Submitted:
13 June 2023
Posted:
16 June 2023
You are already at the latest version
Abstract
Keywords:
Introduction
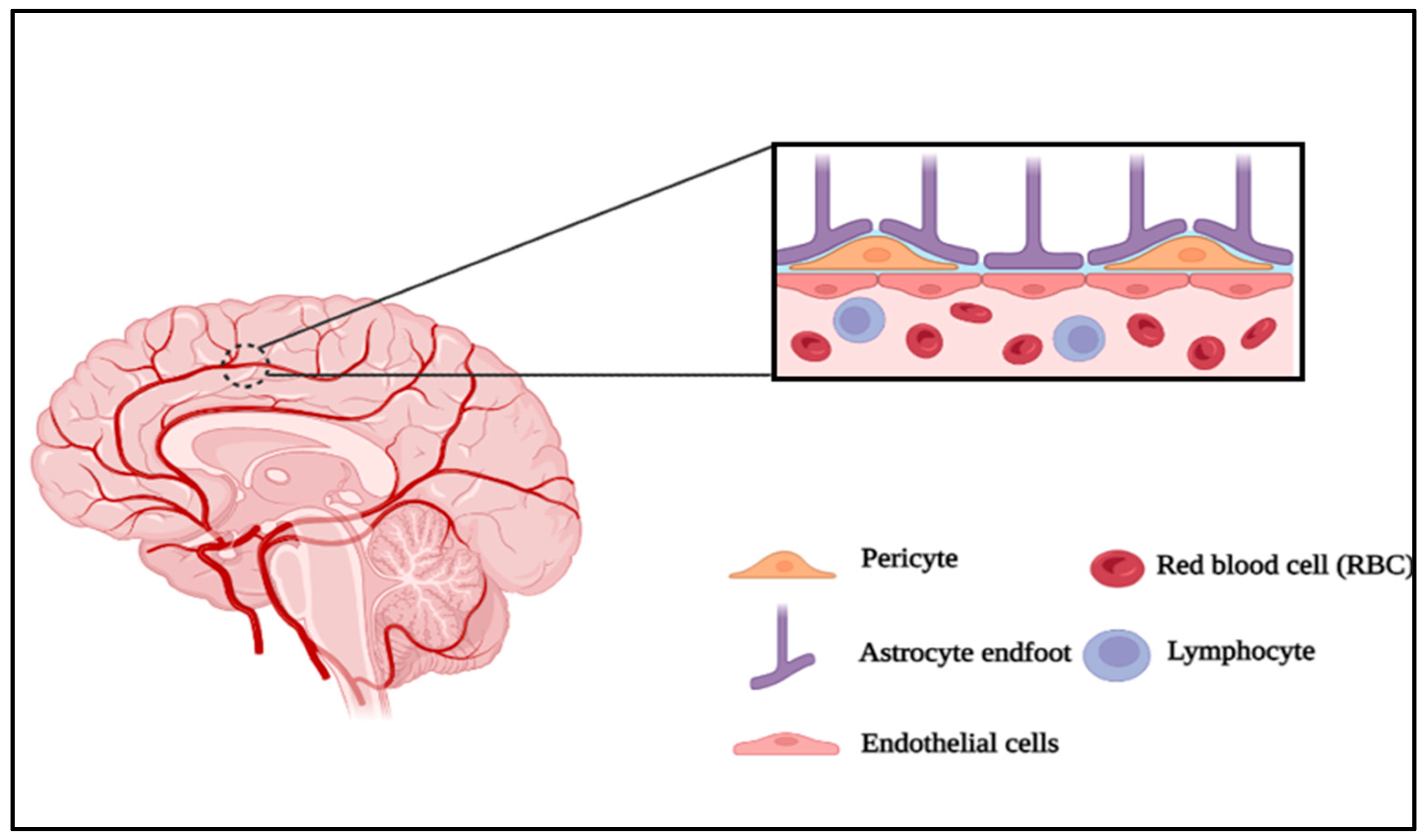
Neurovascular unit and Microfluidics:
1. Molecular and Cellular BBB Players: Roles of NVU non-endothelial cells in BBB formation and function
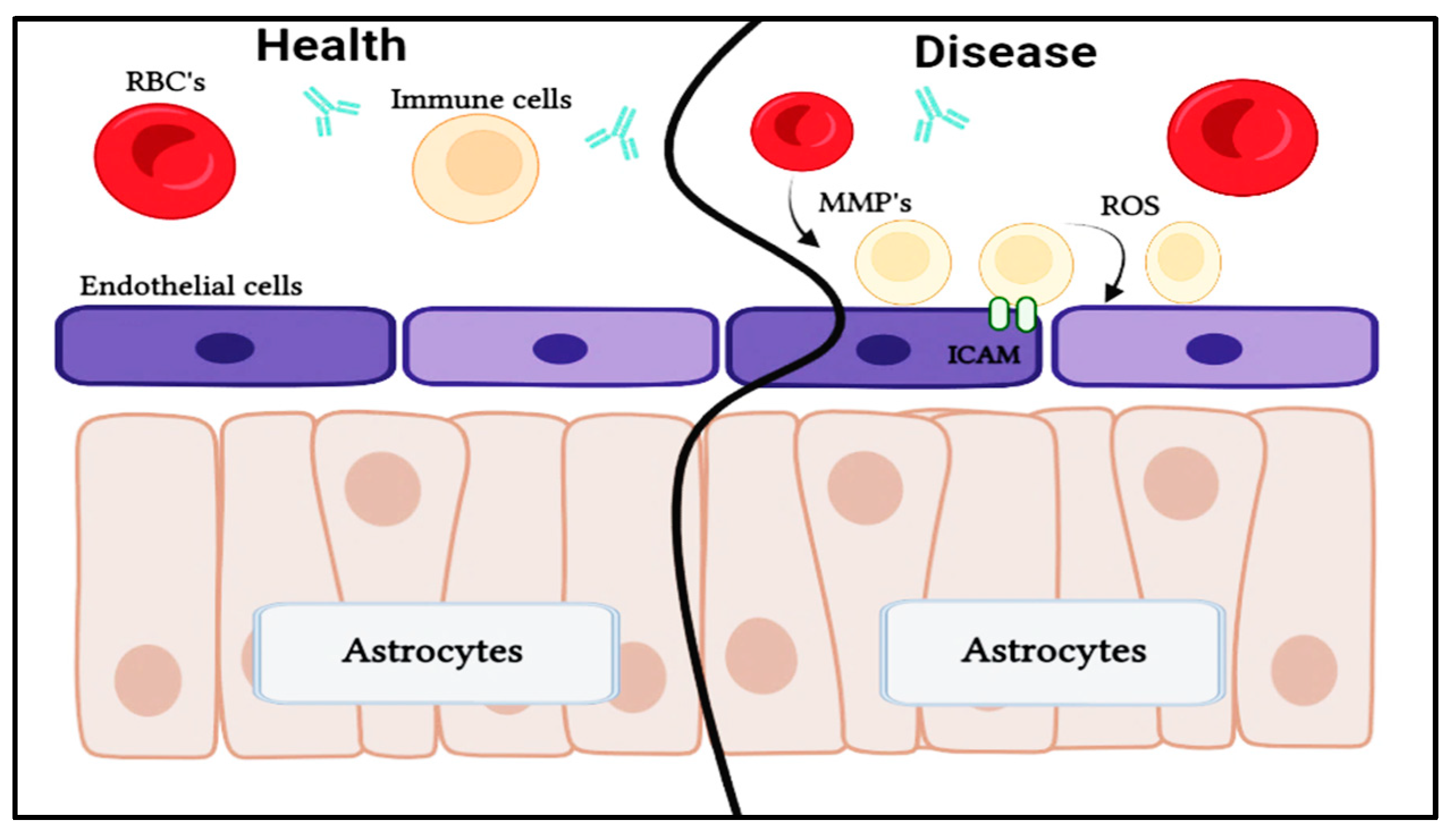
Astrocytes
Endothelial cells
Tight junctions
Transporters
Soluble Lipid Carrier (SLC) Transporters
| Category | Property | Relevance | Validation | References |
|---|---|---|---|---|
| Tight junctions | Occludin claudin-5 ZO-1 | Transendothelial transport and uptake investigations, tight junction exploration, and cell polarization research | mRNA and protein expression localization | [37] |
| High junctional tightness | TEER and permeability measurements | [38] | ||
| Efflux transporters | P-pg | Drug delivery to/through the BBB, transendothelial transport and absorption studies and toxicity | mRNA and protein expression Cellular uptake or efflux in absence/presence of inhibitors bi-directional transport studies | [39] |
| BCRP | [40] | |||
| Mrp | [41] | |||
| SLC expression | Glut-1 | Drug distribution to/through the BBB: investigations on transendothelial transport and uptake, studies on brain nutrition | mRNA and protein expression – Cellular uptake in absence/presence of inhibitors – transendothelial transport studies | [42] |
| LAT-1 | [43] | |||
| MCT-1 | [44] | |||
| Receptor systems | Transferrin receptor | Findings on brain nutrition and receptor-mediated transport | mRNA and protein expression – transferrin uptake – transendothelial transport of iron | [45] |
| Responsiveness to regulation from NVU cells | Induction by astrocytes | Review on NVU signalling and cell regulation | Regulation of TEER, P-glycoprotein expression and cell morphology | [46] |
| Induction by pericytes | Regulation of TEER, proteins involved in vesicular transport | [47] |
Extracellular matrix proteins
2. Microfluidics
2.1. Microfluidic Systems in the Study of Endothelial Astrocyte Interactions
2.2. Astrocyte-Endothelial Interactions and Regulation
2.3. BBB Function Regulation by Endothelial-Astrocyte-Derived Biochemical Factors
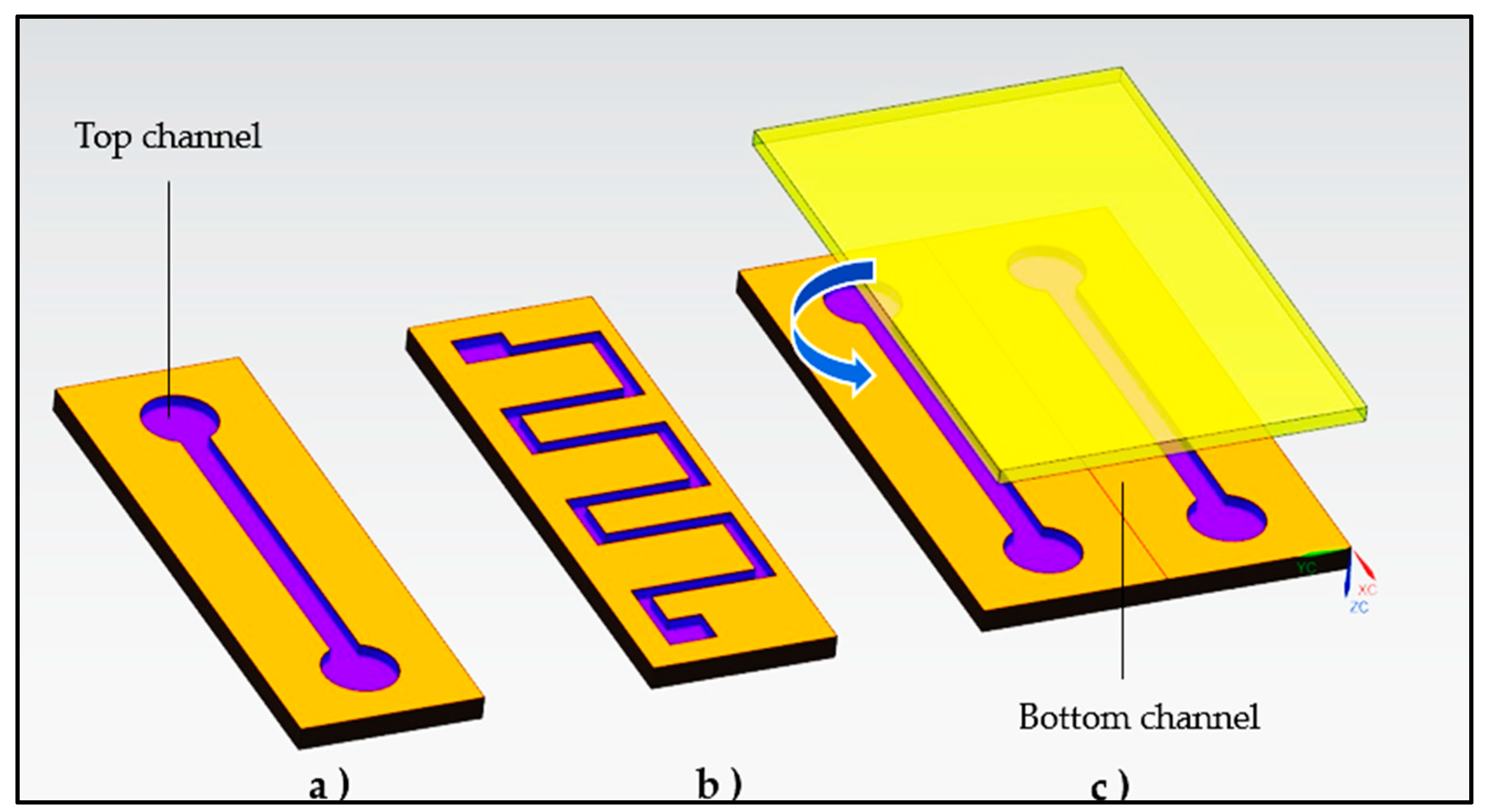
2.4. Most Common Designs and Features of the Chips for Brain Endothelial-Astrocyte Interactions
3. Design Considerations
- The design efficiently mimics the microvascular system of the BBB and to understand the pathophysiological origins of neuro diseases. According to bioengineering theory, an ideal in vitro BBB replica should consist of three main components: A selection of endothelial cells that exhibit the multicellular longitudinal and radial blood vessel architecture. To achieve this, endothelial cells can be ring-shaped or linear in longitudinal section (similar to the radial cross-section of a blood artery). Micropatterning can provide mechanistic information about endothelial cell morphology [83];
- Transwell® chambers that replicate the function of the BBB micro-vessels by maintaining constant and continuous blood flow. The use of a microfluidics-based perfusion system can provide information about disease pathology caused by endothelial functions. In this in vitro NVU, astrocytes maintain apicobasal polarity at the tissue-tissue interface by wrapping their end-feet around the endothelial capillaries on the transluminal surface (i.e., away from the blood vessel lumen) [84].
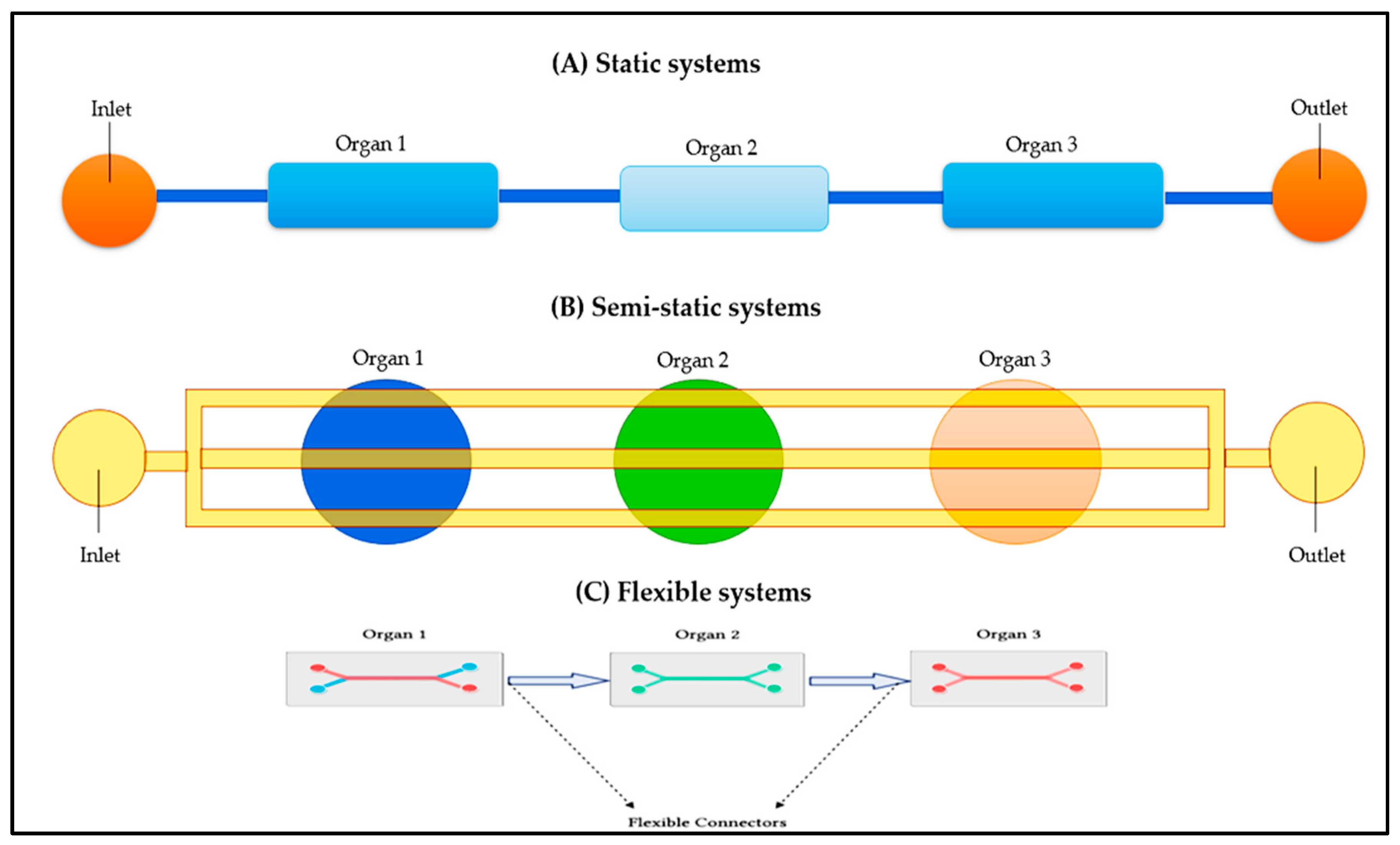
3.1. Various Approaches for the Integration to Multi-Organ Devices
3.2. Fabrication of Microfluidics Devices: Types of Materials
| Material | Advantage | Disadvantage | References |
| Polydimethylsiloxane (PDMS) | Good Gas permeability Good Biocompatibility Good Optical property (Transparency) Good Mechanical property (Elasticity) |
Poor Chemical resistance Expensive |
[89] |
| Poly(methyl methacrylate) (PMMA) | Good Biocompatibility Good Transparency Good Chemical resistance Inexpensive |
Rigid Elasticity | [90] |
| Glass | High Transmittance High processing accuracy |
Extremely fragile Expensive |
[91] |
| Polylactic acid | Biocompatible Transparent Low-cost |
Lower thermal stability | [92] |
| Epoxy resins (Thermosetting polymers) |
Good Biocompatibility Good mechanical Good Chemical resistance Thermal stability |
Expensive Time-consuming |
[93] |
| Polystyrene (Thermoplastic polymer) |
Good Biocompatible Good Transparency |
Poor chemical resistance Poor Elasticity |
[94] |
| Others Polyimide Polycarbonate Cyclic olefin copolymer |
Good Biocompatibility Good Biocompatibility Good Biocompatibility |
Poor Transparency and Elasticity Poor Transparency Poor Transparency |
[95] |
4. In-vitro models of BBB with endothelial astrocytes
Hydrogels for Modeling
Endothelial-Astrocytic Interactions in a microfluidic device:
| Device design | Cells/Co-culture cells | Physiological function | TEER | Fluorescent tracker (probes for permeability assay) | References |
| PDMS | End3 (mouse)/ C8D1A (mouse) |
Co-culture with astrocytes increases BBB integrity | 250–300 Ω cm2 |
1.4 kDa | [106] |
| PDMS sandwich |
hiPS derived BMEC (human)/ primary astrocyte (rat) | Co-culture with astrocyte will enhance BBB integrity | 4000 to 5000 Ω·cm2 | 70 kDa | [107] |
| Transwell® | Endothelial cells directly interfaced with astrocytes via a system of capillaries. | Plays a role in controlling capillary features and BBB permeability | - | 0.16 | [108] |
| 3D ECM gel-based | RBE4, immortalized rat brain micro vessel endothelial cell line | Transmigration of neutrophils | NA | 40 kDa | [109] |
| 3D scaffolds Hydrogel GelMA and PEGDA | hPSC’s | Appropriate mechanical properties and bioactive sites, which are beneficial for cells viability. | - | - | [110] |
| Microfluidics, hydrogel | Human umbilical vein endothelial cells (HUVECs) | Generating a three-dimensional BBB microfluidic platform which presents both structural and functional properties of the BBB in vivo. | - | 10 kDa | [111] |
| PDMS | cEND (immortalized mouse cerebral endothelial cells) | Strong occludin and claudin-5 expression at the tight junctions | 300 -800 | NA | [112] |
