1. Introduction
Osteoarthritis (OA) is a chronic degenerative disease of multifactorial etiology that affects the joints and represents the most frequent cause of pain and disability in adults [
1]. This disease is characterized by the gradual deterioration and weakening of the articular cartilage, resulting in both pain and a decline in joint function. [
1,
2]. Histologically, articular cartilage is a special connective tissue (hyaline cartilage), with a single cell type, the chondrocyte, surrounded by an extracellular matrix composed mainly of water and proteins, among which type II collagen and glycosaminoglycans (mainly aggrecan) stand out due to their abundance [
3]. The first approach to treat OA, which today continue being the main therapeutic option, is focused on relieving pain and inflammation of the affected joint [
1,
2]. It has been published that there are some risk factors that predispose to OA development. Age, genetic factors, obesity, gender (women), and other factors such as professional occupation and physical activity seem to be the most important factors related to OA development [
1,
2,
3].
Recently, a series of biomarkers such as cytokines, enzymes and extracellular matrix molecules have been identified as precursors of collagen degradation associated with OA [
4]. There are several therapeutic strategies for the management of OA, which can be classified into 2 groups: pharmacological treatment and lifestyle modification [
5,
6,
7]. Since pain is the symptom that most frequently appears in patients with OA, pain relievers such as analgesia and non-steroidal anti-inflammatory drugs are the most common treatments used. Inhibition of extracellular matrix degradation is another strategy for OA treatment. In this sense, doxycycline is a potent inhibitor of metalloproteinases but has shown little effect on the improvement of the disease [
8].
Other compounds, such as bisphosphonates, which inhibit the activity of osteoclasts could have clinical benefits. However, clinical trials conducted with risedronate or zoledronic acid have not shown reproducible results, so these drugs are not currently used for the treatment of OA [
9,
10].
Other strategies could be based in the use of biological therapies to modify the ac-tivity of inflammation mediators. As an example, Anankira a recombinant antagonist of the interleukin-1 (IL-1) receptor has clinical benefits, but the response does not last more than 4 days, so its use is very limited [
11]. Other different biological therapies have also been tried without results, such as Adalimumab, a monoclonal antibody with anti-TNF activity (Tumor Necrosis Factor), the Recombinant Bone Morphogenetic Protein or the Recombinant Fibroblast Growth Factor [
12]. Also, therapies based on the use of stem cells have been tested in animals, but, at this moment, no clinical trials have been carried out in patients with OA to find out if they are effective and safe [
13].
Since none of the aforementioned treatments have shown a clear clinical benefit for the treatment of OA, it is necessary to look for other ways to treat or to slow the disease progression. One of the alternatives could be found through the new nutritional and lifestyle modification strategies that, used in prevention and in the early stages of the disease, can help to improve the patient's quality of life and delay implant surgery. Regarding lifestyle modification, obesity control stands out for its importance. Weight loss and exercise to strengthen muscles are two of the most common medical recommendations for the treatment of OA [
5,
6].
Another strategy to treat OA or to delay OA progression could be adding food supplements, concentrated sources of nutrients that have a nutritional and/or physio-logical effect on the inflammatory process. Currently, high-quality and effective sup-plements are available and they represent an effective and convenient help at different stages of life, for different population groups even in people with intense activities or in limiting situations. Chondroitin sulfate and glucosamine, which have shown an-ti-inflammatory properties in vitro are two of the most commonly used nutrients in food supplements, although they seem to show little clinical benefit in osteoarthritic patients [
14].
One of the most interesting food supplements, that is widely used for joint health, is collagen, the most abundant protein in the body. Collagen is a structural protein of connective tissues, present in the skin, tendons, cartilage or bone, giving support and resistance [
15]. Collagen is a fibrous protein composed by fibrils disposed in a triple-helix. Up to 21 types of collagen have been described in mammals, differing among them in the way in which their fibrils are distributed. Type II collagen is the main collagenous component of cartilage. Hydrolyzed collagens are one of the food supplements that are available in the market. They are composed by small collagen peptides that do not seem to have significant clinical benefit [
16]. It is possibly due to the fact that they are more susceptible to degradation during the digestive process, due to the difficulty of reaching the joint tissue, which has very low blood supply. Undenatured type II collagen (UC-II), in which the triple helix of collagen is maintained, is an alternative to hydrolyzed collagen. This compound derived from chicken sternum cartilage and presents an oral tolerance process, since it is adsorbed by a specific part of the intestinal surface known as Peyer's Patches, which are clusters of lymphatic tissue that line the mucous membranes of the intestine [
16,
17,
18]. Uptake of UC-II by Peyer's patches triggers an immune cascade where regulatory T cells (Tregs) are activated. Tregs are T lymphocytes that regulate or suppress other cells of the immune system. These immune cells migrate to areas of inflammation where they secrete anti-inflammatory molecules such as IL-4, IL-10 and TGF-β. This action helps the physiological process of joint recovery and maintenance [
16,
17,
18].
The main objective of this study was to evaluate the impact of oral supplementation with UC-II for 6 months on pain symptomatology in OA patients. To this end, the evolution of pain was observed using the visual analogue scale (VAS). Likewise, the appearance of adverse effects throughout the intervention will be studied and the changes in the prescription of concomitant medication throughout the treatment will be evaluated.
2. Materials and Methods
This was a longitudinal, retrospective study to evaluate the effects on pain of UC-II in patients with OA in the knee who received this food supplement for 6 months. To be eligible for the study, patients of an age between 18 and 80 years should have knee OA of any grade, diagnosed by MR image, without any previous joint surgery that could alter the symptoms of the ongoing OA. The study was performed in accordance to the ethical standards of the Helsinki Declaration of 1964, revised in 2013, was approved by the Ethics Committee of the Institution and patients signed an informed consent.
