1. Introduction
The growing recognition of the environmental impact of traditional plastics and the fast-paced advancements in food innovation have shifted the focus towards smart (active and intelligent systems) biopolymer-based food packaging materials, in a world that seeks more sustainable and diversified food systems.
In this study, the base biopolymer used for developing smart food packaging material was sodium alginate, which is naturally found in seaweeds, readily available, and has high stability, distinct colloidal and good gelling properties, great biocompatibility and non-toxicity, as well as the ability to be chemically or biochemically altered [
1,
2,
3]. However, pure alginate films are water soluble and have poor barrier, antioxidant, and antimicrobial properties [
2,
4,
5]. Improvements in the three-dimensional biopolymer structures can be introduced by crosslinking, which stabilizes the biopolymer mesh, reduces the water vapor/gas permeability, and improves mechanical strength [
6,
7]. For instance, citric acid is known to facilitate the crosslinking of hydroxyl groups within sodium alginate polymers, as a result the covalent intermolecular di-ester links are created between hydroxyl groups of the polysaccharide and the carboxyl groups of the crosslinking agents [
8]. In addition, citric acid acts as an antioxidant synergist [
9,
10] and has been shown to inhibit the growth of foodborne pathogens [
11,
12,
13,
14].
Active and visually responsive food packaging materials able to monitor/flag pH changes during food storage would confer a competitive advantage towards food safety [
15]. However, the synthesis and degradation of traditional synthetic pH dyes (e.g., methyl and cresol red, chlorophenol, bromocresol green and purple, etc.) may lead to the formation of harmful substances and therefore, public health and environmental concerns [
16,
17,
18]. Hence, natural bioactives and pigments are being researched as alternative sources for smart food packaging applications, with premium nutritional/sensory quality and prolonged shelf-life. Phenolic compounds, known for their diverse biological activity and characteristic colour changes in response to pH variations, show great potential for applications in smart food packaging and edible coatings [
19]. Particularly, the antioxidant activity of polyphenolic-rich grape (
Vitis vinifera) seed extracts (GSE) has been attributed to flavonoids, which can scavenge free radicals, have metal-chelating capabilities, and reduce the formation of hydroperoxide [
20,
21,
22]. Moreover, the antimicrobial activity of GSE films has been reported over a broad spectrum of pathogenic bacteria [
23,
24,
25,
26]. Interestingly, colour response as a result of changing pH reported in GSE has been attributed to the phenolic content [
27,
28].
The main aim of this study was to develop a visually responsive alginate-based active food packaging system with good antioxidant and antimicrobial properties without affecting the inherent functional properties of the alginate film. To do so, GSE as an active and intelligent agent and citric acid as a crosslinking agent were added to the film-forming solution. Initially, different citric acid content was added to the alginate solution to determine the best alginate-citric acid combination to achieve the best functional properties in terms of mechanical and barrier properties. Finally, GSE was incorporated into alginate-citric acid films and the antioxidant, antimicrobial, and colour-changing properties were evaluated.
2. Materials and Methods
2.1. Materials
Sodium alginate (alginic acid sodium salt from brown algae, low viscosity), citric acid (99%, 192.12 g/mol), sodium hypophosphite monohydrate (≥ 99%, 105.99 g/mol), Folin-Ciocalteu’s phenol reagent (1.240 g/cm3), and gallic acid (97.5–102.5% titration, 170.12 g/mol) were purchased from Sigma-Aldrich (Germany). Vegetable capsules of grape (Vitis vinifera) seed extract (GSE) were acquired from Swanson Health Products (USA). Sodium hydroxide solution (1 and 0.1 mol/L), hydrochloric acid (1 and 0.1 mol/L), sodium hydrogen carbonate (analysis grade, 84.01 g/mol), Plate Count Agar (PCA) and sodium chloride (analysis grade, 58.44 g/mol) were purchased from Merck (Germany). DPPH powder (2,2-Diphenyl-1-picrohydrazyl free radical, 95%) was acquired from Alfa Aesar, Thermo Fisher Scientific (USA). 96% ethanol (rectified alcohol) was purchased from Antibac (Norway). Mueller-Hinton Agar (MHA) and Tryptone Soy Broth (TSB) were acquired from Oxoid, (UK).
2.2. Preparation of GSE Filtrates
Aqueous GSE filtrates were prepared as described by Silván et al. (2013) [
29] with slight modifications. Vegetable capsules of GSE powder (0.377 g powder each) were stored at room temperature under 95% vacuum (FH-S SuperMAX C V3, Webomatic, Bochum, Germany) and thereafter handled under aseptic conditions. 2 g GSE powder was transferred to a 50 mL Falcon tube (Sarstedt, Germany) with 40 mL sterile distilled water (DW). After 5 min vortexing at room temperature, the suspension was centrifuged at 7,000 rpm and 4°C for 10 min (Heraeus Multifuge X3 FR centrifuge, Thermo Fisher Scientific, Germany). The supernatant was transferred to a 50 mL Falcon tube and centrifuged under the above-mentioned conditions. The resulting supernatant was filter-sterilised through a 0.45 μm pore size membrane filter (Labolytic, Norway) using a Buchner filtration system coupled to a vacuum/pressure piston pump (VWR VCP80, VWR International, Belgium). The GSE filtrates were stored at 4°C for up to 6 h before use.
2.3. Preparation of Film-Forming Solutions
The film-forming solutions were prepared following a modified version of the method described by Sharmin et al. (2021) [
8].
To make an alginate film-forming solution (alg) 2.0 g sodium alginate was dissolved in 100 mL DW by stirring for 1 h at 500 rpm and room temperature using a magnetic stirrer (MR Hei-Tec magnetic stirrer, Heidolph Instruments, Germany).
For alginate and citric acid films, at first, alginate was homogenised with DW by stirring for 1 h at 500 rpm. Afterwards, sodium hypophosphite monohydrate (2.5, 5.0, 7.5, and 10.0% w/w alginate) and citric acid (5, 10, 15, and 20% w/w alginate) at the respective concentrations were stirred for 2 h at 500 rpm and room temperature (
Table 1).
At 4°C, aqueous GSE filtrates were diluted 1:2 in sterile DW. 100 mL of the diluted solution was used to dissolve 2.0 g alginate by stirring for 1 h at 500 rpm (GSE_alg). 1.0 g sodium hypophosphite monohydrate and 0.2 g citric acid were added afterward (GSE_alg_ca10). The resulting solution was stirred for 2 h at 500 rpm and 4°C.
The composition of film-forming solutions is presented in
Table 1. Film-forming solutions were used as samples for FTIR analysis and antimicrobial assays.
2.4. Preparation of Films
Films were prepared by the casting/solvent-evaporation method. To prepare the films, 20 mL of film-forming solution was poured out into polystyrene Petri dishes with a diameter of 90 mm (Sigma-Aldrich, Germany) and set aside to dry. The films were dried without the Petri dish lid either at room temperature or at 12°C until the liquid evaporated. The film was retrieved from the dish when the whole surface area became firm enough (approximately 3 days at room temperature and 5 days at 12°C). The samples stored at 12°C were additionally left at room temperature on a bench in a Petri dish with the lid on for overnight water evaporation. While the room temperature drying occasionally led to the adherence of the film to the dish surface, all films prepared at 12°C were retrieved satisfactorily. The mechanical, barrier, and antioxidant properties of the films were evaluated and no significant differences were found for the films dried using one or the other method.
2.5. Mechanical Properties
To determine the mechanical properties (tensile strength, elongation at break, and tensile modulus), the films were cut into 60 × 15 mm (length × width) rectangular pieces [
8] right after the whole surface area of a film became firm enough to remove it from the plate. The strips of films dried at 12°C were weighted from the top to avoid coiling, in other words, to ensure straightness, and left overnight on the bench to stabilize the structure. The thickness was measured with a 0.01 mm resolution digital calliper. TA.XT Plus texture analyser with Exponent software (Stable Micro Systems, UK) was used to measure the maximum force required to break the film and the change in length at the point of rupture. 50 kg load cell was attached to the texture analyser, the span distance between tensile grips was fixed at 25 mm, test speed was set at 1 mm/s [
30]. More than six pieces of each film type were tested.
2.6. FTIR
The FTIR spectra of the samples were measured using the Bruker INVENIO® Spectrometer (Bruker Optics, Germany), which was equipped with a high-throughput extension HTS-XT for precise analysis. The OPUS v8.5 software was employed to control the data acquisition process. Spectra were acquired in transmission mode, covering a range of 4000-600 cm-1 (4 cm-1 resolution). To establish a baseline, a background spectrum of the empty 96-well Silicon plate was collected before each sample measurement. The background scans were repeated 40 times. Subsequently, the 5 replicate spectra of samples were averaged and baseline corrected using the UnscramblerTM v11 software from Camo Analytics AS (Oslo, Norway).
2.7. Barrier Properties
Water vapor transmission rate (WVTR) was used to evaluate the water molecules’ permeability through the barrier of the film. The method described by Sarwar et al. (2018) [
31] was slightly adjusted to work with sodium alginate films. A glass test tube with an inner diameter (d) of 13.5 mm was filled with 10 mL DW and the opening was closed with 15 × 15 mm film fragments tightened using Laboratory Parafilm PM996 sealing film (American National Can, USA). Test tubes with samples were weighted using Fisher Scientific Precision Series balance (Thermo Fisher Scientific, USA) before (
Wi) and after (
Wt) heating in a 45°C oven for 24 h (
t). More than four replicates of each film composition were tested. The WVTR was determined by the equation:
WVRT is the water vapor transmission rate (g/m2h), Wi is the initial weight of test tube (g), Wt is the final weight of test tube (g), A is the area of tube opening (0.25 ∙ π ∙ d2)(m2), t is 24 h.
2.8. Total phenolic content
The Folin-Ciocalteu micro-method [
29,
32] was used, with slight modifications, to determine the total phenolic content (TPC) of aqueous GSE filtrates prepared at 50 mg GSE/mL (2 g GSE powder in 40 mL DW).
10 μL sample aliquots at an adequate dilution were added in triplicate to a 96-well microplate (Greiner Bio-One, Germany) followed by 150 μL of the Folin-Ciocalteu solution (0.5 mL reagent in 7 mL DW). After 3 min incubation, each well of the plate was filled with 50 μL of sodium bicarbonate solution (0.96 g in 10 mL DW, filtered through 11 μm pore size Whatman filter paper and diluted 2:3 in DW), and subsequently, the plate was carefully kept in a dark environment at room temperature for 2 h. Absorbance was measured at 725 nm using Synergy H1 multi-mode reader and Gen5 software for data acquisition (BioTek Instruments, USA). Gallic acid was the standard used for the calibration curve.
2.9. Antioxidant properties
DPPH radical scavenging activity was measured as described by Dysjaland et al. (2022) [
33] with some modifications. The film was dissolved in DW to a concentration range of 0.01-3.00 mg/mL sodium alginate. A 0.15 mM DPPH and 96% ethanol solution was stirred for 15 min in a darkened flask and stored at 4°C for at least of 1 h before use. GSE_alg and GSE_alg_ca10 solutions were centrifuged at 7,000 rpm and 4°C for 10 min to obtain clear supernatant. The sample was mixed with an equal part of the DPPH solution, and kept in the dark at room temperature for 1 h. The absorbance of the mixture was measured at 517 nm in triplicate using a multi-mode spectrophotometer (Synergy H1, BioTek Instruments, USA). DPPH solution was used as a control, and blank was DW. DPPH radical scavenging activity (%) was expressed using the equation:
AbsS is the absorbance of the sample, AbsB is the absorbance of the blank (DW), and AbsC is the absorbance of the control (0.15 mmol/L DPPH solution).
