1. Cardiac imaging
Cardiovascular diseases constitute the leading cause of global mortality and are a major contributor to reduced quality of life. The diagnosis and the management of cardiovascular diseases is heavily reliant on diagnostic imaging. Since 1920, when the early days of cardiac imaging relied on fluoroscopy, orthodiagraphy and radiography, the diagnosis and treatment of acquired and congenital heart disease (CHD) has been progressively revolutionized by new technologies such as transthoracic echocardiography (TTE), stress echo (SE), transesophageal echocardiography (TEE), nuclear myocardial perfusion imaging (NMPI). In recent years, the rates of use of TTE, SE, TEE have decreased 3% since 2010, while MPI decreased by 36%. These data probably also reflect the advances in cardiac CT and MR technology that have come to the forefront, in the same time period. The use of cardiac computed tomography (CCT) has increased by 84%, and cardiac magnetic resonance imaging (CMR) by 125%(Reeves et al., 2021).
1.1. Computed tomography
In the early 1970s, the first cardiac computed tomography (CCT) was performed. Considering the motion as the main factor that predominantly affected image quality in Cardiac Imaging, by the implementation of electrocardiographically (ECG) gated CCT it was possible to assess a wide range of heart disease, such as left ventricular aneurysms, enhancement of scars, thrombus, and aortic dissection. Since 1998, advances in multi–detector row CT technology with shorter acquisition times and tailored contrast injection protocols let CCT evaluate coronary anatomy and coronary artery disease (CAD). Currently, there are technologies that oriented technical development into a heavily time-resolved equipment (e.g., dual source CT with 256/384 slice) while others focused more on increasing the width of the detector (e.g., 256/320/ 640-slice CT)(Cademartiri et al., 2021). Moreover, with the advent of the new photon-counting CT, coronary CCT showed an improved image quality and diagnostic confidence in humans orienting also toward a tissue characterization that has always been a drawback in comparison to CMR (Si-Mohamed et al., 2022). Nowadays in Italy, the number of imaging centers that perform CCT investigations has been constantly growing as is the number of patients that undergo the test (Cademartiri et al., 2015).
In Italy, as all over the world, the principal indications for CCT are the exclusion of obstructive CAD in symptomatic patients at intermediate clinical likelihood (class I B) with high probability for a good image quality, to improve their referral for angiography thanks to the high negative predict value of the technique, and the calcium score assessment in asymptomatic individual as a risk modifier in the cardiovascular risk assessment (class IIb B) (Knuuti et al., 2020) [
Figure 1].
In the setting of the emergency room, the request for urgent CCT in patients with acute chest pain is showing a growing trend [
Figure 2] and more and more hospitals have machines and personnel skilled to perform the “CT triple rule out protocol” for obstructive CAD, aortic dissection, and pulmonary embolism.
On the other side, given the “CCT based opportunity” for a stronger preventive treatment in patients with non-obstructive CAD and high-risk plaques, there is an increase in CCT request in asymptomatic patients with CAD risk factors at low-intermediate likelihood, as a screening tool (Natale et al., 2023).
CCT is also increasingly requested as a roadmap before minimally invasive intervention: transcatheter valve repair/replacement, prosthetic valve assessment in suspected dysfunction or endocarditis or pre-left atrial ablation (Natale et al., 2023).
The optimal balance between image quality and radiation dose is fundamental in CCT imaging, not only in the scan acquisition protocols, which are increasingly tailored to the clinical query, but also in image analysis. Post-processing requires a standardized execution with a chosen software package to make sure consistent and accurate measures are obtained(Natale et al., 2023). Post-processing software are developing constantly following the direction of progressive automation and are all able to quantify plaque’s stenosis severity and plaque burden(Cury et al., 2022). Newer techniques include semiautomated volumetric quantification of the non-calcified, low-attenuation coronary plaque component (<30 HU being the most frequently applied cutoff), which together with the presence of positive remodeling and spotty calcifications can improve prediction of myocardial infarction in patients with stable chest pain (Ferencik et al., 2018) . Moreover, the peri-coronary-fat-attenuation-index (pFAI- cut off >-70.1 Hounsfield Unit), could provide a quantitative measure of coronary inflammation as indicator of major cardiac adverse events (Oikonomou et al., 2018). Software able to perform the above-mentioned quantifications are still committed in clinical research, but their large-scale use will likely spread as further data become available.
Recently, Positron emission tomography (PET) with newly adopted radiotracers (18F-Sodium Fluoride) provided unique insights into coronary atheroma activity, acting as a powerful independent predictor of myocardial infarctions (Kwiecinski et al., 2023).
The need to standardize the report and to guide the steps in improving the patient management drove the efforts of radiologic societies towards the creation of structured Reporting and Data System, in particular the Coronary Artery Disease Reporting and Data System (CAD-RADS). The updated 2022 CAD-RADS (2.0) improved the initial reporting system for CCTA by considering new technical developments in Cardiac CT, including the assessment of lesion-specific ischemia using CT fractional-flow-reserve (CT-FFR) or myocardial CT perfusion (CTP), where (Cury et al., 2022). The spreading of state-of-the-art CCT with a 16 cm volume of coverage is mandatory to routinely performed static and dynamic myocardial perfusion imaging by CT in order to quantify the myocardial blood flow and to evaluate the lesion specific ischemia. The one shot stop CT (coronary anatomy and ischemia) is the main road to save additional functional exam (i.e., stress echo, MR NMPI or FFR) in the consistent population with a moderate CAD.
Despite descriptive conclusions are still common in CCT reports, most centers in Italy use CAD RADS and the trend is increasing, although CAD-RADS cannot be considered a substitute for the impression provided by the reading physician, called always to interpret the CCT findings based on the more detailed patient-specific information.
1.2. Magnetic Resonance
Cardiovascular Magnetic Resonance (CMR) is the primary imaging modality for non-invasive myocardial tissue characterization, and it is considered the gold standard test for quantifying cardiac function, myocardial volumes, and mass (Ordovas et al., 2021). Since its first applications in the cardiovascular field at the end of the last century, MR imaging has recognized its specific diagnostic strength in its ability to characterize myocardial tissue, through non-parametric sequences evaluated qualitatively or semi-quantitatively, specifically for the identification of oedema, fat, iron, amyloid, necrosis and fibrosis in basal conditions and ischemia in stress settings. Thanks to the possibility of evaluating myocardial tissue by exploiting the magnetic properties of the myocardial structures, it is increasingly applied in different clinical scenarios with a huge impact on the management of the patients with heart involvement (Habib et al., 2017; Mavrogeni et al., 2022).
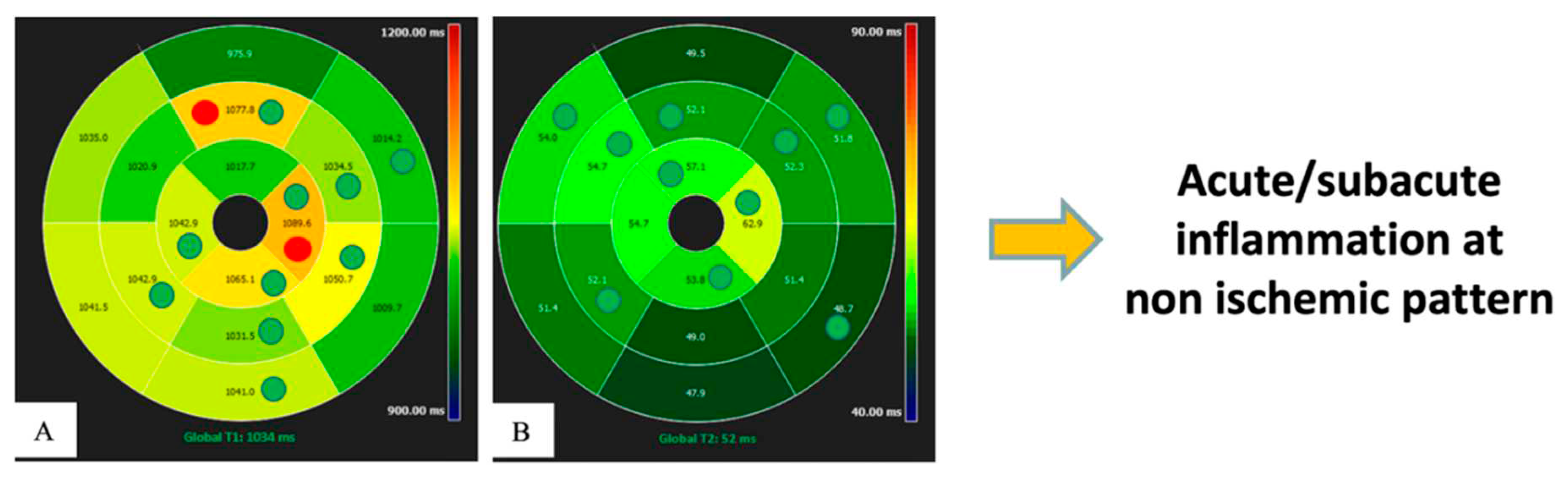
In recent years, the introduction of parametric mapping techniques has permitted the evaluation of quantitative changes in myocardium, based on T1, T2, T2* and extracellular volume (ECV) parameters , which are linked to tissue characteristics, as proved by bioptic correlation studies (Messroghli et al., 2017). This represents a significant step forward in the quantitative diagnostic capacity of various myocardial pathologies, which in the past often required invasive investigations. The main advantages of a quantitative evaluation are objective differentiation between pathology and normal conditions, grading of disease severity, and the possibility of comparing different groups of patients and normal subjects.
Thus, mapping has introduced a new frontier in cardiovascular radiology, enabling to quantify properties of myocardium comparing them with normal reference values acquired under the same scanning conditions (Meloni, Gargani, et al., 2023; Meloni, Pistoia, et al., 2023). Previously, diffuse myocardial disease had always been difficult to measure or even appreciate without invasive procedures. Thus, this advance is of utmost importance, because focal and diffuse changes often directly reflect pathophysiologic processes of various diseases from their preclinical phase up to the end stage, and it promises advancement in the treatment of cardiological patients through better quantitative diagnostics and inter- and intra-patient comparability, allowing patients to be followed over time. Consequently, mapping techniques have the potential to act as biomarkers to help diagnostic decision making in several pathologies (Meloni et al., 2015), leading to a significant improvement in the prognosis of patients with cardiac diseases by tailoring the therapy. Furthermore, the use of mapping in the context of medical research allows quantitative endpoints to be set in phase 2 and 3 clinical trials without the need for invasive testing.
Initially, much of the research published about parametric imaging was focused on progress in acquisition methodology. This allowed to achieve numerous fast and robust mapping software, nowadays commercially available on the state-of-the-art CMR systems. And the evidence on the clinical value of CMR mapping from large clinical outcomes trials is rapidly growing. Thus, parametric mapping can be considered as a natural extension of comprehensive CMR protocols for a deep and quantitative myocardial assessment (Messroghli et al., 2017). Moreover, thanks to technological improvement, it is now possible to analyze the entire left ventricular myocardium with a global or segmental that showed the intrinsic advantage of higher sensitivity [
Figure 3] (Meloni, Gargani, et al., 2023; Meloni, Pistoia, et al., 2023; Pepe et al., 2022).
The main limitation for spreading the mapping techniques in the routine clinical and research arena is due to its high reliance on the single scanner, on the type of sequence used and on imaging acquisition modality. This is the reason why normal reference values based on sex and age are recommended for each center considering that these parameters can be partially influenced by age and sex (Meloni et al., 2021, 2022; Messroghli et al., 2017) [
Figure 4].
