Introduction
Since the beginning of human civilization, plants have been one of the most important sources of medicines. Despite the great advancements made in the field of allopathy during the 20th century, plants continue to be one of the primary sources used in both modern and traditional medical systems that are practised by people all over the world. Among these medicinal plants, kalmegh is getting more significance in present day situations owing to its medicinal and curative properties (Farooqi and Sreeramu, 2010). Andrographis is one of the most important genera of the family Acanthaceae. Kalmegh plays a significant role in 26 Indian ayurvedic formulas and holds a vital position in the Indian Pharmacopoeia. (Verma et al., 2018). Its antipyretic and antiviral property is immense and thus, it is potential to fight against Covid-19 (Verma et al., 2021).
A wide variety of bioactive substances, including andrographolides and polyphenols, are produced by Kalmegh. One of the pharmacologically significant compounds is andrographolides. A minimum of 26 Ayurvedic remedies used to treat liver problems contain a strongly bitter flavoured andrographolide, a labdane diterpenoid produced from Andrographis paniculata (Pandey and Rao, 2018). Andrographolide is important anticancer and immunomodulatory pharmacophore and has potential to be developed as an anticancer chemotherapeutic agent (Mishra et al., 2007). In India, China and other South East Asian nations, kalmegh is used to treat throat infection, fever, colds, and a number of infectious diseases like dysentery, diarrhoea and malaria. The plant also possesses antibacterial, antithrombotic, anti-inflammatory and immunological properties (Verma et al., 2021).
Elicitation is the process of enhancing or inducing the production of metabolites by adding small amounts of elicitors. “Elicitor may be defined as a substance for stress factors which, when applied in small quantity to a living system, induces or improves the biosynthesis of specific compound which do have an important role in the adaptations of plants to a stressful condition” (Radman et al., 2003). In comparison with many applications for enhanced productivity, elicitation is recognised as the most practically possible method for enhancing the synthesis of desirable secondary metabolites from plants without compromising quality (Poornananda and Jameel, 2016).
For pharmaceutical industries, bulk herb accompanied by high quantity and quality of principle component in the herb is important for ease and worth full extraction. In recent years, studies on use of elicitors, both biotic and abiotic for quality improvement in medicinal plants is increasing but, these studies are confined to in-vitro level and only a smaller number of researches were done on ex-vitro spray of elicitors. Elicitation is known to increase secondary metabolite. Thus, use of elicitors fulfils both high yield of herb with quality and this in line supports both farmers and buyers for cost effective production and high returns. However, there is scarcity of research to prove yield increment through elicitation. Thus, there is a basic need to study elicitors mediated response of growth, yield and quality in kalmegh. The biosynthesis of bioactive chemicals in medicinal plants and the creation of biomass are both significantly impacted by nutrients and direct or indirect exposure of herbs to foreign molecules (biotic or abiotic) i.e., elicitors. Therefore, studies on efficacy of organic nutrients and elicitors on growth yield and quality of medicinal herbs is required. The emerging global scenario in respect of demand for herbs in various ayurvedic preparations and pharmaceutical industries suggests that kalmegh may become one of the very important crops in the near future. The purpose of the present research work was to acquire insight into the impacts of elicitors [biotic (chitosan and yeast extract) and abiotic (jasmonate and salicylic acid)] on the enhancement of growth, yield, and quality production of kalmegh while keeping in mind the significance of this crop.
Materials and Methods
Elicitation studies for quality production in kalmegh are meagre and only few studies were done in laboratory scale. Exogenous spray of elicitors to enhance the secondary metabolites and also growth and yield in medicinal plants and other crops as reviewed earlier. To extend this application of elicitors in kalmegh crop for quality production and also to check if any variations in growth and yield attribute, the present experiment was done. Extensive reviewing of earlier literatures showed that different concentrations of biotic (0.2 to 2 g L-1 or 200 to 2000 ppm) and abiotic elicitors (0.5 to 1 mM or 100 to 200 ppm) were influenced the crop response in positive direction. So, after applying extrapolative and qualitative approach of research the following treatments were fixed and evaluated viz., T1; chitosan @ 500 ppm, T2; chitosan @ 1000 ppm, T3; yeast extract @ 500 ppm, T4; yeast extract @ 1000 ppm, T5; jasmonic acid @ 100 ppm, T6; jasmonic acid @ 200 ppm, T7; salicylic acid @ 100 ppm, T8; salicylic acid @ 200 ppm, T9; control (water spray). Elicitors were sprayed at 30 and 60 days after kalmegh was sown. Approximately 100 ml of solution were sprayed on each plant.
Chitosan was dissolved in glacial acetic acid to create a solution (Malekpooret al., 2016), accordingly, 0.50 and 1.00 g of chitosan (500 and 1000 ppm) were combined with distilled water and swirled for 10 minutes. The mixture was then supplemented with 0.50 and 1.00 ml of glacial acetic acid and agitated for another two hours before being diluted to a volume of 1000 ml with distilled water. To obtain 500 and 1000 ppm yeast extract solution, 0.5 and 1 g of yeast extract powder were dissolved in some distilled water, then the volume was increased up to 1000 ml with distilled water. (Maqsood and Abdul, 2017). The different quantity of salicylic acid @ 0.1 and 0.2 g (100 and 200 ppm) were dissolved at first in small quantity of ethanol then distilled water was added for volume made up to one litre (Gorni and Pacheco, 2016; Singh et al., 2020).
At the Bidhan Chandra Krishi Viswavidyalaya's herbal garden in Mohanpur, Nadia, West Bengal, India, which is located at 23.5oN latitude and 89oE longitude and has an average elevation of 9.75 m above mean sea level, the investigation was conducted for two consecutive years in 2021 and 2022. The area is classified as subtropical humid. The average annual rainfall is 1500 mm, with summer months having an average temperature range of 25oC to 36.5oC and winter months having an average temperature range of 12oC to 25oC. The experiment's soil was organic Gangetic alluvial soil (Entisol), which had a sandy clay loam texture, good water holding capacity, was well-drained, and had a moderate level of soil fertility. A pot culture experiment with nine treatments and three replications was established using a completely randomised approach. CIM-Megha variety of kalmegh from CIMAP, Lucknow was used for this research.
A total of nine pots per treatment were maintained. 20 cm × 20 cm size plastic containers were used to plant the seeds during January and the crop was harvested during June in both the years. A basal dose of well rotten FYM @ 20 t ha-1 and 75:75:50 kg NPK ha-1 was incorporated into the soil, the ratio and proportion method wasused in computing the amount of fertilizers and FYM needed in pot experiment (Imakumbili, 2019). Observations on all parameters were recorded in all plants in each replication and the averages were computed. The International Rice Research Institute's Statistical Tool for Agricultural Research (STAR) software was used to pooled analysis on the mean data from two seasons. Each pair of means was compared using Duncan's multiple range test (DMRT) (Duncun, 1955). Utilising the Karl-Pearson (1948) formula, correlations were calculated. By comparing correlation coefficients with table values, the significance of correlation coefficients was examined (Fisher and Yates, 1963). The grouping of treatments was done by using UPGMA clustering method as given by Michener and Sokal (1957).
At harvest, the chlorophyll content of leaves from each plant from each replication was randomly selected, and an average was determined as per procedure given by Sadasivam and Manickam (1996). Carotenoid content (mg g-1) was calculated using the formula given by Bajracharya (1996).The extraction and estimation of the andrographolides from the methanol extract of powdered kalmegh sample was done through HPLC at Floriculture and Medicinal Crops Division , ICAR-Indian Institute of Horticultural Research, Bengaluru, Karnataka.The HPLC studies were completed using a Shimadzu Series LC-10A system (Shimadzu, Kyoto, Japan), which includes a liquid chromatography linked to a UV-VIS detector (10 A) and a binary pump, and the system was controlled by Shimadzu Class VP Workstation software. Gemini, 250 x 4.6 mm, 5 μm C18 (Phenomenex, USA) was the type of column used, and the security guard column was also made of the same material. Shimadzu model SIL-20 A HT autosampler was used to inject the samples. The thermostat was set to 320C for the column and guard column. The mobile phase contained phosphate buffer (solvent A) and acetonitrile (solvent B), and the flow rate was 1.5 ml/min. A linear gradient mode was used to operate the equipment. The gradient conditions were 0 to 18 min, 5 to 60 % B, and 18 to 25 min, 47 to 74 % B. At 223 nm the detection was monitored.
Results and Discussion
Plant growth agents are now being used more frequently to improve crop quality and yield. Plants naturally produce some organic molecules during stress conditions, which will help to combat stress by production of secondary metabolites. External application of some substances enhances the production of metabolites and these substances are known as elicitors. Some of the organic substances like chitosan, yeast extract, salicylic acid and jasmonic acid are being used in recent days by farmers in the name of elicitors for increasing growth and yield of crop along with quality. However, application of elicitors for enhancing secondary metabolites is a known factor but combined and full knowledge on effect of elicitors on overall development of plant with respect to growth, yield and quality at a time has rarely been studied. One of the most crucial ways to boost the yield and production of secondary metabolites in plants is by the application of elicitors. Elicitors are the chemical compounds that increase the formation of secondary metabolites in response to stress (Zhao et al., 2005). According to research by Poornananda and Jameel (2016), elicitors increase the bioaccumulation of andrographolide in kalmegh.
Growth Parameters
The growth parameters of pooled data
viz., height and spread of the plant, primary and secondary branches number per plant changed significantly when elicitors were used compare to control treatment (
Table 1,
Table 2,
Table 3,
Table 4 and
Table 5;
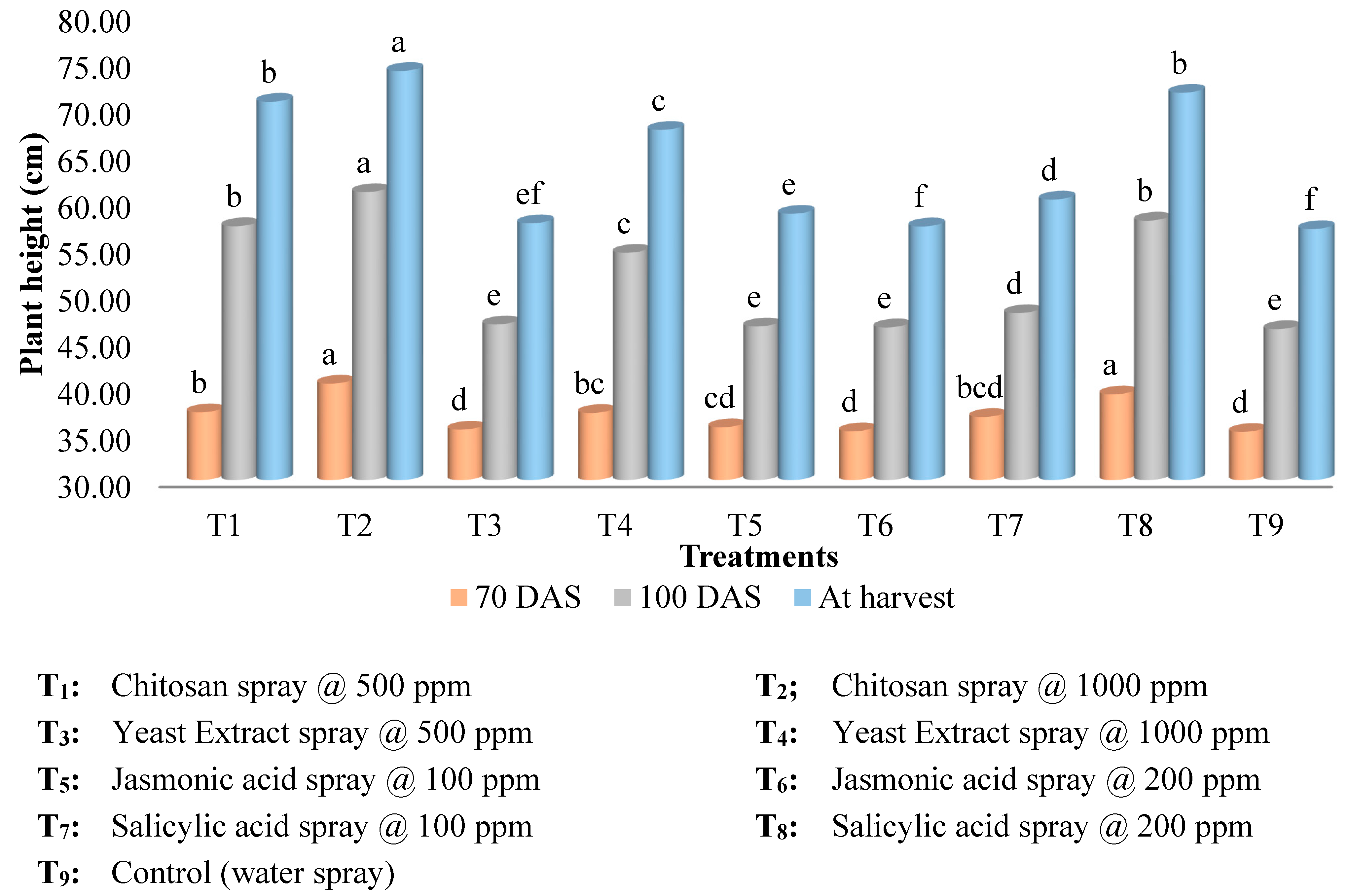
Figure 1). Chitosan @ 1000 ppm spray exhibited highest height of plant (73.91 cm) and secondary branches per plant (29.07) at harvest followed by salicylic acid @ 200 ppm spray (71.57 cm and 27.41) whereas, the lowest (56.93 and 23.35 cm) plant height and secondary branches was recorded for control. Plant spread was highest in salicylic acid @ 200 ppm spray (35.46 cm
2) which was
on par with 1000 ppm chitosan (35.11 cm
2), primary branches number per plant was maximum in chitosan @ 1000 ppm spray (26.36) which was
on par with 200 ppm salicylic acid (26.28) and control recorded minimum values both the parameters.