2.10. Antimicrobial Properties
The evaluation of antimicrobial properties of the film-forming solutions was conducted according to the modified method described by Sharmin et al. (2021) [
34] and Dysjaland et al. (2022) [
33]. The antimicrobial studies focused on
Escherichia coli CCUG 10979 (Gram-negative) and
Staphylococcus aureus CCUG 1828 (Gram-positive) reference strains, obtained from the Culture Collection of the University of Gothenburg (Sweden). These strains were stored in Microbank
TM porous beads (Microbank, Pro-lab Diagnostics, Canada) at -80°C.
To initiate the antimicrobial studies, E. coli and S. aureus microbank beads were streaked onto PCA plates and incubated overnight at 37°C in a Sanyo MIR-154 incubator (SANYO Commercial Solutions, USA). Subsequently, a singled-out colony was transferred into 5 mL of Tryptic Soy Broth (TSB) and incubated at 37°C for 24 h. The stationary-phase cultures were diluted in TSB to inoculate the film-forming solutions (≈ 107 CFU/mL).
For the antimicrobial studies, 250 µL of the selected film-forming solutions or 250 µL of TSB as control were added to 1.5 mL Eppendorf tubes along with 50 µL of the corresponding TSB-diluted E. coli or S. aureus cell suspensions. The Eppendorf tubes were incubated at 37°C and 300 rpm for 24 h using a VorTemp 56 incubator (Labnet International, USA) to simulate temperature abuse conditions. Viable plate counts were determined for both the test and control samples immediately after inoculation and following the incubation period. The pH measurements were performed using a pH electrode LE422 and FiveEasy Plus pH-meter (Mettler-Toledo, Switzerland).
2.11. Colour Characteristics
The effect of pH on the colour of GSE_alg_ca10 film-forming solutions containing diluted GSE filtrates (1:2 in DW) was assessed. The pH of film-forming solutions was measured using glass electrode LE410 with a temperature sensor for aqueous samples (pH-meter FiveEasy Plus, Mettler-Toledo, USA). The pH was adjusted to 2.0, 4.0, 6.0, 8.0, or 10.0 with either sodium hydroxide (0.1 or 1.0 M) or hydrochloric acid (0.1 or 1.0 M), and the solutions were then centrifuged at 7,000 rpm and 4°C for 10 min. 250 μL of clear supernatant was pipetted in triplicate in a 96-well microplate (Greiner Bio-One, Germany). UV/VIS absorption spectra for the above-mentioned GSE solutions were recorded at 230–800 nm wavelength with 10 nm steps using a Synergy H1 multi-mode reader and Gen5 data acquisition software (BioTek Instruments, USA).
2.12. Statistical Analyses
The experiments were performed in triplicate or more (specific number of replicates is mentioned for each experiment). Statistical analysis was carried out using the T-test and analysis of variance (ANOVA) in SPSS software (SPSS statistics, version 26, IBM). Tukey's multiple comparison tests were used as a post hoc test, and a significance level of p < 0.05 was considered statistically significant. It was assumed that the variances were equal. The graphical data are presented as the mean value along with the standard deviation.
3. Results
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
3.1. Citric Acid Concentration
3.1.1. Effect of Citric Acid Concentration on Mechanical Properties
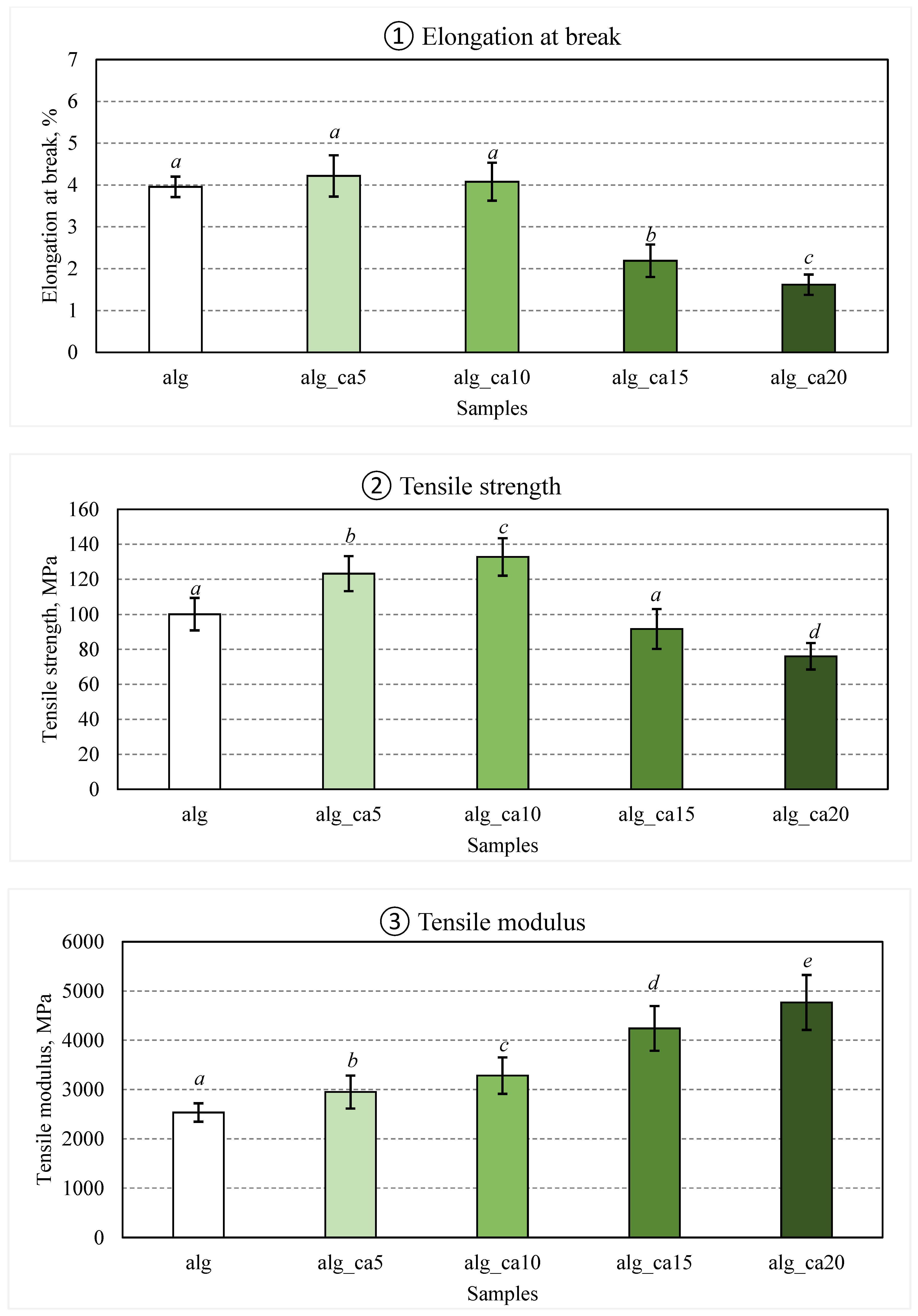
Alginate films were prepared with various citric acid concentrations (0, 5, 10, 15, and 20% w/w alginate), and their mechanical properties are presented in
Figure 1.
No significant change in the elongation at break was observed for the alginate films containing 0, 5, and 10% citric acid. For further increase in the citric acid content up to 20%, the elongation at break of films dropped significantly (p < 0.001) as compared to the control alginate films. The clear decrease in elongation at break could be caused by the crosslinking reactions, which connect the biopolymer molecules and, hence, reduce the mobility of the structure. Similar results were reported after crosslinking 1% (w/w) citric acid with peanut proteins [
36] or 1.5% (w/w) with starch/polyester polymers [
35]. The effect was attributed to the improved intermolecular connections, which limit the flexibility and increase rigidity.
Concerning the tensile strength as a function of the citric acid concentration, the tensile strength of the films with 5% and 10% citric acid increased significantly by 23% (p < 0.001) and 33% (p < 0.001), respectively, as compared to the uncrosslinked control alginate films. With the further increase of the citric acid concentration from 10% to 15 and 20%, the tensile strength reduced significantly (p < 0.001) from 133 MPa to 92 and 76 MPa, respectively. It has been reported previously, that citric acid can act as crosslinking agent by participating in intra- and inter-molecular interactions within the biopolymer reticulum, hence contributing to a more compact structure and thereby improved tensile strength up to certain citric acid concentration [
37,
38,
39,
40]. However, the presence of extra/not-reacted citric acid at higher concentrations may function as a plasticizing agent, which can reduce the interactions between the macromolecules, leading to tensile strength reduction [
8,
41,
42]. In this study, the tensile strength of alginate-citric acid films increased with increasing citric acid content, and reached a maximum at 10% citric acid concentration, then declined with a further increase in the citric acid concentration.
The tensile modulus of the films with increasing citric acid concentrations increased throughout the experiment. Such findings suggest that the citric acid functioned as a crosslinking agent, thus film formulations with lower citric acid concentrations formed less rigid films due to a lower amount of crosslinked junctions. These results are in line with previous studies which showed that higher content of crosslinking points resulted in high tensile modulus [
43,
44,
45,
46].
3.1.2. FTIR and the Effect of Citric Acid
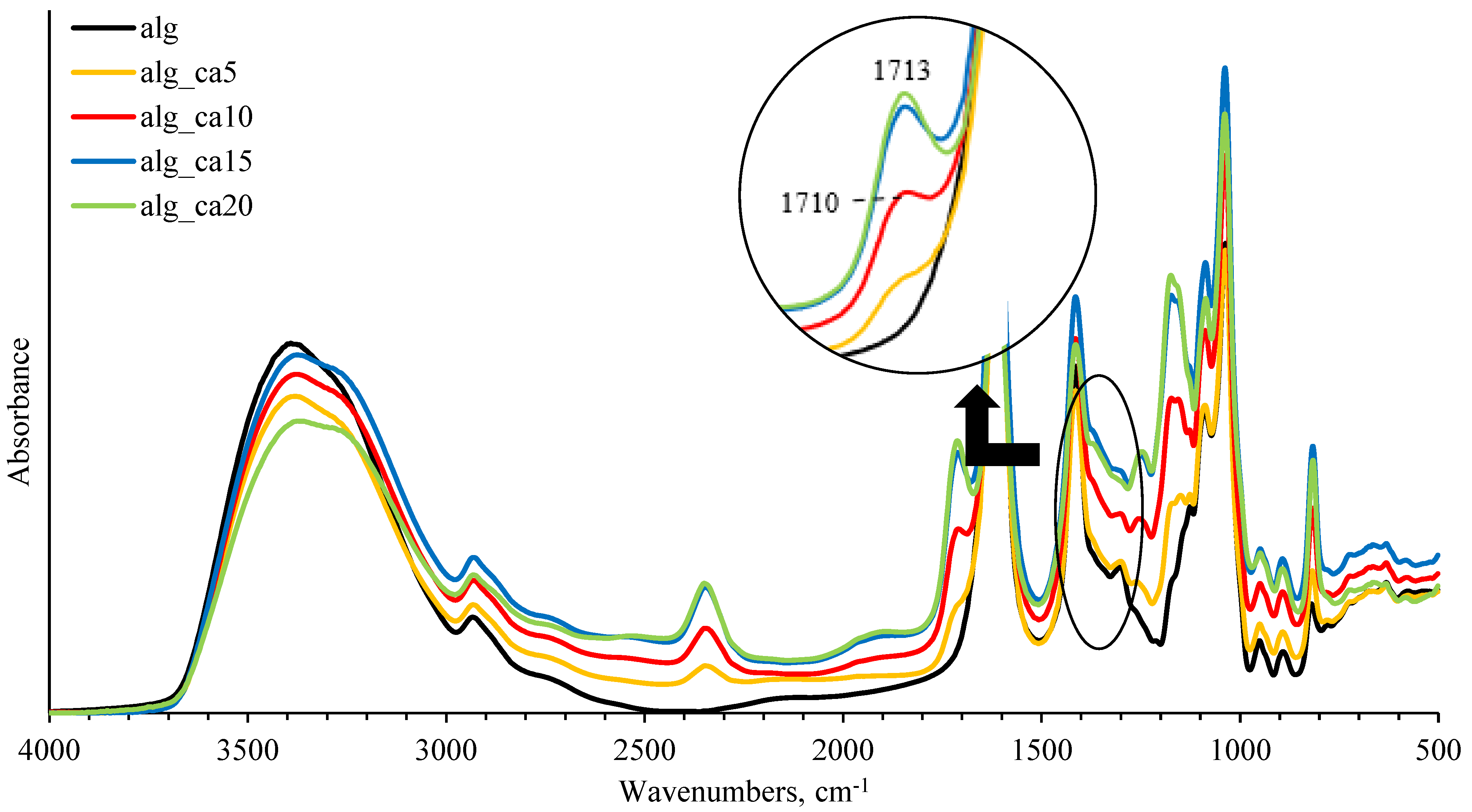
Figure 2 shows the FTIR spectra of sodium alginate crosslinked with 0, 5, 10, 15, and 20% (w/w alginate) citric acid. When citric acid is incorporated into the alginate matrix, carboxylic groups of citric acid react with –OH groups on pyranose rings of sodium alginate backbone [
8,
47]. A sharp absorption in 1690–1750 cm
−1 region is characteristic of the C=O group in citric acid [
48]. In this study, the peak was found at around 1710 cm
−1 in the samples containing citric acid, which was not present in pure alginate samples.