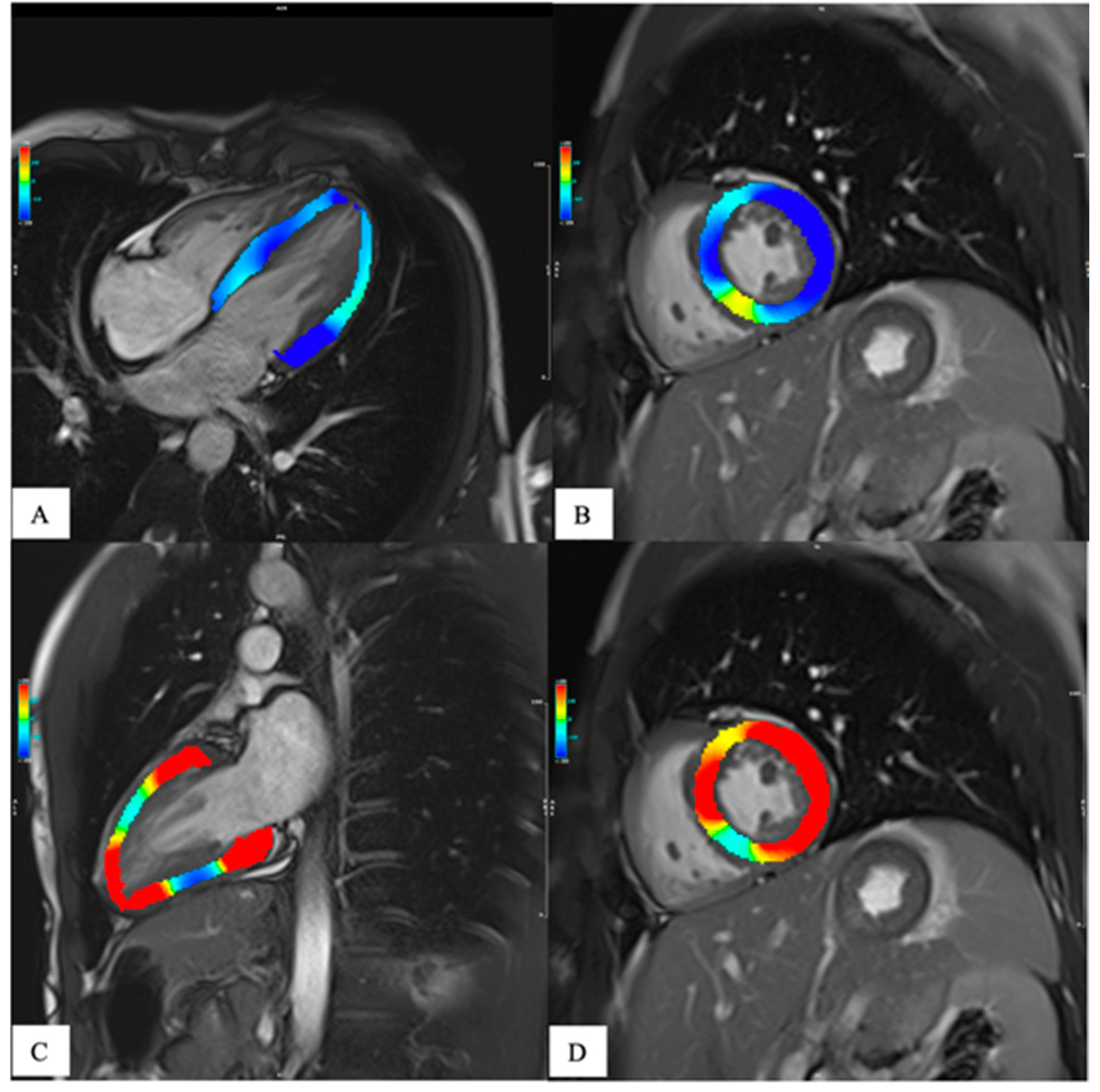
The need to a more in-depth characterization of heart function has led, in the last years, to the development of tissue tracking analysis. This technique provides a quantitative assessment of the global and regional kinetics of the myocardium, [
Figure 3] giving adjunctive information about heart function other than those obtained by the grossly ejection fraction (EF) alone(J. Xu et al., 2022). In fact, the EF is a parameter of overall ventricular systolic function and does not provide information about regional myocardial kinetics and contractility nor allows evaluation of the diastole. Moreover, in many heart diseases, the EF is altered only late in the progression, leaving a diagnostic gap in asymptomatic or subclinical conditions. Since its introduction in the late 1980s, tissue tracking has been extensively developed and is now gaining popularity due to the development of software for fast and robust analysis and to research trends towards generating evidence of the adjunctive value of strain in early and differential diagnosis, risk stratification and prognosis determination of many heart diseases.(J. Xu et al., 2022) Recent evidence has given strength to this technique so that it is starting to be included in CMR assessment. As for mapping, the determination of normative values both for healthy subjects and disease specific population is important to avoid misdiagnosis.
Another promising development in CMR imaging is the quantitative assessment of the functional mean of the coronary artery disease (CAD)(Quinaglia et al., 2019). The quantitative stress perfusion imaging seems to allow greater reproducibility and improving diagnostic accuracy compared to the qualitative stress CMR methods, thus improving the patient management. The quantitative perfusion technique could be carried out with different protocols, like dual-bolus, pre-bolus, and single bolus with dual sequence, and has been proven to be able to detect functionally significant coronary stenosis, with a higher cost-effectiveness compared also to anatomical assessment with Computed Tomography coronary angiography(Knuuti et al., 2020). Also in this case, some considerations should be made, specially about the limited availability of quantitative perfusion CMR.
Last but not least, topic worthy of mention is the introduction of 4-dimensional (4D) flow MRI, which provide a “time-resolved” form enabling a comprehensive visualization of blood flow in heart and throughout the human circulatory system, with the possibility to determine defects and abnormalities (Dyverfeldt et al., 2015).This promising tool is expecting to be increasingly applied, providing new possibilities through non-ionizing imaging method.
Advancing CMR technologies allowing for quantitative evaluations are having a significant impact on clinical management, in optimizing diagnostic process and evaluating prognostic factors, with the aim of guiding new and personalized therapeutic approaches. The identification of new biomarkers within the context of a precision medicine will also lead to evidence of and therapeutic efficacy.
1.3. Future perspective: national networking for big data and artificial intelligence, cardio-radiologist in the heart team by a specific training
Through the technological transfer of innovative quantitative cardiac CT and RM approaches, further developments in efficiency will be possible, also by external collaborations among different centers and with bioengineers for cardiac CT and MR image analysis, and hardware and software development, leading to the creation of national and international networks. The formation of a quantitative imaging network as a comparison and communication tool, allowing remote consultation between specialists and the sharing of clinical-diagnostic protocols, may promote new initiatives and side projects, through which research, knowledge, technological innovation, and high-quality care for the patient are produced. Moreover, the development and analysis of large national high-quality databases, hopefully on behalf of the scientific societies, would pose the basis for the application of artificial intelligence deep learning techniques.
Thus, the application of advanced knowledge and skills for research and clinical purposes may implement multidisciplinary projects and collaborations to test innovative precision therapeutic strategies through quantitative cardiac CT and MRI imaging protocol, placing the cardiac radiologist as pivotal factor.
A close interaction among the other physicians involved in the management of the patients with cardiac disease (cardiologists, cardiac surgeons, reference physician for the systemic disease involving the heart, and nuclear medicine physicians) is crucial, because the sharing of specific knowledge and competence is of outmost importance for the choice of management, pharmacological, interventional and surgery planning (Natale et al., 2023). Aligned with the development of multidisciplinary tumor boards, the heart team has become a clinical necessity in an optimized patient center-based management.
The involvement of dedicated cardiac radiologists within the multidisciplinary team brings many advantages including multimodality knowledge, expertise in 2D and 3D image interpretation, detailed knowledge of radiation exposure/-control and safe procedures, and expertise in the interpretation of non- cardiac findings (Natale et al., 2023).
By the principle of maintaining full respect for each other’s expertise area, in Italy the radiologist is the unique report’s legal manager (D Lgs 101, 2020 and DM 14 gennaio 2021). Thus, expanding education and training opportunities starting from residency, together with certified advanced cardiac life support (ACLS) courses, is the first aim to achieve quality improvement and high standards of clinical service in cardiac imaging in our country.
Just as today’s, cardiac imaging with its ability to provide diagnostic, prognostic, and risk assessment for tailored therapy were unimaginable just two decades ago. Dedicated cardiac radiologists with a specific training and a legal recognition on the model of the path taken in neuroradiology are the main road to further expand the future in cardiac imaging.
2. Vascular imaging
The imaging of the vasculature is an old discipline going back to the fifties, when angiography was the preferred method. The development of computed tomography (CT) in later1970, progressively superseded angiography becoming the standard imaging modality to assess vascular diseases (K. L. Hansen & Carlsen, 2021).
By the introduction of ECG-triggered acquisition, the need of additional scans due to pulsation artifacts was prevented and a high accuracy in the imaging of aorta and nearby vessels was progressively achieved in 1990s (Wielandner et al., 2016).
