Submitted:
12 June 2023
Posted:
15 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
Transcriptome Analysis of MTs Induction
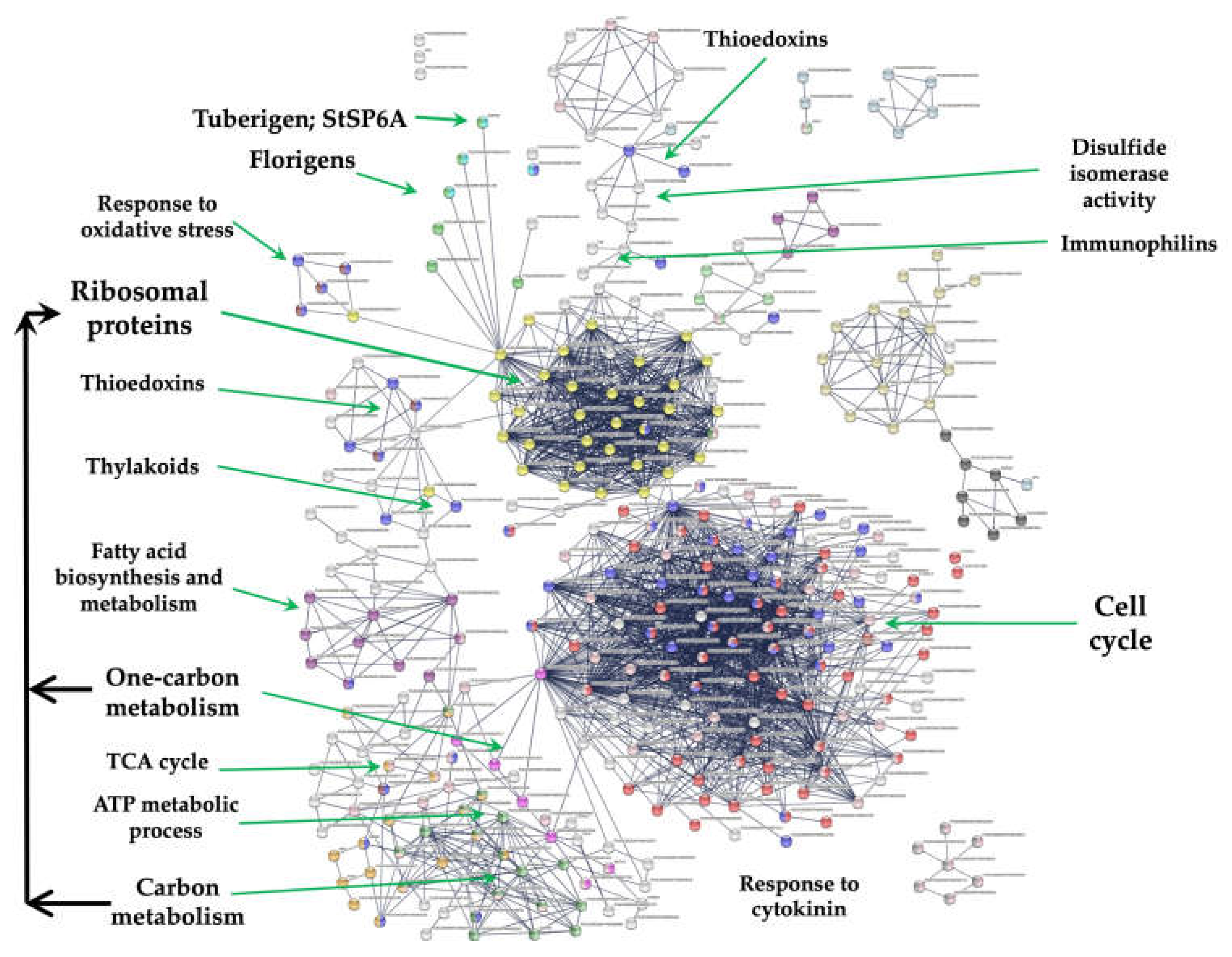
What is the Importance of Ribosome Biogenesis?
Lethal Mutants
Overexpression of Ribosomal Proteins and Ribosome Biogenesis Factors
Are RPs good candidate genes for improving of multiple abiotic stress tolerance in potato?
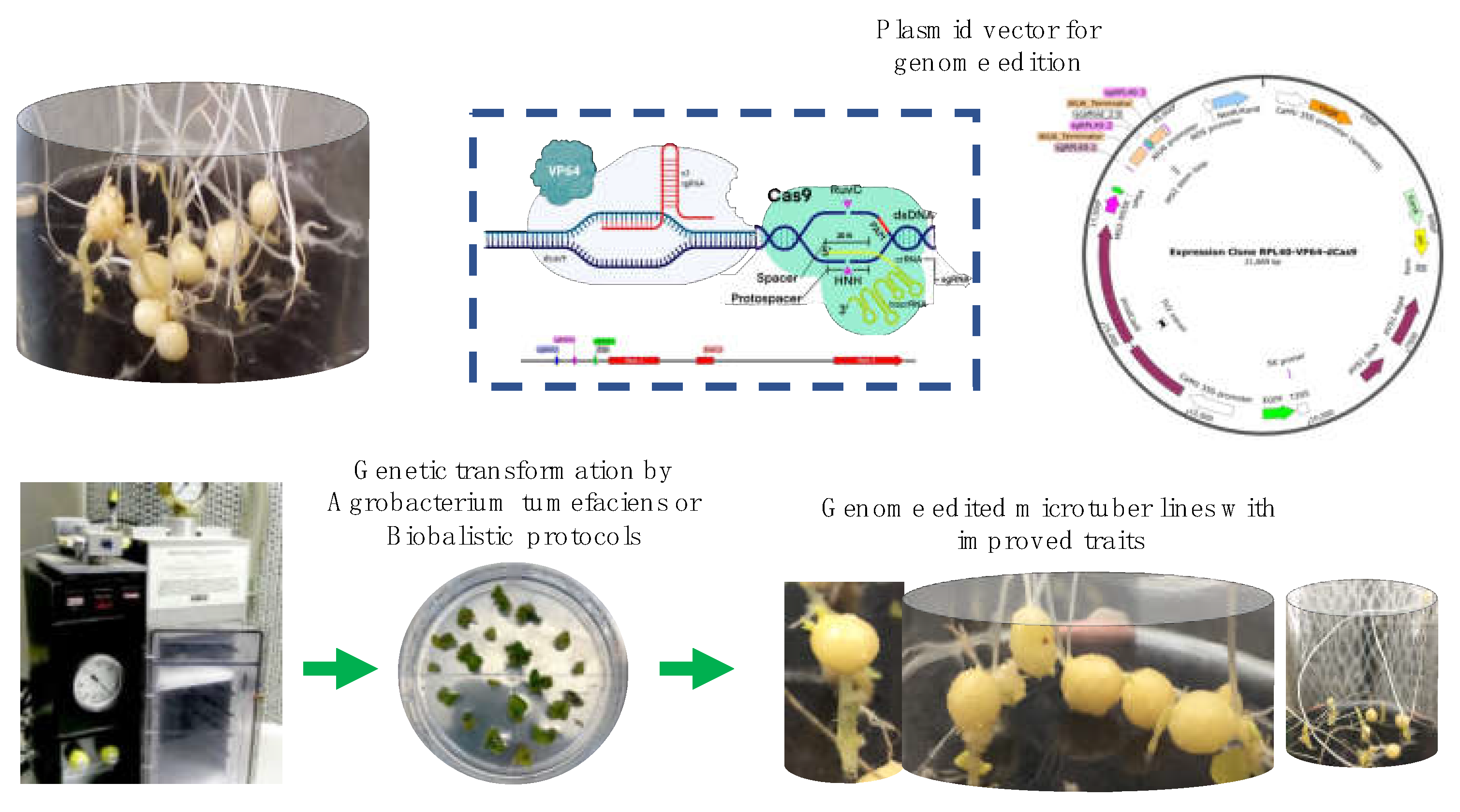
How to produce potato microtubers tolerant to biotic and abiotic stress?
