Submitted:
14 June 2023
Posted:
15 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction:
2. Production of AWLC hydraulic binders
- Heating the raw mixture at a rate R1= 25ºC/min to reach the required melting temperature (T1).
- Maintain temperature T1 in the liquid region for a period of t1= 60 min to allow the homogenization of the composition.
- Quenching the system to room temperature at a rate of at least 300 ºC/min.
3. Characterization of the AWLC
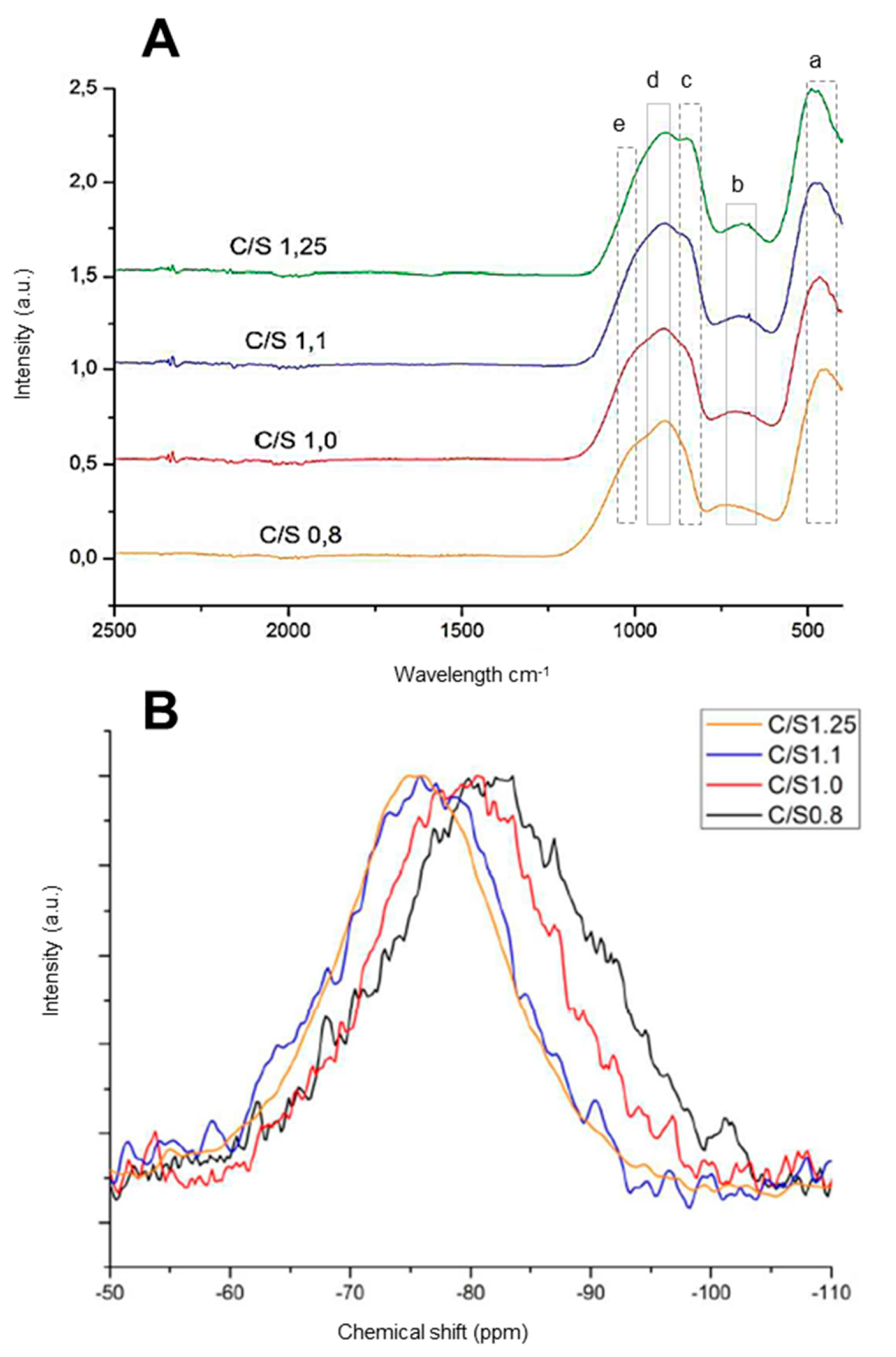
3.1. Anhydrous material
- 400 to 500 cm-1;
- 600 to 750 cm-1;
- 780 to 850 cm-1 870 to 900 cm-1;