Participants who signed their informed consent form to take part in the present study were required to consume 40 mg of UC-II (CondroArtil®) as a dietary supplement on a daily basis for duration of 6 months. In addition to UC-II, the complex CondroArtil® also contains some other compounds: 160 mg Vitamin C, 10 µg Vitamin D3, 45 µg Vitamin K2, 1mg Copper and 2 mg Manganese.
The pain level, measured using the VAS, was evaluated twice. The first time, before pre-scribing UC-II (basal evaluation), and the second, as a ending evaluation, after a period of 6 months. Additionally, the impact of various factors on pain reduction was examined. These factors encompassed demographic variables, as well as the intake of medications or food supplements and participation in physical therapy.
Statistical analysis was carried out with the the IBM® SPSS® Statistics Version 22. Software. Categorical variables were expressed as counts and/or percentages and were analyzed with the Fisher’s exact or Pearson’s χ2 tests. In the case of quantitative variables, normality was assumed for n>30, and in those cases, they were expressed as the mean as a measure of central tendency and standard deviation as a measure of dispersion. For variables with n<30, it was first studied whether they followed a normal distribution or not using the Kolmogorov-Smirnov test. In the case of non-normally distributed variables, the median was used as a measure of central tendency, and the maximum and minimum were used as measures of dispersion. The existence of correlation between different variables was determined using Pearson's R coefficient and its 95% confidence interval. For normally distributed variables, means were compared using the Student's t-test, with Levene's test used to demonstrate homoscedasticity. Non-normally distributed variables were compared using the Mann-Whitney U test. The Kruskal-Wallis test was used to compare pain among different grades of OA. The G*Power Version 3.1.9.2 software was used to estimate post-hoc power in all statistically significant comparisons following univariate analysis. For statistical acceptability, a power of ≥80% was deemed necessary. To study the impact of all factors simultaneously, multivariate analysis utilizing a repeated measures analysis of variance was conducted. The hypothesis of equality between means was tested using Wilks' lambda, and sphericity was tested using Mauchly's W statistic. All statistical comparisons were two-tailed, with a p-value<0.05 considered statistically significant.
3. Results
3.1. Patient characteristics
A total of 100 patients agreed to take part in the current study.
Table 1 shows the demographic characteristics of the patients. Most patients were males, 62.0%, who were under the age of 50, and had a BMI that was either normal or slightly high.
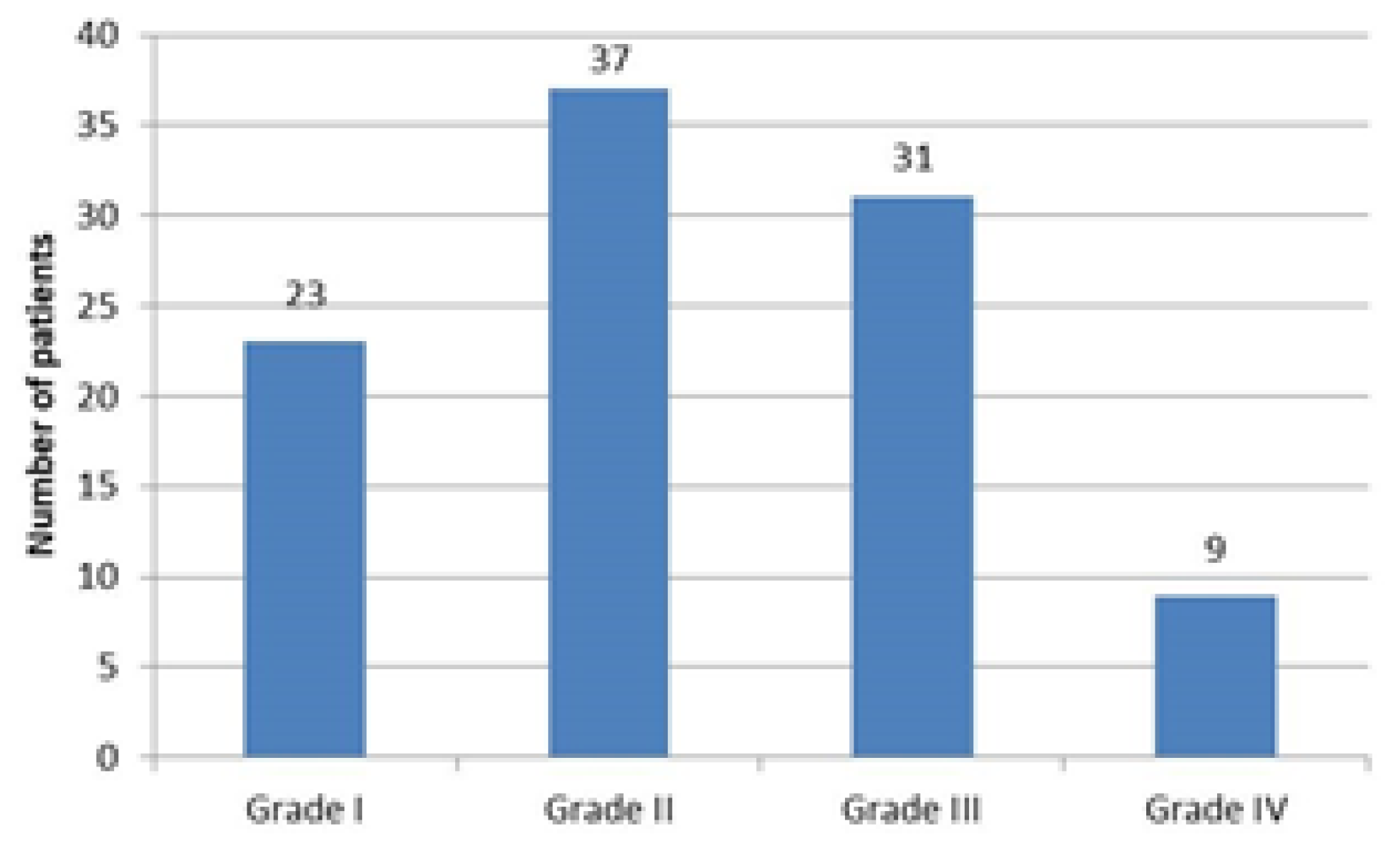
Most of the patients (60%) had either grade I or II OA, with 23 and 37 patients respectively, while only 9 patients had grade IV OA, as illustrated in
Figure 1. Among the patients, 68 had received some form of treatment for OA. This treatment typically involved analgesia alone or in combination with nonsteroidal anti-inflammatory drugs (NSAIDs) and was administered to 55 patients. Other minor treatments included NSAIDs alone, hyaluronic acid, corticoids, ozone, and platelet-rich plasma. Physical therapy was received by 14 patients, while 5 took food supplements such as calcium, vitamins B or D, or curcumin.