The findings of the elicitor treatments made it clear that there is a substantial correlation between the plant height and the number of primary branches, which is then correlated with the number of secondary branches and plant spread. Treatments were seen to have an acceptable number of branches and plant spread when they reached their maximum height at maturity. This can be explained by the fact that the taller plants could bear more number of branching nodes and were able to show a high plant spread. Increased availability and uptake of water and vital nutrients through adjusting cell osmotic pressure and reducing the accumulation of harmful free radicals by increasing antioxidant and enzyme activities are likely the causes of chitosan's stimulatory effect on plant growth (Nithin, 2020). Additionally, it might be credited to improved nitrogen transfer to functional leaves, greater photosynthesis, and increased enzymatic nitrogen metabolism activities (nitrate reductase, glutamine synthetase, and protease), all of which facilitated plant growth and development (Mondal et al., 2012). Additionally, Chitosan boosted pathways for auxin production via a route tryptophan independent pathway may have increased plant height (Dhakad, 2019). The results obtained from the study are in concurrence with results of Gornik et al. (2008), Abdel-Mawgoud et al. (2010), Yin et al. (2012), Sultana et al. (2015), Ahmed et al. (2016) and Malekpoor et al. (2016).
The longer and more numerous branches that resulted in a tilting outward, which boosted plant spread, may be responsible for the increased plant spread in the chitosan spray treatment. Chitosan increases nitrogen transport in the functioning leaves of kalmegh plants by enhancing the enzyme activities of nitrogen metabolism (nitrate reductase, glutamine synthetase, and protease) (Mondal et al.,2012). The findings of Gorniket al. (2008), Abdel-Mawgoud et al. (2010), Pirbalouti et al. (2017), and Kra et al. (2019) are in agreement with these findings.
Salicylic acid also has a significant impact on the morphology and physiology of the Kalmegh plant. The use of salicylic acid in the current investigation may have increased the photosynthetic activities of the kalmegh plant. These outcomes are consistent with researches of Hashemabadi and Zarchini, Farouk and Osman (2011), Jadhav and Bhamburdekar (2012) and Sharma (2012).Salicylic acid increased the chlorophyll content, which resulted into large quantity of photosynthesis and increased plant growth (Kazemi, 2014)and also due to increased length of internodes more number of internodes which directly increased the height of plant, branches number (Sharma, 2012).Ali and Mahmoud (2013), Rahimi et al. (2013) and Mulgir et al. (2014) also reported similar results.Increased plant spread by salicylic acid application may be due to higher vegetative growth, chlorophyll content, and branches number as well as root growth, which could result in increased plant spread. The results also support from earlier works of Asgari and Moghadam (2015), Kumar et al. (2015), Abd-Elkader (2016), Koppad et al. (2017), Nangare (2017) and Sathiyamurthy et al. (2017).
Pooled analysis of two years data of single leaf parameters like its length, width and area of leaf showed that control treatment with highest values (4.53 cm, 1.35 cm and 6.04 cm
2) whereas least length and width was recorded by chitosan 1000 ppm spray which was
on par with 500 ppm chitosan and 200 ppm salicylic acid. In case of leaf area, all the elicitor’s treatments recorded
on par results in both the years.Leaf parameters of plants showed significant difference with elicitor treatment (
Table 7;
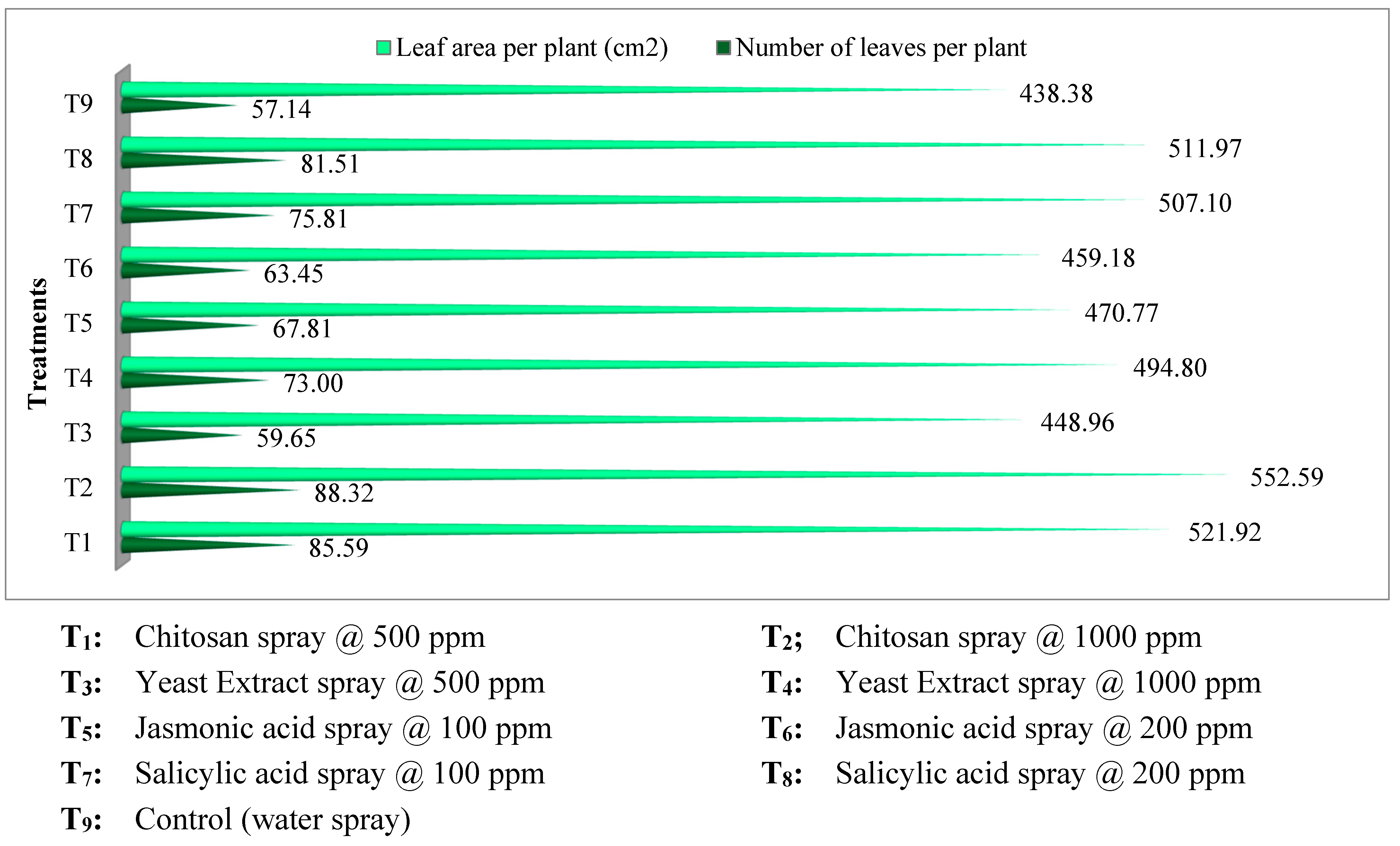
Figure 2). Leaves number per plant was highest in chitosan spray @ 1000 ppm (88.32) which was
on par with chitosan @ 500 ppm (85.59) and salicylic acid @ 200 ppm (81.51). Leaf area of plant and leaf area index recorded maximum value in chitosan @ 1000 ppm (552.59 cm
2 and 1.38) which was
on par with chitosan @ 500 ppm (521.92 cm
2 and 1.30). The control treatment recorded least values for above three parameters.
In this study, the leaves number was in higher rank in those treatments having prime position in branches number and, in turn, leaves number appeared to have governed the total leaf area of plant. The treatments with more leaves per plant had produced more dry substance simultaneously. This is perhaps due to their higher photo-assimilation capacity due to maximum green area of individual plants actively synthesising carbohydrates through photosynthesis. The probable increase in leaves number per plant is owing to increased nutrient absorption. The foliar application of chitosan stimulate molecular signals which served as plant growth promoter that induced higher rate of cell division and cell elongation in sub apical meristems of kalmegh shoots increasing the amount of leaves that each plant produces (Ahmed, 2015). Mondal et al. (2012) and Abdel-Mawgoud et al. (2010) also obtained the similar results. The likely cause of the increase in leaf area per plant can be linked to an increase in epidermal parenchyma cells and an increase in the number of functional leaves. Furthermore, foliar application of chitosan improved cell osmotic pressure, which improved water availability and nutrient uptake (Mondal et al., 2016) and nitrogen transfer in functional leaves promoted photosynthesis, which in turn improved plant growth and development and increased the leaf area naturally (Mondal et al., 2012). These outcomes are consistent with the work of Gornik et al. (2008) and Abdel-Mawgoud et al. (2010), Xu and Mou (2018), Thengumpally (2019) and Ashwini (2020).
Salicylic acid administration enhanced the number of leaves because the number of leaves positively associated with the number of nodes and primary branches per plant (Bhasker et al., 2020). Andrey et al. (2012), Mona et al. (2012), Ram et al. (2012), Anwar et al. (2014), Padmalatha et al. (2014), Mahammad (2016) and Manoj (2017) found similar result. The increased number of leaves per plant in Kalmegh as a result of the spraying of salicylic acid @ 200 ppm may be attributable to the peridined division and expansion of the central cell in the leaf axis that would be made possible by the salicylic acid's morphactin-like properties, as a result the number of leaves per plant in Kalmegh might also increase. Meena et al. (2016), Koppad et al. (2017), Sathiyamurthy et al. (2017) and Vitthal (2018) reported similar results.
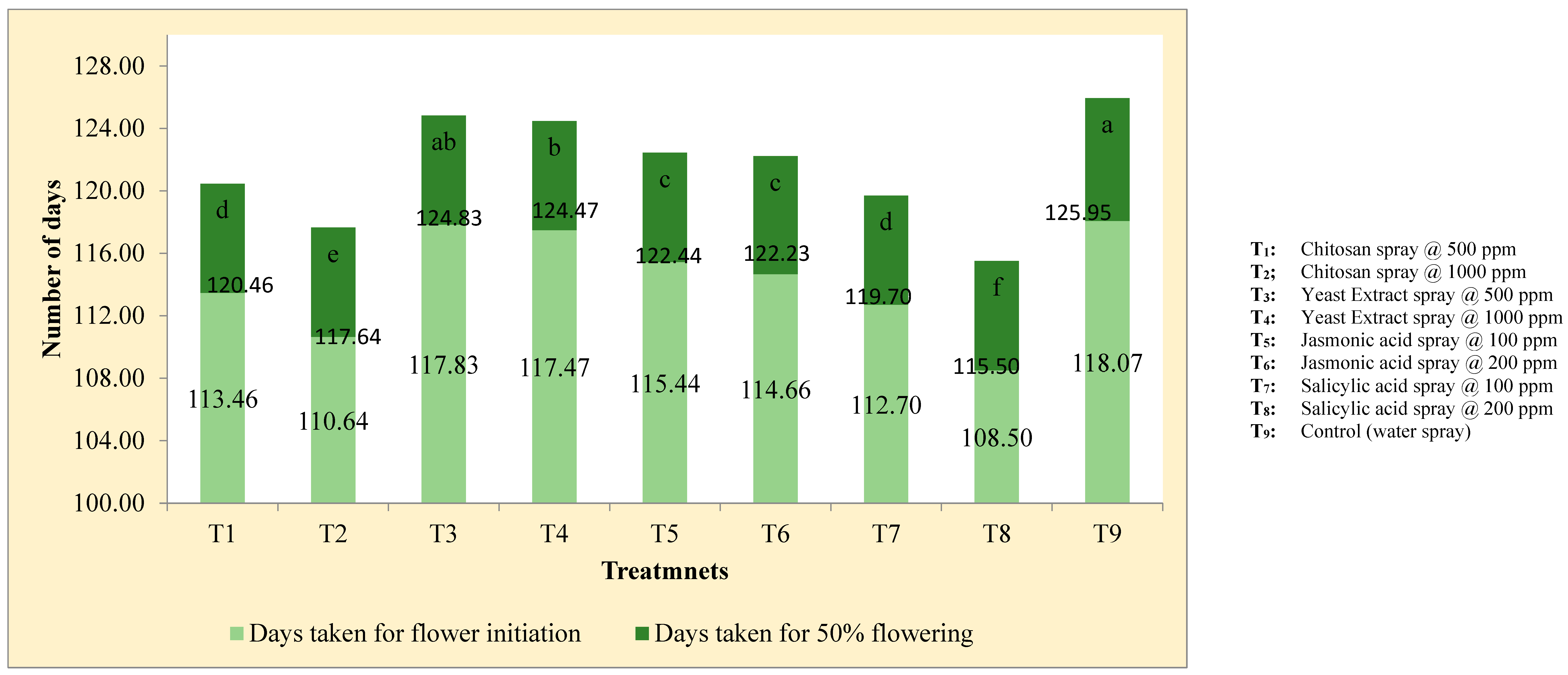
In pooled analysis, plants sprayed with salicylic acid @ 200 ppm followed by chitosan @ 1000 ppm came for early flowering and harvest (
Table 8;
Figure 3). Maximum days for flower initiation and harvest were taken by control treatment, which was
on par with yeast extract elicitation. It might be due to salicylic acid promotes flowers, sexual development which results enhancement in flower production (Kumar and Reddy, 2008). It may be also due to induction of early flowering through transition from vegetative to reproductive growth. Maniram
et al. (2012) and Ram
et al. (2012) have confirmed this. Salicylic acid's florigenic activity, or more likely its effect on the ratio between flower-promoting and flower-inhibiting components, boosted the synthesis of floral stimuli in an inductive cycle (Padmalatha
et al., 2014). Several researchers have reported that salicylic acid causes flower induction in annual crops (Narayanan
et al., 2015, Qureshi
et al., 2015). Choudhary
et al. (2016) concluded that salicylic acid induced flowering by acting as floral stimulus in leaves, which may regulate flowering. Salicylic acid serves as an internal growth regulator for flowering and the florigenic response (Pawar
et al., 2018).