With increasing citric acid concentration, the characteristic peaks in the 1710–1713 cm
−1 region increased in intensity and shifted towards higher wavenumbers. The bands could be assigned to the overlapping peaks from ester bonds joining biopolymer chains with citric acid and unreacted carboxyl groups of citric acid [
13,
35,
37,
41,
49]. The effect of the addition of organic acid to starch/poly-butylene-adipate-co-terephthalate (PBAT) films was studied and it was found that the peaks observed at 1715 cm
−1 were an overlap of the ester bonds in the native PBAT molecules and the crosslinking points with the citric acid [
35]. In addition, the peak at 1730 cm
−1 was ascribed to ester bonds between wheat straw hemicellulose and citric acid and the peak observed at 1717 cm
−1 was ascribed to the free carboxylic acid groups [
41]. Moreover, it has been determined that the intensity of the peaks observed at 1711–1722 cm
−1 increased at higher citric acid concentrations in the thermoplastic starch matrix, therefore the increment was related to the increasing degree of esterification [
49]. The shift of the peaks towards higher vibrational wavelengths can as well indicate the changes in microstructures [
50]. For example, the crosslinked starch and citric acid films were washed from free citric acid and catalyst residues and the band at 1724 cm
-1 was found in FTIR spectra, confirming ester linkages between citric acid and starch [
37]. Although, it must be mentioned that the C=O stretch of the carboxylic acid on alginate at pH 1.2 has been determined at 1734 cm
-1 for the alginate hydrogel blended with NaCl [
51]. In this study, the lowest pH was 3.7 ± 0.1 (20% citric acid), hence not all alginate molecules were protonated, but the acidification of the medium could have promoted the shift of wavenumbers towards the higher values.
Furthermore, the intensity of peak at 3000–3600 cm
−1 related to the –OH vibrations decreased with increasing citric content. Similar results were found for polyvinyl alcohol (PVA)/starch films crosslinked with 5–30% (w/w) citric content, and the results indicated that the –OH groups of biopolymers were consumed by citric acid to create ester linkages [
13].
The peaks observed in the range of 1300–1000 cm
−1 wavenumbers have been attributed to the C–O stretching vibrations [
52]. Therefore, the shifting bands within 1302–1126 cm
−1 range may represent the changes in the alginate backbone and/or citric acid structure. Interestingly, with increasing citric acid concentration, the shift in the peaks toward lower wavenumbers of 822 cm
-1 and increments in its amplitude were measured. This peak has been previously assigned to the mannuronic acids in the alginate structure [
53]. Hence, these residues might have been affected by crosslinking. However, the 812 cm
-1 band has also been observed in the FTIR spectra of SHP [
54]. In this work, the SHP concentration increased with increasing citric acid concentration, which could have influenced the already-mentioned changes.
3.1.3. Effect of Citric Acid on Barrier Properties
As presented in
Table 1, the addition of 5, 10, 15, and 20% citric acid to alginate films, caused a significant (p < 0.001) decrease in the WVTR compared to pure alginate films, in particular WVTR was reduced by 29, 34, 36, and 44%, respectively. Although, between 5-20% citric acid samples, only WVTR of 20% citric acid films was significantly reduced (p ≤ 0.033). It had been reported that the WVTR of a film is dependent on the number of available polar groups of polymer chains [
40]. Hence, the decrease in WVTR could be due to the replacement of hydrophilic –OH groups present in alginate with hydrophobic ester groups from citric acid [
8,
41]. Also, the addition of citric acid is likely to have created a complex path for water molecules to travel through, because a denser film structure with strong inter- and intra-molecular interactions was created by crosslinking action [
55].
3.2. GSE
3.2.1. Effect of GSE on Mechanical Properties
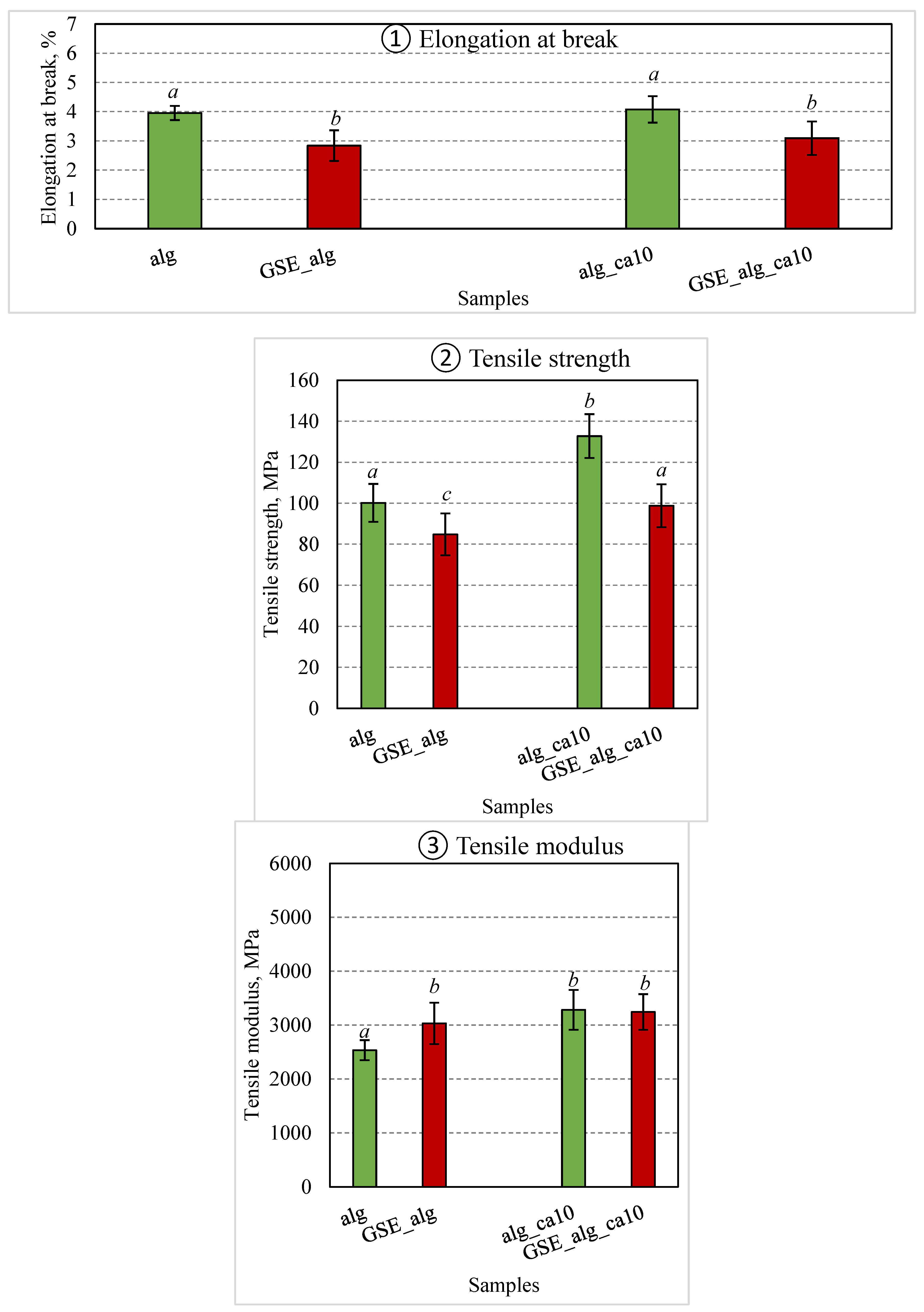
The GSE was used to improve the antioxidant and antimicrobial properties of alginate films. Results of previously described experiments showed that a 10% citric acid addition to alginate films led to the highest tensile strength of homogenous films, therefore this composition was chosen for GSE studies. The comparative results of elongation at break, tensile strength, and modulus of optimum film compositions prepared using DW and GSE solvents without citric acid (alg and GSE_alg, respectively) and with 10% citric acid (alg_ca10 and GSE_alg_ca10, respectively) are presented in
Figure 3.
The results of the current study showed that GSE significantly affected the mechanical properties of alginate films. Particularly, GSE_alg samples exhibited a significant decrease in tensile strength (p = 0.034) plus elongation at break (p < 0.001) while a significant increase in tensile modulus (p = 0.044) values was observed as compared to corresponding pure alginate films. The samples with polyphenolic GSE might be affected by the interactions of phenolic compounds with biopolymer molecules by hydrogen bonds and/or hydrophilic interactions, resulting in increased tensile modulus and decreased elongation [
56]. On the contrary, the decrease in the tensile strength could be the result of the weakened inter-molecular and physical polymer-polymer interactions leading to the breakage of the film [
26]. Also, it is worth mentioning that the addition of extract increased the solid content of the film and GSE films were thicker than DW-alginate films (0.06 ± 0.01 and 0.04 ± 0.00 mm, correspondingly), indicating the expansion of the alginate matrix [
52].
The tensile strength of GSE films was significantly improved (p = 0.015) by 14% with the addition of 10% citric acid. The obtained results are in good agreement with a previous study by Ounkaew et al. (2018) [
13], where polyvinyl alcohol/starch films enriched with active compound (spent coffee ground) and citric acid content up to 10% increased tensile strength compared to control samples without citric acid.