5. CNS dysfunction in disorders
Alzheimer's disease
Parkinson’s Disease
Multiple Sclerosis
Huntington’s Disease
Autism Spectrum Disorder
Neurofibromatosis
Guillain-Barre Syndrome
Microfluidics: A powerful tool for studying the intricate aspects of rare neurological conditions at a microscale level
Outlook
Acknowledgments
References
- Kovarik, M. L.; Gach, P. C.; Ornoff, D. M.; Wang, Y.; Balowski, J.; Farrag, L.; Allbritton, N. L. Micro Total Analysis Systems for Cell Biology and Biochemical Assays. Anal. Chem. 2012, 84, 516–540. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Hassan, S.; Miri, A. K.; Maharjan, S.; Al-kharboosh, R.; Quiñones-Hinojosa, A.; Zhang, Y. S. Mimicking Human Pathophysiology in Organ-on-Chip Devices. Adv. Biosyst. 2018, 2, 1–25. [Google Scholar] [CrossRef]
- Yildirimer, L.; Zhang, Q.; Kuang, S.; Cheung, C. W. J.; Chu, K. A.; He, Y.; Yang, M.; Zhao, X. Engineering Three-Dimensional Microenvironments towards in Vitro Disease Models of the Central Nervous System. Biofabrication 2019, 11. [Google Scholar] [CrossRef]
- Villabona-Rueda, A.; Erice, C.; Pardo, C. A.; Stins, M. F. The Evolving Concept of the Blood Brain Barrier (BBB): From a Single Static Barrier to a Heterogeneous and Dynamic Relay Center. Front. Cell. Neurosci. 2019, 13. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R. M. Development, Maintenance and Disruption of the Blood-Brain Barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Lippmann, E. S.; Azarin, S. M.; Kay, J. E.; Nessler, R. A.; Wilson, H. K.; Al-Ahmad, A.; Palecek, S. P.; Shusta, E. V. Derivation of Blood-Brain Barrier Endothelial Cells from Human Pluripotent Stem Cells. Nat. Biotechnol. 2012, 30, 783–791. [Google Scholar] [CrossRef]
- Wei, W.; Cardes, F.; Hierlemann, A.; Modena, M. M. 3D In Vitro Blood-Brain-Barrier Model for Investigating Barrier Insults. Adv. Sci. 2023, 2205752, 1–15. [Google Scholar] [CrossRef]
- Pijuan, J.; Barceló, C.; Moreno, D. F.; Maiques, O.; Sisó, P.; Marti, R. M.; Macià, A.; Panosa, A. In Vitro Cell Migration, Invasion, and Adhesion Assays: From Cell Imaging to Data Analysis. Front. Cell Dev. Biol. 2019, 7, 1–16. [Google Scholar] [CrossRef]
- Leung, C.M.; de Haan, P.; Ronaldson-Bouchard, K.; Kim, G.A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S.; et al. A Guide to the Organ-on-a-Chip. Nat. Rev. Methods Prim. 2022, 2. [Google Scholar] [CrossRef]
- Stewart, P. A.; Wiley, M. J. Developing Nervous Tissue Induces Formation of Blood-Brain Barrier Characteristics in Invading Endothelial Cells: A Study Using Quail--Chick Transplantation Chimeras. Dev. Biol. 1981, 84, 183–192. [Google Scholar] [CrossRef]
- Janzer, R. C.; Raff, M. C. Astrocytes Induce Blood-Brain Barrier Properties in Endothelial Cells. Nature 1987, 325, 253–257. [Google Scholar] [CrossRef]
- Etc, M.C.S.; Das, C.; Lucia, M.S.; H.K. and T.J. 乳鼠心肌提取 HHS Public Access. Physiol. Behav. 2019, 176, 139–148. [Google Scholar]
- Tontsch, U.; Bauer, H. C. Glial Cells and Neurons Induce Blood-Brain Barrier Related Enzymes in Cultured Cerebral Endothelial Cells. Brain Res. 1991, 539, 247–253. [Google Scholar] [CrossRef]
- Wang, C.-W.; Fischer, W. B. Rotational Dynamics of The Transmembrane Domains Play an Important Role in Peptide Dynamics of Viral Fusion and Ion Channel Forming Proteins-A Molecular Dynamics Simulation Study. Viruses 2022, 14. [Google Scholar] [CrossRef]
- Kubotera, H.; Ikeshima-Kataoka, H.; Hatashita, Y.; Allegra Mascaro, A. L.; Pavone, F. S.; Inoue, T. Astrocytic Endfeet Re-Cover Blood Vessels after Removal by Laser Ablation. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Lin, L.; Yee, S. W.; Kim, R. B.; Giacomini, K. M. SLC Transporters as Therapeutic Targets: Emerging Opportunities. Nat. Rev. Drug Discov. 2015, 14, 543–560. [Google Scholar] [CrossRef]
- Guarino, V.; Zizzari, A.; Bianco, M.; Gigli, G.; Moroni, L.; Arima, V. Advancements in Modelling Human Blood Brain-Barrier on a Chip. Biofabrication 2023, 15. [Google Scholar] [CrossRef]
- Potjewyd, G.; Kellett, K. A. B.; Hooper, N. M. 3D Hydrogel Models of the Neurovascular Unit to Investigate Blood-Brain Barrier Dysfunction. Neuronal Signal. 2021, 5, NS20210027. [Google Scholar] [CrossRef]
- Ge, S.; Pachter, J. S. Isolation and Culture of Microvascular Endothelial Cells from Murine Spinal Cord. J. Neuroimmunol. 2006, 177, (1–2). [Google Scholar] [CrossRef]
- Sofroniew, M. V.; Vinters, H. V. Astrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 7. [Google Scholar] [CrossRef]
- Chan, C. Y.; Goral, V. N.; DeRosa, M. E.; Huang, T. J.; Yuen, P. K. A Polystyrene-Based Microfluidic Device with Threedimensional Interconnected Microporous Walls for Perfusion Cell Culture. Biomicrofluidics 2014, 8, 1–7. [Google Scholar] [CrossRef]
- Jiang, R.; Du, X.; Brink, L.; Lönnerdal, B. The Role of Orally Ingested Milk Fat Globule Membrane on Intestinal Barrier Functions Evaluated with a Suckling Rat Pup Supplementation Model and a Human Enterocyte Model. J. Nutr. Biochem. 2022, 108, 109084. [Google Scholar] [CrossRef]
- Middelkamp, H.H.T.; Verboven, A.H.A.; De Sá Vivas, A.G.; Schoenmaker, C.; Klein Gunnewiek, T.M.; Passier, R.; Albers, C.A.; ’t Hoen, P.A.C.; Nadif Kasri, N.; van der Meer, A.D. Cell Type-Specific Changes in Transcriptomic Profiles of Endothelial Cells, IPSC-Derived Neurons and Astrocytes Cultured on Microfluidic Chips. Sci. Rep. 2021, 11, 2281. [Google Scholar] [CrossRef]
- A, L.; C, A.; F, G.; K, G.; P, C. Tight Junctions at the Blood-Brain Barrier: Physiological Architecture and Disease-Associated Dysregulation. Fluids Barriers CNS 2012, 9, 23. [Google Scholar]
- Haseloff, R. F.; Dithmer, S.; Winkler, L.; Wolburg, H.; Blasig, I. E. Transmembrane Proteins of the Tight Junctions at the Blood-Brain Barrier: Structural and Functional Aspects. Semin. Cell Dev. Biol. 2015, 38, 16–25. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, L.; Bao, L.; Liu, J.; Zhu, H.; Lv, Q.; Liu, R.; Chen, W.; Tong, W.; Wei, Q.; et al. SARS-CoV-2 Crosses the Blood-Brain Barrier Accompanied with Basement Membrane Disruption without Tight Junctions Alteration. Signal Transduct. Target. Ther. 2021, 6, 337. [Google Scholar] [CrossRef]
