Submitted:
15 June 2023
Posted:
15 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
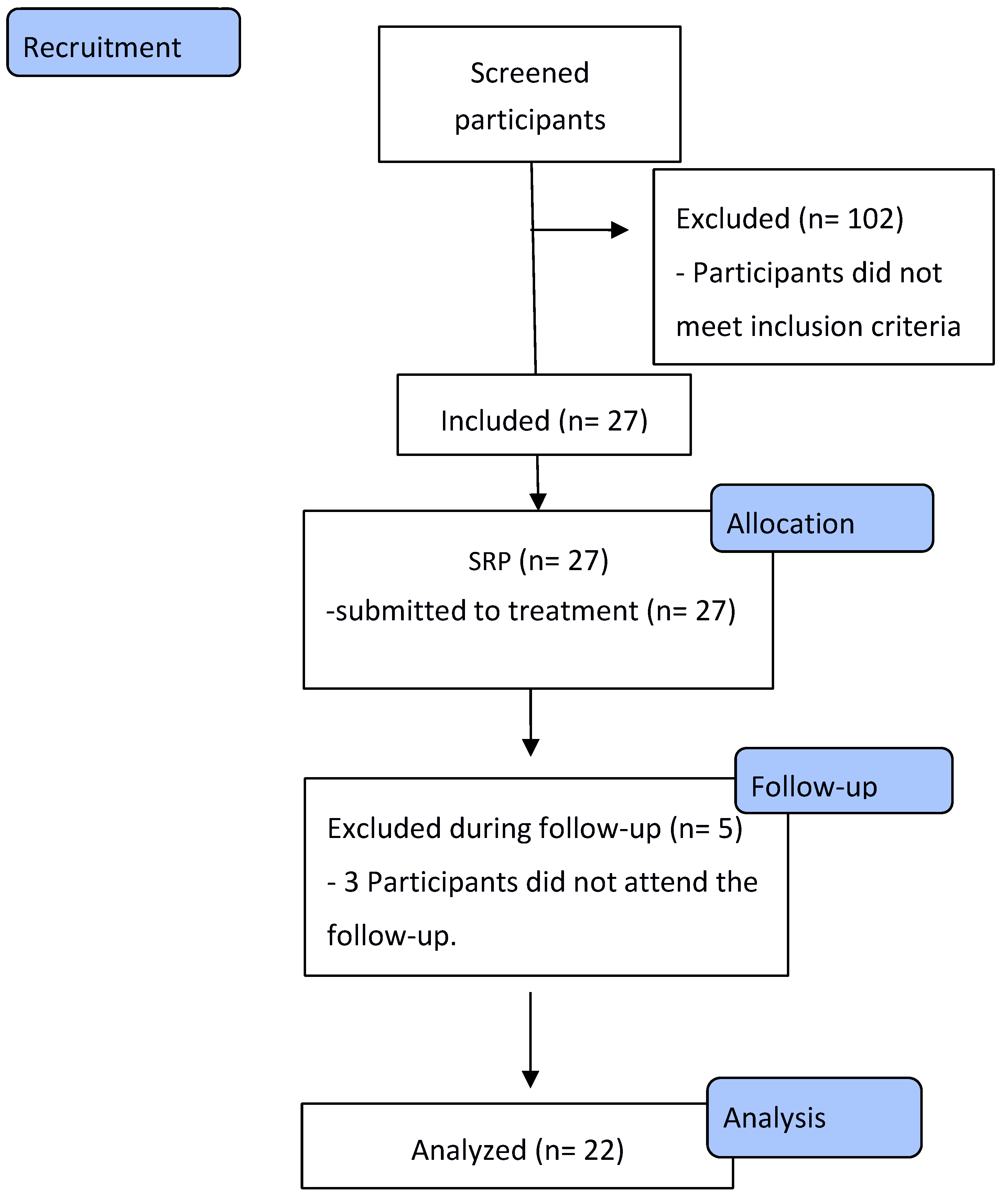
2.1. Study Population
2.2. Participants
2.3. Primary and Secondary Clinical Outcomes
2.4. Blood Pressure Analysis
2.5. Bodyweight Analysis
2.6. Blood Analysis
2.7. Experimental Design and Treatment Protocol
2.8. Statistical Analysis
3. Results
3.1. Examiner Calibration
3.2. Participants sample
3.3. Clinical Parameters
3.4. Blood Pressure
3.5. Blood Analysis
3.6. Bodyweight
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Czopek, A.; Moorhouse, R.; Guyonnet, L.; Farrah, T.; Lenoir, O.; Owen, E.; van Bragt, J.; Costello, H.M.; Menolascina, F.; Baudrie, V.; et al. A novel role for myeloid endothelin-B receptors in hypertension. Eur. Hear. J. 2019, 40, 768–784. [Google Scholar] [CrossRef]
- Pimenta, E.; Gaddam, K.K.; Oparil, S. Mechanisms and Treatment of Resistant Hypertension. J. Clin. Hypertens. 2008, 10, 239–244. [Google Scholar] [CrossRef]
- Roth GA, Mensah GA and Fuster V. The Global Burden of Cardiovascular Diseases and Risks: A Compass for Global Action. J Am Coll Cardiol. 2020, 76, 2980–2981. [CrossRef]
- Razo, C.; Welgan, C.A.; Johnson, C.O.; McLaughlin, S.A.; Iannucci, V.; Rodgers, A.; Wang, N.; LeGrand, K.E.; Sorensen, R.J.D.; He, J.; et al. Effects of elevated systolic blood pressure on ischemic heart disease: a Burden of Proof study. Nat. Med. 2022, 28, 2056–2065. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol. 2018, 45 Suppl 20, S162–S170.