- 900 to 1000 cm-1 and
- 1100 to 1200 cm-1.
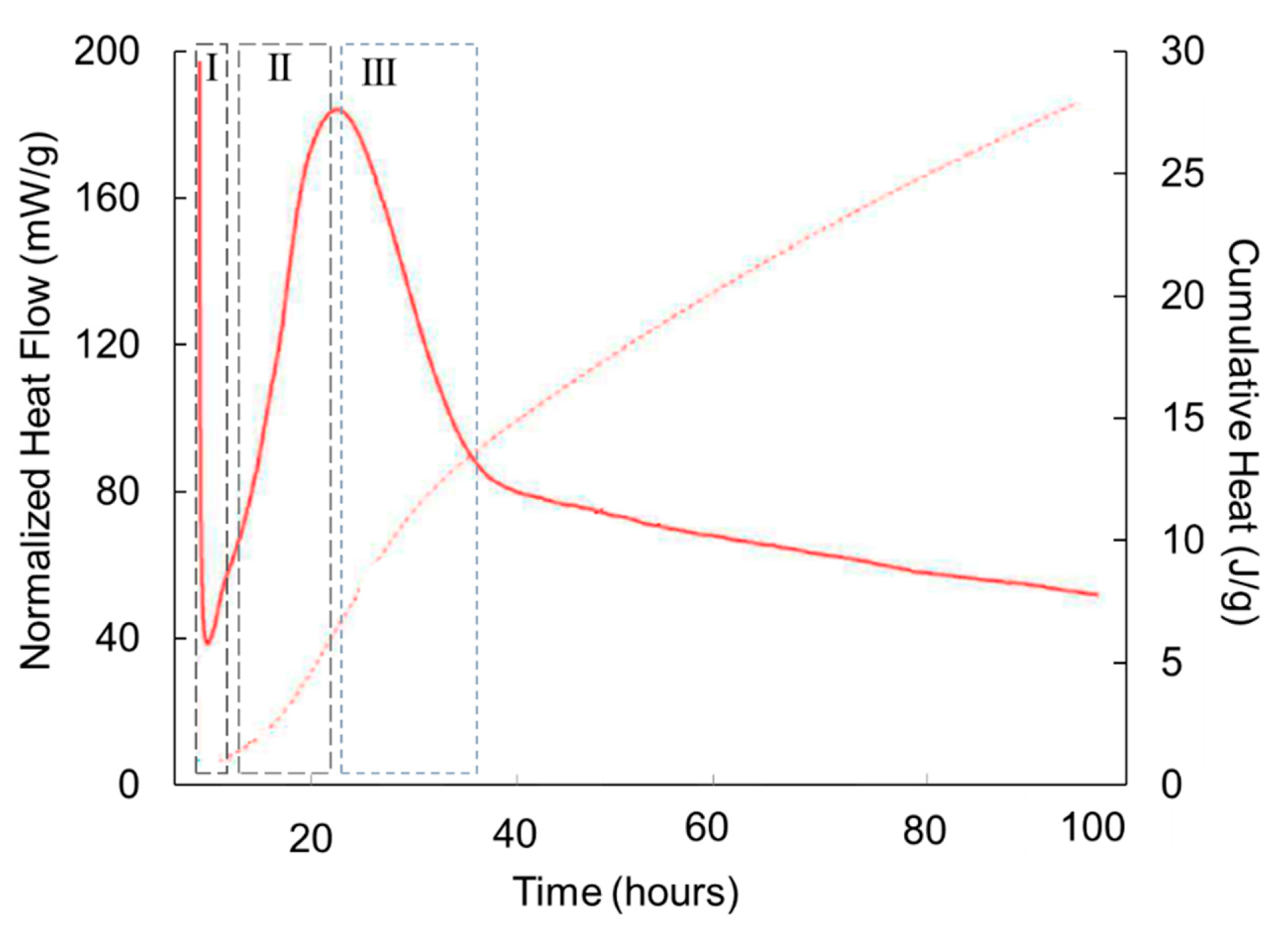
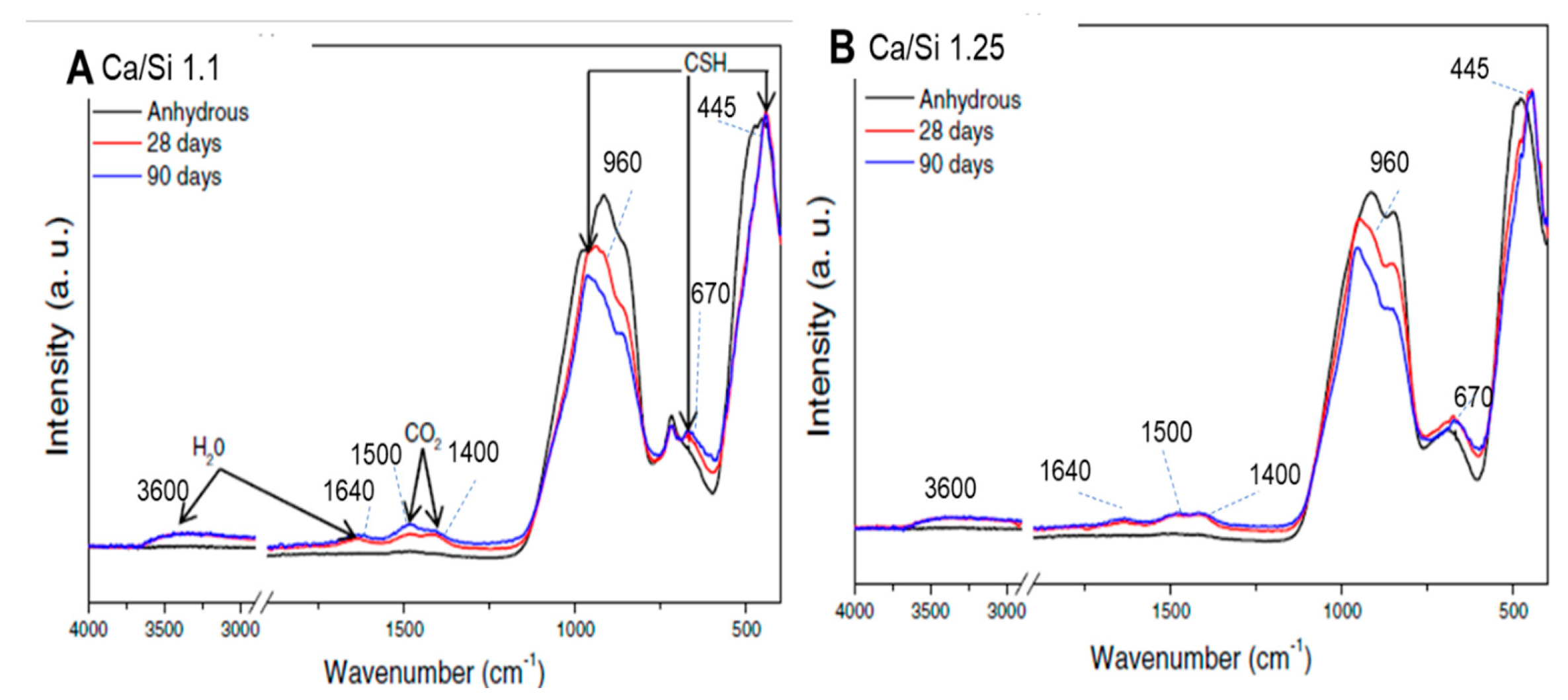
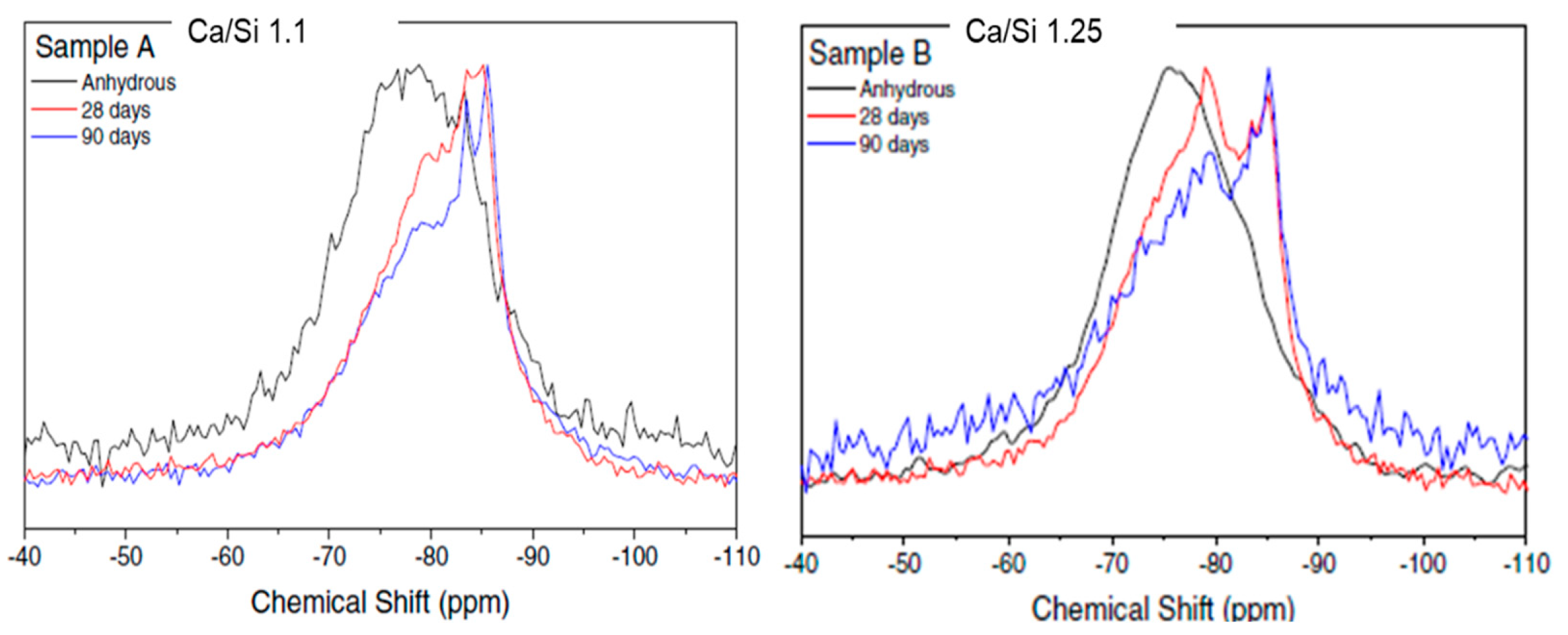
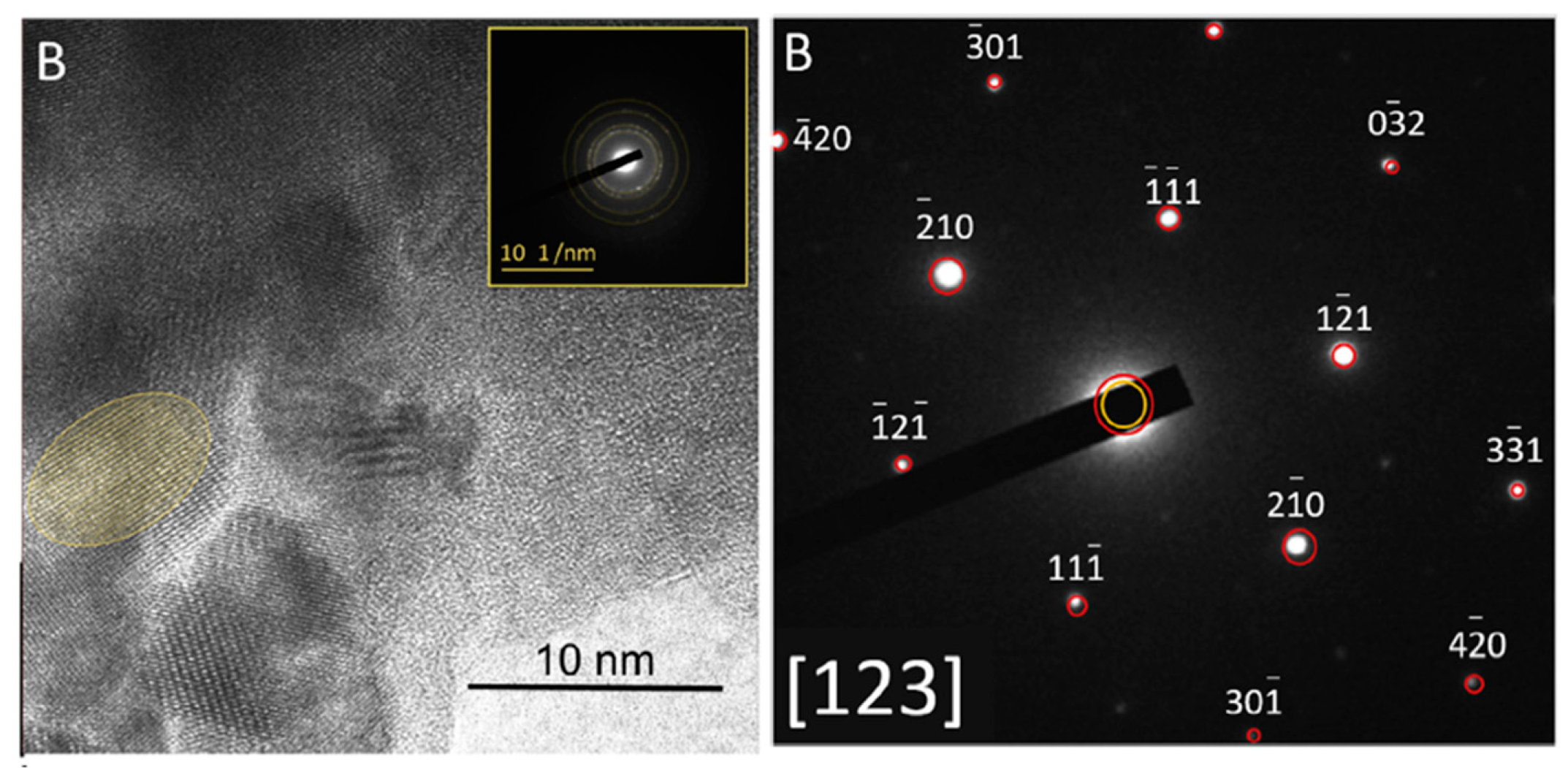
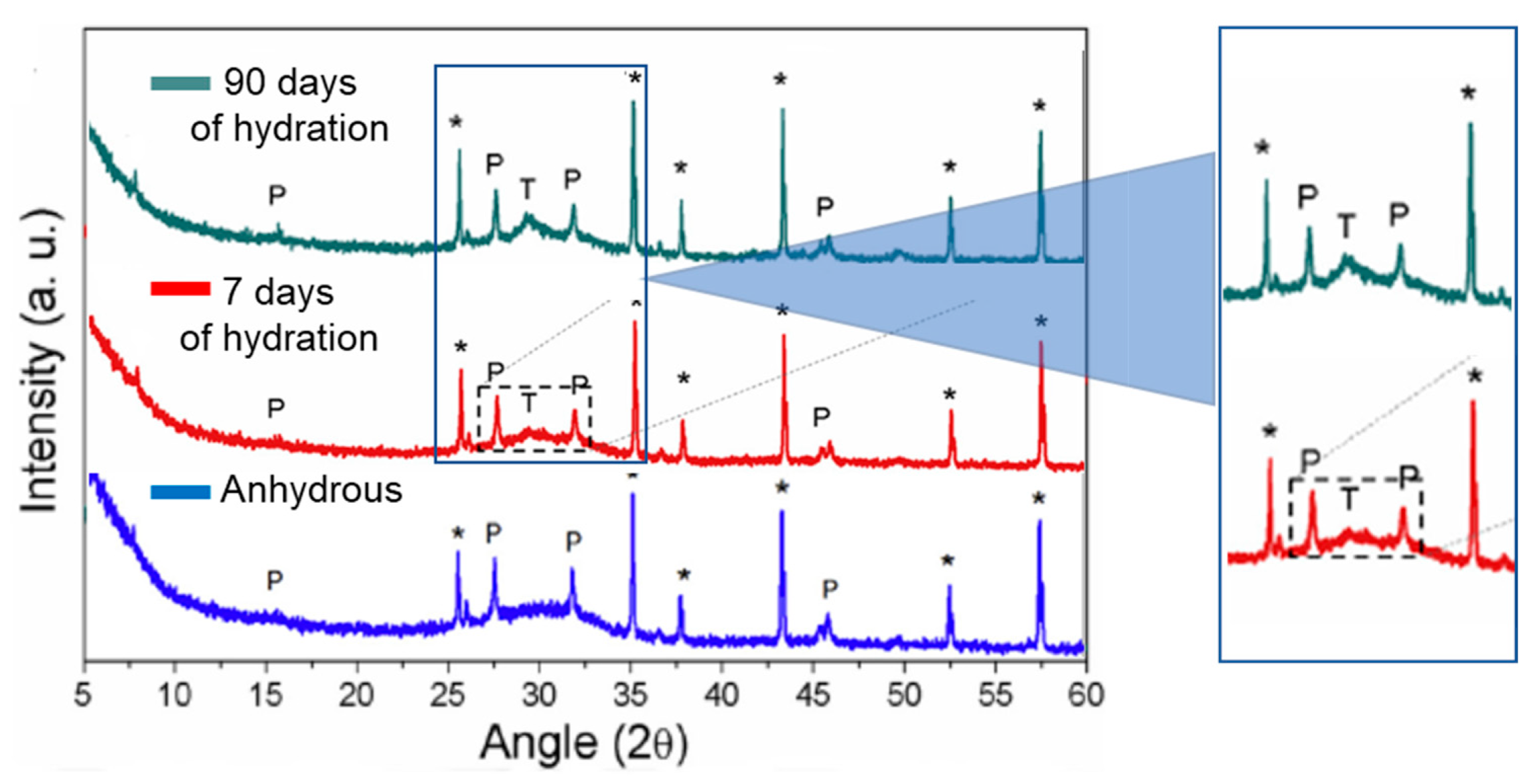
3.2. Hydrated material
- I.
- A first stage of the hydration, characterized by a slow reaction kinetics, also observed in OPC, is usually attributed to species ionic dissolution [35].
- II.
- An acceleration period characterized by a high rate of heat release [35,36]. In OPC this stage is usually attributed to the precipitation