Submitted:
14 June 2023
Posted:
15 June 2023
You are already at the latest version
Abstract
Keywords:
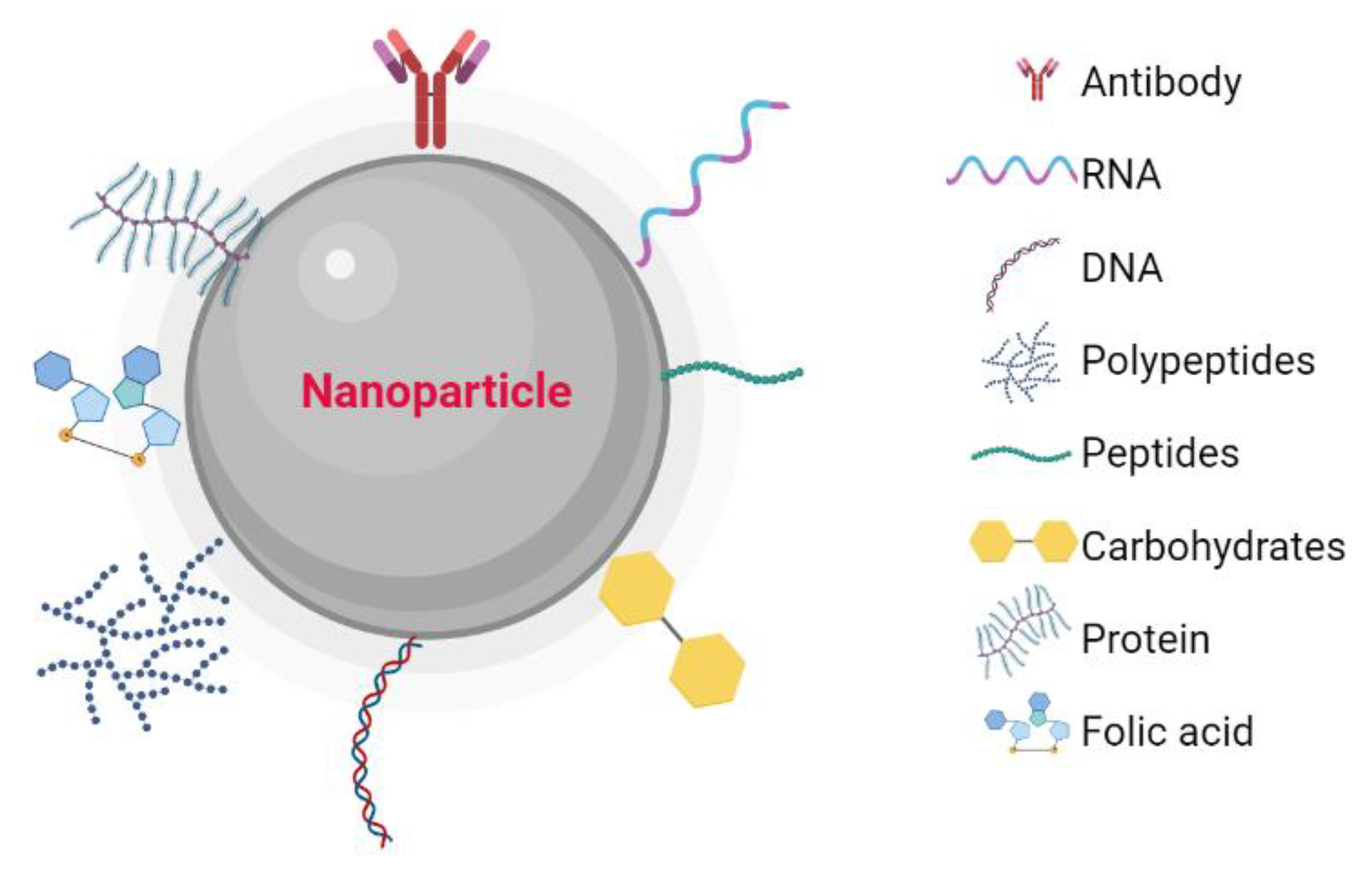
1. Introduction
2. Metallic nanoparticles
2.1. Palladium nanoparticles
2.2. Gold nanoparticles
2.3. Silver nanoparticles
2.4. Platinum nanoparticles
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Gutschner, T.; Diederichs, S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012. 9, 703–719. [CrossRef]
- Graván, P.; Aguilera-Garrido, A.; Marchal, J.A. Navarro-Marchal S.A, Galisteo-González F. Lipid-core nanoparticles: Classification, preparation methods, routes of administration and recent advances in cancer treatment. Adv. Colloid. Interface. Sci. 2023, 314, 102871. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cong, X. Surface-engineered nanoparticles in cancer immune response and immunotherapy: Current status and future prospects. Biomed. Pharmacother. 2023, 157, 113998. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: Anoverview. Int. J. Cancer. 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.; Sathiyamoorthy, P.; Park, I.K. Metallic Nanoparticle-Mediated Immune Cell Regulation and Advanced Cancer Immunotherapy. Pharmaceutics 2021, 13, 1867. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.W.; Chan, L.W.; Li,W. ; Wong, T.W. Critical clinical gaps in cancer precision nanomedicine development. J. Control. Release. 2022, 345, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Mundekkad, D.; Cho, W.C. Nanoparticles in Clinical Translation for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 1685. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Onco.l 2021, 14, 85. [Google Scholar] [CrossRef]
- Khan, Y.; Sadia, H.; Ali Shah, S.Z.; Khan, M.N.; Shah, A.A.; Ullah, N.; Ullah, M.F.; Bibi, H.; Bafakeeh, O.T.; Khedher, N.B.; Eldin, S.M.; Fadhl, B.M.; Khan, M.I. Classification, Synthetic, and Characterization Approaches to Nanoparticles, and Their Applications in Various Fields of Nanotechnology: A Review. Catalysts 2022, 12, 1386. [Google Scholar] [CrossRef]
- Ealia, S.A.M.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser.: Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar] [CrossRef]
- Patel, K.D.; Singh, R.K.; Kim, H.W. Carbon-based nanomaterials as an emerging platform for theranostics. Mater. Horiz. 2019, 6, 434–469. [Google Scholar] [CrossRef]
- Asad, S.; Jacobsen, A.C.; Teleki, A. Inorganic nanoparticles for oral drug delivery: opportunities, barriers, and future perspectives. Curr. Opin. Chem. Eng. 2022, 38, 100869. [Google Scholar] [CrossRef]
- Rytov, R.A.; Bautin, V. A.; Usov, N.A. Towards optimal thermal distribution in magnetic hyperthermia. Sci. Rep. 2022, 12, 3023. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug. Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Nagi, N.M.S.; Khair, Y.A.M.; Abdalla, A.M.E. Capacity of gold nanoparticles in cancer radiotherapy. Jpn. J. Radiol. 2017, 35, 555–561. [Google Scholar] [CrossRef]
