Introduction
Reproduction is a costly phenomenon that shapes the life history of a species through phenomena like decreased survival and a lower number of future reproductive opportunities (Harshman & Zera, 2007; G. C. Williams, 1966). Animals have limited energy resources, which results in trade-offs between different physiological functions, behavior, and reproduction (Harshman & Zera, 2007). Specifically, the reproductive costs to the female can be grouped into resource-allocation costs, for instance, production of the yolk protein, and non-resource-based costs, which include hormonal changes to the female body (T. D. Williams, 2005). Egg production and oviposition are major contributors to this costly phenomenon in females (Azevedo et al., 1997; Rosenheim et al., 2000). Therefore, egg-laying behavior needs to be closely regulated by the female’s physiology and neuronal systems. Hormones and peptides have a long-term effect and change the physiology of the female animal. For example, insulin-like peptides in Drosophila play a key role in oogenesis by regulating stem cell division in response to nutrition intake (LaFever & Drummond-Barbosa, 2005), resulting in a more long-term effect in the female body. In contrast, neurons provide a more immediate response. For example, recent studies have discovered specific neuronal candidates affecting oviposition in female Drosophila and artificial activation of some of these neurons results in the animal exhibiting oviposition sequence (F. Wang, Wang, Forknall, Patrick, et al., 2020).
Egg-laying behavior needs to provide enough flexibility for the female to produce offspring while encountering variable external environments (Kienzle et al., 2020; Reguera, 2002; Roy et al., 2018; Van Wielendaele et al., 2013) and physiological changes (Roy et al., 2018; J. Xu et al., 2010). For instance, unlike Drosophila melanogaster, D. suzukii prefers healthy unwounded fruit as an egg-laying substrate. Kienzle et al. (2020) showed that D. suzukii will lay its eggs on wounded fruit when the healthy fruit becomes less available. Conversely, change in external environmental factors affects the internal physiology of the female. In mosquitos, the expression of microRNA-309 is dependent on the blood meal, and disruption of microRNA-309 results in failure of ovarian development (Y. Zhang et al., 2016). These regulations on egg-laying, whether promoted by hormones or neurons, correlate directly with the female’s fitness and ensure that the female’s genes reach the next generation.
Since the early days of D. melanogaster as one of the model organisms for genetics, egg-laying behavior has been a phenotype that caught the interest of biologists. Egg-laying has been used as a phenotype to study hybridization (Engels & Preston, 1979; Price et al., 2001; Sturtevant, 1920), as an indicator of fecundity (H. Shapiro, 1932; Tantawy & Vetukhiv, 1960), and for quantitative genetics (McMillan et al., 1970). Egg-laying has been studied in connection with female life-span (Partridge et al., 1986, 1987) later came into light as a proxy for cryptic female choice (Frazee and Masly, 2015; Frazee et al., 2021; LeVasseur-Viens et al., 2015) and decision-making (Yang et al., 2008). Studying the egg-laying of Drosophila using genetic tools has always been a subject of interest for researchers, and many studies have focused on the production of the egg itself (Hall & Greenspan, 1979; Wieschaus, 1980). Early studies on female Drosophila focused on reproductive behavior holistically (Manning, 1967) or used decapitated females to assess the neuronal control of egg-laying between closely related Drosophila species (Grossfield & Sakri, 1972). In recent years, the neurobiology of egg-laying and how females control their reproductive output has received more attention in insects, including oriental tobacco budworm (C. Wang et al., 2020) and bean bug (Hasebe & Shiga, 2021). More in-depth studies have been conducted on specific neurons affecting fruit-fly egg-laying behavior (Rezával et al., 2012, 2014; Shao et al., 2019; F. Wang, Wang, Forknall, Patrick, et al., 2020; K. Wang et al., 2021; Yang et al., 2008).
Egg-laying involves different neuronal components (
Figure 1) that overlap with other aspects of female reproductive behavior and motivation (Ishimoto & Kamikouchi, 2021). Ovipositor extension can be part of mating, rejection, and egg-laying behavior. During mating, the female extends her ovipositor horizontally, whereas, during egg-laying, the female extends her ovipositor toward the substrate and bends her abdomen (K. Kimura et al., 2015). This stereotypical pattern lasts around six seconds and is called the “ovipositor motor program” (Yang et al., 2008). Internally, ovarioles need to be stimulated to produce more oocytes, the mature egg needs to move from the ovariole to the uterus via the oviduct, and the female needs to decide on a good location to lay her eggs; all of these components involve neuronal control (Rodríguez-Valentín et al., 2006; White et al., 2021; Yang et al., 2008).
Studying reproduction without also considering hormonal control is impossible. There are studies in many groups of dipterans that hormonal control plays a significant role in egg-laying. Hormonal control has been studied extensively in
Aedes aegypti since the 1970’s (Gwadz & Spielman, 1973; Wheelock et al., 1988). For instance, blocking the decrease in juvenile hormone (JH) levels after a blood meal results in abnormal egg development and decreased egg hatching (A. B. Shapiro et al., 1986). JH (J. P. Shapiro & Hagedorn, 1982), and ovarian ecdysogenic hormone (OEH) (Matsumoto et al., 1989) are two of the neurosecretory hormones that modulate follicle maturation and vitellogenesis in
A. aegypti (Nijhout, 1998). In the case of OEH,
D. melanogaster sister species offer a curious case: they lack the gene
neuroparsin, which is the homolog of OEH and widely conserved among many insects (Veenstra, 2010). Instead of OEH specifying egg maturation, in
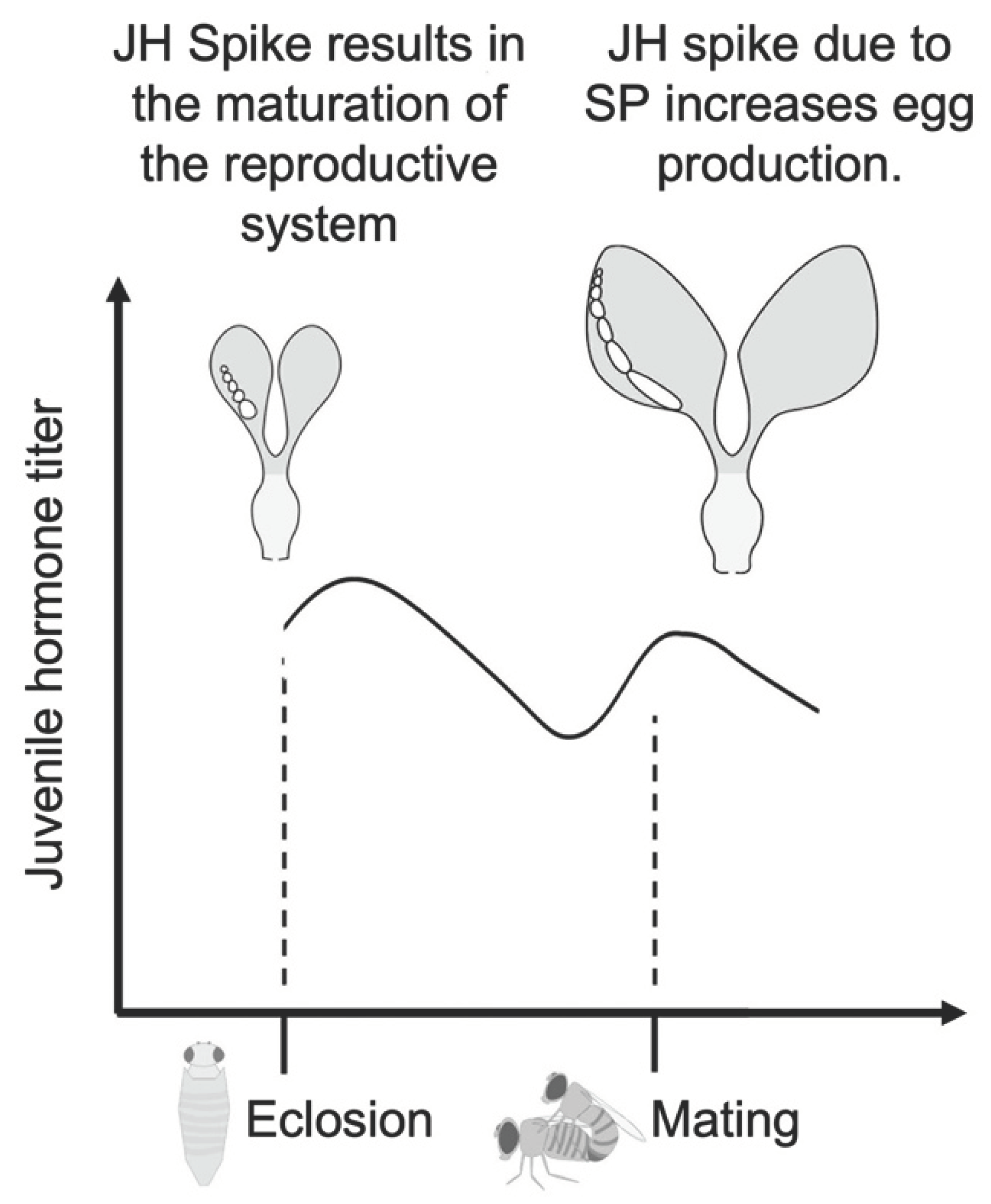
D. melanogaster, JH modulates egg maturation (Bilen et al., 2013). After eclosion, the corpus allatum produces JH that stimulates the yolk protein production in the fat body (Jowett & Postlethwait, 1980) (
Figure 2). The fat body and follicle cells synthesize yolk proteins that are vital for the development of the oocyte (Mirth et al., 2019). With the sexual maturation of the female fruit fly, JH levels fall. Yolk production starts again with the stimulation provided by sex peptide (SP), an accessory gland protein that is transferred from the male to the female reproductive tract during mating (Soller et al., 1997). It has been shown that hormones can change the firing properties and ion currents of neurons in mice (DeFazio & Moenter, 2002). However, the effect of hormones and SP on
Drosophila’s neurons is understudied. Experiments with JH suggest that this hormone plays an active role in dimorphic behavior between males and females. For instance, inhibiting JH activity by feeding the flies Fluvastatin, a drug that inhibits JH, results in the feminization of the walking behavior of male flies (Belgacem & Martin, 2002). The intertwined nature of hormonal and neuronal control calls for more in-depth studies of hormone titer’s effect in female
Drosophila's neurons.
Some aspects of the environment modulate D. melanogaster’s fecundity. For example, the nutritional environment affects the egg-laying output and site selection. Lifetime egg-laying of female D. melanogaster increases with an increase in the food quality (Tracey Chapman & Linda Partridge, 1996). The egg-laying behavior is also affected by the site-selection decision-making process. Drosophila females lay fewer eggs when no appropriate sites are available (Eisses, 1997). The presence of chemical cues in the food affects the egg-laying output. For instance, Geosmin-sensing neurons express Odorant receptor 56a (Or56a) and target a brain region called DA2 glomerulus to inhibit oviposition and feeding (Stensmyr et al., 2012). The presence of external and internal pathogens modulates egg-laying as well. Flies avoid laying eggs on contaminated food by an olfactory circuit in the brain that represses the egg-laying circuit (Stensmyr et al., 2012). Further, bacterial infection reduces the rate of egg-laying in female Drosophila through the NF-kB signaling pathway (Kurz et al., 2017). For example, Schwenke and Lazzaro (2017) have shown that JH affects both increased egg laying and decreased infection resistance, which results in immunosuppression in mated females (Schwenke & Lazzaro, 2017), and thus illustrates a trade-off between a physiological function (immune response) and reproduction.
The light environment is another factor that affects egg-laying decisions. Markow (1975) showed that genetic variation exists for light dependent behaviors and established that some fly strains lay more eggs in the dark while others lay more eggs in light treatment. Female flies display UV aversion for egg-laying site selection. They sense this specific light environment using their R7 photoreceptors (Zhu et al., 2014). Gr66a-expressing neurons on the leg induce aversion, while Gr66a-expressing neurons in the pharynges induce attraction to the chemical lobeline as an egg-laying site (Joseph & Heberlein, 2012). Gr66a-expressing bitter-tasting neurons that are sensitive to H2O2 also promote UV avoidance in the egg-laying site selection (Guntur et al., 2017).
In addition, the social environment affects the fruit fly’s behavior. Mating is known to increase aggression in females (Bath et al., 2017), and field studies of oriental fruit flies have shown that females increase their aggression to defend their egg-laying sites (Shelly, 1999). However, increased aggression in the social environment does not directly affect egg-laying (Bath et al., 2017, 2018). A recent study found that non-egg excrements of other females, combined with the female’s social exposure before the mating, can modulate the number of fertilized eggs by the female (Fowler et al., 2022). The presence of a male-produced pheromone affects female egg-laying site selection by activation of Or7a+ neurons. Additionally, Or7a mutant and Or7a ablated flies lay a higher number of eggs (Lin et al., 2015). Chemical cues by other mated females also guide the female egg-laying site selection (Duménil et al., 2016). Olfactory cues are one of the key players in D. suzukii. Orco-expressing sensory neurons are necessary for the egg-laying site selection of this species (Karageorgi et al., 2017).
Until this point, I covered external factors and hormones that affect egg-laying. From here, I focus on central and peripheral neurons that affect egg-laying. I will explore major sex determination genes that are commonly used in reproductive neurobiology in Drosophila. I will discuss the sensory neurons, with a focus on the abdomen, and explore neurons that detect important signals during and after mating that have been shown to affect egg-laying. Next, I will move to the neurons in the abdominal neuromere (abdominal ganglion) and brain, where a significant part of processing sensory clues and regulating egg-laying behavior occurs, and briefly explore the motor neurons. Lastly, I explore the field’s future and areas that show potential for expansion. Neurobiology of egg laying in Drosophila offers exciting avenues to study evolutionary forces like cryptic female choice using genetic tools already available in this classic model.