3.2.2. FTIR and the Effect of GSE
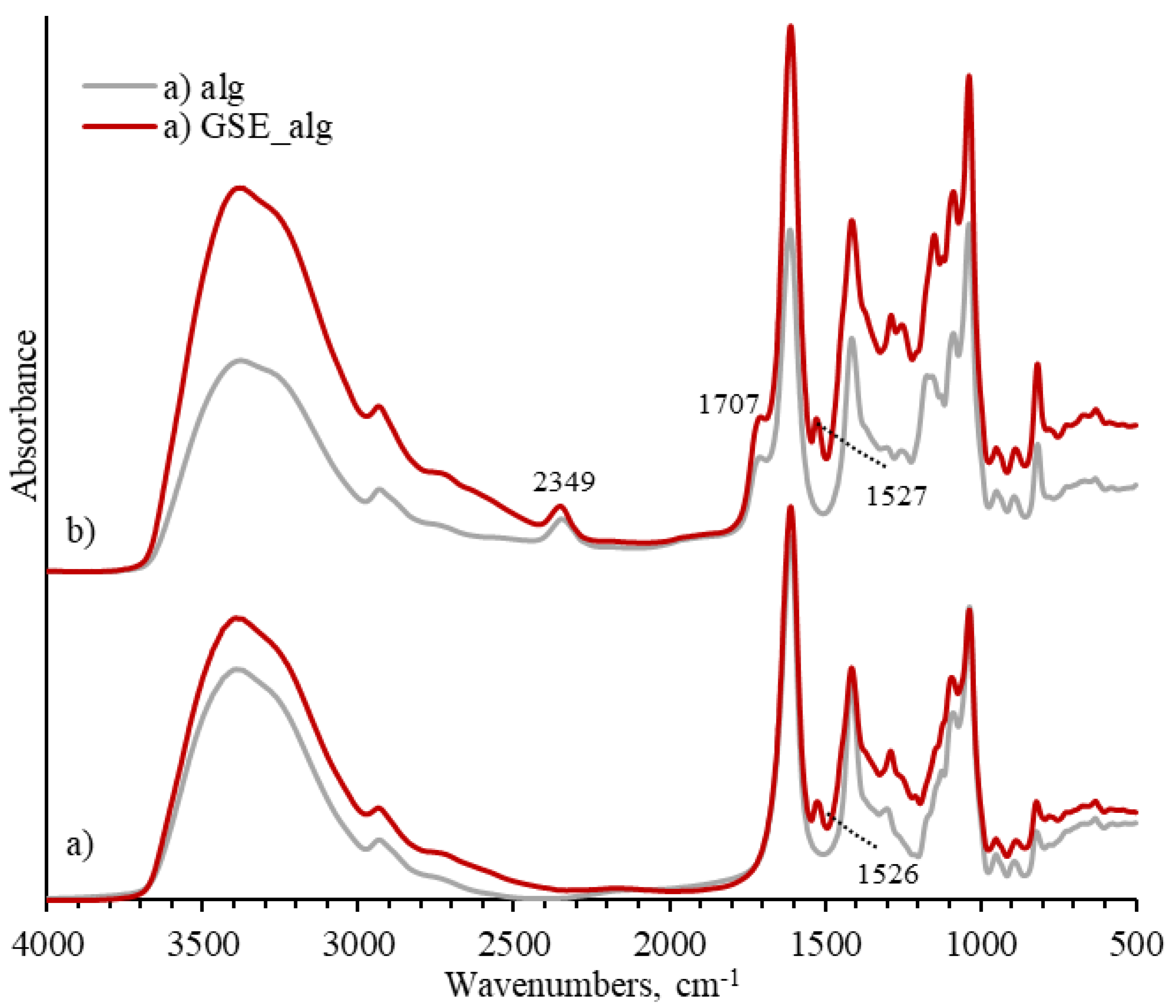
A new peak at 1526 cm
−1 was observed in the FTIR spectra of alginate solution containing GSE, which can be assigned to the vibration of the cyclobenzene ring of phenolic compounds (
Figure 4) [
57,
58]. In the GSE_alg_ca10 sample a peak at 1707 cm
−1 peak was observed, indicating that crosslinking with citric acid happened in the GSE solution as well.
3.2.3. Effect of GSE on Barrier Properties
In comparison with pure alginate films, the WVTR significantly decreased when GSE was incorporated into alginate-based films (p < 0.001;
Table 1), which is in good agreement with previous studies [
24,
56,
59]. Polyphenolic compounds present in GSE are characterized by the presence of one or more hydroxyl groups linked to aromatic rings [
60]. The increased number of crosslinks via hydrogen and hydrophobic interactions in the polymeric matrix likely caused the decrease of WVTR in films containing GSE compared to native biopolymers [
24]. The phenols in the GSE films interact with some –OH groups of alginates and reduce the hydrophilic characteristics of the film matrix related to water vapor permeability [
59].
The WVTR significantly decreased with the addition of 10% citric acid (p < 0.001). Such results indicate the crosslinking between alginate and citric acid. The WVTR of GSE_alg_ca10 was similar to alg_ca10 films (91 ± 5 and 92 ± 4 g/m2h, respectively).
3.2.4. Effect of GSE on Antioxidant Properties
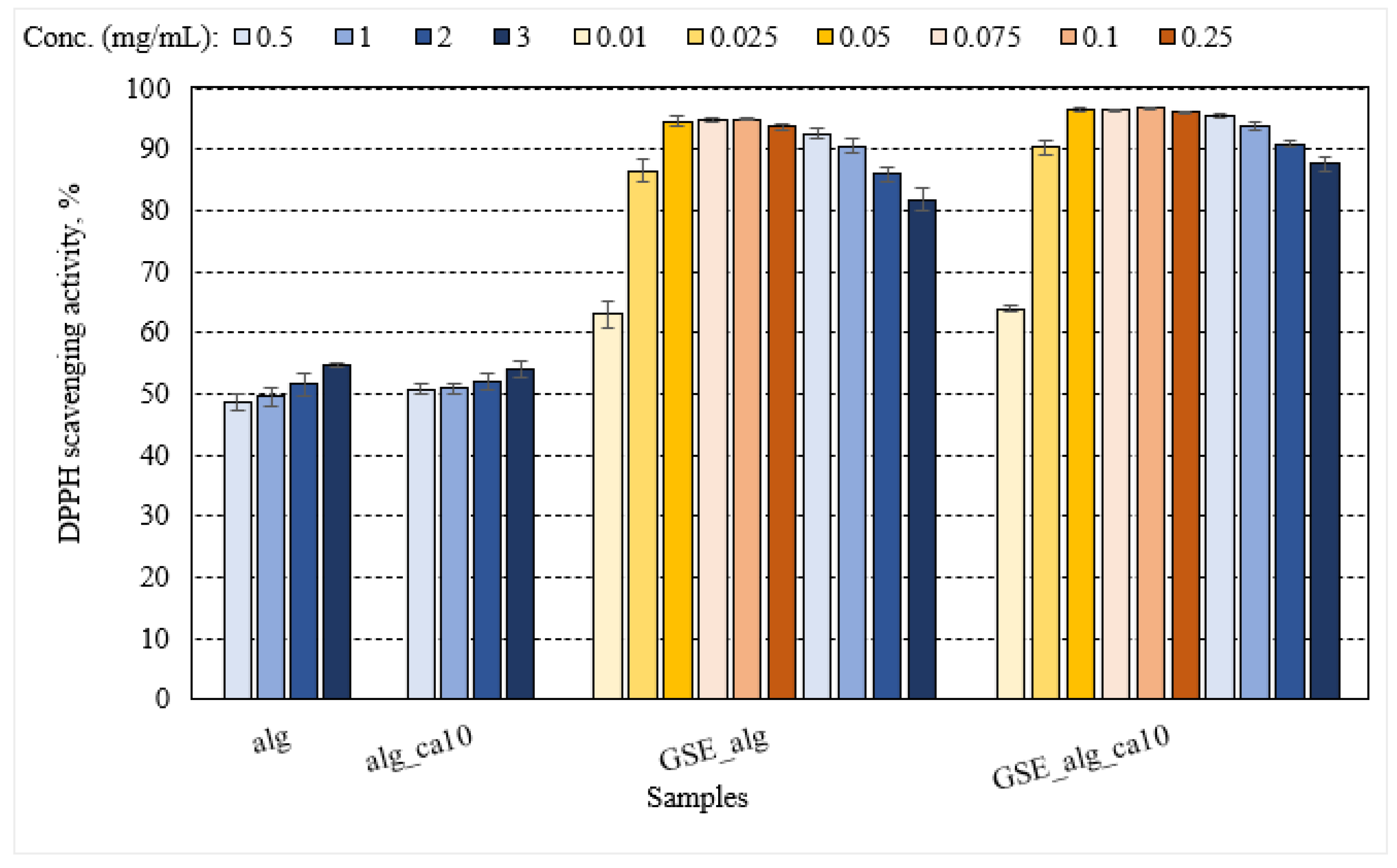
The addition of GSE into the alginate film significantly improved the DPPH scavenging activity by 1.7-fold as compared to pristine alginate film regardless of alginate concentration (0.5–3.0 mg/mL) (
Figure 5). GSE is rich in polyphenols (TPC = 0.22 ± 0.03 g GAE/g), particularly in proanthocyanidins. Several biopolymer films have shown increased antioxidant properties upon GSE incorporation, including chitosan [
56], fish skin gelatine [
20], alginate [
59], and pectin/pullulan blend films [
22], as affected by GSE type, TPC, concentrations, and the method used in the analytical determination, as well as potential contributions of the dominant biopolymer. Typically, DPPH scavenging activity in edible films increases with increasing concentrations of the antioxidant agent [
26,
59]. However, the DPPH scavenging activity of the GSE_alg and GSE_alg_ca10 samples decreased with the increasing GSE concentrations (0.093–0.557 mg GAE/mL). This could be attributed to the aggregation of GSE at high concentrations limiting the compatibility of biopolymer and GSE, as observed in gelatin-GSE-glycerol films at high concentrations (5 mg/mL) [
20]. Alternatively, lower DPPH activity detected in GSE_alg and GSE_alg_ca10 samples may be a result of reversibility in the DPPH reaction, where DPPH∙ radical receives a hydrogen atom H and becomes colourless DPPH-H upon exposure to the antioxidant agent [
61]. Due to the chemical equilibrium of the reaction solution, some of the reduced form DPPH-H could convert back into the free DPPH∙ radical. At low antioxidant concentrations, the re-conversion is small, hence the effects on absorbance are negligible. However, at high concentrations of the antioxidant, the amount of re-converted DPPH∙ would be higher, which could lead to the underestimation of the antioxidant activity [
61]. Considering this possibility, DPPH scavenging capacity was measured at a wider range of low concentrations, 0.01–0.1 mg alginate/mL (0.002–0.019 mg GAE/mL) (
Figure 5). The DPPH activity of GSE_alg and GSE_alg_ca10 films significantly increased with the increasing concentrations between 0.01 and 0.05 mg alginate/mL (0.002–0.009 mg GAE/mL). However, there was no statistically significant increase in the DPPH activity with concentrations between 0.05 and 0.1 mg alginate/mL (0.009–0.019 mg GAE/mL). Regardless of GSE concentration, the presence of GSE in alginate films increased their antioxidant properties compared to pristine alginate films.
GSE-alginate antioxidant activity was significantly enhanced by crosslinking with 10% citric acid, although the effect size was small ≈ 4%, p < 0.001 (
Figure 5). Because of its ability to chelate metal ions [
9], citric acid has been shown to increase the antioxidant properties of phenolic-rich extracts [
10,
62,
63].