3.2. Pain reduction study: univariate analysis
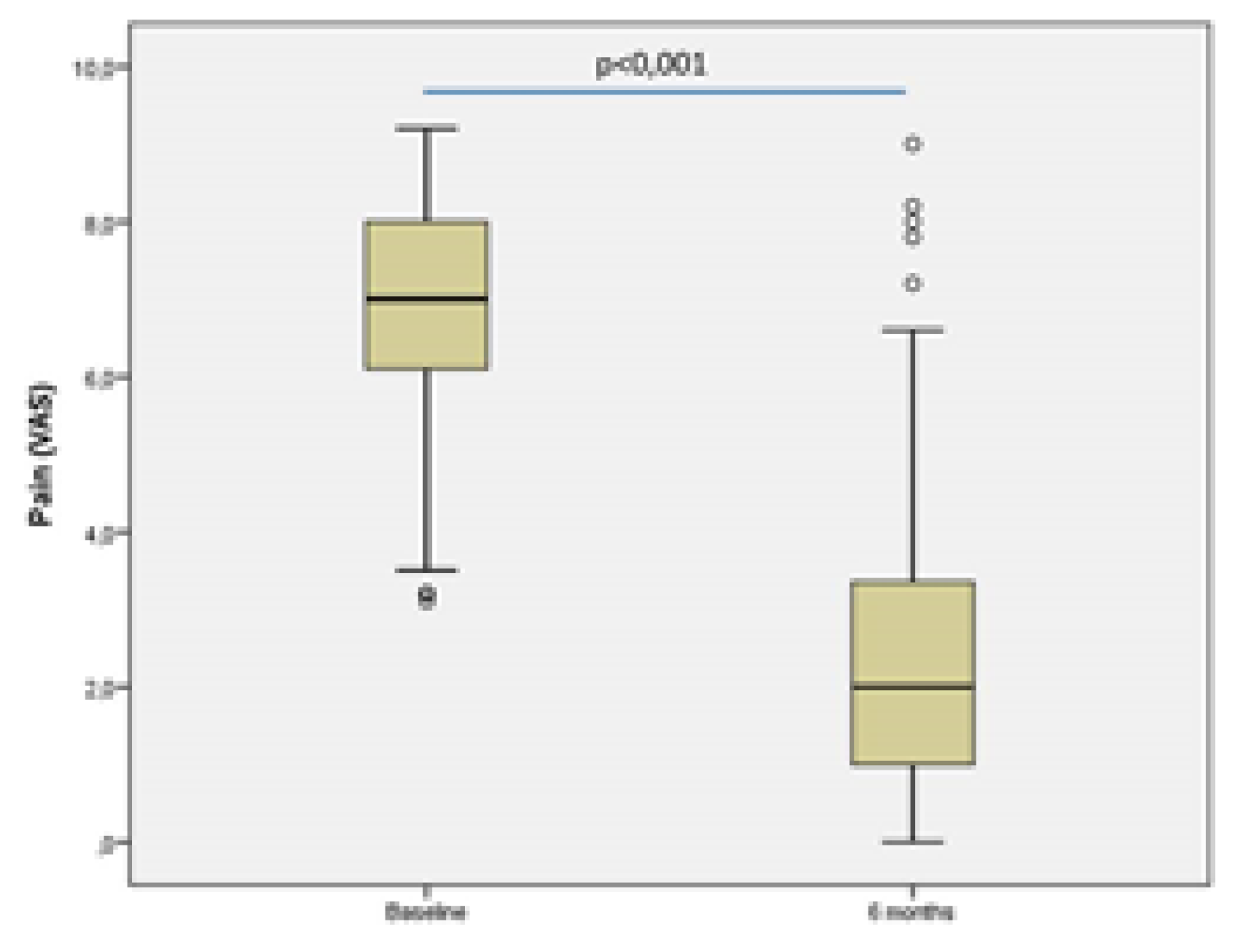
The results of the VAS are shown in
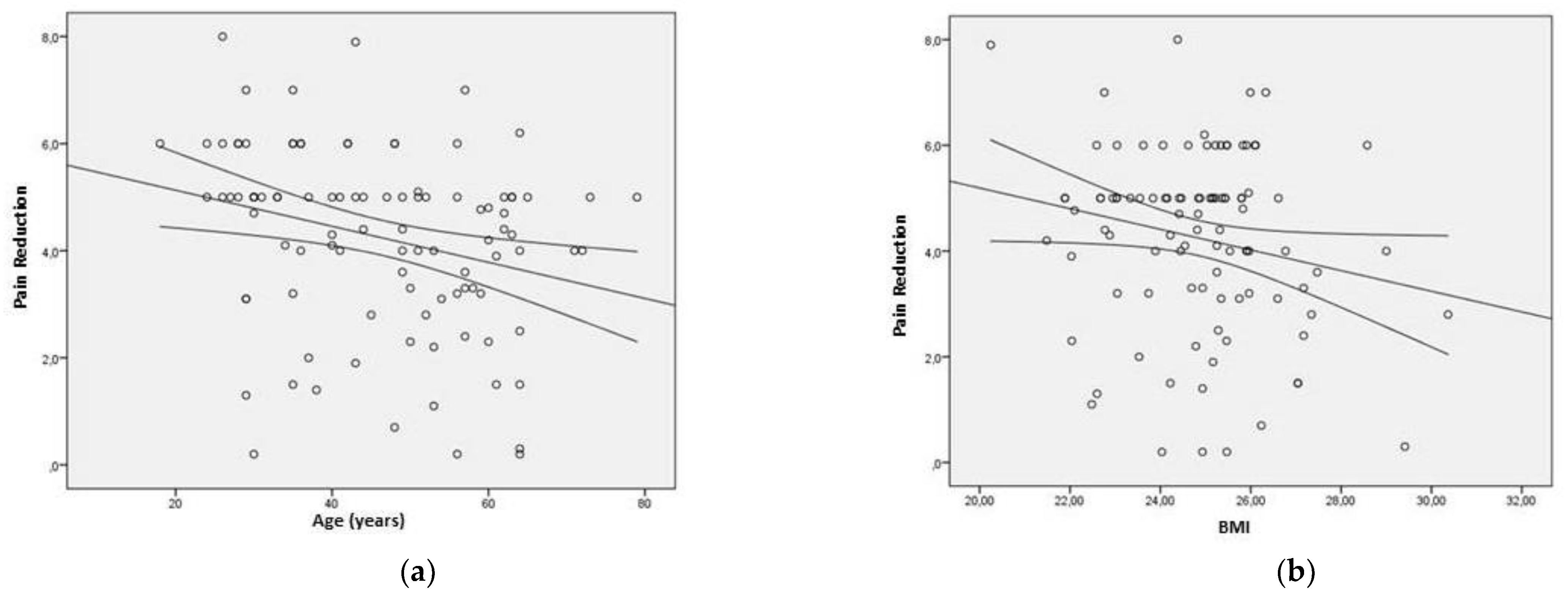
Figure 2. demonstrates a significant reduction in pain levels, as evidenced by the decrease in mean value from 6.9 ± 1.5 during the basal assessment to 2.6 ± 2.0 after six months of taking UC-II. This difference was found to be statistically significant (p<0.001; paired student’s t-test). To determine the pain reduction, the difference between the VAS at 6 months and the baseline was assessed. A negative correlation was observed between pain reduction and age (Pearson's R coefficient=-0.273; p=0.006) (
