Submitted:
15 June 2023
Posted:
16 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and discussion
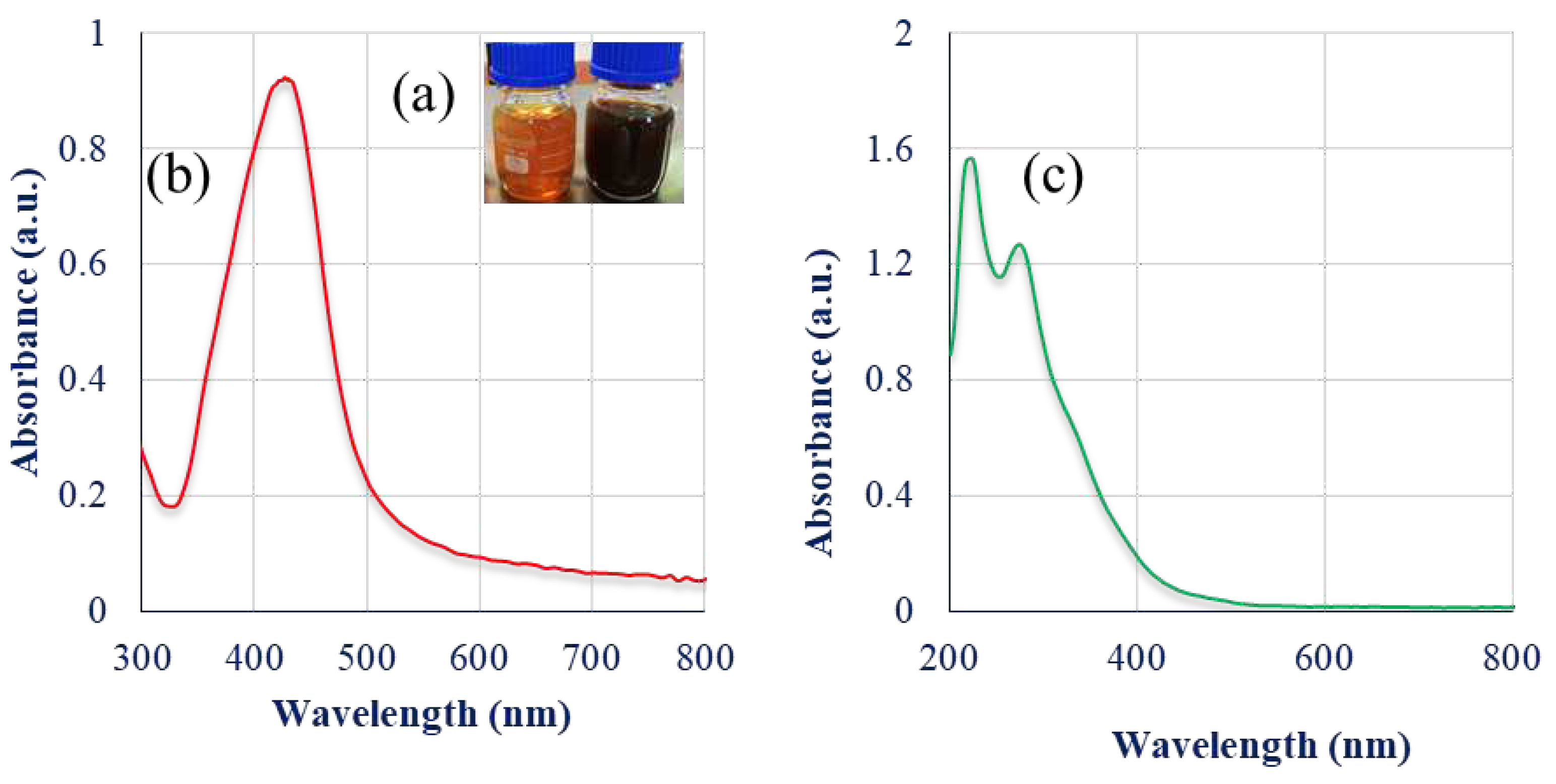
2.1. Biosynthesis and characterization of SmL-Ag-NPs using UV-visible spectroscopy analysis
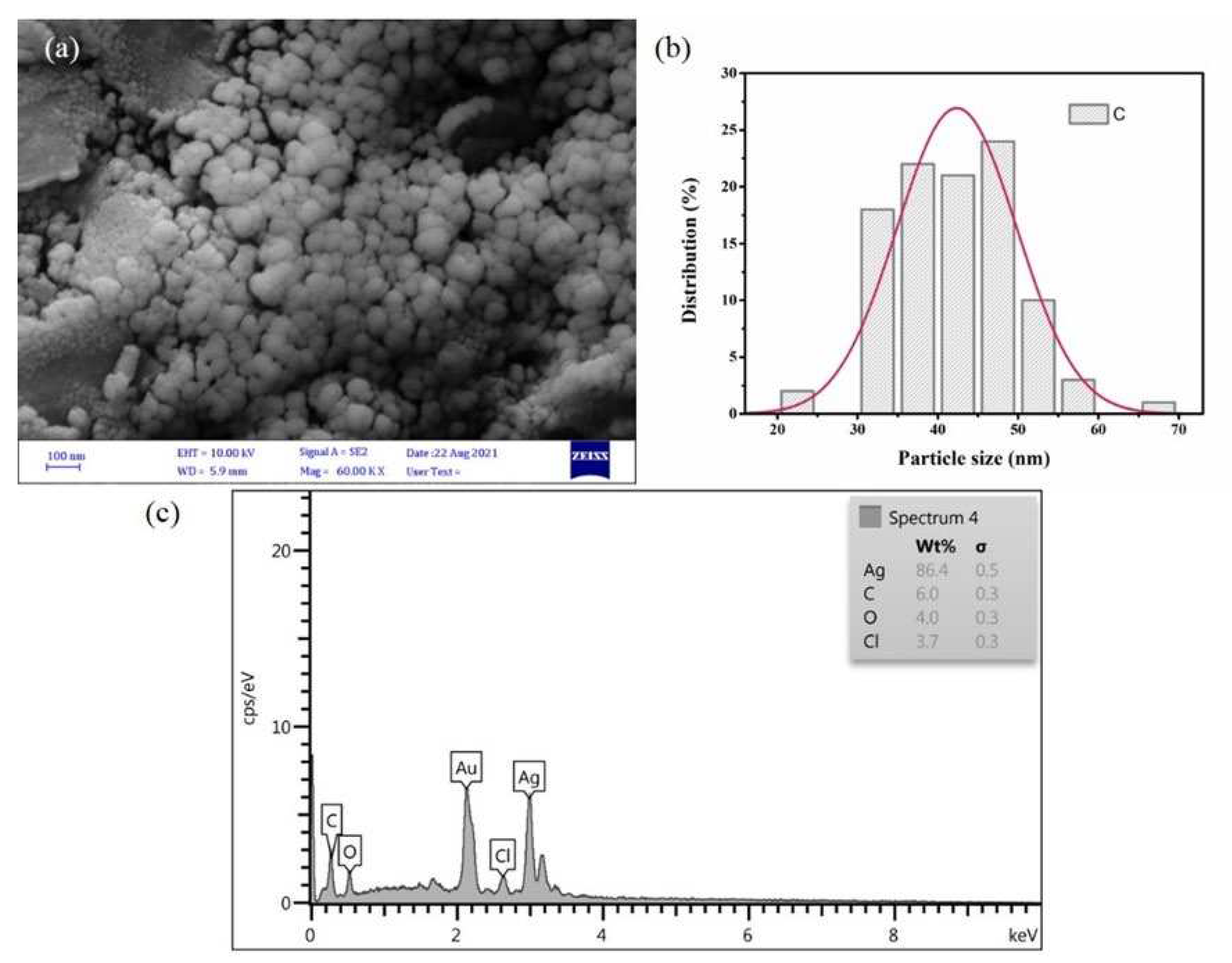
2.2. FESEM and EDX analysis of SmL-Ag-NPs
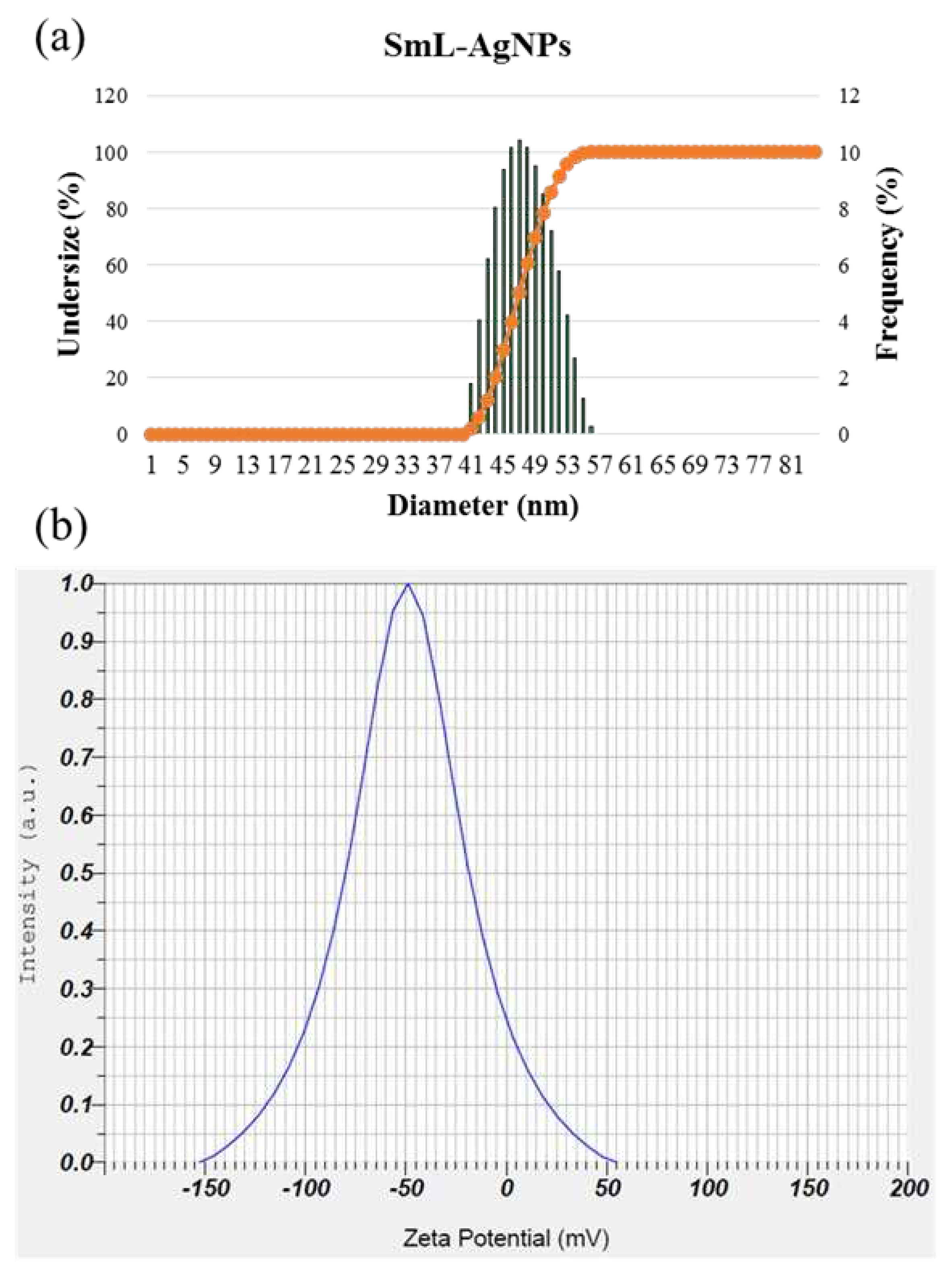
2.3. Size Distribution Analysis by Dynamic Light Scattering (DLS) method
2.4. Zeta potential (ζ) measurement of SmL-Ag-NPs
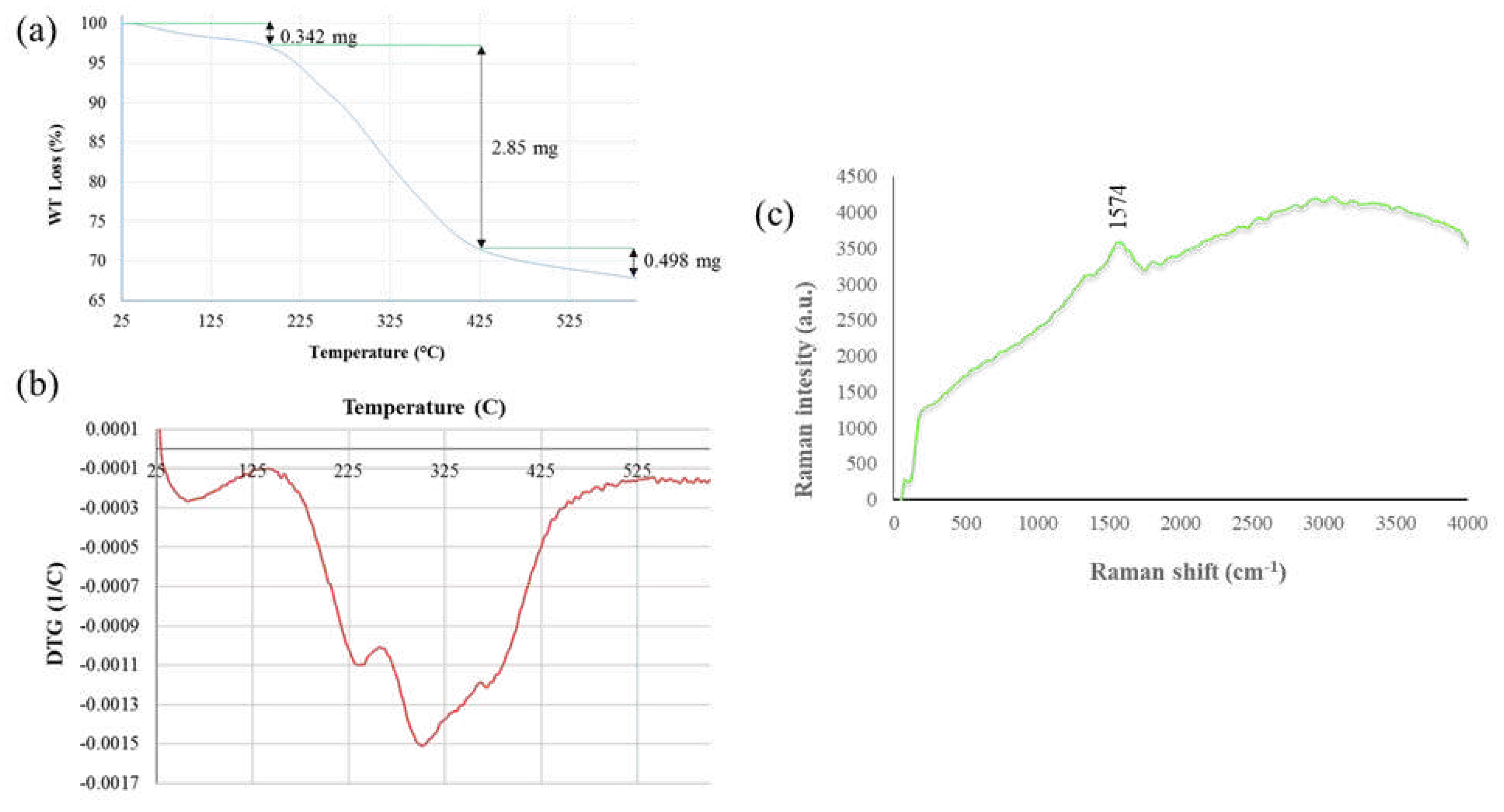
2.5. Thermal stability analysis of SmL-Ag-NPs using TGA and DTG
2.6. Surface-enhanced Raman scattering (SERS) analysis of SmL-Ag-NPs
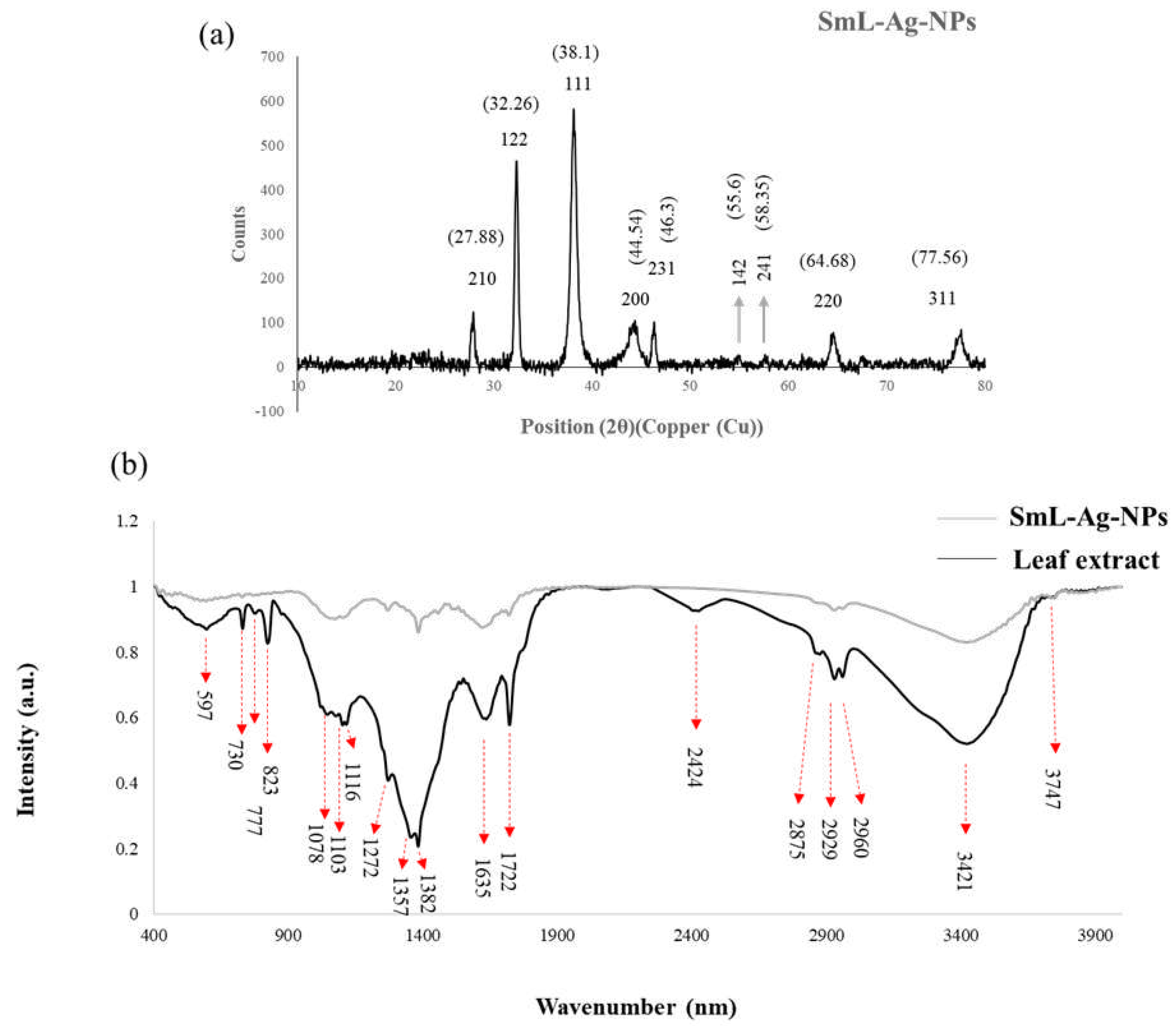