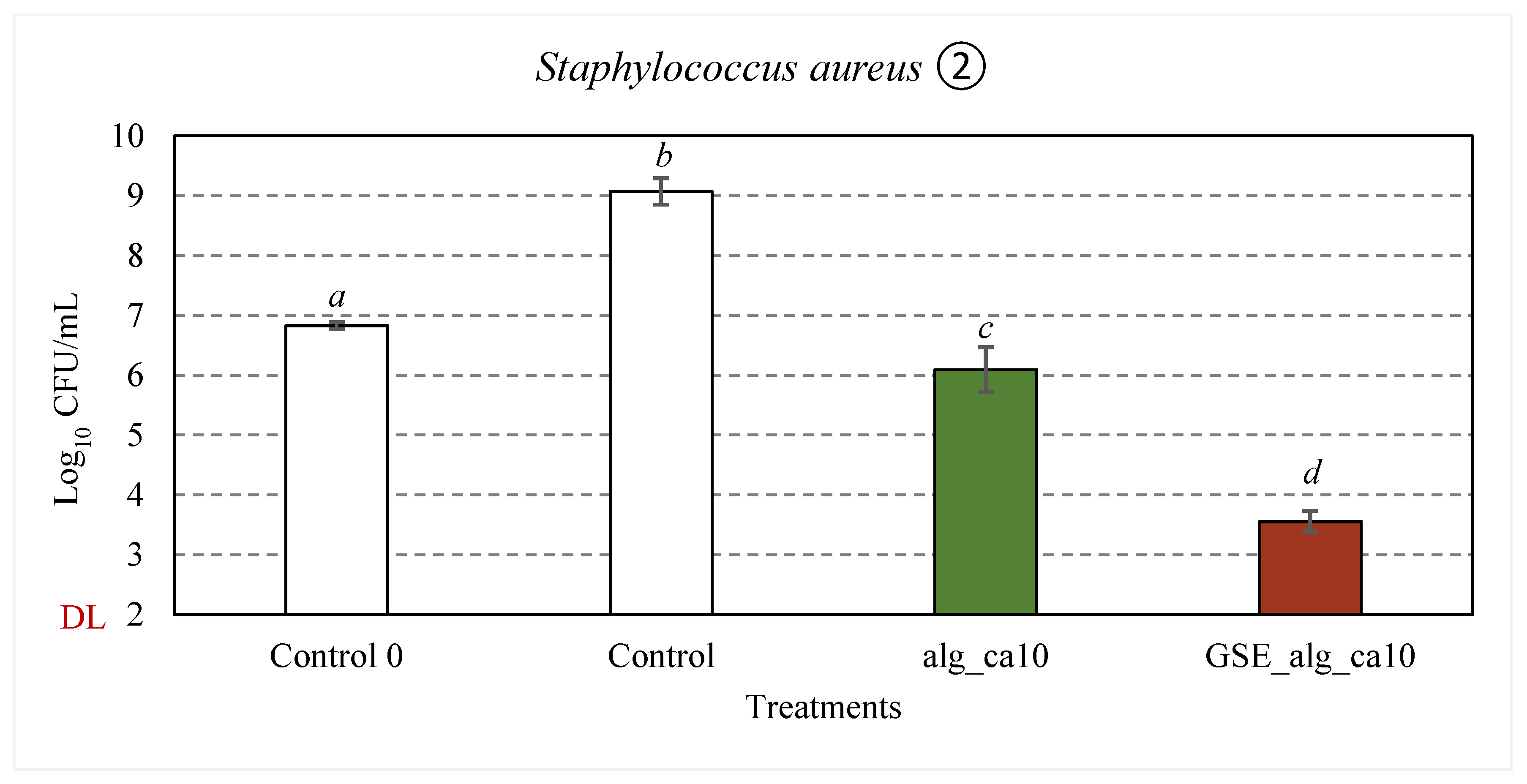
3.2.5. Effect of GSE on Antimicrobial Properties
The effect of GSE on the antimicrobial properties of 2% (w·v
-1) alginate crosslinked with 10% (w/w alginate) citric acid was assessed against
E. coli (
Figure 6(1)) and
S. aureus (
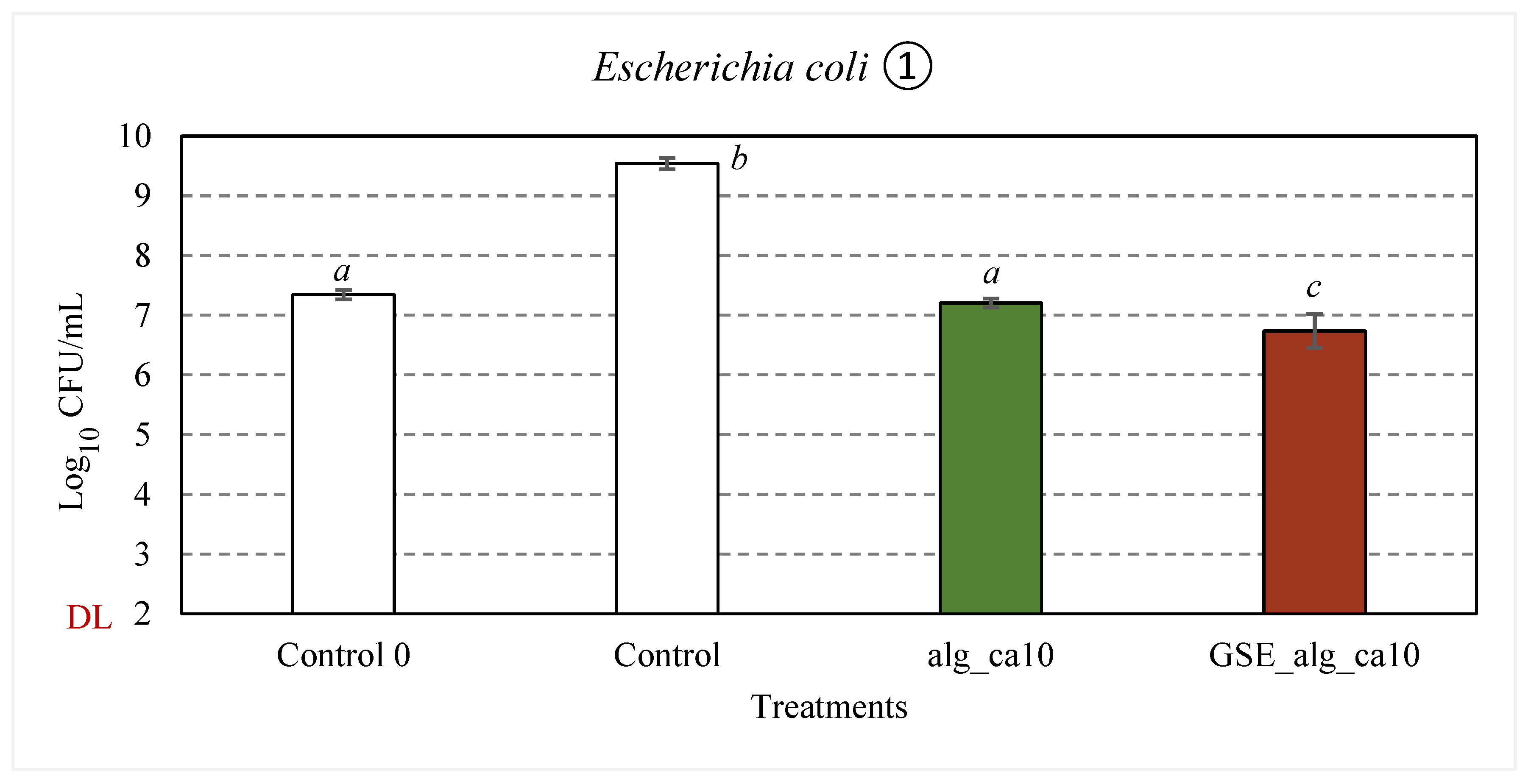
Figure 6(2)). The respective test items (83.3% v/v) were incubated with the cell suspension (≈ 10
7 CFU/mL) at 37°C and 300 rpm for 24 h. For control samples, a significant increase (p < 0.001) in the bacterial concentration and a decrease in pH (from 6.9 ± 0.2 and 7.0 ± 0.2 to 6.2 ± 0.2 and 6.4 ± 0.2, respectively for
E. coli and
S. aureus) were observed after the incubation period.
Compared to the control samples, about 2.3 and 2.8-log
10 reductions in viable counts of
E. coli (p < 0.001) and
S. aureus (p < 0.001), respectively, were observed in alg_ca10 samples after 24 h incubation at 37°C. The observed inhibitory effect can be attributed to the effect of citric acid and the pH of the treatment solutions (4.2 ± 0.1), given the growth limits of
E. coli (pH 4.3–10) and
S. aureus (pH 4.5–9.3) [
64] and the non-present antimicrobial activity reported for alginate [
4,
5]. Due to the differences in the cell wall structure, Gram-positive bacteria are more susceptible than Gram-negative to the inhibitory activity of citric acid, i.e. disruption of substrate/electron transport through the permeabilised cell membrane due to cation chelation and induction of intracellular reactive oxygen species leading to lipid peroxidation in the cell membrane [
12,
14,
65,
66,
67].
The treatment solutions with aqueous GSE filtrate (GSE_alg_ca10) resulted in 2.8-log10 (p < 0.001) and 5.5-log10 (p < 0.001) reductions in viable counts of E. coli and S. aureus, respectively.
The TPC of the GSE used in this study was 0.22 ± 0.03 g gallic acid equivalent (GAE)/g GSE powder or 11.66 ± 1.32 g GAE/L GSE filtrate. According to the manufacturer, the GSE powder consists of 90% (w/w) polyphenols, including proanthocyanidins. The addition of GSE into a variety of films to improve antimicrobial properties has been broadly reported in the literature [
22,
23,
25,
68,
69,
70,
71,
72]. Particularly, several authors [
22,
24,
72] have reported a moderate (or even absent) inhibitory effect of GSE incorporated in different films on Gram-negative bacteria, as compared to Gram-positive, which was attributed to their lipidic cell wall acting as a barrier for polyphenols to enter the bacterial cytoplasm, and could also explain the differences observed in this work. Interestingly, Gadang et al. (2008) [
69] reported enhanced inhibition of
Listeria monocytogenes in GSE-whey protein isolate films when adding 1% malic acid due to the decrease in the intracellular pH and enhanced susceptibility of the cell membrane to antimicrobial agents, which can be related to the synergistic effect of GSE, alginate and citric acid found in this work, particularly for
S. aureus.
3.2.6. Effect of pH on the Colour of GSE-Based Film-Forming Solutions
In this section, the potential of GSE towards visually responsive (intelligent) food packaging materials was investigated, inspired by the patterned colour responses to pH variations reported in phenolic compounds [74,75].