- Núñez, C.; Estévez, S.V.; del Pilar Chantada, M. Inorganic nanoparticles in diagnosis and treatment of breast cancer. J. Biol. Inorg. Chem. 2018, 23, 331–345. [Google Scholar] [CrossRef]
- Gutiérrez de la Rosa, S.Y.; Muñiz Diaz, R.; Villalobos Gutiérrez, P.T.; Patakfalvi, R.; Gutiérrez Coronado, Ó. Functionalized Platinum Nanoparticles with Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 9404. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on metal nanoparticles as nanocarriers: current challenges and perspectives in drug delivery systems. Emergent Mater. 2022, 5, 1593–1615 (). [Google Scholar] [CrossRef]
- Wu, J.; Wang, M.; Pan, Y.; Pang, Y.; Tang, Y.; Song, C.; Zhu, J.; Zhang, X.; Huang, Q. Synthesis of manganese-oxide and palladium nanoparticles co-decorated polypyrrole/graphene oxide (MnO2@Pd@PPy/GO) nanocomposites for anti-cancer treatment. RSC Adv. 2022, 12, 23786–23795. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.K.; Soe, Z.C. , Ou, W.; Poudel, K.; Jeong, J.H.; Jin, S.G.; Ku, S.K.; Choi, H.G.; Lee, Y.M.; Yong, C.S.; Kim, J.O. Palladium nanoparticle-decorated 2-D graphene oxide for effective photodynamic and photothermal therapy of prostate solid tumors. Colloids Surf. B. Biointerfaces. 2018, 169, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Bangde, P. , Pant, T. ( 211, 112287. [CrossRef] [PubMed]
- Bharathiraja, S.; Bui, N.Q.; Manivasagan, P.; Moorthy, M.S.; Mondal, S.; Seo, H.; Phuoc, N.T.; Vy Phan, T.T.; Kim, H.; Lee, K.D.; Oh, J. Multimodal tumor-homing chitosan oligosaccharide-coated biocompatible palladium nanoparticles for photo-based imaging and therapy. Sci. Rep. 2018, 8, 500. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Chen, M.; Zheng, N. Multifunctional ultrasmall Pd nanosheets for enhanced near-infrared photothermal therapy and chemotherapy of cancer. Nano Res. 2015, 8, 165–174. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Soe, Z.C.; Yang, K.Y.; Phung, C.D.; Nguyen, L.T.; Jeong, J.H.; Jin, S.G.; Choi, H.G.; Ku, S.K.; Yong, C.S.; Kim, J.O. Transferrin-conjugated pH-sensitive platform for effective delivery of porous palladium nanoparticles and paclitaxel in cancer treatment. Colloids Surf. B. Biointerfaces. 2019, 176, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, R.; Wang, D.; Sun, X.; Xu, Z. Pd nanoparticle-decorated hydroxy boron nitride nanosheets as a novel drug carrier for chemo-photothermal therapy. Colloids Surf. B. Biointerfaces. 2019, 176, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Bloise, N.; Strada, S.; Dacarro, G.; Visai, L. Gold Nanoparticles Contact with Cancer Cell: A Brief Update. Int. J. Mol. Sci. 2022, 23, 7683. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.-M.; Tan, E.-Z.; Yang, S.-H.; Li, L.-D.; Guo, L. Uniform arrays of gold nanoparticles with different surface roughness for surface enhanced Raman scattering. J. Chin. Chem. Lett. 2015, 26, 1426–1430. [Google Scholar] [CrossRef]