of CSH products and portlandite. Since in AWLC, there is no precipitation of portlandite, this second stage should correspond to the formation of CSH and, most probably, to the formation of tobermorite, which is present in the hydrated product [16] as we will see in the following points.
- III.
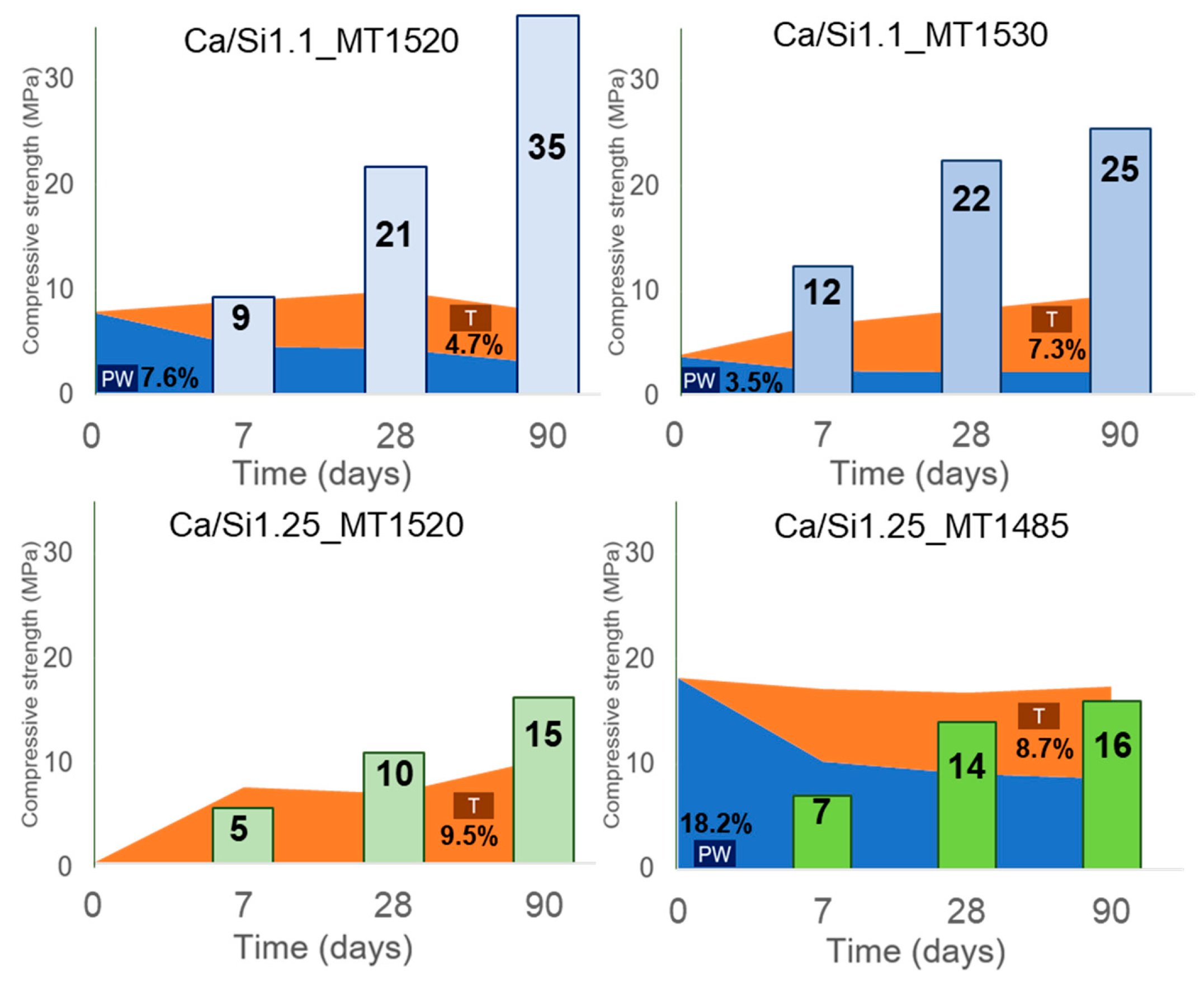
3.3. Relation between the tobermorite and pseudowollastonite content and the mechanical performance
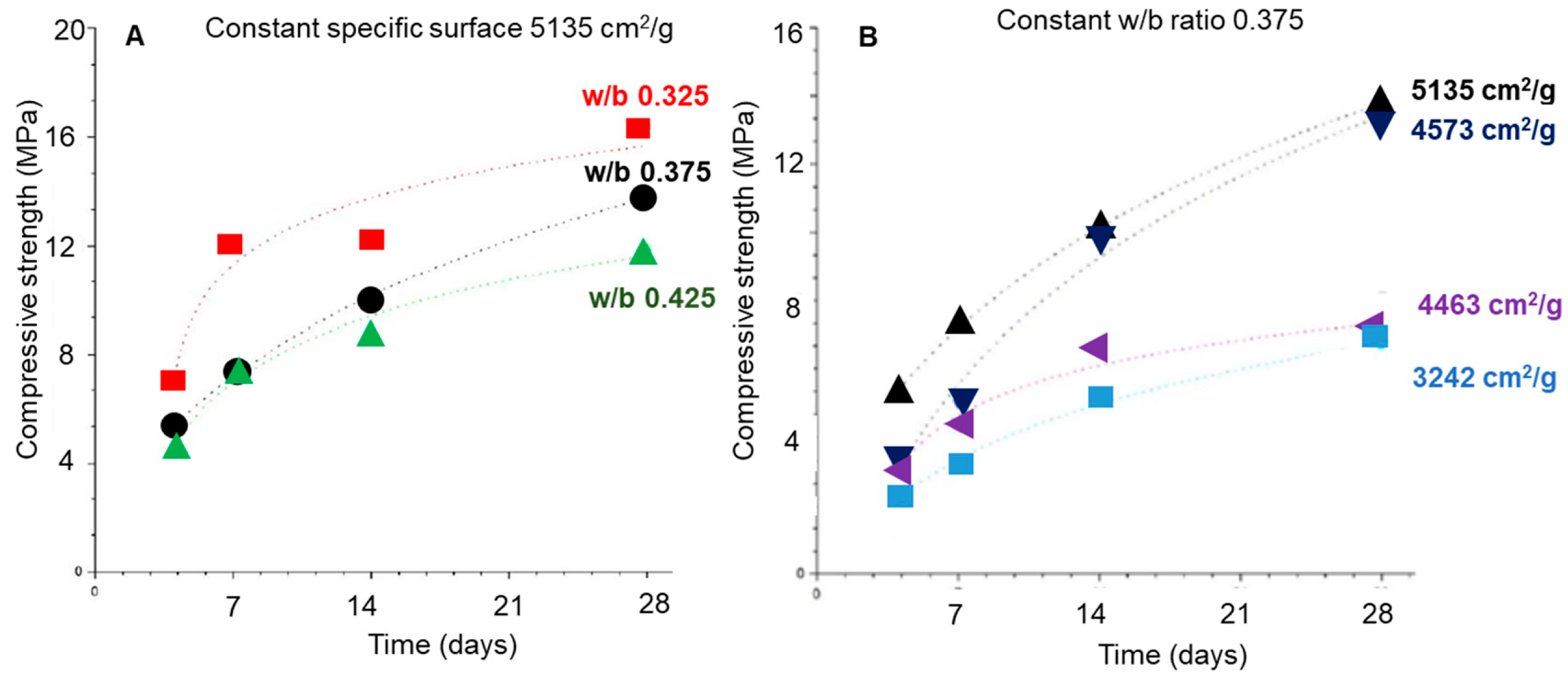
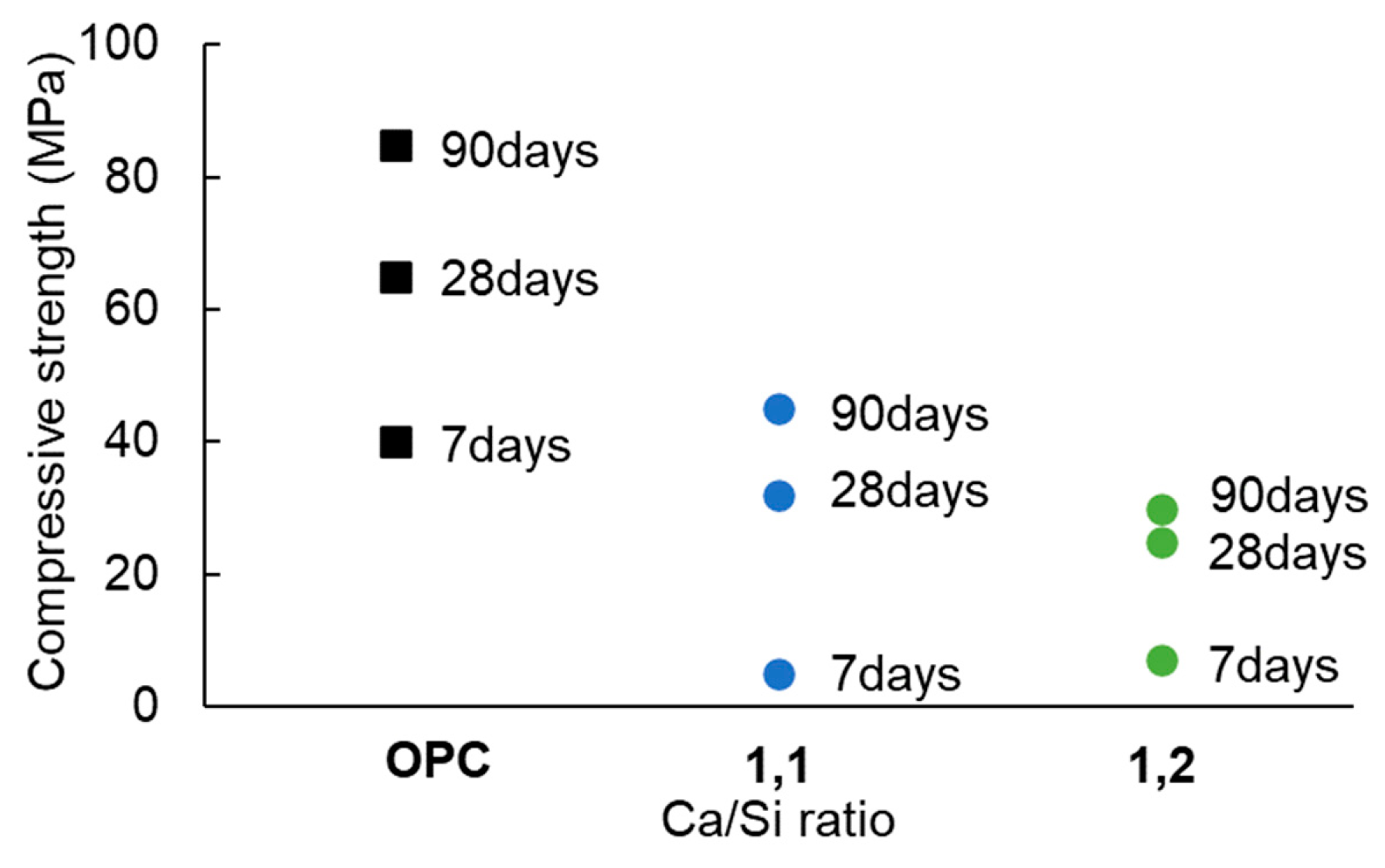
3.4. Influence of water/binder ratio, granulometry, and Ca/Si ratio on the mechanical performance