2.7. XRD analysis of SmL-Ag-NPs
2.8. Fourier Transform Infrared Spectroscopic (FTIR) analysis
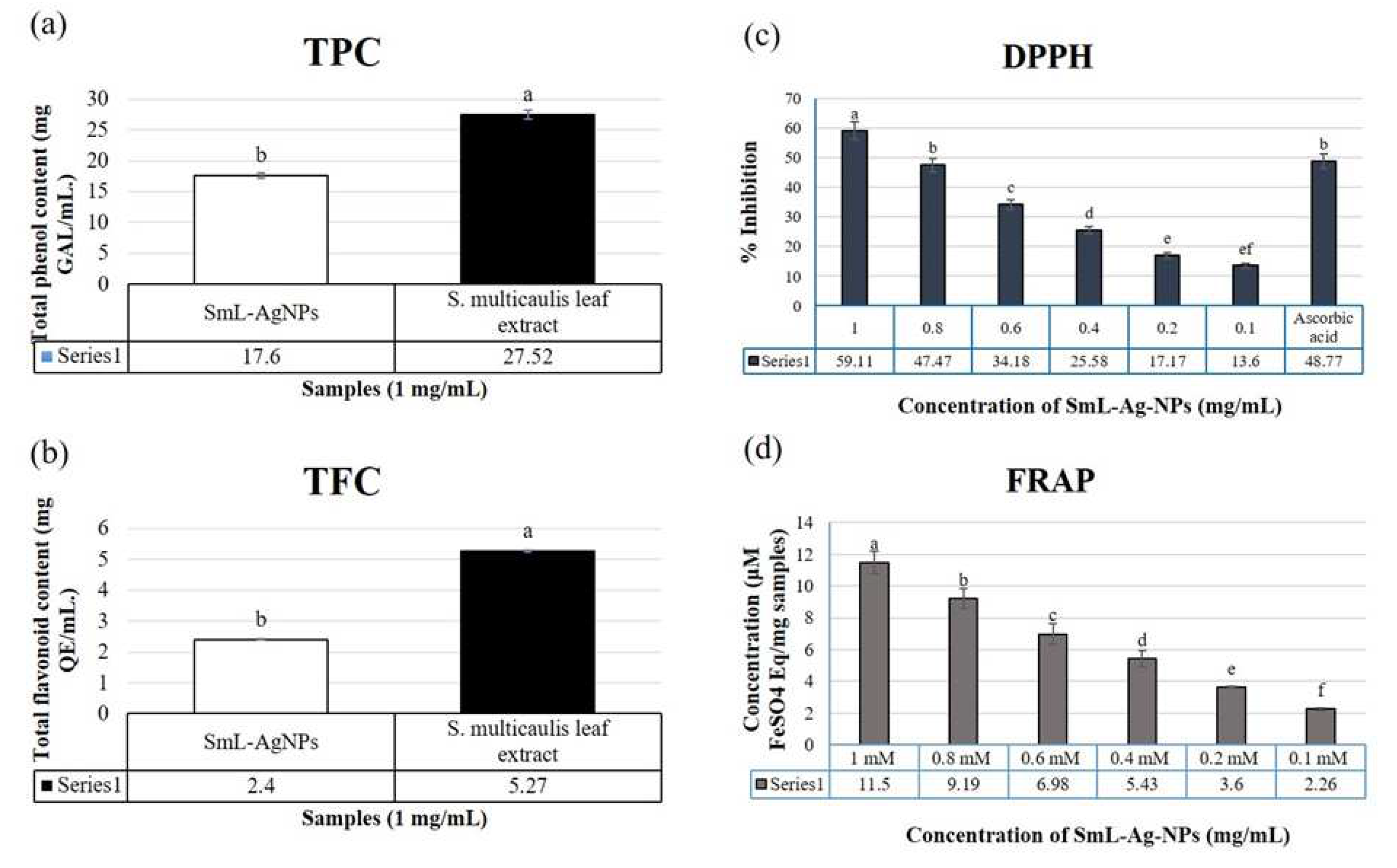
2.9. Phytochemical composition assay of SmL-Ag-NPs and S. multicaulis leaves extract
2.10. Antioxidant activity of S. multicaulis leave extract-mediated synthesized SmL-Ag-NPs
2.11. Antibacterial activity of SmL-Ag-NPs
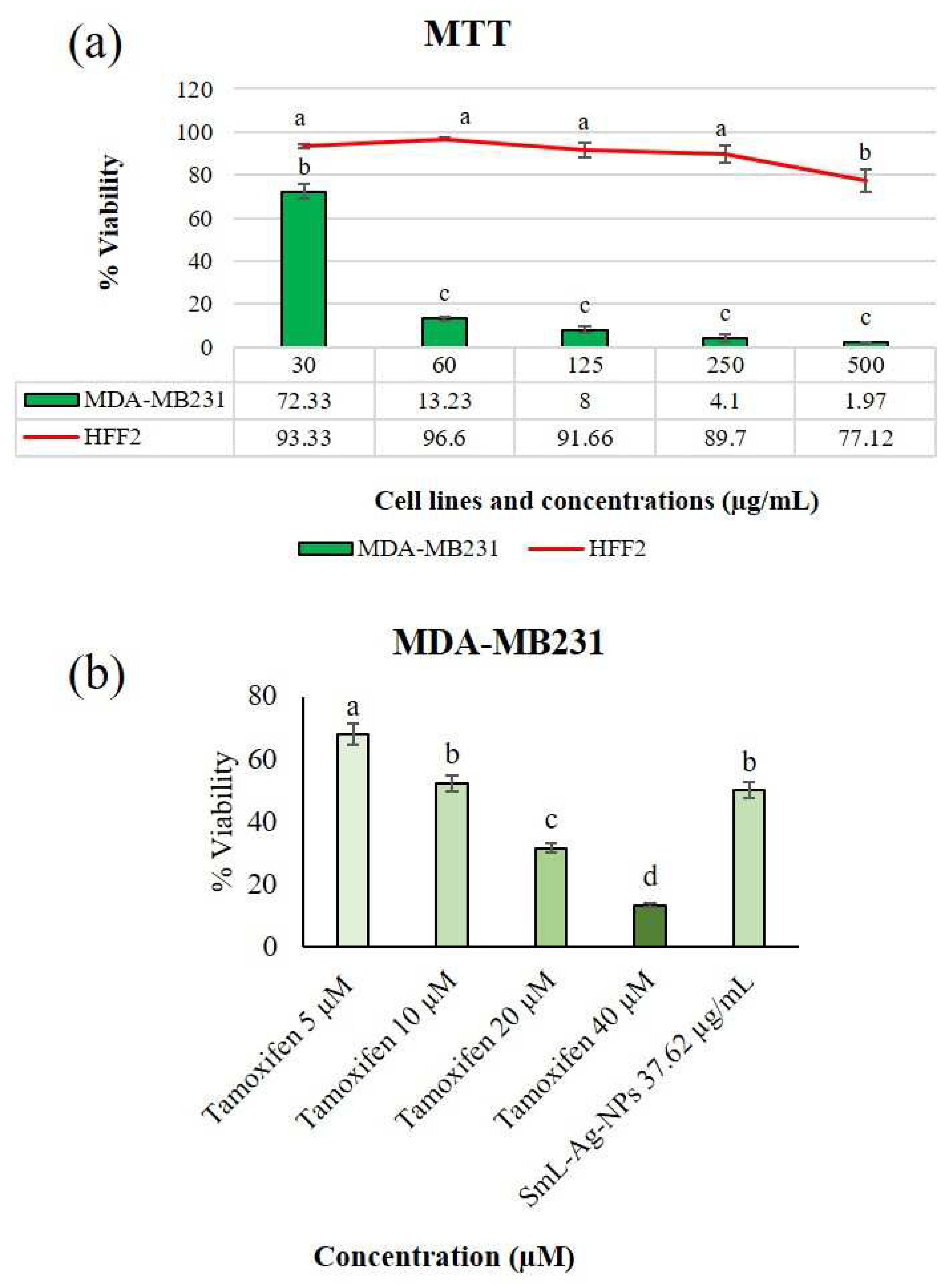
2.11. Cytotoxicity of SmL-Ag-NPs against MDA-MB231 cells
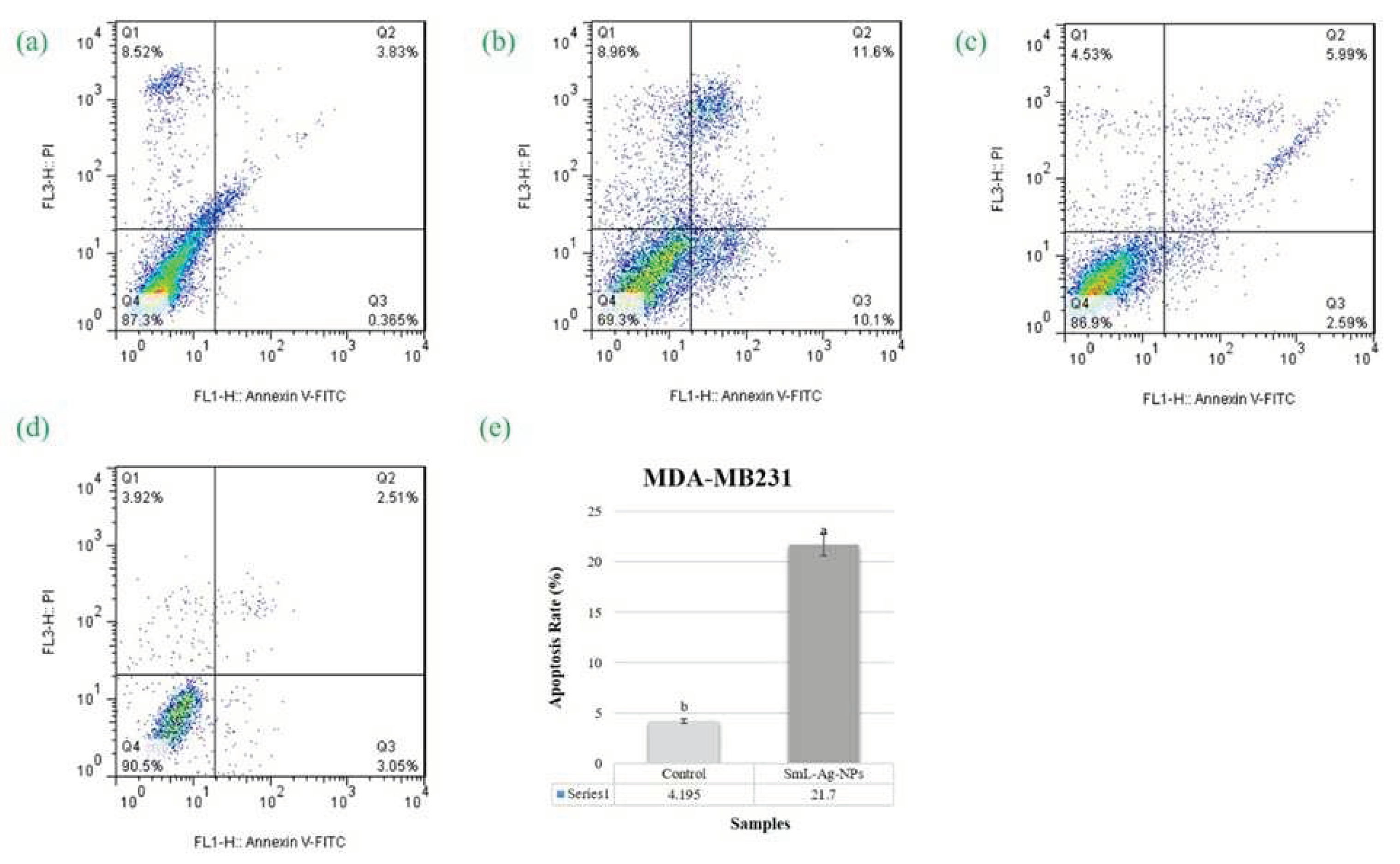
2.12. Apoptotic effect of SmL-Ag-NPs on MDA-MB231 and HFF2 cells
3. Materials and Methods
3.1. Collection of Plant Material and preparation of Plant Extract