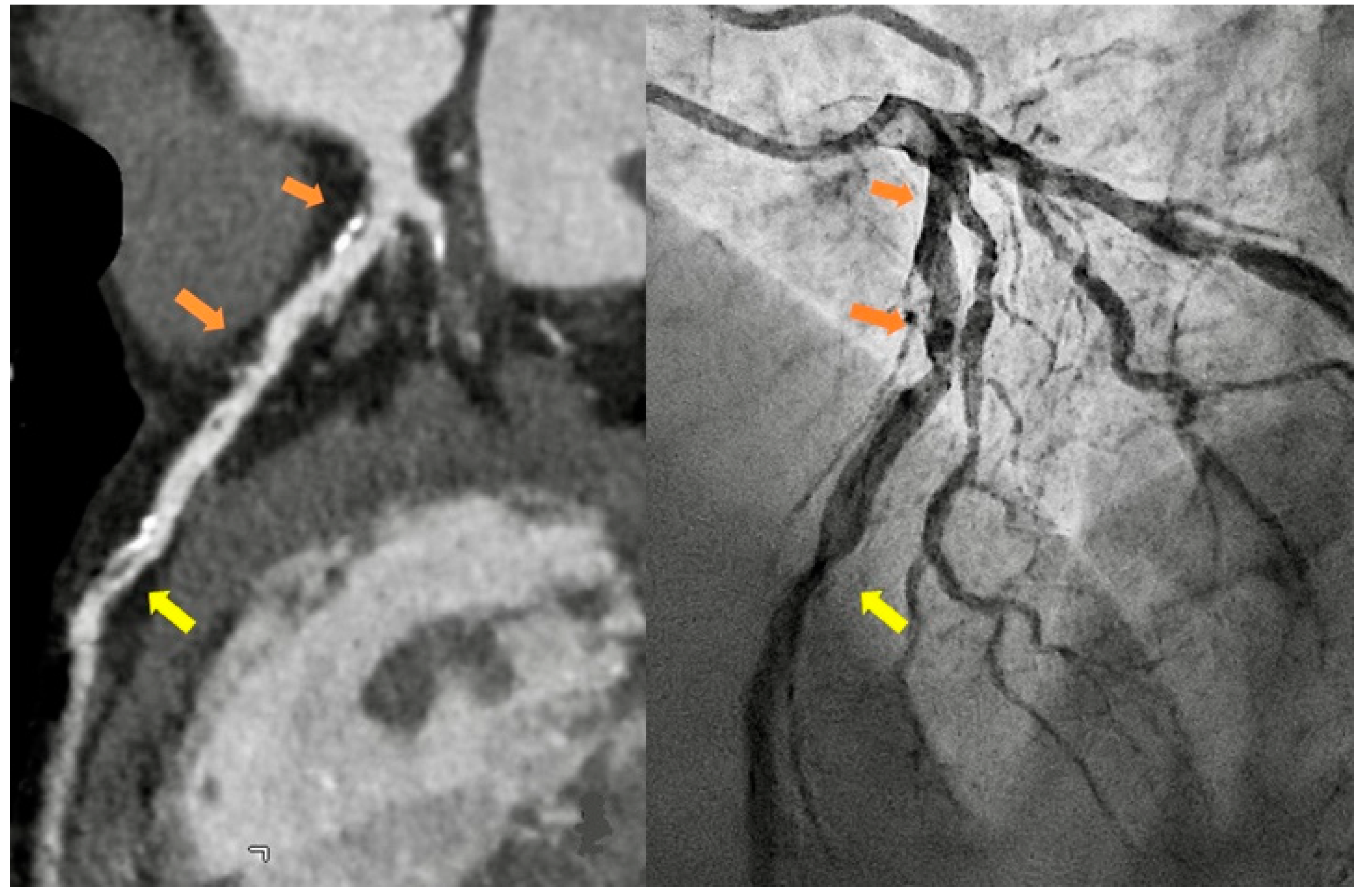
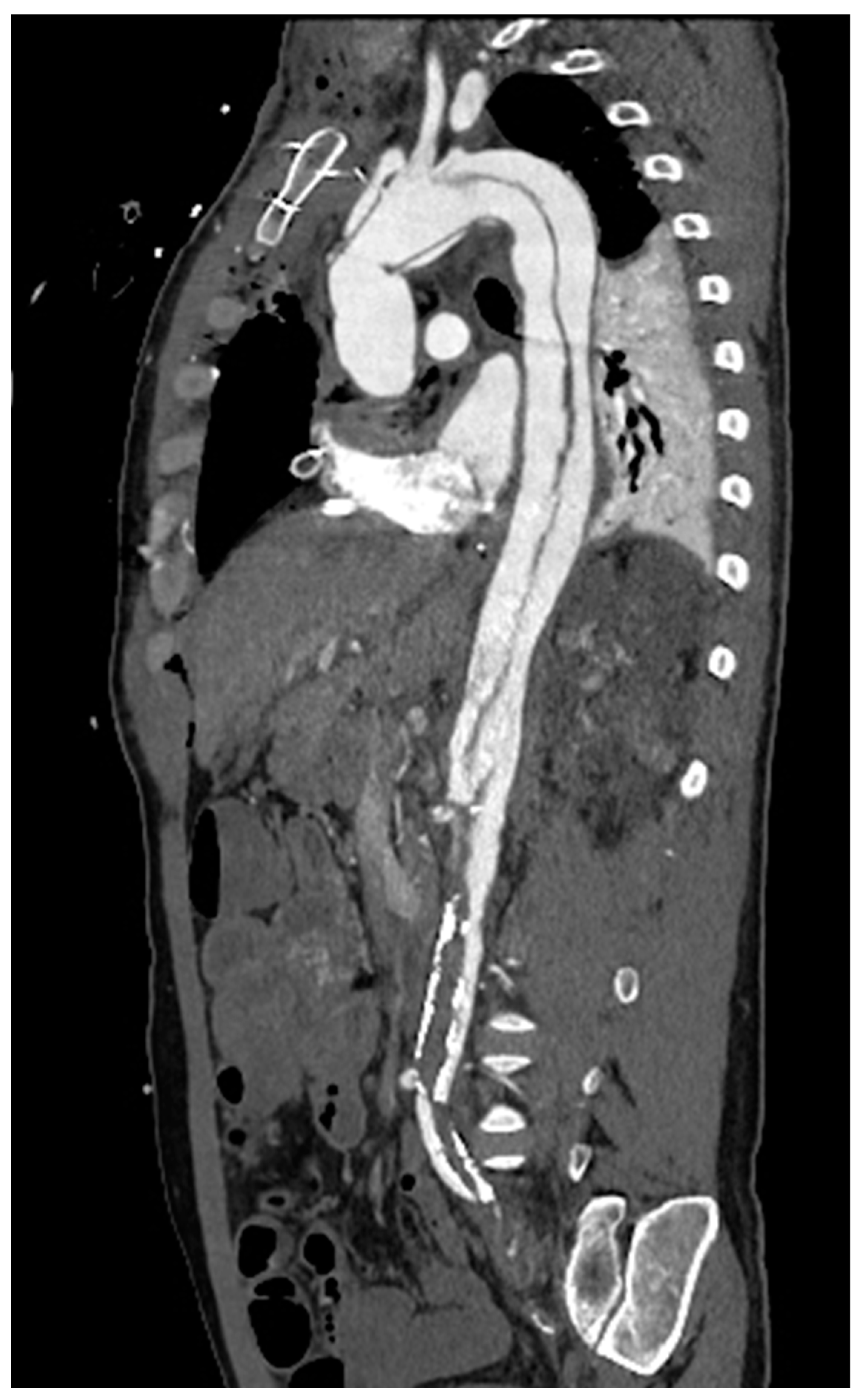
In Italy, CTA is the technique routinely employed in aortic imaging. Nowadays, multidetector row CT (MDCT) scans are highly efficient, widely available, and are highly sensitive and specific in diagnosing acute aortic syndromes (AAS) such as dissections, intramural hematomas, penetrating ulcers. Moreover, MDCT provides detailed insights into the vessel’s thickness and composition, allowing the evaluation of vasculitis and, in the emergency setting, it has been more and more employed to localize the sources of gastrointestinal bleeding(Geffroy et al., 2011). Maximum intensity projection (MIP) reconstruction provides global assessment and rapid detection of vascular stenosis and occlusion(Hyde et al., 2007). Pre-procedural MDCT is crucial for percutaneous interventions, such as transcatheter-aortic-valve-replacement and transcatheter -mitral-valve-replacement. While transthoracic echocardiography is often employed in monitoring post-procedure results with a limited acoustic window, potential complications are generally investigated by CT scans(Francone et al., 2020). In routine surgical scenarios, post-processing techniques, such as multiplanar reformats and segmented volume-rendered (VR) reconstructions, have greatly improved the assessment of vascular anatomy and they are generally requested by cardiac surgeons [
Figure 5].
Although confined in third level care centers, 3D-printed-models have increasingly been used in preoperative setting to improve surgical approach, particularly in congenital heart disease (CHD)(Zhao et al., 2018). Future advancements include improvements in hardware, computer-aided diagnosis (CAD) software, and functional and physiological imaging. Artificial intelligence and deep-learning-based automated software have increasingly been integrated in CT workstations to reduce operator dependence(Santos et al., 2019).
New CT systems, such as dual-energy CT (DECT) and photon-counting CT (PCCT) have been recently introduced. DECT surpasses conventional CT scans by attenuation measurements from different energy spectra, quantifying material composition (So et al., 2012). This novel method facilitates the separation and quantification of iodine concentrations, enabling a better assessment of tissue perfusion,and it is particularly interesting in the study of acute aortic syndrome, where extravasation, intramural hematoma, and endoleak are challenging to accurately image by conventional CT(Jacobsen et al., 2020). PCCT offers complete spectral multi-energy data information, with small detector pixel design, and better spatial resolution facilitating the assessment of iodine density and the quantification of diverse virtual monoenergetic images (Leng et al., 2019). PCCT guarantees that patients reduce noise and artifacts along with minimal radiation doses for evaluating disease processes and optimal dose efficiency. This ensures a superior CT angiographic examination and enhances patient safety (Counseller & Aboelkassem, 2023). By intravenous administration of two contrast agents, PCCT can capture endoleak dynamics and discriminate endoleaks from intra-aneurysmatic calcifications in a single scan reducing the radiation exposure. It also has the potential to improve the visualization of the stent deployment (Meloni, Frijia, et al., 2023).
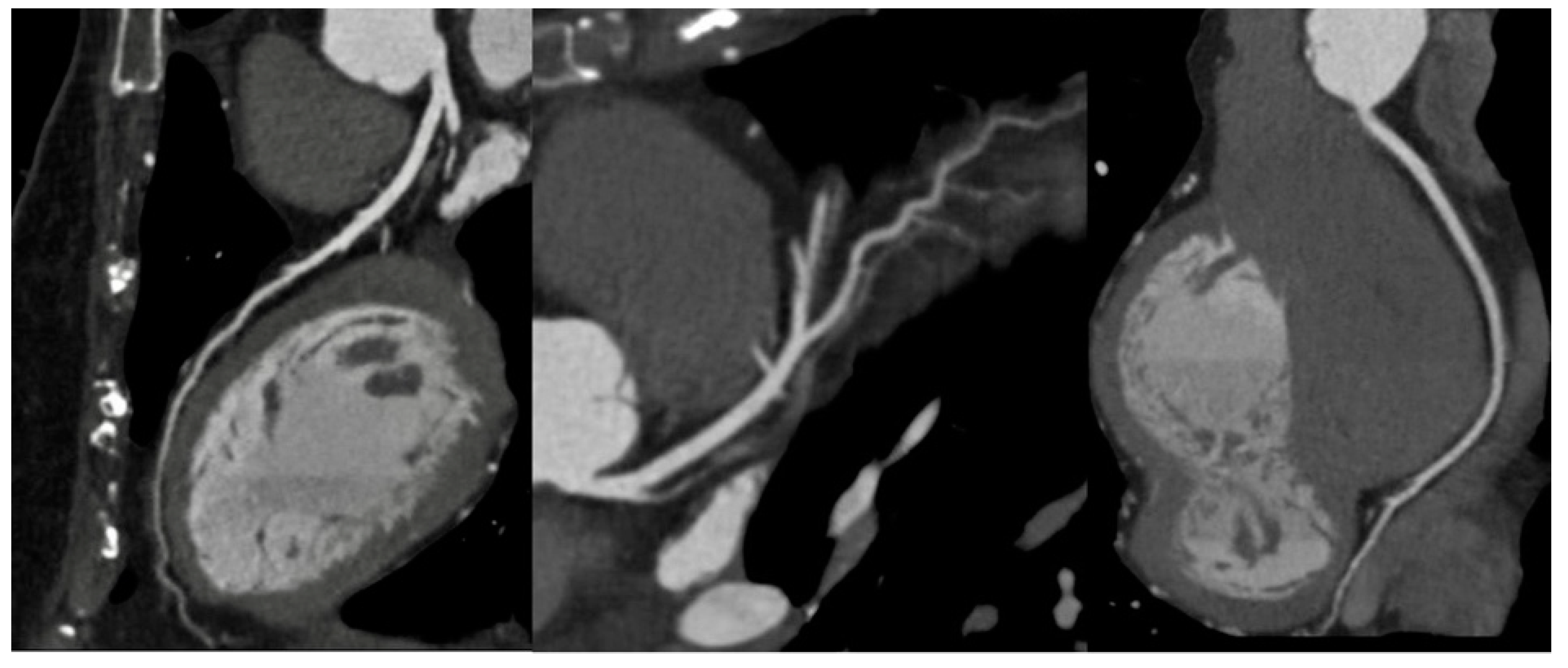
In a non-acute setting, MDCT has been traditionally preferred to Magnetic Resonance (MRI) because of its time consumption, inferior spatial resolution, and lower availability. However, the assessment of large vessels by Gadolinium-enhanced MRI is exponentially growing in the follow up in both pediatric and adult population, thanks to the latest advancements in MRI technology thanks to faster acquisitions and the advantage of being a ionizing free technique, with a specific diagnostic strength for tissue characterization (Pushparajah et al., 2019). Furthermore, MRI can give additional information on ventricular, valvular and vascular function and flow dynamics by using cine Steady-State-Free-Precession (SSFP) and Phase contrast sequences. Advancements in Fast-Spin-Echo (FSE) black-blood T1, Proton Density and Short-Tau-Inversion-Recovery (STIR) T2 sequences have improved the assessment of vessel, making them a valuable tool for aortic wall pathology [
Figure 6](McNally et al., 2021).
