Submitted:
15 June 2023
Posted:
16 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
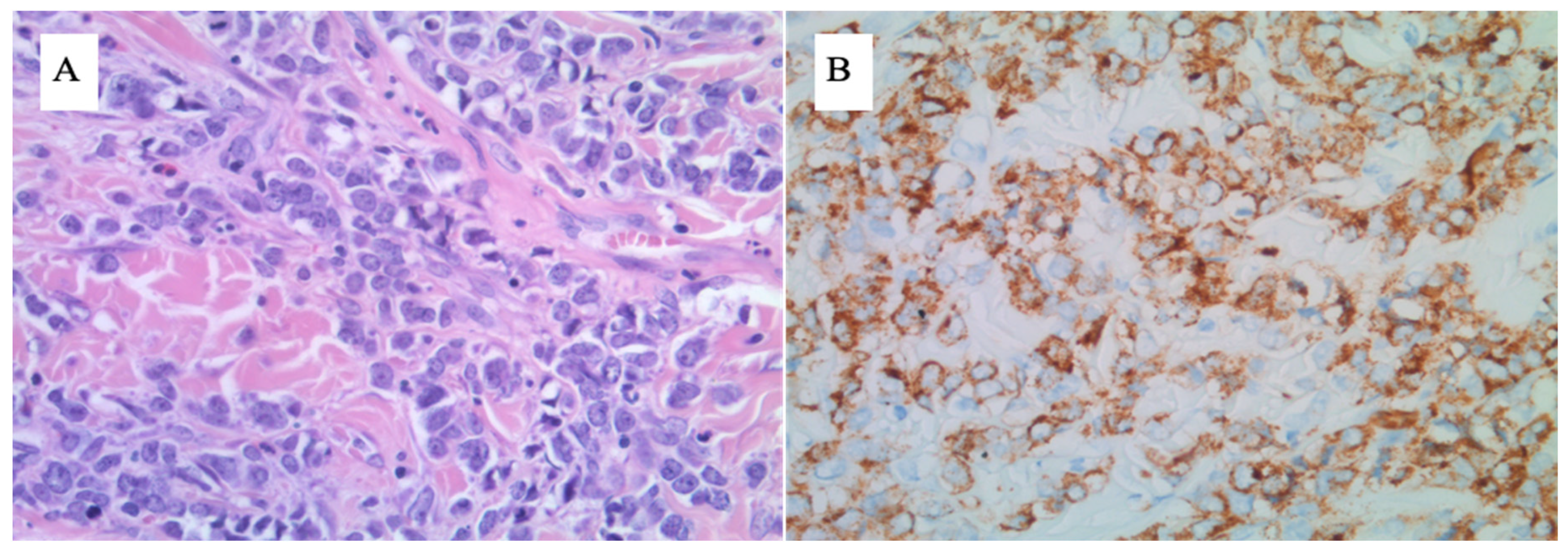
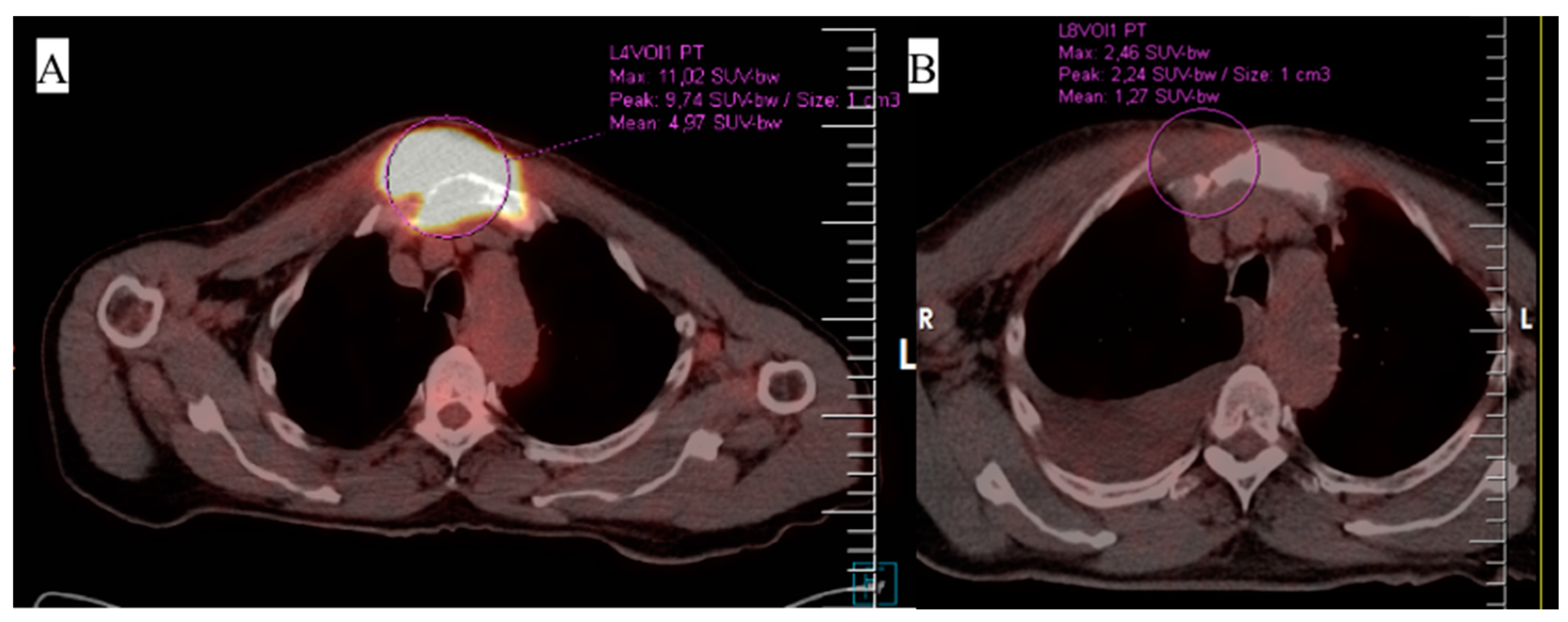
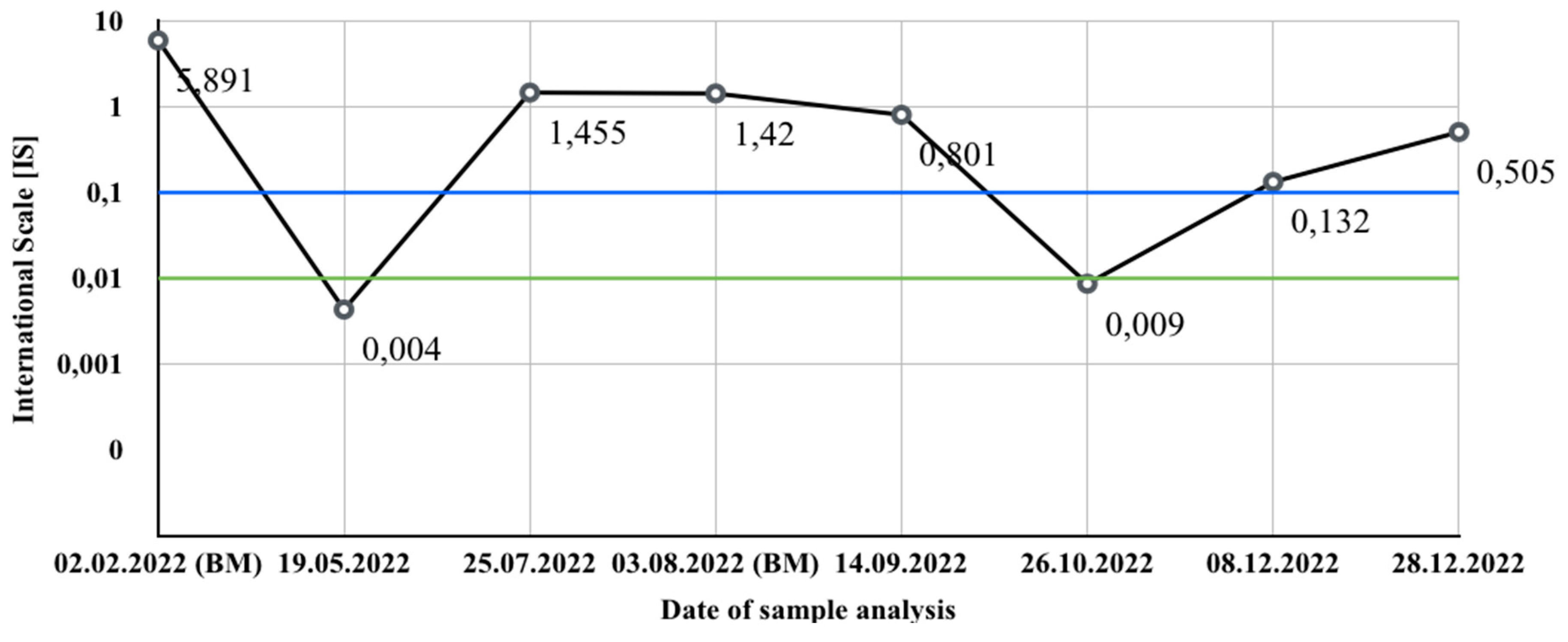
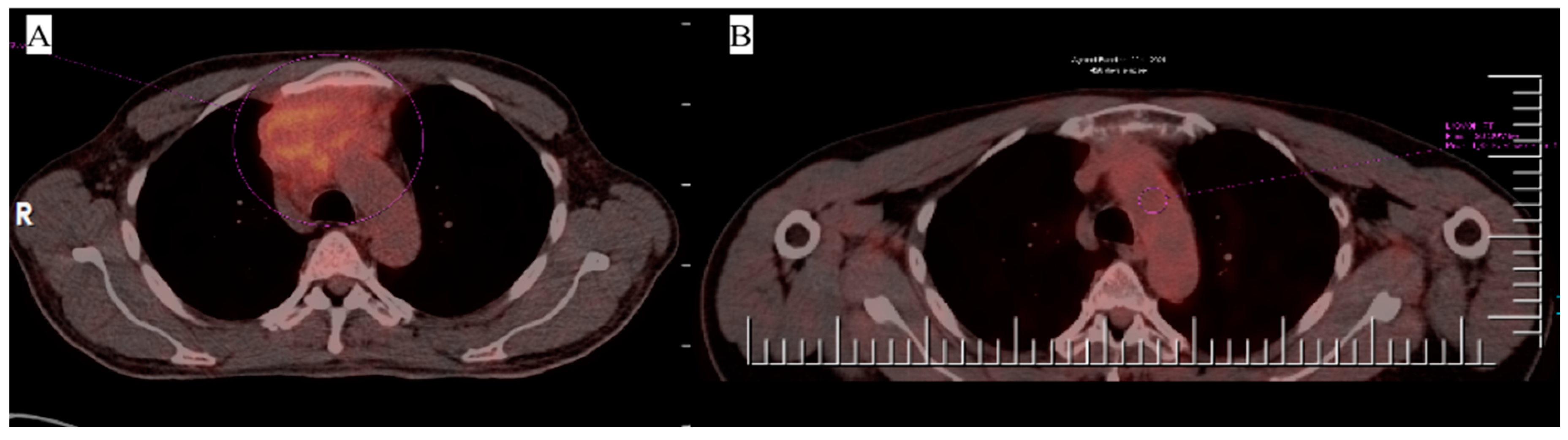
3. Case 1
- 43% had 4 fusion signals, 1 red signal for ABL1 (9q34) and 1 green for BCR (22q11);
- 4% had 3 fusion signals, 1 red signal for ABL1 (9q34) and 1 green for BCR (22q11);
- 3% had 2 fusion signals, 1 red signal for ABL1 (9q34) and 1 green for BCR (22q11).
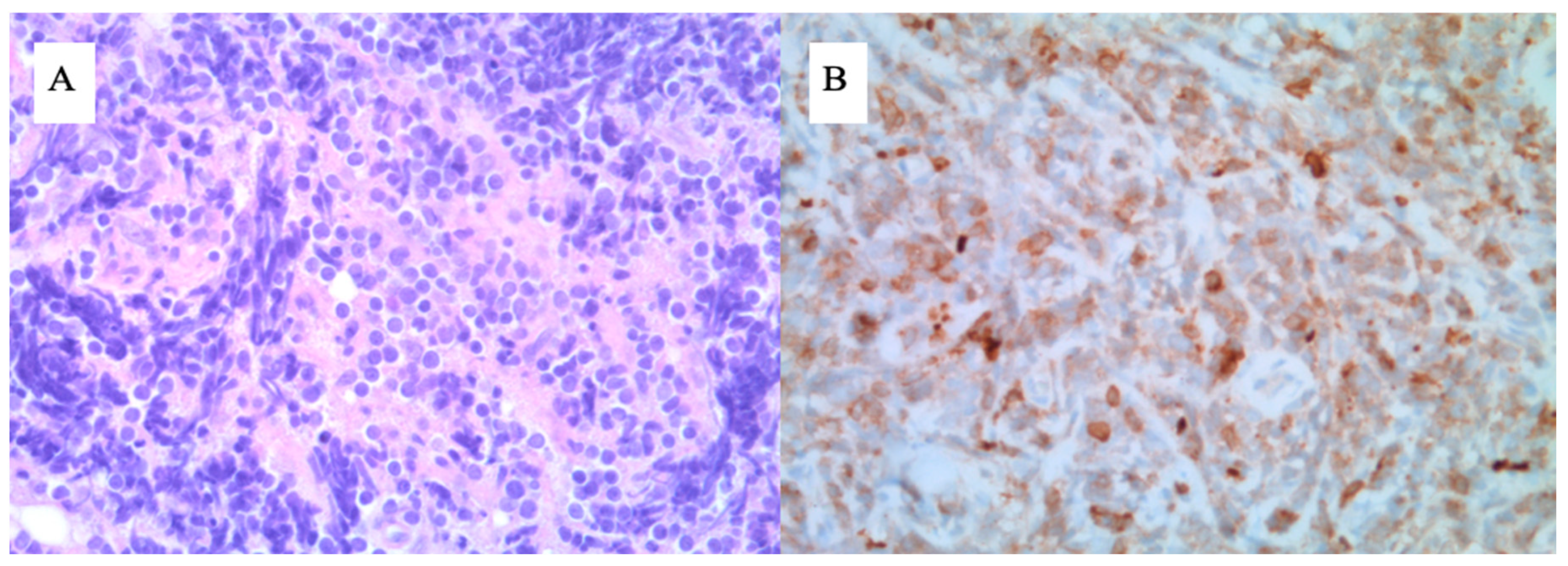
4. Case 2
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
CARE Checklist (2016) statement
References
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th Edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022 36:7 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Goyal, G.; Bartley, A.C.; Patnaik, M.M.; Litzow, M.R.; Al-Kali, A.; Go, R.S. Clinical Features and Outcomes of Extramedullary Myeloid Sarcoma in the United States: Analysis Using a National Data Set. Blood Cancer J 2017, 7. [Google Scholar] [CrossRef]
- Werstein, B.; Dunlap, J.; Cascio, M.J.; Ohgami, R.S.; Fan, G.; Press, R.; Raess, P.W. Molecular Discordance between Myeloid Sarcomas and Concurrent Bone Marrows Occurs in Actionable Genes and Is Associated with Worse Overall Survival. J Mol Diagn 2020, 22, 338–345. [Google Scholar] [CrossRef]
- Greenland, N.Y.; Van Ziffle, J.A.; Liu, Y.C.; Qi, Z.; Prakash, S.; Wang, L. Genomic Analysis in Myeloid Sarcoma and Comparison with Paired Acute Myeloid Leukemia. Hum Pathol 2021, 108, 76–83. [Google Scholar] [CrossRef]
- Engel, N.W.; Reinert, J.; Borchert, N.M.; Panagiota, V.; Gabdoulline, R.; Thol, F.; Heuser, M.; Fiedler, W. Newly Diagnosed Isolated Myeloid Sarcoma-Paired NGS Panel Analysis of Extramedullary Tumor and Bone Marrow. Ann Hematol 2021, 100, 499–503. [Google Scholar] [CrossRef]
- Almond, L.M.; Charalampakis, M.; Ford, S.J.; Gourevitch, D.; Desai, A. Myeloid Sarcoma: Presentation, Diagnosis, and Treatment. Clin Lymphoma Myeloma Leuk 2017, 17, 263–267. [Google Scholar] [CrossRef]
- Yilmaz, A.F.; Saydam, G.; Sahin, F.; Baran, Y. Granulocytic Sarcoma: A Systematic Review. Am J Blood Res 2013, 3, 265. [Google Scholar]