- Van Dyke, T.E.; Bartold, P.M.; Reynolds, E.C. The Nexus Between Periodontal Inflammation and Dysbiosis. Front. Immunol. 2020, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef]
- Richards, D. Review finds that severe periodontitis affects 11% of the world population. Evidence-Based Dent. 2014, 15, 70–1. [Google Scholar] [CrossRef]
- Masi, S.; D’aiuto, F.; Deanfield, J. Cardiovascular prevention starts from your mouth. Eur. Hear. J. 2019, 40, 1146–1148. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T.; Lambris, J.D. Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontology 2000 2020, 84, 14–34. [Google Scholar] [CrossRef]
- Tonetti, M.S.; D’Aiuto, F.; Nibali, L.; Donald, A.; Storry, C.; Parkar, M.; Suvan, J.; Hingorani, A.D.; Vallance, P.; Deanfield, J. Treatment of Periodontitis and Endothelial Function. New Engl. J. Med. 2007, 356, 911–920. [Google Scholar] [CrossRef]
- D’Aiuto, F.; Gkranias, N.; Bhowruth, D.; Khan, T.; Orlandi, M.; Suvan, J.; Masi, S.; Tsakos, G.; Hurel, S.; Hingorani, A.D.; et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol. 2018, 6, 954–965. [Google Scholar] [CrossRef]
- de Molon RS, Rossa C, Jr., Thurlings RM, et al. Linkage of Periodontitis and Rheumatoid Arthritis: Current Evidence and Potential Biological Interactions. Int J Mol Sci. 2019, 20.