The clinical use of 4D-flow MRI has only recently become available. With ECG-gated time-resolved acquisition, a 3D image of blood flow in all three spatial directions can now be captured, allowing visualization of blood flow in the vessels extending from the brain to the lower extremities(Dyverfeldt et al., 2015). Additionally, assessments of wall shear stress, pulse-wave velocity, and pressure gradients can be performed alongside blood flow estimation. MRA combined with 4D flow MRI offers an advantage over dynamic CTA in imaging the whole aorta with no radiation burden; however, its sensitivity in detecting endoleaks has yet to be determined, and further large-scale studies are necessary to determine its optimal use(Qin et al., 2023).
Doppler ultrasound, which is approximately 35 years of age, remains the least invasive and cost-effective technique in cardiovascular radiology; however, its use is confined to specific anatomical sites. Vector Flow Imaging (VFI), a new technique for angle-independent velocity estimation using ultrasound, surpassed traditional spectral Doppler ultrasound in terms of accuracy and precision. VFI can grade stenosis by evaluating the flow complexity of the ascending aorta, carotid, and femoral arteries and enables real-time quantification of vortices in any part of the vessel. VFI studies have focused on assessing vortex formation in children with CHD, and in adults with straight or complex geometries and have concluded that flow is much more intricate than previously assessed by conventional Doppler ultrasound (K. Hansen et al., 2019).
Due to the increasing requests in vascular imaging in our Country, there is a general attitude to provide hospitals with sophisticated machines able to improve spatial and temporal resolution, conspicuity, signal-to-noise ratio, and field of view. Even if qualitative assessment of vessels anatomy is still the most routinary approach, quantitative measurements such as flow complexity, vorticity, perfusion, vessel density, and tortuosity are being increasingly considered. Under these assumptions, the future scenario in our Country will be more and more based on dynamic imaging, such as 4D flow MRI and dynamic CTA which will provide on a large-scale information about vessels’ anatomy and composition, hemodynamic patterns and wall shear stress. Finally, interesting results are coming from Computed Tomography Texture Analysis (CTTA). Through Texture Analysis, it is possible to obtain precise quantitative data from CT scans, which can offer a thorough understanding of the correlation between phenotyping and tissue pathology. CTTA has shown potential for improving the diagnostic accuracy of aortic dissection(Guo et al., n.d.), aortic aneurysms(Wang et al., 2023), and their assessment after EVAR(Charalambous et al., 2022). Moreover, by quantifying periaortic fat and aortic wall calcifications, TA could be a potential tool for identifying patients at a higher risk of developing cardiovascular diseases(Tharmaseelan et al., 2022). In summary, implementing standardized CT protocols across multiple centers and utilizing computer-aided software are crucial steps in improving the visualization quality of vascular systems and reducing radiation dosage and operator variability.
3. Rectal imaging
Latest researches on rectal cancer are focusing mainly on identification of residual disease after neoadjuvant chemo-radiotherapy (nCRT).
This because a complete or major clinical response of locally advanced rectal cancer (LARC) after nCRT, jointly with the absence of loco-regional nodal metastases, allows the patients to be enrolled in rectum preserving approaches.(Dossa et al., 2017)
Since few years ago, the rectum sparing approaches after nCRT were reserved only for those patients that were not fit for classical surgical interventions because of other systemic disease or for patients that refused surgery. There is a growing body of scientific evidence which supports the feasibility and safety of rectum sparing strategies in patients that had a major or complete clinical response to nCRT, since there is a reduction of the side effects and acceptable oncological outcomes(Dossa et al., 2017). Basically, the two main strategies that have been proposed and tested to preserve the organ are the watch-and-wait protocols, where surgery is avoided and the patient is strictly monitored with MRI and endoscopy, and the transanal local excision, where the residual scar/lesion is removed and a histopathology report of the primary tumor can be obtained thus allowing a quantification of the risk of mesorectal metastases(Dossa et al., 2017). The latter approach is not free of complications, since it has been reported that in one-third of patients that underwent local excision a subsequent total mesorectal excision (TME) was required and the bowel function in this group of patients was worse than those who had directly TME (Pucciarelli et al., 2016).
Anyway, these rectum-preserving strategies proved to reduce the side effects that are associated to the classical surgical approaches such as TME or the abdominal-perineal resection, granting a better quality of life to the patients(Dossa et al., 2017).
The role of medical imaging in this field is mainly divided in the evaluation of the residual of local tumor (T staging) and the identification of nodal metastases (N staging).
3.1. T staging
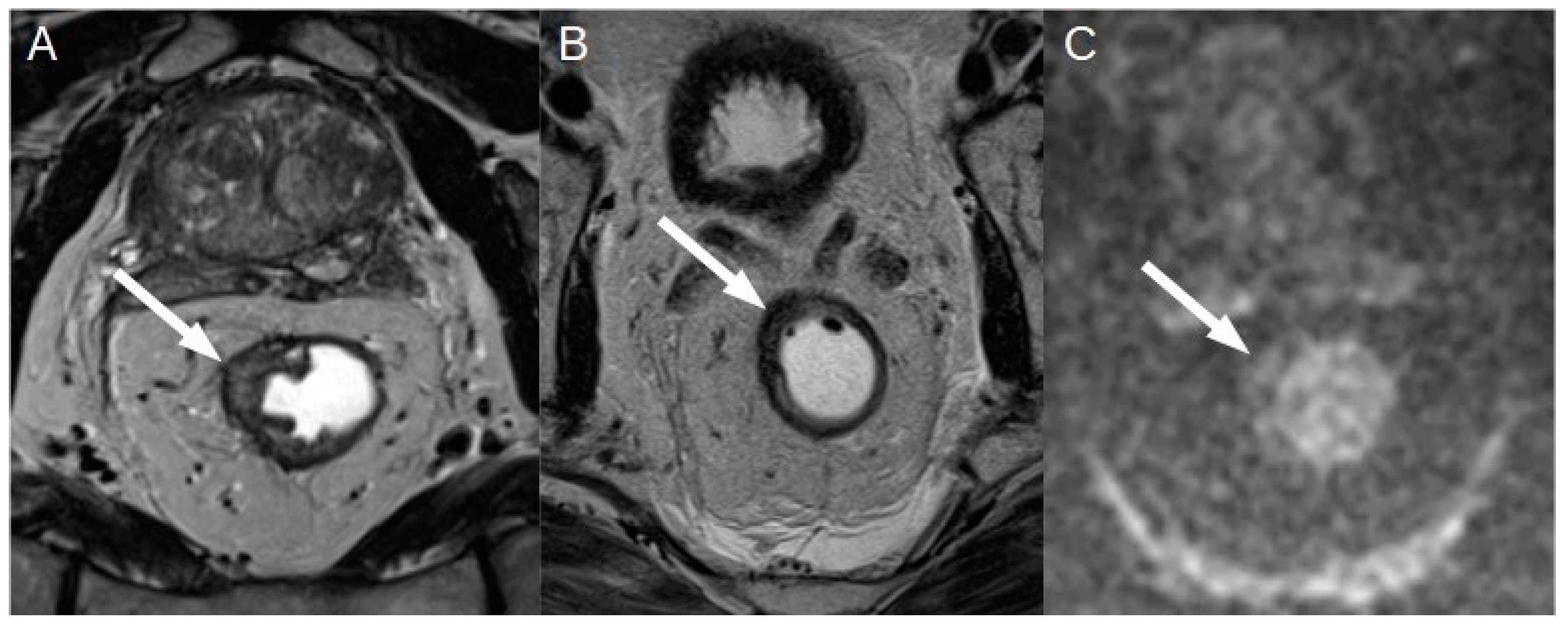
In 2018 the European Society of Gastrointestinal and Abdominal Radiology (ESGAR) published an expert consensus on MRI rectal cancer staging and restaging (Beets-Tan et al., 2018). The suggestion of ESGAR for the evaluation of local tumor after nCRT was to distinguish among: i) a complete normalization of the rectal wall; ii) a fibrotic wall thickening without clear residual mass; iii) residual mass. The normalization of rectal wall is appreciable in 5% of cases(Dresen et al., 2009) . Some studies have proposed an analogue to histopathological tumor regression grade (TRG), that has been called mrTRG, first of all by the Magnetic Resonance Imaging and Rectal Cancer European Equivalence Study (MERCURY) (Horvat et al., 2023a) [
Figure 7].
With this score the tumor response to nCRT is divided in five classes from mrTRG1 (no residual tumor signal) to mrTRG5 (no changes in the tumor after chemoradiation), but it showed a poor correlation with the histopathological evaluation, even if the agreement between radiologist was moderate (Horvat et al., 2023b).
Other authors proposed morphological evaluation of the local tumor based on T2-weighted sequences. The whole tumor volume after nCRT and the percentage of volume reduction have been shown to be effective in the prediction of complete response to nCRT, more than one or two-dimensional measurements, with accuracies up to 85%(Q. Xu et al., 2021) . The main drawback of this method is that it is time consuming and difficulty applicable in a routinary clinical setting.
The use of diffusion weighted imaging (DWI) sequences has also been exploited in the identification of complete response of the tumor to nCRT. Many studies underlined how DWI sequences combined to T2-weighted images can help the radiologist in detecting area of residual tumor after chemo-radiation in the tumoral mass, even if in a 2019 review by Norvat et al. they are not officially recommended and listed as a controversial practice (“maybes”) (Horvat et al., 2019; Q. Xu et al., 2021) . Bates et al proposed the used of high b value (up to b1500), such as in MRI prostate evaluation, underlying how this helped in the identification of residual rectal cancer after nCRT (Bates et al., 2020).
The evaluation of the tumoral volume with DWI sequences have been also evaluated demonstrating a good accuracy in complete response detection (for Δvolume sensitivity and specificity of around 70-80% and 84-93%), anyway suffering of the same technical difficulties of volume measurement on T2-weighted images(Q. Xu et al., 2021). Moreover, the evaluation of the apparent diffusion coefficient (ADC) maps derived from DWI images were tested in complete response to nCRT detection(Q. Xu et al., 2021). The best results were obtained in the evaluation of the percentage change of ADC values more than in the measurement of ADC values before and after nCRT.
While the role of dynamic contrast-enhanced (DCE)-MRI with the evaluation of different parameters derived from the contrast in-flow and out-flow curves in the lesion [Vp (plasma volume,) Ktrans (plasma-to-extravascular volume transfer), Kep (extravascular-to-plasma volume transfer constant), and Ve (extravascular extracellular volume fraction per unit of tissue volume)], is still debated with discording results in literature(Q. Xu et al., 2021).
3.2. N staging
A meta-analysis published in 2020 underlined how the accuracy of MRI for nodal staging after nCRT is suboptimal, with a sensitivity of 77%, a specificity of 77%, a positive likelihood ratio of 3.40 and a negative likelihood ratio of 0.30 (Wei et al., 2020).
Obviously, the presence of loco-regional nodal metastases makes the TME mandatory since a rectum-sparing approach would put the patient at high risk of local recurrence or distant metastases.
On the other hand, the suspect of loco-regional metastases, not confirmed by histopathology, would expose the patients to an over-treatment.
Hence, since the publication of the ESGAR criteria for MRI stating and restaging of rectal cancer, where the criterion that has been proposed for the identification of loco-regional nodal metastases was the presence of lymph nodes with a short axis greater or equal than 5 mm (Beets-Tan et al., 2018) ,different studies tried to improve the accuracy of MRI for identification of loco-regional metastases, mainly focusing on dimensional or morphological evaluation of the lymph nodes.