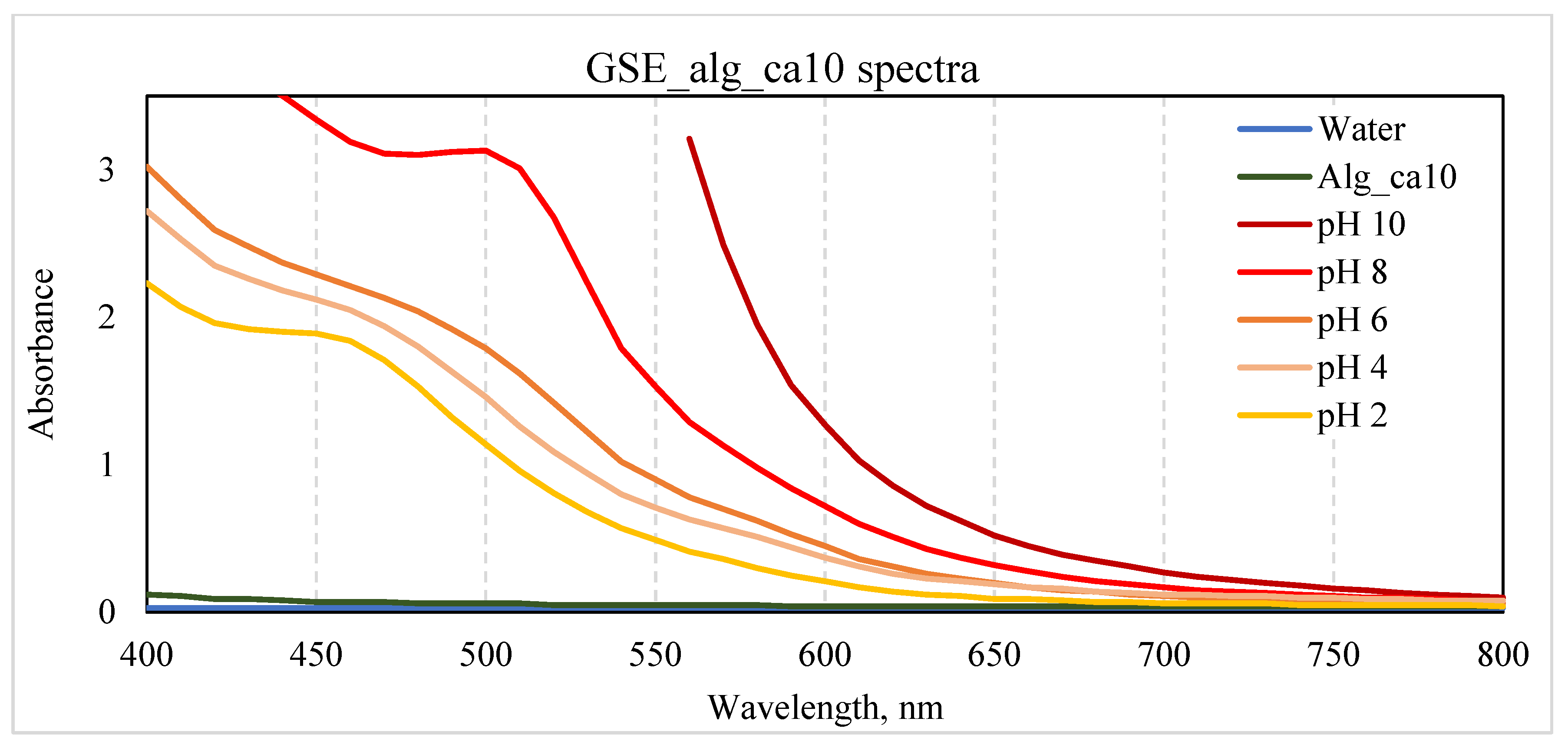
The UV/VIS spectra of (undiluted) GSE_alg_ca10 film-forming solutions adjusted to pH 2.0-10.0 are presented in
Figure 7. Absorption peaks were observed at 450 nm (GSE_alg_ca10 at pH 2.0), 490 nm (GSE_alg_ca10 at pH 8.0), and 500 nm (GSE filtrate at pH 10.0), although they were not clearly distinguishable for other tested pH values. Interestingly, the pH adjustment of the GSE_alg_ca10 film-forming solution from 2.0 to 10.0 shifted the UV/VIS spectra absorption peak from 450 to 500 nm, which resulted in a colour change from yellow to red. Guo et al. (2021) [
27] attributed the shift in UV/VIS absorption peaks (278-502 nm) and the associated colour change (from orange-yellow to dark red) of proanthocyanidins, which according to the manufacturer are present in the GSE used in this study, to changes in their molecular structure as a result of the pH variation (3.0-11.0). Particularly, due to the introduced protons or hydroxide radicals, the molecular polarisation increases, and the mobility of π electrons changes, influencing the variation in the colour of the compound.
4. Conclusions
In conclusion, the findings of this study have showcased the potential of combining the natural active agent GSE with citric acid to enhance the functionality and bioactivity of alginate films for applications in smart food packaging. The incorporation of 10% citric acid as a crosslinking agent significantly increased the tensile strength of the alginate film and decreased the WVTR. With further addition of GSE, the alginate-10% citric acid films exhibited improved antioxidant and antimicrobial properties, while keeping good mechanical and barrier properties. Utilization of GSE in crosslinked films introduced the colour sensitivity to pH, which could be a promising feature for the development of intelligent packaging systems. These developed GSE-alginate-citric acid films has a potential to be used in the food industry to enhance food safety, quality, shelf-life, etc.
Disclaimer
The author, Estefanía Noriega Fernández, is employed with the European Food Safety Authority (EFSA) at the Nutrition and Food Innovation Unit that provides scientific and administrative support to the Panel on “Nutrition, Novel Foods and Food Allergens” in the area “Safety Assessment of Novel Foods”. However, the present article is published under the sole responsibility of the authors Hege Dysjaland, Izumi Sone, Estefanía Noriega Fernández, Morten Sivertsvik and Nusrat Sharmin and may not be considered as an EFSA scientific output. The positions and opinions presented in this article are those of the author/s alone and are not intended to represent the views/any official position or scientific works of EFSA. For more information about the views or scientific outputs of EFSA, please consult its website under
http://efsa.europa.eu.
Author Contributions
Conceptualization, N.S.; methodology, A.P, N.S, I.S.O, and E.N.F; software, A.P. and N.S.; validation, A.P. and N.S.; formal analysis, A.P. and N.S.; writing- original draft preparation, A.P..; writing-review and editing, , N.S, I.S.O, and E.N.F.; supervision, N.S, I.S.O, M.S. and E.N.F.; project administration, N.S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank Nofima Strategic Programme “PackTech” (project no. 12596) and FutureFoodControl (grant no. 314743) for funding the work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abdul Khalil, H.P.S.; Chong, E.W.N.; Owolabi, F.A.T.; Asniza, M.; Tye, Y.Y.; Rizal, S.; Nurul Fazita, M.R.; Mohamad Haafiz, M.K.; Nurmiati, Z.; Paridah, M.T. Enhancement of Basic Properties of Polysaccharide-Based Composites with Organic and Inorganic Fillers: A Review. J. Appl. Polym. Sci. 2019, 136, 47251. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-Based Films and Coatings for Food Packaging: A Review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Cha, D.S.; Chinnan, M.S. Biopolymer-Based Antimicrobial Packaging: A Review. Crit. Rev. Food Sci. Nutr. 2004, 44, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, J.; Li, F.; Shi, Y.; Li, D.; Huang, Q. Chitosan-Sodium Alginate Nanoparticle as a Delivery System for ε-Polylysine: Preparation, Characterization and Antimicrobial Activity. Food Control 2018, 91, 302–310. [Google Scholar] [CrossRef]
- Zhao, X.; Xia, Y.; Li, Q.; Ma, X.; Quan, F.; Geng, C.; Han, Z. Microwave-Assisted Synthesis of Silver Nanoparticles Using Sodium Alginate and Their Antibacterial Activity. Colloids Surf. A Physicochem. Eng. Asp. 2014, 444, 180–188. [Google Scholar] [CrossRef]
- Draget, K.I.; Smidsrød, O.; Skjåk-Bræk, G. Algintes from Algae. In Polysaccharides and polyamides in the food industry: Properties, production, and patents; Wiley-VCH: Weinheim, Germany, 2005; pp. 1–30. ISBN 978-3-527-31345-7. [Google Scholar]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the Integrity of Natural Biopolymer Films Used in Food Packaging by Crosslinking Approach: A Review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef]
- Sharmin, N.; Sone, I.; Walsh, J.L.; Sivertsvik, M.; Fernández, E.N. Effect of Citric Acid and Plasma Activated Water on the Functional Properties of Sodium Alginate for Potential Food Packaging Applications. Food Packag. Shelf Life 2021, 29, 100733. [Google Scholar] [CrossRef]
- Azevedo, V.M.; Dias, M.V.; de Siqueira Elias, H.H.; Fukushima, K.L.; Silva, E.K.; de Deus Souza Carneiro, J.; de Fátima Ferreira Soares, N.; Borges, S.V. Effect of Whey Protein Isolate Films Incorporated with Montmorillonite and Citric Acid on the Preservation of Fresh-Cut Apples. Food Res. Int. 2018, 107, 306–313. [Google Scholar] [CrossRef]
- Menzel, C. Improvement of Starch Films for Food Packaging through a Three-Principle Approach: Antioxidants, Cross-Linking and Reinforcement. Carbohydr. Polym. 2020, 250, 116828. [Google Scholar] [CrossRef]
- Eliuz, E. Antimicrobial Activity of Citric Acid against Escherichia coli, Staphylococcus aureus and Candida albicans as a Sanitizer Agent. Eurasian J. For. Sci. 2020, 8, 295–301. [Google Scholar] [CrossRef]
- In, Y.-W.; Kim, J.-J.; Kim, H.-J.; Oh, S.-W. Antimicrobial Activities of Acetic Acid, Citric Acid and Lactic Acid against Shigella Species: Organic Acids on Shigella. J Food Saf. 2013, 33, 79–85. [Google Scholar] [CrossRef]
- Ounkaew, A.; Kasemsiri, P.; Kamwilaisak, K.; Saengprachatanarug, K.; Mongkolthanaruk, W.; Souvanh, M.; Pongsa, U.; Chindaprasirt, P. Polyvinyl Alcohol (PVA)/Starch Bioactive Packaging Film Enriched with Antioxidants from Spent Coffee Ground and Citric Acid. J. Polym. Environ. 2018, 26, 3762–3772. [Google Scholar] [CrossRef]
- Skřivanová, E.; Marounek, M.; Benda, V.; Brezina, P. Susceptibility of Escherichia coli, Salmonella sp. and Clostridium perfringens to Organic Acids and Monolaurin. Vet. Med. (Praha) 2006, 51. [Google Scholar] [CrossRef]
- Han, J.H.; Ho, C.H.L.; Rodrigues, E.T. Intelligent Packaging. In Innovations in food packaging; Food Science and Technology International Series; Academic Press an imprint of Elsevier: Amsterdam, The Netherlands, 2014; pp. 138–155. ISBN 978-0-12-311632-1. [Google Scholar]
- Aljerf, L. High-Efficiency Extraction of Bromocresol Purple Dye and Heavy Metals as Chromium from Industrial Effluent by Adsorption onto a Modified Surface of Zeolite: Kinetics and Equilibrium Study. J. Environ. Manag. 2018, 225, 120–132. [Google Scholar] [CrossRef]
- Igbinosa, E.O.; Odjadjare, E.E.; Chigor, V.N.; Igbinosa, I.H.; Emoghene, A.O.; Ekhaise, F.O.; Igiehon, N.O.; Idemudia, O.G. Toxicological Profile of Chlorophenols and Their Derivatives in the Environment: The Public Health Perspective. Sci. World J. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nor, N.M.; Hadibarata, T.; Zubir, M.M.F.A.; Lazim, Z.M.; Adnan, L.A.; Fulazzaky, M.A. Mechanism of Triphenylmethane Cresol Red Degradation by Trichoderma harzianum M06. Bioprocess Biosyst. Eng. 2015, 38, 2167–2175. [Google Scholar] [CrossRef] [PubMed]
- Balbinot-Alfaro, E.; Craveiro, D.V.; Lima, K.O.; Costa, H.L.G.; Lopes, D.R.; Prentice, C. Intelligent Packaging with PH Indicator Potential. Food Eng. Rev. 2019, 11, 235–244. [Google Scholar] [CrossRef]
- Li, J.-H.; Miao, J.; Wu, J.-L.; Chen, S.-F.; Zhang, Q.-Q. Preparation and Characterization of Active Gelatin-Based Films Incorporated with Natural Antioxidants. Food Hydrocoll. 2014, 37, 166–173. [Google Scholar] [CrossRef]
- Perumalla, A.V.S.; Hettiarachchy, Navam. S. Green Tea and Grape Seed Extracts—Potential Applications in Food Safety and Quality. Food Res. Int. 2011, 44, 827–839. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Riahi, Z.; Rhim, J.-W. Antioxidant Pectin/Pullulan Edible Coating Incorporated with Vitis vinifera Grape Seed Extract for Extending the Shelf Life of Peanuts. Postharvest Biol. Technol. 2022, 183, 111740. [Google Scholar] [CrossRef]
- Corrales, M.; Han, J.H.; Tauscher, B. Antimicrobial Properties of Grape Seed Extracts and Their Effectiveness after Incorporation into Pea Starch Films. Int. J. Food Sci. Technol. 2009, 44, 425–433. [Google Scholar] [CrossRef]
- Shahbazi, Y. The Properties of Chitosan and Gelatin Films Incorporated with Ethanolic Red Grape Seed Extract and Ziziphora clinopodioides Essential Oil as Biodegradable Materials for Active Food Packaging. Int. J. Biol. Macromol. 2017, 99, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Sivarooban, T.; Hettiarachchy, N.S.; Johnson, M.G. Physical and Antimicrobial Properties of Grape Seed Extract, Nisin, and EDTA Incorporated Soy Protein Edible Films. Food Res. Int. 2008, 41, 781–785. [Google Scholar] [CrossRef]
- Sogut, E.; Seydim, A.C. The Effects of Chitosan and Grape Seed Extract-Based Edible Films on the Quality of Vacuum Packaged Chicken Breast Fillets. Food Packag. Shelf Life 2018, 18, 13–20. [Google Scholar] [CrossRef]
- Guo, B.; Lv, K.; Hu, Y.; Du, J.; Lv, Y. Study on Optical Properties and Biological Activity of Proanthocyanidins at Different PH and Alkalinit. IOP Conf. Ser. Earth Environ. Sci. 2021, 706, 012040. [Google Scholar] [CrossRef]
- Guo, L.; Yang, Z.-Y.; Tang, R.-C.; Yuan, H.-B. Preliminary Studies on the Application of Grape Seed Extract in the Dyeing and Functional Modification of Cotton Fabric. Biomolecules 2020, 10, 220. [Google Scholar] [CrossRef]
- Silván, J.M.; Mingo, E.; Hidalgo, M.; de Pascual-Teresa, S.; Carrascosa, A.V.; Martinez-Rodriguez, A.J. Antibacterial Activity of a Grape Seed Extract and Its Fractions against Campylobacter spp. Food Control 2013, 29, 25–31. [Google Scholar] [CrossRef]
- ASTM. D638-99 Standard Test Method for Tensile Properties of Plastics; Pennsylvania, USA, 1999. Available online: https://www.astm.org/d0638-99.html (accessed on 15 March 2022).