From this study, it was noticed that chitosan treated plants starts flowering early as compared to other, which indicates that chitosan stimulates the nutrient absorption and regulates the plant signaling pathways that helped to induce early flowering in kalmegh. While, greater number of days were needed for the first flower bud to appear on water-sprayed plants because of hormonal imbalance that promote vegetative growth only instead of flowering. These outcomes are consistent with those of Farouk and Amany (2012), Salachna and Zawadzinska (2014), Mutka et al. (2017), Dhakad (2019) and Nithin (2020). The highest chlorophyll concentration was found in the plants sprayed with salicylic acid and chitosan, which led to greater generation of photosynthates and their accumulation. Additionally, the stimulatory effects of salicylic acid and chitosan on absorption of nutrients may aid in timely nutrient supply with simple uptake throughout overall plant growth and result in early maturity by flowering.
Yield Parameters
The application of elicitors resulted in an increasing trend for the yield parameters, including fresh and dry herbage yield and dry matter content per plant, as compared to the control (
Table 9). For both the years and the pooled study, chitosan and salicylic acid sprays produced results that were similar in terms of yield characteristics. The yield data for salicylic acid at 200 ppm, chitosan at 1000 ppm, salicylic acid at 100 ppm, and salicylic acid at 500 ppm were reported on par, but significantly better than those for other elicitors treatment and control. Any agricultural plant's production is influenced by the assimilatory surface of the plant system. A reliable source with regard to plant height, LAI, number of branches and leaves is logically capable of increasing the dry matter, and its distribution in various regions is crucial for determining the crop's overall output. In case the treatments are able to perform similarly for holding moisture and dry matter content, they show more or less similar trend in both dry and fresh weight of whole plant. Those treatments having maximum leaf area could exhibit greater values of fresh as well as dry weight of whole plant. Similarly, in the present study, the fresh weight and dry weight values among the treatments are ranked similarly or on par.
Yield is a composite trait, governed by polygenes, and associated with several other traits, which contribute in increments. In kalmegh, it is well established that yield is related to improvement in height of plant, leaf area, primary and secondary branches (Jadhav and Bhamburdekar, 2012). Salicylic acid and chitosan were found to considerably improve plant height, spread, number of leaves, branches and leaf area per plant in the current study. Similar to this, during harvest, treatments with salicylic acid and chitosan applications dramatically increased the generation of photosynthates, boosting biomass output and dry matter content accumulation. Salicylic acid and chitosan treatment resulted in an overall increase in growth and yield qualities, which led to better fresh and dried herbage yields (Manoj, 2017).
The plant's ability to regenerate itself is boosted by salicylic acid's inhibition of ethylene synthesis, which may have contributed to the enhanced herbage yield (Pawar et al., 2018). It is generally known that SA promotes cell elongation and cell division (Ahmed et al., 2013).According to reports, SA can boost other yield in many plant species by improving plant growth factors including height, number of branches, leaves, and leaf area per plant. (Gharib, 2007, Sayyariet al., 2013, Mohsen et al., 2014, Karimian et al., 2015, Pradhan et al., 2016, Koppad et al., 2017 and Sathiyamurthyet al., 2017). Besides, foliar spray of SA and chitosan helped to activate the signalling pathways of growth regulating substances like gibberellins and auxins which might also contributed to increased bio mass of plants by increasing number and size of cells (Supriya, 2015, Gorni and Pacheco, 2016, Youssef et al., 2017, Vitthal, 2018, Forouzandeh et al., 2019 and Nithin, 2020). The stimulation of physiological processes, improved vegetative growth, active translocation of photoassimilates from source to sink tissues, increased number of leaves and branches per plant, and accumulation of dry matter may all be contributing factors to the increase in yield per plant in the treatment receiving chitosan spray. While, frequent foliar application of water creates moisture stress, which affects the physiological processes of plants, including uptake and translocation of nutrients results in hormonal imbalance, which accounts for reduction of yield per plant in water sprayed treatment. The current findings are consistent with those of Asghari-Zakaria et al. (2009), Ghonameet al. (2010) and Chookhongkhaet al. (2013).
The increased plant total dry weight was linked to the maximum vegetative growth characteristics, including a rise in plant height, leaf count, leaf area, and plant spread. This resulted in production and accumulation of a greater amount of photosynthates in plants leading to increased bio mass accumulation, which in turn yields higher dry matter content. Increments in dry matter by chitosan application were in line with Ahmed et al. (2016), Bistgani et al. (2017), Thengumpally (2019) and Ashwini (2020). Jayalakshmi et al. (2010), Bekheta and Iman (2009), Mahammad (2016), Bhasker et al. (2020), Mohammadi et al. (2020) and Priya (2021) compiled the results on dry matter and their interdependence by salicylic acid.
Quality Parameters
Chlorophyll pigments in leaf varied significantly with elicitor application in kalmegh (
Table 10). Pooled analysis of two years data showed that highest chlorophyll a, b and total (2.529, 1.909 and 4.459 mg g
-1) in salicylic acid 200 ppm spray and the least recorded by control. Carotenoid pigment in leaf was highest in salicylic acid 200 ppm spray (3.674 mg g
-1) which was
on par with 100 ppm SA (3.574 mg g
-1) and the control recorded minimum. Maximum chlorophyll content in leaves was found when 200 ppm of salicylic acid was sprayed over the leaves.These outcomes may be explained by regulating the plant water, which carries nutrient components, particularly nitrogen and phosphorus, which increased the overall chlorophyll concentration. (Maity and Bera, 2009) Similar finding was also reported by Bayat
et al. (2012), Divya
et al. (2014) and Choudhary
et al. (2016).
Chlorophyll and carotenoid levels rose after SA applications in plants, which was attributed to the hormone's beneficial effects on nutrient uptake (Kaydan et al., 2007). This is because higher levels of Fe, Mg, and Ca can stimulate the biosynthesis of chlorophylls (Kong et al., 2014). Additionally, SA's stimulatory influence on the activity of the Rubisco enzyme and photosynthesis may be the cause of the rise in photosynthetic pigments (Kaur et al., 2015). A decrease in stomatal and non-stomatal transpiration, as well as an increase in chlorophyll concentration, can all be attributed to exogenous salicylic acid application on leaf surfaces, which reduces abiotic stressors and improves water usage efficiency. Salicylic acid applied topically to leaves helps to stimulate the enzymes required for the production of chlorophyll in biological processes. These outcomes are in line with those of Mahammad (2016), Pradhan et al. (2016), Kirtikumar (2018) and Gothwal (2021).
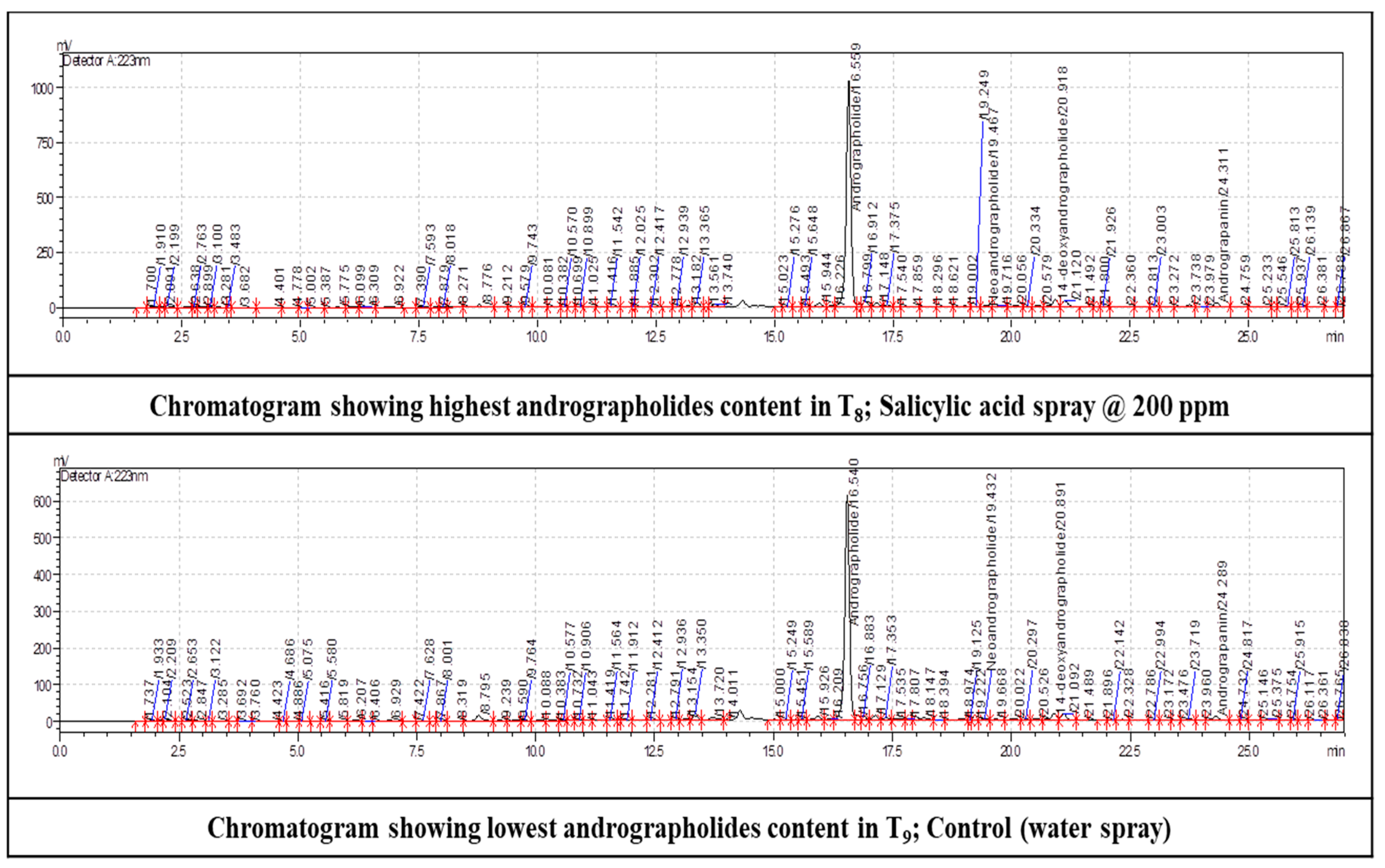
Quantification of secondary metabolites exhibited varied trends in highest and lowest values of carotene and andrographolides content by different treatments (
Table 11;
Figure 4). Pooled analysis of total andrographolides content in herb showed that salicylic acid 200 ppm spray with highest value (3.494%) this was far more effective than any other treatments. The least value recorded by control (2.263%).The enhanced overall plant growth and metabolism identified in the present investigation may be the cause of the beneficial effect of foliar spray of elicitors on alkaloid synthesis. Through improved plant growth, photosynthesis, and general plant metabolism in the current study, it appears that the elicitor may have enhanced the intrinsic genetic ability of the kalmegh plants to produce an additional yield with improved alkaloid quality via biochemical pathways. Elicitor had the ability to increase metabolic activity, which in turn caused the accumulation of secondary metabolites in kalmegh plants by terpenoid synthesis. According to Rivas-San and Plasencia (2011), metabolic alterations at the chloroplast level (Rubisco enzyme activity, photosystem II efficiency and supply of ATP and NADPH for the carbon reduction cycle) can be attributed to the observed increase in photosynthesis rate in plants sprayed with SA. The stimulatory effect of SA on gas exchange parameters and plant development, however, depends on a number of variables, including the application method, exposure duration, and ontogenetic stage (Pacheco
et al., 2013).
The andrographolide is a diterpenoid compound. The terpenoid biosynthesis occurs through mevalonate isoprenoid pathway in glands present in the leaves. The improvement in leaf and herbage development had increased the terpenoid yield. In fact, the trichomes in leaves are the major site for biosynthesis of andrographolide alkaloid. Terpenoid biosynthesis is an integration of several steps such as continuous production of precursors, their transport and translocation to active sites for alkaloid biosynthesis. The metabolic pathway for biosynthesis of alkaloid had improved in plants in response to elicitor application especially with salicylic acid.Increased endogenous salicylic acid (SA) levels can activate cell-signaling pathways, which control the expression of genes encoding enzymes connected to the phenylpropanoid pathway (which produces flavonoids) and the mevalonate system (which produces terpinoids).IPP synthase and DMAPP synthase activity, enzymes that diverge from the MVA and MEP pathway to generate terpinoids in plants, are two examples of enzymes whose activity is improved by SA. (Ghasemzadeh and Jaafar 2012, Tounekti et al., 2013).In Salvia miltiorhiza and Michelia chapensis, it has been demonstrated that SA transcriptionally upregulates the genes involved in isoprenoid production (Cao et al., 2011, Yan et al., 2015). It has also been demonstrated that SA increases the expression of three prenyl transferases from the primary pathway for the biosynthesis of terpenoids in several species (Shabani et al., 2009 in liquorice and Kai et al., 2010 in Arabidopsis). Similar to how SA levels rise during times of drought or salt stress, numerous isoprenoids have also been demonstrated to be upregulated. Despite the fact that the full mechanism of SA in plants is still not fully known, everyone agrees on the crucial role that SA plays in plants (Cappellari et al., 2019).