- Lochhead, J. J.; Yang, J.; Ronaldson, P. T.; Davis, T. P. Structure, Function, and Regulation of the Blood-Brain Barrier Tight Junction in Central Nervous System Disorders. Front. Physiol. 2020, 11, 914. [Google Scholar] [CrossRef]
- Visigalli, R.; Rotoli, B. M.; Ferrari, F.; Di Lascia, M.; Riccardi, B.; Puccini, P.; Dall’Asta, V.; Barilli, A. Expression and Function of ABC Transporters in Human Alveolar Epithelial Cells. Biomolecules 2022, 12. [Google Scholar] [CrossRef]
- Rees, D. C.; Johnson, E.; Lewinson, O. ABC Transporters: The Power to Change. Nat. Rev. Mol. Cell Biol. 2009, 10, 218–227. [Google Scholar] [CrossRef]
- De Stefani, D.; Patron, M.; R. R. 乳鼠心肌提取 HHS Public Access. Physiol. Behav. 2015, 176, 139–148. [Google Scholar]
- Xiao, Y.; Zeng, B.; Berner, N.; Frishman, D.; Langosch, D.; Teese, M. G. Experimental Determination and Data-Driven Prediction of Homotypic Transmembrane Domain Interfaces. Comput. Struct. Biotechnol. J. 2020, 18, 3230–3242. [Google Scholar] [CrossRef]
- Wilkens, S. Structure and Mechanism of ABC Transporters. F1000Prime Rep. 2015, 7, 1–9. [Google Scholar] [CrossRef]
- Sharpe, H. J.; Stevens, T. J.; Munro, S. A Comprehensive Comparison of Transmembrane Domains Reveals Organelle-Specific Properties. Cell 2010, 142, 158–169. [Google Scholar] [CrossRef]
- Geier, E. G.; Chen, E. C.; Webb, A.; Papp, A. C.; Yee, S. W.; Sadee, W.; Giacomini, K. M. Profiling Solute Carrier Transporters in the Human Blood-Brain Barrier. Clin. Pharmacol. Ther. 2013, 94, 636–639. [Google Scholar] [CrossRef]
- Mizuno, N.; Niwa, T.; Yotsumoto, Y.; Sugiyama, Y. Impact of Drug Transporter Studies on Drug Discovery and Development. Pharmacol. Rev. 2003, 55, 425–461. [Google Scholar] [CrossRef]
- Hur, J.; Smith-Warner, S.A.; Rimm, E.B.; Willett, W.C.; Wu, K.; Cao, Y. E. G. 乳鼠心肌提取 HHS Public Access. J. Int. Soc. Burn Inj. 2017, 43, 909–932. [Google Scholar]
- Nitta, T.; Hata, M.; Gotoh, S.; Seo, Y.; Sasaki, H.; Hashimoto, N.; Furuse, M.; Tsukita, S. Size-Selective Loosening of the Blood-Brain Barrier in Claudin-5-Deficient Mice. J. Cell Biol. 2003, 161, 653–660. [Google Scholar] [CrossRef]
- Smith, Q. R.; Rapoport, S. I. Cerebrovascular Permeability Coefficients to Sodium, Potassium, and Chloride. J. Neurochem. 1986, 46, 1732–1742. [Google Scholar] [CrossRef]
- Cordon-Cardo, C.; O’Brien, J. P.; Casals, D.; Rittman-Grauer, L.; Biedler, J. L.; Melamed, M. R.; Bertino, J. R. Multidrug-Resistance Gene (P-Glycoprotein) Is Expressed by Endothelial Cells at Blood-Brain Barrier Sites. Proc. Natl. Acad. Sci. U. S. A. 1989, 86, 695–698. [Google Scholar] [CrossRef]
- Zhang, W.; Mojsilovic-Petrovic, J.; Andrade, M. F.; Zhang, H.; Ball, M.; Stanimirovic, D. B. The Expression and Functional Characterization of ABCG2 in Brain Endothelial Cells and Vessels. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2003, 17, 2085–2087. [Google Scholar] [CrossRef]
- Miller, D. S.; Nobmann, S. N.; Gutmann, H.; Toeroek, M.; Drewe, J.; Fricker, G. Xenobiotic Transport across Isolated Brain Microvessels Studied by Confocal Microscopy. Mol. Pharmacol. 2000, 58, 1357–1367. [Google Scholar] [CrossRef]
- Nualart, F.; Godoy, A.; Reinicke, K. Expression of the Hexose Transporters GLUT1 and GLUT2 during the Early Development of the Human Brain. Brain Res. 1999, 824, 97–104. [Google Scholar] [CrossRef]
- Zheng, P.-P.; Romme, E.; van der Spek, P. J.; Dirven, C. M. F.; Willemsen, R.; Kros, J. M. Glut1/SLC2A1 Is Crucial for the Development of the Blood-Brain Barrier in Vivo. Ann. Neurol. 2010, 68, 835–844. [Google Scholar] [CrossRef]
- Gerhart, D. Z.; Enerson, B. E.; Zhdankina, O. Y.; Leino, R. L.; Drewes, L. R. Expression of Monocarboxylate Transporter MCT1 by Brain Endothelium and Glia in Adult and Suckling Rats. Am. J. Physiol. 1997, 273 Pt 1, E207–13. [Google Scholar] [CrossRef]
- Yu, Y.J.; Atwal, J.K.; Zhang, Y.; Tong, R.K.; Wildsmith, K.R.; Tan, C.; Bien-Ly, N.; Hersom, M.; Maloney, J.A.; Meilandt, W.J.; et al. Therapeutic Bispecific Antibodies Cross the Blood-Brain Barrier in Nonhuman Primates. Sci. Transl. Med. 2014, 6, 261ra154. [Google Scholar] [CrossRef]
- Hayashi, Y.; Nomura, M.; Yamagishi, S.; Harada, S.; Yamashita, J.; Yamamoto, H. Induction of Various Blood-Brain Barrier Properties in Non-Neural Endothelial Cells by Close Apposition to Co-Cultured Astrocytes. Glia 1997, 19, 13–26. [Google Scholar]
- Daneman, R.; Zhou, L.; Kebede, A. A.; Barres, B. A. Pericytes Are Required for Blood-Brain Barrier Integrity during Embryogenesis. Nature 2010, 468, 562–566. [Google Scholar] [CrossRef]
- Menezes, M. J.; McClenahan, F. K.; Leiton, C. V; Aranmolate, A.; Shan, X.; Colognato, H. The Extracellular Matrix Protein Laminin A2 Regulates the Maturation and Function of the Blood-Brain Barrier. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 15260–15280. [Google Scholar] [CrossRef]
- Barraza-Flores, P.; Bates, C. R.; Oliveira-Santos, A.; Burkin, D. J. Laminin and Integrin in LAMA2-Related Congenital Muscular Dystrophy: From Disease to Therapeutics. Front. Mol. Neurosci. 2020, 13, 1–9. [Google Scholar] [CrossRef]
- Xu, L.; Nirwane, A.; Xu, T.; Kang, M.; Devasani, K.; Yao, Y. Fibroblasts Repair Blood-Brain Barrier Damage and Hemorrhagic Brain Injury via TIMP2. Cell Rep. 2022, 41, 111709. [Google Scholar] [CrossRef] [PubMed]
- Cameron, T.; Bennet, T.; Rowe, E. M.; Anwer, M.; Wellington, C. L.; Cheung, K. C. Review of Design Considerations for Brain-on-a-chip Models. Micromachines 2021, 12. [Google Scholar] [CrossRef]
- Pummi, K. P.; Aho, H. J.; Laato, M. K.; Peltonen, J. T. K.; Peltonen, S. A. Tight Junction Proteins and Perineurial Cells in Neurofibromas. J. Histochem. Cytochem. 2006, 54, 53–61. [Google Scholar] [CrossRef]
- Wilson, E. R.; Della-Flora Nunes, G.; Weaver, M. R.; Frick, L. R.; Feltri, M. L. Schwann Cell Interactions during the Development of the Peripheral Nervous System. Dev. Neurobiol. 2021, 81, 464–489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.; Jia, J. Mechanisms of Smoothened Regulation in Hedgehog Signaling. Cells 2021, 10. [Google Scholar] [CrossRef]