Interaction of RPs cluster with immunophilines
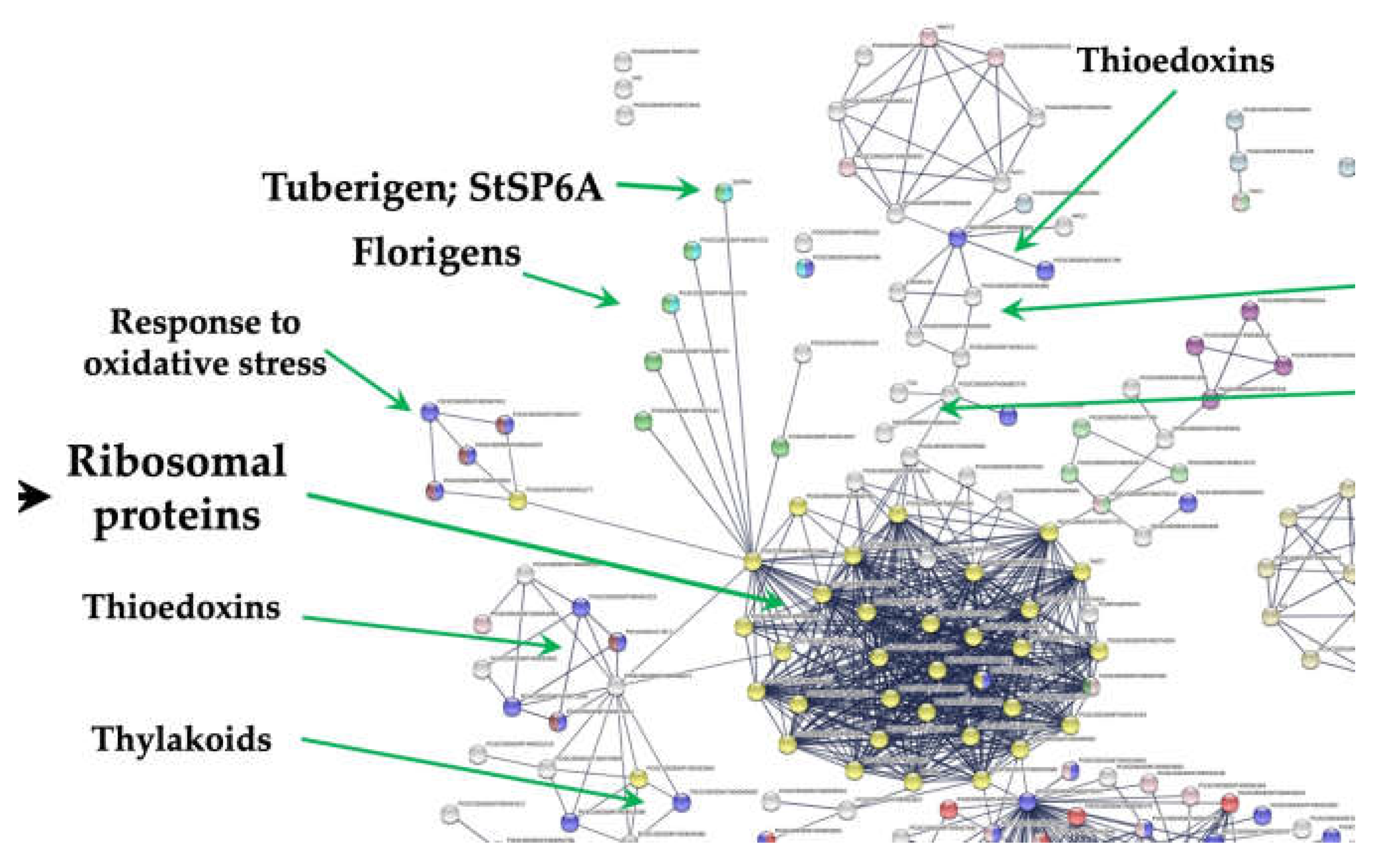
Redoxins
Response to oxidative stress
RPs interacting with Carbon metabolism, one-carbon metabolism, TCA-cycle.
Carbon metabolism
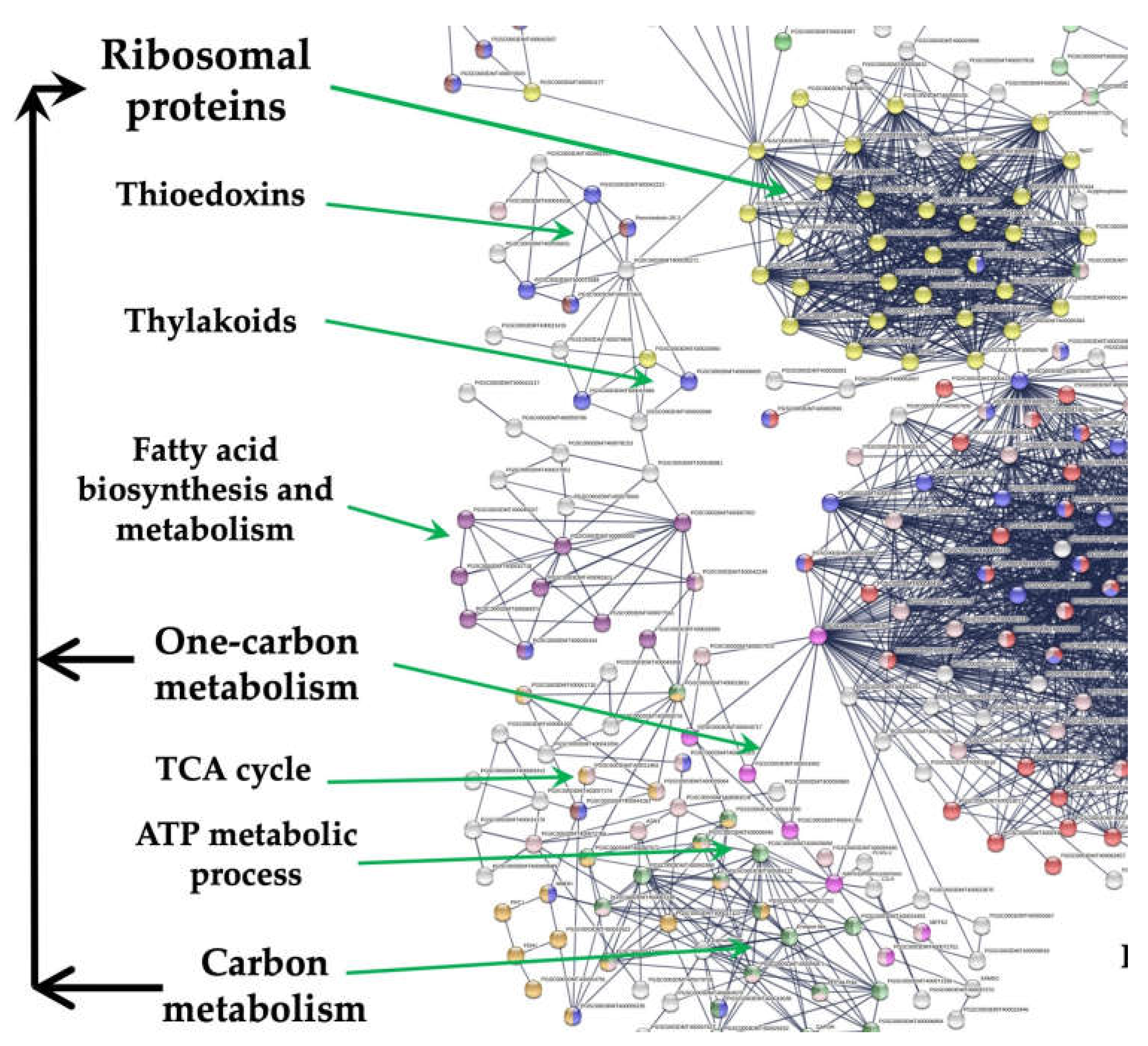
One-carbon-metabolism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food, E. I. T. (2019). About EIT food.