- Graczyk, A.; Pawlowska, R.; Chworos, A. Gold Nanoparticles as Carriers for Functional RNA Nanostructures. Bioconjugate Chem. 2021, 32, 1667–1674. [Google Scholar] [CrossRef]
- Bai, X.; Wang, Y.; Song, Z.; Feng, Y.; Chen, Y.; Zhang, D.; Feng, L. The Basic Properties of Gold Nanoparticles and their Applications in Tumor Diagnosis and Treatment. Int. J. Mol. Sci. 2020, 21, 2480. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, N.; Cai, Z.; Kwon, Y.L.; Lechtman, E.; Pignol, J.P.; Reilly, R.M. Molecularly targeted gold nanoparticles enhance the radiation response of breast cancer cells and tumor xenografts to X-radiation. Breast Cancer Res. Treat. 2013, 137, 81–91. [Google Scholar] [CrossRef]
- Chattopadhyay, N.; Cai, Z.; Pignol, J.P.; Keller, B.; Lechtman, E.; Bendayan, R.; Reilly, R.M. Design and characterization of HER-2-targeted gold nanoparticles for enhanced X-radiation treatment of locally advanced breast cancer. Mol. Pharm. 2010, 7, 2194–2206. [Google Scholar] [CrossRef] [PubMed]
- Yook, S.; Cai, Z.; Jeong, J.J.; Lu, Y.; Winnik, M.A.; Pignol, J.P.; Reilly, R.M. Dual-Receptor-Targeted (DRT) Radiation Nanomedicine Labeled with 177Lu Is More Potent for Killing Human Breast Cancer Cells That Coexpress HER2 and EGFR Than Single-Receptor-Targeted (SRT) Radiation Nanomedicines. Mol. Pharmaceutics. 2020, 17, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Falzone, N.; Vallis, K.A. EGF-coated gold nanoparticles provide an efficient nano-scale delivery system for the molecular radiotherapy of EGFR-positive cancer. Int. J. Radiat. Biol. 2016, 92, 716–723. [Google Scholar] [CrossRef]
- Hameed, M.; Panicker, S.; Abdallah, S. H.; Khan, A.A.; Han, C.; Chehimi, M.M.; Mohamed, A.A. Protein-Coated Aryl Modified Gold Nanoparticles for Cellular Uptake Study by Osteosarcoma Cancer Cells. Langmuir. 2020, 36, 11765–11775. [Google Scholar] [CrossRef] [PubMed]
- Al-Jawad, S.M.H.; Taha, A.A.; Al-Halbosiy, M.M.F.; Al-Barram, L.F.A. Synthesis and characterization of small-sized gold nanoparticles coated by bovine serum albumin (BSA) for cancer photothermal therapy. Photodiagn. Photodyn. Ther. 2018, 21, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Roma-Rodrigues, C.; Heuer-Jungemann, A.; Fernandes, A.R.; Kanaras, A.G.; Baptista, P.V. Peptide-coated gold nanoparticles for modulation of angiogenesis in vivo. Int. J. Nanomedicine. 2016, 11, 2633–2639. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Sun, Z.; Tong, Z.; Zhang, S.; Min, J.; Xu, Q.; Zhou, L.; Mao, Z.; Xia, H.; Wang, W. Tumor microenvironment-responsive multifunctional peptide coated ultrasmall gold nanoparticles and their application in cancer radiotherapy. Theranostics. 2020, 10, 5195–5208. [Google Scholar] [CrossRef]