- Akhshi, T.; Shannon, R.; Trimble, W. S. The Complex Web of Canonical and Non-Canonical Hedgehog Signaling. Bioessays 2022, 44, e2100183. [Google Scholar] [CrossRef]
- Sackmann, E. K.; Fulton, A. L.; Beebe, D. J. The Present and Future Role of Microfluidics in Biomedical Research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef]
- Wang, J. D.; Khafagy, E.-S.; Khanafer, K.; Takayama, S.; ElSayed, M. E. H. Organization of Endothelial Cells, Pericytes, and Astrocytes into a 3D Microfluidic in Vitro Model of the Blood-Brain Barrier. Mol. Pharm. 2016, 13, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Kwon, K. W.; Park, M. C.; Lee, S. H.; Kim, S. M.; Suh, K. Y. Soft Lithography for Microfluidics: A Review. Biochip J. 2008, 2, 1–11. [Google Scholar]
- Gong, M. M.; Sinton, D. Turning the Page: Advancing Paper-Based Microfluidics for Broad Diagnostic Application. Chem. Rev. 2017, 117, 8447–8480. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, N. P.; Cabot, J. M.; Smejkal, P.; Guijt, R. M.; Paull, B.; Breadmore, M. C. Comparing Microfluidic Performance of Three-Dimensional (3D) Printing Platforms. Anal. Chem. 2017, 89, 3858–3866. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Cook, B. S.; Fang, Y.; Tentzeris, M. M. Fully Inkjet-Printed Microfluidics: A Solution to Low-Cost Rapid Three-Dimensional Microfluidics Fabrication with Numerous Electrical and Sensing Applications. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Q.; Gu, M. Three-Dimensional Direct Laser Writing of Biomimetic Neuron Structures. Opt. Express 2018, 26, 32111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, H. R. A Review on Continuous-Flow Microfluidic PCR in Droplets: Advances, Challenges and Future. Anal. Chim. Acta 2016, 914, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Guo, M. T.; Rotem, A.; Heyman, J. A.; Weitz, D. A. Droplet Microfluidics for High-Throughput Biological Assays. Lab Chip 2012, 12, 2146–2155. [Google Scholar] [CrossRef]
- Adriani, G.; Ma, D.; Pavesi, A.; Kamm, R. D.; Goh, E. L. K. A 3D Neurovascular Microfluidic Model Consisting of Neurons, Astrocytes and Cerebral Endothelial Cells as a Blood-Brain Barrier. Lab Chip 2017, 17, 448–459. [Google Scholar] [CrossRef]
- Lauranzano, E.; Rasile, M.; Matteoli, M. Integrating Primary Astrocytes in a Microfluidic Model of the Blood-Brain Barrier. Methods Mol. Biol. 2022, 2492, 225–240. [Google Scholar] [CrossRef]
- Sreekanthreddy, P.; Gromnicova, R.; Davies, H.; Phillips, J.; Romero, I. A.; Male, D. A Three-Dimensional Model of the Human Blood-Brain Barrier to Analyse the Transport of Nanoparticles and Astrocyte/Endothelial Interactions. F1000Research 2015, 4, 1279. [Google Scholar] [CrossRef]
- Ding, J.; Li, H.-Y.; Zhang, L.; Zhou, Y.; Wu, J. Hedgehog Signaling, a Critical Pathway Governing the Development and Progression of Hepatocellular Carcinoma. Cells 2021, 10. [Google Scholar] [CrossRef]
- Kim, H.; Leng, K.; Park, J.; Sorets, A.G.; Kim, S.; Shostak, A.; Embalabala, R.J.; Mlouk, K.; Katdare, K.A.; Rose, I.V.L.; et al. Reactive Astrocytes Transduce Inflammation in a Blood-Brain Barrier Model through a TNF-STAT3 Signaling Axis and Secretion of Alpha 1-Antichymotrypsin. Nat. Commun. 2022, 13. [Google Scholar] [CrossRef]
- Yang, H.; Feng, G.-D.; Olivera, C.; Jiao, X.-Y.; Vitale, A.; Gong, J.; You, S.-W. Sonic Hedgehog Released from Scratch-Injured Astrocytes Is a Key Signal Necessary but Not Sufficient for the Astrocyte de-Differentiation. Stem Cell Res. 2012, 9, 156–166. [Google Scholar] [CrossRef]
- Osaki, T.; Shin, Y.; Sivathanu, V.; Campisi, M.; Kamm, R. D. In Vitro Microfluidic Models for Neurodegenerative Disorders. Adv. Healthc. Mater. 2018, 7, 1–29. [Google Scholar] [CrossRef]
- Wosik, K.; Cayrol, R.; Dodelet-Devillers, A.; Berthelet, F.; Bernard, M.; Moumdjian, R.; Bouthillier, A.; Reudelhuber, T. L.; Prat, A. Angiotensin II Controls Occludin Function and Is Required for Blood-Brain Barrier Maintenance: Relevance to Multiple Sclerosis. J. Neurosci. 2007, 27, 9032–9042. [Google Scholar] [CrossRef]
- Kuo, Y. M.; Lee, Y. H. Epoxyeicosatrienoic Acids and Soluble Epoxide Hydrolase in Physiology and Diseases of the Central Nervous System. Chin. J. Physiol. 2022, 65, 1–11. [Google Scholar] [CrossRef]
- Eilam, R.; Segal, M.; Malach, R.; Sela, M.; Arnon, R.; Aharoni, R. Astrocyte Disruption of Neurovascular Communication Is Linked to Cortical Damage in an Animal Model of Multiple Sclerosis. Glia 2018, 66, 1098–1117. [Google Scholar] [CrossRef]
- Wurdeman, S. R.; Stevens, P. M.; Campbell, J. H.; Davie-Smith, F.; Coulter, E.; Kennon, B.; Wyke, S.; Paul, L.; Ingegneria, F.; Magistrale, L.; et al. 乳鼠心肌提取 HHS Public Access. Physiol. Behav. 2017, 176, 498–503. [Google Scholar]
- Michinaga, S.; Koyama, Y. Dual Roles of Astrocyte-Derived Factors in Regulation of Blood-Brain Barrier Function after Brain Damage. Int. J. Mol. Sci. 2019, 20, 1–22. [Google Scholar] [CrossRef]
- Galan, E. A.; Zhao, H.; Wang, X.; Dai, Q.; Huck, W. T. S.; Ma, S. Intelligent Microfluidics: The Convergence of Machine Learning and Microfluidics in Materials Science and Biomedicine. Matter 2020, 3, 1893–1922. [Google Scholar] [CrossRef]
- Nakatsu, M. N.; Sainson, R. C. A.; Pérez-Del-Pulgar, S.; Aoto, J. N.; Aitkenhead, M.; Taylor, K. L.; Carpenter, P. M.; Hughes, C. C. W. VEGF121 and VEGF165 Regulate Blood Vessel Diameter Through Vascular Endothelial Growth Factor Receptor 2 in an in Vitro Angiogenesis Model. Lab. Investig. 2003, 83, 1873–1885. [Google Scholar] [CrossRef]
- Shin, Y.; Jeon, J. S.; Han, S.; Jung, G. S.; Shin, S.; Lee, S. H.; Sudo, R.; Kamm, R. D.; Chung, S. In Vitro 3D Collective Sprouting Angiogenesis under Orchestrated ANG-1 and VEGF Gradients. Lab Chip 2011, 11, 2175–2181. [Google Scholar] [CrossRef] [PubMed]
- Sivanand. 乳鼠心肌提取 HHS Public Access. Physiol. Behav. 2019, 176, 139–148. [Google Scholar]