3.2. Biosynthesis and characterization of SmL-Ag-NPs Using S. multicaulis leave extract
3.3. Characterization of biosynthesized SmL-Ag-NPs
3.4. Phytochemical composition, antioxidant potential and antimicrobial activity of SmL-Ag-NPs
3.5. In vitro cell viability assay of MDA-MB231 and HFF2 cells treated with SmL-Ag-NPs
3.6. Cell apoptotic effect of SmL-Ag-NPs
3.7. Statistical analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kreso, A.; O’Brien, C.A.; Van Galen, P.; Gan, O.I.; Notta, F.; Brown, A.M.; Ng, K.; Ma, J.; Wienholds, E.; Dunant, C.; Pollett, A.; Gallinger, S., McPherson, J.; Mullighan, C.G.; Shibata, D.; Dick, J. Variable clonal repopulation dynamics influence chemotherapy response in colorectal cancer. Science 2013, 339, 543-548. [CrossRef]
- Jin, C.; Wang, K.; Oppong-Gyebi, A.; Hu, J. Application of nanotechnology in cancer diagnosis and therapy-a mini-review. Int. J. Med. Sci 2020, 17, 2964-2973. [CrossRef]
- Simon, S.; Sibuyi, N.R.S.; Fadaka, A.O.; Meyer, S.; Josephs, J.; Onani, M.O.; Meyer, M.; Madiehe, A.M. Biomedical Applications of Plant Extract-Synthesized Silver Nanoparticles. Biomedicines 2022, 10, 2792. [CrossRef]
- Gharari, Z.; Hanachi, P.; Sadeghinia, H.; Walker, T.R. Cichorium intybus bio-callus synthesized silver nanoparticles: A promising antioxidant, antibacterial and anticancer compound. Int. J. Pharm 2022, 625, 122062. [CrossRef]