- Gonzalez-Febles J and Sanz, M. Periodontitis and rheumatoid arthritis: What have we learned about their connection and their treatment? Periodontol 2000. 2021, 87, 181–203. [Google Scholar] [CrossRef] [PubMed]
- Genco, R.J.; Borgnakke, W.S. Diabetes as a potential risk for periodontitis: association studies. Periodontology 2000 2020, 83, 40–45. [Google Scholar] [CrossRef]
- Nibali, L.; Gkranias, N.; Mainas, G.; Di Pino, A. Periodontitis and implant complications in diabetes. Periodontology 2000 2022, 90, 88–105. [Google Scholar] [CrossRef]
- Morita, T.; Yamazaki, Y.; Fujiharu, C.; Ishii, T.; Seto, M.; Nishinoue, N.; Sasaki, Y.; Nakai, K.; Tanaka, H.; Kawato, T.; et al. Association Between the Duration of Periodontitis and Increased Cardiometabolic Risk Factors: A 9-Year Cohort Study. Metab. Syndr. Relat. Disord. 2016, 14, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Lee JH, Oh JY, Youk TM, et al. Association between periodontal disease and non-communicable diseases: A 12-year longitudinal health-examinee cohort study in South Korea. Medicine (Baltimore) 2017, 96, e7398. [CrossRef]
- Martin-Cabezas, R.; Seelam, N.; Petit, C.; Agossa, K.; Gaertner, S.; Tenenbaum, H.; Davideau, J.-L.; Huck, O. Association between periodontitis and arterial hypertension: A systematic review and meta-analysis. Am. Hear. J. 2016, 180, 98–112. [Google Scholar] [CrossRef]
- Czesnikiewicz-Guzik, M.; Osmenda, G.; Siedlinski, M.; Nosalski, R.; Pelka, P.; Nowakowski, D.; Wilk, G.; Mikolajczyk, T.P.; Schramm-Luc, A.; Furtak, A.; et al. Causal association between periodontitis and hypertension: evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur. Hear. J. 2019, 40, 3459–3470. [Google Scholar] [CrossRef]
- Del Pinto R, Pietropaoli D, Munoz-Aguilera E, et al. Periodontitis and Hypertension: Is the Association Causal? High Blood Press Cardiovasc Prev. 2020, 27, 281–289. [CrossRef] [PubMed]
- Munoz Aguilera E, Suvan J, Buti J, et al. Periodontitis is associated with hypertension: a systematic review and meta-analysis. Cardiovasc Res. 2020, 116, 28–39. [CrossRef] [PubMed]
- D’Aiuto, F.; Parkar, M.; Nibali, L.; Suvan, J.; Lessem, J.; Tonetti, M.S. Periodontal infections cause changes in traditional and novel cardiovascular risk factors: Results from a randomized controlled clinical trial. Am. Hear. J. 2006, 151, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Vidal, F.; Cordovil, I.; Figueredo, C.M.S.; Fischer, R.G. Non-surgical periodontal treatment reduces cardiovascular risk in refractory hypertensive patients: a pilot study. J. Clin. Periodontol. 2013, 40, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Escobar Arregoces FM, Del Hierro Rada M, Saenz Martinez MJ, et al. Systemic inflammatory response to non-surgical treatment in hypertensive patients with periodontal infection. Medicine (Baltimore) 2021, 100, e24951. [CrossRef] [PubMed]