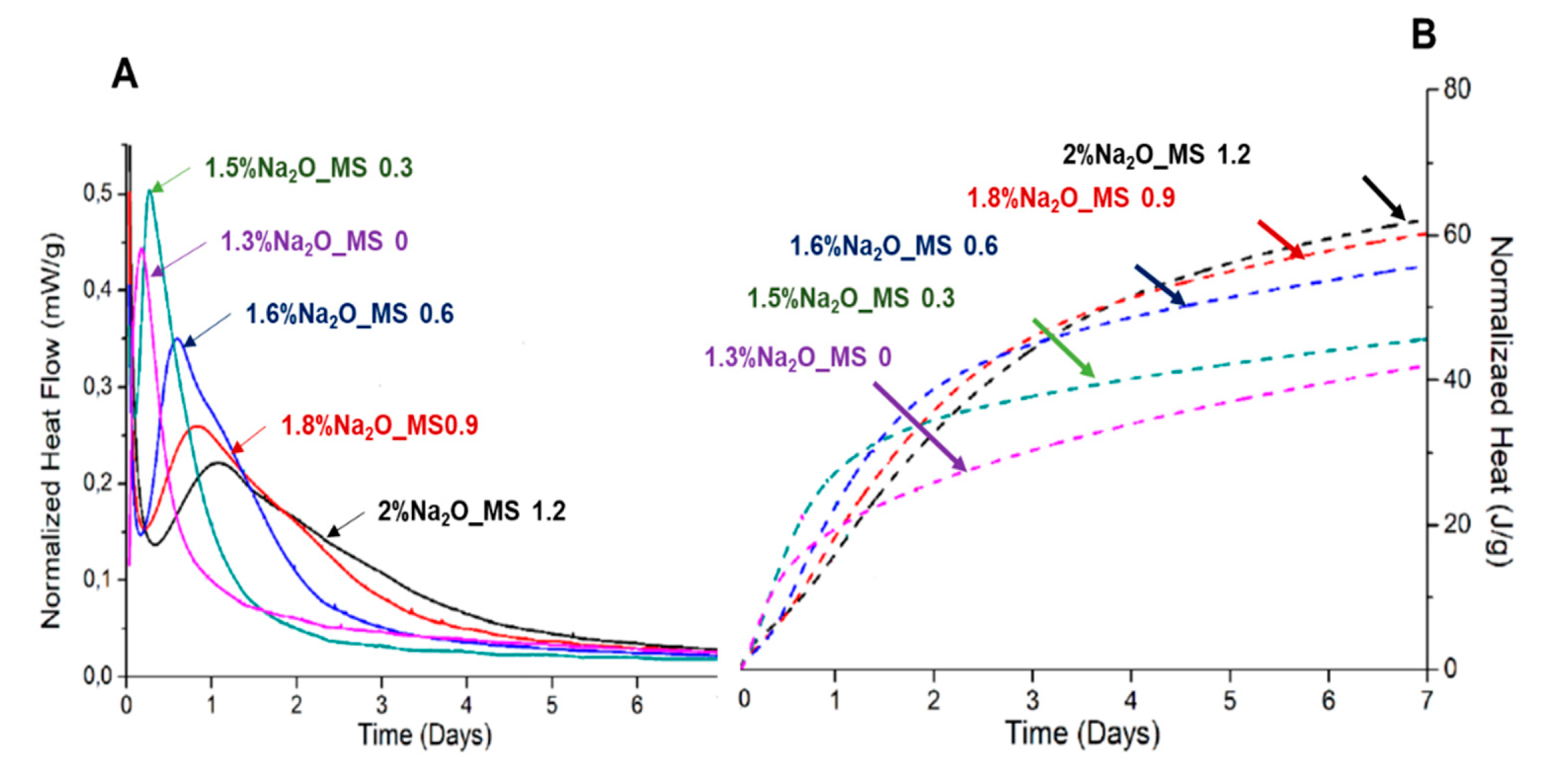
4. Alkaline activation of the binder
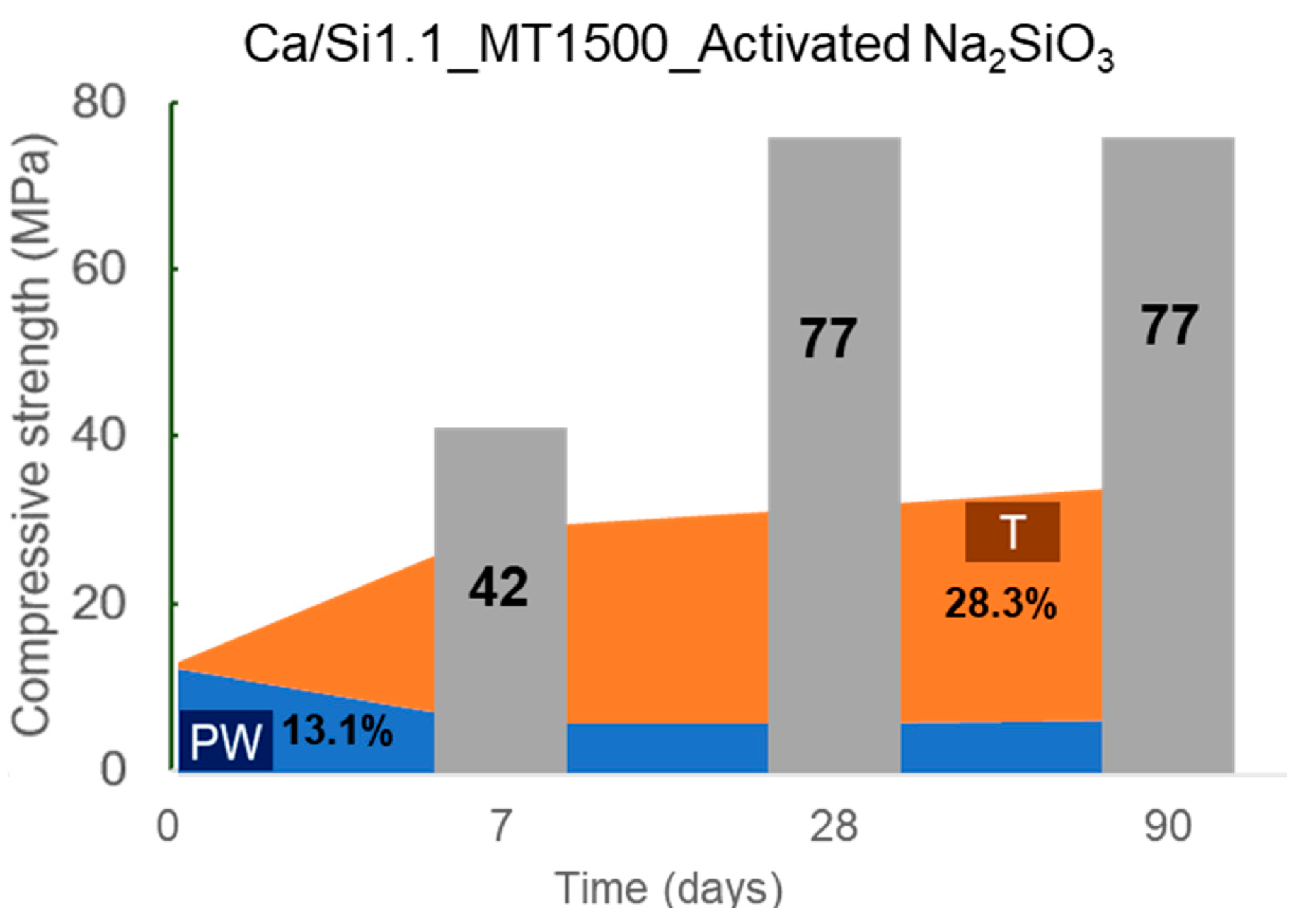
5. Correlation between bonded water and compressive strength
6. Conclusions
- a)
- To obtain a binder with good mechanical performance, the raw materials should be heated to at least 1500ºC and the quenching should be done preferentially in water.
- b)
- With the rise in calcium content in the raw material, with C/S between 0.8 and 1.25 an increase in Q0 structures was observed, reaching a maximum value at a Ca/Si ratio of 1.1. Moreover, pastes prepared with this ratio showed an increase in compressive strength
- c)
- The tests performed on the hydrated product revealed that the only products formed during the hydration of this AWLC were an amorphous CSH with a Ca/Si ratio of 1.1 and an MCL of 5, and a crystalline tobermorite 9Å phase. Furthermore, no portlandite was identified.
- d)
- With the increase in hydration time, a reduction in pseudowollastonite and an increase in tobermorite were observed. On water-hydrated pastes, the optimum content of pseudowollastonite on the anhydrous binder was ~7.6%.