Figure 3a), as well as between BMI and pain reduction (Pearson's R coefficient=-0.197; p=0.049) (
Figure 3b). Post-hoc statistical powers were 79% and 50%, respectively.
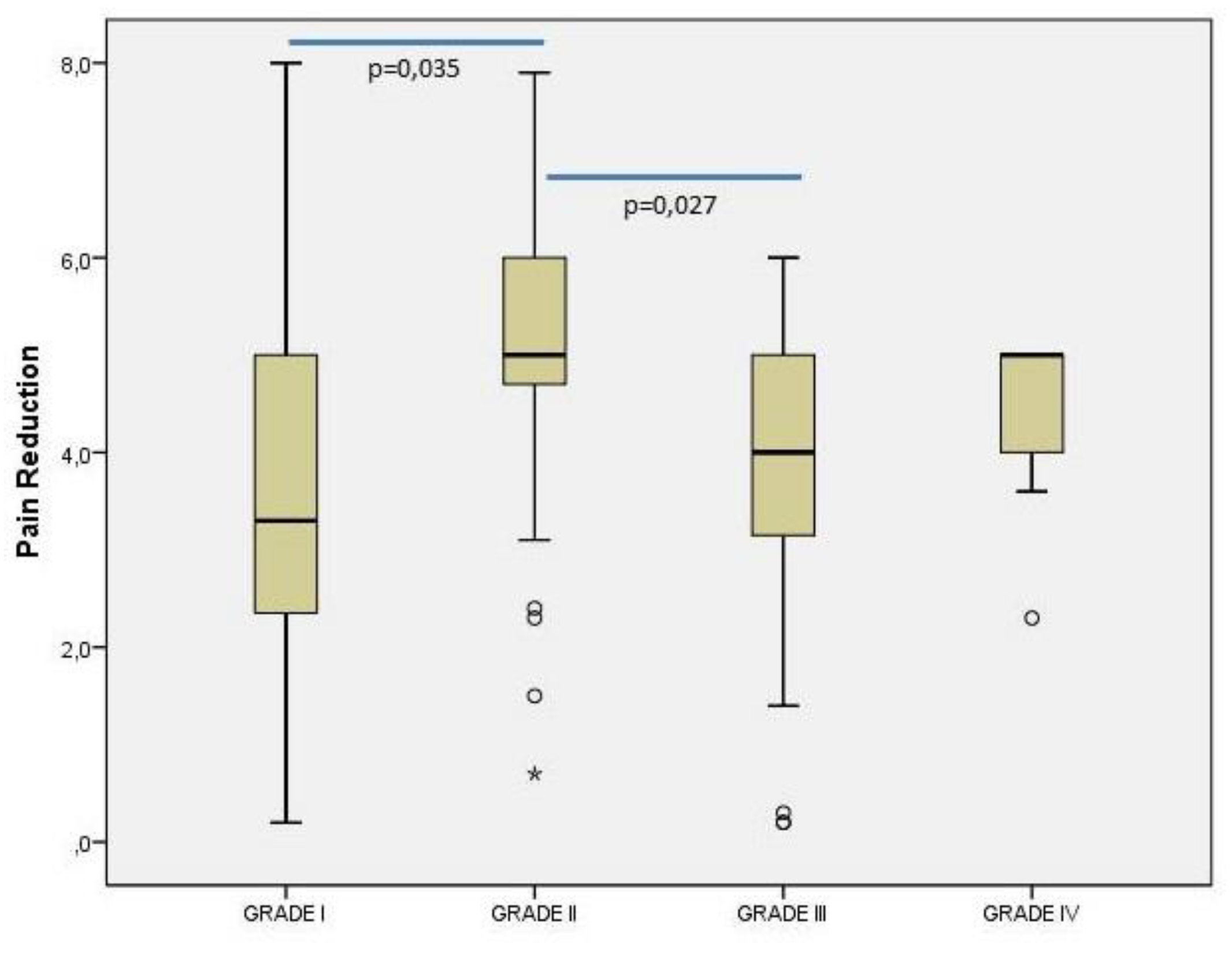
The
Figure 4 illustrates the effect of OA severity on pain reduction. A significant relationship was observed between the severity grade and the difference in visual analog scale (VAS) scores at 6 months and baseline (p=0.011; Kruskal-Wallis Test; post-hoc power=95%). The results of pairwise comparisons revealed that the median (minimum – maximum) VAS score was significantly higher in patients with grade II [5.0 (0.7 – 7.9)] compared to those with grade I [3.3 (0.2 – 8.0)] (p=0.035) or grade III [4.0 (0.2 – 6.0)] (p=0.027).
