de Deus Mendonça, R.; Laclaustra, M.; Moreno-Franco, B.; Åkesson, A.; Guallar-Castillón, P.; et al. The intake of flavonoids, stilbenes, and tyrosols, mainly consumed through red wine and virgin olive oil, is associated with lower carotid and femoral subclinical atherosclerosis and coronary calcium. Eur J Nutr. 2022, 61, 2697–709. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Llorach, R.; Rotches-Ribalta, M.; Guillén, M.; Casas, R.; et al. Differential effects of polyphenols and alcohol of red wine on the expression of adhesion molecules and inflammatory cytokines related to atherosclerosis: a randomized clinical trial. Am J Clin Nutr. 2011, 95, 326–34. [Google Scholar] [CrossRef]
- Canali, R.; Comitato, R.; Ambra, R.; Virgili, F. Red wine metabolites modulate NF-κB, activator protein-1 and cAMP response element-binding proteins in human endothelial cells. Br J Nutr. 2010, 103, 807–14. [Google Scholar] [CrossRef]
- Nallasamy, P.; Kang, Z.Y.; Sun, X.; Anandh Babu, P.V.; Liu, D.; Jia, Z. Natural compound resveratrol attenuates TNF-alpha-induced vascular dysfunction in mice and human endothelial cells: The involvement of the NF-κB signaling pathway. Int J Mol Sci. 2021, 22, 22. [Google Scholar] [CrossRef] [PubMed]
- Kechagias, S.; Zanjani, S.; Gjellan, S.; Leinhard, O.D.; Kihlberg, J.; Smedby, Ö.; et al. Effects of moderate red wine consumption on liver fat and blood lipids: a prospective randomized study. Ann Med. 2011, 43, 545–54. [Google Scholar] [CrossRef]
- Roth, I.; Casas, R.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Consumption of aged white wine modulates cardiovascular risk factors via circulating endothelial progenitor cells and inflammatory biomarkers. Clin Nutr. 2019, 38, 1036–44. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.H.; Chen, Y.H.; Tsai, H.Y.; Chen, J.S.; Wu, T.C.; Lin, F.Y.; et al. Intake of Red Wine Increases the Number and Functional Capacity of Circulating Endothelial Progenitor Cells by Enhancing Nitric Oxide Bioavailability. Art Thromb Vasc Biol. 2010, 30, 869–77. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; Yáñez-Gascón, M.J.; García-Almagro, F.J.; Ruiz-Ros, J.A.; et al. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: A triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc Drugs Ther. 2013, 27, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Cosmi, F.; Di Giulio, P.; Masson, S.; Finzi, A.; Marfisi, R.M.; Cosmi, D.; et al. Regular Wine Consumption in Chronic Heart Failure: Impact on Outcomes, Quality of Life, and Circulating Biomarkers. Circ Heart Fail. 2015, 8, 428–37. [Google Scholar] [CrossRef]
- Downer, M.K.; Kenfield, S.A.; Stampfer, M.J.; Wilson, K.M.; Dickerman, B.A.; Giovannucci, E.L.; Rimm, E.B.; Wang, M.; Mucci, L.A.; Willett, W.C.; Chan, J.M.; Van Blarigan, E.L. Alcohol Intake and Risk of Lethal Prostate Cancer in the Health Professionals Follow-Up Study. J Clin Oncol. 2019, 37, 1499–1511. [Google Scholar] [CrossRef]
- Crockett, S.D.; Long, M.D.; Dellon, E.S.; Martin, C.F.; Galanko, J.A.; Sandler, R.S. Inverse relationship between moderate alcohol intake and rectal cancer: analysis of the North Carolina Colon Cancer Study. Dis Colon Rectum. 2011, 54, 887–94. [Google Scholar] [CrossRef]
- Liu, Z.; Song, C.; Suo, C.; Fan, H.; Zhang, T.; Jin, L.; Chen, X. Alcohol consumption and hepatocellular carcinoma: novel insights from a prospective cohort study and nonlinear Mendelian randomization analysis. BMC Med. 2022, 20, 413. [Google Scholar] [CrossRef]
- Wang, X.; Jia, M.; Mao, Y.; Jia, Z.; Liu, H.; Yang, G.; Wang, S.; Sun, B.; Zhang, H. Very-light alcohol consumption suppresses breast tumor progression in a mouse model. Food Funct. 2022, 13, 3391–3404. [Google Scholar] [CrossRef]
- Schaefer, S.M.; Kaiser, A.; Behrendt, I.; Eichner, G.; Fasshauer, M. Association of alcohol types, coffee and tea intake with mortality: prospective cohort study of UK Biobank participants. Br J Nutr. 2022, 129, 1–11. [Google Scholar] [CrossRef]
- Turati, F.; Carioli, G.; Bravi, F.; Ferraroni, M.; Serraino, D.; Montella, M.; et al. Mediterranean Diet and Breast Cancer Risk. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Shufelt, C.; Merz, C.N.; Yang, Y.; Kirschner, J.; Polk, D.; Stanczyk, F.; Paul-Labrador, M.; Braunstein, D. Red versus white wine as a nutritional aromatase inhibitor in premenopausal women: a pilot study. J Womens Health. 2012, 21, 281–4. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Mellinger, J.L.; Trivedi, P.J. Alcohol Consumption in Patients with Non-alcoholic Fatty Liver Disease: Convenient vs. Inconvenient Truths. Am J Gastroenterol. 2018, 113, 1437–1439. [Google Scholar] [CrossRef]
- Zhu, W.; Qin, W.; Zhang, K.; Rottinghaus, G.E.; Chen, Y.C.; Kliethermes, B.; et al. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr Cancer. 2012, 64, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Bulló, M.; Salas-Salvadó, J.; Corella, D.; et al. Moderate red wine consumption is associated with a lower prevalence of the metabolic syndrome in the PREDIMED population. Br J Nutr. 2015, 113, S121–30. [Google Scholar] [CrossRef]
- Larsen, B.A.; Klinedinst, B.S.; Le, S.T.; Pappas, C.; Wolf, T.; Meier, N.F.; Lim, Y.L.; Willette, A.A. Beer, wine, and spirits differentially influence body composition in older white adults-a United Kingdom Biobank study. Obes Sci Pract. 2022, 8, 641–656. [Google Scholar] [CrossRef]
- Gepner, Y.; Golan, R.; Harman-Boehm, I.; Henkin, Y.; Schwarzfuchs, D.; Shelef, I.; Durst, R.; Kovsan, J.; Bolotin, A.; Leitersdorf, E.; Shpitzen, S.; Balag, S.; Shemesh, E.; Witkow, S.; Tangi-Rosental, O.; Chassidim, Y.; Liberty, I.F.; Sarusi, B.; Ben-Avraham, S.; Helander, A.; Ceglarek, U.; Stumvoll, M.; Blüher, M.; Thiery, J.; Rudich, A.; Stampfer, M.J.; Shai, I. Effects of Initiating Moderate Alcohol Intake on Cardiometabolic Risk in Adults With Type 2 Diabetes: A 2-Year Randomized, Controlled Trial. Ann Intern Med. 2015, 163, 569–79. [Google Scholar] [CrossRef] [PubMed]
- Golan, R.; Shai, I.; Gepner, Y.; Harman-Boehm, I.; Schwarzfuchs, D.; Spence, J.D.; Parraga, G.; Buchanan, D.; Witkow, S.; Friger, M.; Liberty, I.F.; Sarusi, B.; Ben-Avraham, S.; Sefarty, D.; Bril, N.; Rein, M.; Cohen, N.; Ceglarek, U.; Thiery, J.; Stumvoll, M.; Blüher, M.; Stampfer, M.J.; Rudich, A.; Henkin, Y. Effect of wine on carotid atherosclerosis in type 2 diabetes: a 2-year randomized controlled trial. Eur J Clin Nutr. 2018, 72, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, X.; Li, X.; Heianza, Y.; Qi, L. Moderate alcohol drinking with meals is related to lower incidence of type 2 diabetes. Am J Clin Nutr. 2022, 116, 1507–14. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Valderas-Martinez, P.; Casas, R.; Arranz, S.; et al. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: a randomized clinical trial. Clin Nutr. 2013, 32, 200–6. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Stagling, S.; Lundberg, A.; Nystrom, F.H. A cross-over study of postprandial effects from moist snuff and red wine on metabolic rate, appetite-related hormones and glucose. Drug Alcohol Depend. 2022, 236, 109479. [Google Scholar] [CrossRef] [PubMed]
- Sattarinezhad, A.; Roozbeh, J.; Shirazi Yeganeh, B.; Omrani, G.R.; Shams, M. Resveratrol reduces albuminuria in diabetic nephropathy: A randomized double-blind placebo-controlled clinical trial. Diabetes Metab. 2019, 45, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Liu, Y.; Ren, G.; Yin, L.; Liang, X.; Geng, T.; Dang, H.; An, R. Resveratrol limits diabetes-associated cognitive decline in rats by preventing oxidative stress and inflammation and modulating hippocampal structural synaptic plasticity. Brain Res. 2016, 1650, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Melo van Lent, D.; Wolfsgruber, S.; Weinhold, L.; Kleineidam, L.; Bickel, H.; Scherer, M.; Eisele, M.; van den Bussche, H.; Wiese, B.; König, H.H.; Weyerer, S.; Pentzek, M.; Röhr, S.; Maier, W.; Jessen, F.; Schmid, M.; Riedel-Heller, S.G.; Wagner, M. Prospective Associations between Single Foods, Alzheimer's Dementia and Memory Decline in the Elderly. Nutrients. 2018, 10, 852. [Google Scholar] [CrossRef] [PubMed]