- Akbarzadeh Khiavi, M.; Safary, A.; Barar, J.; Farzi-Khajeh, H.; Barzegari, A.; Mousavi, R.; Somi, M.H.; Omidi, Y. PEGylated gold nanoparticles-ribonuclease induced oxidative stress and apoptosis in colorectal cancer cells. BioImpacts: 2020, 10, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Bloise, N. , Massironi, A.; Della Pina, C.; Alongi, J.; Siciliani, S.; Manfredi, A.; Biggiogera, M.; Rossi, M.; Ferruti, P.; Ranucci, E.; Visai, L. Extra-Small Gold Nanospheres Decorated With a Thiol Functionalized Biodegradable and Biocompatible Linear Polyamidoamine as Nanovectors of Anticancer Molecules. Front. Bioeng. Biotechnol, 2020, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Kuroda, S.; Kanaya, N.; Morihiro, T.; Aoyama, K.; Kakiuchi, Y.; Kikuchi, S.; Nishizaki, M.; Kagawa, S.; Tazawa, H.; Fujiwara, T. HER2-targeted gold nanoparticles potentially overcome resistance to trastuzumab in gastric cancer. Nanomedicine. 2018, 14, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- El Hallal, R.; Lyu, N.; Wang, Y. Effect of Cetuximab-Conjugated Gold Nanoparticles on the Cytotoxicity and Phenotypic Evolution of Colorectal Cancer Cells. Molecules. 2021, 26, 567. [Google Scholar] [CrossRef] [PubMed]
- Liszbinski, R.B.; Romagnoli, G.G.; Gorgulho, C. M.; Basso, C.R.; Pedrosa, V.A.; Kaneno, R. Anti-EGFR-Coated Gold Nanoparticles In Vitro Carry 5-Fluorouracil to Colorectal Cancer Cells. Materials. 2020, 13, 375. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Qiu, M.; Wu, Q.; Tian, Y.; Zhang, Y.; Gu, N.; Li, S.; Xu, L.; Yin, R. Enhanced cytotoxic activity of cetuximab in EGFR-positive lung cancer by conjugating with gold nanoparticles. Sci Rep. 2014, 4, 7490. [Google Scholar] [CrossRef] [PubMed]
- Takata, M.; Chikumi, H.; Miyake, N.; Adachi, K.; Kanamori, Y.; Yamasaki, A.; Igishi, T.; Burioka, N.; Nanba, E.; Shimizu, E. Lack of AKT activation in lung cancer cells with EGFR mutation is a novel marker of cetuximab sensitivity. Cancer Biol. Ther. 2012, 13, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Yang, P.; Cai, J.; Wang, J.; Liu, M. Photothermal effects of folate-conjugated Au nanorods on HepG2 cells. Appl. Microbiol. Biotechnol. 2012, 94, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, G.A.; Brandenburg, K.S.; Shakeri-Zadeh, A. A comparative study of two folate-conjugated gold nanoparticles for cancer nanotechnology applications. Cancers. 2010, 2, 1911–1928. [Google Scholar] [CrossRef]
- Minassian, G.; Ghanem, E.; Hage, R.E.; Rahme, K. Gold Nanoparticles Conjugated with Dendrigraft Poly-L-lysine and Folate-Targeted Poly (ethylene glycol) for siRNA Delivery to Prostate cancer. Nanotheranostics. 2023, 7, 152–166. [Google Scholar] [CrossRef]