- Mou, L.; Jiang, X. Materials for Microfluidic Immunoassays: A Review. Adv. Healthc. Mater. 2017, 6, 1–20. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Chen, G. Fabrication, Modification, and Application of Poly(Methyl Methacrylate) Microfluidic Chips. Electrophoresis 2008, 29, 1801–1814. [Google Scholar] [CrossRef] [PubMed]
- Osipova, E. D.; Komleva, Y. K.; Morgun, A. V.; Lopatina, O. L.; Panina, Y. A.; Olovyannikova, R. Y.; Vais, E. F.; Salmin, V. V.; Salmina, A. B. Designing in Vitro Blood-Brain Barrier Models Reproducing Alterations in Brain Aging. Front. Aging Neurosci. 2018, 10, 1–14. [Google Scholar] [CrossRef]
- Saunders, N. R.; Dziegielewska, K. M.; Unsicker, K.; Ek, C. J. Delayed Astrocytic Contact with Cerebral Blood Vessels in FGF-2 Deficient Mice Does Not Compromise Permeability Properties at the Developing Blood-Brain Barrier. Dev. Neurobiol. 2016, 76, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Sollier, E.; Murray, C.; Maoddi, P.; Di Carlo, D. Rapid Prototyping Polymers for Microfluidic Devices and High Pressure Injections. Lab Chip 2011, 11, 3752–3765. [Google Scholar] [CrossRef] [PubMed]
- Borók, A.; Laboda, K.; Bonyár, A. PDMS Bonding Technologies for Microfluidic Applications: A Review. Biosensors 2021, 11. [Google Scholar] [CrossRef]
- Wang, J.; Yang, L.; Wang, H.; Wang, L. Application of Microfluidic Chips in the Detection of Airborne Microorganisms. Micromachines 2022, 13, 1–18. [Google Scholar] [CrossRef]
- Azizipour, N.; Avazpour, R.; Rosenzweig, D. H.; Sawan, M.; Ajji, A. Evolution of Biochip Technology: A Review from Lab-on-a-Chip to Organ-on-a-Chip. Micromachines 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Gao, Y.; Broussard, J.; Haque, A.; Revzin, A.; Lin, T. Functional Imaging of Neuron–Astrocyte Interactions in a Compartmentalized Microfluidic Device. Microsystems Nanoeng. 2016, 2, 1–9. [Google Scholar] [CrossRef]
- Persson, H.; Park, S.; Mohan, M.; Cheung, K. K.; Simmons, C. A.; Young, E. W. K. Rapid Assembly of PMMA Microfluidic Devices with PETE Membranes for Studying the Endothelium. Sensors Actuators B Chem. 2022, 356, 131342. [Google Scholar] [CrossRef]
- Wang, J. D.; Khafagy, E. S.; Khanafer, K.; Takayama, S.; Elsayed, M. E. H. Organization of Endothelial Cells, Pericytes, and Astrocytes into a 3D Microfluidic in Vitro Model of the Blood-Brain Barrier. Mol. Pharm. 2016, 13, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Jarad, N. A.; Khan, S.; F Didar, T. Bio-Functionalization of Microfluidic Platforms Made of Thermoplastic Materials: A Review. Anal. Chim. Acta 2022, 1209, 339283. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M. Polymeric Microfluidic Devices Fabricated Using Epoxy Resin for Chemically Demanding and Day-Long Experiments. Biosensors 2022, 12. [Google Scholar] [CrossRef]
- Apelgren, P.; Amoroso, M.; Säljö, K.; Montelius, M.; Lindahl, A.; Stridh Orrhult, L.; Gatenholm, P.; Kölby, L.; Arulkumar, S.; Parthiban, S.; et al. Accepted Muspt. Mater. Today Proc. 2019, 27, 1–31. [Google Scholar]
- McCall, J. G.; Jeong, J.-W. Minimally Invasive Probes for Programmed Microfluidic Delivery of Molecules in Vivo. Curr. Opin. Pharmacol. 2017, 36, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W. M. The Blood-Brain Barrier: Bottleneck in Brain Drug Development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Prabhakarpandian, B.; Shen, M. C.; Nichols, J. B.; Mills, I. R.; Sidoryk-Wegrzynowicz, M.; Aschner, M.; Pant, K. SyM-BBB: A Microfluidic Blood Brain Barrier Model. Lab Chip 2013, 13, 1093–1101. [Google Scholar] [CrossRef]
- Sivandzade, F.; Cucullo, L. In-Vitro Blood–Brain Barrier Modeling: A Review of Modern and Fast-Advancing Technologies. J. Cereb. Blood Flow Metab. 2018, 38, 1667–1681. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N. J. Astrocyte-Endothelial Interactions and Blood-Brain Barrier Permeability. J. Anat. 2002, 200, 629–638. [Google Scholar] [CrossRef]
- Dewi, B. E.; Takasaki, T.; Kurane, I. In Vitro Assessment of Human Endothelial Cell Permeability: Effects of Inflammatory Cytokines and Dengue Virus Infection. J. Virol. Methods 2004, 121, 171–180. [Google Scholar] [CrossRef]
- Wu, Y. C.; Sonninen, T. M.; Peltonen, S.; Koistinaho, J.; Lehtonen, Š. Blood–Brain Barrier and Neurodegenerative Diseases—Modeling with Ipsc-derived Brain Cells. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef]
- Castillo Ransanz, L.; Van Altena, P. F. J.; Heine, V. M.; Accardo, A. Engineered Cell Culture Microenvironments for Mechanobiology Studies of Brain Neural Cells. Front. Bioeng. Biotechnol. 2022, 10, 1–26. [Google Scholar] [CrossRef]
- Insperger, T.; Stépán, G. Engineering Applications. Appl. Math. Sci. 2011, 178, 93–149. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, D.; Ren, Y.; Guo, S.; Li, J.; Ma, S.; Yao, M.; Guan, F. Injectable Hyaluronic Acid Hydrogel Loaded with BMSC and NGF for Traumatic Brain Injury Treatment. Mater. Today Bio 2022, 13. [Google Scholar] [CrossRef]
- Spampinato, S. F.; Merlo, S.; Costantino, G.; Sano, Y.; Kanda, T.; Sortino, M. A. Decreased Astrocytic CCL2 Accounts for BAF - 312 Effect on PBMCs Transendothelial Migration Through a Blood Brain Barrier in Vitro Model. J. Neuroimmune Pharmacol. 2022, 427–436. [Google Scholar] [CrossRef]
- Helms, H.C.; Abbott, N.J.; Burek, M.; Cecchelli, R.; Couraud, P.O.; Deli, M.A.; Förster, C.; Galla, H.J.; Romero, I.A.; Shusta, E. V.; et al. In Vitro Models of the Blood-Brain Barrier: An Overview of Commonly Used Brain Endothelial Cell Culture Models and Guidelines for Their Use. J. Cereb. Blood Flow Metab. 2015, 36, 862–890. [Google Scholar] [CrossRef]
- Förster, C.; Silwedel, C.; Golenhofen, N.; Burek, M.; Kietz, S.; Mankertz, J.; Drenckhahn, D. Occludin as Direct Target for Glucocorticoid-Induced Improvement of Blood-Brain Barrier Properties in a Murine in Vitro System. J. Physiol. 2005, 565, 475–486. [Google Scholar] [CrossRef]
- Hickman, J. Transepithelial/Endothelial Electrical Resistance (TEER) Theory and Applications for Microfluidic Body-on-a-Chip Devices. J. Rare Dis. Res. Treat. 2016, 1, 46–52. [Google Scholar] [CrossRef]