- Dutt, S.; Manjul, A.S.; Raigond, P.; Singh, B.; Siddappa, S.; Bhardwaj, V.; Kawar, P.G.; Patil, V.U.; Kardile, H.B. Key players associated with tuberization in potato: potential candidates for genetic engineering. Crit. Rev. Biotechnol. 2017, 37, 942–957. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, D. J., Coleman, W. K., & Coleman, S. E. (2003). Potato microtuber production and performance: A review. American journal of potato research, 80, 103-115.
- Hannapel, D. J. (2007). Signalling the induction of tuber formation. In Potato biology and biotechnology (pp. 237-256). Elsevier Science BV. [CrossRef]
- Vinterhalter, D., Dragicevic, I., & Vinterhalter, B. (2008). Potato in vitro culture techniques and biotech-nology. Fruit, vegetable and cereal science and biotechnology, 2(1), 16-45.
- Herrera-Isidron, L., Valencia-Lozano, E., Rosiles-Loeza, P. Y., Robles-Hernández, M. G., Napsuciale-Heredia, A., & Cabrera-Ponce, J. L. (2021). Gene expression analysis of microtubers of potato Solanum tu-berosum L. induced in cytokinin containing medium and osmotic stress. Plants, 10(5), 876.
- Valencia-Lozano, E.; Herrera-Isidrón, L.; Flores-López, J.A.; Recoder-Meléndez, O.S.; Barraza, A.; Cabre-ra-Ponce, J.L. Solanum tuberosum Microtuber Development under Darkness Unveiled through RNAseq Transcriptomic Analysis. Int. J. Mol. Sci. 2022, 23, 13835. [Google Scholar] [CrossRef] [PubMed]
- Purwestri, Y. A., Susanto, F. A., & Tsuji, H. (2017). Hd3a florigen recruits different proteins to reveal its function in plant growth and development. In Plant Engineering. IntechOpen. [CrossRef]
- Wang, E., Liu, T., Sun, X., Jing, S., Zhou, T., Liu, T., & Song, B. (2022). Profiling of the Candidate Interact-ing Proteins of SELF-PRUNING 6A (SP6A) in Solanum tuberosum. International Journal of Molecular Scien-ces, 23(16), 9126.
- Abelenda, J. A. , Navarro, C., & Prat, S. (2011). From the model to the crop: genes controlling tuber for-mation in potato. Current Opinion in Biotechnology, 22(2), 287-292.
- Abelenda, J. A., Bergonzi, S., Oortwijn, M., Sonnewald, S., Du, M., Visser, R. G., ... & Bachem, C. W. (2019). Source-sink regulation is mediated by interaction of an FT homolog with a SWEET protein in po-tato. Current Biology, 29(7), 1178-1186.
- Teo, C.-J.; Takahashi, K.; Shimizu, K.; Shimamoto, K.; Taoka, K.-I. Potato Tuber Induction is Regulated by Interactions Between Components of a Tuberigen Complex. Plant Cell Physiol. 2016, 58, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Lin, T.; Hannapel, D.J. Targets of the StBEL5 Transcription Factor Include the FT Ortholog StSP6A. Plant Physiol. 2015, 170, 310–324. [Google Scholar] [CrossRef]
- Salvato, F.; Havelund, J.F.; Chen, M.; Rao, R.S.P.; Rogowska-Wrzesinska, A.; Jensen, O.N.; Gang, D.R.; Thelen, J.J.; Møller, I.M. The Potato Tuber Mitochondrial Proteome. Plant Physiol. 2013, 164, 637–653. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, J.P.; Cankar, K.; Scheffer, S.J.; Beenen, H.G.; Shepherd, L.V.T.; Stewart, D.; Davies, H.V.; Wilko-ckson, S.J.; Leifert, C.; Gruden, K.; et al. Transcriptome Analysis of Potato TubersEffects of Different Agricultural Practices. J. Agric. Food Chem. 2009, 57, 1612–1623. [Google Scholar] [CrossRef]
- Shan, J.; Song, W.; Zhou, J.; Wang, X.; Xie, C.; Gao, X.; Xie, T.; Liu, J. Transcriptome analysis reveals novel genes potentially involved in photoperiodic tuberization in potato. Genomics 2013, 102, 388–396. [Google Scholar] [CrossRef]
- Vulavala, V. K., Fogelman, E., Faigenboim, A., Shoseyov, O., & Ginzberg, I. (2019). The transcriptome of potato tuber phellogen reveals cellular functions of cork cambium and genes involved in periderm for-mation and maturation. Scientific reports, 9(1), 10216.
- Zhou, K., Zhang, C., Xia, J., Yun, P., Wang, Y., Ma, T., & Li, Z. (2021). Albino seedling lethality 4; Chloro-plast 30S ribosomal protein S1 is required for chloroplast ribosome biogenesis and early chloroplast de-velopment in rice. Rice, 14(1), 1-12.
- Gong, X.; Jiang, Q.; Xu, J.; Zhang, J.; Teng, S.; Lin, D.; Dong, Y. Disruption of the Rice Plastid Ribosomal Protein S20 Leads to Chloroplast Developmental Defects and Seedling Lethality. G3 Ge-nes|Genomes|Genetics 2013, 3, 1769–1777. [Google Scholar] [CrossRef]
- Ma, Z.; Dooner, H.K. A mutation in the nuclear-encoded plastid ribosomal protein S9 leads to early em-bryo lethality in maize. Plant J. 2004, 37, 92–103. [Google Scholar] [CrossRef]
- Schultes, N.P.; Sawers, R.J.H.; Brutnell, T.P.; Krueger, R.W. Maize high chlorophyll fluorescent 60 muta-tion is caused by an Ac disruption of the gene encoding the chloroplast ribosomal small subunit protein 17. Plant J. 2000, 21, 317–327. [Google Scholar] [CrossRef]
- Tsugeki, R.; Kochieva, E.Z.; Fedoroff, N.V. A transposon insertion in the Arabidopsis SSR16 gene causes an embryo-defective lethal mutation. Plant J. 1996, 10, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Revenkova, E.; Masson, J.; Koncz, C.; Afsar, K.; Jakovleva, L.; Paszkowski, J. Involvement of Arabidopsis thaliana ribosomal protein S27 in mRNA degradation triggered by genotoxic stress. EMBO J. 1999, 18, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Rogalski, M. , Ruf, S. ( 34(16), 4537–4545. [PubMed]
- Lee, J.; Jang, S.; Ryu, S.; Lee, S.; Park, J.; Lee, S.; An, G.; Park, S.K. Mutation of Plastid Ribosomal Protein L13 Results in an Albino Seedling-Lethal Phenotype in Rice. Plant Breed. Biotechnol. 2019, 7, 395–404. [Google Scholar] [CrossRef]
- Lee, J., Jang, S., Ryu, S., Lee, S., Park, J., Lee, S., ... & Park, S. K. (2019). Impaired plastid ribosomal pro-tein L3 causes albino seedling lethal phenotype in rice. Journal of Plant Biology, 62, 419-428.