- Vidal, F.; Figueredo, C.M.S.; Cordovil, I.; Fischer, R.G. Periodontal Therapy Reduces Plasma Levels of Interleukin-6, C-Reactive Protein, and Fibrinogen in Patients With Severe Periodontitis and Refractory Arterial Hypertension. J. Periodontol. 2009, 80, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Vidal, F.; Figueredo, C.; Cordovil, I.; Fischer, R. Higher prevalence of periodontitis in patients with refractory arterial hypertension: a case–control study. Oral Dis. 2011, 17, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Pietropaoli, D.; Del Pinto, R.; Ferri, C.; Marzo, G.; Giannoni, M.; Ortu, E.; Monaco, A. Association between periodontal inflammation and hypertension using periodontal inflamed surface area and bleeding on probing. J. Clin. Periodontol. 2019, 47, 160–172. [Google Scholar] [CrossRef]
- Cao Q, Yu S, Xiong W, et al. Waist-hip ratio as a predictor of myocardial infarction risk: A systematic review and meta-analysis. Medicine (Baltimore) 2018, 97, e11639. [CrossRef]
- Paddmanabhan, P.; Gita, B.; Chandrasekaran, S. Association between chronic periodontitis and hypertension in South Indian population: A cross-sectional study. J. Pharm. Bioallied Sci. 2015, 7, 543–S547. [Google Scholar] [CrossRef]
- Kablak-Ziembicka A, Przewlocki T, Sokolowski A, et al. Carotid intima-media thickness, hs-CRP and TNF-alpha are independently associated with cardiovascular event risk in patients with atherosclerotic occlusive disease. Atherosclerosis. 2011, 214, 185–190. [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S.; Consultants, E.W.P.A.M.; Consultants, T.E.W.P.A.M.; et al. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki M, Kimura Y, Ogawa H, et al. Periodontitis, periodontal inflammation, and mild cognitive impairment: A 5-year cohort study. J Periodontal Res. 2019, 54, 233–240. [CrossRef] [PubMed]
- Theodoro, L.H.; Lopes, A.B.; Nuernberg, M.A.A.; Cláudio, M.M.; Miessi, D.M.J.; Alves, M.L.F.; Duque, C.; Mombelli, A.; Garcia, V.G. Comparison of repeated applications of aPDT with amoxicillin and metronidazole in the treatment of chronic periodontitis: A short-term study. J. Photochem. Photobiol. B: Biol. 2017, 174, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Hosadurga, R.; Soe, H.H.K.; Lim, A.T.P.; Adl, A.; Mathew, M. Association between tooth loss and hypertension: A cross-sectional study. J. Fam. Med. Prim. Care 2020, 9, 925–932. [Google Scholar] [CrossRef]
- Pietropaoli, D.; Del Pinto, R.; Ferri, C.; Wright, J.T., Jr.; Giannoni, M.; Ortu, E.; Monaco, A. Poor Oral Health and Blood Pressure Control Among US Hypertensive Adults. Hypertension 2018, 72, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.B.; Razan, K.K.; Raed, A.D.M. Effect of surgical and non-surgical periodontal debridement on vascular thrombotic markers in hypertensives. J. Indian Soc. Periodontol. 2013, 17, 324–9. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Almaghlouth, A.; Cionca, N.; Courvoisier, D.S.; Giannopoulou, C. Differential Benefits of Amoxicillin–Metronidazole in Different Phases of Periodontal Therapy in a Randomized Controlled Crossover Clinical Trial. J. Periodontol. 2015, 86, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.M. Clinical significance of non-surgical periodontal therapy: an evidence-based perspective of scaling and root planing. J. Clin. Periodontol. 2002, 29 (Suppl. 2), 22–32. [Google Scholar] [CrossRef]