The morphological criteria that were deemed to be useful at baseline MRI 70 proved to be inaccurate in the restaging setting with a low agreement between different readers (Horvat et al., 2023a; Pomerri et al., 2017).
Considering these evidences, different researches focused exclusively on lymph nodes size to detect nodal metastases.
Heijnen LA et al measured all the lymph nodes visible on a 3-dimensional T1-weighted gradient echo sequence in the mesorectal fat in 39 patients affected by LARC after nCRT and identified 2.5 mm of short-axis as the best cut-off for nodal metastases detection with a sensitivity of 75% and specificity of 64% (Heijnen et al., 2016) .
Pomerri et al evaluated the global mesorectal lymph node reduction rate between staging and restaging MRI (i.e. the reduction of the sum of all the short axis of the lymph nodes within the mesorectum) and identified a 70% percentage of reduction rate as the best cut-off to identify patients without nodal metastases71. This method showed a very good negative predictive value (95%) and sensitivity (90%) but is suffered of a low specificity, moreover, the measurement of the short axis of all the mesorectal lymph nodes both at staging and restaging MRI is time-consuming and hardly applicable in a routinary clinical setting (Horvat et al., 2023a; Pomerri et al., 2017).
The DWI sequences also have been proposed for the detection of nodal metastases within mesorectum in LARC after nCRT for the detection of nodal metastases (van Heeswijk et al., 2017) .
van Heeswijk MM et al showed how the absence of mesorectal lymph-nodes at DWI sequences in a post chemo-radiation setting had a 100% negative predictive value for the differentiation between patients with and without nodal metastases at histopathology, thus suggesting a role also for this kind of sequences in the selection of patients that should be addressed to rectum sparing approaches (van Heeswijk et al., 2017). The main drawbacks of this method were a very low specificity (14%) and positive predictive value was (24%).
Another issue is the presence of latero-pelvic metastatic lymph nodes (i.e., obturator, internal iliac or external iliac) since lateral pelvic lymph nodes dissection is not performed routinely in Italy, as recommended by the European Society of Medical Oncology guidelines (Glynne-Jones et al., 2017) . Indeed, it is still debated whether this intervention is worth the expense in surgical risks and collateral damages for the patients. In 2022 Kroon HM et al. published a systematic review of literature showing that the survival of patients treated with lateral lymph nodes dissection was not significantly different from that of those whose performed standard TME, even if a lower rate of local recurrence was reported (Kroon et al., 2022).
Based on these assumptions, the imaging evaluation of lateral pelvic lymph nodes is pivotal to address patients toward the best surgical approach.
In 2019 the Lateral Node Study Consortium through a study including more than 700 patients restaged after nCRT suggested 4 mm and 6 mm of short axis as cut-offs for obturator and internal iliac nodes in order to identify metastatic lymph nodes and to address patients toward lateral pelvic dissection(Ogura et al., 2019).
3.3. New Techniques and Applications
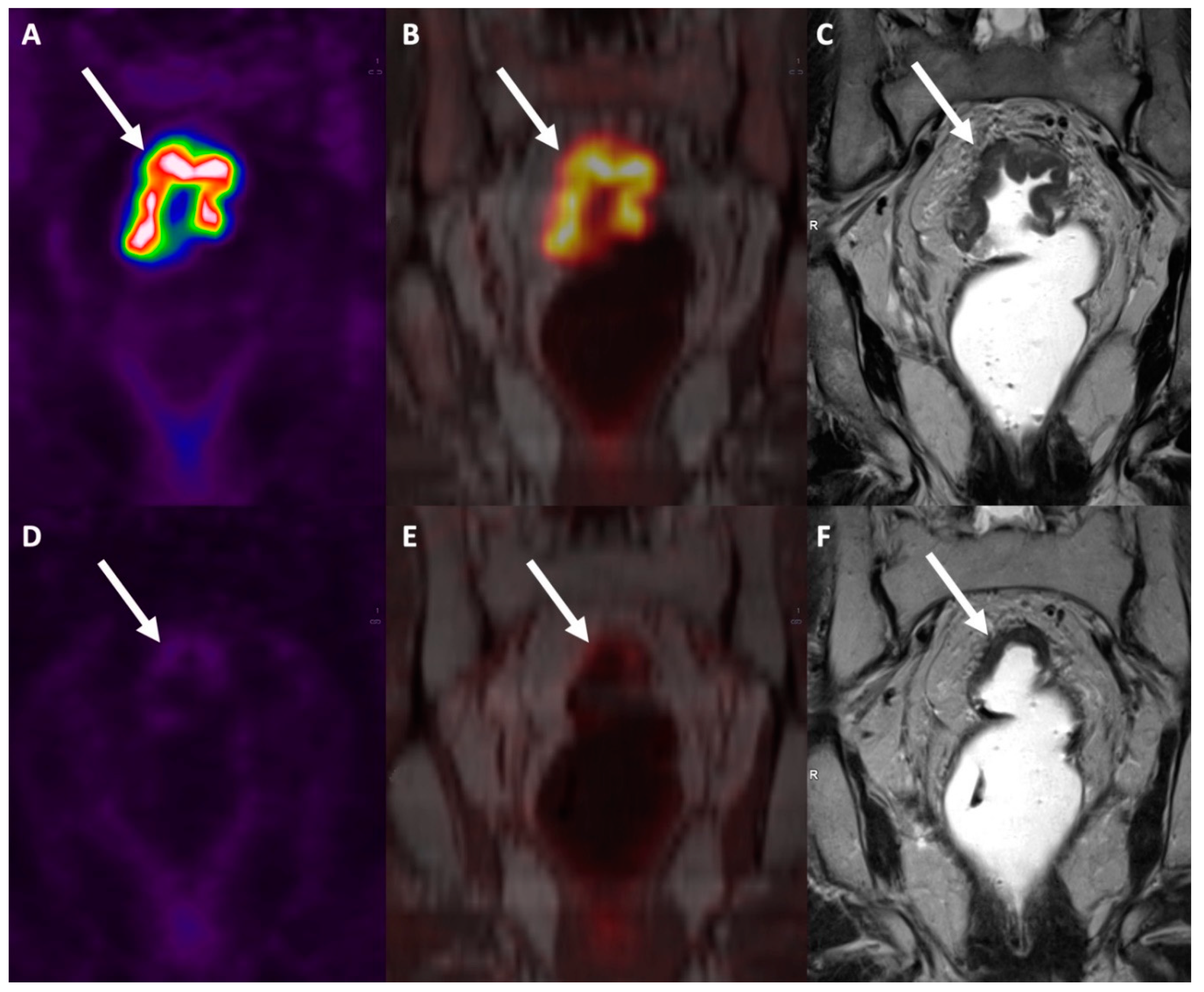
Combined [18F]FDG PET/MRI has recently been proposed as an effective imaging modality for rectal cancer patients, capable of generating high-resolution anatomical and functional data. This combined imaging modality can also spare patients the radiation exposure associated with the CT component of PET/CT. PET/MRI achieves a high soft-tissue contrast that is useful for delineating local tumor extent, and it can be implemented with ‘functional’ MR sequences like diffusion-weighted imaging (DWI) (Jayaprakasam et al., 2023) .
In a recent meta-analysis, the sensitivity and specificity for T staging, N staging and M staging in colorectal patients was 95%/79%, 81%/88% and 97%/93%, respectively (Mirshahvalad et al., 2022). While focusing on rectal cancer, PET/MRI thanks to its good accuracy in T and N staging showed to be a good tool for restaging after nCRT [
Figure 8] and to address patients toward rectum-sparing techniques, while for M staging there is an intrinsic weakness for the lung parenchyma evaluation (Crimì et al., 2021).
Another interesting field is the application of radiomics and texture analysis in prediction of response to nCRT. Several studies tested, with promising results, the capability of different textural T2-weighted, DWI and contrast enhanced parameters to identify the complete pathological response after nCRT 79. The prediction models proposed with the radiomics feature showed an area under the ROC curves ranging from 0.71 to 0.93 for complete response identification(Stanzione et al., 2021). Recently, some research groups proposed models combining both radiomics features and clinical/MRI data obtaining very good results (Cui et al., 2019; Liu et al., 2017) . It is worth to underline that in certain cases the radiomics analysis of the MR images did not show a desirable accuracy for complete response detection 91. The texture analysis was applied on PET/MR images also, combining both MR and PET textural features, and the accuracy of the reported model was 74%, even if the sample size of the study was small (50 patients) (Capelli et al., 2022).
In conclusion, the data present in literature are encouraging even if there are differences among protocols and models that make the results difficulty generalizable.
4. Liver imaging
Epidemiological changes in liver diseases, advances in technologies and treatment opportunities, and the pressing need for non-invasive and fast assessment of diffuse and focal liver diseases have had a huge impact on the approach of abdominal radiologists to liver imaging in the last decades.
4.1. Diffuse Liver Diseases
4.1.1. Clinical setting
Chronic liver diseases are highly prevalent worldwide, and develop as a wound healing response to acute or chronic liver injury. After years of hepatic inflammation triggering progressive fibrosis, cirrhosis may occur constituting the most important risk factor for hepatocellular carcinoma (HCC). The majority of chronic liver diseases in the developed world include alcoholic liver disease, chronic viral hepatitis, including hepatitis B and C, non-alcoholic fatty liver disease (NAFLD), and hemochromatosis; among these, the prevalence of NAFLD in the general population has significantly increased over the last decades. NAFLD may evolve into nonalcoholic steatohepatitis (NASH) with development of inflammation and fibrosis, which may lead eventually to cirrhosis, liver cancer, end-stage liver disease and death.(Lazarus et al., 2022)
4.1.2. Imaging Approach
Most patients at risk of chronic liver diseases are seen in primary care. Therefore, given the alarming epidemiological trend and the potential adverse patient outcomes, the adoption of use some non-invasive tests including ultrasound (US) is suggested in the primary care setting in at-risk groups, primarily those with type 2 diabetes and obesity and those who use alcohol(Berzigotti et al., 2021) . The NAFLD Consensus Consortium has proposed to use non-invasive test for risk-stratifying patients in primary care, and then diagnosing and staging non-alcoholic steatohepatitis (NASH) in terms of steatosis and fibrosis burden in secondary care and has indicated also imaging techniques as important tools(Lazarus et al., 2022).
4.1.3. Ultrasound
US-based modalities for the quantification of fat and fibrotic liver content have moderate diagnostic accuracy but may be useful as a screening strategy for its wide availability(Vernuccio, Cannella, et al., 2021). Controlled attenuation parameter, attenuation imaging coefficient, sound speed estimation, calibrated US, US elastography have proved to be promising for the assessment of steatosis and fibrosis severity (Berzigotti et al., 2021). US-based methods are however limited in monitoring liver changes over time.
4.1.4. Computed Tomography
The adoption of the multimaterial decomposition algorithm on contrast-enhanced dual-energy CT and the use of photon-counting CT (PCCT) seem very promising for quantification of liver fat, although these data need further validation (Schwartz et al., 2023; Vernuccio, Cannella, et al., 2021).
4.1.5. Magnetic Resonance Imaging
MRI-based tools such as MRI- proton density fat fraction (PDFF), MR elastography or MRI corrected T1 allow to further risk stratify NAFLD patients in whom other non-invasive tools are deemed indeterminate or not reflective of clinical suspicion (Berzigotti et al., 2021; Lazarus et al., 2022). Among MRI tools, fat quantification by MRI-proton density fat fraction is superior to controlled attenuation parameter and is being increasingly used at tertiary care centers for hepatic steatosis quantification or in clinical trials (Berzigotti et al., 2021; Dioguardi Burgio et al., 2016; Lazarus et al., 2022; Vernuccio, Cannella, et al., 2021). MR elastography is considered the most accurate non-invasive method for detecting advanced fibrosis and for differentiating the various stages of fibrosis with moderately high accuracy (Vernuccio, Cannella, et al., 2021) . However, costs, patient acceptance and the disadvantage of not being a point-of-care technique still limit the wide adoption of MRI in the primary setting.