Major sex Determination Genes as Indicators of Central Neurons Involved in Female Reproduction
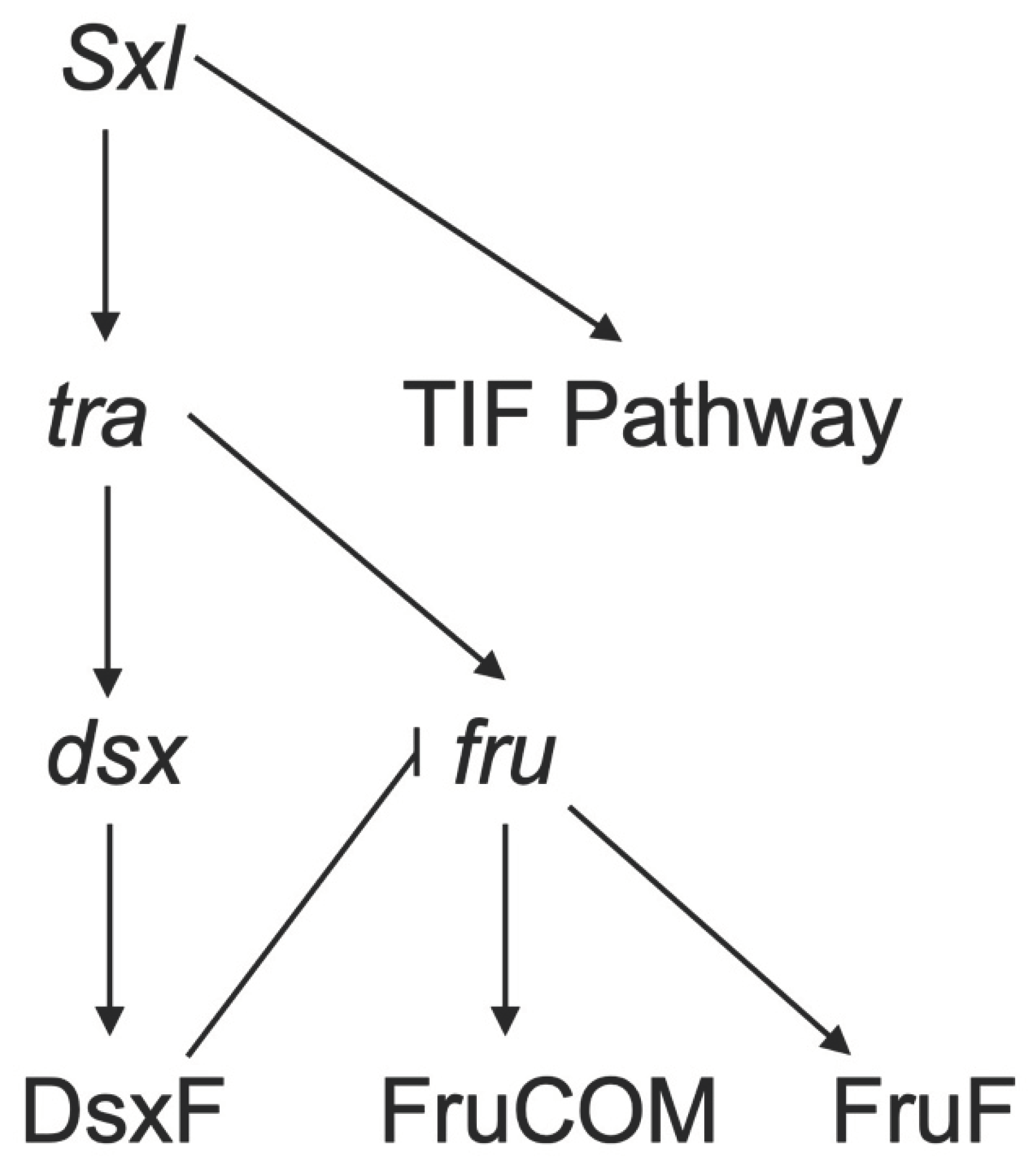
Doublesex (
dsx) and
fruitless (
fru) are two major sex determination genes (Billeter et al., 2006) (
Figure 3). The gene
dsx is involved in male and female developmental differentiation and regulates sex-specific behavior in both sexes (Waterbury et al., 1999). The gene
fru is a major player in male courtship behavior and is involved in the development of male-specific muscle of Lawrence (Billeter et al., 2006). Neurons expressing either or both of these genes are usually important for specifying reproductive behavior (K. Kimura et al., 2008; Sanders & Arbeitman, 2008; K. Wang et al., 2021; Waterbury et al., 1999; Yamamoto & Koganezawa, 2013). For instance, the elimination of synaptic transmission with tetanus toxin in
dsx-expressing neurons results in no egg laying in female
D. melanogaster (Rideout et al., 2010). Further dissection of this phenotype shows that sperm were transferred normally to these females, and inseminated females produced mature oocytes. This result suggests that infertility is due to mature oocytes not being moved into the uterus and being fertilized (Rideout et al., 2010), indicating an essential role for
dsx neurons in egg laying.
The gene dsx encodes an mRNA that is differentially-spliced to produce sex-specific protein isoforms, one of which is the DsxF protein in female Drosophila. DsxF protein is crucial for sexual differentiation, including the production of female pheromones and yolk proteins (Baker & Wolfner, 1988; Waterbury et al., 1999). Dsx-expressing neurons have a wide distribution in the female central nervous system (CNS). In adult females, dsx is expressed in 700 CNS neurons, and there are 30-40 dsx-expressing neurons in each brain hemisphere of female Drosophila, specifically around 16 cells in the pC1 and pC2 regions (Sanders & Arbeitman, 2008). Palavicino-Maggio et al. (2019) suggest that pC1, pC2, and pC3 clusters are the hubs of sex-specific behavioral patterns. For instance, pC2l control the ovipositor (K. Kimura et al., 2015). The pC1 region houses the most studied region between the three that modulate egg-laying (See brain section). Dsx-expressing neurons that affect egg-laying are not limited to the brain. Dsx-expressing neurons in the ventral nerve cord are sufficient to elicit an ovipositor extrusion and egg ejection behavior (K. Kimura et al., 2015). Other genes affecting dsx expression might have an effect on egg-laying as a result. For instance, deletion of mir-iab-4/8 and homothorax (hth) mutants result in increased egg-laying and decreased receptivity, thus showing that mir-iab-4/8 and hth regulate dsx expression levels during development (Garaulet et al., 2021). Other studies have shown that co-expression of the gene dissatisfaction (dsf) and dsx in abdominal neuromere interneurons modulates vaginal plate opening, ovipositor extrusion and movements of uterus walls (Duckhorn et al., 2022). Taken together, these studies suggest a critical role for dsx-expressing neurons in female reproductive behavior, including egg-laying.
The other major sex-determination gene fru has sex-specific splice isoforms as well as common isoforms. Fru is a complex locus that has at least four promoters (P1-4), and only transcripts produced from P1 are sex-specific. Female-specific splice of Fru (FruF) has no known function (Demir & Dickson, 2005). It has been shown that the neurons with transcription from the P1 promoter play a role in female sexual behaviour (Usui-Aoki et al., 2000). Studies have shown that the non-sex-specific Fru proteins (known as FruCOM) are expressed throughout the developing nervous system (Sato & Yamamoto, 2022). Moreover, Chowdhury et al. (2020) show that FruCOM, especially from P2 transcript, affects female receptivity and heterospecific mating. Other studies have shown that there specifically are five fru-expressing mAL interneurons present in the female brain (Goto et al., 2011; K.-I. Kimura et al., 2005). Many of the recently described neurons in the egg-laying circuit are fru-expressing, including oviDNs (F. Wang, Wang, Forknall, Patrick, et al., 2020). These results suggest that even though there is no known function for FruF protein in the female Drosophila, fru+ neurons are involved in egg-laying and other reproductive behaviors.
Fru and
dsx are not the only genes that regulate sexual differentiation and behavior (
Figure 3). Other genes that have been shown to affect female behavior include
Sex-lethal (
Sxl)(Evans & Cline, 2013; Sawala & Gould, 2017),
retained (
retn)(Shirangi et al., 2006), and
transformer (
tra) (Castellanos et al., 2013) and may prove valuable in further determining the pathways involved in female egg-laying.
Sxl has been shown to have targets other than transformer and through a branch of downs stream genes, collectively called
tra-insufficient feminization (TIF) pathway, controls the development of
fru+ neurons that regulate the passage of the mature egg to the oviduct (Evans & Cline, 2013).
The interplay between the sex determination genes plays a crucial role in orchestrating the complex behaviors associated with reproduction in Drosophila. The vast body of work available on both dsx and fru is a gem for neurobiology, which in many cases is underappreciated. The results from papers in fields other than neurobiology might hold the key that leads to a better understanding of circuits. For example, Keisman et al. (2001) described the effect of dsx in female genital disc development that will eventually give rise to reproductive tract and external terminalia. As mentioned above, the extensive distribution of dsx-expressing neurons in the female central nervous system indicates their pivotal role in shaping sex-specific behavioral patterns. The fru gene emerges as a player in e reproductive behaviors in Drosophila which include important neurons like oviDNs. Additionally, the involvement of other genes further emphasizes the complexity of the regulatory network governing female reproductive behaviors. As researchers continue to understand the intricacies of these genetic pathways, more resources will become available to study single neurons and their effect on reproductive behavior.
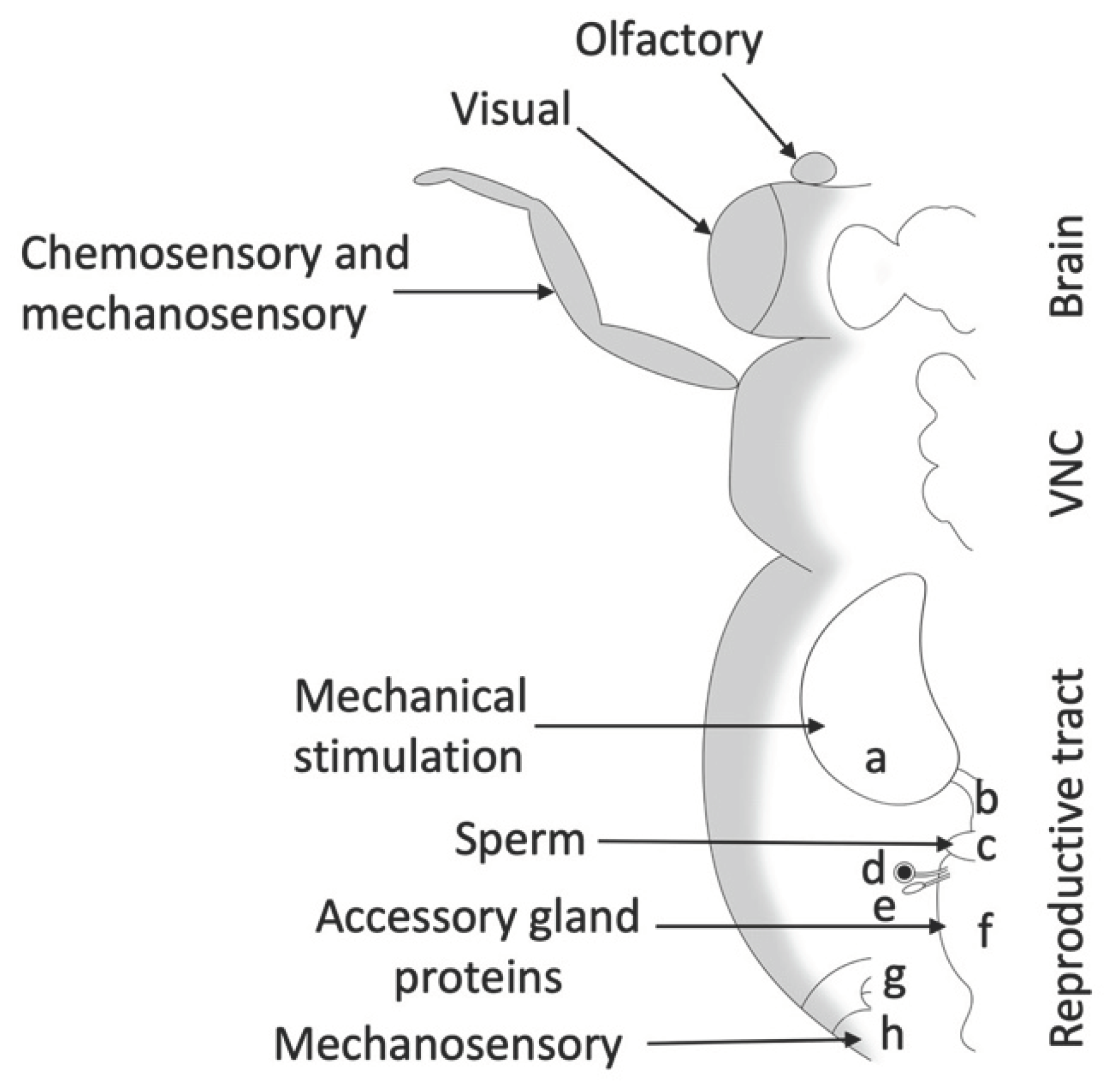
Peripheral Nervous System: Sensory Neurons that Influence Egg-Laying
Drosophila possesses numerous sensory neurons throughout its body, including sensory organs located on the head and legs. The relationship between sensory organs on the head or legs and egg-laying has been extensively discussed in a review by Ishimoto and Kamikouchi in 2021. For this review, I will focus specifically on sensory cues received by the sensory organs located in or on the abdomen (
Figure 4).
Sensory Cues Triggered by Mating
The process of mating changes female fruit flies’ behavior. It reduces receptivity and increases ovulation, egg-laying (Avila et al., 2011), and food intake (Ribeiro & Dickson, 2010). These changes in female behavior are modulated by components of mating, including pheromonal cues (Billeter & Wolfner, 2018), seminal fluid proteins (SFPs) (Haussmann et al., 2013; Ribeiro & Dickson, 2010), and the physical act of mating itself (Shao et al., 2019). Mating and its components modulate vesicle release at the nerve terminals of the reproductive tract (Heifetz & Wolfner, 2004). The act of mating and some components of the seminal fluid change the status of the lower reproductive tract, while SPFs modulate the vesicles released from neurons, innervating the seminal receptacle, while sperm alters the neurons innervating the upper reproductive tract (Heifetz & Wolfner, 2004). Furthermore, SFPs are responsible for the conformational change of the lower reproductive tract (Adams & Wolfner, 2007). In addition, mating affects gene expression in different tissues. For example, gene expression changes in the female D. melanogaster’s head after mating are strongly correlated with metabolic pathways (Newell et al., 2020).