- Mendes, D.; Oliveira, M.M.; Moreira, P.I.; Coutinho, J.; Nunes, F.M.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Videira, R.A. Beneficial effects of white wine polyphenols-enriched diet on Alzheimer's disease-like pathology. J Nutr Biochem. 2018, 55, 165–177. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Wan, Y.; Tan, C.; Li, J.; Tan, L.; Yu, J.T. Alcohol consumption and dementia risk: a dose-response meta-analysis of prospective studies. Eur J Epidemiol. 2017, 32, 31–42. [Google Scholar] [CrossRef]
- Ho, L.; Ferruzzi, M.G.; Janle, E.M.; Wang, J.; Gong, B.; Chen, T.Y.; Lobo, J.; Cooper, B.; Wu, Q.L.; Talcott, S.T.; Percival, S.S.; Simon, J.E.; Pasinetti, G.M. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer's disease. FASEB J. 2013, 27, 769–81. [Google Scholar] [CrossRef]
- Valls-Pedret, C.; Lamuela-Raventós, R.M.; Medina-Remón, A.; Quintana, M.; Corella, D.; Pintó, X.; Martínez-González, M.Á.; Estruch, R.; Ros, E. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J Alzheimers Dis. 2012, 29, 773–82. [Google Scholar] [CrossRef]
- Smyth, A.; O'Donnell, M.; Rangarajan, S.; Hankey, G.J.; Oveisgharan, S.; Canavan, M.; McDermott, C.; Xavier, D.; Zhang, H.; Damasceno, A.; Avezum, A.; Pogosova, N.; Oguz, A.; Ryglewicz, D.; Iversen, H.K.; Lanas, F.; Rosengren, A.; Yusuf, S.; Langhorne, P. INTERSTROKE Investigators. Alcohol Intake as a Risk Factor for Acute Stroke: The INTERSTROKE Study. Neurology. 2023, 100, e142–e153. [Google Scholar] [CrossRef] [PubMed]
- Kaluza, J.; Harris, H.R.; Linden, A.; Wolk, A. Alcohol Consumption and Risk of Chronic Obstructive Pulmonary Disease: A Prospective Cohort Study of Men. Am J Epidemiol. 2019, 188, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Castellano, S.; Ray, S.; Grosso, G.; Galvano, F. ; Dietary Polyphenol Intake and Depression: Results from the Mediterranean Healthy Eating, Lifestyle and Aging (MEAL) Study. Molecules. 2018, 23, 999. [Google Scholar] [CrossRef] [PubMed]
- Barbería-Latasa, M.; Bes-Rastrollo, M.; Pérez-Araluce, R.; Martínez-González, M.Á.; Gea, A. Mediterranean Alcohol-Drinking Patterns and All-Cause Mortality in Women More Than 55 Years Old and Men More Than 50 Years Old in the "Seguimiento Universidad de Navarra" (SUN) Cohort. Nutrients. 2022, 14, 5310. [Google Scholar] [CrossRef] [PubMed]
- Carballo-Casla, A.; Ortolá, R.; García-Esquinas, E.; Oliveira, A.; Sotos-Prieto, M.; Lopes, C.; Lopez-Garcia, E.; Rodríguez-Artalejo, F. The Southern European Atlantic Diet and all-cause mortality in older adults. BMC Med. 2021, 19, 36. [Google Scholar] [CrossRef]
- Grønbaek, M.; Becker, U.; Johansen, D.; Gottschau, A.; Schnohr, P.; Hein, H.O.; et al. Type of alcohol consumed and mortality from all causes, coronary heart disease, and cancer. Ann Intern Med. 2000, 133, 411–9. [Google Scholar] [CrossRef] [PubMed]
- Noguer, M.A.; Cerezo, A.B.; Donoso Navarro, E.; Garcia-Parrilla, M.C. Intake of alcohol-free red wine modulates antioxidant enzyme activities in a human intervention study. Pharmacol Res. 2012, 65, 609–14. [Google Scholar] [CrossRef]
- Giovannucci, E.; Stampfer, M.J.; Colditz, G.A.; Manson, J.E.; Rosner, B.A.; Longnecker, M.P.; Speizer, F.E.; Willett, W.C. ; Recall and selection bias in reporting past alcohol consumption among breast cancer cases. Cancer Causes Control. 1993, 4, 441–8. [Google Scholar] [CrossRef]
- Wu, S.; Li, W.Q.; Qureshi, A.A.; Cho, E. Alcohol consumption and risk of cutaneous basal cell carcinoma in women and men: 3 prospective cohort studies. Am J Clin Nutr. 2015, 102, 1158–66. [Google Scholar] [CrossRef]
- Papadimitriou, N.; Bouras, E.; van den Brandt, P.A.; Muller, D.C.; Papadopoulou, A.; Heath, A.K.; et al. A Prospective Diet-Wide Association Study for Risk of Colorectal Cancer in EPIC. Clin Gastroenterol Hepatol. 2022, 20, 864–873.e13. [Google Scholar] [CrossRef] [PubMed]
- Mahamat-Saleh, Y.; Al-Rahmoun, M.; Severi, G.; Ghiasvand, R.; Veierod, M.B.; Caini, S.; Palli, D.; Botteri, E,; et al. Baseline and lifetime alcohol consumption and risk of skin cancer in the European Prospective Investigation into Cancer and Nutrition cohort (EPIC). Int J Cancer. 2023, 152, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Heath, A.K.; Muller, D.C.; van den Brandt, P.A.; Papadimitriou, N.; Critselis, E.; et al. Nutrient-wide association study of 92 foods and nutrients and breast cancer risk. Breast Cancer Res. 2020, 22, 5. [Google Scholar] [CrossRef] [PubMed]
- Naudin, S.; Li, K.; Jaouen, T.; Assi, N.; Kyrø, C.; Tjønneland, A.; Overvad, K.; Boutron-Ruault, M.C.; et al. Lifetime and baseline alcohol intakes and risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition study. Int J Cancer. 2018, 143, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Seidenberg, A.B.; Wiseman, K.P.; Klein, W.M.P. Do Beliefs about Alcohol and Cancer Risk Vary by Alcoholic Beverage Type and Heart Disease Risk Beliefs? Cancer Epidemiol Biomarkers Prev. 2023, 32, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Hay, J.L.; Kiviniemi, M.T.; Orom, H.; Waters, E.A. Moving beyond the "Health Halo" of Alcohol: What Will it Take to Achieve Population Awareness of the Cancer Risks of Alcohol? Cancer Epidemiol Biomarkers Prev. 2023, 32, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Duell, E.J.; Travier, N.; Lujan-Barroso, L.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; Morois, S.; et al. Alcohol consumption and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Am J Clin Nutr. 2011, 94, 1266–75. [Google Scholar] [CrossRef] [PubMed]
- Cote, D.J.; Smith, T.R.; Kaiser, U.B.; Laws, E.R. Jr.; Stampfer, M.J. Alcohol intake and risk of pituitary adenoma. Cancer Causes Control. 2022, 33, 353–361. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Liu, Y.L.; Zheng, Y.; Ivey, K.; Willett, W.C.; Stampfer, M.J.; Rimm, E.B.; Cassidy, A. Change in habitual intakes of flavonoid-rich foods and mortality in US males and females. BMC Med. 2023, 21, 181. [Google Scholar] [CrossRef]
- Duan, J.; Guo, H.; Fang, Y.; Zhou, G. The mechanisms of wine phenolic compounds for preclinical anticancer therapeutics. Food Nutr Res. 2021, 65. [Google Scholar] [CrossRef]
- O'Keefe, J.H.; Bybee, K.A.; Lavie, C.J. Alcohol and cardiovascular health: the razor-sharp double-edged sword. J Am Coll Cardiol. 2007, 50, 1009–14. [Google Scholar] [CrossRef] [PubMed]
- O'Keefe, E.L.; DiNicolantonio, J.J.; O'Keefe, J.H.; Lavie, C.J. Alcohol and CV Health: Jekyll and Hyde J-Curves. Prog Cardiov Dis. 2018, 61, 68–75. [Google Scholar] [CrossRef]
- Poli, A. Is drinking wine in moderation good for health or not? Eur Heart J Suppl. 2022, 24, I119–I122. [Google Scholar] [CrossRef] [PubMed]
- Rifler, J.P. Is a Meal without Wine Good for Health? Diseases. 2018, 6, 105. [Google Scholar] [CrossRef]
- Gerardi, G.; Cavia-Saiz, M.; Muñiz, P. From winery by-product to healthy product: bioavailability, redox signaling and oxidative stress modulation by wine pomace product. Crit Rev Food Sci Nutr. 2022, 62, 7427–7448. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wen, J.Y.; Deng, Z.X.; Pan, X.Q.; Xie, X.F.; Peng, C. Effective utilization of food wastes: Bioactivity of grape seed extraction and its application in food industry. J. Funct. Foods 2020, 73, 104113. [Google Scholar] [CrossRef]