- Zhao, D. S., Zhang, C. Q., Li, Q. F., Yang, Q. Q., Gu, M. H., & Liu, Q. Q. (2016). A residue substitution in the plastid ribosomal protein L12/AL1 produces defective plastid ribosome and causes early seedling le-thality in rice. Plant molecular biology, 91, 161-177.
- Lin, D., Jiang, Q., Zheng, K., Chen, S., Zhou, H., Gong, X., ... & Dong, Y. (2015). Mutation of the rice ASL 2 gene encoding plastid ribosomal protein L21 causes chloroplast developmental defects and seedling death. Plant biology, 17(3), 599-607.
- Dupouy, G.; McDermott, E.; Cashell, R.; Scian, A.; McHale, M.; Ryder, P.; de Groot, J.; Lucca, N.; Brych-kova, G.; McKeown, P.C.; et al. Plastid ribosome protein L5 is essential for post-globular embryo develo-pment in Arabidopsis thaliana. Plant Reprod. 2022, 35, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Devis, D., Firth, S. M., Liang, Z., & Byrne, M. E. (2015). Dosage sensitivity of RPL9 and concerted evolu-tion of ribosomal protein genes in plants. Frontiers in Plant Science, 6, 1102.
- Ferreyra, M. L. F., Pezza, A., Biarc, J., Burlingame, A. L., & Casati, P. (2010). Plant L10 ribosomal proteins have different roles during development and translation under ultraviolet-B stress. Plant physiology, 153(4), 1878-1894.
- Pesaresi, P.; Varotto, C.; Meurer, J.; Jahns, P.; Salamini, F.; Leister, D. Knock-out of the plastid ribosomal protein L11 in Arabidopsis: effects on mRNA translation and photosynthesis. Plant J. 2001, 27, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Yin, J., Ibrahim, S., Petersen, F., & Yu, X. (2021). Autoimmunomic signatures of aging and age-related neurodegenerative diseases are associated with brain function and ribosomal proteins. Frontiers in Aging Neuroscience, 13, 679688.
- Romani, I.; Tadini, L.; Rossi, F.; Masiero, S.; Pribil, M.; Jahns, P.; Kater, M.; Leister, D.; Pesaresi, P. Versati-le roles of Arabidopsis plastid ribosomal proteins in plant growth and development. Plant J. 2012, 72, 922–934. [Google Scholar] [CrossRef]
- Horváth, B.M.; Magyar, Z.; Zhang, Y.; Hamburger, A.W.; Bakó, L.; Visser, R.G.F.; Bachem, C.W.B.; Bögre, L. EBP1 regulates organ size through cell growth and proliferation in plants. EMBO J. 2006, 25, 4909–4920. [Google Scholar] [CrossRef]
- Yang, L.; Xie, C.; Li, W.; Zhang, R.; Jue, D.; Yang, Q. Expression of a wild eggplant ribosomal protein L13a in potato enhances resistance to Verticillium dahliae. Plant Cell, Tissue Organ Cult. (PCTOC) 2013, 115, 329–340. [Google Scholar] [CrossRef]
- Moin, M.; Saha, A.; Bakshi, A.; Madhav, M.; Kirti, P. Constitutive expression of Ribosomal Protein L6 modulates salt tolerance in rice transgenic plants. Gene 2021, 789, 145670. [Google Scholar] [CrossRef] [PubMed]
- Moin, M.; Bakshi, A.; Madhav, M.S.; Kirti, P.B. Expression Profiling of Ribosomal Protein Gene Family in Dehydration Stress Responses and Characterization of Transgenic Rice Plants Overexpressing RPL23A for Water-Use Efficiency and Tolerance to Drought and Salt Stresses. Front. Chem. 2017, 5, 97. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, S.; Yanagisawa, S. Ribosome biogenesis factor OLI2 and its interactor BRX1-2 are associated with morphogenesis and lifespan extension in Arabidopsis thaliana. Plant Biotechnol. 2021, 38, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Yu, H., Kong, X., Huang, H., Wu, W., Park, J., Yun, D. J., ... & Zhu, J. K. (2020). STCH4/REIL2 confers cold stress tolerance in Arabidopsis by promoting rRNA processing and CBF protein translation. Cell Reports, 30(1), 229-242.
- Kappachery, S.; Yu, J.W.; Baniekal-Hiremath, G.; Park, S.W. Rapid identification of potential drought tolerance genes from Solanum tuberosum by using a yeast functional screening method. Comptes Rendus Biol. 2013, 336, 530–545. [Google Scholar] [CrossRef]
- Lei, Y.; Lu, L.; Liu, H.-Y.; Li, S.; Xing, F.; Chen, L.-L. CRISPR-P: A Web Tool for Synthetic Single-Guide RNA Design of CRISPR-System in Plants. Mol. Plant 2014, 7, 1494–1496. [Google Scholar] [CrossRef]
- Liu, H.; Ding, Y.; Zhou, Y.; Jin, W.; Xie, K.; Chen, L.-L. CRISPR-P 2.0: An Improved CRISPR-Cas9 Tool for Genome Editing in Plants. Mol. Plant 2017, 10, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Lowder, L. G., Zhang, D., Baltes, N. J., Paul III, J. W., Tang, X., Zheng, X., ... & Qi, Y. (2015). A CRISPR/Cas9 toolbox for multiplexed plant genome editing and transcriptional regulation. Plant physio-logy, 169(2), 971-985.
- García-Murillo, L.; Valencia-Lozano, E.; Priego-Ranero, N.A.; Cabrera-Ponce, J.L.; Duarte-Aké, F.P.; Vizuet-De-Rueda, J.C.; Rivera-Toro, D.M.; Herrera-Ubaldo, H.; de Folter, S.; Alvarez-Venegas, R. CRIS-PRa-mediated transcriptional activation of the SlPR-1 gene in edited tomato plants. Plant Sci. 2023, 329, 111617. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Ponce, J. L., López, L., Assad-Garcia, N., Medina-Arevalo, C., Bailey, A. M., & Herrera-Estrella, L. (1997). An efficient particle bombardment system for the genetic transformation of asparagus (Aspara-gus officinalis L.). Plant cell reports, 16, 255-260.
- Mahajan, S.; Pandey, G.K.; Tuteja, N. Calcium- and salt-stress signaling in plants: Shedding light on SOS pathway. Arch. Biochem. Biophys. 2008, 471, 146–158. [Google Scholar] [CrossRef]
- Alavilli, H., Lee, H., Park, M., Yun, D. J., y Lee, B. H. (2018). Enhanced multiple stress tolerance in Ara-bidopsis by overexpression of the polar moss peptidyl prolyl isomerase FKBP12 gene. Plant cell reports, 37(3), 453–465.
- Park, H.J.; Lee, A.; Lee, S.S.; An, D.-J.; Moon, K.-B.; Ahn, J.C.; Kim, H.-S.; Cho, H.S. Overexpression of Golgi Protein CYP21-4s Improves Crop Productivity in Potato and Rice by Increasing the Abundance of Mannosidic Glycoproteins. Front. Plant Sci. 2017, 8, 1250. [Google Scholar] [CrossRef]
- Andème Ondzighi, C., Christopher, D. A., Cho, E. J., Chang, S. C., & Staehelin, L. A. (2008). Arabidopsis protein disulfide isomerase-5 inhibits cysteine proteases during trafficking to vacuoles before pro-grammed cell death of the endothelium in developing seeds. The Plant Cell, 20(8), 2205-222.