SP is one of the SFPs secreted by male accessory glands and, in its mature form, is composed of 36 amino acids (Kubli, 2003). SP is sensed by pickpocket (ppk)+ and fru+ neurons in the female reproductive tract, which are called sex peptide sensory neurons (SPSNs)(Yapici et al., 2008). Silencing SPSNs in virgin female flies results in increased egg laying that is comparable to mated females (Häsemeyer et al., 2009; Rezával et al., 2012). SPSNs express a G protein-coupled receptor called the sex peptide receptor (SPR). The binding of SP to SPR results in a cascade that blocks the activity of SPSN neurons. The silencing of SPSNs by SP results in behaviors specific to post-mating response, including increased egg-laying and rejection (Häsemeyer et al., 2009; Rezával et al., 2012). SPR is also expressed by spermatheca secretory cells and aids in sperm release (Avila et al., 2015). Knockdown of the SPR gene with RNA interference reduces egg laying significantly (Yapici et al., 2008). SP is not only detected by neurons and cells in the reproductive tract but also passes through hemolymph and is sensed by neurons in the CNS and VNC (Yapici et al., 2008); specifically, fru+ neurons in the brain (Chow et al., 2010). SP stimulates JH synthesis in the corpus allatum resulting in the development of oocytes (Moshitzky et al., 1996).
SPR expressed in the neurons has a similar structure to myo-inhibitory peptide (MIP) receptors. SP is more stable than MIPs and can survive the harsh environment of the female reproductive tract (Isaac et al., 2014). There are 5 MIPs in Drosophila, and they have been known to act as neuropeptides (Carlsson et al., 2010). One early study showed that pan-neuronal knockdown of MIP does not affect receptivity and remating, but at least in one fly line, there was a significant decrease of the egg-laying (Kim et al., 2010). Furthermore, Poels et al. (2010) show that neither RNAi knockdown of MIPs nor direct injection of MIPs in the fruit fly’s abdomen has an effect on the egg-laying. More recent studies have shown that Mip-expressing neurons in the CNS are essential contributors to female receptivity and copulation effect (Jang et al., 2017; Shao et al., 2019).
Other seminal fluid proteins affect the female egg-laying as well. Acp26Aa, also known as ovulin, has a small increasing effect on egg-laying (Herndon & Wolfner, 1995). Specifically, it helps release the mature oocyte from the ovary after mating (Heifetz et al., 2000) by increasing the activity of octopamine-releasing neurons (Rubinstein & Wolfner, 2013). Studies show that ovulin is a protein that interacts with itself. However, the pathway by which this molecule interacts with octopaminergic neurons, whether as a monomer or dimer, has not yet been identified (A. Wong et al., 2006). In a systematic study of gustatory receptors (Grs), Park and Kwon (2011) found the expression of 21 Grs in the abdomen that send their projections to the ANm. Most of these Grs are expressed in multidendritic neurons on the abdominal wall, while 4 are expressed in the reproductive tract. Gr28b.b, Gr28b.c, Gr32a (involved in pheromone detection), and Gr64c (a sugar receptor) are expressed in the female’s upper reproductive tract (Park & Kwon, 2011) and can be candidates for modulation of ovulation. Other studies have shown that uterine Gr32a-expressing and ppk+ neurons, increase aggressive behavior after mating. Similarly, activation of Gr32a-expressing uterus neurons in virgin females reduced their sexual receptivity (Liu et al., 2020). These results suggest that Gr32a-expressing neurons might be potential candidates that affect egg-laying behavior as well. In males, Gr32a-expressing neurons detect synomones and enforce reproductive isolation (Fan et al., 2013), and affect aggressive behavior (Andrews et al., 2014).
Sensory Cues Triggered by Ovulation and Egg Delivery
Ovulation and release of the egg are done by both virgin and mated females. As mentioned above, the release of the oocyte into the uterus and uterine contractions are modulated by octopamine. However, the movement of oocytes into the uterus triggers mechanosensory neurons and provides feedback to the insect (Chiang et al., 1991). The ppk- and dsx+ neurons not only sense sex peptide, but also can act as stretch receptors (Rezával et al., 2012). Silencing of a group of two to three ppk and CG3542 expressing neurons residing on the upper oviduct reduces egg-laying but does not affect the mating receptivity (Lee et al., 2016), thus hinting that these neurons might be involved in stretch sensation in the oviduct. Other studies suggest that fru+ neurons are developmentally controlled by TIF pathway and might also be involved in the release of egg from the ovary (Evans & Cline, 2013). Cury and Axel (2023) have identified a pair of sensory neurons in the posterior uterus, called PU neurons. PU neurons sense the movement of the egg to the vagina and ovipositor and are necessary for transition into egg burrowing behavior (Cury & Axel, 2023). Taken together, studies suggest that defects in the neuronal control of mature egg release from the ovary and its movement through the reproductive tract can reduce the number of eggs significantly.
Mechanosensory Cues from Mating Direct Egg-Laying Behavior
Mechanosensation provides valuable external information and internal feedback to egg-laying females. The mechanical stimulus on the
D. melanogaster body is sensed by two types of devices: ciliated type I neurons, which include mechanosensory bristles, and multidendritic neurons (Hehlert et al., 2021). Female
Drosophila genitalia have many mechanosensory bristles arising from the genital disc during development (McQueen et al., 2022). It has been shown that the genes
invected and
engrailed are necessary for the development of normal bristle phenotype of the female genitalia, and mutants of these two genes lack the bristle phenotype (
Figure 3 of Casares et al., 1997). Specification of these genitalia bristles is under the genetic control of
Hairless, and a mutation in this gene results in female genitalia with almost no bristles (
Figure 3 of Bang et al., 1991). A system that affects the development and innervation of the bristle is the
achaete-scute system (García-Bellido & Santamaria, 1978; Simpson, 1990) and its adjacent genes like
asense (Domínguez & Campuzano, 1993)
Daughterless, Senseless (Jafar-Nejad, 2006) and
musashi (Nakamura et al., 1994). However, their effects on the bristles of the female genitalia have not been characterized extensively. The mechanosensation modulated by the Piezo mechanoreceptor affects the reproductive behavior of
D. melanogaster and optogenetic activation of
Piezo-expressing
ppk- neurons in the female abdomen increases neuronally in LSANs, in turn affect egg-laying (Shao et al., 2019). The bristles directly on the ovipositor have also been studied in
D. suzukii and resemble mechanosensory neurons in
D. melanogaster and express mechanosensory channels, including
Piezo (Crava et al., 2020). A recent study shows that the bristles on female
D.
melanogaster genitalia express the
nompC (Cury & Axel, 2023) as well as
Piezo. Cury and Axel (2023) show that the mechanosensory bristle on the posterior end of the abdomen are necessary for assessing the firmness of the substrate for egg-laying.
Mechanosensation of the genitalia affects the female’s egg-laying in other insect species and can provide basis for further discovery in D. melanogaster. For example, in female crickets, Gryllus bimaculatus the mechanosensory hairs on the vulva send the signal to the terminal abdominal ganglion and result in a change in the motor pattern of the vulva after the egg is laid (Ogawa et al., 2011). Elimination of sensory feedback from these mechanoreceptors resulted in the cricket staying in the egg-deposition pattern (Ogawa et al., 2011). Similar studies have not been conducted in D. melanogaster and are a puzzle piece of many unknown aspects of the effect of mechanosensation on the egg-laying behavior of Drosophila.
Central Nervous System: The Abdominal Neuromere in the Ventral Nerve Cord
In adult
Drosophila, the neuromeres of the ventral nerve cord (VNC) are merged to form segmental thoracic neuromeres and a smaller fused neuromere for the abdominal sections (Court et al., 2020). The
Drosophila VNC has around 20,000 neurons (Bates et al., 2019) and the abdominal neuromere (ANm) or abdominal ganglion (AG) is the most posterior segment of
Drosophila’s VNC (Court et al., 2020) (
Figure 5). Unlike its name, this fusion of abdominal neuromeres resides completely in the thorax and connects to the abdomen through the main nerve called the abdominal nerve trunk (AbNT). There are four other bilaterally-paired nerves coming out of ANm or its adjacent regions (AbN1-4)(Court et al., 2020), but for the purpose of this review, I will focus only on ANm and AbNT. Most of the cell bodies of neurons reside on the outer cortex of ANm and project toward the center (Court et al., 2020). Some subsets of ANm neurons have been studied regarding egg-laying behavior and regulation. There are roughly 300
dsx+ neurons in the ANm (K. Kimura et al., 2015). Studies have shown that the activation of
dsx+ neurons in the ventral nerve cord results in ovipositor extension and egg ejection in some, but not all, decapitated female flies (K. Kimura et al., 2015).
SP abdominal ganglion (SAG) neurons (
Figure 5), as the name suggests, are
dsx+ and
Fru- female specific cholinergic neurons that reside within ANm and receive information from sex peptide sensory neurons (SPSNs) (Feng et al., 2014) (
Figure 5). There are two SAG neurons, one on each side of ANm, and they send their projections bilaterally to the dorsal protocerebrum and ipsilaterally to the periesophageal regions in the brain (Feng et al., 2014). SAG neurons have excitatory synapses with neuropeptide allatostatin-C (AstC)-producing neurons in the brain; mating silences the SAG-Ast-C axis and results in the production of JH and vitellogenesis (C. Zhang et al., 2022). Reduction of the activity of SAG neurons reduces female receptivity and increases the number of eggs laid by the female fly (Feng et al., 2014) and increases the appetite for salt and yeast (Walker et al., 2015). The SPSN-SAG axis modulates post-mating decrease in sleep (Garbe et al., 2016). Studies suggest that SAGs are not the only pathway regulating female behavior downstream of SPSNs (Feng et al., 2014). There are four
dsx/ETFLP250 neurons in the abdominal neuromere with the opposite effect of SAG neurons that send their axons to SOG as well (K. Kimura et al., 2015; Rezával et al., 2012).
SPSNs come into contact with other neurons in the ANm. The ventral abdominal lateral (vAL) (
dsx+) neurons and ventral abdominal medial (vAM neurons) are
Mip-expressing interneurons in the abdominal neuromere that relay the signal from SPSNs to neurons that send their signal to the brain (SAG neurons in case of vAM) (Jang et al., 2017) (
Figure 5). A pair of ascending neurons in ANm, called LSANs, convey the information of the act of copulation sensed by
Piezo-expressing neurons in the abdomen to the brain (Shao et al., 2019). Shao et al. (2019) further showed that artificial activation of LSANs suppresses egg-laying (
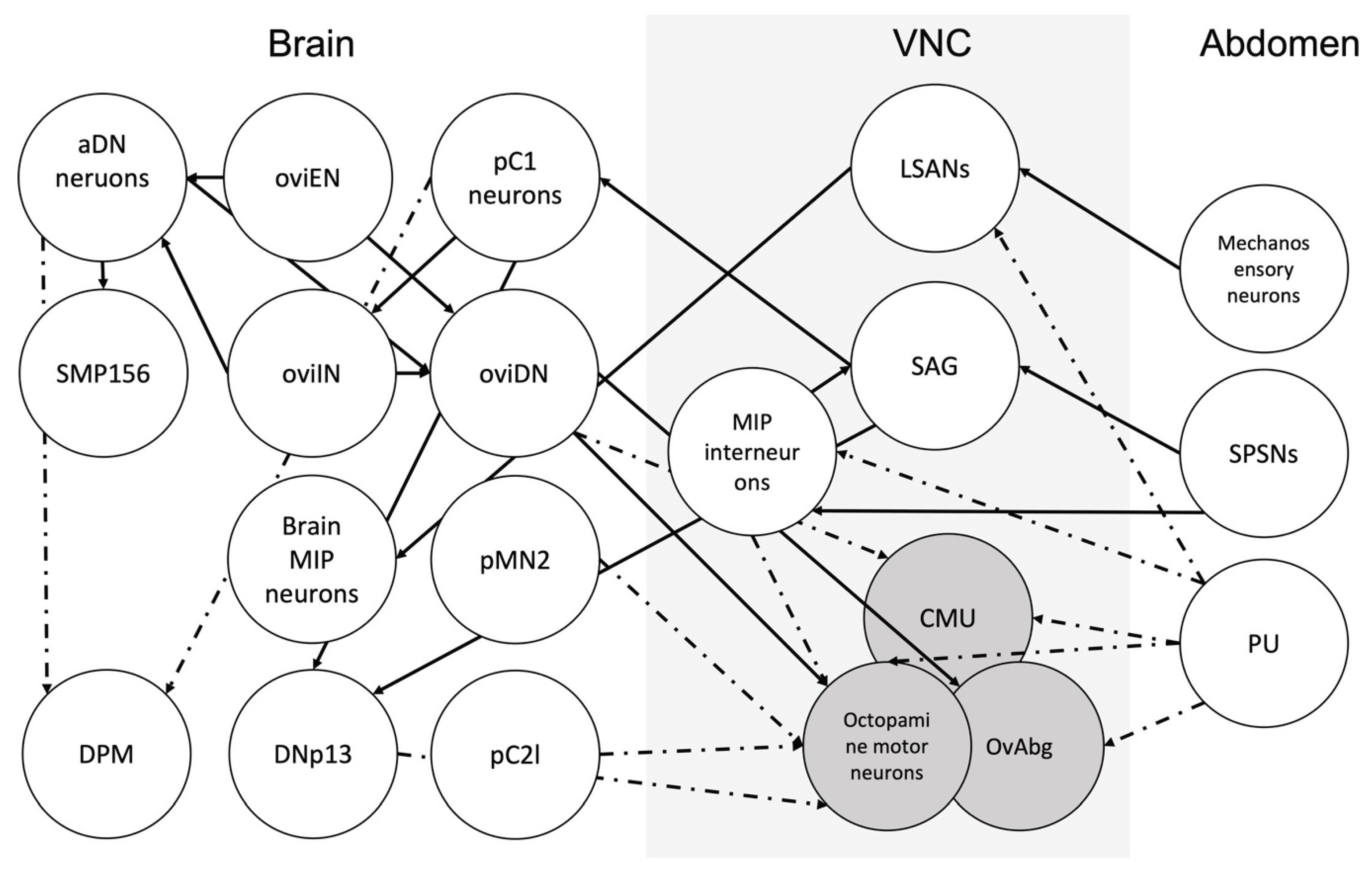
Figure 5). While the connection of some neurons in the ANm, for instance, SAG, is well characterized, potential synapses between other neurons involved in the egg-laying circuit and the nature of these connections are waiting to be discovered (Figure 7).