- Sackett, S.D.; Tremmel, D.M.; Ma, F.; Feeney, A.K.; Maguire, R.M.; Brown, M.E.; Zhou, Y.; Li, X.; O’Brien, C.; Li, L.; et al. Extracellular Matrix Scaffold and Hydrogel Derived from Decellularized and Delipidized Human Pancreas. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Cui, K.; Guo, Y.; Zhang, X.; Qin, J. Advances in Hydrogels in Organoids and Organs-on-a-Chip. Adv. Mater. 2019, 31, 1–28. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, S.; Zhong, A.X.; Shelton, S.E.; Campisi, M.; Sundararaman, S.K.; Offeddu, G.S.; Ko, E.; Ibrahim, L.; Coughlin, M.F.; et al. A Robust Vasculogenic Microfluidic Model Using Human Immortalized Endothelial Cells and Thy1 Positive Fibroblasts. Biomaterials 2021, 276, 121032. [Google Scholar] [CrossRef] [PubMed]
- Sivarapatna, A.; Ghaedi, M.; Xiao, Y.; Han, E.; Aryal, B.; Zhou, J.; Fernandez-Hernando, C.; Qyang, Y.; Hirschi, K. K.; Niklason, L. E. Engineered Microvasculature in PDMS Networks Using Endothelial Cells Derived from Human Induced Pluripotent Stem Cells. Cell Transplant. 2017, 26, 1365–1379. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lutz, M. W.; Xing, Y. A Systems-Based Model of Alzheimer’s Disease. Alzheimers. Dement. 2019, 15, 168–171. [Google Scholar] [CrossRef]
- Fan, L.; Mao, C.; Hu, X.; Zhang, S.; Yang, Z.; Hu, Z.; Sun, H.; Fan, Y.; Dong, Y.; Yang, J.; Shi, C.; Xu, Y. New Insights Into the Pathogenesis of Alzheimer’s Disease. Front. Neurol. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Verma, M.; Wills, Z.; Chu, C. T. Excitatory Dendritic Mitochondrial Calcium Toxicity: Implications for Parkinson’s and Other Neurodegenerative Diseases. Front. Neurosci. 2018, 12, 523. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Koo, E. H. Biology and Pathophysiology of the Amyloid Precursor Protein. Mol. Neurodegener. 2011, 6, 1–16. [Google Scholar] [CrossRef]
- Haapasalo, A.; Hiltunen, M. A report from the 8th Kuopio Alzheimer Symposium. Neurodegener. Dis. Manag. 2018, 18, 289–299. [Google Scholar] [CrossRef]
- Kim, H. Detection of Severity in Alzheimer’s Disease (AD) Using Computational Modeling. Bioinformation 2018, 14, 259–264. [Google Scholar] [CrossRef]
- Salmina, A. B.; Inzhutova, A. I.; Malinovskaya, N. A.; Petrova, M. M. Endothelial Dysfunction and Repair in Alzheimer-Type Neurodegeneration: Neuronal and Glial Control. J. Alzheimer’s Dis. 2010, 22, 17–36. [Google Scholar] [CrossRef]
- González-Reyes, R. E.; Nava-Mesa, M. O.; Vargas-Sánchez, K.; Ariza-Salamanca, D.; Mora-Muñoz, L. Involvement of Astrocytes in Alzheimer’s Disease from a Neuroinflammatory and Oxidative Stress Perspective. Front. Mol. Neurosci. 2017, 10, 1–20. [Google Scholar] [CrossRef]
- Ávila-Villanueva, M.; Marcos Dolado, A.; Gómez-Ramírez, J.; Fernández-Blázquez, M. Brain Structural and Functional Changes in Cognitive Impairment Due to Alzheimer’s Disease. Front. Psychol. 2022, 13, 1–6. [Google Scholar] [CrossRef]
- Khoury, R.; Grysman, N.; Gold, J.; Patel, K.; Grossberg, G. T. The Role of 5 HT6-Receptor Antagonists in Alzheimer’s Disease: An Update. Expert Opin. Investig. Drugs 2018, 27, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Hussain, B.; Fang, C.; Chang, J. Blood–Brain Barrier Breakdown: An Emerging Biomarker of Cognitive Impairment in Normal Aging and Dementia. Front. Neurosci. 2021, 15, 1–22. [Google Scholar] [CrossRef]
- Devadhasan, J. P.; Kim, S.; An, J. Fish-on-a-Chip: A Sensitive Detection Microfluidic System for Alzheimer’s Disease. J. Biomed. Sci. 2011, 18, 1–11. [Google Scholar] [CrossRef]
- Sugama, S.; Takenouchi, T.; Cho, B. P.; Joh, T. H.; Hashimoto, M.; Kitani, H. Possible Roles of Microglial Cells for Neurotoxicity in Clinical Neurodegenerative Diseases and Experimental Animal Models. Inflamm. Allergy - Drug Targets 2009, 8, 277–284. [Google Scholar] [CrossRef]
- Yi, Y. Y.; Park, J. S.; Lim, J.; Lee, C. J.; Lee, S. H. Central Nervous System and Its Disease Models on a Chip. Trends Biotechnol. 2015, 33, 762–776. [Google Scholar] [CrossRef]
- Wu, J.W.; Hussaini, S.A.; Bastille, I.M.; Rodriguez, G.A.; Mrejeru, A.; Rilett, K.; Sanders, D.W.; Cook, C.; Fu, H.; Boonen, R.A.C.M.; et al. Neuronal Activity Enhances Tau Propagation and Tau Pathology in Vivo. Nat. Neurosci. 2016, 19, 1085–1092. [Google Scholar] [CrossRef]
- Alexoudi, A.; Alexoudi, I.; Gatzonis, S. Parkinson’s Disease Pathogenesis, Evolution and Alternative Pathways: A Review. Rev. Neurol. (Paris). 2018, 174, 699–704. [Google Scholar] [CrossRef]
- Cai, P.; Zheng, Y.; Sun, Y.; Zhang, C.; Zhang, Q.; Liu, Q. New Blood-Brain Barrier Models Using Primary Parkinson’s Disease Rat Brain Endothelial Cells and Astrocytes for the Development of Central Nervous System Drug Delivery Systems. ACS Chem. Neurosci. 2021, 12, 3829–3837. [Google Scholar] [CrossRef]
- Wang, C.; Yang, T.; Liang, M.; Xie, J.; Song, N. Astrocyte Dysfunction in Parkinson’s Disease: From the Perspectives of Transmitted α-Synuclein and Genetic Modulation. Transl. Neurodegener. 2021, 10, 1–17. [Google Scholar] [CrossRef]
- Yang, Y.; Song, J.J.; Choi, Y.R.; Kim, S.H.; Seok, M.J.; Wulansari, N.; Darsono, W.H.W.; Kwon, O.C.; Chang, M.Y.; Park, S.M.; et al. Therapeutic Functions of Astrocytes to Treat α-Synuclein Pathology in Parkinson’s Disease. Proc. Natl. Acad. Sci. U. S. A. 2022, 119, 1–12. [Google Scholar] [CrossRef]
- Gratton, C.; Koller, J. M.; Shannon, W.; Greene, D. J.; Maiti, B.; Snyder, A. Z.; Petersen, S. E.; Perlmutter, J. S.; Campbell, M. C. Emergent Functional Network Effects in Parkinson Disease. Cereb. Cortex 2019, 29, 2509–2523. [Google Scholar] [CrossRef]
- Creaby, M. W.; Cole, M. H. Gait Characteristics and Falls in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Parkinsonism Relat. Disord. 2018, 57, 1–8. [Google Scholar] [CrossRef]
- Wanneveich, M.; Moisan, F.; Jacqmin-Gadda, H.; Elbaz, A.; Joly, P. Projections of Prevalence, Lifetime Risk, and Life Expectancy of Parkinson’s Disease (2010-2030) in France. Mov. Disord. 2018, 33, 1449–1455. [Google Scholar] [CrossRef]
- Eggers, C.; Dano, R.; Schill, J.; Fink, G. R.; Timmermann, L.; Voltz, R.; Golla, H.; Lorenzl, S. Access to End-of Life Parkinson’s Disease Patients Through Patient-Centered Integrated Healthcare. Front. Neurol. 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Hughes, R. C. Parkinson’s Disease and Its Management. Bmj 1994, 308, 281. [Google Scholar] [CrossRef]