- Eggert, E., Obata, T., Gerstenberger, A., Gier, K., Brandt, T., Fernie, A. R., ... & Kühn, C. (2016). A sucrose transporter-interacting protein disulphide isomerase affects redox homeostasis and links sucrose parti-tioning with abiotic stress tolerance. Plant, cell & environment, 39(6), 1366-1380.
- Kim, M. D., Kim, Y. H., Kwon, S. Y., Jang, B. Y., Lee, S. Y., Yun, D. J., ... & Lee, H. S. (2011). Overexpres-sion of 2-cysteine peroxiredoxin enhances tolerance to methyl viologen-mediated oxidative stress and high temperature in potato plants. Plant Physiology and Biochemistry, 49(8), 891-897.
- Che, Y.; Zhang, N.; Zhu, X.; Li, S.; Wang, S.; Si, H. Enhanced tolerance of the transgenic potato plants overexpressing Cu/Zn superoxide dismutase to low temperature. Sci. Hortic. 2020, 261, 108949. [Google Scholar] [CrossRef]
- Shafi, A.; Pal, A.K.; Sharma, V.; Kalia, S.; Kumar, S.; Ahuja, P.S.; Singh, A.K. Transgenic Potato Plants Overexpressing SOD and APX Exhibit Enhanced Lignification and Starch Biosynthesis with Improved Salt Stress Tolerance. Plant Mol. Biol. Rep. 2017, 35, 504–518. [Google Scholar] [CrossRef]
- Ahmad, R., Kim, Y. H., Kim, M. D., Kwon, S. Y., Cho, K., Lee, H. S., & Kwak, S. S. (2010). Simultaneous expression of choline oxidase, superoxide dismutase and ascorbate peroxidase in potato plant chloro-plasts provides synergistically enhanced protection against various abiotic stresses. Physiologia Plantarum, 138(4), 520-533.
- Watkinson, J. I., Hendricks, L., Sioson, A. A., Vasquez-Robinet, C., Stromberg, V., Heath, L. S., ... & Grene, R. (2006). Accessions of Solanum tuberosum ssp. andigena show differences in photosynthetic recovery after drought stress as reflected in gene expression profiles. Plant Science, 171(6), 745-758.
- Joshi, R., Karan, R., Singla-Pareek, S. L., & Pareek, A. (2016). Ectopic expression of Pokkali phospho-glycerate kinase-2 (OsPGK2-P) improves yield in tobacco plants under salinity stress. Plant Cell Reports, 35, 27-41.
- Suzuki, Y., Konno, Y., Takegahara-Tamakawa, Y., Miyake, C., & Makino, A. (2022). Effects of suppression of chloroplast phosphoglycerate kinase on photosynthesis in rice. Photosynthesis Research, 153(1-2), 83-91.
- Lei, J., Teng, X., Wang, Y., Jiang, X., Zhao, H., Zheng, X., Ren, Y., Dong, H., Wang, Y., Duan, E., Zhang, Y., Zhang, W., Yang, H., Chen, X., Chen, R., Zhang, Y., Yu, M., Xu, S., Bao, X., Zhang, P., Liu, S., Tian, Y., Jiang, L., Wang, Y. & Wan, J. (2022). Plastidic pyruvate dehydrogenase complex E1 component subunit Alpha1 is involved in galactolipid biosynthesis required for amyloplast development in rice. Plant Bio-technology Journal, 20(3), 437-453.
- Guo, P., Baum, M., Grando, S., Ceccarelli, S., Bai, G., Li, R., ... & Valkoun, J. (2009). Differentially ex-pressed genes between drought-tolerant and drought-sensitive barley genotypes in response to drought stress during the reproductive stage. Journal Of Experimental Botany, 60(12), 3531-3544.
- Huang, S.; Xin, S.; Xie, G.; Han, J.; Liu, Z.; Wang, B.; Zhang, S.; Wu, Q.; Cheng, X. Mutagenesis reveals that the rice OsMPT3 gene is an important osmotic regulatory factor. Crop. J. 2020, 8, 465–479. [Google Scholar] [CrossRef]
- He, K.; Zhao, Z.; Ren, W.; Chen, Z.; Chen, L.; Chen, F.; Mi, G.; Pan, Q.; Yuan, L. Mining genes regulating root system architecture in maize based on data integration analysis. Theor. Appl. Genet. 2023, 136, 127. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Tu, B.; Yang, W.; Yuan, H.; Li, J.; Guo, L.; Zheng, L.; Chen, W.; Zhu, X.; Wang, Y.; et al. Mitochon-dria-Associated Pyruvate Kinase Complexes Regulate Grain Filling in Rice. Plant Physiol. 2020, 183, 1073–1087. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y., Li, S., Jiao, G., Sheng, Z., Wu, Y., Shao, G., Xie, L., Peng, C., Xu, J., Tang, S., Wei, X. & Hu, P. (2018). Os PK 2 encodes a plastidic pyruvate kinase involved in rice endosperm starch synthesis, com-pound granule formation and grain filling. Plant Biotechnology Journal, 16(11), 1878-1891.
- Awana, M.; Jain, N.; Samota, M.K.; Rani, K.; Kumar, A.; Ray, M.; Gaikwad, K.; Praveen, S.; Singh, N.K.; Singh, A. Protein and gene integration analysis through proteome and transcriptome brings new insight into salt stress tolerance in pigeonpea (Cajanus cajan L. ). Int. J. Biol. Macromol. 2020, 164, 3589–3602. [Google Scholar] [CrossRef] [PubMed]
- Salekdeh, G. H., Siopongco, J., Wade, L. J., Ghareyazie, B., & Bennett, J. (2002). Proteomic analysis of rice leaves during drought stress and recovery. PROTEOMICS: International Edition, 2(9), 1131-1145.
- Riccardi, F., Gazeau, P., de Vienne, D., & Zivy, M. (1998). Protein changes in response to progressive water deficit in maize: quantitative variation and polypeptide identification. Plant physiology, 117(4), 1253-1263.
- Xing, Q.; Bi, G.; Cao, M.; Belcour, A.; Aite, M.; Mo, Z.; Mao, Y. Comparative Transcriptome Analysis Pro-vides Insights into Response of Ulva compressa to Fluctuating Salinity Conditions. J. Phycol. 2021, 57, 1295–1308. [Google Scholar] [CrossRef] [PubMed]
- Perveen, N.; Dinesh, M.R.; Sankaran, M.; Ravishankar, K.V.; Krishnajee, H.G.; Hanur, V.S.; Alamri, S.; Kesawat, M.S.; Irfan, M. Comparative transcriptome analysis provides novel insights into molecular re-sponse of salt-tolerant and sensitive polyembryonic mango genotypes to salinity stress at seedling stage. Front. Plant Sci. 2023, 14. [Google Scholar] [CrossRef]
- Uematsu, K.; Suzuki, N.; Iwamae, T.; Inui, M.; Yukawa, H. Increased fructose 1,6-bisphosphate aldolase in plastids enhances growth and photosynthesis of tobacco plants. J. Exp. Bot. 2012, 63, 3001–3009. [Google Scholar] [CrossRef]
- Talebi, A. F., Tohidfar, M., Bagheri, A., Lyon, S. R., Salehi-Ashtiani, K., & Tabatabaei, M. (2014). Manipu-lation of carbon flux into fatty acid biosynthesis pathway in Dunaliella salina using AccD and ME genes to enhance lipid content and to improve produced biodiesel quality. Biofuel Research Journal, 1(3), 91-97.