In this study we also examined the influence of the demographics and other variables that could affect the pain reduction. Univariate analysis results are presented in
Table 2. Both sex and the affected knee did not have a statistically significant effect on the mean pain reduction (p=0.354 and p=0.303, respectively). Conversely, other factors such as dominance, previous treatment, physical therapy sessions, and food supplement intake had a statistically significant impact on pain reduction (
Table 2). It is worth noting that the post-hoc statistical power for dominance, previous physical therapy sessions, and food supplement intake was 73%, 75%, and 79%, respectively. Regarding previous treatment, patients who received treatment before had a statistically higher mean pain reduction than those who did not (p<0.001, post-hoc power=92%) (
Table 2).
3.2. Pain reduction study: multivariate analysis
After conducting multivariate analysis and exploring all potential interactions among variables, a statistically significant model was obtained by including basal and 6-month pain as dependent variables and discarding all nonsignificant variables. The results, presented in
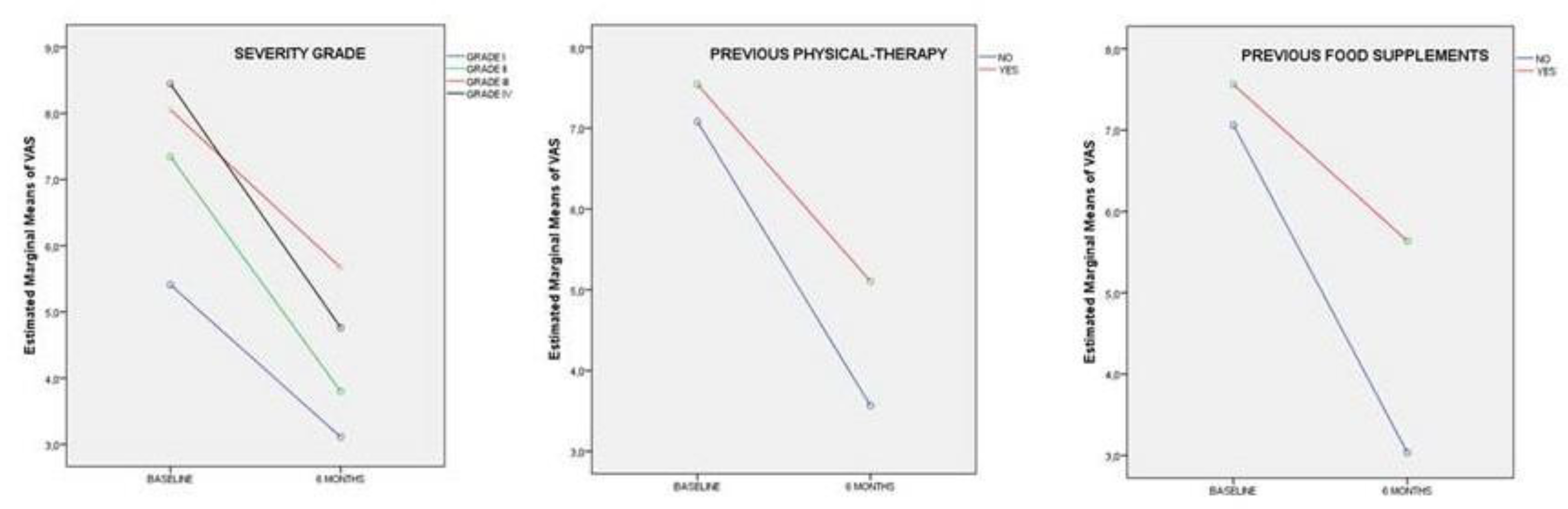
Table 3, indicate that OA severity grade with previous physical therapy treatment, and prior intake of food supplements had a significant impact on pain reduction from baseline to the final of the supplementation with UC-II.
Figure 5 demonstrates that after 6 months of UC-II treatment, there was a reduction in VAS from the baseline value, regardless of the severity grade of OA or whether patients had previously undergone physical therapy or added food supplements to their diet. However, the decrease was more pronounced in patients with higher severity grades of OA and those who had not undergone previous physical therapy or added food supplements to their diet.