- Yang, C.; Han, Y.; Tian, X.; Sajid, M.; Mehmood, S.; Wang, H.; Li, H. Phenolic composition of grape pomace and its metabolism. Crit Rev Food Sci Nutr. 2022, 17, 1–17. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. ;. The Potential of Grape Pomace Varieties as a Dietary Source of Pectic Substances. Foods 2021, 10, 867. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; and Napolitano, A. Bioactive Phenolic Compounds From Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef]
- Ilyas, T.; Chowdhary, P.; Chaurasia, D.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Sustainable green processing of grape pomace for the production of value-added products: An overview. Envir Tech Innov 2021, 23, 101592. [Google Scholar] [CrossRef]
- Chowdhary, P.; Gupta, A.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Current trends and possibilities for exploitation of Grape pomace as a potential source for value addition. Envir Pollution 2021, 278. [Google Scholar] [CrossRef]
- Drevelegka, I.; Goula, A.M. Recovery of grape pomace phenolic compounds through optimized extraction and adsorption processes. Chem Engin Proc - Process Intens 2020, 149, 107845. [Google Scholar] [CrossRef]
- Barba, F.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci Tech 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Jokić, S.; Velić, D.; Bilić, M.; Bucić-Kojić, A.; Planinić, M.; Tomas, S. Modelling of the Process of Solid-Liquid Extraction of Total Polyphenols from Soybeans. Czech J. Food Sci. 2010, 28, 7. [Google Scholar] [CrossRef]
- Yang, C.L.; Shang, K.; Lin, C.C.; Wang, C.; Shi, X.Q.; Wang, H.; Li, H. Processing technologies, phytochemical constituents, and biological activities of grape seed oil (gso): A review. Trends Food Sci. Technol. 2021, 116, 1074–1083. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical fluid extraction: Recent advances and applications. J Chromatogr A, Extraction Techniques 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [PubMed]
- Pinelo, M.; Ruiz-Rodríguez, A.; Sineiro, J.; Señoráns, F.J.; Reglero, G.; Núñez, M.J. Supercritical fluid and solid–liquid extraction of phenolic antioxidants from grape pomace: a comparative study. Eur Food Res Technol 2007, 226, 199–205. [Google Scholar] [CrossRef]
- Jeong, J.; Jung, H.; Lee, S.; Lee, H.; Hwang, K.; Kim, T. Anti-oxidant, anti-proliferative and anti-inflammatory activities of the extracts from black raspberry fruits and wine. Food Chem 2010, 123, 338–344. [Google Scholar] [CrossRef]
- Casas, L.; Mantell, C.; Rodríguez, M.; Ossa, E.J.M. de la; Roldán, A.; Ory, I.D.; Caro, I.; Blandino, A. Extraction of resveratrol from the pomace of Palomino fino grapes by supercritical carbon dioxide. J Food Engin 2010, 96, 304–308. [Google Scholar] [CrossRef]
- Liazid, A.; Guerrero, R.F.; Cantos, E.; Palma, M.; Barroso, C.G. Microwave assisted extraction of anthocyanins from grape skins. Food Chem 2011, 124, 1238–1243. [Google Scholar] [CrossRef]
- Yu, H.B.; Ding, L.F.; Wang, Z.; Shi, L.X. Study on Extraction of Polyphenol from Grape Peel Microwave-Assisted Activity. Adv Mater Res 2014, 864–867, 520–525. [Google Scholar] [CrossRef]
- Al Bittar, S.; Périno-Issartier, S.; Dangles, O.; Chemat, F. An innovative grape juice enriched in polyphenols by microwave-assisted extraction. Food Chem 2013, 141, 3268–3272. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, C.; Porretto, E.; Decorti, D. Comparison of ultrasound-assisted extraction with conventional extraction methods of oil and polyphenols from grape (Vitis vinifera L.) seeds. Ultrasonics Sonochem 2013, 20, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Industrial Crops and Products, Advances in Industrial Crops and Products Worldwide: AAIC 2014 international conference 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Wang, J.; Wang, K.; Wang, Y.; Lin, S.; Zhao, P.; Jones, G. A novel application of pulsed electric field (PEF) processing for improving glutathione (GSH) antioxidant activity. Food Chem 2014, 161, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Jeyamkondan, S.; Jayas, D.S.; Holley, R.A. Pulsed electric field processing of foods: a review. J Food Prot 1999, 62, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Brianceau, S.; Turk, M.; Vitrac, X.; Vorobiev, E. Combined densification and pulsed electric field treatment for selective polyphenols recovery from fermented grape pomace. Innov Food Sci Emerg Tech, App Food Process 2015, 29, 2–8. [Google Scholar] [CrossRef]
- Wan, J.; Coventry, J.; Swiergon, P.; Sanguansri, P.; Versteeg, C. Advances in innovative processing technologies for microbial inactivation and enhancement of food safety – pulsed electric field and low-temperature plasma. Trends Food Sci Tech, Natural Safe Foods 2009, 20, 414–424. [Google Scholar] [CrossRef]
- Barba, F.J.; Brianceau, S.; Turk, M.; Boussetta, N.; Vorobiev, E. Effect of Alternative Physical Treatments (Ultrasounds, Pulsed Electric Fields, and High-Voltage Electrical Discharges) on Selective Recovery of Bio-compounds from Fermented Grape Pomace. Food Bioprocess Technol 2015, 8, 1139–1148. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov Food Sci Emerg Tech 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Dordoni, R.; Duserm Garrido, G.; Marinoni, L.; Torri, L.; Piochi, M.; Spigno, G. Enrichment of Whole Wheat Cocoa Biscuits with Encapsulated Grape Skin Extract. Int J Food Sci 2019, 2019, 9161840. [Google Scholar] [CrossRef]
- Pasqualone, A.; Bianco, A.M.; Paradiso, V.M. Production trials to improve the nutritional quality of biscuits and to enrich them with natural anthocyanins. CyTA – J Food 2013, 11, 301–308. [Google Scholar] [CrossRef]
- Mildner-Szkudlarz, S.; Bajerska, J.; Zawirska-Wojtasiak, R.; Górecka, D. White grape pomace as a source of dietary fibre and polyphenols and its effect on physical and nutraceutical characteristics of wheat biscuits. J. Sci. Food Agric. 2013, 93, 389–395. [Google Scholar] [CrossRef]
- Acun, S.; Gül, H. Effects of grape pomace and grape seed flours on cookie quality. Qual Assur Saf Crops Foods 2014, 6, 81–88. [Google Scholar] [CrossRef]
- Bender, A.B.B.; Speroni, C.S.; Salvador, P.R.; Loureiro, B.B.; Lovatto, N.M.; Goulart, F.R.; Lovatto, M.T.; Miranda, M.Z.; Silva, L.P.; Penna, N.G. Grape Pomace Skins and the Effects of Its Inclusion in the Technological Properties of Muffins. J Culinary Sci Tech 2017, 15, 143–157. [Google Scholar] [CrossRef]
- Šporin, M.; Avbelj, M.; Kovač, B.; Možina, S.S. Quality characteristics of wheat flour dough and bread containing grape pomace flour. Food Sci. Technol. Int. 2018, 24, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Tolve, R.; Simonato, B.; Rainero, G.; Bianchi, F.; Rizzi, C.; Cervini, M.; Giuberti, G. Wheat Bread Fortification by Grape Pomace Powder: Nutritional, Technological, Antioxidant, and Sensory Properties. Foods 2021, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Lomuscio, E.; Rizzi, C.; Simonato, B. Predicted Shelf-Life, Thermodynamic Study and Antioxidant Capacity of Breadsticks Fortified with Grape Pomace Powders. Foods 2021, 10, 2815. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.Z. Oxidative stress, polyunsaturated fatty acids-derived oxidation products and bisretinoids as potential inducers of CNS diseases: focus on age-related macular degeneration. Pharmacol Rep 2013, 65, 288–304. [Google Scholar] [CrossRef] [PubMed]
- Hoye, C.; Ross, C. Total Phenolic Content, Consumer Acceptance, and Instrumental Analysis of Bread Made with Grape Seed Flour. J Food Sci 2011, 76, S428–S436. [Google Scholar]
- Lavelli, V.; Kerr, W.L.; García-Lomillo, J.; González-SanJosé, M.L. Applications of Recovered Bioactive Compounds in Food Products, In Handbook of Grape Processing By-Products, Galanakis, C.M., Ed.; Academic Press, 2017, Volume 10, pp. 233–266.