- Guan, Z.; Ying, S.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [CrossRef]
- Pokrajac, L.; Abbas, A.; Chrzanowski, W.; Dias, G.M.; Eggleton, B.J.; Maguire, S.; Maine, E.; Malloy, T.; Nathwani, J.; Nazar, L. Nanotechnology for a sustainable future: Addressing global challenges with the international network4sustainable nanotechnology. ACS Nano 2021, 12, 18608-18623. [CrossRef]
- Gharari, Z.; Hanachi, P.; Walker, T.R. Green synthesized Ag-nanoparticles using Scutellaria multicaulis stem extract and their selective cytotoxicity against breast cancer. Anal. Biochem 2022, 653, 114786. [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci 2016, 17, 1534. [CrossRef]
- Govaerts, R. World checklist of selected plant families’ database in ACCESS. In The board of trustees of the Royal Botanic Gardens,1-216203, Kew, England, 2003. [CrossRef]
- Gharari, Z.; Bagheri, K.; Khodaeiaminjan, M.; Sharafi, A. Potential therapeutic effects and bioavailability of wogonin, the flavone of Baikal skullcap. J Nutr Med Diet Care 2019, 5, 039. [CrossRef]
- Gharari, Z.; Bagheri, K.; Danafar, H.; Sharafi, A. Chemical Composition and Antimicrobial Activity of Scutellaria araxensis Essential Oil from Iran. Chem. Nat. Compd 2020, 56, 745-747. [CrossRef]
- Gharari, Z.; Aghajanzadeh, M.; Sharafi, A. Scutellaria orientalis subsp. Bornmuelleri: phytochemical composition and biological activities. Nat. Prod. Res 2022, 36, 1385-1390. [CrossRef]
- Chen, L.; Huo, Y.; Han, Y.X.; Li, J.F.; Ali, H.; Batjikh, I.; Hurh, J.; Pu, J.Y.; Yang, D.C. Biosynthesis of gold and silver nanoparticles from Scutellaria baicalensis roots and in vitro applications. Appl. Phys. A 2020, 126, 424. [CrossRef]
- Shermatova, I.B.; Ismailova, M.G. Obtaining and Studying Technology of Dry Substance Silver Nanoparticles Obtained by" Green Synthesis" Method Using Scutellaria Iscandaria L. Indian J. Forensic Med. Toxicol 2020, 14, 7624-7635. [CrossRef]
- Ismailova, M.; Shermatova, I.; Ismailova, P.; Ishimov, U. Study of the role of some Scutellaria Iscandaria L. extract’s flavonoids on nanosilver synthesis; World J. Pharm. Res 2020, 8, 19-25.