- Nanote, V.A.; Mahajan, O.A.; Gaidhane, S.A.; Parve, S.D.; Raut, L. Chronic Myeloid Leukemia: Chronic Phase Presenting as Extramedullary Presentation. Cureus 2022, 14. [Google Scholar] [CrossRef]
- Ali, E.A.; Abu-Tineh, M.; Abdelrazek, M.; Petkar, M.; Karzoun, M.; Subahi, E.A.; Szabados, L.; Yassin, M.A. Myeloid Sarcoma Mimicking Dental Abscess in a Patient with Chronic Myeloid Leukemia: Diagnostic and Therapeutic Dilemma. Case Rep Oncol 2022, 15, 755–761. [Google Scholar] [CrossRef]
- Han, S.; Li, Y.; Niu, T.; Wang, X.; Li, Z.; Ren, X.; Gao, J. Granulocytic Sarcoma Causing Long Spinal Cord Compression: Case Report and Literature Review. J Spinal Cord Med 2022, 45, 481–485. [Google Scholar] [CrossRef]
- Zhang, Y.; Swoboda, D.M.; Grover, A.; Nodzon, L.; Zhang, L.; Pinilla-Ibarz, J. T315I-Mutated Myeloid Sarcoma. Leuk Res Rep 2019, 12. [Google Scholar] [CrossRef]
- Palejwala, A.H.; O’Connor, K. P.; Shi, H.; Villeneuve, L.; Scordino, T.; Glenn, C.A. Chronic Myeloid Leukemia Manifested as Myeloid Sarcoma: Review of Literature and Case Report. J Clin Neurosci 2019, 64, 269–276. [Google Scholar] [CrossRef]
- AI, D.; LIU, W.; LU, G.; PATEL, K.P.; CHEN, Z. Extramedullary Blast Crisis as Initial Presentation in Chronic Myeloid Leukemia with the E1a2 BCR-ABL1 Transcript: A Case Report. Mol Clin Oncol 2015, 3, 1319–1322. [Google Scholar] [CrossRef]
- Kwatra, K.S.; Prabhakar, B.R.; Arora, Y. Bilateral Granulocytic Sarcoma (Chloroma) of the Breast in CML in Blast Crisis: A Case Report. Indian J Pathol Microbiol 2004, 47, 66–68. [Google Scholar]
- Amiraian, D.; McDonough, M.; Geiger, X. Bilateral Myeloid Sarcoma of the Breast: A Case Report With Radiological and Pathological Correlation. Cureus 2022, 14. [Google Scholar] [CrossRef]
- Mullen, C.; Beverstock, S.; Roddie, H.; Campbell, V.L.; Al-Qsous, W. Myeloid Sarcoma of Uterine Cervix: A Case Report with Review of the Literature. Gynecol Oncol Rep 2022, 39, 100931. [Google Scholar] [CrossRef]
- Park, J.H.; Son, Y.; Hyon, J.Y.; Lee, J.Y.; Jeon, H.S. Relapsed Acute Myeloid Leukemia Presenting as Conjunctival Myeloid Sarcoma: A Case Report. BMC Ophthalmol 2022, 22. [Google Scholar] [CrossRef] [PubMed]
- Athukuri, P.; Khan, A.B.; Gadot, R.; Haque, M.; Lee, S.; Gallagher, K.K.; Mims, M.P.; Rivero, G.A.; Barbieri, A.; Patel, A.J.; et al. Myeloid Sarcoma of the Skull Base: A Case Report and Systematic Literature Review. Surg Neurol Int 2022, 13. [Google Scholar] [CrossRef]