Central Nervous System: The Brain
The Drosophila brain is one of the well-studied animal brains in terms of circuits and neuronal connectivity (Dorkenwald et al., 2022; Schlegel et al., 2017; C. S. Xu et al., 2020). The adult fly brain has around 135,000 neurons (Bates et al., 2019) and shows a strong sexual dimorphism both in the number of neurons and the presence and absence of them (Cachero et al., 2010). Anatomically, the Drosophila brain has multiple regions, some of which have a more prominent role in the modulation of reproductive behavior.
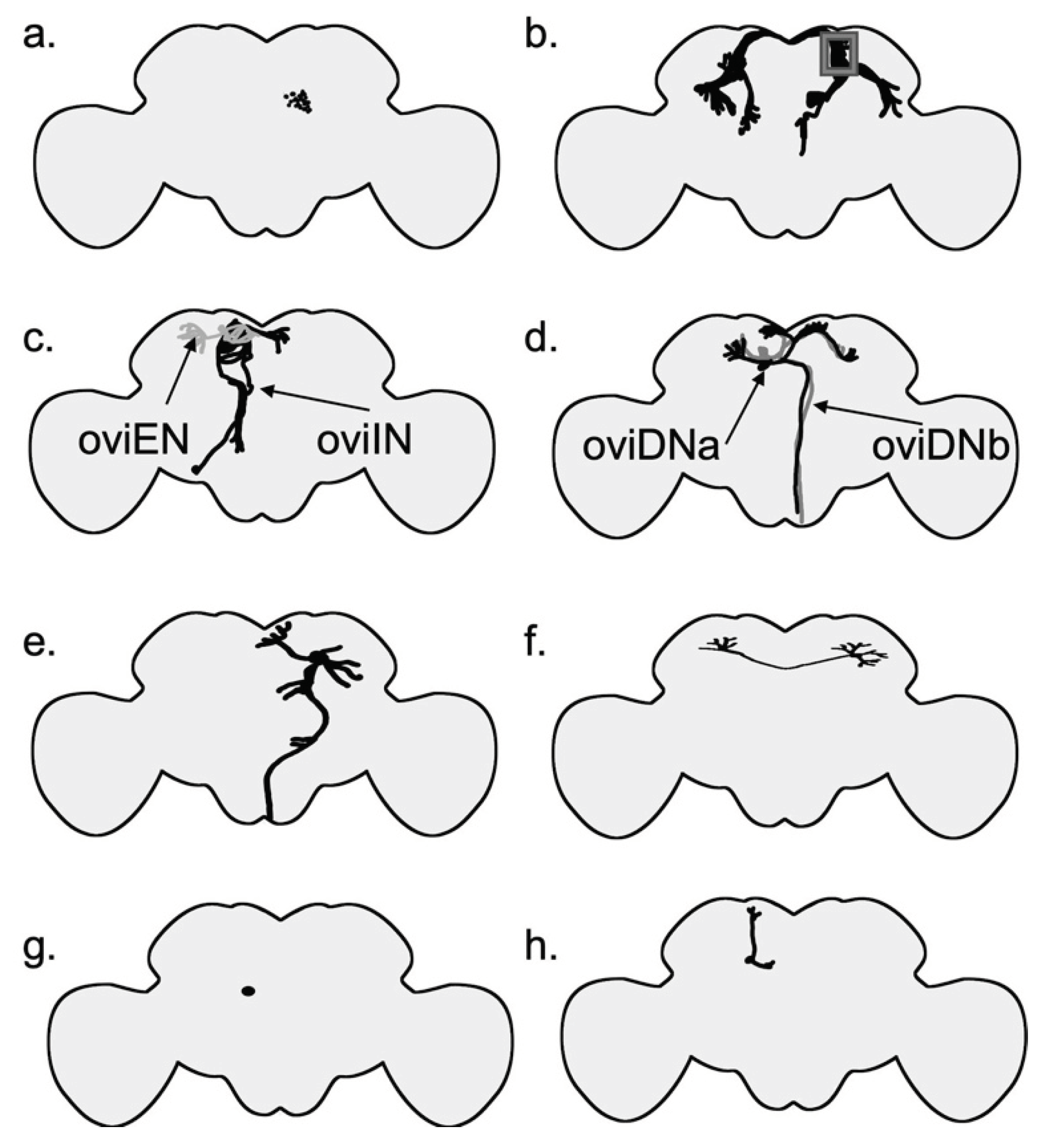
The female brain has several distinct groups of neurons that are important in egg-laying (
Figure 6). For example, pC1 neurons are a group of neurons in the brain that express
dsx (Zhou et al., 2014). P1 neurons are a male-specific subset of pC1 neurons that express both
fru and
dsx genes (K. Kimura et al., 2008). P1 neurons are located near the mushroom body and are composed of 20 interneurons. In females, all P1 neurons die as a result of the female-specific
dsx transcript (K. Kimura et al., 2008). It has been suggested that pC1 neurons receive information of the female reproductive state and regulate the output accordingly (F. Wang, Wang, Forknall, Patrick, et al., 2020) (
Figure 6,
Figure 7). Sex peptide abdominal ganglion (SAG) neurons are one type of these neurons that send information on the female reproductive state to the brain (Feng et al., 2014). In a recent study, Wang et al. (2020) have shown that out of five morphologically different pC1 neurons (pC1a-e) in each hemisphere, three (pC1a-c) have an amplitude of synapses with SAG neurons and the other two have synapses to a lesser degree. Out of the five pC1 neurons, optogenetic activation of SAG neurons induces the strongest activation in pC1a neurons. The neurons in the pC1 cluster have synapses with each other. Experiments have shown that virgin females with ablated pC1 neurons lay more eggs (F. Wang, Wang, Forknall, Parekh, et al., 2020). These findings, along with the fact that cholinergic pC1 neurons have only a few synapses with oviposition descending neurons, suggest that pC1 neurons suppress egg laying in virgin females by an indirect connection. (F. Wang, Wang, Forknall, Patrick, et al., 2020).
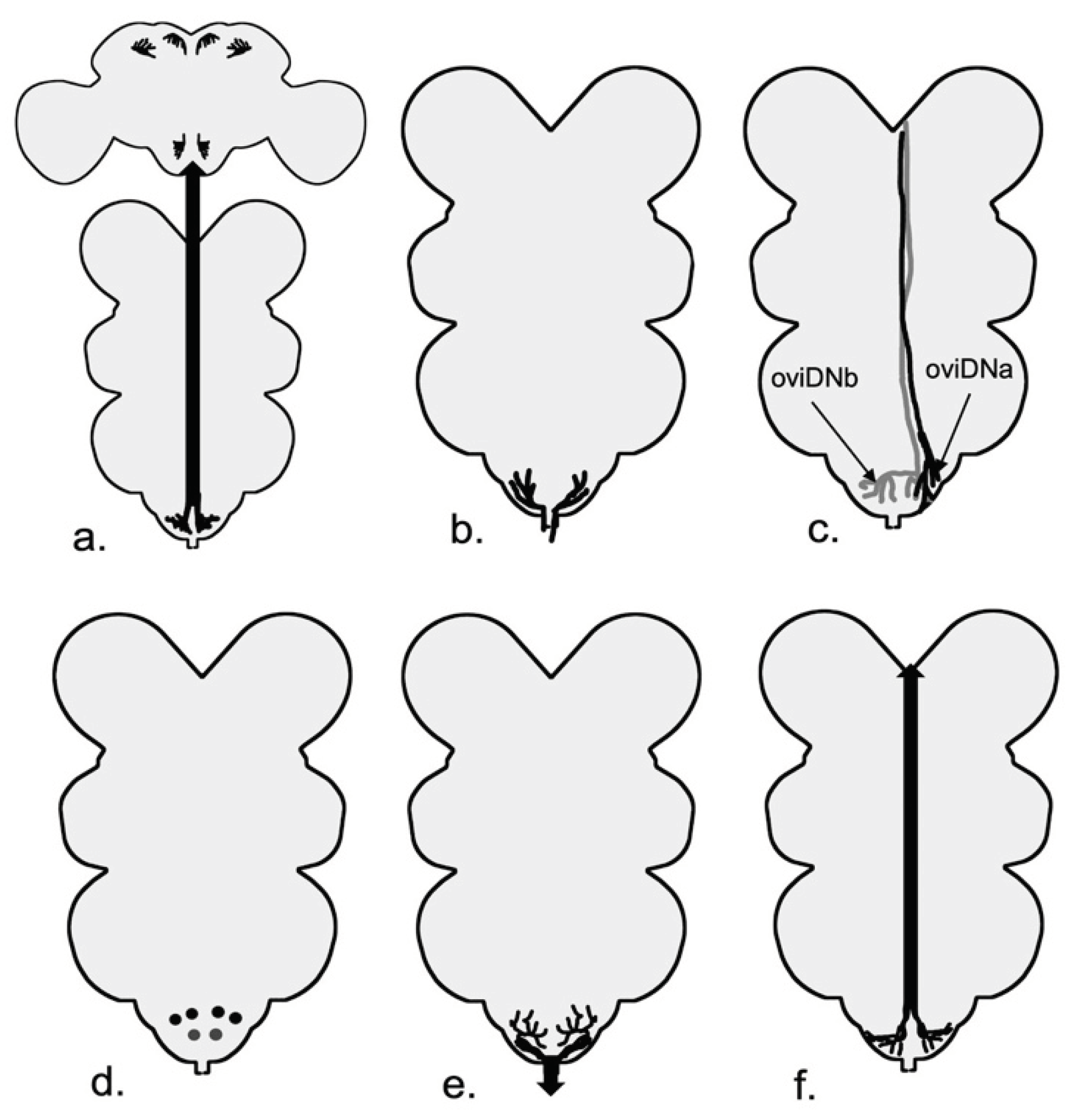
SAG neurons connect directly to pC1 neurons; pC1 neurons, in turn, are connected to oviposition inhibitory neurons (oviINs) (K. Wang et al., 2021) (
Figure 5). There is one oviIN per hemisphere and silencing their activity results in higher egg-laying in virgins, whereas increasing their activity in mated females reduces their egg-laying (K. Wang et al., 2021). The activity of oviposition descending neurons (oviDNs) induces egg deposition behavior (F. Wang, Wang, Forknall, Patrick, et al., 2020). The cell body of these
fru+ dsx- cholinergic neurons resides in the brain, and they innervate the abdominal ganglion in the ventral nerve cord (
Figure 6 and
Figure 7). The two types of oviDNs, ovDNa and oviDNb, have similar arborization in the brain, but differ in their axonal branching in the abdominal ganglion (F. Wang, Wang, Forknall, Patrick, et al., 2020). Furthermore, Vijayan et al. (2023) show that the cell state of oviDNs is a context-dependent phenomenon and relies on both the quality of the substrate and the stage of ovulation in the reproductive tract (
Figure 6). The cholinergic oviposition excitatory neurons (oviENs) send excitatory signals to oviDNs playing a key role in the regulation of egg laying in the process (F. Wang, Wang, Forknall, Patrick, et al., 2020) (
Figure 6).
The dsx-expressing anterior dorsal neuron (aDN) cluster has two glutaminergic (Milyaev et al., 2012) neurons per hemisphere. The aDN has dimorphic dendritic arborizations between the sexes and gives rise to distinct behaviours. Specifically, in the females it is involved in egg-laying site selection using olfactory signals (Nojima et al., 2021). Male aDN receives visual information, whereas the female receives multiple sensory signals, with the main senses being olfactory, thermosensory, and hygrosensory (Nojima et al., 2021). Interestingly, oviposition excitatory neurons (oviENs) have the most synaptic input, including axo-axonic connections to the aDNs. Oviposition inhibitory neurons (oviINs) also form connections with axons of aDNs. Optogenetic activation of olfactory neurons has been shown to increase the calcium signal in the female aDN neurons. Further, this calcium signal is more intense in mated females than in virgin females. Axons of aDN neurons are restricted to the superior medial protocerebrum (SMP) and specifically have a direct output to oviDNa. Another one of the postsynaptic targets is a neuron called SMP156, which suggests that SMP156 might be a neuron joining together different parts of the egg-laying circuit (Nojima et al., 2021). The discovery of aDNs not only provides evidence for the use of sexually divergent sensory input and behavior output by the same neurons in males and females, but also sheds light on another piece of the egg-laying circuit puzzle.
Other noteworthy neurons include pC2l and pMN2 neurons (K. Kimura et al., 2015) (
Figure 6). pMN2 are female-specific
dsx-expressing neurons that are responsible for the extrusion of the ovipositor during oviposition, while pC2l neurons are responsible for the extrusion of the ovipositor during mating (K. Kimura et al., 2015). Kimura et al., (2015) suggest that pMN2 neurons direct egg laying and activate the VNC motor program. Other studies suggest that oviposition command was mis-assigned to pMN2 (Wang et al., 2021 supplement). DNp13 (pMN1) are
dsx+ neurons that control the ovipositor extrusion in mated females and form synaptic connections with SAG neurons and pC1 neurons. Silencing the activity of DNp13 neurons does affect the number of eggs laid (Mezzera et al., 2020) (
Figure 6). The activity of two dorsal paired medial (DPM) neurons is important for maintaining the egg-laying substrate choice, and expression of the gene
amnesiac is necessary for the correct function of these two neurons (Wu et al., 2015) (
Figure 6).