- Sarwar, M.S.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A. Preparation and Characterization of PVA/Nanocellulose/Ag Nanocomposite Films for Antimicrobial Food Packaging. Carbohydr. Polym. 2018, 184, 453–464. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144. [Google Scholar] [CrossRef]
- Dysjaland, H.; Sone, I.; Noriega Fernández, E.; Sivertsvik, M.; Sharmin, N. Mechanical, Barrier, Antioxidant and Antimicrobial Properties of Alginate Films: Effect of Seaweed Powder and Plasma-Activated Water. Molecules 2022, 27, 8356. [Google Scholar] [CrossRef]
- Sharmin, N.; Pang, C.; Sone, I.; Walsh, J.L.; Fernández, C.G.; Sivertsvik, M.; Fernández, E.N. Synthesis of Sodium Alginate–Silver Nanocomposites Using Plasma Activated Water and Cold Atmospheric Plasma Treatment. Nanomaterials 2021, 11, 2306. [Google Scholar] [CrossRef] [PubMed]
- Olivato, J.B.; Grossmann, M.V.E.; Bilck, A.P.; Yamashita, F. Effect of Organic Acids as Additives on the Performance of Thermoplastic Starch/Polyester Blown Films. Carbohydr. Polym. 2012, 90, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Jiang, Q.; Yang, Y. Preparation and Properties of Peanut Protein Films Crosslinked with Citric Acid. Ind. Crops Prod. 2012, 39, 26–30. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric Acid Cross-Linking of Starch Films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef]
- Shi, R.; Bi, J.; Zhang, Z.; Zhu, A.; Chen, D.; Zhou, X.; Zhang, L.; Tian, W. The Effect of Citric Acid on the Structural Properties and Cytotoxicity of the Polyvinyl Alcohol/Starch Films When Molding at High Temperature. Carbohydr. Polym. 2008, 74, 763–770. [Google Scholar] [CrossRef]
- Wang, S.; Ren, J.; Li, W.; Sun, R.; Liu, S. Properties of Polyvinyl Alcohol/Xylan Composite Films with Citric Acid. Carbohydr. Polym. 2014, 103, 94–99. [Google Scholar] [CrossRef]
- Wu, H.; Lei, Y.; Lu, J.; Zhu, R.; Xiao, D.; Jiao, C.; Xia, R.; Zhang, Z.; Shen, G.; Liu, Y.; et al. Effect of Citric Acid Induced Crosslinking on the Structure and Properties of Potato Starch/Chitosan Composite Films. Food Hydrocoll. 2019, 97, 105208. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Kontou-Vrettou, C.; Moates, G.K.; Wellner, N.; Cross, K.; Pereira, P.H.F.; Waldron, K.W. Wheat Straw Hemicellulose Films as Affected by Citric Acid. Food Hydrocoll. 2015, 50, 1–6. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Sauraj; Kumar, B. ; Negi, Y.S. Chitosan Film Incorporated with Citric Acid and Glycerol as an Active Packaging Material for Extension of Green Chilli Shelf Life. Carbohydr. Polym. 2018, 195, 329–338. [Google Scholar] [CrossRef]
- Costa, M.J.; Marques, A.M.; Pastrana, L.M.; Teixeira, J.A.; Sillankorva, S.M.; Cerqueira, M.A. Physicochemical Properties of Alginate-Based Films: Effect of Ionic Crosslinking and Mannuronic and Guluronic Acid Ratio. Food Hydrocoll. 2018, 81, 442–448. [Google Scholar] [CrossRef]
- Kaygusuz, H.; Evingür, G.A.; Pekcan, Ö.; von Klitzing, R.; Erim, F.B. Surfactant and Metal Ion Effects on the Mechanical Properties of Alginate Hydrogels. Int. J. Biol. Macromol. 2016, 92, 220–224. [Google Scholar] [CrossRef]
- Shitrit, Y.; Davidovich-Pinhas, M.; Bianco-Peled, H. Shear Thinning Pectin Hydrogels Physically Cross-Linked with Chitosan Nanogels. Carbohydr. Polym. 2019, 225, 115249. [Google Scholar] [CrossRef] [PubMed]
- Sinha-Ray, S.; Khansari, S.; Yarin, A.L.; Pourdeyhimi, B. Effect of Chemical and Physical Cross-Linking on Tensile Characteristics of Solution-Blown Soy Protein Nanofiber Mats. Ind. Eng. Chem. Res. 2012, 51, 15109–15121. [Google Scholar] [CrossRef]
- Singh, P.; Baisthakur, P.; Yemul, O.S. Synthesis, Characterization and Application of Crosslinked Alginate as Green Packaging Material. Heliyon 2020, 6, e03026. [Google Scholar] [CrossRef] [PubMed]
- Kanatt, S.R.; Makwana, S.H. Development of Active, Water-Resistant Carboxymethyl Cellulose-Poly Vinyl Alcohol-Aloe vera Packaging Film. Carbohydr. Polym. 2020, 227, 115303. [Google Scholar] [CrossRef]
- Niazi, M.B.K.; Broekhuis, A.A. Surface Photo-Crosslinking of Plasticized Thermoplastic Starch Films. Eur. Polym. J. 2015, 64, 229–243. [Google Scholar] [CrossRef]
- Awadhiya, A.; Kumar, D.; Verma, V. Crosslinking of Agarose Bioplastic Using Citric Acid. Carbohydr. Polym. 2016, 151, 60–67. [Google Scholar] [CrossRef]
- Liang, H.-F.; Hong, M.-H.; Ho, R.-M.; Chung, C.-K.; Lin, Y.-H.; Chen, C.-H.; Sung, H.-W. Novel Method Using a Temperature-Sensitive Polymer (Methylcellulose) to Thermally Gel Aqueous Alginate as a PH-Sensitive Hydrogel. Biomacromolecules 2004, 5, 1917–1925. [Google Scholar] [CrossRef]
- Kazayawoko, M.; Balatinecz, J.J.; Woodhams, R.T. Diffuse Reflectance Fourier Transform Infrared Spectra of Wood Fibers Treated with Maleated Polypropylenes. J. Appl. Polym. Sci. 1997, 66, 1163–1173. [Google Scholar] [CrossRef]
- Leal, D.; Matsuhiro, B.; Rossi, M.; Caruso, F. FT-IR Spectra of Alginic Acid Block Fractions in Three Species of Brown Seaweeds. Carbohydr. Res. 2008, 343, 308–316. [Google Scholar] [CrossRef]
- Xiaohong, G.; Yang, C.Q. FTIR Spectroscopy Study of the Formation of Cyclic Anhydride Intermediates of Polycarboxylic Acids Catalyzed by Sodium Hypophosphite. Text. Res. J. 2000, 70, 64–70. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H.; Entezami, A.A. Improving the Barrier and Mechanical Properties of Corn Starch-Based Edible Films: Effect of Citric Acid and Carboxymethyl Cellulose. Ind. Crops Prod. 2011, 33, 229–235. [Google Scholar] [CrossRef]
- Moradi, M.; Tajik, H.; Razavi Rohani, S.M.; Oromiehie, A.R.; Malekinejad, H.; Aliakbarlu, J.; Hadian, M. Characterization of Antioxidant Chitosan Film Incorporated with Zataria multiflora Boiss Essential Oil and Grape Seed Extract. LWT 2012, 46, 477–484. [Google Scholar] [CrossRef]
- Borges-Vilches, J. Graphene Oxide/Polyethylene Glycol Aerogel Reinforced with Grape Seed Extracts as Wound Dressing. J. Mater. Sci. 2021, 16082–16096. [Google Scholar] [CrossRef]
- Xu, Y.; Tian, Y.; Ma, R.; Liu, Q.; Zhang, J. Effect of Plasma Activated Water on the Postharvest Quality of Button Mushrooms, Agaricus bisporus. Food Chem. 2016, 197, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Fabra, M.J.; Falcó, I.; Randazzo, W.; Sánchez, G.; López-Rubio, A. Antiviral and Antioxidant Properties of Active Alginate Edible Films Containing Phenolic Extracts. Food Hydrocoll. 2018, 81, 96–103. [Google Scholar] [CrossRef]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- Yeo, J.; Shahidi, F. Revisiting DPPH (2,2-Diphenyl-1-Picrylhydrazyl) Assay as a Useful Tool in Antioxidant Evaluation: A New IC100 Concept to Address Its Limitations. JFB 2019, 7. [Google Scholar] [CrossRef]
- Akaranta, O.; Akaho, A.A. Synergic Effect of Citric Acid and Peanut Skin Extract on the Oxidative Stability of Vegetable Oil. J. Appl. Sci. Environ. Manag. 2013, 16, 345–351. [Google Scholar]
- Hraš, A.R.; Hadolin, M.; Knez, Ž.; Bauman, D. Comparison of Antioxidative and Synergistic Effects of Rosemary Extract with α-Tocopherol, Ascorbyl Palmitate and Citric Acid in Sunflower Oil. Food Chem. 2000, 71, 229–233. [Google Scholar] [CrossRef]
- Bintsis, T. Foodborne Pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef] [PubMed]
- Helander, I.M.; Mattila-Sandholm, T. Fluorometric Assessment of Gram-Negative Bacterial Permeabilization. J. Appl. Microbiol. 2000, 88, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-W.; Lee, H.-Y.; Kang, D.-H. Synergistic Bactericidal Effect of Hot Water with Citric Acid against Escherichia coli O157:H7 Biofilm Formed on Stainless Steel. Food Microbiol. 2021, 95, 103676. [Google Scholar] [CrossRef]
- Raftari, M.; Jalilian, F.A.; Abdulamir, A.S.; Son, R.; Sekawi, Z.; Fatimah, A.B. Effect of Organic Acids on Escherichia coli O157:H7 and Staphylococcus aureus Contaminated Meat. Open Microbiol. J. 2009, 3, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Chedea, V.S.; Echim, C.; Braicu, C.; Andjelkovic, M.; Verhe, R.; Socaciu, C. Composition In Polyphenols And Stability Of The Aqueous Grape Seed Extract From The Romanian Variety “Merlot Recas”: Polyphenols From The “Merlot Recas” Grape Seeds. J. Food Biochem. 2011, 35, 92–108. [Google Scholar] [CrossRef]
- Gadang, V.P.; Hettiarachchy, N.S.; Johnson, M.G.; Owens, C. Evaluation of Antibacterial Activity of Whey Protein Isolate Coating Incorporated with Nisin, Grape Seed Extract, Malic Acid, and EDTA on a Turkey Frankfurter System. J. Food Sci. 2008, 73, M389–M394. [Google Scholar] [CrossRef]
- Memar, M.Y.; Adibkia, K.; Farajnia, S.; Kafil, H.S.; Yekani, M.; Alizadeh, N.; Ghotaslou, R. The Grape Seed Extract: A Natural Antimicrobial Agent against Different Pathogens. Rev. Med. Microbiol. 2019, 30, 173–182. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in Grape Seeds—Biochemistry and Functionality. J. Med. Food 2003, 6, 291–299. [Google Scholar] [CrossRef]
- Sogut, E.; Seydim, A.C. The Effects of Chitosan and Grape Seed Extract-Based Edible Films on the Quality of Vacuum Packaged Chicken Breast Fillets. Food Packag. Shelf Life 2018, 18, 13–20. [Google Scholar] [CrossRef]
- Nopwinyuwong, A.; Trevanich, S.; Suppakul, P. Development of a Novel Colorimetric Indicator Label for Monitoring Freshness of Intermediate-Moisture Dessert Spoilage. Talanta 2010, 81, 1126–1132. [Google Scholar] [CrossRef]
- Wahyuningsih, S.; Wulandari, L.; Wartono, M.W.; Munawaroh, H.; Ramelan, A.H. The Effect of PH and Color Stability of Anthocyanin on Food Colorant. IOP Conf. Ser. Mater. Sci. Eng. 2017, 193, 012047. [Google Scholar] [CrossRef]
Figure 1.