- e)
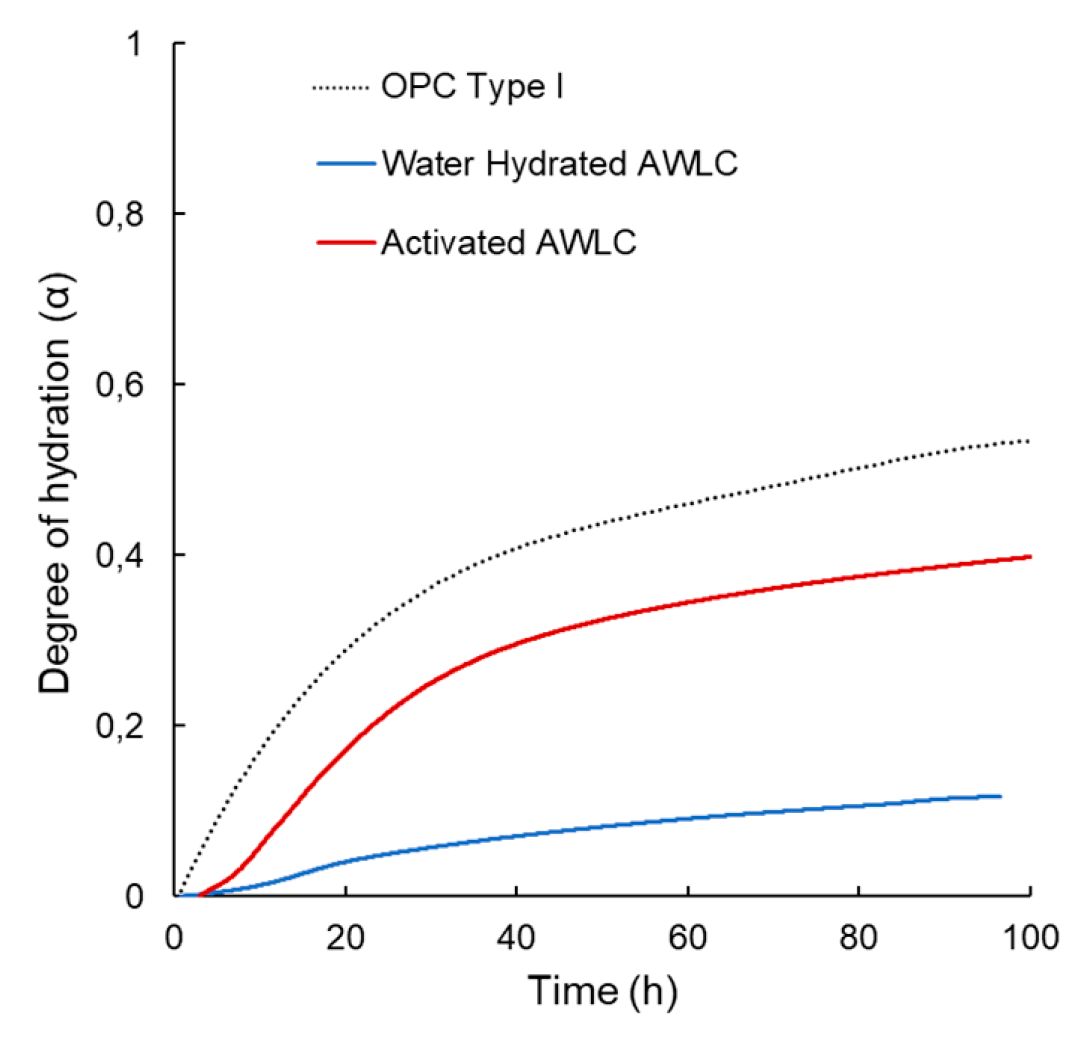
- The degree of hydration rate of the AWLCs binder was established and compared with type I OPC, the results showed that even though the hydration rate of the binder is lower than that of OPC, by activating the material a significant enhancement in the in the degree of hydration was observed, suggesting a potential improved in its performance.
- f)
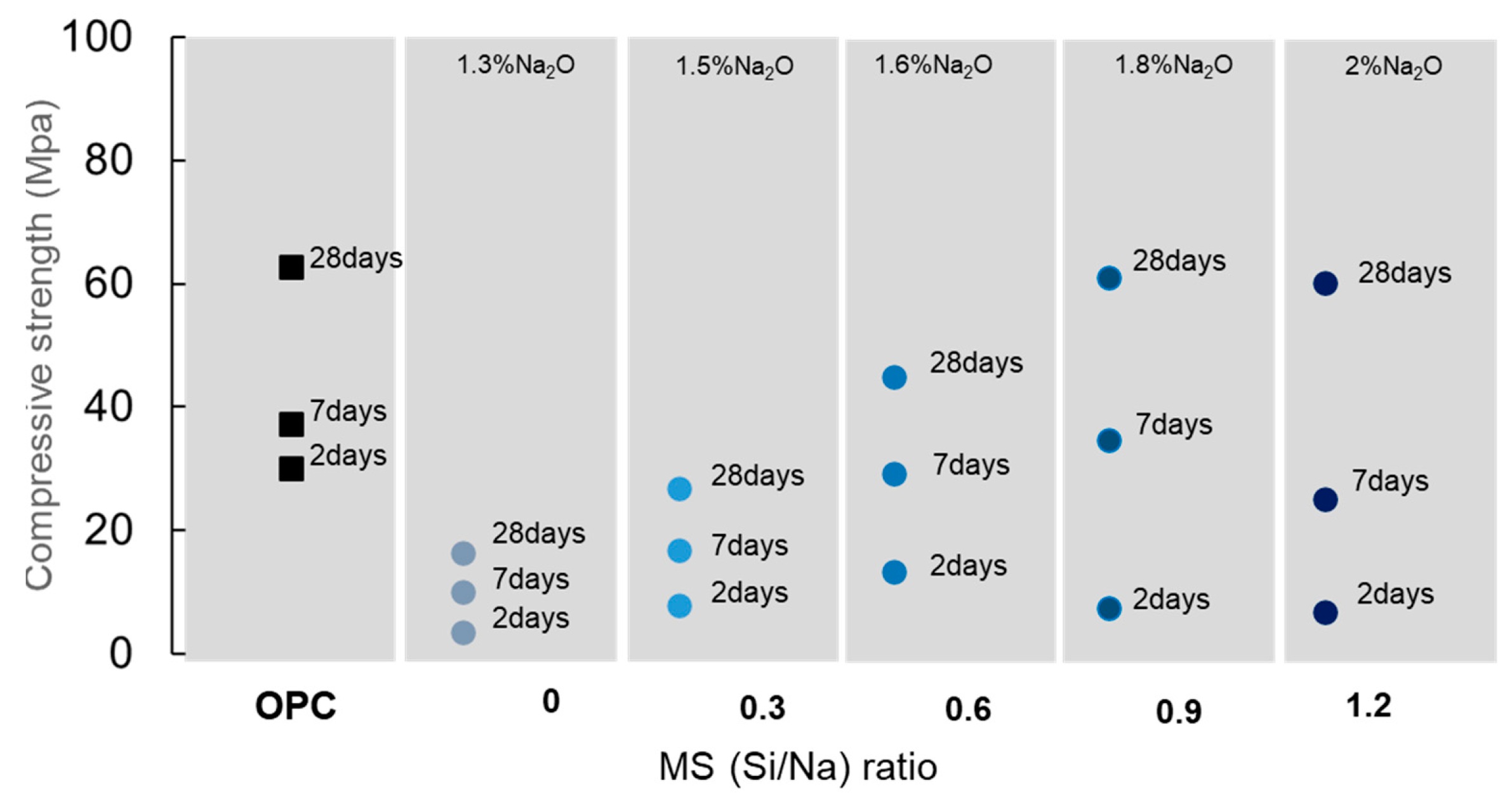
- Competitive strength on pastes was only obtained when the binder was hydrated with a Na2SiO3 solution with at least 1.8 Na2O%wt content. When activated, the pseudowollastonite content does not seem to be as relevant to the performance of the binder. An important parameter observed was that the AWLC binder exhibited a much lower heat release than traditional type I OPCs, even when activated.
- g)
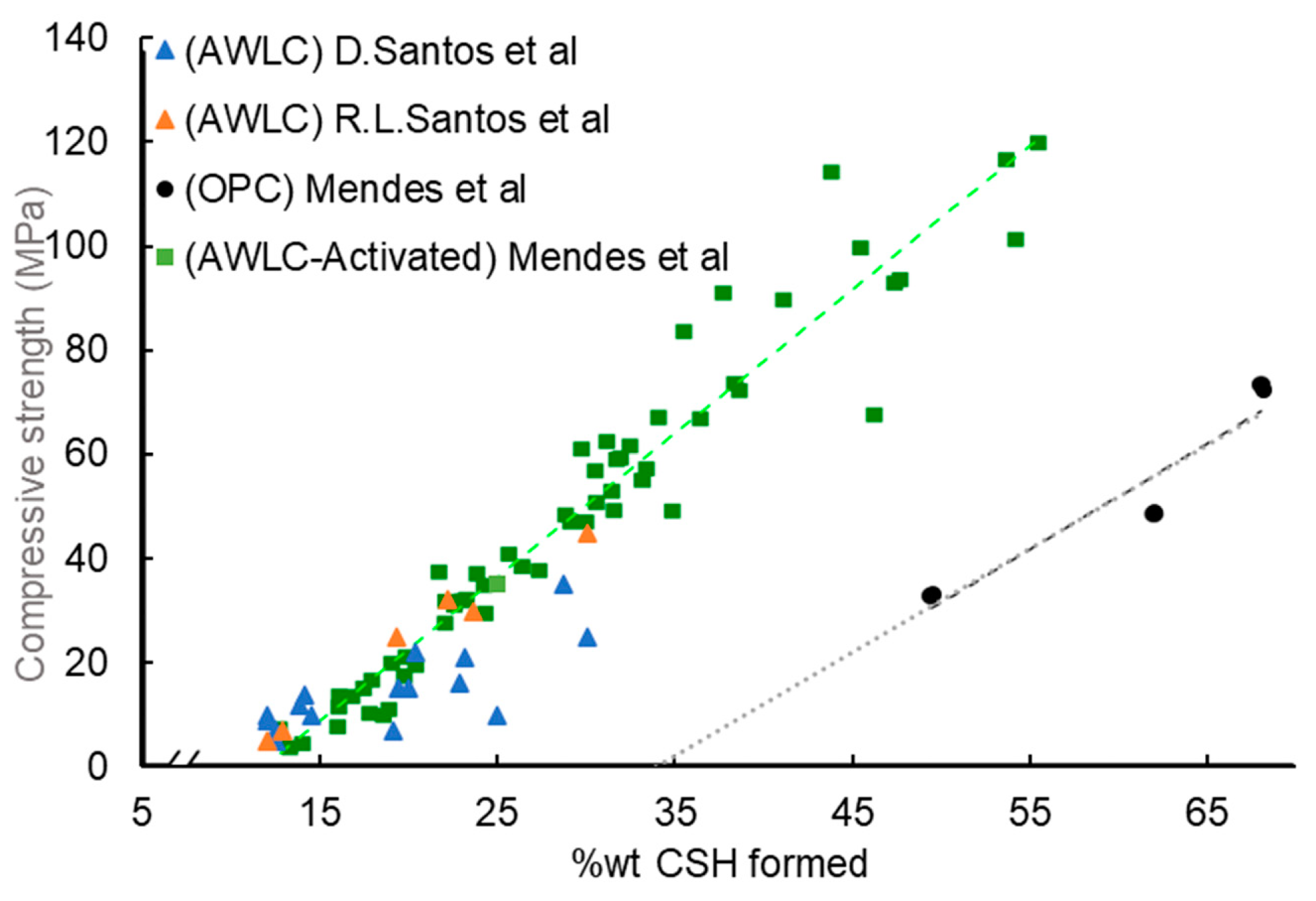
- Finally, a correlation between bonded water and the formation of CSH chains with compressive strength was established by different authors.