- Taminishi-Katsuragawa, Y.; Shimura, Y.; Inoue, Y.; Matsumura-Kimoto, Y.; Tsukamoto, T.; Mizutani, S.; Kobayashi, T.; Takeda-Miyata, N.; Nishimura, A.; Takatsuka, K.; et al. Gastric Myeloid Sarcoma Mimicking a Scirrhous Gastric Cancer. Intern Med 2022, 61, 1231–1235. [Google Scholar] [CrossRef]
- Asawa, P.; Vusqa, U.; Vashistha, K.; Din, M.T.U.; Karna, R.; Chen, F.; Dutton, N.; Samhouri, Y.; Fazal, S. Polyserositis as a Unique Presentation of Isolated Myeloid Sarcoma: A Case Report. Anticancer Res 2022, 42, 3595–3599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, S.; Wang, F.; Shen, Q.; Jia, Y. Microtransplantation for Myeloid Sarcoma: Two Case Reports. Leuk Res Rep 2022, 17, 100326. [Google Scholar] [CrossRef] [PubMed]
- Tuna, R.; Karaman, S.; Oktar, T.; Anak, S.; Doğan, Ö.; Ünüvar, A.; Tuğcu, D.; Bayramoğlu, Z.; Kılıç, S.Ç.; Çelik, A.İ.; et al. Bladder Granulocytic Sarcoma in a Child: Case Report and Literature Review. Turk J Pediatr 2022, 64, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Jiang, Y. Granulocytic Sarcoma of Cervix: A Rare Case Report. Medicine 2022, 101, E29419. [Google Scholar] [CrossRef] [PubMed]
- Shash, H.A.; Khairy, A.M. Successful Treatment of Pediatric Acute Myeloid Leukemia Presenting with Hyperbilirubinemia Secondary to Myeloid Sarcoma: A Case Report. Children (Basel) 2022, 9. [Google Scholar] [CrossRef]
- Wang, V.; Van Perre, K.; Pu, L.; Liu, Y.; Wang, J.; Choo, E.; Moyers, J.; Cao, H.; Lau, E. Concurrent Epstein Barr Virus-Associated Smooth Muscle Tumor and Myeloid Sarcoma of the Liver and Acute Myeloid Leukemia in a Patient Post Kidney Transplant: A Case Report and Review of the Literature. J Gastrointest Oncol 2022, 13, 3329–3335. [Google Scholar] [CrossRef]
- Cross, A.; Chajewski, O.S.; Rutland, C.; Smith, K.; Woodham, P.; Skipper, D.; Lindsey, K.G. Myeloid Sarcoma Diagnosed on Pleural Effusion Cytology: A Case Report and Literature Review. Diagn Cytopathol 2021, 49, E316–E319. [Google Scholar] [CrossRef]
- Gosavi, A.Y.; Puranik, A.; Agrawal, A.; Purandare, N.C.; Shah, S.; Rangarajan, V.; Thomas, A. Myeloid Sarcoma of the Prostatic Tissue Diagnosed on 18F-FDG PET/CT in Treated Case of Acute Myeloid Leukemia. Indian J Nucl Med 2021, 36, 193. [Google Scholar] [CrossRef]