Character association between yield, quality and its component traits in elicitor treatments and UPGMA based grouping of treatments
Correlation analysis among different traits in elicitor treatments (
Table 12) revealed that fresh herbage yield of plant having highly significant positive association with plant’s height (0.80), spread (0.95), primary (0.91) and secondary branches (0.87), leaves number (0.88), leaf area (0.87), leaf area index (0.87), dry herb yield(0.99) and dry matter content (0.97) and positive significant association with total chlorophyll (0.81) and total andrographolide contents (0.82). Similar positive association was reported by Jadhav and Bhamburdekar (2012), Sayyari
et al. (2013), Mohsen
et al. (2014), Karimian
et al. (2015), Pradhan
et al. (2016), Koppad
et al. (2017), Manoj (2017) and Pawar
et al, (2018). Plant’s dry herb yield exhibited highly significant positive correlation with plant’s height (0.82), spread (0.96), primary (0.92) and secondary branches (0.89), leaves number (0.89), leaf area (0.88), leaf area index (0.88), fresh herb yield (0.99), dry matter content (0.97), total chlorophyll (0.81) and total andrographolide content (0.82). Similar results were also reported by Ghoname
et al. (2010), Chookhongkha
et al. (2013), Supriya (2015), Gorni and Pacheco (2016), Youssef
et al. (2017), Vitthal (2018), Forouzandeh
et al. (2019) and Nithin (2020).Total andrographolides content in kalmegh plant in elicitor treatments showed positive and significant association with plant’s leaves number (0.72), leaf area (0.69) and leaf area index (0.69). Positive and highly significant association was found with plant’s fresh (0.82) and dry herb yields (0.82), dry matter content (0.82) and total chlorophyll content (0.98). Manoj (2017) recorded the similar positive relation between growth, yield and quality components by elicitor treatment in turmeric and Nithin (2020) in strawberry.
According to a correlation study of elicitor treatment parameters, plant height, number of primary and secondary branches, leaves number, leaf area per plant, LAI, dry matter content per plant, total chlorophyll content in leaves and total andrographolides content in plants were all positively and significantly correlated with the fresh and dry yield of herb. With the exception of plant height and the branches number, the total andrographolides content in the kalmegh plant was likewise found to be related to the same factors, regardless of level of significance. Therefore, it can be inferred that elicitors achieved improvement in herbage yield and quality of kalmegh by improving above characters in kalmegh production.
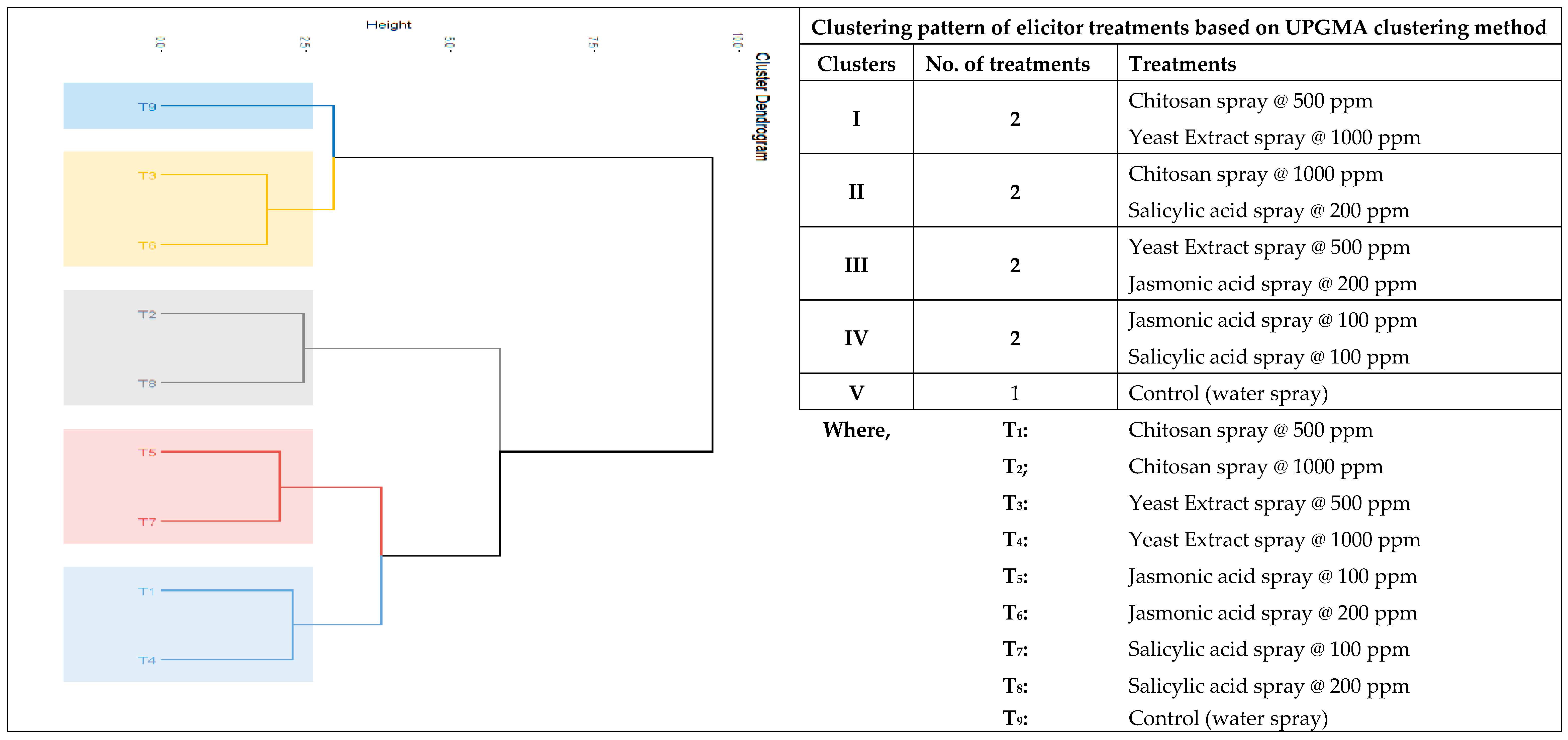
Clustering pattern of elicitor treatments based on UPGMA clustering method employing twelve important characters showed five clusters (
Figure 5). Cluster I consist of chitosan @ 500 ppm and yeast extract @ 1000 ppm spray, cluster II contained chitosan @ 1000 ppm and salicylic acid @ 200 ppm spray. Cluster III with yeast extract @ 500 ppm and jasmonic acid @ 200 ppm spray. Treatments100 ppmjasmonic acid and 100 ppm salicylic acid spray belongs to cluster IV. Control (water spray) treatment falls in cluster V. Treatments belongs to same clusters have relatively same or
on par effects on yield and quality of kalmegh.
Significant and positive improvement in growth, yield and quality of kalmegh was noticed with chitosan and salicylic acid spraying. 200 ppm salicylic acid spray at 30 and 60 DAS on kalmegh exhibited best results in quantity and quality production of kalmegh signifying its use in cultivation of kalmegh for high productivity, quality and better returns for farmers and processors. The effect of elicitors as foliar spray on improving the kalmegh quality is being reported for the first time. This novel and useful finding needs further understanding of how, when and where exactly these chemicals elicit accumulation of andrographolides. As concentration and frequency of elicitor application significantly affects various characters under study, still different other concentrations may be tried depending on method of application like seed treatment, seedling dip etc. on kalmegh.
References
- Abd-Elkader, D.Y. Effect of foliar spraying of micronutrients and salicylic acid on growth, yield and quality of garlic plants. Alexandria Journal of Agricultural Sciences, 2016, 61, 649–658. [Google Scholar]
- Abdel-Mawgoud, A.M.R.; Tantawy, T.A.; El-Nemr, M.A.; Sassine, Y.N. Growth and yield responses of strawberry plants to chitosan application. European Journal of Scientific Research 2010, 39, 170–177. [Google Scholar]
- Ahmed, A.H.; Nesiem, M.R.A.; Allam, H.A.; El-Wakil, A.F. Effect of pre-harvest chitosan application on growth, yield and chemical composition of Washington navel orange trees grown in two different regions. African Journal of Biochemistry Research 2016, 10, 59–69. [Google Scholar] [CrossRef]
- Ahmed, F.; Baloch, D.M.; Hassan, M.J.; Ahmed, N. Role of plant growth regulators in improving oil quantity and quality of sunflower hybrids in drought stress. Biologia(Pakistan) 2013, 59, 315–322. [Google Scholar]
- Ahmed, M.E.M. Response of garlic plants (Allium sativum L.) to foliar application of some bio-stimulants. Egyptian Journal of Horticulture 2015, 42, 613–625. [Google Scholar]
- Ali, E.A.; Mahmoud, A.M. Effect of foliar spray of different salicylic acid and zinc concentrations on seed and yield components of mungbean in sandy soils. Asian Journal of Crop Sciences 2013, 5, 33–40. [Google Scholar] [CrossRef]
- Andrey, Y.R.; Anna, A.R.; Marina, G.A.; Airat, R.K.; Mikhail, I.B.; Maxim, V.T. Effect of lead and salicylic acid on some plant growth parameters in Pisum sativum L. World Applied Sciences Journal 2012, 19, 1157–1159. [Google Scholar]
- Anwar, M.; Sahito, H.A.; Hassan, I.; Abbasi, N.A.; Ahmed, H.A.; Bhatti, M.A.; Abro, A. Effect of pre harvest treatment of salicylic on growth and vase life of tuberose with aroma environment. Journal of Agricultural Research 2014, 3, 50–57. [Google Scholar]
- Asgari, M.O.N.A.; Moghadam, A.R.L. Comparison of different salicylic acid application ways as a preservative on postharvest life of gerbera cut flowers. Agricultural Communications 2015, 3, 1–8. [Google Scholar]
- Asghari-Zakaria, R.; Maleki-zanjani, B.; Sedghi, E. Effect of in vitro chitosan application on growth and minituber yield of Solanum tuberosum L. Plant Soil Environment 2009, 55, 252–256. [Google Scholar] [CrossRef]
- Ashwini, S. Harvesting stages and chitosan sprayes on curcumin yield in turmeric. M.Sc. Thesis, Kerala Agricultural University, Kerala, India, 2020. [Google Scholar]
- Bajracharya, D. Experiments in plant physiology: A laboratory manual. Narosa Publication, (1996). pp. 51–52.
- Bekheta, M.A.; Iman, M.T. Physiological response of mungbean to some bioregulators. Journal of Applied Botany and Food Quality, 2009, 83, 76–84. [Google Scholar]
- Bhasker, P.; Gupta, P.K.; Sharma, H.P. Role of salicylic acid on growth, yield, quality and disease pest reaction of onion (Allium cepa L.) cv. Agrifound Light Red. SAARC Journal of Agriculture 2020, 18, 39–49. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Siadat, S.A.; Bakhshandeh, A.; Pirbalouti, A.G.; Hashemic, M. Interactive effects of drought stress and chitosan application on physiological characteristics and essential oil yield of Thymus daenensisCelak. The Crop Journal, 2017, 5, 407–415. [Google Scholar] [CrossRef]
- Cao, X.Y.; Li, C.G.; Miao, Q.; Zheng, Z.J.; Jiang, J.H. Molecular cloning and expression analysis of a leaf-specific expressing 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase gene from Micheliachapensis Dandy. Journalof Medicinal Plants Research. 2011, 5, 3868–3875. [Google Scholar]
- Cappellari, L.D.R.; Santoro, M.V.; Schmidt, A.; Gershenzon, J.; Banchio, E. Improving phenolic total content and monoterpene in Mentha x piperita by using salicylic acid or methyl jasmonate combined with rhizobacteria inoculation. International Journal of Molecular Sciences 2019, 21, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Chookhnogkha, N.; Sopondilok, T.; Photchanachai, S. Effect of chitosan and chitosan nanoparticles on fungal growth and chilli seed quality. Acta Horticulture, 2013, 973, 231–237. [Google Scholar] [CrossRef]
- Choudhary, A.; Mishra, A.; Bola, P.K.; Moond, S.K.; Dhayal, M. Effect of foliar application of zinc and Salicylic acid on growth, flowering and chemical constitute of African marigold cv. PusaNarangiGainda (Targetserecta L.). Journal of Applied and Natural Science 2016, 8, 1467–1470. [Google Scholar] [CrossRef]
- Dhakad, L. Study on the effect of plant growth enhancer chitosan on physiological efficiency, growth, quality and productivity of transplanted rice (Oryza sativa L.). M.Sc. Thesis, Jawaharlal Nehru Krishi Vishwavidyalaya, Jabalpur, India.(2019).
- Divya, P.; Puthusseri, B.; Neelwarne, B. The effect of plant regulators on the concentration of carotenoids and phenolic compounds in foliage of coriander. LWT-Food Science and Technology 2014, 56, 101–110. [Google Scholar] [CrossRef]
- Duncun, D.; B. Multiple range and multiple F tests. Biometrics. 1955, 11, 1–42.