There are Mip-expressing neurons in the brain, as well as VNC, that affect egg-laying. There are an average of 24 Mip-expressing neurons in each hemisphere (Shao et al., 2019). Previously, it has been shown that some MIP neurons affect satiety and affect body weight (Min et al., 2016). There are fru+ MIP neurons in the female brain. However, these neurons show no effect on female receptivity or remating (Jang et al., 2017). Interestingly, Shao et al. (2019) showed that acute photoactivation of brain Mip-expressing neurons can result in complete suppression of egg-laying. Additionally, the act of copulation induces MIP release from some Mip-expressing neurons in the brain (Shao, 2019). These neurons await further characterization of their connection to other key neurons in the circuit. Previously, I discussed that MIPs and SP could activate the same receptor, and that specific Mip-expressing neurons in the brain and VNC modulate egg-laying. But the question remains: Even though MIPs do not induce egg-laying, why are Mip-expressing neurons important in the egg-laying circuit? Could they be remnants of an old circuit that is now highjacked by SP?
Motor Neurons
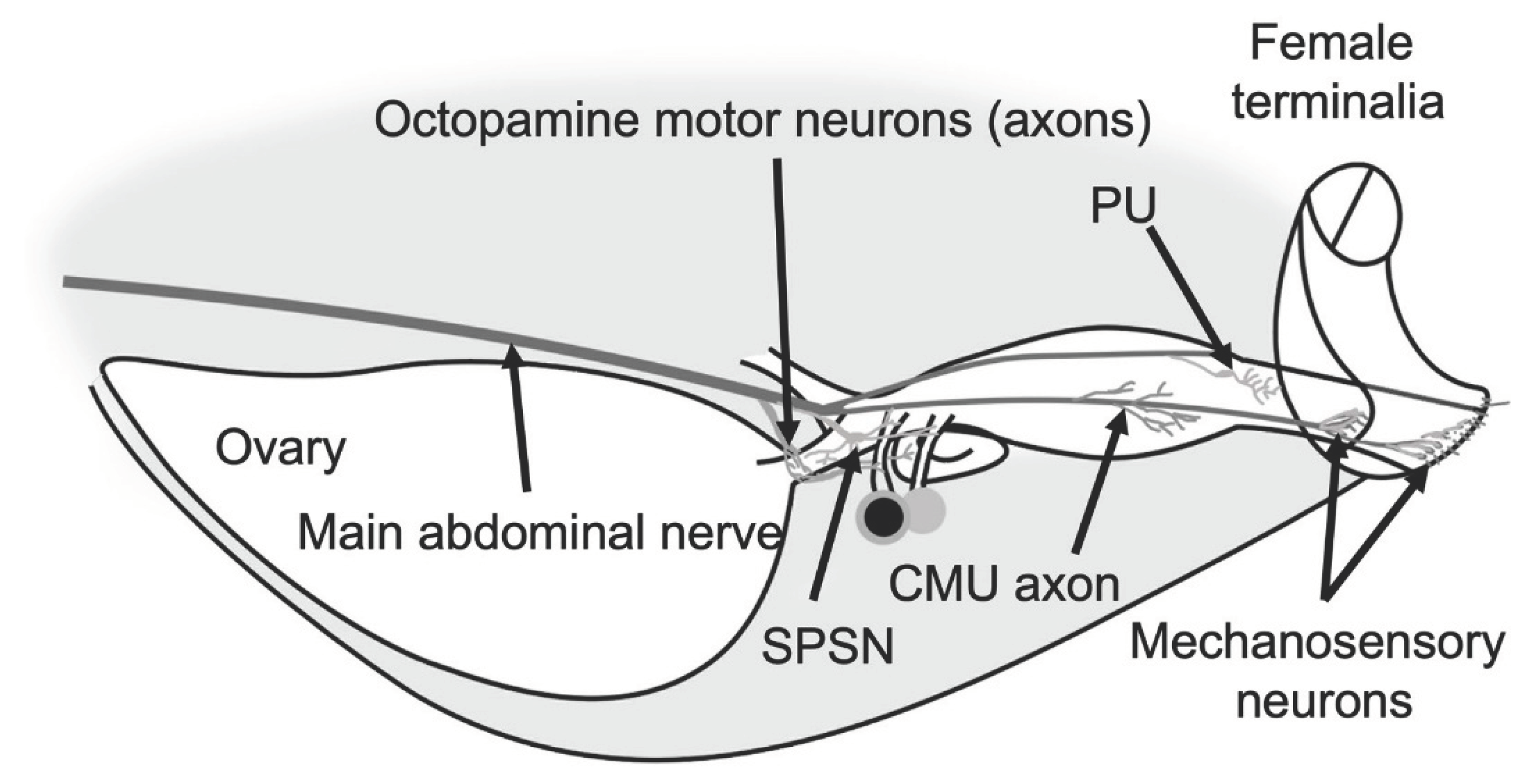
Octopaminergic neurons regulate female reproduction in many insects (White et al., 2021). In the locust,
Locusta migratoria, digging and egg retention are under the control of coordinated central pattern generators (CPGs) in the abdominal ganglion. Octopamine modulates egg laying by inhibiting digging behavior and relaxing the oviducts (R. Wong & Lange, 2014). Ovipositor valve contractions are the result of rhythmic activity of the ovipositor muscle, which in turn is controlled by the initiator and oscillator interneurons in the terminal abdominal ganglion (Ogawa et al., 2011). Even though CPG’s are not commonly studied in terms of female
Drosophila reproduction, studies on other species provide insight on how fruit fly’s egg-laying is modulated. In
D. melanogaster, oviduct contraction is the product of a set of neurons in the abdominal neuromere that is both glutamate and octopamine-releasing. Octopaminergic neurons are inhibitory, whereas glutaminergic neurons excite the muscles (Rodríguez-Valentín et al., 2006). The ring-like muscles rhythmically contract and relax to push the egg forward in the oviduct (Hudson et al., 2008) and subset of
Insulin-like peptide 7 (
Ilp7) expressing neurons are glutaminergic motor neurons that innervate the oviduct and are necessary for egg-laying (Castellanos et al., 2013). Studies have shown that female flies that lack octopamine retain their developed eggs (Monastirioti et al., 1996), while additional studies have shown that the octopamine neurons innervate ovaries and oviducts and trigger ovulation (Monastirioti, 2003). A pair of motor neurons innervating the uterus were recently discovered. The pair of circular muscle of the uterus (CMU) neurons residing in ANm are necessary for uterus constriction during egg-laying sequence and egg expulsion and silencing the CMU neurons reduces the egg-laying (Cury & Axel, 2023) (
Figure 1 and
Figure 7). Whether CMU neurons also incorporate octopamine as a neurotransmitter has yet to be confirmed. Oliveira-Ferreira et al. (2023) identified a broader group of aNM neurons called OvAbg that have sex specific arborizations and modulate egg-laying behaviour in the female. They further clustered the OvAbgs based on the neurotransmitters and show that as a group OvAbgs promote egg deposition and are functionally downstream of oviDNs (Oliveira-Ferreira et al., 2023).
Why Is Neuronal Control of Egg-Laying Important in Light of Evolution?
The neuronal basis of female Drosophila reproductive behavior is less explored compared to some male reproductive behaviors such as courtship. The male reproductive phenotype has been studied extensively with regard to evolutionary pressures. Female reproductive phenotype and, by extension, egg-laying behavior is under the control of natural and sexual selection as well and have direct consequences on not only the individual’s fitness, but also its mating partner. However, males and females have different evolutionary interests, and sexual conflict happens when the optimal outcome of male-female interaction is different for one sex compared to the other (Chapman et al., 2003; Parker, 1979). In Drosophila, sexual conflict can occur due to SFPs and their effect on egg-laying. Females can respond to this conflict by controlling the activity and longevity of SFPs (Sirot et al., 2015) or their sensitivity to them. For example, SP can result in increased mating costs in females by increasing egg production (Wigby & Chapman, 2005). Wensing and Fricke (2018) showed that the egg-laying response to SP varies across wild populations of D. melanogaster. Therefore, the response to SP and the subsequent egg-laying phenotype can be used to measure conflict in Drosophila.
Eberhard brought about the study of cryptic female choice, a process that allows the female to alter a male’s chances to father her offspring (Eberhard, 1996). However, experimental evidence for and against cryptic female choice is difficult to obtain due to its complex nature and overlap with other evolutionary processes like sexual conflict. Egg-laying may be used as an indicator of cryptic female choice. Shining light on the neural control of egg-laying can elucidate the neuronal basis of cryptic female choice. The female nervous system plays an important role in sperm storage in D. melanogaster. For example, Arthur et al. (1998) have shown that females with a masculinized nervous system or isolated abdomens store much less sperm, suggesting evidence for female control at the sperm storage level. Other studies have shown that females egg-laying output is affected by the morphology of a novel trait on male genitalia suggesting a sensory component in the females that assesses mate quality and modulates the egg-laying output based on this signal (Frazee et al., 2021; Frazee & Masly, 2015).
D. melanogaster as a species provides an excellent resource for studying female behavior, and the genus Drosophila offers a wide diversity of reproductive traits that are ideal for studying the evolution of reproductive behaviors (Anholt et al., 2020). This field can benefit from more in-depth studies on neuronal control within D. melanogaster sister species and other members of this genus. Two ideal candidates to study comparative female reproductive behavior are D. suzukii and D. sechellia. These two species present two cases of evolutionary adaptations on egg laying. D. suzukii shows a shift of preference in egg-laying substrate compared to D. melanogaster. D. suzukii prefers ripe fruit, which provides a firm egg-laying substrate compared to rotten fruit. This indicates a shift in the mechanosensory and chemosensory preferences (Durkin et al., 2021; Karageorgi et al., 2017), and it is coupled with a change in the ovipositor morphology (Atallah et al., 2014). In contrast, D. sechellia has a specialized food source and habitat, the Morinda citrifolia shrub, which repels other members of the genus Drosophila (Grunwald Kadow & Gompel, 2020). D. sechellia has the largest eggs size amount the among the D. melanogaster sister species. Its number of ovarioles and rate of egg production has also been reduced when compared to its sister species, each with separate genetic mechanisms (R’Kha et al., 1997). In laboratory cultures D. sechellia egg-laying can be stimulated by adding Morinda to their food. The neuronal basis of D. sechellia’s attraction to morinda has been well studied (Auer et al., 2020; Dekker et al., 2006). However, the underlying neuronal control of egg-laying in D. sechellia is unknown. Due to D. sechellia long retention of the fertilized egg in the uterus (Markow et al., 2009), researchers have struggled with incorporating modern genetic tools such as CRISPR-Cas9 in studying this species but recent studies have successfully incorporated these methods in D. sechellia (Auer et al., 2020).
In conclusion, egg-lying behavior in the fruit fly offers new and exciting avenues to study evolution, reproduction, and decision-making. Special attention needs to be paid to sensory neurons residing in and on the female’s abdomen that sense mating and environmental signals and affect egg-laying. With the newer tools developed for this model organism and their decreased cost, the topic of neuronal control of egg-laying behavior promises new discoveries.
Funding
M.A. was supported in part by funding from NSF IOS 1453642 to J.P. Masly.