- Kim, H.J.; Takemoto, H.; Yi, Y.; Zheng, M.; Maeda, Y.; Chaya, H.; Kataoka, K. Precise Engineering of siRNA Delivery Vehicles to Tumors Using Polyion Complexes and Gold Nanoparticles. ACS Nano. 2014, 8, 8979–8991. [Google Scholar] [CrossRef]
- Kong, L.; Wu, Y.; Alves, C.S.; Shi, X. Efficient delivery of therapeutic siRNA into glioblastoma cells using multifunctional dendrimer-entrapped gold nanoparticles. Nanomedicine (Lond). 2016, 11, 3103–3115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.; Machado, V.; Dias, F.; Figueiredo, P.; Palmeira, C.; Martins, G.; Fernandes, R.; Malheiro, A.R.; Mikkonen, K.S.; Teixeira, A.L.; Medeiros, R. Glucose-Functionalized Silver Nanoparticles as a Potential New Therapy Agent Targeting Hormone-Resistant Prostate Cancer cells. Int. J. Nanomedicine. 2022, 17, 4321–4337. [Google Scholar] [CrossRef]
- Pollok, N.E.; Rabin, C.; Smith, L.; Crooks, R.M. Orientation-Controlled Bioconjugation of Antibodies to Silver Nanoparticles. Bioconjugate Chem. 2019, 30, 3078–3086. [Google Scholar] [CrossRef]
- Valdez, J.; Bawage, S.; Gomez, I.; Singh, S.R. Facile and rapid detection of respiratory syncytial virus using metallic nanoparticles. J Nanobiotechnology. 2016, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Majhi, R.K.; Singh, A.; Mishra, M.; Tiwari, A.; Chawla, S.; Guha, P.; Satpati, B.; Mohapatra, H.; Goswami, L.; Goswami, C. Carbohydrate-Coated Gold-Silver Nanoparticles for Efficient Elimination of Multidrug Resistant Bacteria and in Vivo Wound Healing. ACS Appl. Mater. Interfaces. 2019, 11, 42998–43017. [Google Scholar] [CrossRef]
- Sur, I.; Cam, D.; Kahraman, M.; Baysal, A.; Culha, M. Interaction of multi-functional silver nanoparticles with living cells. Nanotechnology. 2010, 21, 175104. [Google Scholar] [CrossRef] [PubMed]
- Boca, S.C.; Potara, M.; Gabudean, A.-M.; Juhem, A.; Baldeck, P.L.; Astilean, S. Chitosan-coated triangular silver nanoparticles as a novel class of biocompatible, highly effective photothermal transducers for in vitro cancer cell therapy. Cancer Lett. 2011, 311(2), 131–140. [Google Scholar] [CrossRef]
- Brahmkhatri, V.P.; Chandra, K.; Dubey, A.; Atreya, H.S. An ultrastable conjugate of silver nanoparticles and protein formed through weak interactions. Nanoscale. 2015, 7, 12921–12931. [Google Scholar] [CrossRef]
- Durán, N.; Silveira, C.P.; Durán, M.; Martinez, D.S. Silver nanoparticle protein corona and toxicity: a mini-review. J. Nanobiotechnol. 2015, 13, 55. [Google Scholar] [CrossRef]
- Karuppaiah, A.; Rajan, R.; Hariharan, S.; Balasubramaniam, D.K.; Gregory, M.; Sankar, V. Synthesis and Characterization of Folic Acid Conjugated Gemcitabine Tethered Silver Nanoparticles (FA-GEM-AgNPs) for Targeted Delivery. Curr. Pharm. Des. 2020, 26, 3141–3146. [Google Scholar] [CrossRef] [PubMed]