- Huang, C.; Fei, S.; Yao, J.; Chen, P.; Luo, J.; Wang, Y.; Li, J.; Wang, W. Breast Myeloid Sarcoma Presenting as a Palpable Breast Lump after Allogeneic Stem Cell Transplantation for Acute Myelomonocytic Leukemia: A Rare Case Report. World J Surg Oncol 2021, 19. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, X.; Zhang, B. Myeloid Sarcoma of the Pancreas: A Case Report and Literature Review. Medicine 2021, 100, E24913. [Google Scholar] [CrossRef]
- Thomson, A.; Timm, B.; Nazaretian, S.; Liodakis, P.; Bolton, D. Rare Presentation of Isolated Bilateral Testicular Myeloid Sarcoma: A Case Report. Urol Case Rep 2021, 36. [Google Scholar] [CrossRef]
- Liu, R.; Du, J.; Gao, L.; Liu, Y.; Liu, S. Myeloid Sarcoma of the Nasal Cavity in a 15-Month-Old Child: A Case Report. Medicine 2020, 99, e21119. [Google Scholar] [CrossRef]
- Hernández, E.M.P.; Vázquez, L.A.Z.; Herevia, A.E.J.; Ugarte, D.H.; Moreno, E.O. Intestinal Chloroma. Cureus 2020, 12. [Google Scholar] [CrossRef]
- Sun, X.; Rong, X.; Nie, H.; Yan, X. Isolated Retro-Orbital Granulocytic Sarcoma Relapse of Acute Myeloid Leukemia after Allogeneic Hematopoietic Stem Cell Transplantation: A Case Report. Eur J Ophthalmol 2020, 32, NP94–NP98. [Google Scholar] [CrossRef]
- Slusarenko da Silva, Y.; Naclério-Homem, M. da G. Myeloid Sarcoma on the Temporal Region before the Onset of the Acute Myeloid Leukemia: An Extremely Rare Case Report. Oral Maxillofac Surg 2020, 24, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sonia, F.; Hamadani, S.M.; Abbas, S.A. A Rare Case of Acute Cord Compression From Spinal Myeloid Sarcoma: A Complication of Acute Myeloid Leukemia. Cureus 2020, 12. [Google Scholar] [CrossRef]
- Pi, Y.; Wang, B.; Wang, L.; Ren, H. Polyserositis as a Primary Clinical Manifestation of CD7+ Acute Myelogenous Leukemia with Myeloid Sarcoma: A Case Report. Medicine 2020, 99, E23615. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Dadu, T.; Bhalla, V.P.; Malhotra, V. Myeloid Sarcoma of Bile Ducts Presenting as Obstructive Jaundice - A Case Report. Indian J Pathol Microbiol 2019, 62, 602–604. [Google Scholar] [CrossRef]
- Marwah, N.; Bhutani, N.; Budhwar, A.; Sen, R. Isolated Myeloid Sarcoma of the Temporal Bone: As the First Clinical Manifestation of Acute Myeloid Leukemia in a Patient of down’s Syndrome. Int J Surg Case Rep 2019, 58, 77–80. [Google Scholar] [CrossRef]