- Czesnikiewicz-Guzik, M.; Nosalski, R.; Mikolajczyk, T.P.; Vidler, F.; Dohnal, T.; Dembowska, E.; Graham, D.; Harrison, D.G.; Guzik, T.J. Th1-type immune responses to Porphyromonas gingivalis antigens exacerbate angiotensin II-dependent hypertension and vascular dysfunction. Br. J. Pharmacol. 2018, 176, 1922–1931. [Google Scholar] [CrossRef]
- Yuan, M.; Wang, B.; Tan, S. Mecobalamin and early functional outcomes of ischemic stroke patients with H-type hypertension. Front. Public Heal. 2018, 64, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Munoz Aguilera E, Leira Y, Miro Catalina Q, et al. Is systemic inflammation a missing link between periodontitis and hypertension? Results from two large population-based surveys. J Intern Med. 2021, 289, 532–546. [CrossRef]
- Ide, M.; McPartlin, D.; Coward, P.; Crook, M.; Lumb, P.; Wilson, R. Effect of treatment of chronic periodontitis on levels of serum markers of acute-phase inflammatory and vascular responses. J. Clin. Periodontol. 2003, 30, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.; Honda, T.; Oda, T.; Ueki-Maruyama, K.; Nakajima, T.; Yoshie, H.; Seymour, G.J. Effect of periodontal treatment on the C-reactive protein and proinflammatory cytokine levels in Japanese periodontitis patients. J. Periodontal Res. 2004, 40, 53–58. [Google Scholar] [CrossRef]
- D’Isidoro, O.; Perrotti, V.; Hui, W.L.; Piattelli, A.; Iaculli, F.; Quaranta, A. The impact of non-surgical therapy of periodontal disease on surrogate markers for cardiovascular disease: A literature review. Am J Dent. 2019, 32, 191–200. [Google Scholar] [PubMed]
- Higashi, Y.; Goto, C.; Hidaka, T.; Soga, J.; Nakamura, S.; Fujii, Y.; Hata, T.; Idei, N.; Fujimura, N.; Chayama, K.; et al. Oral infection-inflammatory pathway, periodontitis, is a risk factor for endothelial dysfunction in patients with coronary artery disease. Atherosclerosis 2009, 206, 604–610. [Google Scholar] [CrossRef]
- Higashi, Y.; Goto, C.; Jitsuiki, D.; Umemura, T.; Nishioka, K.; Hidaka, T.; Takemoto, H.; Nakamura, S.; Soga, J.; Chayama, K.; et al. Periodontal Infection Is Associated With Endothelial Dysfunction in Healthy Subjects and Hypertensive Patients. Hypertension 2008, 51, 446–453. [Google Scholar] [CrossRef]
- Jain, A.; Jain, A.; Gupta, J.; Bansal, D.; Sood, S.; Gupta, S. Effect of scaling and root planing as monotherapy on glycemic control in patients of Type 2 diabetes with chronic periodontitis: A systematic review and meta-analysis. J. Indian Soc. Periodontol. 2019, 23, 303–310. [Google Scholar] [CrossRef]
- Baeza, M.; Morales, A.; Cisterna, C.; Cavalla, F.; Jara, G.; Isamitt, Y.; Pino, P.; Gamonal, J. Effect of periodontal treatment in patients with periodontitis and diabetes: systematic review and meta-analysis. J. Appl. Oral Sci. 2020, 28, e20190248. [Google Scholar] [CrossRef]