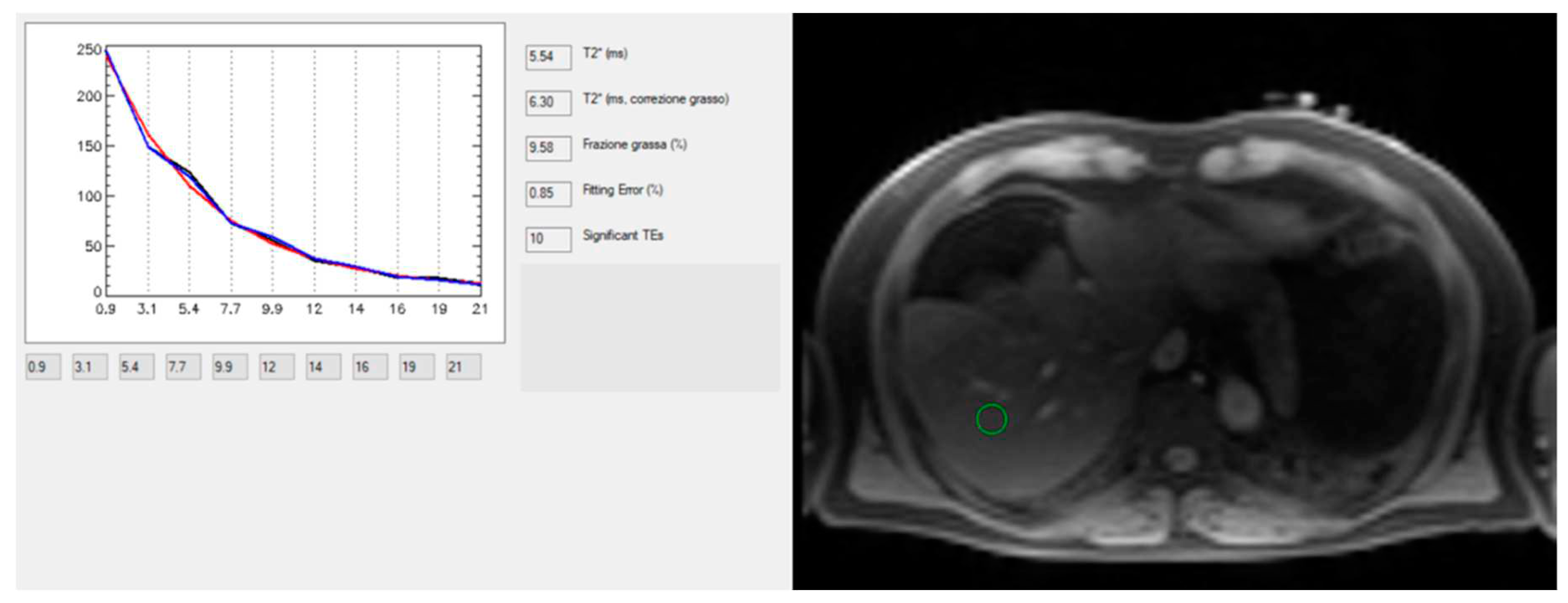
Technological advances of MRI for the study of chronic liver diseases have been proved also for the quantification of hepatic iron concentration, which may occur in hereditary hemochromatosis (i.e. the most prevalent genetic disease in the Caucasian population of North European origin), or secondary hemochromatosis (i.e. Italy is in the black belt of high prevalence for hemoglobinopathies). MRI provides a quantitative and safe, noninvasive approach to determine liver iron concentration (LIC). LIC may be calculated using signal intensity ratio (SIR), and R2- and R2*-based relaxometry methods. But nowadays, the last one is recognized as state-of-the-art method for accurate, validated, reproducible, and fast quantification of LIC (Positano et al., 2009) at both 1.5 T and 3 T (Meloni et al., 2012) .
MRI does not enable direct measurement of iron, but rather helps estimating the iron concentration indirectly through the effects of iron on the rate of proton signal decay. Therefore, any effect that alters the apparent signal decay rate, such as fat, may be introduce a bias for iron estimation. In the recent years, methods for simultaneous fat-water separation and R2* mapping have been introduced that allow unconfounded R2* relaxometry in the presence of fat (Meloni et al., 2012; Positano et al., 2009) also providing simultaneous quantification of liver fat, which is advantageous in NAFLD [
Figure 9].
Therefore, commercially available confounder-corrected R2*-based LIC quantification should be preferred for their accuracy and reproducibility in quantification of LIC, being also strengthened by presence of regulatory approval, and rapid acquisition time (Reeder et al., 2023).
4.1.6. Future imaging trends
One of the limitations in the adoption of MRI tools is the limited availability of MRI scanners, and the need for expert radiologists in the interpretation. CT scanner are more widely available, but limited by exposure to ionizing radiation. However, in the setting of patients with chronic liver disease at high risk of HCC, either CT or MRI are indicated with CT being more commonly adopted. In addition, many patients undergo CT for other reasons which may include the emergency or the oncological setting. As such, postprocessing software have been developed to extract quantitative data from CT scans for liver imaging to quantify steatosis and the hepatic morphologic fibrotic changes occurring in chronic liver diseases. Several promising CT techniques have been proposed for quantifying steatosis, iron and staging hepatic fibrosis with post-processing software, including radiomics and deep learning (Positano et al., 2023; Vernuccio, Cannella, et al., 2021). Lots of research is being published using these novel tools, and many of them still need validation in different populations and using different scanners.
4.2. Focal liver diseases
4.2.1. Clinical setting
Focal liver lesions encompass a large spectrum of entities with different pathogenesis, clinical presentations, imaging features, outcome and management. Incidentally detected solid liver lesions in healthy patients are mostly benign. The most common benign liver lesions are hepatic cysts and hemangiomas, while focal nodular hyperplasia and hepatocellular adenomas are far less common. Hepatic cysts, hemangiomas, and focal nodular hyperplasia have a benign course and do not require any follow-up(“EASL Clinical Practice Guidelines on the Management of Benign Liver Tumours,” 2016a). Conversely, hepatocellular adenomas may increase in size over time, and develop complications including hemorrhage and malignancy, and, therefore, imaging follow-up is needed and surgery may be indicated in selected cases(“EASL Clinical Practice Guidelines on the Management of Benign Liver Tumours,” 2016b; Vernuccio et al., 2020).
Pathology subtyping of hepatocellular adenomas has been updated in 2017 and the identification of the different subtypes is clinically relevant in terms of potential complications that may occur based on the subtype (Nault et al., 2017).
With regard to imaging diagnosis of malignancy, metastases are the most common malignancies with colorectal, pancreatic, lung, or breast cancers being usually the primary tumors. Primary malignancies usually originate in the setting of chronic liver disease with advanced fibrosis or cirrhosis and most commonly include HCC and intra-hepatic cholangiocarcinoma. HCC accounts for almost 90% of all primary liver cancers in cirrhosis(Galle et al., 2018). The diagnosis and management of HCC is complex, due to the underlying chronic liver disease, different imaging presentations, and multiple treatment options (Galle et al., 2018).
4.2.2. Imaging Approach
In noncirrhotic patients, most lesions are benign; hence, lesion characterization should be performed at the minimum cost and with high specificity to avoid unnecessary treatment. Imaging diagnosis of most benign focal liver lesions is oftentimes straightforward on US, CT and MRI. However, in case of atypical presentation or uncommon evolutions of benign liver lesions, current imaging techniques may not be considered definitive and further work-up, sometimes including biopsy, may be needed(Vernuccio et al., 2018).With regard to malignancies, HCC is the only neoplasm that can be confidently diagnosed at imaging without the need of biopsy confirmation whenever specific imaging criteria are present on contrast-enhanced CT or MRI in patients at high risk of HCC (Galle et al., 2018). Therefore, current gap-in-knowledge include the diagnosis of HCC when specific imaging criteria are not present or outside the context of at-risk patients and the imaging diagnosis of non-HCC malignancies, in order to reduce the need of biopsy for characterization.
4.2.3. Ultrasound
US elastography, contrast-enhanced US (CEUS) and new Doppler techniques have recently provided some tips as additional US tools to differentiate between benign and malignant lesion, as well as for monitoring after locoregional therapies.
CEUS is currently recommended as the first-line imaging technique for the characterization of incidentally detected focal liver lesions, indeterminate at US in non-cirrhotic, non-oncological patients and in patients with renal insufficiency, as well as a helpful tool in patients with inconclusive findings on CT or MRI (Dietrich et al., 2020). CEUS is also recommended for differentiation between benign and malignant portal vein thrombosis (Dietrich et al., 2020), as the differentiation may be tricky sometimes on CT or MRI 113 , but also for the evaluation of the treatment effect after ablation, guidance for immediate US-guided re-treatment of residual tumors and in the follow-up after ablation treatment at appropriate time intervals(Dietrich et al., 2020).
With regard to Doppler techniques, relatively recent advances in Doppler technology have seen the development of advanced Doppler US techniques, including microvascular or microflow imaging (MVI/MFI) and superb microvascular imaging (SMI) (Wilson & Lim, 2022). MVI/MFI nowadays permits high-resolution assessment of “microvessel” architecture, to approximately 0.5 mm and speeds <0/1 cm/s without the need for intravenous contrast medium (Wilson & Lim, 2022). Benign and malignant focal liver lesions have a different vasculature appearance on MVI/MFI and SMI which may help in their differentiation; however, the scientific literature on the role of these advanced Doppler US techniques for focal liver lesions is currently limited(Wilson & Lim, 2022) .
4.2.4. Computed Tomography
The adoption of dual energy CT (DECT) for liver imaging has shown some value in selected cases particularly with virtual monochromatic images (VMC), and material-specific images, namely the iodine images, and with deep-learning reconstruction techniques applied to DECT images(Vernuccio, Cannella, et al., 2021). Low keV VMC images and iodine maps can improve the conspicuity of liver lesions and may serve as imaging biomarker of tumor treatment response(Patel et al., 2018; Reginelli et al., 2023; Seo et al., 2022). PCCT may also provide additional help in improving diagnostic accuracy for liver lesion characterization, although until now studies have been performed in small cohorts and this technology is not yet widely available.(Sartoretti et al., 2023)
4.2.5. Magnetic Resonance Imaging
MRI techniques are steadily improving over time for temporal resolution and noise reduction. In addition, abbreviated MRI strategies have been investigated for oncological and HCC screening in cirrhotic patients(Vernuccio, Cannella, et al., 2021). All these efforts are aimed at allowing a wider routine use of MRI as an alternative to CT, given the higher accuracy for focal liver lesion characterization.
4.2.6. Future imaging trends
Similar to the current need of quantitative data for the diagnosis of diffuse liver diseases, postprocessing software have been developed to extract quantitative data from CT and MRI scans for liver imaging to differentiate among benign and malignant lesion in the overall population, and to timely and accurately diagnose HCC in the cirrhotic population. On one hand, perfusion images have been obtained both on CT and MRI with the aim of calculating quantitative parameters that might help in the differential diagnosis of focal liver lesions and assessment of tumor response (Pahwa et al., 2018).
A growing number of studies is also being published on the use of radiomics applied to CT and MRI studies for the differential diagnosis of benign and malignant liver lesions and to improve accuracy for the diagnosis of malignant lesions; however, the clinical routine adoption is still limited.