- Almalki, A.M.J.; Alotaibi, F.A.; Jabr, H.F.; Mastan, A.R. Unilateral Proptosis As An Initial Sign Of Acute Myeloid Leukemia In A Child: A Case Report. Int Med Case Rep J 2019, 12, 319–323. [Google Scholar] [CrossRef]
- Abdelnabi, M.H.; Almaghraby, A.; Saleh, Y.; ElSharkawy, E. Cardiac Chloroma or Cardiac Myeloid Sarcoma: Case Report. Echocardiography 2019, 36, 1594–1595. [Google Scholar] [CrossRef]
- Bubulac, L.; Bardaş, A.; Popa, D.C.; Didona Vasilache, E.; Ionescu, B.O.; Coriu, D.; Varady, Z.; Dobrea, C.-M. Breast Myeloid Sarcoma after Allogeneic Stem Cell Transplantation for Acute Myelomonocytic Leukemia-Case Report. Rom J Morphol Embryol 2019, 707–711. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, L.; Cheng, N.; Lai, W.; Chen, Y.; Liu, J. A Case of Granulocytic Sarcoma of the Lung in Acute Myeloid Leukemia. Clin Lab 2019, 65, 1355–1359. [Google Scholar] [CrossRef]
- Nguyen, R.; Sayar, H. Primary Myeloid Sarcoma of the Prostate: A Case Report and Literature Review. Case Rep Hematol 2018, 2018, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Khaja, M.; Omesh, T.; Niazi, M.; Shaikh, D.; Mudunuru, S.A.; Reyes, O.A.; Malik, S. Co-Occurrence of Myeloid Sarcoma of the Lymph Node and Acute Monocytic Myeloid Leukemia: A Case Report and Literature Review. Case Rep Oncol 2018, 11, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Siraj, F.; Kaur, M.; Dalal, V.; Khanna, A.; Khan, A.A. Myeloid Sarcoma: A Report of Four Cases at Unusual Sites. Ger Med Sci 2017, 15. [Google Scholar] [CrossRef]
- Huang, B.; You, P.; Zhu, P.; Du, Z.; Wu, B.; Xu, X.; Chen, Z. Isolated Duodenal Myeloid Sarcoma Associated with the CBFβ/MYH11 Fusion Gene Followed by Acute Myeloid Leukemia Progression: A Case Report and Literature Review. Oncol Lett 2014, 8, 1261. [Google Scholar] [CrossRef] [PubMed]
- Kini, S.; Amarapurkar, A.; Balasubramanian, M. Small Intestinal Obstruction with Intussusception Due to Acute Myeloid Leukemia: A Case Report. Case Rep Gastrointest Med 2012, 2012, 1–3. [Google Scholar] [CrossRef]
- Cash, T.; Becton, D.; Mian, A. Cardiac Myeloid Sarcoma: A Case Report and Review of Literature. J Pediatr Hematol Oncol 2011, 33. [Google Scholar] [CrossRef]