- Farooqi, A.A.; Sreeramu, B.S. Cultivation of Medicinal and Aromatic Crops, Universities Press Private Ltd., Pp. 1-8.(2010).
- Farouk, S.; Amany, A.R. Improving growth and yield of cowpea by foliar application of chitosan under water stress. Egyptian Journal of Biology, 2012, 14, 14–26. [Google Scholar] [CrossRef]
- Farouk, S.; Osman, M.A. The effect of plant defence elicitors on common bean (Phaseolus vulgaris L.) growth and yield in absence or presence of spider mite (Tetranychusurticae Koch) infestation. Journal of Stress Physiology Biochemistry 2011, 7, 5–22. [Google Scholar]
- Fisher, R.A.; Yates, F. (1963). Statistical Table for Biological, Agricultural and Medical Research, 6th ed. Longmans, London.
- Forouzandeh, M.; Mohkami, Z.; Fazelinasab, B. Evaluation of biotic elicitors foliar application on functional changes, physiological and biochemical parameters of fennel (Foeniculum vulgare). Journal of Plant Production Research 2019, 25, 49–65. [Google Scholar]
- Gharib, F.A.E.L. Effect of salicylic acid on the growth, metabolic activities and oil content of basil and majoram. International Journal of Agriculture and Biology 2007, 9, 294–301. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z. Effect of salicylic acid application on biochemical changes in ginger (Zingiber officinale R.). Journal of Medicinal Plants Research 2012, 6, 790–795. [Google Scholar] [CrossRef]
- Ghoname, A.A.; El-Nemr, M.A.; Abdel-Mawgoud, A.M.R.; El-Tohamy, W.A. Enhancement of sweet pepper crop growth and production by application of biological, organic and nutritional solutions. Research Journal of Agriculture and Biological Sciences 2010, 6, 349–355. [Google Scholar]
- Gorni, P.H.; Pacheco, A.C. Growth promotion and elicitor activity of salicylic acid in Achillea millefolium L. African Journal of Biotechnology 2016, 15, 657–665. [Google Scholar]
- Gornik, K.; Grzesik, M.; Romanowska-Duda, B. The effect of chitosan on rooting of grapevine cuttings and on subsequent plant growth under drought and temperature stress. Journal of Fruit and Ornamental Plant Research, 2008, 16, 333–343. [Google Scholar]
- Gothwal, J. Effect of methyl jasmonate and salicylic acid on growth, flowering and corm production of gladiolus (Gladiolushybridus Hort.) cv. American Beauty. M.Sc Thesis, Agriculture University, Kota, India.(2021).
- Hashemabadi, D.; Zarchini, M. Effect of some plant growth regulators on growth and flowering of Rosahybrida cv. Poison. Plant Omics Journal 2010, 3, 167–171. [Google Scholar]
- Imakumbili, M.L.E. (2019) Adding solid fertilisers to soil in pot experiments. Protocols.io. [CrossRef]
- Jadhav, S.H.; Bhamburdekar, S.B. Physiological studies of Arachis hypogaeaL. under the influence of sulfosalicylic acid. International Journal of Applied Biology and Pharmaceutical Technology 2012, 3, 389–393. [Google Scholar]
- Jayalakshmi, P.; Devi, S.P.; Prasanna, N.D.; Revathi, G.; Shaheen, S.K. Morphological and physiological changes of groundnut plants by foliar application with salicylic acid. The Bioscan 2010, 5, 193–195. [Google Scholar]
- Kai, M.; Crespo, E.; Cristescu, S.M.; Harren, F.J.M.; Francke, W.; Piechulla, B. Serratia odorifera: Analysis of volatile emission and biological impact of volatile compounds on Arabidopsis thaliana. Applied Microbiology and Biotechnology. 2010, 88, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Karimian, M.A.; Dahmardeh, M.; Bidarnamani, F.; Forouzandeh. Assessment quantitative and qualitative factors of peanut (Arachis hypogaeaL.) under drought stress and salicylic acid treatments. Biological Forum- An International Journal 2015, 7, 871–878. [Google Scholar]
- Pearson, K. Early Statistical Papers. Cambridge, England: University Press.(1948).
- Kaur, J.; Ram, H.; Gill, B.S.; Kaur, J. Agronomic performance and economic analysis of soybean (Glycinemax) in relation to growth regulating substances in Punjab, India. Legume Research 2015, 38, 603–608. [Google Scholar]
- Kaydan, D.; Mehmet, Y.; Okut, N. Effect of salicylic acid on the growth and some physiological characters in salt stressed wheat (Triticumaestivum L. ) TarimBilimleriDergisi, Ankara UniversitesiZiraatFakultesi 2007, 13, 114–119. [Google Scholar]
- Kazemi, M. Foliar application of salicylic acid and methyl jasmonate on yield, yield components and chemical properties of tomato. Jordan Journal of Agricultural Science 2014, 10, 771–778. [Google Scholar] [CrossRef]
- Kirtikumar, T.S. Effect of exogenous application of salicylic acid on green gram (Vigna radiate (L.) Wilczek) irrigated with saline water. M.Sc. Thesis, (2018). Available online: http://krishikosh.egranth.ac.in/handle/1/5810063893.
- Kong, J.; Dong, Y.; Xu, L.; Liu Bai, X. Effects of foliar application of salicylic acid and nitric oxide in alleviating iron deficiency induced chlorosis of Arachishypogaea L. Botanical Studies, (2014). Available online: http://www.as-botanicalstudies.com/ content/55/1/9.
- Koppad, S.R.; Babaleshwar, S.B.; Dharmatti, P.R.; Math, K.K. Influence of salicylic acid on growth and bulb yield of onion (Alliumcepa L.). International Journal of Current Microbiology and Applied Science 2017, 6, 1732–1737. [Google Scholar] [CrossRef]
- Kra, K.D.; Gogbeu, S.J.; Soro, K.K.; Kouakou, K.J.; Kouassi, K.N.; Dogbo, D.O. Effects of chitosan on vegetative organs growth and peroxidases activities in cassava (Manihot esculentaCrantz) cultivars YACE, 9620A, TMS4 (2)1425 and TMS30572. Tropical Plant Research 2019, 6, 08–14. [Google Scholar]
- Kumar, M.A.; Reddy, Y.N. Preliminary investigations on the effect of foliar spray of chemicals on flowering and fruiting characters of mango cv. Baneshan. Research Journal of Agriculture and Biological Sciences 2008, 7, 150–156. [Google Scholar]
- Kumar, P.K.; Padmalatha, T.; Pratap, M.; Reddy, S.N. Effect of plant bio-regulators on growth, flowering and seed yield in China aster (Callistephuschinensis L.) cv. Kamini. Indian Journal of Agriculture Research 2015, 49, 348–352. [Google Scholar] [CrossRef]
- Mahammad, N.P. Physiological effect of salicylic acid on pod setting and yield of groundnut (Arachis hypogaea L.). M.Sc. Thesis, Acharya N.G. Ranga Agricultural University, India. (2016).
- Maity, U.; Bera, A.K. Effect of exogenous application of brassinolide and salicylic acid on certain physiological and biochemical aspects of green gram (Vigna radiata L. wilczek). Indian Journal of Agricultural Research 2009, 43, 194–199. [Google Scholar]
- Malekpoor, F.; Pirbalouti, A.G.; Salimi, A. Effect of foliar application of chitosan on morphological and physiological characteristics of basil under reduced irrigation. Research on Crops 2016, 17, 354–359. [Google Scholar] [CrossRef]
- Maniram, K.M.; Malik, S.; Singh, M.K.; Pal, V. Impact of spacing, doses of vermicompost and foliar application of Salicylic acid on growth and flowering of gladiolus (Gladiolushybridus L.) cv. “White Prosperity”. Annals of Horticulture 2012, 5, 272–279. [Google Scholar]
- Manoj, M.R. Effect of metabolite elicitors on curcumin content in turmeric (Curcuma longa L.) cv. Mydukur. M.Sc. Thesis,Dr. Y.S.R. Horticultural University, India.(2017).
- Maqsood, M.; Abdul, M. Yeast extract elicitation increases vinblastine and vincristine yield in protoplast derived tissues and plantlets in Catharanthus roseus. Brazilian Journal of Pharmacognosy 2017, 27, 549–556. [Google Scholar] [CrossRef]
- Meena, B.; Arvindakshan, K.; Singh, P.; Yadav, I.; Patidar, D.K. Effect of soaking and foliar application of salicylic acid and enthral on growth, yield and biochemical traits of garlic (Alliumsativum L) cv. “G-282”. International Journal of Farm Sciences 2016, 6, 61–66. [Google Scholar]
- Michener, C.D.; Sokal, R.R. A quantitative approach to a classification. Evolution 1957, 11, 490–99. [Google Scholar] [CrossRef]
- Mishra, S.K.; Sangwan, N.S.; Sangwan, R.S. Andrographis paniculata (Kalmegh): A Review. Pharmacognosy Reviews 2007, 01, 283–298. [Google Scholar]
- Mohammadi, H.; Javad, N.M.; Hazrati, S.; Hashempour, H. Improvement of yield and phytochemical compounds of Thymus vulgaris through foliar application of salicylic acid under water stress. Agriculture and Forestry 2020, 66, 129–142. [Google Scholar] [CrossRef]
- Mohsen, J.; Yousef, N.; Hamed, Z.; Mohsen, K.A.; Naser, S. Effect of manure and foliar application of growth regulators on Lentil (Lens culinaris) performance in semi-arid high land environment. Botanica Lithuanica 2014, 20, 99–108. [Google Scholar]
- Mona, G.D.; Sadak, M.S.; Hozayen, M. Physiological role of salicylic acid in improving performance, yield and some biochemical aspects of sunflower plant grown under newly reclaimed sandy soil. Australian Journal of Basic and Applied Sciences 2012, 6, 82–89. [Google Scholar]
- Mondal, M.A.; Puteh, A.B.; Dafader, N.C. Foliar application of chitosan improved morpho-physiological attributes and yield in summer tomato (Solanumlycopersicum). Pakistan Journal of Agricultural Scince 2016, 53, 339–344. [Google Scholar] [CrossRef]
- Mondal, M.M.A.; Malek, M.A.; Puteh, A.B.; Ismail, M.R.; Ashrafuzzaman, M.; Naher, L. Effect of foliar application of chitosan on growth and yield in okra. Australian Journal of Crop Science 2012, 6, 918–921. [Google Scholar]
- Mulgir, V.B.; Joshi, A.K.; Dhutmal, R.R.; More, A.W.; Sharma, V. Effect of exogenous application of brassinosteroide and salicylic acid on growth and yield parameters of groundnut (Arachis hypogea L.). The Ecoscan, 2014, VI: 157-62.
- Mutka, J.A.; Rahman, M.; Sabir, A.A.; Gupta, D.R.; Surovy, M.Z.; Rahman, M.; Tofazzal-Islam, M. Chitosan and plant probiotics application enhance growth and yield of strawberry. Biocatalysis and Agricultural Biotechnology 2017, 11, 9–18. [Google Scholar]
- Nangare, S.B. Effect of salicylic acid on onion (Alliumcepa L.). M.Sc. Thesis, MPKV, Rahuri, India, 2017. [Google Scholar]
- Narayanan, G.S.; Prakash, M.; Reka, M. Influence of seed hardening cum foliar spray treatments on biometric, physiological and yield parameters in black gram under dry land condition. Agriculture Science Digest 2015, 35, 1–6. [Google Scholar] [CrossRef]
- Nithin, K.M. Effect of chitosan foliar application on growth, yield and fruit quality of strawberry (Fragaria × ananassaDuch.) under naturally ventilated polyhouse. M.Sc. Thesis, University of Agricultural and Horticultural Sciences, Shivmogga, Karnataka.
- Pacheco, A.C.; Cabral, C.S.; Fermino, E.S.S.; Aleman, C.C. Salicylic acid-induced changes to growth, flowering and flavonoids production in marigold plants. Journal of Medicinal Plant Research 2013, 7, 3158–3163. [Google Scholar]
- Padmalatha, T.; Reddy, G.S.; Chandrasekhar, R.; Siva Shankar, A.; Chaturvedi, A. Effect of foliar sprays of bioregulators on growth and flowering in Gladiolus plants raised from cormels. ProgressiveHorticulture 2014, 46, 288–294. [Google Scholar]
- Pandey, G.; Rao, C.H. Andrographolide: its pharmacology, natural bioavailability and current approaches to increase its content in Andrographis paniculata. International Journal of Complementary & Alternative Medicine 2018, 11, 355–360. [Google Scholar]
- Pawar, A.; Chopde, N.; Nikam, B. Effect of thiourea and salicylic acid on growth, yield and quality of gladiolus. Journal of Pharmacognosy and Phytochemistry 2018, 7, 970–972. [Google Scholar]
- Pirbalouti, A.G.; Malekpoor, F.; Salimi, A.; Golparvar, A. Exogenous application of chitosan on biochemical and physiological characteristics, phenolic content and antioxidant activity of two species of basil (Ocimumciliatumand Ocimumbasilicum) under reduced irrigation. Scientia Horticulturae 2017, 217, 114–122. [Google Scholar] [CrossRef]
- Poornananda, M.N.; Jameel, M.A. Abiotic and biotic elicitors–role in secondary metabolites production through in vitro culture of medicinal plants. Abiotic and Biotic Stress in Plants - Recent Advances and Future Perspectives, chapter 10. (2016).pp: 247-77.