- Chen, D. , Yuan, X., Liang, L., Liu, K., Ye, H., Liu, Z.,... & Xue, T. (2019). Overexpression of acetyl-CoA carboxylase increases fatty acid production in the green alga Chlamydomonas reinhardtii. Biotechnology letters, 41, 1133-1145.
- Ma, C.; Ren, H.; Xing, D.; Xie, G.; Ren, N.; Liu, B. Mechanistic understanding towards the effective lipid production of a microalgal mutant strain Scenedesmus sp. Z-4 by the whole genome bioinformation. J. Hazard. Mater. 2019, 375, 115–120. [Google Scholar] [CrossRef]
- Madoka, Y., Tomizawa, K. I., Mizoi, J., Nishida, I., Nagano, Y., & Sasaki, Y. (2002). Chloroplast transfor-mation with modified accD operon increases acetyl-CoA carboxylase and causes extension of leaf longev-ity and increase in seed yield in tobacco. Plant and cell physiology, 43(12), 1518-1525.
- Zhang, Y.; Zeng, D.; Liu, Y.; Zhu, W. SlSPS, a Sucrose Phosphate Synthase Gene, Mediates Plant Growth and Thermotolerance in Tomato. Horticulturae 2022, 8, 491. [Google Scholar] [CrossRef]
- Liu, H., Xiu, Z., Yang, H., Ma, Z., Yang, D., Wang, H., & Tan, B. C. (2022). Maize Shrek1 encodes a WD40 protein that regulates pre-rRNA processing in ribosome biogenesis. The Plant Cell, 34(10), 4028-4044.
- Nguyen-Quoc, B.; N'Tchobo, H.; Foyer, C.H.; Yelle, S. Overexpression of sucrose phosphate synthase increases sucrose unloading in transformed tomato fruit. J. Exp. Bot. 1999, 50, 785–791. [Google Scholar] [CrossRef]
- Ishimaru, K., Hirotsu, N., Kashiwagi, T., Madoka, Y., Nagasuga, K., Ono, K., & Ohsugi, R. (2008). Over-expression of a maize SPS gene improves yield characters of potato under field conditions. Plant Produc-tion Science, 11(1), 104-107.
- Wang, K.; Bai, Z.-Y.; Liang, Q.-Y.; Liu, Q.-L.; Zhang, L.; Pan, Y.-Z.; Liu, G.-L.; Jiang, B.-B.; Zhang, F.; Jia, Y. Transcriptome analysis of chrysanthemum (Dendranthema grandiflorum) in response to low tempera-ture stress. BMC Genom. 2018, 19, 319. [Google Scholar] [CrossRef]
- Anur, R. M., Mufithah, N., Sawitri, W. D., Sakakibara, H., & Sugiharto, B. (2020). Overexpression of su-crose phosphate synthase enhanced sucrose content and biomass production in transgenic sugarcane. Plants, 9(2), 200.
- Galtier, N., Foyer, C. H., Murchie, E., Aired, R., Quick, P., Voelker, T. A., ... & Betsche, T. (1995). Effects of light and atmospheric carbon dioxide enrichment on photosynthesis and carbon partitioning in the leaves of tomato (Lycopersicon esculentum L.) plants over-expressing sucrose phosphate synthase. Journal of Exper-imental Botany, 46(special issue), 1335-1344.
- Signora, L.; Galtier, N.; Skøt, L.; Lucas, H.; Foyer, C.H. Over-expression of sucrose phosphate synthase in Arabidopsis thaliana results in increased foliar sucrose/starch ratios and favours decreased foliar carbo-hydrate accumulation in plants after prolonged growth with CO2 enrichment. J. Exp. Bot. 1998, 49, 669–680. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Meng, Y.; Ma, X.; Lai, Y.; Si, E.; Yang, K.; Ren, P.; Shang, X.; Wang, H. Transcriptomic profiling of the salt-stress response in the halophyte Halogeton glomeratus. BMC Genom. 2015, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Stevanato, P.; Lv, C.; Li, R.; Geng, G. Comparative Physiological and Proteomic Analysis of Two Sugar Beet Genotypes with Contrasting Salt Tolerance. J. Agric. Food Chem. 2019, 67, 6056–6073. [Google Scholar] [CrossRef] [PubMed]
- Kappachery, S.; Baniekal-Hiremath, G.; Yu, J.W.; Park, S.W. Effect of over-and under-expression of glyceraldehyde 3-phosphate dehydrogenase on tolerance of plants to water-deficit stress. Plant Cell, Tis-sue Organ Cult. (PCTOC) 2014, 121, 97–107. [Google Scholar] [CrossRef]
- Zhao, X., Hong, H., Wang, J., Zhan, Y., Teng, W., Li, H., ... & Han, Y. (2022). Genome-wide identification and analysis of glyceraldehyde-3-phosphate dehydrogenase family reveals the role of GmGAPDH14 to improve salt tolerance in soybean (Glycine max L.).
- Lim, H.; Hwang, H.; Kim, T.; Kim, S.; Chung, H.; Lee, D.; Kim, S.; Park, S.; Cho, W.; Ji, H.; et al. Tran-scriptomic Analysis of Rice Plants Overexpressing PsGAPDH in Response to Salinity Stress. Genes 2021, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.-J.; Park, S.-C.; Byun, M.-O. Improvement of Salt Tolerance in Transgenic Potato Plants by Glyceraldehyde-3 Phosphate Dehydrogenase Gene Transfer. Mol. Cells 2001, 12, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Kuai, P.; Ye, M.; Zhou, S.; Lu, J.; Lou, Y. Overexpression of a Cytosolic 6-Phosphogluconate Dehydrogenase Gene Enhances the Resistance of Rice to Nilaparvata lugens. Plants 2020, 9, 1529. [Google Scholar] [CrossRef]
- Witzel, K., Weidner, A., SURABHI, G. K., Varshney, R. K., Kunze, G., BUCK-SORLIN, G. H., ... & MOCK, H. P. (2010). Comparative analysis of the grain proteome fraction in barley genotypes with contrasting sa-linity tolerance during germination. Plant, Cell & Environment, 33(2), 211-222.
- Spielbauer, G., Li, L., Römisch-Margl, L., Do, P. T., Fouquet, R., Fernie, A. R., ... & Settles, A. M. (2013). Chloroplast-localized 6-phosphogluconate dehydrogenase is critical for maize endosperm starch accumu-lation. Journal of experimental botany, 64(8), 2231-2242.
- Tesfaye, M., Temple, S. J., Allan, D. L., Vance, C. P., & Samac, D. A. (2001). Overexpression of malate dehydrogenase in transgenic alfalfa enhances organic acid synthesis and confers tolerance to aluminum. Plant physiology, 127(4), 1836-1844.
- Zhang, Y.; Wang, Y.; Sun, X.; Yuan, J.; Zhao, Z.; Gao, J.; Wen, X.; Tang, F.; Kang, M.; Abliz, B.; et al. Ge-nome-Wide Identification of MDH Family Genes and Their Association with Salt Tolerance in Rice. Plants 2022, 11, 1498. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.-X.; Dong, Q.-L.; Zhai, H.; You, C.-X.; Hao, Y.-J. The functions of an apple cytosolic malate dehy-drogenase gene in growth and tolerance to cold and salt stresses. Plant Physiol. Biochem. 2011, 49, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q. J., Sun, H., Dong, Q. L., Sun, T. Y., Jin, Z. X., Hao, Y. J., & Yao, Y. X. (2016). The enhancement of tolerance to salt and cold stresses by modifying the redox state and salicylic acid content via the cytosolic malate dehydrogenase gene in transgenic apple plants. Plant biotechnology journal, 14(10), 1986-1997.