Mechanical properties of alginate-citric acid films: (1) Elongation at break, (2) Tensile strength, (3) Tensile modulus. White columns depict controls (i.e. sodium alginate). Light to dark green columns display 5, 10, 15, and 20% citric acid addition to alginate films. Error bars represent standard deviation (n ≥ 5). Statistical significance (p < 0.05) is illustrated by different letters (a-e).
Figure 1.
Mechanical properties of alginate-citric acid films: (1) Elongation at break, (2) Tensile strength, (3) Tensile modulus. White columns depict controls (i.e. sodium alginate). Light to dark green columns display 5, 10, 15, and 20% citric acid addition to alginate films. Error bars represent standard deviation (n ≥ 5). Statistical significance (p < 0.05) is illustrated by different letters (a-e).
Figure 2.
Intensity differences in FTIR spectra of alginate-citric acid samples presented by black (alg), yellow (alg_ca5), red (alg_ca10), blue (alg_ca15), and green (alg_ca20) colours.
Figure 2.
Intensity differences in FTIR spectra of alginate-citric acid samples presented by black (alg), yellow (alg_ca5), red (alg_ca10), blue (alg_ca15), and green (alg_ca20) colours.
Figure 3.
Mechanical properties of DW and GSE films: (1) Elongation at break, (2) Tensile strength, (3) Tensile modulus. The green and red columns depict DW and GSE films, respectively. Colum groups from left to right: without citric acid (alg, GSE_alg), with 10% citric acid (alg_ca10, GSE_alg_ca10). Error bars represent standard deviation (n ≥ 6). Statistical significance (p < 0.05) is illustrated by different letters (a – c).
Figure 3.
Mechanical properties of DW and GSE films: (1) Elongation at break, (2) Tensile strength, (3) Tensile modulus. The green and red columns depict DW and GSE films, respectively. Colum groups from left to right: without citric acid (alg, GSE_alg), with 10% citric acid (alg_ca10, GSE_alg_ca10). Error bars represent standard deviation (n ≥ 6). Statistical significance (p < 0.05) is illustrated by different letters (a – c).
Figure 4.
FTIR spectra of GSE-alginate and their combination with 10% citric acid. Bottom (a) spectra represent controls, i.e. alginate (alg) in grey and GSE-alginate (GSE_alg) in red. Top (b) spectra illustrate 10% citric acid addition to alginate (alg_ca10 in grey), and to GSE-alginate (GSE_alg_ca10 in red).
Figure 4.
FTIR spectra of GSE-alginate and their combination with 10% citric acid. Bottom (a) spectra represent controls, i.e. alginate (alg) in grey and GSE-alginate (GSE_alg) in red. Top (b) spectra illustrate 10% citric acid addition to alginate (alg_ca10 in grey), and to GSE-alginate (GSE_alg_ca10 in red).
Figure 5.
Free radical DPPH∙ scavenging activity of alginate films (alg) and their combination with GSE (GSE_alg); and alginate films treated with 10% citric acid (alg_ca10) and their combination with GSE (GSE_alg_ca10). The darkening of the colour tone illustrates the increasing alginate concentration: 0.5, 1.0, 2.0, 3.0 mg/mL (from light to dark blue, respectively), 0.01, 0.025, 0.05 mg/mL (from light to dark yellow, respectively), 0.075, 0.1, 0.25 mg/mL (from light to dark orange, respectively). The GSE_alg and GSE_alg_ca10 dilutions recalculated to mg GAE/mL from highest to lowest: 0.557, 0.372, 0.186, 0.093, 0.046, 0.019, 0.014, 0.009, 0.005, 0.002 mg GAE/mL. Error bars represent standard deviation (n ≥ 3).
Figure 5.
Free radical DPPH∙ scavenging activity of alginate films (alg) and their combination with GSE (GSE_alg); and alginate films treated with 10% citric acid (alg_ca10) and their combination with GSE (GSE_alg_ca10). The darkening of the colour tone illustrates the increasing alginate concentration: 0.5, 1.0, 2.0, 3.0 mg/mL (from light to dark blue, respectively), 0.01, 0.025, 0.05 mg/mL (from light to dark yellow, respectively), 0.075, 0.1, 0.25 mg/mL (from light to dark orange, respectively). The GSE_alg and GSE_alg_ca10 dilutions recalculated to mg GAE/mL from highest to lowest: 0.557, 0.372, 0.186, 0.093, 0.046, 0.019, 0.014, 0.009, 0.005, 0.002 mg GAE/mL. Error bars represent standard deviation (n ≥ 3).
Figure 6.
Effect of GSE (in red) on the inhibitory activity against E. coli (1) and S. aureus (2) of alginate and citric acid film-forming solutions (in green). In white, control samples (TSB). “Control 0” represents the average initial viable counts for all the assayed conditions (≈ 107 CFU/mL). “DL” is the detection limit of the colony counting method (102 CFU/mL). Error bars represent standard deviation (n ≥ 3). Statistical significance (p < 0.05) is illustrated by different letters a – d.
Figure 6.
Effect of GSE (in red) on the inhibitory activity against E. coli (1) and S. aureus (2) of alginate and citric acid film-forming solutions (in green). In white, control samples (TSB). “Control 0” represents the average initial viable counts for all the assayed conditions (≈ 107 CFU/mL). “DL” is the detection limit of the colony counting method (102 CFU/mL). Error bars represent standard deviation (n ≥ 3). Statistical significance (p < 0.05) is illustrated by different letters a – d.
Figure 7.
Effect of pH (2.0-10.0) on the UV/VIS spectra and colour of GSE-based film-forming solution (GSE_alg_ca10). Controls: water and alg_ca10 film-forming solutions at original pH values.
Figure 7.
Effect of pH (2.0-10.0) on the UV/VIS spectra and colour of GSE-based film-forming solution (GSE_alg_ca10). Controls: water and alg_ca10 film-forming solutions at original pH values.
Table 1.
Overview of the composition and pH of film-forming solutions, as well as thickness and barrier properties of tested films. Statistical significance (p < 0.05) within a column is illustrated by different letters (a – e). alg: alginate, CA: citric acid, ca5: 5% (w/w) citric acid, ca10: 10% (w/w) citric acid, ca15: 15% (w/w) citric acid, ca20: 20% (w/w) citric acid, GSE: grape seed extract, SHP: sodium hypophosphite monohydrate, WVTR: water vapor transition rate.
Table 1.
Overview of the composition and pH of film-forming solutions, as well as thickness and barrier properties of tested films. Statistical significance (p < 0.05) within a column is illustrated by different letters (a – e). alg: alginate, CA: citric acid, ca5: 5% (w/w) citric acid, ca10: 10% (w/w) citric acid, ca15: 15% (w/w) citric acid, ca20: 20% (w/w) citric acid, GSE: grape seed extract, SHP: sodium hypophosphite monohydrate, WVTR: water vapor transition rate.
| Sample code |
Composition (%w·v-1) |
pH |
Thickness (mm) |
WVTR, (g/m2h) |
| Solvent |
alg |
SHP |
CA |
| alg |
DW |
2.00 |
- |
- |
5.9 ± |
0.1a
|
0.04 ± 0.01a
|
138.56 ± |
4.87a
|
| alg_ca5 |
DW |
2.00 |
0.05 |
0.10 |
4.4 ± |
0.2b
|
0.04 ± 0.00a
|
98.49 ± |
9.46c
|
| alg_ca10 |
DW |
2.00 |
0.10 |
0.20 |
4.0 ± |
0.1c
|
0.04 ± 0.00a
|
91.99 ± |
4.41c
|
| alg_ca15 |
DW |
2.00 |
0.15 |
0.30 |
3.8 ± |
0.1d
|
0.05 ± 0.00a
|
89.66 ± |
9.74c
|
| alg_ca20 |
DW |
2.00 |
0.20 |
0.40 |
3.7 ± |
0.1e
|
0.05 ± 0.00a
|
78.04 ± |
3.17d
|
| GSE_alg |
GSE |
2.00 |
- |
- |
5.9 ± |
0.2a
|
0.06 ± 0.01b
|
116.02 ± |
5.16b
|
| GSE_alg_ca10 |
GSE |
2.00 |
0.10 |
0.20 |
4.2 ± |
0.2b
|
0.07 ± 0.00b
|
91.49 ± |
5.00c
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).