- Pradhan, M.; Tripathy, P.; Mandal, P.; Sahoo, B.B.; Pradhan, R.; Mishra, S.P.; Mishra, H.N. (2016). Effect of salicylic acid on growth and bulb yield of onion (Alliumcepa L.). International Journal of Bio-resource and Stress Management 2021, 7, 960–963. [Google Scholar] [CrossRef]
- Priya. Effect of salicylic acid on morpho-physiological and biochemical attributes of summer mungbean (Vignaradiata L. Wilczek) under different irrigation regimes. M.Sc. Thesis. Available online: https://krishikosh.egranth.ac.in/handle/1/5810174259.
- Qureshi, U.S.; Izhar, S.; Chughtai, S.; Mir, A.R.; Qureshi, A.R. Efficiency of boron and salicylic acid on quality production of sim carnation (Dianthus caryophyllus) International Journal of Biosciences 2015, 7, 14–21. 7.
- Radman, R.; Saez, T.; Bucke, C.; Keshavarz, T. Elicitation of plant and microbial systems. Biotechnology and Applied Biochemistry 2003, 37, 91–102. [Google Scholar] [CrossRef]
- Rahimi, A.R.; Rokhzadi, A.; Amini, S.; Karami, E. Effect of salicylic acid and methyl jasmonate on growth and secondary metabolites in Cuminum cyminum L. Journal of Biodiversity and Environmental Sciences 2013, 3, 140–149. [Google Scholar]
- Ram, M.; Pal, V.; Singh, M.K.; Kumar, M. Response of different spacing and salicylic acid levels on growth and flowering of gladiolus (Gladiolusgrandiflora L.). Hort Flora Research Spectrum 2012, 1, 270–273. [Google Scholar]
- Rivas-San, V.M.; Plasencia, J. Salicylic acid beyond defence: its role in plant growth and development. Journal of Experimental Botany 2011, 62, 3321–3338. [Google Scholar] [CrossRef]
- Sadasivam, S.; Manicham, A. (1996). Biochemical methods II Edition. New Age International Publication, Pp. 190-191.
- Salachna, P.; Zawadzinska, A. Effect of chitosan on plant growth, flowering and croms yield in potted freesia. Journal of Ecological Engineering 2014, 15, 97–102. [Google Scholar]
- Sathiyamurthy, V.A.T.; Saraswathi, N.A.; Tamilselvi, K.; Thingalmanian, S.A. Rohini,B.N. and Arumugam, T. (2017).Effect of salicylic acid on growth, yield and storage quality of onion (Alliumcepa L.). International Journal of Current Microbiology and Applied Sciences, Special Issue-4: 78-86.
- Sayyari, M.; Ghavami, M.; Ghanbari, F.; Kordi, S. Assessment of salicylic acid impacts on growth rate and some physiological parameters of lettuce plants under drought stress conditions. International Journal of Agriculture and Crop Sciences 2013, 5, 1951–1957. [Google Scholar]
- Shabani, L.; Ehsanpour, A.A.; Asghari, G.; Emami, J. Glycyrrhizin production by in vitro cultured Glycyrrhizaglabra elicited by methyl jasmonate and salicylic acid. Russian Journal of Plant Physiology 2009, 56, 621–626. [Google Scholar] [CrossRef]
- Sharma, R.K. Effect of salicylic acid and gibberellic acid on seed germination and growth of pea. International Journal of Plant Sciences 2012, 7, 322–324. [Google Scholar]
- Singh, M.; Poddar, N.K.; Singh, D.; Agarwal, S. Foliar application of elicitors enhanced the yield of withanolide contents in Withaniasomnifera (L.) Dunal (variety, Poshita). 3 Biotech 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Sulthana, S.; Islam, M.; Khatun, M.A.; Hassaim, M.A.; Huque, R. Effect of foliar application of oligo-chitosan on growth, yield and quality of tomato and eggplant. Asian Journal of Agricultural Research 2017, 11, 36–42. [Google Scholar] [CrossRef]
- Supriya, A.T. Influence of pre-harvest foliar sprays on storage life and quality of onion var. Arkakalyan. M.Sc. Thesis, UHS, Bagalkot, India, 2015. [Google Scholar]
- Thengumpally, N.J. Chitosan mediated metabolite elicitation and growth responses in kasturi turmeric (Curcuma aromaticaSalisb.). M.Sc. Thesis, Kerala Agricultural University, Kerala, India.(2019).
- Tounekti, T.; Hernández, I.; Munne-Bosch, S. Salicylic acid biosynthesis and role in modulating terpenoid and flavonoid metabolism in plant responses to abiotic stress. (2013). Book chapter in springer online. [CrossRef]
- Verma, H.; Negi, M.S.; Joshi, A.; Belal, B.; Shukla, A.; Mahapatra, B. Jaipaul. Growth Attributes of Kalmegh [Andrographis paniculata (Burm. F.) Wall Ness] as influenced by integrated nutrient management under tarai conditions of Uttarakhand. International Journal of Chemical Studies 2018, 6,2947-2949.
- Verma, R.; Misra, V.; Tieari, D.; Bisen, P.S. Potential of selected Indian herbs for covid-19. (2021). www.researchnet.net/publication/344878741.
- Vitthal, S.P. Influence of salicylic acid on the growth, yield and quality in onion Cv. (Akola safed). M.Sc. Thesis, Dr.Panjabrao Deshmukh Krishi Vidyapeeth, Akola.
- Xu, C.; Mou, B. Chitosan as soil amendment affects lettuce growth, photochemical efficiency and gas exchange. Horticulture Technology 2018, 28, 476–479. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, L.; Wang, J. Molecular characterization and expression of 1-deoxy- d-xylulose 5-phosphate reductoisomerase (DXR) gene from Salvia miltiorrhiza. Acta Physiologiae Plantarum 2015, 31, 1015. [Google Scholar] [CrossRef]
- Yin, H.; Frette, X.C.; Christensen, L.P.; Grevsen, Y. Chitosan oligosaccharides promote the content of polyphenols in Greek oregano (Oregano vulgare ssp. Hirtum). Journal of Agriculture and Food Chemistry 2012, 60, 136–143. [Google Scholar] [CrossRef]
- Youssef, S.M.S.; Abd El Hady, S.A.; Abu El-Azm, N.A.I.; El- Shinawy, M.Z. Foliar application of salicylic acid and calcium chloride enhances growth and productivity of lettuce (Lactucasativa). Egyptian Journal of Horticulture 2017, 44, 1–16. [Google Scholar] [CrossRef]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnological Advances 2005, 23, 283–333. [Google Scholar] [CrossRef]
Figure 1.
Elicitors effect on plant height of kalmegh.
Figure 1.
Elicitors effect on plant height of kalmegh.
Figure 2.
Elicitors effect on leaf parameters of kalmegh.
Figure 2.
Elicitors effect on leaf parameters of kalmegh.
Figure 3.
Elicitors effect on days to flower initiation and fifty per cent flowering of kalmegh.
Figure 3.
Elicitors effect on days to flower initiation and fifty per cent flowering of kalmegh.
Figure 4.
Chromatograms showing highest and lowest andrographolides among elicitor treatments in kalmegh.
Figure 4.
Chromatograms showing highest and lowest andrographolides among elicitor treatments in kalmegh.
Figure 5.
Grouping of nine elicitor treatments in clusters presented in the form of Dendrogram.
Figure 5.
Grouping of nine elicitor treatments in clusters presented in the form of Dendrogram.
Table 1.
Details of elicitors used for the experiment.
Table 1.
Details of elicitors used for the experiment.
| S.No. |
Elicitors used |
Specification |
Procured from |
| 1 |
Chitosan |
Chitosan (Low MW) extrapure, 90% DA |
Sisco Research Laboratories Pvt. Ltd., Kolkata |
| 2 |
Yeast extract |
Brownish yellow powder, Laboratory grade prepared from baker’s yeast |
| 3 |
Salicylic acid |
Salicylic Acid extrapure AR grade, 99.9% |
| 4 |
Jasmonic acid |
Technical grade jasmonic acid powder 99% pure, for agriculture use |
Aquatic Chemicals, Mumbai |
Table 2.
Response of plant height to elicitors in kalmegh.
Table 2.
Response of plant height to elicitors in kalmegh.
| Treatments |
Plant height (cm) |
| 70 DAS |
100 DAS |
At harvest |
| 2021 |
2022 |
Pooled |
2021 |
2022 |
Pooled |
2021 |
2022 |
Pooled |
|
T1: Chitosan spray @ 500 ppm |
36.95bc
|
37.59bc
|
37.27b
|
56.84b
|
57.64b
|
57.24b
|
70.66b
|
70.53b
|
70.60b
|
|
T2: Chitosan spray @ 1000 ppm |
39.91a
|
40.79a
|
40.35a
|
60.60a
|
61.20a
|
60.90a
|
73.97a
|
73.85a
|
73.91a
|
|
T3: Yeast extract spray @ 500 ppm |
35.55c
|
35.27cd
|
35.41d
|
46.80d
|
46.60d
|
46.70e
|
57.59e
|
57.51ef
|
57.55ef
|
|
T4: Yeast extract spray @ 1000 ppm |
36.88bc
|
37.52bc
|
37.20bc
|
54.24c
|
54.54c
|
54.39c
|
67.41c
|
67.75c
|
67.58c
|
|
T5:Jasmonic acid spray @ 100 ppm |
35.54c
|
35.79cd
|
35.66cd
|
46.63d
|
46.33d
|
46.48e
|
58.30de
|
58.85de
|
58.57e
|
|
T6:Jasmonic acid spray @ 200 ppm |
35.37c
|
35.09d
|
35.23d
|
46.48d
|
46.28d
|
46.38e
|
57.26e
|
57.18ef
|
57.22f
|
|
T7: Salicylic acidspray @ 100 ppm |
36.52c
|
37.02bcd
|
36.77bcd
|
47.81d
|
48.01d
|
47.91d
|
59.95d
|
60.25d
|
60.10d
|
|
T8: Salicylic acid spray @ 200 ppm |
39.07ab
|
39.32ab
|
39.19a
|
57.59b
|
58.09b
|
57.84b
|
71.69b
|
71.44b
|
71.57b
|
|
T9: Control (Water spray) |
35.30c
|
35.02d
|
35.16d
|
46.30d
|
46.10d
|
46.20e
|
56.97e
|
56.89f
|
56.93f
|
| S Em± |
0.72 |
0.75 |
0.51 |
0.58 |
0.58 |
0.41 |
0.59 |
0.59 |
0.41 |
| CD (0.05) |
2.14 |
2.15 |
1.46 |
1.74 |
1.74 |
1.18 |
1.75 |
1.75 |
1.20 |
| CV (%) |
3.41 |
3.38 |
3.39 |
1.97 |
1.97 |
1.97 |
1.61 |
1.60 |
1.60 |
Table 3.
Response of plant spread to elicitors in kalmegh.
Table 3.
Response of plant spread to elicitors in kalmegh.
| Treatments |
Plant spread (cm2) |
| 70 DAS |
100 DAS |
At harvest |
| 2021 |
2022 |
Pooled |
2021 |
2022 |
Pooled |
2021 |
2022 |
Pooled |
|
T1: Chitosan spray @ 500 ppm |
15.72bc
|
15.42c
|
15.57c
|
23.82b
|
23.96c
|
23.89c
|
33.13b
|
33.29b
|
33.21b
|
|
T2: Chitosan spray @ 1000 ppm |
17.19a
|
17.42a
|
17.31a
|
26.76a
|
27.38a
|
27.07a
|
35.66a
|
34.56a
|
35.11a
|
|
T3: Yeast extract spray @ 500 ppm |
13.58ef
|
13.06ef
|
13.32g
|
20.30d
|
19.68f
|
19.99f
|
29.45de
|
30.59e
|
30.02c
|
|
T4: Yeast extract spray @ 1000 ppm |
15.24c
|
15.01c
|
15.13d
|
22.94c
|
23.14d
|
23.04d
|
32.86bc
|
32.89b
|
32.88b
|
|
T5:Jasmonic acid spray @ 100 ppm |
14.05de
|
13.53e
|
13.79f
|
20.79d
|
20.17f
|
20.48f
|
29.93d
|
31.07c
|
30.50c
|
|
T6:Jasmonic acid spray @ 200 ppm |
13.43ef
|
12.93ef
|
13.18g
|
19.16e
|
18.56g
|
18.86g
|
28.39ef
|
29.49d
|
28.94d
|
|
T7: Salicylic acidspray @ 100 ppm |
14.40d
|
14.19d
|
14.29e
|
22.56c
|
22.26e
|
22.41e
|
31.91c
|
32.42b
|
32.16b
|
|
T8: Salicylic acid spray @ 200 ppm |
16.23b
|
16.44b
|
16.34b
|
24.39b
|
24.96b
|
24.67b
|
35.85a
|
35.07a
|
35.46a
|
|
T9: Control (Water spray) |
13.18f
|
12.68f
|
12.93g
|
18.92e
|
18.32g
|
18.62g
|
27.76f
|
28.86d
|
28.31d
|
| S Em± |
0.21 |
0.21 |
0.15 |
0.21 |
0.21 |
0.15 |
0.36 |
0.36 |
0.25 |
| CD (0.05) |
0.64 |
0.64 |
0.43 |
0.64 |
0.64 |
0.43 |
1.06 |
1.06 |
0.72 |
| CV (%) |
2.52 |
2.57 |
2.55 |
1.68 |
1.69 |
1.69 |
1.97 |
1.94 |
1.95 |
Table 4.
Response of number of primary branches to elicitors in kalmegh.