- Tolve, R.; Pasini, G.; Vignale, F.; Favati, F.; Simonato, B. Effect of Grape Pomace Addition on the Technological, Sensory, and Nutritional Properties of Durum Wheat Pasta. Foods 2020, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, V.; Christiano, F.D.P.; Marczak, L.D.F.; Tessaro, I.C.; Thys, R.C.S. The effect of the incorporation of grape marc powder in fettuccini pasta properties. LWT - Food Sci Techn 2014, 58, 497–501. [Google Scholar] [CrossRef]
- Lavelli, V.; Torri, L.; Zeppa, G.; Fiori, L.; Spigno, G. Recovery Of Winemaking By-Products For Innovative Food Applications No. 4 (): Ital J Food Sci. 2016, 28. [Google Scholar]
- Balli, D.; Cecchi, L.; Innocenti, M.; Bellumori, M.; Mulinacci, N. Food by-products valorisation: Grape pomace and olive pomace (pâté) as sources of phenolic compounds and fiber for enrichment of tagliatelle pasta. Food Chem 2021, 355, 129642. [Google Scholar] [CrossRef]
- Codină, G.G.; Zaharia, D.; Stroe, S.-G.; Ropciuc, S. Influence of calcium ions addition from gluconate and lactate salts on refined wheat flour dough rheological properties. CyTA - J Food 2018, 16, 884–891. [Google Scholar] [CrossRef]
- Mironeasa, S.; Codina, G.; Mironeasa, C. The effects of wheat flour substitution with grape seed flour on the rheological parameters of the dough assessed by Mixolab. J Texture Studies 2012, 43. [Google Scholar] [CrossRef]
- Rashwan, A.K.; Osman, A.I.; Chen, W. Natural nutraceuticals for enhancing yogurt properties: a review. Environ Chem Lett 2023, 21, 1907–1931. [Google Scholar] [CrossRef]
- Barakat, H.; Y. Hassan, M. Chemical, Nutritional, Rheological, and Organoleptical Characterizations of Stirred Pumpkin-Yoghurt. Food Nutr Sci 2017, 8, 746–759. [Google Scholar]
- O’Connell, J.E.; Fox, P.F. Significance and applications of phenolic compounds in the production and quality of milk and dairy products: a review. Int Dairy J 2001, 11, 103–120. [Google Scholar] [CrossRef]
- Demirkol, M.; Tarakci, Z. Effect of grape (Vitis labrusca L.) pomace dried by different methods on physicochemical, microbiological and bioactive properties of yoghurt. LWT Food Sci Technol 2018, 97, 770–777. [Google Scholar] [CrossRef]
- Tseng, A. ; Zhao. Y.Wine grape pomace as antioxidant dietary fibre for enhancing nutritional value and improving storability of yogurt and salad dressing Food Chem., 2013, 138, 356–365. [Google Scholar]
- Silva, F.A.; et al. Incorporation of phenolic-rich ingredients from integral valorization of Isabel grape improves the nutritional, functional and sensory characteristics of probiotic goat milk yogurt. Food Chem 2022, 369, 130957. [Google Scholar] [CrossRef] [PubMed]
- Marchiani, R.; Bertolino, M.; Ghirardello, D.; McSweeney, P.L.H.; Zeppa, G. Physicochemical and nutritional qualities of grape pomace powder-fortified semi-hard cheeses. J Food Sci Technol 2016, 53, 1585–1596. [Google Scholar] [CrossRef]
- Tami, S.H.; et al. Buffalo stirred yoghurt fortified with grape seed extract: new insights into its functional properties. Food Biosci 2022, 47, 101752. [Google Scholar] [CrossRef]
- Gaglio, R.; Restivo, I.; Barbera, M.; Barbaccia, P.; Ponte, M.; Tesoriere, L.; Bonanno, A.; Attanzio, A.; Di Grigoli, A.; Francesca, N.; Moschetti, G.; Settanni, L. Effect on the Antioxidant, Lipoperoxyl Radical Scavenger Capacity, Nutritional, Sensory and Microbiological Traits of an Ovine Stretched Cheese Produced with Grape Pomace Powder Addition. Antioxidants 2021, 10, 306. [Google Scholar] [CrossRef]
- Chouchouli, V.; Kalogeropoulos, N.; Konteles, S.J.; Karvela, E.; Makris, D.P.; Karathanos, V.T. Fortification of yoghurts with grape (Vitis vinifera) seed extracts. LWT - Food Sci Techn 2013, 53, 522–529. [Google Scholar] [CrossRef]
- Marchiani, R.; Bertolino, M.; Belviso, S.; Giordano, M.; Ghirardello, D.; Torri, L.; Piochi, M.; Zeppa, G. Yogurt Enrichment with Grape Pomace: Effect of Grape Cultivar on Physicochemical, Microbiological and Sensory Properties. J Food Qual 2016, 3977–89. [Google Scholar] [CrossRef]
- Karaaslan, M.; Ozden, M.; Vardin, H.; Turkoglu, H. Phenolic fortification of yogurt using grape and callus extracts. LWT - Food Sci Techn 2011, 44, 1065–1072. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Wang, Q.Y.; Wang, S.J. Effects of rosemary extract, grape seed extract and green tea polyphenol on the formation of n-Nitrosamines and quality of western-Style smoked sausage. J. Food Process. Preserv. 2020, 44, e14459. [Google Scholar] [CrossRef]
- García-Lomillo, J.; González-SanJosé, M.L. Applications of Wine Pomace in the Food Industry: Approaches and Functions. Compreh Rev Food Sci Food Safety 2017, 16, 3–22. [Google Scholar] [CrossRef]
- García-Lomillo, J.; Gonzalez-SanJose, M.L.; Del Pino-García, R.; Ortega-Heras, M.; Muñiz-Rodríguez, P. Antioxidant effect of seasonings derived from wine pomace on lipid oxidation in refrigerated and frozen beef patties. LWT 2017, 77, 85–91. [Google Scholar] [CrossRef]
- Ryu, K.; Shim, K.; Shin, D. Effect of Grape Pomace Powder Addition on TBARS and Color of Cooked Pork Sausages during Storage. Kor J Food Sci An Res 2014, 34. [Google Scholar] [CrossRef] [PubMed]
- Riazi, F.; Zeynali, F.; Hoseini, E.; Behmadi, H. Effect of Dry Red Grape Pomace as a Nitrite Substitute on the Microbiological and Physicochemical Properties and Residual Nitrite of Dry-cured Sausage. Nutr Food Sci Res 2016, 3, 37–44. [Google Scholar] [CrossRef]