4. Discussion
Undenatured type II collagen (UC-II) is a form of collagen that retains its native triple helix structure, unlike hydrolyzed collagen, which is broken down into smaller peptides. UC-II has gained attention as a potential alternative for managing OA due to its unique properties [
19]. In vitro studies have been based on elucidating the role that UC-II collagen exerts on regulatory T cells, and the role of these cells as an effective therapy in the management of OA, as well as the possible regulation of inflammation factors, such as interleukins, TNF-α and TGF-β [
20,
21]. Animal studies showed how UC-II collagen supplementation reduced pain and improved movement in joint injury animal models [
22,
23,
24,
25,
26,
27]. There are some studies in which the role of UC-II in joint degradation decrease has been demonstrated [
28]. The present study aimed to evaluate the effect of UC-II supplementation on pain reduction in patients with knee OA. The results showed that UC-II supplementation led to a significant reduction in pain levels, as evidenced by the decrease in mean VAS scores from 6.9 ± 1.5 during the baseline assessment to 2.6 ± 2.0 after six months of treatment. This finding is consistent with the results of several other studies that have investigated the efficacy of UC-II in reducing joint pain and inflammation. Numerous studies have shown that a reduction in Visual Analog Scale (VAS) scores serves as a reliable indicator for assessing pain reduction across various medical conditions (29,30). These findings highlight the widespread adoption of VAS as a valuable tool to measure the effectiveness of interventions and evaluate changes in pain intensity. Clinical trials in humans confirm all the results obtained in the in vitro and in vivo studies. [
15,
31,
32,
33,
34,
35,
36], being more effective in managing pain and mobility than the combination of glucosamine and sulphate-chondroitin [
19].
A randomized, double-blind, placebo-controlled study conducted by Crowley et al. (2009) [
15] found that UC-II supplementation significantly reduced joint pain and stiffness in patients with knee OA. Similarly, a randomized, double-blind, placebo-controlled trial conducted by Lugo et al. (2013) [
36] found that UC-II supplementation resulted in a significant reduction in joint pain and stiffness in healthy individuals who performed high-intensity exercise.
In the current study, a negative correlation was observed between pain reduction and both age and BMI. Although this aligns with previous research that has linked age and BMI to joint pain, the statistical power of these findings was low (79% and 50%) and may not be considered significant. However, a systematic review and meta-analysis by Zhang et al. (2018) [
37] revealed a significant association between higher BMI and an increased risk of knee OA, as well as more severe joint pain. Additionally, van Dijk et al. (2016) [
38] found that older age was associated with a higher risk of developing knee OA and experiencing more severe joint pain.
The severity grade of OA was also found to have a significant impact on pain reduction in the present study (post-hoc statistical power = 94%). In that sense, the patients with higher severity grades of OA had a greater reduction in pain levels after six months of UC-II treatment compared to those with lower severity grades. This finding is consistent with the results of a study conducted by Kumar et al. (2016) [
39], which found that UC-II supplementation was effective in reducing pain and inflammation in patients with moderate to severe knee OA.
Previous treatment, physical therapy, and food supplement intake were also found to have a significant impact on pain reduction in the present study. Patients who received treatment before had a statistically higher mean pain reduction than those who did not (post-hoc statistical power = 92%). This finding suggests that previous treatment for OA with antinflammatory substances, NSAIDs or corticosteroids, may enhance the effectiveness of UC-II supplementation in reducing joint pain. Physical therapy sessions and food supplement intake also had a statistically significant impact on pain reduction, further supporting the role of non-pharmacological interventions in the management of OA. However, these results should be taken cautiously since statistical powers were 75% and 79%, respectively.
Multivariate analysis revealed that OA severity grade, previous physical therapy treatment, and prior intake of food supplements had a significant impact on pain reduction from baseline to the end of the treatment with UC-II. These findings suggest that the effectiveness of UC-II supplementation in reducing joint pain may be influenced by various factors, including the severity of OA and the use of other non-pharmacological interventions.
The present study has some limitations that should be considered when interpreting the results. The first one is that the study did not have a control group, which limits the ability to draw conclusions about the efficacy of UC-II supplementation compared to a placebo. Secondly, the study did not investigate the long-term effects of UC-II supplementation on pain reduction, which is an important area for future research. On the third limitation we can not offer the mechanisms underlying the observed effects of UC-II supplementation on pain reduction as it was not designed initially for that purpose, and finally, the study did not evaluate long-term outcomes, which is important given the chronic nature of knee OA.
5. Conclusions
In conclusion, the results of this study suggests that UC-II can be used as an effective treatment for reducing pain in patients with knee OA, mainly if other medical therapy has been used before. The findings highlight the importance of early intervention and the potential benefits of UC-II for patients with more severe OA. Future studies with larger sample sizes and longer follow-up periods are needed to further evaluate the efficacy and safety of UC-II for knee OA.
Author Contributions
Conceptualization, T.F.F.J. and P.B.; methodology, P.G.G., T.F.F.J. and C.G.V.; validation, P.S., P.B., E.R.I. and J.M.L.A.; formal analysis, J.M.L.A..; investigation, P.G.G., T.F.F.J. and C.G.V.; data curation, E.R.I.; writing—original draft preparation, P.S. and J.M.L.A.; writing—review and editing, P.B., E.R.I, F.D.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Clínica CEMTRO Research Committee (protocol code CDI 015/22, date of approval: July 5, 2022).
Informed Consent Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Clínica CEMTRO Research Committee (protocol code CDI 015/22, date of approval: July 5, 2022).
Informed Consent Statement
Written informed consent has been obtained from the patient(s).