- Vanitha Kumari, G.; Ananth, N.; Asha, T.M.; Rajan, M.A.J. Synthesis and characterization of folic acid conjugated silver/gold nanoparticles for biomedical applications. Mater. Today. Proc. 2016, 3, 4215–4219. [Google Scholar] [CrossRef]
- Ray Chowdhuri, A.; Tripathy, S.; Haldar, C.; Chandra, S.; Das, B.; Roy, S.; Sahu, S.K. Theoretical and experimental study of folic acid conjugated silver nanoparticles through electrostatic interaction for enhance antibacterial activity. RSC Adv. 2015, 5, 21515–21524. [Google Scholar] [CrossRef]
- Kovács, D.; Igaz, N.; Gopisetty, M.K.; Kiricsi, M. Cancer Therapy by Silver Nanoparticles: Fiction or Reality? Int. J. Mol. Sci. 2022, 23, 839. [Google Scholar] [CrossRef]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef]
- Farah, M.A.; Ali, M.A.; Chen, S.-M.; Li, Y.; Al-Hemaid, F.M.; Abou-Tarboush, F.M.; Al-Anazi, K.M.; Lee, J. Silver Nanoparticles Synthesized from Adenium Obesum Leaf Extract Induced DNA Damage, Apoptosis and Autophagy via Generation of Reactive Oxygen Species. Colloids Surf. B. Biointerfaces 2016, 141, 158–169. [Google Scholar] [CrossRef]
- Sanpui, P.; Chattopadhyay, A.; Ghosh, S.S. Induction of apoptosis in cancer cells at low silver nanoparticle concentrations using chitosan nanocarrier. ACS Appl Mater Interfaces. 2011, 3, 218–228. [Google Scholar] [CrossRef]
- Oladipo, A.O.; Unuofin, J.O.; Lebelo, S.L.; Msagati, T.A.M. Phytochemical-Stabilized Platinum-Decorated Silver Nanocubes INHIBIT Adenocarcinoma Cells and Enhance Antioxidant Effects by Promoting Apoptosis via Cell Cycle Arrest. Pharmaceutics 2022, 14, 2541. [Google Scholar] [CrossRef]
- El-Naggar, N.E.; Hussein, M.H.; El-Sawah, A.A. Bio-fabrication of silver nanoparticles by phycocyanin, characterization, in vitro anticancer activity against breast cancer cell line and in vivo cytotxicity. Sci. Rep. 2017, 7, 10844. [Google Scholar] [CrossRef]
- Piao, M.J.; Kang, K.A.; Lee, I.K.; Kim, H.S.; Kim, S.; Choi, J.Y.; Choi, J.; Hyun, J.W. Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol Lett. 2011, 201, 92–100. [Google Scholar] [CrossRef]
- De Matteis, V.; Malvindi, M.A.; Galeone, A.; Brunetti, V.; De Luca, E.; Kote, S.; Kshirsagar, P.; Sabella, S.; Bardi, G.; Pompa, P.P. (). Negligible particle-specific toxicity mechanism of silver nanoparticles: the role of Ag+ ion release in the cytosol. Nanomedicine: NBM. 2015, 11, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Takáč, P.; Michalková, R.; Čižmáriková, M.; Bedlovičová, Z.; Balážová, Ľ.; Takáčová, G. The Role of Silver Nanoparticles in the Diagnosis and Treatment of Cancer: Are There Any Perspectives for the Future? Life 2023, 13, 466. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, Y.; Lu, H.; Zhao, D. Silver nanoparticles coupled to anti-EGFR antibodies sensitize nasopharyngeal carcinoma cells to irradiation. Mol. Med. Rep. 2017, 16, 9005–9010. [Google Scholar] [CrossRef] [PubMed]