An interesting potential opportunity that is still under initial investigation is the possibility of simultaneous biphasic liver imaging in a single multienergy PCD-CT acquisition using a dual-contrast injection protocol with both a iodine-based and a gadolinium- based contrast; this would have a clear significant benefit for lesion characterization allowing to obtain functional and quantitative information from both contrast agents at the same time(Ren et al., 2022).
References
- Bates, D. D. B., Golia Pernicka, J. S., Fuqua, J. L., Paroder, V., Petkovska, I., Zheng, J., Capanu, M., Schilsky, J., & Gollub, M. J. (2020). Diagnostic accuracy of b800 and b1500 DWI-MRI of the pelvis to detect residual rectal adenocarcinoma: a multi-reader study. Abdominal Radiology, 45(2), 293–300. [CrossRef]
- Beets-Tan, R. G. H., Lambregts, D. M. J., Maas, M., Bipat, S., Barbaro, B., Curvo-Semedo, L., Fenlon, H. M., Gollub, M. J., Gourtsoyianni, S., Halligan, S., Hoeffel, C., Kim, S. H., Laghi, A., Maier, A., Rafaelsen, S. R., Stoker, J., Taylor, S. A., Torkzad, M. R., & Blomqvist, L. (2018). Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. European Radiology, 28(4), 1465–1475. [CrossRef]
- Berzigotti, A., Tsochatzis, E., Boursier, J., Castera, L., Cazzagon, N., Friedrich-Rust, M., Petta, S., & Thiele, M. (2021). EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis – 2021 update. Journal of Hepatology, 75(3), 659–689. [CrossRef]
- Brown, G., Richards, C. J., Bourne, M. W., Newcombe, R. G., Radcliffe, A. G., Dallimore, N. S., & Williams, G. T. (2003). Morphologic Predictors of Lymph Node Status in Rectal Cancer with Use of High-Spatial-Resolution MR Imaging with Histopathologic Comparison. Radiology, 227(2), 371–377. [CrossRef]
- Cademartiri, F., Casolo, G., Clemente, A., Seitun, S., Mantini, C., Bossone, E., Saba, L., Sverzellati, N., Nistri, S., Punzo, B., Cavaliere, C., La Grutta, L., Gentile, G., & Maffei, E. (2021). Coronary CT angiography: a guide to examination, interpretation, and clinical indications. Expert Review of Cardiovascular Therapy, 19(5), 413–425. [CrossRef]
- Cademartiri, F., Di Cesare, E., Francone, M., Ballerini, G., Ligabue, G., Maffei, E., Romagnoli, A., Argiolas, G. M., Russo, V., Buffa, V., Marano, R., Guzzetta, M., Belgrano, M., Carbone, I., Macarini, L., Borghi, C., Di Renzi, P., Barile, V., & Patriarca, L. (2015). Italian Registry of Cardiac Computed Tomography. La Radiologia Medica, 120(10), 919–929. [CrossRef]
- Capelli, G., Campi, C., Bao, Q. R., Morra, F., Lacognata, C., Zucchetta, P., Cecchin, D., Pucciarelli, S., Spolverato, G., & Crimì, F. (2022). 18F-FDG-PET/MRI texture analysis in rectal cancer after neoadjuvant chemoradiotherapy. Nuclear Medicine Communications, 43(7), 815–822. [CrossRef]
- Charalambous, S., Klontzas, M. E., Kontopodis, N., Ioannou, C. V., Perisinakis, K., Maris, T. G., Damilakis, J., Karantanas, A., & Tsetis, D. (2022). Radiomics and machine learning to predict aggressive type 2 endoleaks after endovascular aneurysm repair: a proof of concept. Acta Radiologica, 63(9), 1293–1299. [CrossRef]
- Counseller, Q., & Aboelkassem, Y. (2023). Recent technologies in cardiac imaging. Frontiers in Medical Technology, 4. [CrossRef]
- Crimì, F., Capelli, G., Spolverato, G., Bao, Q. R., Florio, A., Milite Rossi, S., Cecchin, D., Albertoni, L., Campi, C., Pucciarelli, S., & Stramare, R. (2020). MRI T2-weighted sequences-based texture analysis (TA) as a predictor of response to neoadjuvant chemo-radiotherapy (nCRT) in patients with locally advanced rectal cancer (LARC). La Radiologia Medica, 125(12), 1216–1224. [CrossRef]
- Crimì, F., Valeggia, S., Baffoni, L., Stramare, R., Lacognata, C., Spolverato, G., Albertoni, L., Spimpolo, A., Evangelista, L., Zucchetta, P., Cecchin, D., & Pucciarelli, S. (2021). [18F]FDG PET/MRI in rectal cancer. Annals of Nuclear Medicine, 35(3), 281–290. [CrossRef]
- Cui, Y., Yang, X., Shi, Z., Yang, Z., Du, X., Zhao, Z., & Cheng, X. (2019). Radiomics analysis of multiparametric MRI for prediction of pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. European Radiology, 29(3), 1211–1220. [CrossRef]
- Cury, R. C., Leipsic, J., Abbara, S., Achenbach, S., Berman, D., Bittencourt, M., Budoff, M., Chinnaiyan, K., Choi, A. D., Ghoshhajra, B., Jacobs, J., Koweek, L., Lesser, J., Maroules, C., Rubin, G. D., Rybicki, F. J., Shaw, L. J., Williams, M. C., Williamson, E., … Blankstein, R. (2022). CAD-RADSTM 2.0 - 2022 Coronary Artery Disease-Reporting and Data System. Journal of Cardiovascular Computed Tomography, 16(6), 536–557. [CrossRef]
- Dietrich, C. F., Nolsøe, C. P., Barr, R. G., Berzigotti, A., Burns, P. N., Cantisani, V., Chammas, M. C., Chaubal, N., Choi, B. I., Clevert, D.-A., Cui, X., Dong, Y., D’Onofrio, M., Fowlkes, J. B., Gilja, O. H., Huang, P., Ignee, A., Jenssen, C., Kono, Y., … Zheng, R. (2020). Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver–Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound in Medicine & Biology, 46(10), 2579–2604. [CrossRef]
- Dioguardi Burgio, M., Bruno, O., Agnello, F., Torrisi, C., Vernuccio, F., Cabibbo, G., Soresi, M., Petta, S., Calamia, M., Papia, G., Gambino, A., Ricceri, V., Midiri, M., Lagalla, R., & Brancatelli, G. (2016). The cheating liver: imaging of focal steatosis and fatty sparing. Expert Review of Gastroenterology & Hepatology, 10(6), 671–678. [CrossRef]
- Dossa, F., Chesney, T. R., Acuna, S. A., & Baxter, N. N. (2017). A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. The Lancet Gastroenterology & Hepatology, 2(7), 501–513. [CrossRef]
- Dresen, R. C., Beets, G. L., Rutten, H. J. T., Engelen, S. M. E., Lahaye, M. J., Vliegen, R. F. A., de Bruïne, A. P., Kessels, A. G. H., Lammering, G., & Beets-Tan, R. G. H. (2009). Locally Advanced Rectal Cancer: MR Imaging for Restaging after Neoadjuvant Radiation Therapy with Concomitant Chemotherapy Part I. Are We Able to Predict Tumor Confined to the Rectal Wall? Radiology, 252(1), 71–80. [CrossRef]
- Dyverfeldt, P., Bissell, M., Barker, A. J., Bolger, A. F., Carlhäll, C.-J., Ebbers, T., Francios, C. J., Frydrychowicz, A., Geiger, J., Giese, D., Hope, M. D., Kilner, P. J., Kozerke, S., Myerson, S., Neubauer, S., Wieben, O., & Markl, M. (2015). 4D flow cardiovascular magnetic resonance consensus statement. Journal of Cardiovascular Magnetic Resonance, 17(1), 72. [CrossRef]
- EASL Clinical Practice Guidelines on the management of benign liver tumours. (2016a). Journal of Hepatology, 65(2), 386–398. [CrossRef]
- EASL Clinical Practice Guidelines on the management of benign liver tumours. (2016b). Journal of Hepatology, 65(2), 386–398. [CrossRef]
- Ferencik, M., Mayrhofer, T., Bittner, D. O., Emami, H., Puchner, S. B., Lu, M. T., Meyersohn, N. M., Ivanov, A. V., Adami, E. C., Patel, M. R., Mark, D. B., Udelson, J. E., Lee, K. L., Douglas, P. S., & Hoffmann, U. (2018). Use of High-Risk Coronary Atherosclerotic Plaque Detection for Risk Stratification of Patients With Stable Chest Pain. JAMA Cardiology, 3(2), 144. [CrossRef]
- Francone, M., Budde, R. P. J., Bremerich, J., Dacher, J. N., Loewe, C., Wolf, F., Natale, L., Pontone, G., Redheuil, A., Vliegenthart, R., Nikolaou, K., Gutberlet, M., & Salgado, R. (2020). CT and MR imaging prior to transcatheter aortic valve implantation: standardisation of scanning protocols, measurements and reporting—a consensus document by the European Society of Cardiovascular Radiology (ESCR). European Radiology, 30(5), 2627–2650. [CrossRef]
- Galle, P. R., Forner, A., Llovet, J. M., Mazzaferro, V., Piscaglia, F., Raoul, J.-L., Schirmacher, P., & Vilgrain, V. (2018). EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. Journal of Hepatology, 69(1), 182–236. [CrossRef]
- Geffroy, Y., Rodallec, M. H., Boulay-Coletta, I., Jullès, M.-C., Ridereau-Zins, C., & Zins, M. (2011). Multidetector CT Angiography in Acute Gastrointestinal Bleeding: Why, When, and How. RadioGraphics, 31(3), E35–E46. [CrossRef]
- Glynne-Jones, R., Wyrwicz, L., Tiret, E., Brown, G., Rödel, C., Cervantes, A., & Arnold, D. (2017). Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 28, iv22–iv40. [CrossRef]
- Guo, Y., Chen, X., Lin, X., Chen, L., Shu, J., Pang, P., Cheng, J., Xu, M., & Sun, Z. (n.d.). Non-contrast CT-based radiomic signature for screening thoracic aortic dissections: a multicenter study. [CrossRef]
- Habib, G., Bucciarelli-Ducci, C., Caforio, A. L. P., Cardim, N., Charron, P., Cosyns, B., Dehaene, A., Derumeaux, G., Donal, E., Dweck, M. R., Edvardsen, T., Erba, P. A., Ernande, L., Gaemperli, O., Galderisi, M., Grapsa, J., Jacquier, A., Klingel, K., Lancellotti, P., … Vijayaraghavan, G. (2017). Multimodality Imaging in Restrictive Cardiomyopathies: An EACVI expert consensus document In collaboration with the “Working Group on myocardial and pericardial diseases” of the European Society of Cardiology Endorsed by The Indian Academy of Echocardiography. European Heart Journal - Cardiovascular Imaging, 18(10), 1090–1121. [CrossRef]
- Hansen, K., Hansen, P., Ewertsen, C., Lönn, L., Jensen, J., & Nielsen, M. (2019). Vector Flow Imaging Compared with Digital Subtraction Angiography for Stenosis Assessment in the Superficial Femoral Artery – A Study of Vector Concentration, Velocity Ratio and Stenosis Degree Percentage. Ultrasound International Open, 05(02), E53–E59. [CrossRef]
- Hansen, K. L., & Carlsen, J. F. (2021). New Trends in Vascular Imaging. Diagnostics, 11(1), 112. [CrossRef]