Acknowledgments
I thank J.P. Masly and L. Weider for helpful comments on the manuscript, and M. Markham, C. Siler, C. Bourne, and members of the Masly lab for helpful discussion during the course of this work.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Adams, E.M.; Wolfner, M.F. Seminal proteins but not sperm induce morphological changes in the Drosophila melanogaster female reproductive tract during sperm storage. J. Insect Physiol. 2007, 53, 319–331. [Google Scholar] [CrossRef]
- Andrews, J.C.; Fernández, M.P.; Yu, Q.; Leary, G.P.; Leung, A.K.W.; Kavanaugh, M.P.; Kravitz, E.A.; Certel, S.J. Octopamine Neuromodulation Regulates Gr32a-Linked Aggression and Courtship Pathways in Drosophila Males. PLoS Genet. 2014, 10, e1004356. [Google Scholar] [CrossRef]
- Anholt, R.R.H.; O’grady, P.; Wolfner, M.F.; Harbison, S.T. Evolution of Reproductive Behavior. Genetics 2020, 214, 49–73. [Google Scholar] [CrossRef] [PubMed]
- Atallah, J.; Teixeira, L.; Salazar, R.; Zaragoza, G.; Kopp, A. The making of a pest: The evolution of a fruit-penetrating ovipositor in Drosophila suzukii and related species. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132840. [Google Scholar] [CrossRef] [PubMed]
- Auer, T.O.; Khallaf, M.A.; Silbering, A.F.; Zappia, G.; Ellis, K.; Álvarez-Ocaña, R.; Arguello, J.R.; Hansson, B.S.; Jefferis, G.S.X.E.; Caron, S.J.C.; et al. Olfactory receptor and circuit evolution promote host specialization. Nature 2020, 579, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Avila, F.W.; Mattei, A.L.; Wolfner, M.F. Sex peptide receptor is required for the release of stored sperm by mated Drosophila melanogaster females. J. Insect Physiol. 2015, 76, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Avila, F.W.; Sirot, L.K.; LaFlamme, B.A.; Rubinstein, C.D.; Wolfner, M.F. Insect Seminal Fluid Proteins: Identification and Function. Annu. Rev. Èntomol. 2011, 56, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.B.R.; French, V.; Partridge, L. Life-History Consequences of Egg Size in Drosophila Melanogaster. Am. Nat. 1997, 150, 250–282. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.S.; Wolfner, M.F. A molecular analysis of doublesex, a bifunctional gene that controls both male and female sexual differentiation in Drosophila melanogaster. Genes Dev. 1988, 2, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Bang, A.G.; Hartenstein, V.; Posakony, J.W. Hairless is required for the development of adult sensory organ precursor cells in Drosophila. Development 1991, 111, 89–104. [Google Scholar] [CrossRef]
- Bates, A.S.; Janssens, J.; Jefferis, G.S.; Aerts, S. Neuronal cell types in the fly: Single-cell anatomy meets single-cell genomics. Curr. Opin. Neurobiol. 2019, 56, 125–134. [Google Scholar] [CrossRef]
- Bath, E.; Bowden, S.; Peters, C.; Reddy, A.; Tobias, J.A.; Easton-Calabria, E.; Seddon, N.; Goodwin, S.F.; Wigby, S. Sperm and sex peptide stimulate aggression in female Drosophila. Nat. Ecol. Evol. 2017, 1, 0154. [Google Scholar] [CrossRef]
- Bath, E.; Morimoto, J.; Wigby, S. The developmental environment modulates mating-induced aggression and fighting success in adult female Drosophila. Funct. Ecol. 2018, 32, 2542–2552. [Google Scholar] [CrossRef]
- Belgacem, Y.H.; Martin, J.-R. Neuroendocrine control of a sexually dimorphic behavior by a few neurons of the pars intercerebralis in Drosophila. Proc. Natl. Acad. Sci. USA 2002, 99, 15154–15158. [Google Scholar] [CrossRef] [PubMed]
- Bilen, J.; Atallah, J.; Azanchi, R.; Levine, J.D.; Riddiford, L.M. Regulation of onset of female mating and sex pheromone production by juvenile hormone in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2013, 110, 18321–18326. [Google Scholar] [CrossRef] [PubMed]
- Billeter, J.-C.; Rideout, E.J.; Dornan, A.J.; Goodwin, S.F. Control of Male Sexual Behavior in Drosophila by the Sex Determination Pathway. Curr. Biol. 2006, 16, R766–R776. [Google Scholar] [CrossRef] [PubMed]
- Billeter, J.-C.; Wolfner, M.F. Chemical Cues that Guide Female Reproduction in Drosophila melanogaster. J. Chem. Ecol. 2018, 44, 750–769. [Google Scholar] [CrossRef] [PubMed]
- Cachero, S.; Ostrovsky, A.D.; Yu, J.Y.; Dickson, B.J.; Jefferis, G.S.X.E. Sexual Dimorphism in the Fly Brain. Curr. Biol. 2010, 20, 1589–1601. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, M.A.; Diesner, M.; Schachtner, J.; Nässel, D.R. Multiple neuropeptides in the Drosophila antennal lobe suggest complex modulatory circuits. J. Comp. Neurol. 2010, 518, 3359–3380. [Google Scholar] [CrossRef]
- Casares, F.; Sánchez, L.; Guerrero, I.; Sánchez-Herrero, E. The genital disc of Drosophila melanogaster. Dev. Genes Evol. 1997, 207, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, M.C.; Tang, J.C.Y.; Allan, D.W. Female-biased dimorphism underlies a female-specific role for post-embryonic Ilp7 neurons inDrosophilafertility. Development 2013, 140, 3915–3926. [Google Scholar] [CrossRef]
- Chapman, T.; Arnqvist, G.; Bangham, J.; Rowe, L. Sexual conflict. Trends Ecol. Evol. 2003, 18, 41–47. [Google Scholar] [CrossRef]
- Chiang, A.-S.; Burns, E.L.; Schal, C. Ovarian regulation of cyclic changes in size and activity of corpus allatum cells in Blattella germanica. J. Insect Physiol. 1991, 37, 907–917. [Google Scholar] [CrossRef]
- Chow, C.Y.; Wolfner, M.F.; Clark, A.G. The Genetic Basis for Male × Female Interactions Underlying Variation in Reproductive Phenotypes of Drosophila. Genetics 2010, 186, 4. [Google Scholar] [CrossRef]
- Court, R., Namiki, S., Armstrong, J. D., Börner, J., Card, G., Costa, M., Dickinson, M., Duch, C., Korff, W., Mann, R., Merritt, D., Murphey, R. K., Seeds, A. M., Shirangi, T., Simpson, J. H., Truman, J. W., Tuthill, J. C., Williams, D. W., & Shepherd, D. A Systematic Nomenclature for the Drosophila Ventral Nerve Cord. Neuron 2020, 107, 1071–1079. [CrossRef] [PubMed]
- Crava, C. M., Zanini, D., Amati, S., Sollai, G., Crnjar, R., Paoli, M., Rossi-Stacconi, M. V., Rota-Stabelli, O., Tait, G., Haase, A., Romani, R., & Anfora, G. Structural and transcriptional evidence of mechanotransduction in the Drosophila suzukii ovipositor. J. Insect Physiol. 2020, 125, 104088. [CrossRef] [PubMed]
- Cury, K.M.; Axel, R. Flexible neural control of transition points within the egg-laying behavioral sequence in Drosophila. Nat. Neurosci. 2023, 26, 1054–1067. [Google Scholar] [CrossRef] [PubMed]
- DeFazio, R.A.; Moenter, S.M. Estradiol Feedback Alters Potassium Currents and Firing Properties of Gonadotropin-Releasing Hormone Neurons. Mol. Endocrinol. 2002, 16, 2255–2265. [Google Scholar] [CrossRef] [PubMed]
- Dekker, T.; Ibba, I.; Siju, K.; Stensmyr, M.C.; Hansson, B.S. Olfactory Shifts Parallel Superspecialism for Toxic Fruit in Drosophila melanogaster Sibling, D. sechellia. Curr. Biol. 2006, 16, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Demir, E.; Dickson, B.J. Fruitless Splicing Specifies Male Courtship Behavior in Drosophila. Cell 2005, 121, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, D., Pacheco, D., Encarnacion-Rivera, L., Pereira, T., Fathy, R., Clemens, J., Girardin, C., Calhoun, A., Ireland, E., Burke, A., Dorkenwald, S., McKellar, C., Macrina, T., Lu, R., Lee, K., Kemnitz, N., Ih, D., Castro, M., Halageri, A., … Murthy, M. The neural basis for a persistent internal state in Drosophila females. eLife 2020, 9, e59502. [CrossRef]
- Domínguez, M.; Campuzano, S. asense, a member of the Drosophila achaete-scute complex, is a proneural and neural differentiation gene. EMBO J. 1993, 12, 2049–2060. [Google Scholar] [CrossRef]
- Dorkenwald, S., McKellar, C. E., Macrina, T., Kemnitz, N., Lee, K., Lu, R., Wu, J., Popovych, S., Mitchell, E., Nehoran, B., Jia, Z., Bae, J. A., Mu, S., Ih, D., Castro, M., Ogedengbe, O., Halageri, A., Kuehner, K., Sterling, A. R., … Seung, H. S. FlyWire: Online community for whole-brain connectomics. Nat. Methods 2022, 19, 119–128. [CrossRef]
- Duckhorn, J.C.; Cande, J.; Metkus, M.C.; Song, H.; Altamirano, S.; Stern, D.L.; Shirangi, T.R. Regulation of Drosophila courtship behavior by the Tlx/tailless-like nuclear receptor, dissatisfaction. Curr. Biol. 2022, 32, 1703–1714.e3. [Google Scholar] [CrossRef]
- Duménil, C.; Woud, D.; Pinto, F.; Alkema, J.T.; Jansen, I.; Van Der Geest, A.M.; Roessingh, S.; Billeter, J.-C. Pheromonal Cues Deposited by Mated Females Convey Social Information about Egg-Laying Sites in Drosophila Melanogaster. J. Chem. Ecol. 2016, 42, 259–269. [Google Scholar] [CrossRef]
- Durkin, S.M.; Chakraborty, M.; Abrieux, A.; Lewald, K.M.; Gadau, A.; Svetec, N.; Peng, J.; Kopyto, M.; Langer, C.B.; Chiu, J.C.; et al. Behavioral and Genomic Sensory Adaptations Underlying the Pest Activity of Drosophila suzukii. Mol. Biol. Evol. 2021, 38, 2532–2546. [Google Scholar] [CrossRef]
- Eberhard, W. G. (1996). Female control: Sexual selection by cryptic female choice. Princeton University Press.
- Eisses, K.T. The influence of 2-propanol and acetone on oviposition rate and oviposition site preference for acetic acid and ethanol of Drosophila melanogaster. Behav. Genet. 1997, 27, 171–180. [Google Scholar] [CrossRef]
- Engels, W.R.; Preston, C.R. HYBRID DYSGENESIS IN DROSOPHILA MELANOGASTER: THE BIOLOGY OF FEMALE AND MALE STERILITY. Genetics 1979, 92, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.S.; Cline, T.W. Drosophila switch gene Sex-lethal can bypass its switch-gene target transformer to regulate aspects of female behavior. Proc. Natl. Acad. Sci. USA 2013, 110. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Manoli, D.S.; Ahmed, O.M.; Chen, Y.; Agarwal, N.; Kwong, S.; Cai, A.G.; Neitz, J.; Renslo, A.; Baker, B.S.; et al. Genetic and Neural Mechanisms that Inhibit Drosophila from Mating with Other Species. Cell 2013, 154, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Palfreyman, M.T.; Häsemeyer, M.; Talsma, A.; Dickson, B.J. Ascending SAG Neurons Control Sexual Receptivity of Drosophila Females. Neuron 2014, 83, 135–148. [Google Scholar] [CrossRef]
- Fowler, E.K.; Leigh, S.; Rostant, W.G.; Thomas, A.; Bretman, A.; Chapman, T. Memory of social experience affects female fecundity via perception of fly deposits. BMC Biol. 2022, 20, 244. [Google Scholar] [CrossRef] [PubMed]
- Frazee, S.R.; Harper, A.R.; Afkhami, M.; Wood, M.L.; McCrory, J.C.; Masly, J.P. Interspecific introgression reveals a role of male genital morphology during the evolution of reproductive isolation in Drosophila. Evolution 2021, 75, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Frazee, S.R.; Masly, J.P. Multiple sexual selection pressures drive the rapid evolution of complex morphology in a male secondary genital structure. Ecol. Evol. 2015, 5, 4437–4450. [Google Scholar] [CrossRef]
- Garaulet, D.L.; Moro, A.; Lai, E.C. A double-negative gene regulatory circuit underlies the virgin behavioral state. Cell Rep. 2021, 36, 109335. [Google Scholar] [CrossRef] [PubMed]
- Garbe, D.S.; Vigderman, A.S.; Moscato, E.; Dove, A.E.; Vecsey, C.G.; Kayser, M.S.; Sehgal, A. Changes in Female Drosophila Sleep following Mating Are Mediated by SPSN-SAG Neurons. J. Biol. Rhythm. 2016, 31, 551–567. [Google Scholar] [CrossRef] [PubMed]
- García-Bellido, A.; Santamaria, P. DEVELOPMENTAL ANALYSIS OF THE ACHAETE-SCUTE SYSTEM OF DROSOPHILA MELANOGASTER. Genetics 1978, 88, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Goto, J.; Mikawa, Y.; Koganezawa, M.; Ito, H.; Yamamoto, D. Sexually Dimorphic Shaping of Interneuron Dendrites Involves the Hunchback Transcription Factor. J. Neurosci. 2011, 31, 5454–5459. [Google Scholar] [CrossRef] [PubMed]
- Grossfield, J.; Sakri, B. Divergence in the neural control of oviposition in Drosophila. J. Insect Physiol. 1972, 18, 237–241. [Google Scholar] [CrossRef]
- Grunwald Kadow, I.C.; Gompel, N. Sensory Evolution: Making Sense of the Noni Scent. Curr. Biol. 2020, 30, R712–R715. [Google Scholar] [CrossRef]
- Guntur, A.R.; Gou, B.; Gu, P.; He, R.; Stern, U.; Xiang, Y.; Yang, C.-H. H2O2-Sensitive Isoforms of Drosophila melanogaster TRPA1 Act in Bitter-Sensing Gustatory Neurons to Promote Avoidance of UV During Egg-Laying. Genetics 2017, 205, 749–759. [Google Scholar] [CrossRef]