- Garrido, J.; Borges, F. Wine and grape polyphenols — A chemical perspective. Food Res Intern 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Bobko, M.; Haščí k, P.; Kročko, M.; Trembecká, L.; Mendelova, A.; Tkáčová, J.; Czako, P.; Tóth, T. Effect of grape seed extract on quality of raw-cooked meat products. Potr Slovak J Food Sci 2017, 11, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Rivas, C.; Vieira, C.; Rubio, B.; Martínez, B.; Gallardo, B.; Mantecón, A.R.; Lavín, P.; Manso. T. Effects of grape pomace in growing lamb diets compared with vitamin E and grape seed extract on meat shelf life. Meat Sci. 2016, 116, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Selani, M.M.; Contreras-Castillo, C.J.; Shirahigue, L.D.; Gallo, C.R.; Plata-Oviedo, M.; Montes-Villanueva, N.D. Wine industry residues extracts as natural antioxidants in raw and cooked chicken meat during frozen storage. Meat Sci 2011, 88, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Shirahigue, L.D.; Plata-Oviedo, M.; De Alencar, S.M.; D’Arce, M.A.B.R.; De Souza Vieira, T.M.F.; Oldoni, T.L.C.; Contreras-Castillo, C.J. Wine industry residue as antioxidant in cooked chicken meat. Int J Food Sci Techn 2010, 45, 863–870. [Google Scholar] [CrossRef]
- Sáyago-Ayerdi, S.G.; Brenes, A.; Goñi. I. Effect of grape antioxidante dietary fibre on the lípido oxidation of raw and cooked Chicken hamburgers. LWT- Food Sci. Technol. 2009, 42, 971–976. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Jiménez-Escrig, A.; Saura-Calixto, F.; Borderías. A.J. Effect of grape antioxidant dietary fibre on the prevention of lipid oxidation in miced fish: Evaluation by different methodologies. Food Chem., 2007, 101, 372–378. [Google Scholar] [CrossRef]
- Özvural, E.; Vural, H. Grape seed flour is a viable ingredient to improve the nutritional profile and reduce lipid oxidation of frankfurters. Meat Sci 2011, 88, 179–83. [Google Scholar] [CrossRef]
- Choi, Y.; Choi, J.; Han, D.; Kim, H.; Lee, M.; Kim, H.; Lee, J.; Chung, H.; Kim. C. Optimization of replacing pork back fat with grape seed oil and rice bran fibre for reduced-fat meat emulsion systems. Meat Sci. 2010, 84, 212–218. [Google Scholar] [CrossRef]
- Özalp Özen, B.; Eren, M.; Pala, A.; Özmen, İ.; Soyer, A. Effect of plant extracts on lipid oxidation during frozen storage of minced fish muscle. Intern J Food Sci Techn 2011, 46, 724–731. [Google Scholar] [CrossRef]
- Cilli, L.; Contini, L.; Sinnecker, P.; Lopes, P.; Andréo, M.; Neiva, C.; Nascimento, M.; Yoshida, C.; Venturini, A. Effects of grape pomace flour on quality parameters of salmon burger. J Food Process Preserv 2019, 44. [Google Scholar] [CrossRef]
- Pazos, M.; Gallardo, J.M.; Torres, J.L.; Medina. I. Activity of grape polyphenols as inhibitors of the oxidation of fish lipids and frozen fish muscle. Food Chem. 2005, 92, 547–557. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Jiménez-Escrig, A.; Saura-Calixto, F.; Borderías. A.J. Antioxidant protection of white grape pomace on restructured fish products during frozen storage. LWT Food Sci. Technol. 2008, 41, 42–50. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Borderías, J.; Larsson, K.; Undeland. I. Inhibition of hemoglobin-mediated oxidation of regular and lipid-fortified washed cod mince by a white grape dietary fibre. J. Agric. Food Chem. 2007, 55, 5299–5305. [Google Scholar] [CrossRef] [PubMed]
- Urquiaga, I.; Troncoso, D.; Mackenna, M.J.; Urzúa, C.; Pérez, D.; Dicenta, S.; de la Cerda, P.M.; Amigo, L.; Carreño, J.C.; Echeverría, G.; Rigotti, A. The Consumption of Beef Burgers Prepared with Wine Grape Pomace Flour Improves Fasting Glucose, Plasma Antioxidant Levels, and Oxidative Damage Markers in Humans: A Controlled Trial. Nutrients. 2018, 10, 1388. [Google Scholar] [CrossRef] [PubMed]
- Lavelli, V.; Sri Harsha, P.S.C.; Torri, L.; Zeppa, G. Use of winemaking by-products as an ingredient for tomato puree: The effect of particle size on product quality. Food Chem 2014, 152, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.A.T.; Bucknall, M.P.; Arcot, J. Interferences of anthocyanins with the uptake of lycopene in Caco-2 cells, and their interactive effects on anti-oxidation and anti-inflammation in vitro and ex vivo. Food Chem 2019, 276, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Dordoni, R.; Cantaboni, S.; Spigno, G. Walnut paste: oxidative stability and effect of grape skin extract addition. Heliyon 2019, 5, e02506. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, Biochemistry, and Safety of Acrylamide. A Review. J. Agric. Food Chem. 2003, 51, 4504–4526. [Google Scholar] [CrossRef]
- Xu, C.; Yagiz, Y.; Marshall, S.; Li, Z.; Simonne, A.; Lu, J.; Marshall, M.R. Application of muscadine grape (Vitis rotundifolia Michx.) pomace extract to reduce carcinogenic acrylamide. Food Chem 2015, 182, 200–208. [Google Scholar] [CrossRef]
- Teng, J.; Hu, X.; Tao, N.; Wang, M. Impact and inhibitory mechanism of phenolic compounds on the formation of toxic Maillard reaction products in food. Front. Agr. Sci. Eng. 2018, 5, 321. [Google Scholar] [CrossRef]
- Rózek, A.; Achaerandio, I.; Güell, C.; López, F.; Ferrando. M. Use of commercial grape phenolic extracts to supplement solid foodstuff. LWT Food Sci. Technol. 2010, 43, 623–631. [Google Scholar] [CrossRef]
- Guerrero, R.L.F.; Smith, P.; Bindon. K.A. Application of insoluble fibres in the fining of wine phenolics. J. Agric. Food Chem. 2013, 61, 4424–4432. [Google Scholar] [CrossRef]
- Shinagawa, F.B.; de Santana, F.C.; Torres, L.R.O.; Mancini, J. Grape seed oil: A potential functional food? Food Sci. Technol. 2015, 35, 399–406. [Google Scholar] [CrossRef]
- Auger, C.; Gérain, P.; Laurent-Bichon, F.; Portet, K.; Bornet, A.; Caporiccio, B.; Rouanet. J.M. Phenolics from commercialized grape extracts prevent early atherosclerotic lesions in hamsters by mechanisms other than antioxidant effect. J. Agric. Food Chem. 2004, 52, 5297–5302. [Google Scholar] [CrossRef]
- Tournour, H.H.; Segundo, M.A.; Magalhães, L.M.; Barreiros, L.; Queiroz, J.; Cunha. L.M. Valorization of grape pomace: Extraction of bioactive phenolics with antioxidant properties. Ind Crops Prod, 2015, 74, 397–406. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, R.; Justo, M.L.; Claro, C.M.; Vila, E.; Parrado, J.; Herrera, M.D.; de Sotomayor, M.A. . Endothelium-dependent vasodilator and antioxidant properties of a novel enzymatic extract of grape pomace from wine industrial waste. Food Chem. 2012, 135, 1044–1051. [Google Scholar] [CrossRef]