Table 4.
Response of number of primary branches to elicitors in kalmegh.
| Treatments |
Primary branches (nos.) |
| 70 DAS |
100 DAS |
At harvest |
| 2021 |
2022 |
Pooled |
2021 |
2022 |
Pooled |
2021 |
2022 |
Pooled |
|
T1: Chitosan spray @ 500 ppm |
17.24b
|
18.76b
|
18.00b
|
21.06c
|
22.26b
|
21.66c
|
24.96b
|
25.58a
|
25.27b
|
|
T2: Chitosan spray @ 1000 ppm |
18.11a
|
19.81a
|
18.96a
|
23.26a
|
23.33a
|
23.30a
|
26.14a
|
26.58a
|
26.36a
|
|
T3: Yeast extract spray @ 500 ppm |
14.27e
|
16.04e
|
15.15e
|
18.53f
|
20.14e
|
19.34f
|
21.78e
|
22.53cd
|
22.16d
|
|
T4: Yeast extract spray @ 1000 ppm |
16.20c
|
18.28c
|
17.24c
|
19.91d
|
21.25c
|
20.58d
|
23.69c
|
24.12b
|
23.91c
|
|
T5:Jasmonic acid spray @ 100 ppm |
14.63de
|
16.40de
|
15.51de
|
18.96ef
|
20.57d
|
19.77ef
|
22.25de
|
23.00bc
|
22.63d
|
|
T6:Jasmonic acid spray @ 200 ppm |
13.37f
|
14.57f
|
13.97f
|
17.47g
|
19.82e
|
18.64g
|
20.59f
|
21.54d
|
21.07e
|
|
T7: Salicylic acidspray @ 100 ppm |
14.80d
|
16.73d
|
15.76d
|
19.19e
|
21.02c
|
20.11e
|
23.11cd
|
22.51cd
|
22.81d
|
|
T8: Salicylic acid spray @ 200 ppm |
17.23b
|
19.51a
|
18.37a
|
22.35b
|
23.01a
|
22.68b
|
26.01a
|
26.54a
|
26.28a
|
|
T9: Control (Water spray) |
13.02f
|
14.22f
|
13.62f
|
16.50h
|
18.85f
|
17.67h
|
20.35f
|
21.29d
|
20.82e
|
| S Em± |
0.16 |
0.16 |
0.11 |
0.17 |
0.14 |
0.11 |
0.31 |
0.40 |
0.25 |
| CD (0.05) |
0.49 |
0.47 |
0.33 |
0.50 |
0.41 |
0.31 |
0.93 |
1.20 |
0.73 |
| CV (%) |
1.88 |
1.62 |
1.74 |
1.51 |
1.16 |
1.33 |
2.35 |
2.95 |
2.67 |
Table 5.
Response of number of secondary branches to elicitors in kalmegh.
Table 5.
Response of number of secondary branches to elicitors in kalmegh.
| Treatments |
Secondary branches (nos.) |
| 70 DAS |
100 DAS |
At harvest |
| 2021 |
2022 |
Pooled |
2021 |
2022 |
Pooled |
2021 |
2022 |
Pooled |
|
T1: Chitosan spray @ 500 ppm |
11.42b
|
12.86ab
|
12.14b
|
22.11b
|
23.79b
|
22.95bc
|
25.43b
|
28.55b
|
26.99b
|
|
T2: Chitosan spray @ 1000 ppm |
12.57a
|
13.65a
|
13.11a
|
24.10a
|
25.41a
|
24.76a
|
27.98a
|
30.16a
|
29.07a
|
|
T3: Yeast extract spray @ 500 ppm |
8.67de
|
10.21e
|
9.44de
|
18.93de
|
20.93de
|
19.93ef
|
22.49ef
|
26.25e
|
24.37e
|
|
T4: Yeast extract spray @ 1000 ppm |
10.87b
|
12.34bc
|
11.60b
|
21.47bc
|
23.18bc
|
22.32c
|
24.58c
|
27.76c
|
26.17c
|
|
T5:Jasmonic acid spray @ 100 ppm |
9.13d
|
10.67de
|
9.90d
|
19.37de
|
21.37de
|
20.37de
|
23.03de
|
26.79de
|
24.91de
|
|
T6:Jasmonic acid spray @ 200 ppm |
8.44e
|
9.65ef
|
9.05ef
|
21.57bc
|
23.25bc
|
22.41c
|
21.90fg
|
25.39f
|
23.65f
|
|
T7: Salicylic acidspray @ 100 ppm |
9.74c
|
11.41cd
|
10.57c
|
20.16cd
|
22.07cd
|
21.11d
|
23.34d
|
26.92d
|
25.13d
|
|
T8: Salicylic acid spray @ 200 ppm |
12.48a
|
13.91a
|
13.20a
|
22.86ab
|
24.21ab
|
23.53b
|
26.02b
|
28.80b
|
27.41b
|
|
T9: Control (Water spray) |
7.86f
|
9.06f
|
8.46f
|
17.89e
|
20.00e
|
18.95f
|
21.61g
|
25.10f
|
23.35f
|
| S Em± |
0.19 |
0.36 |
0.20 |
0.48 |
0.49 |
0.34 |
0.23 |
0.19 |
0.15 |
| CD (0.05) |
0.56 |
1.08 |
0.59 |
1.44 |
1.46 |
0.99 |
0.70 |
0.57 |
0.43 |
| CV (%) |
3.27 |
5.49 |
4.67 |
4.01 |
3.77 |
3.88 |
1.71 |
1.22 |
1.46 |
Table 6.
Response of leaf length, leaf width and leaf area to elicitors in kalmegh.
Table 6.
Response of leaf length, leaf width and leaf area to elicitors in kalmegh.
| Treatments |
Leaf parameters at harvest |
| Leaf length (cm) |
Leaf width (cm) |
Leaf area (cm2) |
| 2021 |
2022 |
Pooled |
2021 |
2022 |
Pooled |
2021 |
2022 |
Pooled |
|
T1: Chitosan spray @ 500 ppm |
5.06a
|
5.14a
|
5.10a
|
1.35e
|
1.35f
|
1.35de
|
6.09a
|
6.20a
|
6.15c
|
|
T2: Chitosan spray @ 1000 ppm |
5.17a
|
5.48a
|
5.32a
|
1.34e
|
1.30g
|
1.32e
|
6.20a
|
6.42a
|
6.31bc
|
|
T3: Yeast extract spray @ 500 ppm |
5.59a
|
5.40a
|
5.50a
|
1.52b
|
1.53b
|
1.52b
|
7.68a
|
7.44a
|
7.56ab
|
|
T4: Yeast extract spray @ 1000 ppm |
5.24a
|
5.27a
|
5.25a
|
1.44c
|
1.45de
|
1.44c
|
6.76a
|
6.85a
|
6.80ab
|
|
T5:Jasmonic acid spray @ 100 ppm |
5.39a
|
5.22a
|
5.31a
|
1.45c
|
1.48cd
|
1.46c
|
7.03a
|
6.92a
|
6.98ab
|
|
T6:Jasmonic acid spray @ 200 ppm |
5.41a
|
5.33a
|
5.37a
|
1.49b
|
1.51bc
|
1.50b
|
7.25a
|
7.23a
|
7.24ab
|
|
T7: Salicylic acidspray @ 100 ppm |
5.23a
|
5.24a
|
5.23a
|
1.43c
|
1.43e
|
1.43c
|
6.71a
|
6.72a
|
6.71bc
|
|
T8: Salicylic acid spray @ 200 ppm |
5.11a
|
5.18a
|
5.14a
|
1.39d
|
1.36f
|
1.37d
|
6.35a
|
6.31a
|
6.33bc
|
|
T9: Control (Water spray) |
5.42a
|
5.13a
|
5.28a
|
1.61a
|
1.65a
|
1.63a
|
7.86a
|
7.57a
|
7.71a
|
| S Em± |
0.28 |
0.34 |
0.22 |
0.01 |
0.01 |
0.01 |
0.44 |
0.50 |
0.33 |
| CD (0.05) |
0.85 |
1.02 |
0.64 |
0.03 |
0.03 |
0.02 |
1.31 |
1.49 |
0.95 |
| CV (%) |
0.30 |
1.10 |
0.80 |
1.65 |
1.73 |
1.69 |
17.12 |
19.10 |
18.14 |
Table 7.
Response of number of leaves plant-1, leaf area plant-1 and leaf area index to elicitors in kalmegh.
Table 7.
Response of number of leaves plant-1, leaf area plant-1 and leaf area index to elicitors in kalmegh.
| Treatments |
Leaf parameters at harvest |
| Number of leaves plant-1
|
Leaf area plant-1(cm2) |
Leaf area index |
| 2021 |
2022 |
Pooled |
2021 |
2022 |
Pooled |
2021 |
2022 |
Pooled |
|
T1: Chitosan spray @ 500 ppm |
85.59a
|
85.59ab
|
85.59a
|
519.32ab
|
524.52ab
|
521.92ab
|
1.30ab
|
1.31ab
|
1.30ab
|
|
T2: Chitosan spray @ 1000 ppm |
88.32a
|
88.32a
|
88.32a
|
545.18a
|
560.00a
|
552.59a
|
1.36a
|
1.40a
|
1.38a
|
|
T3: Yeast extract spray @ 500 ppm |
58.37e
|
60.92de
|
59.65f
|
445.01cd
|
452.91cd
|
448.96e
|
1.11cd
|
1.13cd
|
1.12e
|
|
T4: Yeast extract spray @ 1000 ppm |
72.75bc
|
73.25bcd
|
73.00cd
|
491.73abcd
|
497.87bcd
|
494.80bcd
|
1.23abcd
|
1.24bcd
|
1.24bcd
|
|
T5:Jasmonic acid spray @ 100 ppm |
66.96cd
|
68.66cde
|
67.81de
|
469.71bcd
|
471.83bcd
|
470.77cde
|
1.17bcd
|
1.18bcd
|
1.18cde
|
|
T6:Jasmonic acid spray @ 200 ppm |
63.08de
|
63.82cde
|
63.45ef
|
457.10bcd
|
461.27cd
|
459.18de
|
1.14bcd
|
1.15cd
|
1.15de
|
|
T7: Salicylic acidspray @ 100 ppm |
75.64b
|
75.99abc
|
75.81bc
|
506.97abc
|
507.24abc
|
507.10bc
|
1.27abc
|
1.27abc
|
1.27bc
|
|
T8: Salicylic acid spray @ 200 ppm |
80.29ab
|
82.74ab
|
81.51ab
|
508.97ab
|
514.96abc
|
511.97bc
|
1.27ab
|
1.29abc
|
1.28bc
|
|
T9: Control (Water spray) |
55.87e
|
58.42e
|
57.14f
|
434.88d
|
441.88d
|
438.38e
|
1.09d
|
1.10d
|
1.10e
|
| S Em± |
2.61 |
3.91 |
2.35 |
19.04 |
18.90 |
13.42 |
0.50 |
0.05 |
0.03 |
| CD (0.05) |
7.77 |
11.63 |
6.75 |
56.59 |
56.17 |
38.49 |
1.14 |
0.14 |
0.09 |
| CV (%) |
6.31 |
9.28 |
7.96 |
6.78 |
6.65 |
6.72 |
6.75 |
6.65 |
6.70 |
Table 8.
Response of days taken for flower initiation and fifty per cent flowering of kalmegh to elicitors.
Table 8.
Response of days taken for flower initiation and fifty per cent flowering of kalmegh to elicitors.
| Treatments |
Days taken for flower initiation and harvest |
| Days taken for flower initiation |
Days taken for harvest |
| 2021 |
2022 |
Pooled |
2021 |
2022 |
Pooled |
|
T1: Chitosan spray @ 500 ppm |
113.21d
|
113.71c
|
113.46c
|
120.21d
|
120.71cd
|
120.46d
|
|
T2: Chitosan spray @ 1000 ppm |
110.39e
|
110.89d
|
110.64d
|
117.39e
|
117.89e
|
117.64e
|
|
T3: Yeast extract spray @ 500 ppm |
117.47a
|
118.19a
|
117.83a
|
124.47b
|
125.19a
|
124.83ab
|
|
T4: Yeast extract spray @ 1000 ppm |
117.09a
|
117.86a
|
117.47a
|
124.09b
|
124.86a
|
124.47b
|
|
T5:Jasmonic acid spray @ 100 ppm |
115.40b
|
115.49b
|
115.44b
|
122.40c
|
122.49b
|
122.44c
|
|
T6:Jasmonic acid spray @ 200 ppm |
114.38c
|
114.94b
|
114.66b
|
122.33c
|
122.12bc
|
122.23c
|
|
T7: Salicylic acidspray @ 100 ppm |
112.36d
|
113.04c
|
112.70c
|
119.36d
|
120.04d
|
119.70d
|
|
T8: Salicylic acid spray @ 200 ppm |
108.29f
|
108.71e
|
108.50e
|
115.29f
|
115.71f
|
115.50f
|
|
T9: Control (Water spray) |
117.71a
|
118.43a
|
118.07a
|
125.92a
|
125.97a
|
125.95a
|
| S Em± |
0.31 |
0.32 |
0.22 |
0.31 |
0.53 |
0.31 |
| CD (0.05) |
0.93 |
0.96 |
0.64 |
0.93 |
1.59 |
0.89 |
| CV (%) |
0.48 |
0.48 |
0.48 |
0.45 |
0.76 |
0.62 |
Table 9.
Response of fresh and dry herbage yield per plant and dry matter content per plant in kalmegh to elicitors.
Table 9.