- Gadage, V.; Zutshi, G.; Menon, S.; Shet, T.; Gupta, S. Gastric Myeloid Sarcoma--a Report of Two Cases Addressing Diagnostic Issues. Indian J Pathol Microbiol 2011, 54, 832–835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Zhang, R.; Li, Y. Granulocytic Sarcoma of Abdomen in Acute Myeloid Leukemia Patient with Inv(16) and t(6;17) Abnormal Chromosome: Case Report and Review of Literature. Leuk Res 2010, 34, 958–961. [Google Scholar] [CrossRef]
- Skeete, D.H.A.; Cesar-Rittenberg, P.; Jong, R.; Murray, S.K.; Colgan, T.J. Myeloid Sarcoma of the Vagina: A Report of 2 Cases. J Low Genit Tract Dis 2010, 14, 136–141. [Google Scholar] [CrossRef]
- Mneimneh, W.S.; Bonte, H.; Bernheim, A.; Martin, A. Myeloid Sarcoma of the Prostate Revealed by Urinary Retention: A Case Report. BMJ Case Rep 2009, 2009. [Google Scholar] [CrossRef] [PubMed]
- Shallis, R.M.; Gale, R.P.; Lazarus, H.M.; Roberts, K.B.; Xu, M.L.; Seropian, S.E.; Gore, S.D.; Podoltsev, N.A. Myeloid Sarcoma, Chloroma, or Extramedullary Acute Myeloid Leukemia Tumor: A Tale of Misnomers, Controversy and the Unresolved. Blood Rev 2021, 47. [Google Scholar] [CrossRef]
- Guo, M.; Chao, N.J.; Li, J.Y.; Rizzieri, D.A.; Sun, Q.Y.; Mohrbacher, A.; Krakow, E.F.; Sun, W.J.; Shen, X.L.; Zhan, X.R.; et al. HLA-Mismatched Microtransplant in Older Patients Newly Diagnosed With Acute Myeloid Leukemia: Results From the Microtransplantation Interest Group. JAMA Oncol 2018, 4, 54. [Google Scholar] [CrossRef] [PubMed]
| Year | Author | Age | Sex | Malignancy | Occurrence of MS in relation to leukemia | Location | Follow-up period | Patient status (dead/alive) | Therapy for MS | Clinical outcome of MS |
|---|---|---|---|---|---|---|---|---|---|---|
| 2022 | Nanote et al. [8] | 69 | F | CML | Secondary | Parotid gland | NR | alive | CHTH | NR |
| 2022 | Ali et al. [9] | 26 | M | CML | Secondary | Maxilla | NR | alive | CRT | PR |
| 2020 | Han et al. [10] | 37 | M | CML | Secondary | Spinal canal | 1 year | alive | Surgical, CHTH | CR |
| 2019 | Zhang et al. [11] | 21 | M | CML | Secondary | Femur | NR | alive | CHTH | NR |
| 2019 | Palejwala et al. [12] | 50 | F | CML | Secondary | Frontal lobe | 1 month | alive | Surgical, CRT | PR |
| 2015 | Ai et al. [13] | 23 | F | CML | Secondary | Lymph nodes | 4 years | alive | CHTH, allo-SCT MRD | CR |
| 2004 | Kwatra et al. [14] | 35 | F | CML | Secondary | Breast | N/a | N/a | N/a | N/a |