Data Availability Statement
Data is unavailable due to ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Farooqi, A. Osteoarthritis. Best Pract Res Clin Rheumatol 2008, 22, 657–675. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, X.; Richette, P. Cartílago articular normal: anatomía, fisiología, metabolismo y envejecimiento. EMC - Apar. Locomot. 2005, 38, 1–13. [Google Scholar] [CrossRef]
- van Spil, W.; DeGroot, J.; Lems, W.; Oostveen, J.; Lafeber, F.; Lawrenson, P.R.; Crossley, K.M.; Vicenzino, B.T.; Hodges, P.W.; James, G.; et al. Serum and urinary biochemical markers for knee and hip-osteoarthritis: a systematic review applying the consensus BIPED criteria. Osteoarthr. Cartil. 2010, 18, 605–612. [Google Scholar] [CrossRef]
- Richette, P.; Poitou, C.; Garnero, P.; Vicaut, E.; Bouillot, J.-L.; Lacorte, J.-M.; Basdevant, A.; Clément, K.; Bardin, T.; Chevalier, X. Benefits of massive weight loss on symptoms, systemic inflammation and cartilage turnover in obese patients with knee osteoarthritis. Ann Rheum Dis 2010, 70, 139–144. [Google Scholar] [CrossRef]
- Uthman, O.A.; van der Windt, D.A.; Jordan, J.L.; Dziedzic, K.S.; Healey, E.L.; Peat, G.M.; Foster, N.E. Exercise for lower limb osteoarthritis: systematic review incorporating trial sequential analysis and network meta-analysis. BMJ 2013, 347, f5555. [Google Scholar] [CrossRef]
- Hochberg, M.C.; Altman, R.D.; April, K.T.; Benkhalti, M.; Guyatt, G.; McGowan, J.; Towheed, T.; Welch, V.; Wells, G.; Tugwell, P. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012, 64, 465–474. [Google Scholar] [CrossRef]
- da Costa, B.R.; Nüesch, E.; Reichenbach, S.; Jüni, P.; Rutjes, A.W. Doxycycline for osteoarthritis of the knee or hip. Cochrane Database Syst Rev 2012, 14, CD007323. [Google Scholar] [CrossRef]
- Bingham, C.O., 3rd; Buckland-Wright, J.C.; Garnero, P.; Cohen, S.B.; Dougados, M.; Adami, S.; Clauw, D.J.; Spector, T.D.; Pelletier, J.; Raynauld, J.; et al. Risedronate decreases biochemical markers of cartilage degradation but does not decrease symptoms or slow radiographic progression in patients with medial compartment osteoarthritis of the knee: Results of the two-year multinational knee osteoarthritis structural arthritis study. Arthritis Rheum. 2006, 54, 3494–3507. [Google Scholar] [CrossRef]
- Laslett, L.L.; A Doré, D.; Quinn, S.J.; Boon, P.; Ryan, E.; Winzenberg, T.M.; Jones, G. Zoledronic acid reduces knee pain and bone marrow lesions over 1 year: a randomised controlled trial. Ann Rheum Dis 2012, 71, 1322–1328. [Google Scholar] [CrossRef]
- Chevalier, X.; Goupille, P.; Beaulieu, A.D.; Burch, F.X.; Bensen, W.G.; Conrozier, T.; Loeuille, D.; Kivitz, A.J.; Silver, D.; Appleton, B.E. Intraarticular injection of anakinra in osteoarthritis of the knee: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009, 61, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Knudson, C.B.; Knudson, W. Osteogenic Protein-1 inhibits matrix depletion in a hyaluronan hexasaccharide-induced model of osteoarthritis. Osteoarthr. Cartil. 2004, 12, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Zhu, S.; Tremblay, M.S.; Payette, J.N.; Wang, J.; Bouchez, L.C.; Meeusen, S.; Althage, A.; Cho, C.Y.; Wu, X.; et al. A Stem Cell–Based Approach to Cartilage Repair. Science 2012, 336, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Lambert, C. Chondroitin and Glucosamine in the Management of Osteoarthritis: An Update. Curr. Rheumatol. Rep. 2013, 15. [Google Scholar] [CrossRef] [PubMed]
- Crowley, D.C.; Lau, F.C.; Sharma, P.; Evans, M.; Guthrie, N.; Bagchi, M.; Bagchi, D.; Dey, D.K.; Raychaudhuri, S.P. Safety and efficacy of undenatured type II collagen in the treatment of osteoarthritis of the knee: a clinical trial. Int. J. Med Sci. 2009, 6, 312–321. [Google Scholar] [CrossRef]
- Narayanan, G. Understanding Collagen Supplements in Arthritis – Immunomodulation with Undenatured Collagen II Versus Cartilage Building with Hydrolysed Collagen II. Archives of Orthopedics and Rheumatology 2019, 2, 2639–3654. [Google Scholar] [CrossRef]
- Weiner, H.L.; da Cunha, A.P.; Quintana, F.; Wu, H. Oral tolerance. Immunol. Rev. 2011, 241, 241–259. [Google Scholar] [CrossRef]
- Bagchi, D.; Misner, B.; Bagchi, M.; Kothari, S.C.; Downs, B.W.; Fafard, R.D.; Preuss, H.G. Effects of orally administered undenatured type II chicken collagen against arthritic inflammatory pathologies: a mechanistic exploration. Int J Clin Pharmacol Res 2002, 22, 101–110. [Google Scholar]
- Lugo, J.P.; Saiyed, Z.M.; Lane, N.E. Efficacy and tolerability of an undenatured type II collagen supplement in modulating knee osteoarthritis symptoms: a multicenter randomized, double-blind, placebo-controlled study. Nutr. J. 2016, 15, 14. [Google Scholar] [CrossRef]
- Asnagli, H.; Martire, D.; Belmonte, N.; Quentin, J.; Bastian, H.; Boucard-Jourdin, M.; Fall, P.B.; Mausset-Bonnefont, A.-L.; Mantello-Moreau, A.; Rouquier, S.; et al. Type 1 regulatory T cells specific for collagen type II as an efficient cell-based therapy in arthritis. Arthritis Res. Ther. 2014, 16, R115. [Google Scholar] [CrossRef]
- Tong, T.; Zhao, W.; Wu, Y.-Q.; Chang, Y.; Wang, Q.-T.; Zhang, L.-L.; Wei, W. Chicken type II collagen induced immune balance of main subtype of helper T cells in mesenteric lymph node lymphocytes in rats with collagen-induced arthritis. Inflamm. Res. 2010, 59, 369–377. [Google Scholar] [CrossRef] [PubMed]
- D'Altilio, M.; Peal, A.; Alvey, M.; Simms, C.; Curtsinger, A.; Gupta, R.C.; Canerdy, T.D.; Goad, J.T.; Bagchi, M.; Bagchi, D. Therapeutic Efficacy and Safety of Undenatured Type II Collagen Singly or in Combination with Glucosamine and Chondroitin in Arthritic Dogs. Toxicol. Mech. Methods 2007, 17, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Deparle, L.A.; Gupta, R.C.; Canerdy, T.D.; Goad, J.T.; D’Altilio, M.; Bagchi, M.; Bagchi, D. Efficacy and safety of glycosylated undenatured type-II collagen (UC-II) in therapy of arthritic dogs. J Vet Pharmacol Ther 2005, 28, 385–390. [Google Scholar] [CrossRef]
- Gupta, R.C.; Canerdy, T.D.; Skaggs, P.; Stocker, A.; Zyrkowski, G.; Burke, R.; Wegford, K.; Goad, J.T.; Rohde, K.; Barnett, D.; et al. Therapeutic efficacy of undenatured type-II collagen (UC-II) in comparison to glucosamine and chondroitin in arthritic horses. J. Veter- Pharmacol. Ther. 2009, 32, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Lindley, J.; Barnes, M.; Minniear, J.; Goad, J.T.; Canerdy, T.D.; Bagchi, M.; Bagchi, D. Pain reduction measured by ground force plate in arthritic dogs treated with type-II collagen Baltimore, MD: Society of Toxicology, 2009b.