- Veeraraghavan, V.P.; Periadurai, N.D.; Karunakaran, T.; Hussain, S.; Surapaneni, K.M.; Jiao, X. Green synthesis of silver nanoparticles from aqueous extract of Scutellaria barbata and coating on the cotton fabric for antimicrobial applications and wound healing activity in fibroblast cells (L929); Saudi J. Biol. Sci 2021, 28, 3633-3640. [CrossRef]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2022. CA: Cancer J. Clin 2022, 72, 524-541. [CrossRef]
- Charghadchi, M.; Gharari, Z.; Sadighian, S.; Yazdinezhad, A.; Sharafi, A. Green Synthesized Silver Nanostructure Using Rhus coriaria Fruit Extract Inhibits the Growth of Malignant MCF-7 Cell Line. Braz Arch Biol Technol 2022, 64. [CrossRef]
- Hanachi, P.; Gharari, Z.; Sadeghinia, H.; Walker, T.R. Synthesis of Bioactive Silver Nanoparticles with Eco-Friendly Processes Using Heracleum persicum Stem Extract and Evaluation of Their Antioxidant, Antibacterial, Anticancer and Apoptotic Potential. J. Mol. Struct 2022, 1265, 133325. [CrossRef]
- Jadhav, K.; Deore, S.; Dhamecha, D.; Hr, R.; Jagwani, S.; Jalalpure, S.; Bohara R. Phytosynthesis of silver nanoparticles: characterization, biocompatibility studies, and anticancer activity. ACS Biomater. Sci. Eng 2018, 4, 892-899. [CrossRef]
- Aglin, A.; Shruthi, P.; Subathra, S. Selective Toxicity of Biosynthesised Silver Nanoparticles on MCF-7 and MDA MB-231 Breast Cancer Cell Lines. Int. J. Chemtech Res 2019, 12, 7-16.
- Sun, Q.; Cai, X.; Li J.; Zheng, M.; Chen, Z.; Yu, C.-P. Green synthesis of silver nanoparticles using tea leaf extract and evaluation of their stability and antibacterial activity. Colloids Surf, A Physicochem. Eng. Asp 2014, 444, 226-231. [CrossRef]
- Liu, J.; Yang, T.; Li, C.; Dai, J.; Han, Y. Reversibly switching silver hierarchical structures via reaction kinetics. Sci. Rep 2015, 5, 14942. [CrossRef]
- Vatansever, F.; Hamblin, M.R. Surface-initiated polymerization with poly (n-hexylisocyanate) to covalently functionalize silica nanoparticles. Macromol. Res 2017, 25, 97-107. [CrossRef]
- Wong, C.S.; Badri, K.H. Chemical analyses of palm kernel oil-based polyurethane prepolymer. Mater. sci. appl 2012, 3, 78-81. [CrossRef]
- Aboyewa, J.A.; Sibuyi, N.R.; Meyer, M.; Oguntibeju, O.O. Green synthesis of metallic nanoparticles using some selected medicinal plants from southern africa and their biological applications. Plants 2021, 10, 1929. [CrossRef]
- Aboyewa, J.A.; Sibuyi, N.R.; Meyer, M.; Oguntibeju, O.O. Gold nanoparticles synthesized using extracts of Cyclopia intermedia, commonly known as honeybush, amplify the cytotoxic effects of doxorubicin. Nanomaterials 2021, 11, 132. [CrossRef]
- Majoumouo, M.S.; Sharma, J.R.; Sibuyi, N.R.; Tincho, M.B.; Boyom, F.F.; Meyer, M. Synthesis of biogenic gold nanoparticles from Terminalia mantaly extracts and the evaluation of their in vitro cytotoxic effects in cancer cells. Molecules 2020, 25, 4469. [CrossRef]
- Saumya, S.; Basha, P.M. Antioxidant effect of Lagerstroemia speciosa Pers (Banaba) leaf extract in streptozotocin-induced diabetic mice. Indian J Exp Biol 2011, 49, 125-131.
- Wahab, S.; Khan, T.; Adil, M.; Khan, A. Mechanistic aspects of plant-based silver nanoparticles against multi-drug resistant bacteria. Heliyon 2021, 7, 07448. [CrossRef]
- Lin, W.; Huang, Y.-w.; Zhou, X.-D.; Ma, Y. In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicol. Appl. Pharmacol 2006, 217, 252-259. [CrossRef]
- Henslee, E.A.; Serrano, R.M.T.; Labeed, F.H.; Jabr, R.I.; Fry, C.H.; Hughes, M.P.; Hoettges, K.F. Accurate quantification of apoptosis progression and toxicity using a dielectrophoretic approach. Analyst 2016, 141, 6408-6415. [CrossRef]
- Ullah, I.; Khalil, A.T.; Ali, M.; Iqbal, J.; Ali, W.; Alarifi, S.; Shinwari, Z.K. Green-synthesized silver nanoparticles induced apoptotic cell death in MCF-7 breast cancer cells by generating reactive oxygen species and activating caspase 3 and 9 enzyme activities. Oxid. Med. Cell. Longev 2020, 2020, 1215395. [CrossRef]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Jeyaraj, M.; Kim, J.-H. Cytotoxicity of biologically synthesized silver nanoparticles in MDA-MB-231 human breast cancer cells. Biomed Res. Int 2013, 2013, 535796. [CrossRef]
- Hsin, Y.-H.; Chen, C.-F.; Huang, S.; Shih, T.-S.; Lai, P.-S.; Chueh, P.J. The apoptotic effect of nanosilver is mediated by a ROS-and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol. Lett 2008, 179, 130-139. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