| 2022 | Amiraian et al. [15] | 63 | F | AML | Secondary | Breast | NR | dead | None (passed before therapy) | DP |
| 2022 | Mullen et al. [16] | 26 | F | AML | Primary | Uterine cervix | 18 months | alive | CHTH, allo-SCT MRD | CR |
| 2022 | Park et al. [17] | 50 | M | AML | Secondary | Conjuctiva | 3 years | alive | CRT, allo-SCT | CR |
| 2022 | Athukuri et al. [18] | 30 | M | AML | Secondary | Cranial base | 8 months | alive | Surgical, CHTH | PR |
| 2022 | Taminishi-Katsuragawa et al. [19] | 66 | M | AML | Concomitant | Stomach | NR | alive | CHTH | CR |
| 2022 | Asawa et al. [20] | 20 | M | AML | Primary | Upper lobe of the left lung, Mediastinum | 6 months | alive | CHTH, allo-SCT | CR |
| 2022 | Zhang et al. [21] | 38 | F | AML | Primary | Lymph nodes | 5,5 years | alive | CHTH, microtransplantation[54] | DP |
| 2022 | Zhang et al. [21] | 26 | F | AML | Concomitant | Cranial base, parapharyngeal space | 6 years | alive | CHTH, microtransplantation[54] | CR |
| 2022 | Tuna et al. [22] | 12 | F | AML | Concomitant | Bladder | 5 years | alive | CRT, surgical, allo-SCT MUD | CR |
| 2022 | Ye et al. [23] | 45 | F | AML | Primary | Uterine cervix | 1 year | alive | Surgical, CHTH | CR |
| 2022 | Shash et al. [24] | 4 | M | AML | Primary | Retroperitoneum | 4 years | alive | CHTH | CR |
| 2022 | Wang et al. [25] | 23 | F | AML | Concomitant | Liver | NR | dead | CHTH | CR |
| 2021 | Cross et al. [26] | 45 | M | AML | Primary | Heart, Pancreas | NR | alive | CHTH | PR |
| 2021 | Gosavi et al. [27] | 27 | M | AML | Secondary | Prostate | 6 months | dead | CRT, allo-SCT | DP |
| 2021 | Huang et al. [28] | 34 | F | AML | Secondary | Breast | 1 year | dead | CHTH, allo-SCT MMRD | DP |
| 2021 | Wu et al. [29] | 32 | F | AML | Secondary | Pancreas | NR | alive | CHTH | PR |
| 2021 | Thomson et al. [30] | 67 | M | AML | Primary | Testicles | NR | alive | CHTH | CR |
| 2020 | Liu et al. [31] | 15 months | M | AML | Primary | Nasal cavity | 2 years | alive | Surgical, CHTH | CR |
| 2020 | Hernández et al. [32] | 22 | F | AML | Concomitant | Intestine | 12 days | dead | Surgical | DP |
| 2020 | Sun et al. [33] | 37 | M | AML | Secondary | Retro-orbital space | 6 months | dead | RT, allo-SCT | DP |
| 2020 | Slusarenko da Silva et al. [34] | 29 | M | AML | Primary | Temporal region | N/a | N/a | N/a | N/a |