- Shipunova, V.O.; Belova, M.M.; Kotelnikova, P.A.; Shilova, O.N.; Mirkasymov, A.B.; Danilova, N.V.; Komedchikova, E.N.; Popovtzer, R.; Deyev, S.M.; Nikitin, M.P. Photothermal Therapy with HER2-Targeted Silver Nanoparticles Leading to Cancer Remission. Pharmaceutics 2022, 14, 1013. [Google Scholar] [CrossRef]
- Kim, D.; Amatya, R.; Hwang, S.; Lee, S.; Min, K.A.; Shin, M.C. BSA-Silver Nanoparticles: A Potential Multimodal Therapeutics for Conventional and Photothermal Treatment of Skin Cancer. Pharmaceutics 2021, 13, 575. [Google Scholar] [CrossRef]
- Azizi, M.; Ghourchian, H.; Yazdian, F. Bagherifam, S.; Bekhradnia, S.; Nyström B. Anti-cancerous effect of albumin coated silver nanoparticles on MDA-MB 231 human breast cancer cell line. Sci. Rep. 2017, 7, 5178. [Google Scholar] [CrossRef]
- Frei, E. Albumin binding ligands and albumin conjugate uptake by cancer cells. Diabetol. Metab. Syndr. 2011, 3, 11. [Google Scholar] [CrossRef]
- Ma, L.; Wei, H.L.; Wang, K.J.; Meng, X.Y.; Ni, S.Q.; Zhou, C.; Li, Y.; Yu, R.; Ma, Q. Rhein promotes TRAIL-induced apoptosis in bladder cancer cells by up-regulating DR5 expression. Aging. 2022, 14, 6642–6655. [Google Scholar] [CrossRef]
- Sur-Erdem, I.; Muslu, K.; Pınarbası, N.; Altunbek, M.; Seker-Polat, F.; Cingöz, A.; Bagcı-Önder, T. TRAIL-conjugated silver nanoparticles sensitize glioblastoma cells to TRAIL by regulating CHK1 in the DNA repair pathway. Neurol Res. 2020, 42, 1016–1069. [Google Scholar] [CrossRef]
- Jang, M.; Kim, S.; Lee, J. Cancer cell metabolism: implications for therapeutic targets. Exp. Mol. Med. 2013, 45, e45. [Google Scholar] [CrossRef]
- Morais, M.; Machado, V.; Dias, F.; Figueiredo, P.; Palmeira, C.; Martins, G.; Fernandes, R.; Malheiro, A.R.; Mikkonen, K.S.; Teixeira, A.L.; Medeiros, R. Glucose-Functionalized Silver Nanoparticles as a Potential New Therapy Agent Targeting Hormone-Resistant Prostate Cancer cells. Int J Nanomedicine. 2022, 17, 4321–4337. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.C.; Orts-Gil, G.; Lai, C.H.; Müller, L.; Haase, A.; Luch, A.; Seeberger, P.H. Carbohydrate functionalization of silver nanoparticles modulates cytotoxicity and cellular uptake. J. Nanobiotechnol. 2014, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Reedijk, J. The Mechanism of Action of Platinum Antitumor Drugs. Pure Appl. Chem. 1987, 59, 181–192. [Google Scholar] [CrossRef]
- Khan, M.A.R.; Mamun, M.S.A.; Ara, M.H. Review on platinum nanoparticles: Synthesis, characterization, and applications. Microchem. J. 2021, 171, 106840. [Google Scholar] [CrossRef]