Author Contributions
Funding
Conflicts of Interest
References
- Hewlett, P.C. Lea’s Chemistry of Cement and Concrete; 4th edition.; Butterworth Heinemann: Oxford, UK, 2003; ISBN 9780750662567. [Google Scholar]
- Balonis, M.; Glasser, F.P. The Density of Cement Phases. Cem Concr Res 2009, 39, 733–739. [Google Scholar] [CrossRef]
- Jankovic, A.; Valery, W.; Davis, E. Cement Grinding Optimisation. Miner Eng 2004, 17, 1075–1081. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. ASTM C465 – Standard Specification for Processing Additions for Use in the Manufacture of Hydraulic Cements; 2014. [Google Scholar]
- American Association of State Highway and Transportation Officials AASHTO Designation: M 85-19 Standard Specification for Portland Cement, Technical Subcommitee: 3a, Hydraulic Cement and Lime; Washington, D. C. 2001, 2019.
- Nontananandh, S.; Yoobanpot, N.; Chaysuwan, D.; Thongdaeng, K. Influence of Fineness of Cement Produced from Industrial Wastes on Strength of Mortar. Nat. Sci. 2011, 45, 762–772. [Google Scholar]
- Lehne, J.; Preston, F. Making Concrete Change Innovation in Low-Carbon Cement and Concrete; 1st edition.; The Royal Institute of International Affair: London, UK, 2018. [Google Scholar]
- Energy Agency International. Achieving Net Zero Heavy Industry Sectors in G7 Members; 2022. [Google Scholar]
- International Energy Agency. Technology Roadmap - Low-Carbon Transition in the Cement Industry; Technical Report International Energy Agency: Paris, France, 2018. [Google Scholar]
- Antunes, M.; Santos, R.L.; Pereira, J.; Rocha, P.; Horta, R.B.; Colaço, R. Alternative Clinker Technologies for Reducing Carbon Emissions in Cement Industry: A Critical Review. Materials 2022, 15. [Google Scholar] [CrossRef]
- Andrew, R.M. Global CO2 Emissions from Cement Production,1928–2018. Earth Syst Sci Data 2019, 11, 1675–1710. [Google Scholar] [CrossRef]
- Horta, R.S.B.; Colaço, R.A.C.; Lopes, J.N.A.; Santos, R.L.; Pereira, João Chaves Rocha, P.J.P.; Lebreiro, S.M.M. Amorphous Low-Calcium Content Silicate Hydraulic Binders and Methods for Their Manufacturing. US Patent US10414690B2, 2016.
- Santos, R.L. New Hydraulic Binders with Low Calcium Content. PhD Thesis, Lisbon, Portugal; PhD Thesis, Universidade de Lisboa, 2016. [Google Scholar]
- Santos, D.; Santos, R.L.; Pereira, J.; Horta, R.B.; Colaço, R.; Paradiso, P. Influence of Pseudowollastonite on the Performance of Low Calcium Amorphous Hydraulic Binders. Materials 2019, 12, 1–13. [Google Scholar] [CrossRef]
- Santos, R.L.; Horta, R.B.; Al, E. Alkali Activation of a Novel Calcium-Silicate Hydraulic Binder. American Ceramic Society 2018, 101, 4158–4170. [Google Scholar] [CrossRef]
- Antunes, M.; Santos, R.L.; Pereira, J.; Horta, R.B.; Paradiso, P.; Colaço, R. The Apparent Activation Energy of a Novel Low-Calcium Silicate Hydraulic Binder. Materials 2021. [Google Scholar] [CrossRef]
- Santos, R.L.; Horta, R.B.; Pereira, J.; Nunes, T.G.; Rocha, P.; Lopes, J.N.C.; Colaço, R. Novel High-Resistance Clinkers with 1.10<CaO/SiO2<1.25 : Production Route and Preliminary Hydration Characterization. Cem Concr Res 2016, 85, 39–47. [Google Scholar] [CrossRef]
- Stemmermann, P.; Beuchle, G.; Garbev, K.; Schweike, U. Celitement- A New Sustainable Hydraulic Binder Based on Calcium Hydrosilicates. 13th International Congress on the Chemistry of Cement. 2011, 1–7. [Google Scholar]
- Sahu, S.; Meininger, R.C. Sustainability and Durability of Solidia Cement Concrete. Concrete International 2020, 29–34. [Google Scholar]
- Chi, L.; Zhang, A.; Qiu, Z.; Zhang, L.; Wang, Z.; Lu, S.; Zhao, D. Hydration Activity, Crystal Structural, and Electronic Properties Studies of Ba - Doped Dicalcium Silicate. Nanotechnology Review 2020, 1027–1033. [Google Scholar] [CrossRef]
- Kotsay, G.; Jaskulski, R. Belite Cement as an Ecological Alternative to Portland Cement – a Review. Materials Structures Technology 2020, 2, 70–76. [Google Scholar] [CrossRef]
- Meyer, V.; Cristofaro, N. De; Bryant, J.; Sahu, S. Solidia Cement an Example of Carbon Capture and Utilization. Key Eng Mater 2018, 761, 197–203. [Google Scholar] [CrossRef]
- Bayão, M.I. Mecanismos de Hidratação e Otimização Das Condições de Hidratação de Cimentos de Baixo Teor Em Cálcio. MsC; Lisbon University, Instituto Superior Técnico: LIsbon, Portugal, 2017. [Google Scholar]
- Câmara Santos, D. Desenvolvimento de Novos Cimentos Amigos Do Ambiente. MsC; Lisbon University, Instituto Superior Técnico: Lisbon, Portugal, 2015. [Google Scholar]
- Pardal, M. Desenvolvimento de Novos Cimentos Com Política Sustentável de Baixo Teor Em Cálcio Produção e Caracterização de Amorfos No Sistema CaO-SiO2-Al2O3-Fe2O3. MsC; Lisbon University, Instituto Superior Técnico: Lisbon, Portugal,, 2015. [Google Scholar]
- Cunha, M.I. Mecanismos de Hidratação e Otimização Das Condições de Hidratação de Cimentos de Baixo Teor Em Cálcio. MsC; Lisbon University , Instituto Superior Técnico: Lisbon, Portugal, 2017. [Google Scholar]
- Santos, D. Studies on Low Calcium Amorphous Hydraulic Binders and on the Pseudowollastonite Effect on Their Compressive Strength Performance. MsC; Lisbon University, Instituto Superior Técnico: Lisbon, Portugal, 2018. [Google Scholar]
- Pinha, M. Eco-Efficient Concrete Produced with a New Amorphous Hydraulic Binder with C/S Molar Ratio of 1.1-Proof of Concept in Mortar Mixtures. MsC; Lisbon University, Instituto Superior Técnico: Lisbon, Potugal, 2018. [Google Scholar]
- Faria, B. Hydration of Amorphous Calcium Silicate Clinkers MD Simulations with ReaxFF; Lisbon University, Instituto Superior Técnico: Lisbon, Portugal, 2018. [Google Scholar]
- Mendes, Á. Studies on a New Low Calcium Amorphous Hydraulic Binder. MsC, Lisbon University; Instituto Superior Técnico: Lisbon, Portugal, 2021. [Google Scholar]
- Santos, R.L.; Horta, R.B.; Pereira, J.; Nunes, T.G.; Rocha, P.; Lopes, J.N.; Colaço, R. Microstructural Control and Hydration of Novel Micro-Dendritic Clinkers with CaO/SiO2 = 1.4. Cem Concr Res 2015, 76, 212–221. [Google Scholar] [CrossRef]
- Smith, B. Infrared Spectral Interpretation A Systematic Approach; 1st Edition.; Taylor and Francis Group: Boca Raton, Florida USA, 1999; ISBN 0-8493-2463-7. [Google Scholar]