- Alpert, M.A.; Omran, J.; Mehra, A.; Ardhanari, S. Impact of Obesity and Weight Loss on Cardiac Performance and Morphology in Adults. Prog. Cardiovasc. Dis. 2014, 56, 391–400. [Google Scholar] [CrossRef]
- Zhao, D.; Qi, Y.; Zheng, Z.; Wang, Y.; Zhang, X.-Y.; Li, H.-J.; Liu, H.-H.; Zhang, X.-T.; Du, J.; Liu, J. Dietary factors associated with hypertension. Nat. Rev. Cardiol. 2011, 8, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Sanz M, Del Castillo AM, Jepsen S, et al. Periodontitis and Cardiovascular Diseases. Consensus Report. Glob Heart. 2020, 15, 1. [CrossRef]
- Desvarieux, M.; Demmer, R.T.; Jacobs, D.R.; Rundek, T., Jr.; Boden-Albala, B.; Sacco, R.L.; Papapanou, P.N. Periodontal bacteria and hypertension: the oral infections and vascular disease epidemiology study (INVEST). J. Hypertens. 2010, 28, 1413–1421. [Google Scholar] [CrossRef]
- Graziani, F.; Cei, S.; Tonetti, M.; Paolantonio, M.; Serio, R.; Sammartino, G.; Gabriele, M.; D’Aiuto, F. Systemic inflammation following non-surgical and surgical periodontal therapy. J. Clin. Periodontol. 2010, 37, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Nogami, Y. The effect of regular aquatic exercise on blood pressure: A meta-analysis of randomized controlled trials. Eur. J. Prev. Cardiol. 2017, 25, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Munekawa, C.; Hosomi, Y.; Hashimoto, Y.; Okamura, T.; Takahashi, F.; Kawano, R.; Nakajima, H.; Osaka, T.; Okada, H.; Majima, S.; et al. Effect of coronavirus disease 2019 pandemic on the lifestyle and glycemic control in patients with type 2 diabetes: a cross-section and retrospective cohort study. Endocr. J. 2021, 68, 201–210. [Google Scholar] [CrossRef]
| Demographic characteristics | n= 22 |
|---|---|
| Age - mean (SD) | 59.9 (±8.2) |
| Sex – n, (%) | |
| Men | 10 (45.45%) |
| Women | 12 (54.55%) |
| Evaluated variables | Baseline n=22 |
90 days n=22 |
180 days n=22 |
p-value |
|---|---|---|---|---|
| Periodontal Clinical Parameters - Mean (SD) | ||||
| N° teeth | 22.9 (± 4.1) | 22.9 (± 4.1) | 22.8 (±4.0) | 0.99 |
| PI (% of the sites) | 35.7 (±26.2)a | 16.0 (±9.6)b | 19.8 (±16.5)b | <0.0025 |
| BOP (% of the sites) | 0.39 (±0.18) a | 0.29 (±0.09) b | 0.17 (±0.08) )b | <0.0001 |
| PD ≤4 mm (% of the sites) | 94.1 (±5.74)a | 99.1 (±1.42)b | 99.13 (±1.61)b | <0.0001 |
| PD ≥5 mm (% of the sites) | 5.89 (±5.74)a | 0.87 (±1.42) b | 0.87 (±1.61) b | <0.0001 |
| CAL ≤3 mm (% of the sites) | 6.05 (±5.37)a | 0.89 (±1.45)b | 0.96 (±1.75)b | <0.0001 |
| CAL 4-5 mm(% of the sites) | 65.1 (±20.14) | 74.29 (±18.56) | 74.62 (±18.39) | 0.1172 |
| CAL ≥6 mm (% of the sites) | 28.85(±17.38 | 24.81 (±18.59) | 24.42 (±17.63) | 0.5968 |
| Biochemical and physical exams – Mean (SD) | ||||
| HbA1c (%) | 5.78 (±0.52) | 5.83 (±0.44) | 5.73 (±0.52) | 0.7296 |
| Fasting glucose (mg/dL) | 103.3(±14.89) | 106.0 (±14.20) | 107.0 (±13.12) | 0.6814 |
| Estimated glucose (mg/dL) | 116.9(±16.34) | 114.0 (±13.15) | 114.9 (±13.65) | 0.8091 |
| Total cholesterol(mg/dL) | 178.1(±40.07) | 195.5 (±47.94) | 171.2 (±29.41) | 0.2478 |
| Creatinine (mg/dL) | 0.94 (±0.32) | 0.94 (±0.26) | 0.97 (±0.35) | 0.9222 |