- Bagi, C.; Berryman, E.; Teo, S.; Lane, N. Oral administration of undenatured native chicken type II collagen (UC-II) diminished deterioration of articular cartilage in a rat model of osteoarthritis (OA). Osteoarthr. Cartil. 2017, 25, 2080–2090. [Google Scholar] [CrossRef] [PubMed]
- Gencoglu, H.; Orhan, C.; Sahin, E.; Sahin, K. Undenatured Type II Collagen (UC-II) in Joint Health and Disease: A Review on the Current Knowledge of Companion Animals. Animals 2020, 10, 697. [Google Scholar] [CrossRef]
- Varney, J.L.; Fowler, J.W.; Coon, C.N. Undenatured type II collagen mitigates inflammation and cartilage degeneration in healthy untrained Labrador retrievers after exercise. Transl. Anim. Sci. 2021, 5, txab084. [Google Scholar] [CrossRef]
- Morley, S.; Williams, A.C.; Eccleston, C. Examining the evidence about psychological treatments for chronic pain: Time for a paradigm shift? Pain 2013, 154, 1929–1931. [Google Scholar] [CrossRef]
- Bijur, P.E.; Latimer, C.T.; Gallagher, E.J. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003, 10, 390–392. [Google Scholar] [CrossRef]
- Mehra, A.; Anand, P.; Borate, M.; Pal, P.; Kamble, S.; Mehta, K.D.; Qamra, A.; Shah, A.; Jain, R. A non-interventional, prospective, multicentric real life Indian study to assess safety and effectiveness of un-denatured type 2 collagen in management of osteoarthritis. Int. J. Res. Orthop. 2019, 5, 315–320. [Google Scholar] [CrossRef]
- Costa, A.; Teixeira, V.C.; Pereira, M.; Ferreira, P.M.; Kuplich, P.; Dohnert, M.; Guths, J.d.S.; Daitx, R.B. Associated Strengthening Exercises to Undenatured Oral Type II Collagen UCII. A Randomized Study in Patients Affected by Knee Osteoarthritis. Muscle Ligaments Tendons J. 2020, 10, 481–492. [Google Scholar] [CrossRef]
- Bakilan, F.; Armagan, O.; Ozgen, M.; Tascioglu, F.; Bolluk, O.; Alatas, O. Effects of Native Type II Collagen Treatment on Knee Osteoarthritis: A Randomized Controlled Trial. Eurasian J. Med. 2016, 48, 95–101. [Google Scholar] [CrossRef]
- Saiyed, Z.; Durkee, S.; Bowman, J.; Juturu, V. Efficacy of UC-II ® Undenatured Type II Collagen on Knee Joint Function in Healthy Subjects: An Exploratory Post Hoc Analysis of a Randomized, Double-Blind, Placebo-Controlled Trial. J Clin Trials. 2021, 12, 1000002. [Google Scholar]
- Yatish, R.; L, N.; Bilagi, A.; Joshi, D. Evaluation of clinical efficacy of undenatured type ii collagen in the treatment of osteoarthritis of knee. A randomized controlled study. Int. J. Orthop. Sci. 2020, 6, 497–500. [Google Scholar] [CrossRef]
- Lugo, J.P.; Saiyed, Z.M.; Lau, F.C.; Molina, J.P.L.; Pakdaman, M.N.; Shamie, A.N.; Udani, J.K. Undenatured type II collagen (UC-II®) for joint support: a randomized, double-blind, placebo-controlled study in healthy volunteers. J. Int. Soc. Sports Nutr. 2013, 10, 48–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Niu, J.; Chen, C. Association between body mass index and knee osteoarthritis: a meta-analysis of observational studies. J Clin Rheumatol 2018, 24, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, G.M.; Dekker, J.; Veenhof, C.; Van den Ende, C.H.; Carpa, S.; Captain, K.; Van der Heijden, R.A.; ter Wee, M.M.; Dekker, J.M.; Lems, W.F. Course of functional status and pain in osteoarthritis of the hip or knee: a systematic review of the literature. Arthritis Rheumatol 2016, 68, 632–42. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kothari, P.; Kim, J.; Duan, X.; Bhargava, A.; Yu, B.; Lee, E.J. Knee Osteoarthritis: A Review of Management Options. Korean J Pain 2016, 29, 77–84. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).