- Holloway, P. M.; Willaime-Morawek, S.; Siow, R.; Barber, M.; Owens, R. M.; Sharma, A. D.; Rowan, W.; Hill, E.; Zagnoni, M. Advances in Microfluidic in Vitro Systems for Neurological Disease Modeling. J. Neurosci. Res. 2021, 99, 1276–1307. [Google Scholar] [CrossRef]
- Rahmani Dabbagh, S.; Rezapour Sarabi, M.; Birtek, M. T.; Mustafaoglu, N.; Zhang, Y. S.; Tasoglu, S. 3D Bioprinted Organ-on-chips. Aggregate 2023, 4, 1–26. [Google Scholar] [CrossRef]
- Fanizza, F.; Campanile, M.; Forloni, G.; Giordano, C.; Albani, D. Induced Pluripotent Stem Cell-Based Organ-on-a-Chip as Personalized Drug Screening Tools: A Focus on Neurodegenerative Disorders. J. Tissue Eng. 2022, 13. [Google Scholar] [CrossRef]
- Pediaditakis, I.; Kodella, K.R.; Manatakis, D. V.; Le, C.Y.; Hinojosa, C.D.; Tien-Street, W.; Manolakos, E.S.; Vekrellis, K.; Hamilton, G.A.; Ewart, L.; et al. Modeling Alpha-Synuclein Pathology in a Human Brain-Chip to Assess Blood-Brain Barrier Disruption. Nat. Commun. 2021, 12, 1–17. [Google Scholar] [CrossRef]
- Fernandes, J. T. S.; Chutna, O.; Chu, V.; Conde, J. P.; Outeiro, T. F. A Novel Microfluidic Cell Co-Culture Platform for the Study of the Molecular Mechanisms of Parkinson’s Disease and Other Synucleinopathies. Front. Neurosci. 2016, 10, 1–11. [Google Scholar] [CrossRef]
- Noyes, K.; Weinstock-Guttman, B. Impact of Diagnosis and Early Treatment on the Course of Multiple Sclerosis. Am. J. Manag. Care 2013, 19, s321–31. [Google Scholar]
- Sintzel, M. B.; Rametta, M.; Reder, A. T. Vitamin D and Multiple Sclerosis: A Comprehensive Review. Neurol. Ther. 2018, 7, 59–85. [Google Scholar] [CrossRef]
- Solaro, C.; Trabucco, E.; Messmer Uccelli, M. Pain and Multiple Sclerosis: Pathophysiology and Treatment Topical Collection on Demyelinating Disorders. Curr. Neurol. Neurosci. Rep. 2013, 13. [Google Scholar] [CrossRef]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy Citation: Ghasemi N, Razavi Sh, Nikzad E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1–10. [Google Scholar] [CrossRef]
- Hosmane, S.; Tegenge, M. A.; Rajbhandari, L.; Uapinyoying, P.; Kumar, N. G.; Thakor, N.; Venkatesan, A. Toll/Interleukin-1 Receptor Domain-Containing Adapter Inducing Interferon-β Mediates Microglial Phagocytosis of Degenerating Axons. J. Neurosci. 2012, 32, 7745–7757. [Google Scholar] [CrossRef]
- Kerman, B.E.; Kim, H.J.; Padmanabhan, K.; Mei, A.; Georges, S.; Joens, M.S.; Fitzpatrick, J.A.J.; Jappelli, R.; Chandross, K.J.; August, P.; et al. In Vitro Myelin Formation Using Embryonic Stem Cells. Dev. 2015, 142, 2213–2225. [Google Scholar] [CrossRef]
- Finkbeiner, S. Huntington’s Disease. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef]
- Nopoulos, P. C. Huntington Disease: A Single-Gene Degenerative Disorder of the Striatum. Dialogues Clin. Neurosci. 2016, 18, 91–98. [Google Scholar] [CrossRef]
- Bachoud-Lévi, A.C.; Ferreira, J.; Massart, R.; Youssov, K.; Rosser, A.; Busse, M.; Craufurd, D.; Reilmann, R.; De Michele, G.; Rae, D.; et al. International Guidelines for the Treatment of Huntington’s Disease. Front. Neurol. 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Nix, C.; Ghassemi, M.; Crommen, J.; Fillet, M. Overview on Microfluidics Devices for Monitoring Brain Disorder Biomarkers. TrAC Trends Anal. Chem. 2022, 155, 116693. [Google Scholar] [CrossRef]
- Teixeira, M. I.; Amaral, M. H.; Costa, P. C.; Lopes, C. M.; Lamprou, D. A. Recent Developments in Microfluidic Technologies for Central Nervous System Targeted Studies. Pharmaceutics 2020, 12, 1–37. [Google Scholar] [CrossRef]
- Yochum, A. Autism Spectrum/Pervasive Developmental Disorder. Prim. Care 2016, 43, 285–300. [Google Scholar] [CrossRef]
- Rashighi, M.; Harris, J. E. 乳鼠心肌提取 HHS Public Access. Physiol. Behav. 2017, 176, 139–148. [Google Scholar]
- Feliciano, P. Promoting Resistance Repeat Expansion in ALS and FTD Epiallele Accumulation. Nat. Genet. 2011, 43, 1053. [Google Scholar] [CrossRef]
- Chahboun, S.; Stenseng, F.; Page, A. G. The Changing Faces of Autism: The Fluctuating International Diagnostic Criteria and the Resulting Inclusion and Exclusion—A Norwegian Perspective. Front. Psychiatry 2022, 13. [Google Scholar] [CrossRef]
- Gutmann, D. H.; Ferner, R. E.; Listernick, R. H.; Korf, B. R.; Wolters, P. L.; Johnson, K. J. Neurofibromatosis Type 1. Nat. Rev. Dis. Prim. 2017, 3, 1–18. [Google Scholar] [CrossRef]
- Boyd, K. P.; Korf, B. R.; Theos, A. Neurofibromatosis Type 1. J. Am. Acad. Dermatol. 2009, 61, 1–6. [Google Scholar] [CrossRef]
- Wang, W.; Wei, C. J.; Cui, X. W.; Li, Y. H.; Gu, Y. H.; Gu, B.; Li, Q. F.; Wang, Z. C. Impacts of NF1 Gene Mutations and Genetic Modifiers in Neurofibromatosis Type 1. Front. Neurol. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Hirbe, A. C.; Gutmann, D. H. Neurofibromatosis Type 1: A Multidisciplinary Approach to Care. Lancet Neurol. 2014, 13, 834–843. [Google Scholar] [CrossRef]
- Ferner, R. E. Neurofibromatosis 1. Eur. J. Hum. Genet. 2007, 15, 131–138. [Google Scholar] [CrossRef] [PubMed]
- emov, A.; Sung, H.; Hyland, P.L.; Sloan, J.L.; Ruppert, S.L.; Baldwin, A.M.; Boland, J.F.; Bass, S.E.; Lee, H.J.; Jones, K.M.; et al. Genetic Modifiers of Neurofibromatosis Type 1-Associated ´ -au-Lait Macule Count Identified Using Multi-platform Cafe Analysis. 2014, 10. [Google Scholar] [CrossRef]
- Van Den Berg, B.; Walgaard, C.; Drenthen, J.; Fokke, C.; Jacobs, B. C.; Van Doorn, P. A. Guillain-Barré Syndrome: Pathogenesis, Diagnosis, Treatment and Prognosis. Nat. Rev. Neurol. 2014, 10, 469–482. [Google Scholar] [CrossRef]
- Miny, L.; Maisonneuve, B. G. C.; Quadrio, I.; Honegger, T. Modeling Neurodegenerative Diseases Using In Vitro Compartmentalized Microfluidic Devices. Front. Bioeng. Biotechnol. 2022, 10, 1–17. [Google Scholar] [CrossRef]
- Korhonen, P.; Malm, T.; White, A. R. 3D Human Brain Cell Models: New Frontiers in Disease Understanding and Drug Discovery for Neurodegenerative Diseases. Neurochem. Int. 2018, 120, 191–199. [Google Scholar] [CrossRef]