- Beeler, S.; Liu, H.-C.; Stadler, M.; Schreier, T.; Eicke, S.; Lue, W.-L.; Truernit, E.; Zeeman, S.C.; Chen, J.; Kötting, O. Plastidial NAD-Dependent Malate Dehydrogenase Is Critical for Embryo Development and Heterotrophic Metabolism in Arabidopsis. Plant Physiol. 2014, 164, 1175–1190. [Google Scholar] [CrossRef]
- Behera, D., Swain, A., Karmakar, S., Dash, M., Swain, P., Baig, M. J., & Molla, K. A. (2023). Overexpres-sion of Setaria italica phosphoenolpyruvate carboxylase gene in rice positively impacts photosynthesis and agronomic traits. Plant Physiology and Biochemistry, 194, 169-181.
- Fan, Z.; Li, J.; Lu, M.; Li, X.; Yin, H. Overexpression of phosphoenolpyruvate carboxylase from Jatropha curcas increases fatty acid accumulation in Nicotiana tabacum. Acta Physiol. Plant. 2013, 35, 2269–2279. [Google Scholar] [CrossRef]
- Rolletschek, H.; Borisjuk, L.; Radchuk, R.; Miranda, M.; Heim, U.; Wobus, U.; Weber, H. Seed-specific expression of a bacterial phosphoenolpyruvate carboxylase in Vicia narbonensis increases protein content and improves carbon economy. Plant Biotechnol. J. 2004, 2, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Häusler, R. E., Kleines, M., Uhrig, H., Hirsch, H. J., & Smets, H. (1999). Overexpression of phospho enol pyruvate carboxylase from Corynebacterium glutamicum lowers the CO2 compensation point (Γ*) and enhances dark and light respiration in transgenic potato. Journal of Experimental Botany, 50(336), 1231-1242.
- Wu, D., Hou, Y., Cheng, J., Han, T., Hao, N., Zhang, B., ... & Chen, S. (2022). Transcriptome analysis of lipid metabolism in response to cerium stress in the oleaginous microalga Nannochloropsis oculata. Sci-ence of The Total Environment, 838, 156420.
- Seong, E.S.; Jeon, M.R.; Choi, J.H.; Yoo, J.H.; Lee, J.G.; Na, J.K.; Kim, N.Y.; Yu, C.Y. Overexpression of S-Adenosylmethionine Synthetase Enhances Tolerance to Cold Stress in Tobacco. Russ. J. Plant Physiol. 2020, 67, 242–249. [Google Scholar] [CrossRef]
- Zhu, H.; He, M.; Jahan, M.S.; Wu, J.; Gu, Q.; Shu, S.; Sun, J.; Guo, S. CsCDPK6, a CsSAMS1-Interacting Protein, Affects Polyamine/Ethylene Biosynthesis in Cucumber and Enhances Salt Tolerance by Overex-pression in Tobacco. Int. J. Mol. Sci. 2021, 22, 11133. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. H., Ahn, J. W., Park, E. J., & Choi, J. I. (2023). Overexpression of S-Adenosylmethionine Synthetase in Recombinant Chlamydomonas for Enhanced Lipid Production. Journal of Microbiology and Biotechnolo-gy, 33(3), 310.
- Ma, C.; Wang, Y.; Gu, D.; Nan, J.; Chen, S.; Li, H. Overexpression of S-Adenosyl-l-Methionine Synthetase 2 from Sugar Beet M14 Increased Arabidopsis Tolerance to Salt and Oxidative Stress. Int. J. Mol. Sci. 2017, 18, 847. [Google Scholar] [CrossRef]
- Kim, E. Y., Park, K. Y., Seo, Y. S., & Kim, W. T. (2016). Arabidopsis small rubber particle protein homolog SRPs play dual roles as positive factors for tissue growth and development and in drought stress re-sponses. Plant physiology, 170(4), 2494-2510.
- Gong, B., Li, X., VandenLangenberg, K. M., Wen, D., Sun, S., Wei, M., ... & Wang, X. (2014). Overexpres-sion of S-adenosyl-l-methionine synthetase increased tomato tolerance to alkali stress through polyamine metabolism. Plant biotechnology journal, 12(6), 694-708.
- Godge, M. R., Kumar, D., & Kumar, P. P. (2008). Arabidopsis HOG1 gene and its petunia homolog PET-CBP act as key regulators of yield parameters. Plant cell reports, 27, 1497-1507.
- Yang, Y.; Zhu, G.; Li, R.; Yan, S.; Fu, D.; Zhu, B.; Tian, H.; Luo, Y.; Zhu, H. The RNA Editing Factor SlORRM4 Is Required for Normal Fruit Ripening in Tomato. Plant Physiol. 2017, 175, 1690–1702. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Bhoomika, K.; Dubey, R.S. Differential responses of antioxidative defense system to prolonged salinity stress in salt-tolerant and salt-sensitive Indica rice (Oryza sativa L. ) seedlings. Protoplasma 2011, 250, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Xu, D.; Xiang, D.; Jiang, L.; Hu, H. Serine Hydroxymethyltransferase 1 Is Essential for Primary-Root Growth at Low-Sucrose Conditions. Int. J. Mol. Sci. 2022, 23, 4540. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Rao, Y.; Wang, M.; Li, Y.; Liu, Y.; Xiong, P.; Zeng, L. Characterization of the SWEET Gene Family in Longan (Dimocarpus longan) and the Role of DlSWEET1 in Cold Tolerance. Int. J. Mol. Sci. 2022, 23, 8914. [Google Scholar] [CrossRef] [PubMed]
- Wang, S., Li, X., Zhu, J., Liu, H., Liu, T., Yu, G., & Shao, M. (2021). Covalent interaction between high hydrostatic pressure-pretreated rice bran protein hydrolysates and ferulic acid: Focus on antioxidant ac-tivities and emulsifying properties. Journal of Agricultural and Food Chemistry, 69(27), 7777-7785.
- Gorelova, V., De Lepeleire, J., Van Daele, J., Pluim, D., Meï, C., Cuypers, A., ... & Van Der Straeten, D. (2017). Dihydrofolate reductase/thymidylate synthase fine-tunes the folate status and controls redox ho-meostasis in plants. The Plant Cell, 29(11), 2831-2853.
- Maniga, A. (2017). Studies on the meristematic and E2F-dependent gene expression in Arabidopsis thali-ana plants. Cellular Biology, 17(9), 5077-5086.