- Scola, G.; Conte, D.; Spada, P.W.D.-S.; Dani, C.; Vanderlinde, R.; Funchal, C.; Salvador, M. Flavan-3-ol Compounds from Wine Wastes with in Vitro and in Vivo Antioxidant Activity. Nutrients 2012, 2, 1048–1059. [Google Scholar] [CrossRef]
- Ed Nignpense, B.; Chinkwo, K.A.; Blanchard, C.L.; Santhakumar, A.B. Polyphenols: Modulators of Platelet Function and Platelet Microparticle Generation? Int J Mol Sci 2019, 21, 146. [Google Scholar] [CrossRef]
- de Lange, D.W.; van Golde, P.H.; Scholman, W.L.G.; Kraaijenhagen, R.J.; Akkerman, J.W.N.; Van De Wiel. A. Red wine and red wine polyphenolic compounds but not alcohol inhibit ADP-induced platelet aggregation. Eur J Inter Med 2003, 14, 361–366. [Google Scholar] [CrossRef]
- Olas, B.; Wachowicz, B.; Stochmal, A.; Oleszek, W. The polyphenol-rich extract from grape seeds inhibits platelet signaling pathways triggered by both proteolytic and non-proteolytic agonists. Platelets 2012, 23, 282–289. [Google Scholar] [CrossRef]
- Bijak, M.; Sut, A.; Kosiorek, A.; Saluk-Bijak, J.; Golanski, J. Dual anticoagulant/antiplatelet activity of polyphenolic grape seeds extract. Nutrients 2019, 11, 93. [Google Scholar] [CrossRef]
- Shanmuganayagam, D.; Beahm, M.R.; Osman, H.E.; Krueger, C.G.; Reed, J.D.; Folts, J.D. Grape seed and grape skin extracts elicit a greater antiplatelet effect when used in combination than when used individually in dogs and humans. J Nutr 2002, 132, 3592–3598. [Google Scholar] [CrossRef]
- Moschona, A.; Liakopoulou-Kyriakides. M. Encapsulation of biological active phenolic compounds extracted from wine wastes in alginate-chitosan microbeads. J Microencaps 2018, 35, 229–240. [Google Scholar] [CrossRef]
- Rivera, K.; Salas-Pérez, F.; Echeverría, G.; Urquiaga, I.; Dicenta, S.; et al. Red Wine Grape Pomace Attenuates Atherosclerosis and Myocardial Damage and Increases Survival in Association with Improved Plasma Antioxidant Activity in a Murine Model of Lethal Ischemic Heart Disease. Nutrients. 2019, 11, 2135. [Google Scholar] [CrossRef]
- Hogan, S.; Zhang, L.; Li, J.; Sun, S.; Canning, C.; Zhou. K. Antioxidant rich grape pomace extract suppresses postprandial hyperglycemia in diabetic mice by specifically inhibiting alpha-glucosidase. Nutr. Metab. 2010, 7, 1. [Google Scholar] [CrossRef]
- Urquiaga, I.; D'Acuña, S.; Pérez, D.; Dicenta, S.; Echeverría, G.; Rigotti, A.; Leighton, F. Wine grape pomace flour improves blood pressure, fasting glucose and protein damage in humans: a randomized controlled trial. Biol Res. 2015, 48, 49. [Google Scholar] [CrossRef]
- Draijer, R.; de Graaf, Y.; Slettenaar, M.; de Groot, E.; Wright, C. Consumption of a Polyphenol-Rich Grape-Wine Extract Lowers Ambulatory Blood Pressure in Mildly Hypertensive Subjects. Nutrients 2015, 7, 3138–3153. [Google Scholar] [CrossRef]
- Del Pino-García, R.; Gerardi, G.; Rivero-Pérez, M.D.; González-SanJosé, M.L.; García-Lomillo, J.; Muñiz, P. Wine pomace seasoning attenuates hyperglycaemia-induced endothelial dysfunction and oxidative damage in endothelial cells. J Funct Foods 2016, 22, 431–445. [Google Scholar] [CrossRef]
- Flammer, A.J.; Sudano, I.; Wolfrum, M.; Thomas, R.; Enseleit, F.; Périat, D.; Kaiser, P.; et al. Cardiovascular effects of flavanol-rich chocolate in patients with heart failure. Eur Heart J 2012, 33, 2172–2180. [Google Scholar] [CrossRef]
- Wang, S.; Mateos, R.; Goya, L.; Amigo-Benavent, M.; Sarriá, B.; Bravo. L. A phenolic extract from grape by-products and its main hydroxybenzoic acids protect Caco-2 cells against pro-oxidant induced toxicity. Food Chem. Toxicol. 2016, 88, 65–74. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; García-Almagro, F.J.; Avilés-Plaza, F.; Parra, S.; Yáñez-Gascón, M.J.; Ruiz-Ros, J.A.; García-Conesa, M.T.; Tomás-Barberán, F.A.; Espín, J.C. Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients undergoing primary prevention of cardiovascular disease: A triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol Nutr Food Res 2012, 56, 810–821. [Google Scholar] [CrossRef]
- de Oliveira, W.P.; Biasoto, A.C.T.; Marques, V.F.; dos Santos, I.M.; Magalhães, K.; Correa, L.C.; Shahidi. F. Phenolics from Winemaking By-Products Better Decrease VLDL-Cholesterol and Triacylglycerol Levels than Those of Red Wine in Wistar Rats. J Food Sci 2017, 82, 2432–2437. [Google Scholar] [CrossRef]
- Razavi, S.-M.; Gholamin, S.; Eskandari, A.; Mohsenian, N.; Ghorbanihaghjo, A.; Delazar, A.; Argani. H. Red grape seed extract improves lipid profiles and decreases oxidized low-density lipoprotein in patients with mild hyperlipidemia. J Med Food 2013, 16, 255–258. [Google Scholar] [CrossRef]
- Park, E.; Edirisinghe, I.; Choy, Y.Y.; Waterhouse, A.; Burton-Freeman, B. Effects of grape seed extract beverage on blood pressure and metabolic indices in individuals with pre-Hypertension: A randomised, double-Blinded, two-Arm, parallel, placebo-Controlled trial. Br. J. Nutr. 2016, 115, 226–238. [Google Scholar] [CrossRef]
- Pasini, F.; Chinnici, F.; Caboni, M.F.; Verardo, V. Recovery of oligomeric proanthocyanidins and other phenolic compounds with established bioactivity from grape seed by-Products. Molecules 2019, 24, 677. [Google Scholar] [CrossRef]
- Sapwarobol, S.; Adisakwattana, S.; Changpeng, S.; Ratanawachirin, W.; Tanruttanawong, K.; Boonyarit, W. Postprandial blood glucose response to grape seed extract in healthy participants: A pilot study. Pharmacogn Mag. 2012, 8, 192–6. [Google Scholar] [CrossRef]
- Montagut, G.; Onnockx, S.; Vaqué, M.; Bladé, C.; Blay, M.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, M.J.; Arola, L.; Pirson, I. Oligomers of grape-seed procyanidin extract activate the insulin receptor and key targets of the insulin signaling pathway differently from insulin. J. Nutr. Biochem. 2010, 21, 476–481. [Google Scholar] [CrossRef]
- Kaur, M.; Agarwal, C.; Agarwa. R. Anticancer and cancer chemopreventive potential of grape seed extract and other grape-based products. J. Nutr. 2009, 139, 1806S–1812S. [Google Scholar] [CrossRef]