- Garcia, M.D. Synthesis by Supercritical Fluids Methods of Advanced Additions for Cementitious Materials. PhD Thesis Material chemistry., Université de Bordeaux, 2018; p. 233. [Google Scholar]
- Freitas, A.A.; Santos, R.L.; Colaço, R.; Bayão Horta, R.; Canongia Lopes, J.N. From Lime to Silica and Alumina: Systematic Modeling of Cement Clinkers Using a General Force-Field. Physical Chemistry Chemical Physics 2015, 17, 18477–18494. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Nonat, A. Hydration of Cementitious Materials, Present and Future. Cem Concr Res 2011, 41, 651–665. [Google Scholar] [CrossRef]
- Ouzia, A.; Scrivener, K. The Needle Model: A New Model for the Main Hydration Peak of Alite. Cem Concr Res 2019, 115, 339–360. [Google Scholar] [CrossRef]
- Berodier, E.; Scrivener, K. Understanding the Filler Effect on the Nucleation and Growth of C-S-H. Journal of the American Ceramic Society 2014, 97, 3764–3773. [Google Scholar] [CrossRef]
- Thomas JJ , Ghazizadeh S, M.E.. Kinetic Mechanisms and Activation Energies for Hydration of Standard and Highly Reactive Forms of β – Dicalcium Silicate ( C2S ). Cem Concr Res 2018, 322–328. [CrossRef]
- Thomas, J.J. The Instantaneous Apparent Activation Energy of Cement Hydration Measured Using a Novel Calorimetry-Based Method. Journal of the American Ceramic Society 2012, 95, 3291–3296. [Google Scholar] [CrossRef]
- Ping, Y.; Kirkpatrick, R.J.; Brent, P.; McMillan, P.F.; Cong, X. Structure of Calcium Silicate Hydrate (C-S-H): Near-, Mid-, and Far-Infrared Spectroscopy. Journal of the American Ceramic Society 1999, 82, 742–748. [Google Scholar] [CrossRef]
- Joshi, S.; Kalyanasundaram, S.; Balasubramanian, V. Quantitative Analysis of Sodium Carbonate and Sodium Bicarbonate in Solid Mixtures Using Fourier Transform Infrared Spectroscopy (FT-IR). In Proceedings of the Applied Spectroscopy; August 2013; Vol. 67; pp. 841–845. [Google Scholar]
- Paradiso, P.; Santos, R.L.; Horta, R.B.; Lopes, J.N.C.; Ferreira, P.J.; Colaço, R. Formation of Nanocrystalline Tobermorite in Calcium Silicate Binders with Low C/S Ratio. Acta Mater 2018, 152, 7–15. [Google Scholar] [CrossRef]
- Abdolhosseini Qomi, M.J.; Krakowiak, K.J.; Bauchy, M.; Stewart, K.L.; Shahsavari, R.; Jagannathan, D.; Brommer, D.B.; Baronnet, A.; Buehler, M.J.; Yip, S.; et al. Combinatorial Molecular Optimization of Cement Hydrates. Nat Commun 2014, 5, 4960. [Google Scholar] [CrossRef]
- Richardson, I.G. Model Structures for C-(A)-S-H(I). Structural Science, Crystal Engineering and Materials, Acta Crystallographica Section B: 2014, 70, 903–923. [Google Scholar] [CrossRef]
- Houston, J.R.; Maxwell, R.S.; Carroll, S.A. Transformation of Meta-Stable Calcium Silicate Hydrates to Tobermorite: Reaction Kinetics and Molecular Structure from XRD and NMR Spectroscopy. Geochem Trans 2009, 10. [Google Scholar] [CrossRef]
- Mitra, N.; Sarkar, P.K.; Prasad, D. Intermolecular Dynamics of Ultraconfined Interlayer Water in Tobermorite: Influence on Mechanical Performance. Physical Chemistry Chemical Physics 2019, 21, 11416–11423. [Google Scholar] [CrossRef]
- Tunega, D.; Zaoui, A. Understanding of Bonding and Mechanical Characteristics of Cementitious Mineral Tobermorite from First Principles. J Comput Chem 2011, 32, 306–314. [Google Scholar] [CrossRef]
- Essene, E. High-Pressure Transformations in CaSi03*. Contr. Mineral. and Petrol 1974, 45, 247–250. [Google Scholar] [CrossRef]
- Ashraf, W.; Olek, J.; Sahu, S. Phase Evolution and Strength Development during Carbonation of Low-Lime Calcium Silicate Cement (CSC). Constr Build Mater 2019, 210, 473–482. [Google Scholar] [CrossRef]
- Qian, B.; Li, X.; Shen, X. Preparation and Accelerated Carbonation of Low Temperature Sintered Clinker with Low Ca/Si Ratio. J Clean Prod 2016, 120, 249–259. [Google Scholar] [CrossRef]
- Plattenberger, D.A.; Opila, E.J.; Shahsavari, R.; Clarens, A.F. Feasibility of Using Calcium Silicate Carbonation to Synthesize High-Performance and Low-Carbon Cements. ACS Sustain Chem Eng 2020, 8, 5431–5436. [Google Scholar] [CrossRef]
- Wang, X.; Guo, M.Z.; Ling, T.C. Review on CO2 Curing of Non-Hydraulic Calcium Silicates Cements: Mechanism, Carbonation and Performance. Cem Concr Compos 2022, 133. [Google Scholar] [CrossRef]
- Plattenberger, D.A.; Ling, F.T.; Tao, Z.; Peters, C.A.; Clarens, A.F. Calcium Silicate Crystal Structure Impacts Reactivity with CO2 and Precipitate Chemistry. Environ Sci Technol Lett 2018, 5, 558–563. [Google Scholar] [CrossRef]
- Bentz, D.P. The Hidden Meaning of Water-Cement Ratio. Concrete International 2008, 30, 51–54. [Google Scholar]
- Bentz, D.P. Influence of Water-to-Cement Ratio on Hydration Kinetics: Simple Models Based on Spatial Considerations. Cem Concr Res 2006, 36, 238–244. [Google Scholar] [CrossRef]
- Poole, J.L.; Riding, K.A.; Folliard, K.J.; Juenger, M.C.G.; Schindler, A.K. Methods for Calculating Activation Energy for Portland Cement. ACI Mater J 2007, 104, 86–94. [Google Scholar] [CrossRef]
- Richardson, I.G. Model Structures for C-(A)-S-H(I). Acta Crystallogr B Struct Sci Cryst Eng Mater 2014, 70, 903–923. [Google Scholar] [CrossRef]
| Ca/Si ratio | 0.8 | 1 | 1.1 | 1.25 | |
|---|---|---|---|---|---|
| Qn Area (%) | |||||
| Q0 | 18.1 | 18.4 | 28.1 | 21.5 | |
| Q1 | 26.8 | 37.5 | 40.2 | 50.1 | |
| Q2 | 34.7 | 30.4 | 27.6 | 26.8 | |
| Q3 | 20.4 | 13.6 | 4.1 | 2.6 | |
| OPC | AWLC | |
|---|---|---|
| Production Method | The raw mix is fed into a kiln and fired to a temperature of 1400–1450ºC | Melting at ~1500ºC of the mixture followed by a fast quenching |
| Process-Related CO2 (kg/ton) | 535* | 340** |
| The density of the material (g/cm3) | 3.1 | 2.94±0.05 |
| Main phases of the binder | Alite (50-70%) Belite (15-30%) C3A and C4AF ( <20%) |
Amorphous (90%) Pseudowollastonite (< 18%) |
| Activation Energy (kJ/mol) | 51-55 | 82-85 |
| Cumulative Heat after 72h (J/g) | 250 | 53 when activated with Na2SiO3 solution with 1.8%wt Na2O content |
| Main hydration products | CSH and portlandite | CSH and tobermorite 9A |
| Ca/Si ratio of CSH | ~1.7 | ~1.1 |
| MCL | ~2.4 | ~5 |
| Paste compressive strength 2 days (MPa) | 33 | 11 when activated with Na2SiO3 solution with 1.8%wt Na2O content |
| Paste compressive strength 28 days (MPa) | 65 | 63 when activated with Na2SiO3 solution with 1.8%wt Na2O content |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