- Albani, D.; Giorgetti, L.; Pitto, L.; Luo, M.; Cantoni, R.M.; Pujada, M.E.; Rotino, G.L.; Cella, R. Prolifera-tion-dependent pattern of expression of a dihydrofolate reductase-thymidylate synthase gene from Dau-cus carota. Eur. J. Histochem. 2005, 49, 107–116. [Google Scholar] [PubMed]
| RPS5A | Ribosomal protein. Arabidopsis | Growth retardation and floral and vascular defects and the recessive, embryo lethality | Weijers et al. 2001 [2] |
| RPS9 | Ribosomal protein. Maize | Embryo lethal | Ma and Dooner, 2004 [3] |
| RPS13A | Ribosomal protein | Aberrant leaf and trichome morphology, retarded root growth and late flowering. | Ito et al. 2000 [4] |
| RPS16 | Ribosomal protein. Arabidopsis | Embryo lethal | Tsugeki et al. 1996 [5] |
| RPS17 | Ribosomal protein. Maize | Seedling lethal | Schultes et al. 2000 [6] |
| RPS18A | Ribosomal protein. Tobacco | Essential for survival, lethal. | Rogalski et al. 2006 [7] |
| RPS20 | Ribosomal protein. Rice | Seedling lethal | Gong et al. 2013 [8] |
| RPS21 | Ribosomal protein. | Decreased leaf pigmentation, plant growth and photosynthetic activity | Morita et al. 2004 [9] |
| RPS22 | Ribosomal protein. Arabidopsis | No detectable alteration in growth | Tiller et al. 2012 [10] |
| RPS23 | Ribosomal protein. Arabidopsis | Light-green phenotype and retarded growth severely disrupted mesophyll differentiation | Tiller et al. 2012 [10] |
| RPS27 | Ribosomal protein. Arabidopsis | Embryo lethal | Revenkova et al. 1999 [11] |
| RPL3 | Ribosomal protein. Rice | Seedling lethal | Lee et al. 2019 [12] |
| RPL5C | Ribosomal. Arabidopsis | Embryo lethal | Dupouy et al. 2022 [13] |
| RPL9C, RPL9D | Ribosomal protein. Arabidopsis | Embryo lethal | Devis et al. 2015 [14] |
| RPL10 | Ribosomal protein. Arabidopsis, Maize | Embryo lethal | Falcone et al. 2010 [15] |
| RPL11 | Ribsomal protein. Arabidopsis | Significantly decreased leaf pigmentation, plant growth and photosynthetic activity | Pesaresi et al. 2001 [16] |
| RPL12 | Ribosomal protein. Rice | Seeling lethal | Zhao et al. 2016 [17] |
| RPL13 | Ribsomal protein. Rice | Embryo lethal | Lee et al. 2019 [18] |
| RPL15C | Ribosomal protein. | Embryo lethal | Bobik et al. 2019 [19] |
| RPL21C | Ribosomal protein. Arabidopsis, Rice | Embryo lethal | Yin et al. 2021 [20] , Lin et al. 2015 [21] |
| RPL23a | Ribosomal protein, ribosome biogenesis, Arabidopsis | RPL23aA RNAi: growth delay, irregularities in morphology of leaves, roots, phyllotaxy and vasculature, and loss of apical dominance | Degenhardt and Bonham-Smith, 2008 [22] |
| RPL24B | Ribosomal protein. Arabidopsis | Auxin-related developmental defects, in cotyledon number and vascularization | Zhou et al. 2010. [23] |
| RPL28-1 | Ribosomal protein. Arabidopsis | Embryo lethal | Romani et al. 2012 [24] |
| RPL35-1 | Ribosomal protein. Maize | Embryo lethal | Magnard et al. 2004 [25] |
| RPS20, RPL1, RPL4, RPL27 and RPL35 | Ribosomal proteins. Arabidopsis | Embryo lethal | Romani et al. 2012 [24] |
| Chloroplast/Ribosome biogenesis factors | |||
| EDD1 (GlyRS9) | Glycyl tRNA synthetase. Arabidopsis | Embryo lethal | Uwer et al. 1998 [26] |
| CFG1, CFG2 | Chloroplast development. Arabidopsis | Seedling lethal | Zhu et al. 2020 [27] |
| DCL-M | Defective chloroplast and leaf-mutable. Tomato | Embryo lethal | Bellaoui et al. 2003 [28] |
| CPN21 | Chaperonin: Tomato, Tobacco | Seed abortion | Hanania et al. 2006 [29] |
| AtBRX-1-1, AtBRX-1-2 | Maturation of the large pre-60S ribosomal subunit | Pointed leaf and delay early growth. | Weis et al. 2015 [30] |
| AtNuc-L1-AtNuc-L2 | Ribosome biogenesis. Arabidopsis | Seedling lethal | Durut et al. 2015 [31] |
| AtTHAL | Nucleolar organization | thal2 embryo lethal | Chen et al. 2016 [32] |
| AtNMD3 | Nuclear export adaptor of 60S pre-ribosome export and maturation | Lethal | Chen et al. 2012 [33] |
| 30 Ribosome biogenesis factors | Pre-rDNA transcription, pre-rRNA processing, modification, folding, and assembly with RPs | Gene disruptions: infertility, embryo lethality, impaired growth and gametophyte development, aberrant cotyledon, leaf and root development | Weis et al. 2015 [34] |
| RID1 | DEAH-box RNA helicase, Pre-mRNA Splicing | rid1-1: abnormalities in shoot and root apical meristem maintenance, leaf and root morphogenesis | Ohtani et al. 2013 [35] |
| TIC32 | Translocon of the inner envelope of chloroplasts | Embryo lethal | Hörmann et al. 2007 [36] |
| ATS2 | Phosphatidic acid as intermediate for chloroplast membrane lipid biosynthesis. | Embryo lethal | Yu et al. 2004 [37] |
| TIC110 | Translocon of the inner envelope of chloroplasts | Embryo lethal | Kovacheva et al. 2005 [38] |
| CHL27 | Chlorophyl biosynthesis | Retarded growth and chloroplast developmental defects | Bang et al. 2008 [39] |
| DG1 | Early chloroplast development | Delayed greening phenotype | Chi et al. 2008 [40] |
| OEP80 | Chloroplast Outer Envelope Protein | Embryo lethal | Patel et al. 2008 [41] |
| EMB5067/AKRP | Embryo development chloroplast protein | Embryo lethal | Garcion et al. 2006 [42] |
| SPC1 | Carotenoid biosynthesis | Embryo lethal | Dong et al. 2007 [43] |
| PDS3 | phytoene desaturase gene, | Embryo lethal | Qian et al. 2007 [44] |
| EMB1303-1 | Chloroplast biogenesis | Embryo lethal | Huang et al. 2009 [45] |
| EMB1211 | Chloroplast biogenesis | Seedling lethality | Liang et al. 2010 [46] |
| BPG2 | Chloroplast protein accumulation induced by Brassinazole | Decreased number of stacked grana thylakoids | Komatsu et al. 2010 [47] |
| 119 Nuclear genes-assoc. w/chloroplast | Embryo deffective mutants/ associated to chloroplast | Embryo lethal | Bryant et al. 2011 [48] |
| IRM | Involved in RNA processing | Embryo lethal | Palm et al. 2019 [49] |
| ZMRH3 | The RH3 DEAD Box Helicase | Embryo lethal | Asakura et al. 2012 [50] |
| HSP90C | Chloroplast biogenesis | Embryo lethal | Inoue et al. 2013 [51] |
| FTSHI4 | Thylakoid membrane-associated protein | Embryo lethal | Lu et al. 2014 [52] |
| RNAJ | Ribonuclease J (RNase J) required for chloroplast and embryo development | Embryo lethal | Chen et al. 2015 [53] |
| DER | Chloroplast ribosomal RNA processing | Embryo lethal | Jeon et al. 2014 [54] |
| Rrp5, Pwp2, Nob1, Enp1 and Noc4 | Ribosome biogenesis factors | Embryo lethal | Missbach et al. 2013 [55] |
| SHREK1 | Ribosome biogenesis factor | Embryo lethal | Liu et al. 2022 [56] |
| NOP2A,NOP2B | tRNA and rRNA methylation profiles | Embryo lethal | Burgess et al. 2015 [57] |
| RH22 | RNA helicase22 | Embryo lethal | Chi et al. 2012 [58] |
| MDN1 | The AAA-ATPase MIDASIN 1 functions in ribosome biogenesis | Embryo lethal | Li et al. 2019 [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