| PGT (U/l) | 23.55 (±9.26) | 22.66 (±7.75) | 2196 (±8.67) | 0.8321 |
| OGT (U/l) | 25.10 (±8.77) | 23.24 (±6.67) | 25.27 (±7.36) | 0.8929 |
| Waist hip ratio (cm) | 0.97 (±0.08) | 0.97 (±0.09) | 0.95 (±0.06) | 0.5930 |
| BMI (Kg/m2) | 29.41 (±4.61) | 29.66 (±4.62) | 29.53(±5.21) | 0.9823 |
| C-reactive Protein n, (%) | ||||
| Positive | 5 | 0 | 1 | 0.0213 |
| Negative | 17 | 22 | 21 | |
| Blood pressure parameters - Mean (DP) | ||||
| SBP (mmHg) | 139.6(±15.24) | 132.9 (±15.46) | 136.2 (±10.61) | 0.2878 |
| DBP (mmHg) | 79.50(±12.35) | 77.45 (±17.65) | 83.14 (±9.54) | 0.3912 |
| MBP (mmHg) | 109.5(±11.23) | 103.8 (±10.83) | 109.7(±6.29) | 0.0861 |
| Differential pressure (mmHg) | 60.09(±16.28) | 50.14 (±13.62) | 52.95 (±15.41) | 0.0924 |
| Periodontal Clinical Parameters | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PI | BOP | PD≤4mm | PD≥5mm | CAL≤3mm | CAL≥6mm | |||||||
| β (95% CI) | p-value | β (95% CI) | p-value | β (95% CI) | p-value | β (95% CI) | p-value | β (95% CI) | p-value | β (95% CI) | p-value | |
| 90 days | -0.007(-0.01- -0.001) | 0.021 | -1.80(-2.46- -1.13) | 0.0001 | 0.05(0.02 -0.08) | 0.001 | -0.05(-0.08 - -0.03) | 0.0001 | -0.06(-0.09 - -0.03) | 0.0001 | -0.003(-0.01 -0005) | 0.41 |
| 180 days | -0.008(-0.01 - -0.001) | 0.021 | -1.81(-2.47 - -1.14) | 0.0001 | 0.05(0.03 -0.08) | 0.001 | -0.05(-0.07 - -0.03) | 0.0001 | -0.06(-0.09 - -0.03) | 0.0001 | -0.003(-0.02 -0005) | 0.40 |
| Biochemical exams | ||||||||||||
| HbA 1c | Fasting glucose | Total Cholesterol | C reactive protein | PGT | OCT | |||||||
| 90 days | -0.05(-0.36 – 0.24) | 0.73 | 0.004(-0.006 – 0.02) | 0.39 | -0.001(-0.006 – 0.003) | 0.58 | -0.38(-0.82 0.05) | 0.08 | -0.005(-0.03 -0.01) | 0.56 | 0.0007(-0.2 – 0.21) | 0.94 |
| 180 days | -0.05(-0.36 – 0.25) | 0.73 | 0.005(-0.007 – 0.02) | 0.40 | -0.002(-0.007 – 0.004) | 0.58 | -0.39(-0.82 0.05) | 0.08 | -0.005(-0.03 -0.01) | 0.56 | 0.0007(-0.19 – 0.20) | 0.95 |
| Physical Exams and Blood Pressure Parameters | ||||||||||||
| Hip/Waist ratio | MIB | SBP | DBP | MBP | Differential pressure | |||||||
| 90 days | -0.96(-3.01 -1.06) | 0.34 | 0.001(-0.03 – 0.03) | 0.93 | -0.002(-0.12 -0.009) | 0.79 | 0.008(-0.003 -0.20) | 0.15 | 0.004(-0.009 -0.018) | 0.53 | -0.007(-0.02 – 0.003) | 0.14 |
| 180 days | -0.97(-3.01 -1.06) | 0.34 | 0.001(-0.03 – 0.04) | 0.93 | -0.001(-0.12 -0.009) | 0.74 | 0.008(-0.003 -0.20) | 0.15 | 0.004(-0.009 -0.018) | 0.53 | -0.007(-0.01 – 0.001) | 0.14 |
| . | Time 1 | Time 2 | ||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| BOP (% of sites) | ||||
| <30 % | Ref | Ref | ||
| ≥30 % | 0.02 (0.002 – 0.29) | 0.001 | 0.05 (0.009 – 0.26) | 0.000 |
| CAL ≥6 mm (% of sites) | ||||
| <30 % | Ref | Ref | ||
| ≥30 % | 0.54 (0.18 – 1.68) | 0.28 | 0.61 (0.19 – 1.94) | 0.41 |
| SBP (mmHg) | ||||
| <140 | Ref | Ref | ||
| ≥140 | 0.48 (0.15-1.61) | 0.24 | 0.83 (0.26 – 2.65) | 0.75 |
| DBP (mmHg) | ||||
| <90 | Ref | Ref | ||
| ≥90 | 1.17 (0.34-1.02) | 0.79 | 1.97 (0.59 – 6.63) | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