- Gwadz, R.W.; Spielman, A. Corpus allatum control of ovarian development in Aedes aegypti. J. Insect Physiol. 1973, 19, 1441–1448. [Google Scholar] [CrossRef]
- Hall, J.C.; Greenspan, R.J. GENETIC ANALYSIS OF DROSOPHILA NEUROBIOLOGY. Annu. Rev. Genet. 1979, 13, 127–195. [Google Scholar] [CrossRef]
- Harshman, L.G.; Zera, A.J. The cost of reproduction: The devil in the details. Trends Ecol. Evol. 2007, 22, 80–86. [Google Scholar] [CrossRef]
- Hasebe, M.; Shiga, S. Oviposition-promoting pars intercerebralis neurons show period-dependent photoperiodic changes in their firing activity in the bean bug. Proc. Natl. Acad. Sci. USA 2021, 118, e2018823118. [Google Scholar] [CrossRef]
- Häsemeyer, M.; Yapici, N.; Heberlein, U.; Dickson, B.J. Sensory Neurons in the Drosophila Genital Tract Regulate Female Reproductive Behavior. Neuron 2009, 61, 511–518. [Google Scholar] [CrossRef]
- Haussmann, I.U.; Hemani, Y.; Wijesekera, T.; Dauwalder, B.; Soller, M. Multiple pathways mediate the sex-peptide-regulated switch in female Drosophila reproductive behaviours. Proc. R. Soc. B Biol. Sci. 2013, 280, 20131938. [Google Scholar] [CrossRef] [PubMed]
- Hehlert, P.; Zhang, W.; Göpfert, M.C. Drosophila Mechanosensory Transduction. Trends Neurosci. 2021, 44, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Heifetz, Y.; Lung, O.; Frongillo, E.A.; Wolfner, M.F. The Drosophila seminal fluid protein Acp26Aa stimulates release of oocytes by the ovary. Curr. Biol. 2000, 10, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Heifetz, Y.; Wolfner, M.F. Mating, seminal fluid components, and sperm cause changes in vesicle release in the Drosophila female reproductive tract. Proc. Natl. Acad. Sci. USA 2004, 101, 6261–6266. [Google Scholar] [CrossRef] [PubMed]
- A Herndon, L.; Wolfner, M.F. A Drosophila seminal fluid protein, Acp26Aa, stimulates egg laying in females for 1 day after mating. Proc. Natl. Acad. Sci. USA 1995, 92, 10114–10118. [Google Scholar] [CrossRef]
- Hudson, A.M.; Petrella, L.N.; Tanaka, A.J.; Cooley, L. Mononuclear muscle cells in Drosophila ovaries revealed by GFP protein traps. Dev. Biol. 2008, 314, 329–340. [Google Scholar] [CrossRef]
- Isaac, R.E.; Kim, Y.-J.; Audsley, N. The degradome and the evolution of Drosophila sex peptide as a ligand for the MIP receptor. Peptides 2014, 53, 258–264. [Google Scholar] [CrossRef]
- Ishimoto, H.; Kamikouchi, A. Molecular and neural mechanisms regulating sexual motivation of virgin female Drosophila. Cell. Mol. Life Sci. 2021, 78, 4805–4819. [Google Scholar] [CrossRef]
- Jafar-Nejad, H. Senseless and Daughterless confer neuronal identity to epithelial cells in theDrosophilawing margin. Development 2006, 133, 1683–1692. [Google Scholar] [CrossRef]
- Jang, Y.-H.; Chae, H.-S.; Kim, Y.-J. Female-specific myoinhibitory peptide neurons regulate mating receptivity in Drosophila melanogaster. Nat. Commun. 2017, 8, 1630–1630. [Google Scholar] [CrossRef]
- Joseph, R.M.; Heberlein, U. Tissue-Specific Activation of a Single Gustatory Receptor Produces Opposing Behavioral Responses in Drosophila. Genetics 2012, 192, 521–532. [Google Scholar] [CrossRef]
- Jowett, T.; Postlethwait, J.H. The regulation of yolk polypeptide synthesis in Drosophila ovaries and fat body by 20-hydroxyecdysone and a juvenile hormone analog. Dev. Biol. 1980, 80, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Karageorgi, M., Bräcker, L. B., Lebreton, S., Minervino, C., Cavey, M., Siju, K. P., Grunwald Kadow, I. C., Gompel, N., & Prud’homme, B. Evolution of Multiple Sensory Systems Drives Novel Egg-Laying Behavior in the Fruit Pest Drosophila suzukii. Curr. Biol. 2017, 27, 847–853. [CrossRef] [PubMed]
- Keisman, E.L.; Christiansen, A.E.; Baker, B.S. The Sex Determination Gene doublesex Regulates the A/P Organizer to Direct Sex-Specific Patterns of Growth in the Drosophila Genital Imaginal Disc. Dev. Cell 2001, 1, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Kienzle, R.; Groß, L.B.; Caughman, S.; Rohlfs, M. Resource use by individual Drosophila suzukii reveals a flexible preference for oviposition into healthy fruits. Sci. Rep. 2020, 10, 3132. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Bartalska, K.; Audsley, N.; Yamanaka, N.; Yapici, N.; Lee, J.-Y.; Kim, Y.-C.; Markovic, M.; Isaac, E.; Tanaka, Y.; et al. MIPs are ancestral ligands for the sex peptide receptor. Proc. Natl. Acad. Sci. USA 2010, 107, 6520–6525. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Hachiya, T.; Koganezawa, M.; Tazawa, T.; Yamamoto, D. Fruitless and Doublesex Coordinate to Generate Male-Specific Neurons that Can Initiate Courtship. Neuron 2008, 59, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Sato, C.; Koganezawa, M.; Yamamoto, D. Drosophila Ovipositor Extension in Mating Behavior and Egg Deposition Involves Distinct Sets of Brain Interneurons. PLoS ONE 2015, 10, e0126445. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.-I.; Ote, M.; Tazawa, T.; Yamamoto, D. Fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature 2005, 438, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Kubli, E. Sex-peptides: seminal peptides of the Drosophila male. Cell. Mol. Life Sci. 2003, 60, 1689–1704. [Google Scholar] [CrossRef] [PubMed]
- Kurz, C.L.; Charroux, B.; Chaduli, D.; Viallat-Lieutaud, A.; Royet, J. Peptidoglycan sensing by octopaminergic neurons modulates Drosophila oviposition. eLife 2017, 6, e21937. [Google Scholar] [CrossRef]
- Kvitsiani, D.; Dickson, B.J. Shared neural circuitry for female and male sexual behaviours in Drosophila. Curr. Biol. 2006, 16, R355–R356. [Google Scholar] [CrossRef]
- LaFever, L.; Drummond-Barbosa, D. Direct Control of Germline Stem Cell Division and Cyst Growth by Neural Insulin in Drosophila. Science 2005, 309, 1071–1073. [Google Scholar] [CrossRef]
- Lee, H.; Choi, H.W.; Zhang, C.; Park, Z.-Y.; Kim, Y.-J. A Pair of Oviduct-Born Pickpocket Neurons Important for Egg-Laying in Drosophila melanogaster. Mol. Cells 2016, 39, 573–579. [Google Scholar] [CrossRef]
- LeVasseur-Viens, H.; Polak, M.; Moehring, A.J. No evidence for external genital morphology affecting cryptic female choice and reproductive isolation in Drosophila: GENITAL SHAPE AND SEXUAL SELECTION. Evolution 2015, 69, 1797–1807. [Google Scholar] [CrossRef]
- Lin, C.-C.; A Prokop-Prigge, K.; Preti, G.; Potter, C.J. Food odors trigger Drosophila males to deposit a pheromone that guides aggregation and female oviposition decisions. eLife 2015, 4, e08688. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, B.; Zhang, L.; Yang, T.; Zhang, Z.; Gao, Z.; Zhang, W. A neural circuit encoding mating states tunes defensive behavior in Drosophila. Nat. Commun. 2020, 11, 3962. [Google Scholar] [CrossRef]
- Manning, A. The control of sexual receptivity in female Drosophila. Anim. Behav. 1967, 15, 239–250. [Google Scholar] [CrossRef]
- Markow, T.A.; Beall, S.; Matzkin, L.M. Egg size, embryonic development time and ovoviviparity in Drosophila species: Ovoviviparity in Drosophila species. J. Evol. Biol. 2009, 22, 430–434. [Google Scholar] [CrossRef]
- Matsumoto, S.; Brown, M.R.; Suzuki, A.; Lea, A.O. Isolation and characterization of ovarian ecdysteroidogenic hormones from the mosquito, Aedes aegypti. Insect Biochem. 1989, 19, 651–656. [Google Scholar] [CrossRef]
- McMillan, I.; Fitz-Earle, M.; Robson, D.S. QUANTITATIVE GENETICS OF FERTILITY I. LIFETIME EGG PRODUCTION OF DROSOPHILA MELANOGASTER—THEORETICAL. Genetics 1970, 65, 349–353. [Google Scholar] [CrossRef] [PubMed]
- McQueen, E. W., Afkhami, M., Atallah, J., Belote, J. M., Gompel, N., Heifetz, Y., Kamimura, Y., Kornhauser, S. C., Masly, J. P., O’Grady, P., Peláez, J., Rebeiz, M., Rice, G., Sánchez-Herrero, E., Santos Nunes, M. D., Santos Rampasso, A., Schnakenberg, S. L., Siegal, M. L., Takahashi, A., … Yassin, A. A standardized nomenclature and atlas of the female terminalia of Drosophila melanogaster. Fly 2022, 16, 128–151. [CrossRef] [PubMed]
- Mezzera, C.; Brotas, M.; Gaspar, M.; Pavlou, H.J.; Goodwin, S.F.; Vasconcelos, M.L. Ovipositor Extrusion Promotes the Transition from Courtship to Copulation and Signals Female Acceptance in Drosophila melanogaster. Curr. Biol. 2020, 30, 3736–3748. [Google Scholar] [CrossRef] [PubMed]
- Milyaev, N.; Osumi-Sutherland, D.; Reeve, S.; Burton, N.; Baldock, R.A.; Armstrong, J.D. The Virtual Fly Brain browser and query interface. Bioinformatics 2012, 28, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Chae, H.-S.; Jang, Y.-H.; Choi, S.; Lee, S.; Jeong, Y.T.; Jones, W.D.; Moon, S.J.; Kim, Y.-J.; Chung, J. Identification of a Peptidergic Pathway Critical to Satiety Responses in Drosophila. Curr. Biol. 2016, 26, 814–820. [Google Scholar] [CrossRef]
- Mirth, C.K.; Alves, A.N.; Piper, M.D.W. Turning food into eggs: insights from nutritional biology and developmental physiology of Drosophila. Curr. Opin. Insect Sci. 2019, 31, 49–57. [Google Scholar] [CrossRef]
- Monastirioti, M. Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster. Dev. Biol. 2003, 264, 38–49. [Google Scholar] [CrossRef]
- Monastirioti, M.; Linn, J.C.E.; White, K. Characterization ofDrosophila Tyramine β-HydroxylaseGene and Isolation of Mutant Flies Lacking Octopamine. J. Neurosci. 1996, 16, 3900–3911. [Google Scholar] [CrossRef]
- Moshitzky, P. , Fleischmann, I., Chaimov, N., Saudan, P., Klauser, S., Kubli, E., & Applebaum, S. W. (1996). Sex-peptide activates juvenile hormone biosynthesis in the Drosophila melanogaster corpus allatum. Archives of Insect Biochemistry and Physiology, 32(3–4), 363–374. [CrossRef]
- Nakamura, M.; Okano, H.; Blendy, J.A.; Montell, C. Musashi, a neural RNA-binding protein required for Drosophila adult external sensory organ development. Neuron 1994, 13, 67–81. [Google Scholar] [CrossRef]
- Newell, N.R.; Ray, S.; E Dalton, J.; Fortier, J.C.; Kao, J.Y.; Chang, P.L.; Nuzhdin, S.V.; Arbeitman, M.N. The Drosophila Post-mating Response: Gene Expression and Behavioral Changes Reveal Perdurance and Variation in Cross-Tissue Interactions. G3 Genes|Genomes|Genetics 2020, 10, 967–983. [Google Scholar] [CrossRef]
- Nijhout, H.F. (2021). Insect Hormones. Princeton University Press. [CrossRef]
- Nojima, T.; Rings, A.; Allen, A.M.; Otto, N.; Verschut, T.A.; Billeter, J.-C.; Neville, M.C.; Goodwin, S.F. A sex-specific switch between visual and olfactory inputs underlies adaptive sex differences in behavior. Curr. Biol. 2021, 31, 1175–1191.e6. [Google Scholar] [CrossRef]
- Ogawa, H.; Kagaya, K.; Saito, M.; Yamaguchi, T. Neural mechanism for generating and switching motor patterns of rhythmic movements of ovipositor valves in the cricket. J. Insect Physiol. 2011, 57, 326–338. [Google Scholar] [CrossRef]
- Oliveira-Ferreira, C.; Gaspar, M.; Vasconcelos, M.L. Neuronal substrates of egg-laying behaviour at the abdominal ganglion of Drosophila melanogaster. Sci. Rep. 2023, 13, 21941. [Google Scholar] [CrossRef]
- Palavicino-Maggio, C.B.; Chan, Y.-B.; McKellar, C.; Kravitz, E.A. A small number of cholinergic neurons mediate hyperaggression in female Drosophila. Proc. Natl. Acad. Sci. USA 2019, 116, 17029–17038. [Google Scholar] [CrossRef]
- Park, J.-H.; Kwon, J.Y. A systematic analysis of Drosophila gustatory receptor gene expression in abdominal neurons which project to the central nervous system. Mol. Cells 2011, 32, 375–381. [Google Scholar] [CrossRef]
- Parker, G. A. (1979). SEXUAL SELECTION AND SEXUAL CONFLICT. In Sexual Selection and Reproductive Competition in Insects (pp. 123–166). Elsevier. [CrossRef]
- Partridge, L.; Fowler, K.; Trevitt, S.; Sharp, W. An examination of the effects of males on the survival and egg-production rates of female Drosophila melanogaster. J. Insect Physiol. 1986, 32, 925–929. [Google Scholar] [CrossRef]
- Partridge, L.; Green, A.; Fowler, K. Effects of egg-production and of exposure to males on female survival in Drosophila melanogaster. J. Insect Physiol. 1987, 33, 745–749. [Google Scholar] [CrossRef]
- Price, C.S.C.; Kim, C.H.; Gronlund, C.J.; Coyne, J.A. CRYPTIC REPRODUCTIVE ISOLATION IN THE DROSOPHILA SIMULANS SPECIES COMPLEX. Evolution 2001, 55, 81–92. [Google Scholar] [CrossRef]