- Tan, T.L.; Wang, L.; Zhang, J.; Johnson, D.D.; and Bai, K. Platinum Nanoparticle During Electrochemical Hydrogen Evolution: Adsorbate Distribution, Active Reaction Species, and Size Effect. ACS Catal. 2015, 5, 2376–2383. [Google Scholar] [CrossRef]
- Pawar, A.A.; Sahoo, J.; Verma, A.; Lodh, A.; Lakkakula, J. Usage of Platinum Nanoparticles for Anticancer Therapy over Last Decade: A Review. Part. Part. Syst. Charact. 2021, 38, 2100115. [Google Scholar] [CrossRef]
- Abed, A.; Derakhshan, M.; Karimi, M.; Shirazinia, M.; Mahjoubin-Tehran, M.; Homayonfal, M.; Hamblin, M. R.; Mirzaei, S. A.; Soleimanpour, H.; Dehghani, S.; Dehkordi, F. F.; Mirzaei, H. Platinum Nanoparticles in Biomedicine: Preparation, Anti-Cancer Activity, and Drug Delivery Vehicles. Front. Pharmacol. 2022, 13, 797804. [Google Scholar] [CrossRef]
- Patel, P.; Umapathy, D.; Manivannan, S.; Nadar, V.M.; Venkatesan, R.; Joseph Arokiyam, V.A.; Pappu, S.; Ponnuchamy, K. A doxorubicin-platinum conjugate system: impacts on PI3K/AKT actuation and apoptosis in breast cancer cells. RSC Rdv. 2021, 11, 4818–4828. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kotcherlakota, R.; Haque, S.; Bhattacharya, D.; Kumar, J. M.; Chakravarty, S.; Patra, C. R. Improved delivery of doxorubicin using rationally designed PEGylated platinum nanoparticles for the treatment of melanoma. Mater Sci Eng C Mater Biol Appl. 2020, 108, 110375. [Google Scholar] [CrossRef]
- Hassn Mesrati, M.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules 2021, 11, 1850. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, W.; Zhao, X.; Zhou, Z.; Wang, Y.; Cheng, Y.; Zhang, Q. Hyaluronic Acid-Encapsulated Platinum Nanoparticles for Targeted Photothermal Therapy of Breast Cancer. J. Biomed. Nanotechnol. 2017, 13, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Yang, Yang, X. ; Salado-Leza, D.; Porcel, E.; González-Vargas, C.R.; Savina, F.; Dragoe, D.; Remita, H.; Lacombe, S. A Facile One-Pot Synthesis of Versatile PEGylated Platinum Nanoflowers and Their Application in Radiation Therapy. Int. J. Mol. Sci. 2020, 21, 1619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, D.; Talaei, S.; Abasi, M. Albumin stabilized Pt nanoparticles as radiosensitizer for sensitization of breast cancer cells under X-ray radiation therapy. Inorg. Chem. Commun. 2022, 140, 109423. [Google Scholar] [CrossRef]
- Yaray, K.; Norbakhsh, A.; Rashidzadeh, H.; Mohammadi, A.; Mozafari, F. , Ghaffarlou, M.; Mousazadeh, N.; Ghaderzadeh, R.; Ghorbani, Y.; Nasehi, L.; Danafar, H.; Ertas, Y.N.. Chemoradiation therapy of 4T1 cancer cells with methotrexate conjugated platinum nanoparticles under X-Ray irradiation. Inorg. Chem. Commun. 2023, 150, 110457. [Google Scholar] [CrossRef]
- Teow, Y.; Valiyaveettil, S. Active targeting of cancer cells using folic acid-conjugated platinum nanoparticles. Nanoscala. 2010, 2, 2607–2613. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