Response of fresh and dry herbage yield per plant and dry matter content per plant in kalmegh to elicitors.
| Treatments |
Yield parameters at harvest |
Fresh herbage yield
plant-1 (g) |
Dry herbage yield plant-1 (g) |
Dry matter content
plant-1 (%) |
| 2021 |
2022 |
Pooled |
2021 |
2022 |
Pooled |
2021 |
2022 |
Pooled |
|
T1: Chitosan spray @ 500 ppm |
35.35b
|
39.58abc
|
37.47abc
|
13.64b
|
16.36abc
|
15.00ab
|
38.56b
|
41.13ab
|
39.84ab
|
|
T2: Chitosan spray @ 1000 ppm |
38.74a
|
42.08ab
|
40.41ab
|
16.25a
|
18.59ab
|
17.42a
|
41.93a
|
44.06a
|
43.00a
|
|
T3: Yeast extract spray @ 500 ppm |
30.75d
|
34.84bcd
|
32.79cd
|
11.13c
|
13.60bcd
|
12.36bc
|
36.17c
|
38.83bc
|
37.50b
|
|
T4: Yeast extract spray @ 1000 ppm |
33.75bc
|
38.17abc
|
35.96bcd
|
12.29c
|
14.90abc
|
13.59bc
|
36.40c
|
38.84bc
|
37.62b
|
|
T5:Jasmonic acid spray @ 100 ppm |
32.84cd
|
36.93abc
|
34.89bcd
|
11.96c
|
14.52abcd
|
13.24bc
|
36.40c
|
39.11b
|
37.75b
|
|
T6:Jasmonic acid spray @ 200 ppm |
26.91e
|
32.83cd
|
29.87de
|
9.54d
|
12.52cd
|
11.03cd
|
35.44c
|
37.84bc
|
36.64b
|
|
T7: Salicylic acidspray @ 100 ppm |
35.65b
|
39.68abc
|
37.66abc
|
13.76b
|
16.35abc
|
15.05ab
|
38.58b
|
41.04ab
|
39.81ab
|
|
T8: Salicylic acid spray @ 200 ppm |
40.99a
|
43.68a
|
42.34a
|
17.22a
|
19.37a
|
18.30a
|
42.01a
|
44.25a
|
43.13a
|
|
T9: Control (Water spray) |
22.33f
|
28.25d
|
25.29e
|
6.92e
|
9.76d
|
8.34d
|
30.93d
|
34.11c
|
32.52c
|
| S Em± |
0.80 |
2.59 |
1.32 |
0.43 |
1.52 |
0.79 |
0.42 |
1.49 |
0.77 |
| CD (0.05) |
2.38 |
7.48 |
3.79 |
1.28 |
4.52 |
2.26 |
1.26 |
4.44 |
2.2 |
| CV (%) |
4.21 |
11.68 |
9.20 |
5.98 |
17.44 |
14.02 |
1.96 |
6.50 |
4.93 |
Table 10.
Response of chlorophyll a, b and total chlorophyll content in kalmegh to elicitors.
Table 10.
Response of chlorophyll a, b and total chlorophyll content in kalmegh to elicitors.
| Treatments |
Chlorophyll contents (mg g-1) |
| Chlorophyll a |
Chlorophyll b |
Total chlorophyll |
| 2021 |
2022 |
Pooled |
2021 |
2022 |
Pooled |
2021 |
2022 |
Pooled |
|
T1: Chitosan spray @ 500 ppm |
2.004e
|
2.271e
|
2.138e
|
1.490e
|
1.710e
|
1.600e
|
3.510e
|
3.997e
|
3.753e
|
|
T2: Chitosan spray @ 1000 ppm |
2.051d
|
2.381d
|
2.216d
|
1.542d
|
1.772d
|
1.657d
|
3.611d
|
4.171d
|
3.891d
|
|
T3: Yeast extract spray @ 500 ppm |
1.725h
|
1.975h
|
1.850h
|
1.275h
|
1.474h
|
1.375h
|
3.013h
|
3.463h
|
3.23h8
|
|
T4: Yeast extract spray @ 1000 ppm |
1.838g
|
2.148g
|
1.993g
|
1.325g
|
1.483g
|
1.404g
|
3.178g
|
3.646g
|
3.412g
|
|
T5:Jasmonic acid spray @ 100 ppm |
2.072c
|
2.407c
|
2.240c
|
1.570c
|
1.805c
|
1.687c
|
3.660c
|
4.230c
|
3.945c
|
|
T6:Jasmonic acid spray @ 200 ppm |
1.950f
|
2.233f
|
2.092f
|
1.438f
|
1.648f
|
1.543f
|
3.403f
|
3.896f
|
3.649f
|
|
T7: Salicylic acidspray @ 100 ppm |
2.194b
|
2.554b
|
2.374b
|
1.651b
|
1.911b
|
1.781b
|
3.864b
|
4.484b
|
4.174b
|
|
T8: Salicylic acid spray @ 200 ppm |
2.344a
|
2.714a
|
2.529a
|
1.774a
|
2.044a
|
1.909a
|
4.139a
|
4.779a
|
4.459a
|
|
T9: Control (Water spray) |
1.604i |
1.811i
|
1.707i
|
1.171i
|
1.404i
|
1.288i
|
2.779i
|
3.243i
|
3.011i
|
| S Em± |
0.001 |
0.002 |
0.001 |
0.001 |
0.001 |
0.001 |
0.005 |
0.002 |
0.003 |
| CD (0.05) |
0.003 |
0.006 |
0.003 |
0.003 |
0.002 |
0.003 |
0.014 |
0.007 |
0.008 |
| CV (%) |
0.080 |
0.151 |
0.130 |
0.109 |
0.082 |
0.095 |
0.241 |
0.105 |
0.173 |
Table 11.
Response of carotenoids and andrographolide contents in kalmegh to elicitors.
Table 11.
Response of carotenoids and andrographolide contents in kalmegh to elicitors.
| Treatments |
Quality parameters in kalmegh |
| Carotenoid content (mg g-1) |
Andrographolide content (%) (A) |
Neoandrographolide content (%) (B) |
| 2021 |
2022 |
Pooled |
2021 |
2022 |
Pooled |
2021 |
2022 |
Pooled |
|
T1: Chitosan spray @ 500 ppm |
2.806c
|
3.126b
|
2.966b
|
2.477d
|
2.537d
|
2.507d
|
0.364c
|
0.393cd
|
0.379c
|
|
T2: Chitosan spray @ 1000 ppm |
2.858c
|
3.188b
|
3.023b
|
2.646c
|
2.707c
|
2.676c
|
0.338c
|
0.367d
|
0.352c
|
|
T3: Yeast extract spray @ 500 ppm |
2.577d
|
2.807c
|
2.692c
|
2.026g
|
2.086g
|
2.056g
|
0.368c
|
0.397c
|
0.382c
|
|
T4: Yeast extract spray @ 1000 ppm |
2.594d
|
2.844c
|
2.719c
|
2.268e
|
2.328e
|
2.298e
|
0.348c
|
0.377cd
|
0.363c
|
|
T5:Jasmonic acid spray @ 100 ppm |
3.294b
|
3.629a
|
3.462a
|
2.866b
|
2.926b
|
2.896b
|
0.211d
|
0.240e
|
0.225d
|
|
T6:Jasmonic acid spray @ 200 ppm |
2.666d
|
2.976bc
|
2.821bc
|
2.105f
|
2.165f
|
2.135f
|
0.566a
|
0.595a
|
0.580a
|
|
T7: Salicylic acidspray @ 100 ppm |
3.394ab
|
3.754a
|
3.574a
|
2.688c
|
2.748c
|
2.718c
|
0.400b
|
0.429b
|
0.414b
|
|
T8: Salicylic acid spray @ 200 ppm |
3.489a
|
3.859a
|
3.674a
|
2.936a
|
2.997a
|
2.966a
|
0.221d
|
0.250e
|
0.236d
|
|
T9: Control (Water spray) |
2.140e
|
2.527d
|
2.334d
|
1.834h
|
1.896h
|
1.865h
|
0.236d
|
0.266e
|
0.251d
|
| S Em± |
0.034 |
0.085 |
0.046 |
0.019 |
0.018 |
0.013 |
0.010 |
0.009 |
0.007 |
| CD (0.05) |
0.100 |
0.251 |
0.131 |
0.057 |
0.052 |
0.037 |
0.029 |
0.026 |
0.019 |
| CV (%) |
2.041 |
4.604 |
3.682 |
1.372 |
1.222 |
1.301 |
4.918 |
4.134 |
4.516 |
| Treatments |
Andrographolides content (%) |
14-Deoxy-11,12-didehydro
Andrographolide content(C)
|
Andrograpanin content (%) (D) |
Total Andrographolide content (%) (A+B+C+D) |
| 2021 |
2022 |
Pooled |
2021 |
2022 |
Pooled |
2021 |
2022 |
Pooled |
|
T1: Chitosan spray @ 500 ppm |
0.155b
|
0.182b
|
0.169b
|
0.020cd
|
0.030c
|
0.025c
|
3.016cd
|
3.142cd
|
3.079d
|
|
T2: Chitosan spray @ 1000 ppm |
0.105c
|
0.132c
|
0.119c
|
0.015cd
|
0.024cd
|
0.019cd
|
3.104bc
|
3.230bc
|
3.167cd
|
|
T3: Yeast extract spray @ 500 ppm |
0.054d
|
0.081d
|
0.068d
|
0.014cd
|
0.024cd
|
0.019cd
|
2.462f
|
2.588f
|
2.525g
|
|
T4: Yeast extract spray @ 1000 ppm |
0.041d
|
0.068d
|
0.055d
|
0.016cd
|
0.025cd
|
0.021cd
|
2.673e
|
2.799e
|
2.736f
|
|
T5:Jasmonic acid spray @ 100 ppm |
0.105c
|
0.132c
|
0.119c
|
0.016cd
|
0.025cd
|
0.020cd
|
3.197bc
|
3.323b
|
3.260bc
|
|
T6:Jasmonic acid spray @ 200 ppm |
0.166d
|
0.193b
|
0.179b
|
0.031b
|
0.040b
|
0.035b
|
2.867d
|
2.993d
|
2.930e
|
|
T7: Salicylic acidspray @ 100 ppm |
0.119c
|
0.145c
|
0.132c
|
0.021c
|
0.030c
|
0.026c
|
3.227b
|
3.352b
|
3.290b
|
|
T8: Salicylic acid spray @ 200 ppm |
0.233a
|
0.260a
|
0.246a
|
0.041a
|
0.051a
|
0.046a
|
3.432a
|
3.557a
|
3.494a
|
|
T9: Control (Water spray) |
0.120c
|
0.146c
|
0.133c
|
0.010d
|
0.019d
|
0.015d
|
2.199g
|
2.328g
|
2.263h
|
| S Em± |
0.006 |
0.006 |
0.004 |
0.003 |
0.003 |
0.002 |
0.038 |
0.035 |
0.026 |
| CD (0.05) |
0.019 |
0.017 |
0.012 |
0.009 |
0.009 |
0.006 |
0.114 |
0.105 |
0.074 |
| CV (%) |
9.075 |
6.844 |
7.854 |
26.345 |
16.996 |
20.806 |
2.282 |
2.001 |
2.141 |
Table 12.
Correlation matrix of important parameters of kalmegh (Elicitor treatments).
Table 12.
Correlation matrix of important parameters of kalmegh (Elicitor treatments).
| |
PH |
PS |
NPB |
NSB |
NL |
LAP |
LAI |
FRY |
DRY |
DMAT |
CHLO |
ANDR |
| PH |
|
|
|
|
|
|
|
|
|
|
|
|
| PS |
0.94** |
|
|
|
|
|
|
|
|
|
|
|
| NPB |
0.97** |
0.98** |
|
|
|
|
|
|
|
|
|
|
| NSB |
0.96** |
0.95** |
0.98** |
|
|
|
|
|
|
|
|
|
| NL |
0.91** |
0.92** |
0.93** |
0.93** |
|
|
|
|
|
|
|
|
| LAP |
0.89** |
0.92** |
0.90** |
0.94** |
0.98** |
|
|
|
|
|
|
|
| LAI |
0.89** |
0.91** |
0.90** |
0.94** |
0.98** |
0.99** |
|
|
|
|
|
|
| FRY |
0.80** |
0.95** |
0.91** |
0.87** |
0.88** |
0.87** |
0.87** |
|
|
|
|
|
| DRY |
0.82** |
0.96** |
0.92** |
0.89** |
0.89** |
0.88** |
0.88** |
0.99** |
|
|
|
|
| DMAT |
0.79* |
0.92** |
0.89** |
0.87** |
0.87** |
0.87** |
0.87** |
0.97** |
0.99** |
|
|
|
| CHLO |
0.47 |
0.68* |
0.61 |
0.54 |
0.67* |
0.64* |
0.65* |
0.81** |
0.82** |
0.81** |
|
|
| ANDR |
0.50 |
0.68* |
0.63 |
0.59 |
0.72* |
0.69* |
0.69* |
0.82** |
0.82** |
0.82** |
0.98** |
|
| PH |
: Plant height |
NL |
: Number of leaves |
DRY |
: Dry herbage yield |
| PS |
: Plant spread |
LAP |
: Leaf area per plant |
DMAT |
: Dry matter content |
| NPB |
: Number of primary branches |
LAI |
: Leaf area index |
CHLO |
: Total chlorophyll |
| NSB |
: Number of secondary branches |
FRY |
: Fresh herbage yield |
ANDR |
: Total andrographolides |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).