- Poels J., T. Van Loy, H. P. Vandersmissen, B. Van Hiel, S. Van Soest, et al., Myoinhibiting peptides are the ancestral ligands of the promiscuous Drosophila sex peptide receptor. Cell. Mol. Life Sci. 2010, 67, 3511–3522. [CrossRef]
- Reguera, P. Flexible oviposition behavior in the golden egg bug (Phyllomorpha laciniata) and its implications for offspring survival. Behav. Ecol. 2002, 13, 70–74. [Google Scholar] [CrossRef]
- Rezával, C.; Nojima, T.; Neville, M.C.; Lin, A.C.; Goodwin, S.F. Sexually Dimorphic Octopaminergic Neurons Modulate Female Postmating Behaviors in Drosophila. Curr. Biol. 2014, 24, 725–730. [Google Scholar] [CrossRef]
- Rezával, C.; Pavlou, H.J.; Dornan, A.J.; Chan, Y.-B.; Kravitz, E.A.; Goodwin, S.F. Neural Circuitry Underlying Drosophila Female Postmating Behavioral Responses. Curr. Biol. 2012, 22, 1155–1165. [Google Scholar] [CrossRef]
- Ribeiro, C.; Dickson, B.J. Sex Peptide Receptor and Neuronal TOR/S6K Signaling Modulate Nutrient Balancing in Drosophila. Curr. Biol. 2010, 20, 1000–1005. [Google Scholar] [CrossRef]
- Rideout, E.J.; Dornan, A.J.; Neville, M.C.; Eadie, S.; Goodwin, S.F. Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nat. Neurosci. 2010, 13, 458–466. [Google Scholar] [CrossRef]
- R'Kha, S.; Moreteau, B.; Coyne, J.A.; David, J.R. Evolution of a lesser fitness trait: Egg production in the specialist Drosophila sechellia. Genet. Res. 1997, 69, 17–23. [Google Scholar] [CrossRef]
- Rodríguez-Valentín, R.; López-González, I.; Jorquera, R.; Labarca, P.; Zurita, M.; Reynaud, E. Oviduct contraction in Drosophila is modulated by a neural network that is both, octopaminergic and glutamatergic. J. Cell. Physiol. 2006, 209, 183–198. [Google Scholar] [CrossRef]
- Rosenheim, J.A.; Heimpel, G.E.; Mangel, M. Egg maturation, egg resorption and the costliness of transient egg limitation in insects. Proc. R. Soc. B: Biol. Sci. 2000, 267, 1565–1573. [Google Scholar] [CrossRef]
- Roy, S.; Saha, T.T.; Zou, Z.; Raikhel, A.S. Regulatory Pathways Controlling Female Insect Reproduction. Annu. Rev. Èntomol. 2018, 63, 489–511. [Google Scholar] [CrossRef]
- Rubinstein, C.D.; Wolfner, M.F. Drosophila seminal protein ovulin mediates ovulation through female octopamine neuronal signaling. Proc. Natl. Acad. Sci. USA 2013, 110, 17420–17425. [Google Scholar] [CrossRef]
- Sanders, L.E.; Arbeitman, M.N. Doublesex establishes sexual dimorphism in the Drosophila central nervous system in an isoform-dependent manner by directing cell number. Dev. Biol. 2008, 320, 378–390. [Google Scholar] [CrossRef]
- Sato, K.; Yamamoto, D. Mutually exclusive expression of sex-specific and non-sex-specific fruitless gene products in the Drosophila central nervous system. Gene Expr. Patterns 2022, 43, 119232. [Google Scholar] [CrossRef]
- Sawala, A.; Gould, A.P. The sex of specific neurons controls female body growth in Drosophila. PLoS Biol. 2017, 15, e2002252. [Google Scholar] [CrossRef]
- Schlegel, P.; Costa, M.; Jefferis, G.S. Learning from connectomics on the fly. Curr. Opin. Insect Sci. 2017, 24, 96–105. [Google Scholar] [CrossRef]
- Schwenke, R.A.; Lazzaro, B.P. Juvenile Hormone Suppresses Resistance to Infection in Mated Female Drosophila melanogaster. Curr. Biol. 2017, 27, 596–601. [Google Scholar] [CrossRef]
- Shao, L.; Chung, P.; Wong, A.; Siwanowicz, I.; Kent, C.F.; Long, X.; Heberlein, U. A Neural Circuit Encoding the Experience of Copulation in Female Drosophila. Neuron 2019, 102, 1025–1036. [Google Scholar] [CrossRef]
- Shapiro, A. B., Wheelock, G. D., Hagedorn, H. H., Baker, F. C., Tsai, L. W., & Schooley, D. A. Juvenile hormone and juvenile hormone esterase in adult females of the mosquito Aedes aegypti. J. Insect Physiol. 1986, 32, 867–877. [CrossRef]
- Shapiro, H. THE RATE OF OVIPOSITION IN THE FRUIT FLY, DROSOPHILA. Biol. Bull. 1932, 63, 456–471. [Google Scholar] [CrossRef]
- Shapiro, J.P.; Hagedorn, H.H. Juvenile hormone and the development of ovarian responsiveness to a brain hormone in the mosquito, Aedes aegypti. Gen. Comp. Endocrinol. 1982, 46, 176–183. [Google Scholar] [CrossRef]
- Shelly, T.E. Defense of Oviposition Sites by Female Oriental Fruit Flies (Diptera: Tephritidae). Fla. Èntomol. 1999, 82, 339. [Google Scholar] [CrossRef]
- Shirangi, T.R.; Taylor, B.J.; McKeown, M. A double-switch system regulates male courtship behavior in male and female Drosophila melanogaster. Nat. Genet. 2006, 38, 1435–1439. [Google Scholar] [CrossRef]
- Simpson, P. Lateral inhibition and the development of the sensory bristles of the adult peripheral nervous system of Drosophila. Development 1990, 109, 509–519. [Google Scholar] [CrossRef]
- Sirot, L.K.; Wong, A.; Chapman, T.; Wolfner, M.F. Sexual Conflict and Seminal Fluid Proteins: A Dynamic Landscape of Sexual Interactions. Cold Spring Harb. Perspect. Biol. 2015, 7, a017533. [Google Scholar] [CrossRef]
- Soller, M.; Bownes, M.; Kubli, E. Mating and Sex Peptide Stimulate the Accumulation of Yolk in Oocytes of Drosophila Melanogaster. JBIC J. Biol. Inorg. Chem. 1997, 243, 732–738. [Google Scholar] [CrossRef]
- Stensmyr, M.C.; Dweck, H.K.; Farhan, A.; Ibba, I.; Strutz, A.; Mukunda, L.; Linz, J.; Grabe, V.; Steck, K.; Lavista-Llanos, S.; et al. A Conserved Dedicated Olfactory Circuit for Detecting Harmful Microbes in Drosophila. Cell 2012, 151, 1345–1357. [Google Scholar] [CrossRef]
- Sturtevant, A.H. GENETIC STUDIES ON DROSOPHILA SIMULANS. I. INTRODUCTION. HYBRIDS WITH DROSOPHILA MELANOGASTER. Genetics 1920, 5, 488–500. [Google Scholar] [CrossRef]
- Tantawy, A.O.; Vetukhiv, M.O. Effects of Size on Fecundity, Longevity and Viability in Populations of Drosophila pseudoobscura. Am. Nat. 1960, 94, 395–403. [Google Scholar] [CrossRef]
- Chapman, T.; Partridge, L. Female fitness inDrosophila melanogaster: An interaction between the effect of nutrition and of encounter rate with males. Proc. R. Soc. B: Biol. Sci. 1996, 263, 755–759. [Google Scholar] [CrossRef]
- Usui-Aoki K., H. Ito, K. Ui-Tei, K. Takahashi, T. Lukacsovich, et al., Formation of the male-specific muscle in female Drosophila by ectopic fruitless expression. Nature 2000, 2, 500–506. [CrossRef]
- Van Wielendaele, P.; Badisco, L.; Broeck, J.V. Neuropeptidergic regulation of reproduction in insects. Gen. Comp. Endocrinol. 2013, 188, 23–34. [Google Scholar] [CrossRef]
- Veenstra, J.A. What the loss of the hormone neuroparsin in the melanogaster subgroup of Drosophila can tell us about its function. Insect Biochem. Mol. Biol. 2010, 40, 354–361. [Google Scholar] [CrossRef]
- Vijayan, V.; Wang, F.; Wang, K.; Chakravorty, A.; Adachi, A.; Akhlaghpour, H.; Dickson, B.J.; Maimon, G. A rise-to-threshold process for a relative-value decision. Nature 2023, 619, 563–571. [Google Scholar] [CrossRef]
- Walker, S.J.; Corrales-Carvajal, V.M.; Ribeiro, C. Postmating Circuitry Modulates Salt Taste Processing to Increase Reproductive Output in Drosophila. Curr. Biol. 2015, 25, 2621–2630. [Google Scholar] [CrossRef]
- Wang, C.; Li, G.; Miao, C.; Zhao, M.; Wang, B.; Guo, X. Nonanal modulates oviposition preference in female Helicoverpa assulta (Lepidoptera: Noctuidae) via the activation of peripheral neurons. Pest Manag. Sci. 2020, 76, 3159–3167. [Google Scholar] [CrossRef]
- Wang, F.; Wang, K.; Forknall, N.; Parekh, R.; Dickson, B.J. Circuit and Behavioral Mechanisms of Sexual Rejection by Drosophila Females. Curr. Biol. 2020, 30, 3749–3760. [Google Scholar] [CrossRef]
- Wang, F.; Wang, K.; Forknall, N.; Patrick, C.; Yang, T.; Parekh, R.; Bock, D.; Dickson, B.J. Neural circuitry linking mating and egg laying in Drosophila females. Nature 2020, 579, 101–105. [Google Scholar] [CrossRef]
- Wang, K.; Wang, F.; Forknall, N.; Yang, T.; Patrick, C.; Parekh, R.; Dickson, B.J. Neural circuit mechanisms of sexual receptivity in Drosophila females. Nature 2021, 589, 577–581. [Google Scholar] [CrossRef]
- A Waterbury, J.; Jackson, L.L.; Schedl, P. Analysis of the Doublesex Female Protein in Drosophila melanogaster: Role in Sexual Differentiation and Behavior and Dependence on Intersex. Genetics 1999, 152, 1653–1667. [Google Scholar] [CrossRef]
- Wensing, K.U.; Fricke, C. Divergence in sex peptide-mediated female post-mating responses in Drosophila melanogaster. Proc. R. Soc. B. 2018, 285, 20181563. [Google Scholar] [CrossRef]
- Wheelock, G.D.; Petzel, D.H.; Gillett, J.D.; Beyenbach, K.W.; Hagedorn, H.H. Evidence for hormonal control of diuresis after a blood meal in the mosquito Aedes aegypti. Arch. Insect Biochem. Physiol. 1988, 7, 75–89. [Google Scholar] [CrossRef]
- White, M.A.; Chen, D.S.; Wolfner, M.F. She’s got nerve: Roles of octopamine in insect female reproduction. J. Neurogenetics 2021, 35, 132–153. [Google Scholar] [CrossRef]
- Wieschaus, E. (1980). A Combined Genetic and Mosaic Approach to the Study of Oogenesis in Drosophila. In O. Siddiqi, P. Babu, L. M. Hall, & J. C. Hall (Eds.), Development and Neurobiology of Drosophila (pp. 85–94). Springer US. [CrossRef]
- Wigby, S.; Chapman, T. Sex Peptide Causes Mating Costs in Female Drosophila melanogaster. Curr. Biol. 2005, 15, 316–321. [Google Scholar] [CrossRef]
- Williams, G.C. Natural Selection, the Costs of Reproduction, and a Refinement of Lack's Principle. Am. Nat. 1966, 100, 687–690. [Google Scholar] [CrossRef]
- Williams, T.D. Mechanisms Underlying the Costs of Egg Production. BioScience 2005, 55, 39–48. [Google Scholar] [CrossRef]
- Wong, A.; Albright, S.N.; Wolfner, M.F. Evidence for structural constraint on ovulin, a rapidly evolving Drosophila melanogaster seminal protein. Proc. Natl. Acad. Sci. USA 2006, 103, 18644–18649. [Google Scholar] [CrossRef]
- Wong, R.; Lange, A.B. Octopamine modulates a central pattern generator associated with egg-laying in the locust, Locusta migratoria. J. Insect Physiol. 2014, 63, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-L.; Fu, T.-F.; Chou, Y.-Y.; Yeh, S.-R. A Single Pair of Neurons Modulates Egg-Laying Decisions in Drosophila. PLoS ONE 2015, 10, e0121335. [Google Scholar] [CrossRef]
- Xu, C. S. , Januszewski, M., Lu, Z., Takemura, S., Hayworth, K. J., Huang, G., Shinomiya, K., Maitin-Shepard, J., Ackerman, D., Berg, S., Blakely, T., Bogovic, J., Clements, J., Dolafi, T., Hubbard, P., Kainmueller, D., Katz, W., Kawase, T., Khairy, K. A., … Plaza, S. M. (2020). A Connectome of the Adult Drosophila Central Brain [Preprint]. Neuroscience. [CrossRef]
- Xu, J.; Tan, A.; Palli, S.R. The function of nuclear receptors in regulation of female reproduction and embryogenesis in the red flour beetle, Tribolium castaneum. J. Insect Physiol. 2010, 56, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, D.; Koganezawa, M. Genes and circuits of courtship behaviour in Drosophila males. Nat. Rev. Neurosci. 2013, 14, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-H.; Belawat, P.; Hafen, E.; Jan, L.Y.; Jan, Y.-N. Drosophila Egg-Laying Site Selection as a System to Study Simple Decision-Making Processes. Science 2008, 319, 1679–1683. [Google Scholar] [CrossRef] [PubMed]
- Yapici, N.; Kim, Y.-J.; Ribeiro, C.; Dickson, B.J. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature 2008, 451, 33–37. [Google Scholar] [CrossRef]
- Zhang, C.; Kim, A.J.; Rivera-Perez, C.; Noriega, F.G.; Kim, Y.-J. The insect somatostatin pathway gates vitellogenesis progression during reproductive maturation and the post-mating response. Nat. Commun. 2022, 13, 969. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, B.; Roy, S.; Saha, T.T.; Kokoza, V.A.; Li, M.; Raikhel, A.S. MicroRNA-309 targets the Homeobox gene SIX4 and controls ovarian development in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2016, 113. [Google Scholar] [CrossRef]
- Zhou, C.; Pan, Y.; Robinett, C.C.; Meissner, G.W.; Baker, B.S. Central Brain Neurons Expressing doublesex Regulate Female Receptivity in Drosophila. Neuron 2014, 83, 149–163. [Google Scholar] [CrossRef]
- Zhu, E.Y.; Guntur, A.R.; He, R.; Stern, U.; Yang, C.-H. Egg-Laying Demand Induces Aversion of UV Light in Drosophila Females. Curr. Biol. 2014, 24, 2797–2804. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).