| 2020 | Kumar et al. [35] | 64 | F | AML | Secondary | Thoracic spine | NR | dead | CRT | DP |
| 2020 | Pi et al. [36] | 30 | F | AML | Concomitant | Lymph node | 4 months | dead | CHTH | DP |
| 2019 | Agarwal et al. [37] | 72 | F | AML | Primary | Bile ducts | 2 months | dead | Surgical | DP |
| 2019 | Marwah et al. [38] | 2 | M | AML | Primary | Temporal bone | NR | alive | CHTH | CR |
| 2019 | Almalki et al. [39] | 1 | M | AML | Concomitant | Retro-orbital space | NR | alive | CHTH | N/a |
| 2019 | Abdelnabi et al. [40] | 70 | M | AML | Secondary | Left cardiac ventricle | NR | dead | CRT | DP |
| 2019 | Bubulac et al. [41] | 30 | F | AML | Secondary | Breast | 3 years | dead | CRT, allo-SCT MRD | DP |
| 2019 | Feng et al. [42] | 56 | M | AML | Secondary | Lung | N/a | N/a | CHTH | CR |
| 2018 | Nguyen et al. [43] | 73 | M | AML | Primary | Prostate | NR | alive | Surgical, CHTH | PR |
| 2018 | Khaja et al. [44] | 29 | F | AML | Concomitant | Lymph node | NR | alive | CHTH | NR |
| 2017 | Siraj et al. [45] | 38 | M | AML | Primary | Nasal cavity | NR | N/a | CHTH | N/a |
| 2017 | Siraj et al. [45] | 11 | M | AML | Primary | Retro-orbital space | 18 months | alive | CRT | CR |
| 2017 | Siraj et al. [45] | 49 | M | AML | Primary | Brain | 6 months | dead | Surgical, CHTH | DP |
| 2017 | Siraj et al. [45] | 52 | M | AML | Secondary | Peritoneum | NR | NR | NR | NR |
| 2014 | Huang et al. [46] | 58 | F | AML | Primary | Duodenum | NR | alive | CHTH | CR |
| 2012 | Kini et al. [47] | 25 | M | AML | Concomitant | Gastrointestinal tract | 10 days | dead | Surgical | DP |
| 2011 | Cash et al. [48] | 24 | F | AML | Secondary | Heart | NR | dead | CHTH, allo-SCT | DP |
| 2011 | Gadage et al. [49] | 35 | M | AML | Concomitant | Stomach | 10 months | dead | CHTH | DP |
| 2011 | Gadage et al. [49] | 25 | M | AML | Primary | Stomach | 15 days | dead | Surgical | DP |
| 2010 | Zhang et al. [50] | 16 | M | AML | Concomitant | Abdomen | NR | alive | CHTH | CR |
| 2010 | Skeete et al. [51] | 77 | F | AML | Primary | Vagina | 5 months | dead | RT | DP |
| 2010 | Skeete et al. [51] | 36 | F | AML | Secondary | Vagina | 5 months | dead | RT | DP |
| 2009 | Mneimneh et al. [52] | 65 | M | AML | Concomitant | Prostate | 3 weeks | dead | Surgical, CHTH | DP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