- Ono, K.; Condron, M.M.; Ho, L.; Wang, J.; Zhao, W.; Pasinetti, G.M.; Teplow, D.B. Effects of grape seed-derived polyphenols on amyloid beta-protein self-assembly and cytotoxicity. J Biol Chem. 2008, 283, 32176–87. [Google Scholar] [CrossRef]
- Charradi, K.; Mahmoudi, M.; Bedhiafi, T.; Jebari, K.; El May, M.; Limam, F.; Aouani, E. Safety evaluation, anti-oxidative and anti-inflammatory effects of subchronically dietary supplemented high dosing grape seed powder (GSP) to healthy rat. Biomed Pharmac 2018, 107, 534–546. [Google Scholar] [CrossRef]
- Cádiz-Gurrea, M.D.L.L.; Borrás-Linares, I.; Lozano-Sánchez, J.; Joven, J.; Fernández-Arroyo, S.; Segura-Carretero, A. Cocoa and Grape Seed Byproducts as a Source of Antioxidant and Anti-Inflammatory Proanthocyanidins. Int J Mol Sci 2017, 18, 376. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham,-Ul-Haq. ; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed Pharm 2019, 116, 108999. [Google Scholar]
- Kruger, M.J.; Davies, N.; Myburgh, K.H.; Lecour, S. Proanthocyanidins, anthocyanins and cardiovascular diseases. Food Res Int 2014, 59, 41–52. [Google Scholar] [CrossRef]
- Iriondo-DeHond, M.; Blázquez-Duff, J.M.; Del Castillo, M.D.; Miguel, E. Nutritional Quality, Sensory Analysis and Shelf Life Stability of Yogurts Containing Inulin-Type Fructans and Winery Byproducts for Sustainable Health. Foods. 2020, 9, 1199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-H.; Huang, B.; Choi, S.-K.; Seo, J.-S. Anti-obesity effect of resveratrol-amplified grape skin extracts on 3T3-L1 adipocytes differentiation. Nutr Res Pract 2012, 6, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.F.M.; Moawed, F.S.M.; Mohamed. M.A. Protective mechanism of grape seed oil on carbon tetrachloride-induced brain damage in γ-irradiated rats. J. Photoch. Photobio. 2015, 153, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.F.M.; Salem, A.A.M.; Eassawy. M.M.T. Hepatoprotective effect of grape seed oil against carbon tetrachloride induced oxidative stress in liver of γ-irradiated rat. J. Photoch. Photobio. 2016, 160, 1–10. [Google Scholar] [CrossRef]
- Asadi, F.; Shahriari, A.; Chahardah-Cheric. M. Effect of long-term optimal ingestion of canola oil, grape seed oil, corn oil and yogurt butter on serum, muscle and liver cholesterol status in rats. Food Chem. Toxicol. 2010, 48, 2454–2457. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Martinez, M.; Stamos, M.J.; Moyer, M.P.; Planutis, K.; Hope, C.; Holcombe. R.F. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag. Res. 2009, 1, 25–37. [Google Scholar]
- Elshaer, M.; Chen, Y.; Wang, X.J.; Tang, X. Resveratrol: An overview of its anti-cancer mechanisms. Life Sci 2018, 207, 340–349. [Google Scholar] [CrossRef]
- Wang, L.-S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett 2008, 269, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Lala, G.; Malik, M.; Zhao, C.; He, J.; Kwon, Y.; Giusti, M.M.; Magnuson. B.A. Anthocyanin-rich extracts inhibit multiple biomarkers of colon cancer in rats. Nutr Cancer 2006, 54, 84–93. [Google Scholar] [CrossRef]
- Tian, Q.; Xu, Z.; Sun, X.; Deavila, J.; Du, M.; Zhu, M. Grape pomace inhibits colon carcinogenesis by suppressing cell proliferation and inducing epigenetic modifications. J Nutr Biochem. 2020, 84, 108443. [Google Scholar] [CrossRef]
- Mišković Špoljarić, K.; Šelo, G.; Pešut, E.; Martinović, J.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Antioxidant and antiproliferative potentials of phenolic-rich extracts from biotransformed grape pomace in colorectal Cancer. BMC Complement Med Ther. 2023, 23, 29. [Google Scholar] [CrossRef] [PubMed]
- Singletary, K.W.; Jung, K.J.; Giusti, M. Anthocyanin-rich grape extract blocks breast cell DNA damage. J Med Food. 2007, 10, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Song, S.; Wei, Z.; Huang, Y.; Zhang, Y.; Lu, J. The comparative study among different fractions of muscadine grape 'Noble' pomace extracts regarding anti-oxidative activities, cell cycle arrest and apoptosis in breast cancer. Food Nutr Res. 2017, 61, 1412795. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Silva, V.; Igrejas, G.; Gaivao, I.; Aires, A.; Klibi, N.; Dapkevicius, M.; Valentao, P.; Falco, V.; Poeta, P. Valorization of Winemaking By-Products as a Novel Source of Antibacterial Properties: New Strategies to Fight Antibiotic Resistance. Molecules. 2021, 26, 2331. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. Potential application of spice and herb extracts as natural preservatives in cheese. J Med Food 2011, 14, 284–290. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects – A review. J Funct Foods, Natural Antioxidants 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Mattio, L.M.; Dallavalle, S.; Musso, L.; Filardi, R.; Franzetti, L.; Pellegrino, L.; D’Incecco, P.; Mora, D.; Pinto, A.; Arioli, S. Antimicrobial activity of resveratrol-derived monomers and dimers against foodborne pathogens. Sci Rep 2019, 9, 19525. [Google Scholar] [CrossRef]
- de Vries, K.; Strydom, M.; Steenkamp, V. Bioavailability of resveratrol: Possibilities for enhancement. J Herbal Med 2018, 11, 71–77. [Google Scholar] [CrossRef]
- Tsali, A.; Goula, A.M. Valorization of grape pomace: Encapsulation and storage stability of its phenolic extract. Powder Tech 2018, 340, 194–207. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; McClements, D.J.; Lorenzo, J.M. Encapsulation of Bioactive Phytochemicals in Plant-Based Matrices and Application as Additives in Meat and Meat Products. Molecules 2021, 26, 3984. [Google Scholar] [CrossRef]
- Glampedaki, P.; Dutschk. V. Stability studies of cosmetic emulsions prepared from natural products such as wine, grape seed oil and mastic resin. Colloid. Surf. A, 2014, 460, 306–311. [Google Scholar] [CrossRef]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle. R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef]