- Lee, C. T.; Bendriem, R. M.; Wu, W. W.; Shen, R. F. 3D Brain Organoids Derived from Pluripotent Stem Cells: Promising Experimental Models for Brain Development and Neurodegenerative Disorders Julie Y.H. Chan. J. Biomed. Sci. 2017, 24, 1–12. [Google Scholar] [CrossRef]
- de Mello, C. P. P.; Rumsey, J.; Slaughter, V.; Hickman, J. J. A Human-on-a-Chip Approach to Tackling Rare Diseases. Drug Discov. Today 2019, 24, 2139–2151. [Google Scholar] [CrossRef]
- Mencattini, A.; Giuseppe, D.; D’Orazio, M.; Rizzuto, V.; Pereira, M.; Comes, M. C.; Lopez-Martinez, M.; Samitier, J.; Martinelli, E. A Microfluidic Device for Shape Measurement in Red Blood Cells (RBCs). In Proceedings of the 2020 IEEE International Symposium on Medical Measurements and Applications (MeMeA); 2020. [Google Scholar] [CrossRef]
- Herrmann, K.; Pistollato, F.; Stephens, M. L. Beyond the 3Rs: Expanding the Use of Human-Relevant Replacement Methods in Biomedical Research. ALTEX - Altern. to Anim. Exp. 2019, 36, 343–352. [Google Scholar] [CrossRef]
- Ravi, K.; Paidas, M. J.; Saad, A.; Jayakumar, A. R. Astrocytes in Rare Neurological Conditions: Morphological and Functional Considerations. J. Comp. Neurol. 2021, 529, 2676–2705. [Google Scholar] [CrossRef]
- Gupta, N.; Liu, J. R.; Patel, B.; Solomon, D. E.; Vaidya, B.; Gupta, V. Microfluidics-based 3D Cell Culture Models: Utility in Novel Drug Discovery and Delivery Research. Bioeng. Transl. Med. 2016, 1, 63–81. [Google Scholar] [CrossRef]
- Chiareli, R.A.; Carvalho, G.A.; Marques, B.L.; Mota, L.S.; Oliveira-Lima, O.C.; Gomes, R.M.; Birbrair, A.; Gomez, R.S.; Simão, F.; Klempin, F.; et al. The Role of Astrocytes in the Neurorepair Process. Front. Cell Dev. Biol. 2021, 9, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Strazielle, N.; Ghersi-Egea, J. F. Physiology of Blood-Brain Interfaces in Relation to Brain Disposition of Small Compounds and Macromolecules. Mol. Pharm. 2013, 10, 1473–1491. [Google Scholar] [CrossRef] [PubMed]
- Combedazou, A.; Gayral, S.; Colombié, N.; Fougerat, A.; Laffargue, M.; Ramel, D. Small GTPases Orchestrate Cell-Cell Communication during Collective Cell Movement. Small GTPases 2020, 11, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Mi, G.; Wang, M.; Webster, T. J. Since January 2020 Elsevier Has Created a COVID-19 Resource Centre with Free Information in English and Mandarin on the Novel Coronavirus COVID- 19 . The COVID-19 Resource Centre Is Hosted on Elsevier Connect , the Company ’ s Public News and Information. 2020. [Google Scholar]
- Regmi, S.; Poudel, C.; Adhikari, R.; Luo, K. Q. Applications of Microfluidics and Organ-on-a-Chip in Cancer Research. Biosensors 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Peng, Y.; Li, H.; Chen, W. Organ-on-a-Chip: A New Paradigm for Drug Development. Trends Pharmacol. Sci. 2021, 42, 119–133. [Google Scholar] [CrossRef]

| Method | Advantages | Disadvantages | References |
| Manufacturing molds by soft lithography | Resolution to mm scale is feasible, which enables the development of multi-layered designs or curved channels. | Expensive and time-consuming to acquire the necessary equipment. | [58] |
| Paper based microfluidics | Simple, low-cost devices made of paper or other porous membranes that wick fluids through capillary action. | Low resolution (mm), patterned shape variation | [59] |
| Manufacturing molds by 3D printing | Resolution to tens of mm is possible, inexpensive and simple. | Channel height must be at least 50 mm. | [60] |
| Inkjet Printing | Simple, quick prototyping, and low cost. | It must be treated with a solvent. | [61] |
| 3D-Laser lithography | True 3D-feature generation, including gradually changing channel dimensions. | Expensive, slow, and unsuitable for deep channels (greater than 100 mm). | [62] |
| Continuous-flow microfluidics | High temporal and spatial precision of flow conditions is possible. | The removal of substances from cells or tissues limits temporal resolution. | [63] |
| Droplet microfluidics | High-throughput screening is possible, and commercial systems for some applications are available. | The development of new approaches necessitates the use of costly laboratory equipment and technically skilled personnel. | [64] |
| Dysfunction | Characteristic features | Symptoms | Genes involved |
|---|---|---|---|
| Alzheimer’s disease | The gradual loss of most of the normal brain activity is caused by abnormal alterations in the brain. Senile plaques and neurofibrillary tangles. | Gradual loss of control over mental and physical abilities, dementia & mood swings | Presenilin 1 and 2 are located on chromosome 14 and 1, respectively, and APP is located on chromosome 21. |
| Parkinson’s disease | Loss of pigmented dopaminergic neurons & Lewy bodies | Both motor and nonmotor symptoms. | X PINK1, PRKN, or SNCA gene PARK7 & LRRK2 |
| Multiple Sclerosis | Axons that are demyelinated and transected, inflammatory cells and their byproducts, and astrogliosis form CNS plaques. | Neurological problems as well as severe physical or cognitive impairment | HLA-DRB1 |
| Huntington’s disease | Chorea, dystonia and Slow or unusual eye movements | Impatience, melancholy, and other mood problems, as well as erratic involuntary movements and a dramatic loss in thinking and reasoning skills | Faulty gene on chromosome 4 |
| Autism spectrum disorder | Challenges with social interaction, verbal and nonverbal communication, the appearance of repetitive behavior, and narrowed interests. | Struggling to make friendships and odd reactions to sensory stimuli | ASH1L, CHD2, SHANK3, SYNGAP1 CHD8 & DYRK1A |
| Neurofibromatosis | Meningiomas and bilateral vestibular schwannomas | Tumors on the skin and in the nerve system. | Neurofibromin 1 (NF1) gene |
| Guillain-Barre syndrome | Autoimmune destruction of peripheral nervous system nerves | Numbness, tingling, and weakness that can develop into paralysis | ICAM1, HLA genes, CD-1A, FcGR, NOD, TNF-α & TLR4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