- Marino, A.; Battaglini, M.; Desii, A.; Lavarello, C.; Genchi, G.; Petretto, A.; Ciofani, G. Liposomes loaded with polyphenol-rich grape pomace extracts protect from neurodegeneration in a rotenone-based in vitro model of Parkinson's disease. Biomater Sci. 2021, 9, 8171–8188. [Google Scholar] [CrossRef]
- Davidov-Pardo, G. ; McClements. D.J. Nutraceutical delivery systems: Resveratrol encapsulation in grape seed oil nanoemulsions formed by spontaneous emulsification. Food Chem 2015, 167, 205–212. [Google Scholar]
- Hübner, A.A.; Sarruf, F.D.; Oliveira, C.A.; Neto, A.V.; Fischer, D.C.H.; Kato, E.T.M.; Lourenço, F.R.; Baby, A.R.; Bacchi, E.M. Safety and Photoprotective Efficacy of a Sunscreen System Based on Grape Pomace (Vitis vinifera L.) Phenolics from Winemaking. Pharmaceutics. 2020, 12, 1148. [Google Scholar] [CrossRef]
- Jakobek, L.; Matić, P. Non-covalent dietary fiber - Polyphenol interactions and their influence on polyphenol bioaccessibility. Trends Food Sci Tech 2019, 83, 235–247. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Serrano, J.; Tabernero, M.; Arranz, S.; Díaz-Rubio, M.E.; García-Diz, L.; Goñi, I.; Saura-Calixto, F. Bioavailability of Phenolic Antioxidants Associated with Dietary Fiber: Plasma Antioxidant Capacity After Acute and Long-Term Intake in Humans. Plant Foods Hum Nutr 2009, 64, 102–107. [Google Scholar] [CrossRef]
- Ferreira, C.S.; Pinho, M.N.; Cabral, L. Solid-liquid extraction and concentration with processes of membrane technology of soluble fibers from wine grape pomace. Técnico Lisboa, 2013; 1–9. [Google Scholar]
- Zhang, L.; Zhu, M.; Shi, T.; Guo, C.; Huang, Y.; Chen, Y.; Xie, M. Recovery of dietary fiber and polyphenol from grape juice pomace and evaluation of their functional properties and polyphenol compositions. Food Funct. 2017, 8, 341–351. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Zheng, L.; Li, J. Advance on the bioactivity and potential applications of dietary fibre from grape pomace. Food Chemistry, ISPMF 2015: International Symposium on Phytochemicals in Medicine and Food (Shanghai, China, June 26th –29th, 2015, 186, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Valiente, C.; Arrigoni, E.; Esteban, R.; Amadò, R. Grape Pomace as a Potential Food Fiber. J Food Sci 1995, 60, 818–820. [Google Scholar] [CrossRef]
- Dias, J.F.; Simbras, B.D.; Beres, C.; dos Santos, K.O.; Cabral, L.M.C.; Miguel, M.A.L. Acid Lactic Bacteria as a Bio-Preservant for Grape Pomace Beverage. Front Sust Food Syst 2018, 2. [Google Scholar] [CrossRef]
- Ageyeva, N.; Tikhonova, A.; Burtsev, B.; Biryukova, S.; Globa, E. Grape pomace treatment methods and their effects on storage. Foods Raw Mat 2021, 9, 215–223. [Google Scholar] [CrossRef]
- Ayed, N.; Yu, H.-L.; Lacroix, M. Using gamma irradiation for the recovery of anthocyanins from grape pomace. Rad Phys Chem 2000, 57, 277–279. [Google Scholar] [CrossRef]
- Tseng, A.; Zhao, Y. Effect of different drying methods and storage time on the retention of bioactive compounds and antibacterial activity of wine grape pomace (Pinot Noir and Merlot). J Food Sci 2012, 77, H192–H201. [Google Scholar] [CrossRef]
- Sokač, T.; Gunjević, V.; Pušek, A.; Tušek, A.J.; Dujmić, F.; Brnčić, M.; Ganić, K.K.; Jakovljević, T.; Uher, D.; Mitrić, G.; Redovniković, I.R. Comparison of Drying Methods and Their Effect on the Stability of Graševina Grape Pomace Biologically Active Compounds. Foods 2022, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- MohdMaidin, N.; Oruna-Concha, M.J.; Jauregi, P. Surfactant TWEEN20 provides stabilisation effect on anthocyanins extracted from red grape pomace. Food Chem. 2019, 271, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Torri, L.; Piochi, M.; Marchiani, R.; Zeppa, G.; Dinnella, C.; Monteleone, E. A sensory- and consumer-based approach to optimize cheese enrichment with grape skin powders. J Dairy Sci 2016, 99, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Soares, Susana, Brandão, E. ; Guerreiro, C.; Soares, Sónia, Mateus, N.; de Freitas, V. Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef] [PubMed]
- McRae, J.M.; Kennedy, J.A. Wine and Grape Tannin Interactions with Salivary Proteins and Their Impact on Astringency: A Review of Current Research. Molecules 2011, 16, 2348–2364. [Google Scholar] [CrossRef] [PubMed]
- Canett-Romero, R.; Osuna, A.; Sánchez, M.; Castro, R.; León-Martínez, L.; León-Gálvez, R. Characterization of cookies made with deseeded grape pomace. Arch Latinoam Nutr 2004, 54, 93–9. [Google Scholar] [PubMed]
- Caponio, G.R.; Noviello, M.; Calabrese, F.M.; Gambacorta, G.; Giannelli, G.; De Angelis, M. Effects of Grape Pomace Polyphenols and In Vitro Gastrointestinal Digestion on Antimicrobial Activity: Recovery of Bioactive Compounds. Antioxidants 2022, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- Čuš, F.; Česnik, H.B.; Bolta, Š.V.; Gregorčič, A. Pesticide residues in grapes and during vinification process. Food Control 2010, 21, 1512–1518. [Google Scholar] [CrossRef]
- Hou, X.; Xu, Z.; Zhao, Y.; Liu, D. Rapid analysis and residue evaluation of six fungicides in grape wine-making and drying. J Food Comp Anal 2020, 89, 103465. [Google Scholar] [CrossRef]
- Moncalvo, A.; Marinoni, L.; Dordoni, R.; Duserm Garrido, G.; Lavelli, V.; Spigno, G. Waste grape skins: evaluation of safety aspects for the production of functional powders and extracts for the food sector. Food AddCont: Part A 2016, 33, 1116–1126. [Google Scholar] [CrossRef]
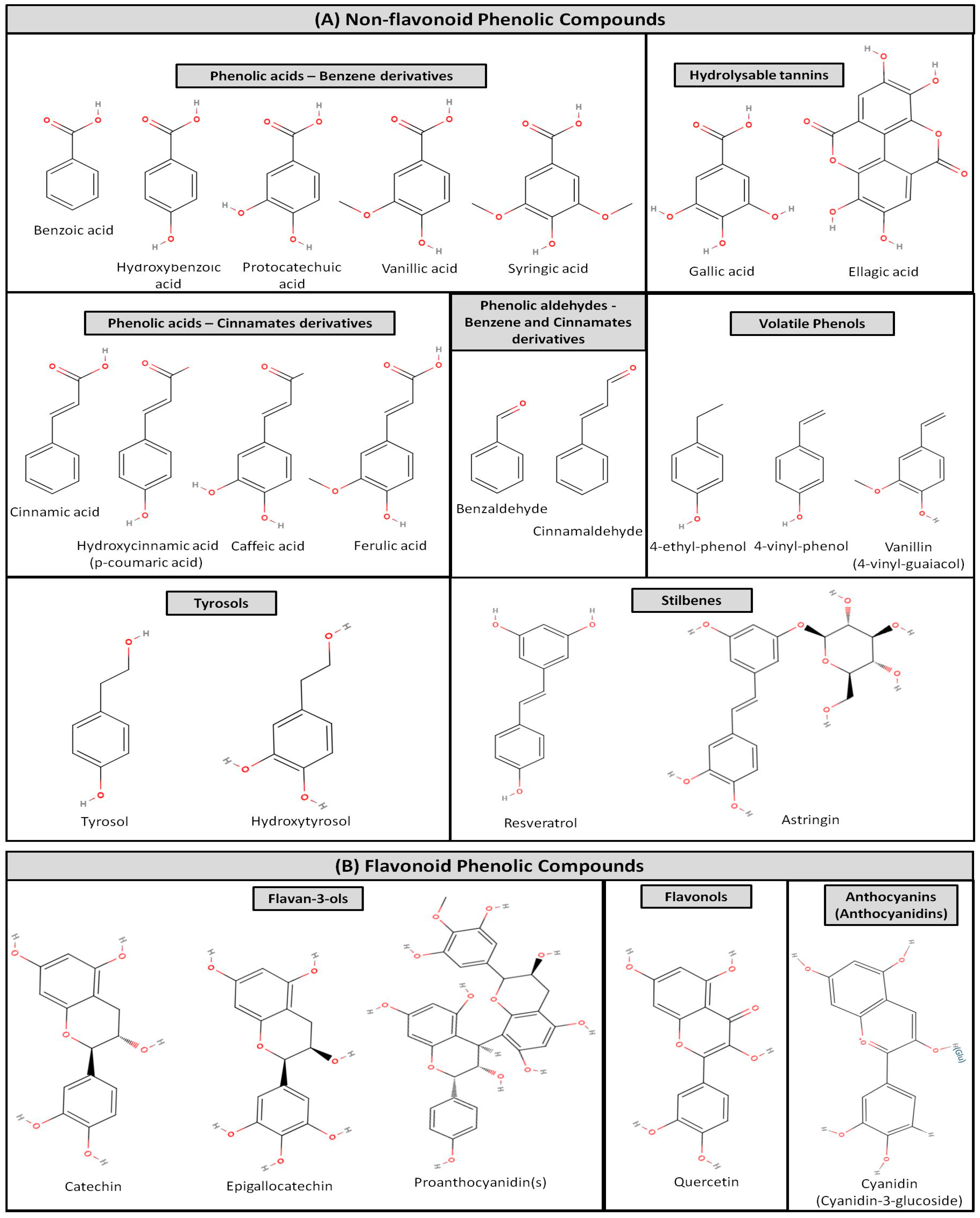
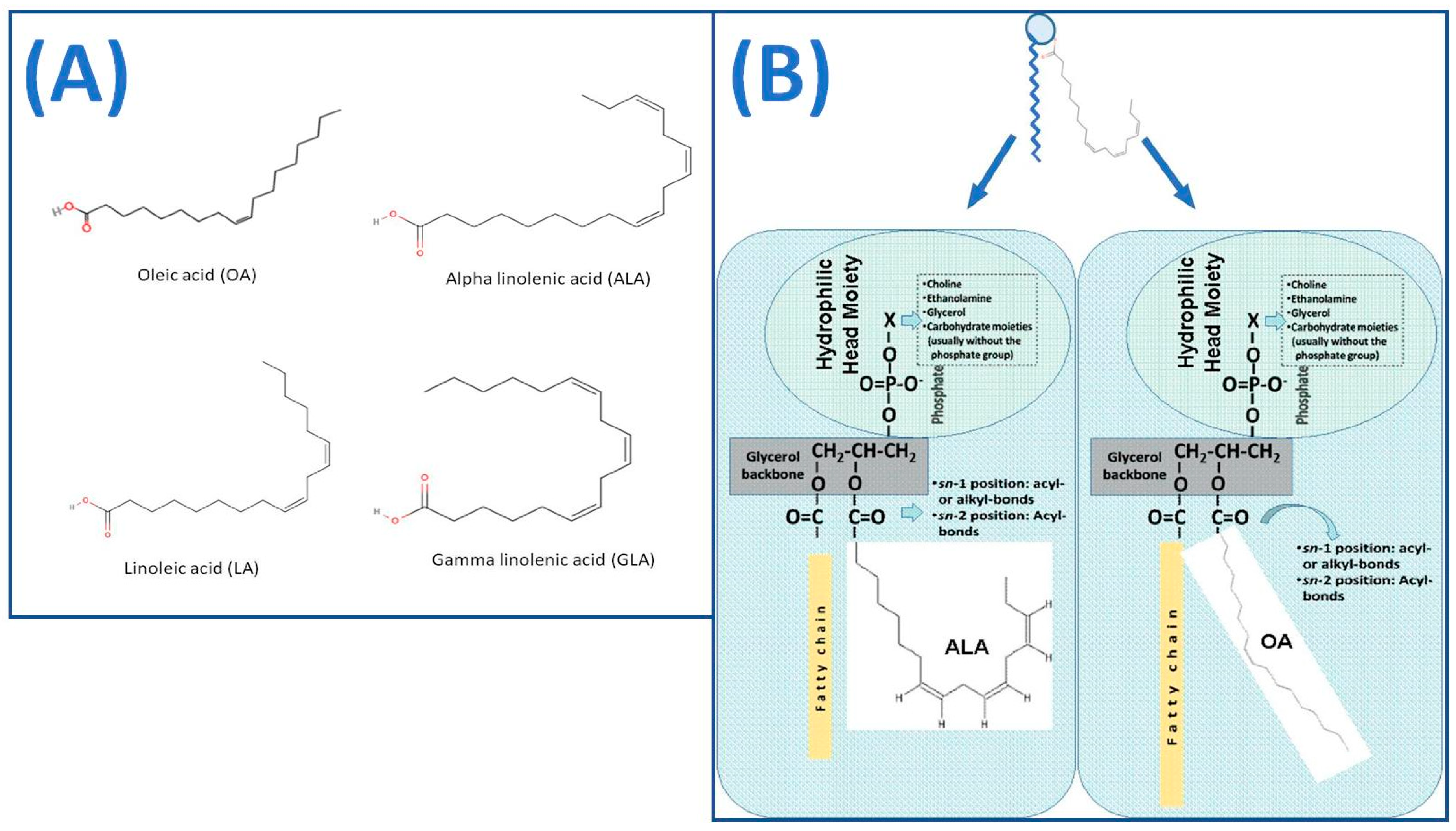
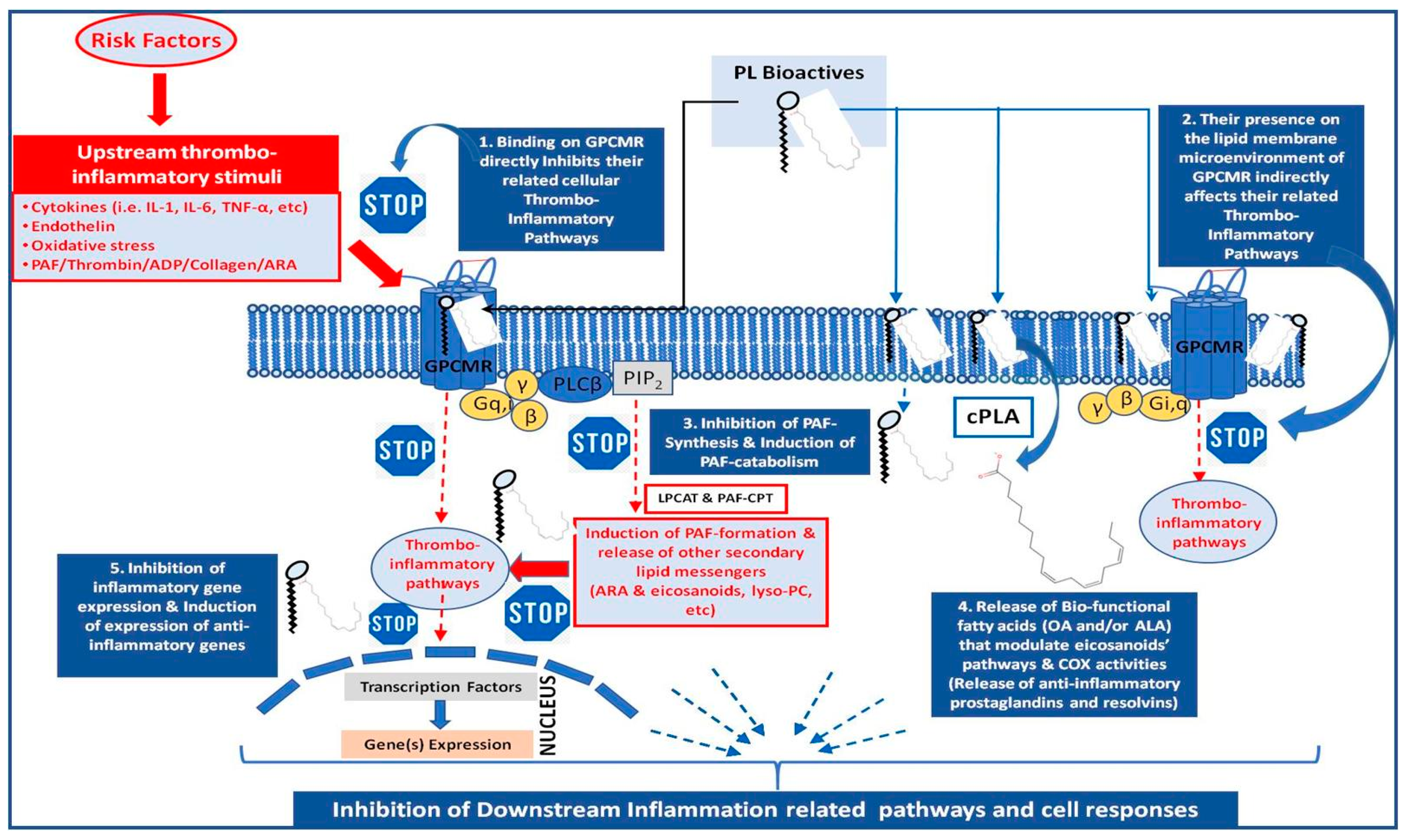
| Compounds | Quantity g/100g | Phenolic compounds | mg GAE/L |
|---|---|---|---|
| Ash | 1.73 – 9.10 | Flavonoids | 1.31 – 8.36 |
| Protein | 3.57-14.17 | Anthocyanins | 0.33 – 6.8 |
| lipids | 1.14-13.90 | Tannins | 0.74 – 6.41 |
| Total dietary fibre | 17.28-88.70 | Proanthocyanins | 0.43 – 12.00 |
| Insoluble fibre | 16.44-63.70 | Catechin | 0.69 – 8.06 |
| Carbohydrates | 12.20-40.53 | Epicatechin | 0.61-0.99 |
| Total polyphenolic content | 0.28-8.70 | Resveratrol | 0.58 – 37.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