- Heijnen, L. A., Maas, M., Beets-Tan, R. G., Berkhof, M., Lambregts, D. M., Nelemans, P. J., Riedl, R., & Beets, G. L. (2016). Nodal staging in rectal cancer: why is restaging after chemoradiation more accurate than primary nodal staging? International Journal of Colorectal Disease, 31(6), 1157–1162. [CrossRef]
- Horvat, N., Carlos Tavares Rocha, C., Clemente Oliveira, B., Petkovska, I., & Gollub, M. J. (2019). MRI of Rectal Cancer: Tumor Staging, Imaging Techniques, and Management. RadioGraphics, 39(2), 367–387. [CrossRef]
- Horvat, N., El Homsi, M., Miranda, J., Mazaheri, Y., Gollub, M. J., & Paroder, V. (2023a). Rectal <scp>MRI</scp> Interpretation After Neoadjuvant Therapy. Journal of Magnetic Resonance Imaging, 57(2), 353–369. [CrossRef]
- Horvat, N., El Homsi, M., Miranda, J., Mazaheri, Y., Gollub, M. J., & Paroder, V. (2023b). Rectal <scp>MRI</scp> Interpretation After Neoadjuvant Therapy. Journal of Magnetic Resonance Imaging, 57(2), 353–369. [CrossRef]
- Hyde, D. E., Habets, D. F., Fox, A. J., Gulka, I., Kalapos, P., Lee, D. H., Pelz, D. M., & Holdsworth, D. W. (2007). Comparison of maximum intensity projection and digitally reconstructed radiographic projection for carotid artery stenosis measurement. Medical Physics, 34(7), 2968–2974. [CrossRef]
- Jacobsen, M. C., Thrower, S. L., Ger, R. B., Leng, S., Court, L. E., Brock, K. K., Tamm, E. P., Cressman, E. N. K., Cody, D. D., & Layman, R. R. (2020). Multi-energy computed tomography and material quantification: Current barriers and opportunities for advancement. Medical Physics, 47(8), 3752–3771. [CrossRef]
- Jayaprakasam, V. S., Ince, S., Suman, G., Nepal, P., Hope, T. A., Paspulati, R. M., & Fraum, T. J. (2023). PET/MRI in colorectal and anal cancers: an update. Abdominal Radiology. [CrossRef]
- Knuuti, J., Wijns, W., Saraste, A., Capodanno, D., Barbato, E., Funck-Brentano, C., Prescott, E., Storey, R. F., Deaton, C., Cuisset, T., Agewall, S., Dickstein, K., Edvardsen, T., Escaned, J., Gersh, B. J., Svitil, P., Gilard, M., Hasdai, D., Hatala, R., … Clapp, B. (2020). 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. European Heart Journal, 41(3), 407–477. [CrossRef]
- Kroon, H. M., Hoogervorst, L. A., Hanna-Rivero, N., Traeger, L., Dudi-Venkata, N. N., Bedrikovetski, S., Kusters, M., Chang, G. J., Thomas, M. L., & Sammour, T. (2022). Systematic review and meta-analysis of long-term oncological outcomes of lateral lymph node dissection for metastatic nodes after neoadjuvant chemoradiotherapy in rectal cancer. European Journal of Surgical Oncology, 48(7), 1475–1482. [CrossRef]
- Kwiecinski, J., Wolny, R., Chwala, A., & Slomka, P. (2023). Advances in the Assessment of Coronary Artery Disease Activity with PET/CT and CTA. Tomography, 9(1), 328–341. [CrossRef]
- Lazarus, J. V., Mark, H. E., Anstee, Q. M., Arab, J. P., Batterham, R. L., Castera, L., Cortez-Pinto, H., Crespo, J., Cusi, K., Dirac, M. A., Francque, S., George, J., Hagström, H., Huang, T. T.-K., Ismail, M. H., Kautz, A., Sarin, S. K., Loomba, R., Miller, V., … Zheng, M.-H. (2022). Advancing the global public health agenda for NAFLD: a consensus statement. Nature Reviews Gastroenterology & Hepatology, 19(1), 60–78. [CrossRef]
- Leng, S., Bruesewitz, M., Tao, S., Rajendran, K., Halaweish, A. F., Campeau, N. G., Fletcher, J. G., & McCollough, C. H. (2019). Photon-counting Detector CT: System Design and Clinical Applications of an Emerging Technology. RadioGraphics, 39(3), 729–743. [CrossRef]
- Liu, Z., Zhang, X.-Y., Shi, Y.-J., Wang, L., Zhu, H.-T., Tang, Z., Wang, S., Li, X.-T., Tian, J., & Sun, Y.-S. (2017). Radiomics Analysis for Evaluation of Pathological Complete Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Clinical Cancer Research, 23(23), 7253–7262. [CrossRef]
- Mavrogeni, S., Pepe, A., Nijveldt, R., Ntusi, N., Sierra-Galan, L. M., Bratis, K., Wei, J., Mukherjee, M., Markousis-Mavrogenis, G., Gargani, L., Sade, L. E., Ajmone-Marsan, N., Seferovic, P., Donal, E., Nurmohamed, M., Cerinic, M. M., Sfikakis, P., Kitas, G., Schwitter, J., … Cosyns, B. (2022). Cardiovascular magnetic resonance in autoimmune rheumatic diseases: a clinical consensus document by the European Association of Cardiovascular Imaging. European Heart Journal - Cardiovascular Imaging, 23(9), e308–e322. [CrossRef]
- McNally, J. S., Sakata, A., Alexander, M. D., Dewitt, L. D., Sonnen, J. A., Menacho, S. T., Stoddard, G. J., Kim, S.-E., & de Havenon, A. H. (2021). Vessel Wall Enhancement on Black-Blood MRI Predicts Acute and Future Stroke in Cerebral Amyloid Angiopathy. American Journal of Neuroradiology, 42(6), 1038–1045. [CrossRef]
- Meloni, A., Frijia, F., Panetta, D., Degiorgi, G., De Gori, C., Maffei, E., Clemente, A., Positano, V., & Cademartiri, F. (2023). Photon-Counting Computed Tomography (PCCT): Technical Background and Cardio-Vascular Applications. Diagnostics, 13(4), 645. [CrossRef]
- Meloni, A., Gargani, L., Bruni, C., Cavallaro, C., Gobbo, M., D’Agostino, A., D’Angelo, G., Martini, N., Grigioni, F., Sinagra, G., De Caterina, R., Quaia, E., Mavrogeni, S., Cademartiri, F., Matucci-Cerinic, M., & Pepe, A. (2023). Additional value of T1 and T2 mapping techniques for early detection of myocardial involvement in scleroderma. International Journal of Cardiology, 376, 139–146. [CrossRef]
- Meloni, A., Martini, N., Positano, V., D’Angelo, G., Barison, A., Todiere, G., Grigoratos, C., Barra, V., Pistoia, L., Gargani, L., Ripoli, A., & Pepe, A. (2021). Myocardial <scp>T1</scp> Values at 1.5 T: Normal Values for General Electric Scanners and Sex-Related Differences. Journal of Magnetic Resonance Imaging, 54(5), 1486–1500. [CrossRef]
- Meloni, A., Nicola, M., Positano, V., D’Angelo, G., Barison, A., Todiere, G., Grigoratos, C., Keilberg, P., Pistoia, L., Gargani, L., Ripoli, A., & Pepe, A. (2022). Myocardial T2 values at 1.5 T by a segmental approach with healthy aging and gender. European Radiology, 32(5), 2962–2975. [CrossRef]
- Meloni, A., Pistoia, L., Positano, V., Martini, N., Borrello, R. L., Sbragi, S., Spasiano, A., Casini, T., Bitti, P. P., Putti, M. C., Cuccia, L., Allò, M., Massei, F., Sanna, P. M. G., De Caterina, R., Quaia, E., Cademartiri, F., & Pepe, A. (2023). Myocardial tissue characterization by segmental T 2 mapping in thalassaemia major: detecting inflammation beyond iron. European Heart Journal - Cardiovascular Imaging. [CrossRef]
- Meloni, A., Positano, V., Keilberg, P., De Marchi, D., Pepe, P., Zuccarelli, A., Campisi, S., Romeo, M. A., Casini, T., Bitti, P. P., Gerardi, C., Lai, M. E., Piraino, B., Giuffrida, G., Secchi, G., Midiri, M., Lombardi, M., & Pepe, A. (2012). Feasibility, reproducibility, and reliability for the T*2 iron evaluation at 3 T in comparison with 1.5 T. Magnetic Resonance in Medicine, 68(2), 543–551. [CrossRef]
- Meloni, A., Positano, V., Ruffo, G. B., Spasiano, A., D’Ascola, D. G., Peluso, A., Keilberg, P., Restaino, G., Valeri, G., Renne, S., Midiri, M., & Pepe, A. (2015). Improvement of heart iron with preserved patterns of iron store by CMR-guided chelation therapy. European Heart Journal – Cardiovascular Imaging, 16(3), 325–334. [CrossRef]
- Messroghli, D. R., Moon, J. C., Ferreira, V. M., Grosse-Wortmann, L., He, T., Kellman, P., Mascherbauer, J., Nezafat, R., Salerno, M., Schelbert, E. B., Taylor, A. J., Thompson, R., Ugander, M., van Heeswijk, R. B., & Friedrich, M. G. (2017). Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). Journal of Cardiovascular Magnetic Resonance, 19(1), 75. [CrossRef]
- Mirshahvalad, S. A., Hinzpeter, R., Kohan, A., Anconina, R., Kulanthaivelu, R., Ortega, C., Metser, U., & Veit-Haibach, P. (2022). Diagnostic performance of [18F]-FDG PET/MR in evaluating colorectal cancer: a systematic review and meta-analysis. European Journal of Nuclear Medicine and Molecular Imaging, 49(12), 4205–4217. [CrossRef]
- Natale, L., Vliegenthart, R., Salgado, R., Bremerich, J., Budde, R. P. J., Dacher, J.-N., Francone, M., Kreitner, K.-F., Loewe, C., Nikolaou, K., Peebles, C., Velthuis, B. K., & Catalano, C. (2023). Cardiac radiology in Europe: status and vision by the European Society of Cardiovascular Radiology (ESCR) and the European Society of Radiology (ESR). European Radiology. [CrossRef]
- Nault, J.-C., Couchy, G., Balabaud, C., Morcrette, G., Caruso, S., Blanc, J.-F., Bacq, Y., Calderaro, J., Paradis, V., Ramos, J., Scoazec, J.-Y., Gnemmi, V., Sturm, N., Guettier, C., Fabre, M., Savier, E., Chiche, L., Labrune, P., Selves, J., … Terris, B. (2017). Molecular Classification of Hepatocellular Adenoma Associates With Risk Factors, Bleeding, and Malignant Transformation. Gastroenterology, 152(4), 880-894.e6. [CrossRef]
- Ogura, A., Konishi, T., Beets, G. L., Cunningham, C., Garcia-Aguilar, J., Iversen, H., Toda, S., Lee, I. K., Lee, H. X., Uehara, K., Lee, P., Putter, H., van de Velde, C. J. H., Rutten, H. J. T., Tuynman, J. B., & Kusters, M. (2019). Lateral Nodal Features on Restaging Magnetic Resonance Imaging Associated With Lateral Local Recurrence in Low Rectal Cancer After Neoadjuvant Chemoradiotherapy or Radiotherapy. JAMA Surgery, 154(9), e192172. [CrossRef]
- Oikonomou, E. K., Marwan, M., Desai, M. Y., Mancio, J., Alashi, A., Hutt Centeno, E., Thomas, S., Herdman, L., Kotanidis, C. P., Thomas, K. E., Griffin, B. P., Flamm, S. D., Antonopoulos, A. S., Shirodaria, C., Sabharwal, N., Deanfield, J., Neubauer, S., Hopewell, J. C., Channon, K. M., … Antoniades, C. (2018). Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. The Lancet, 392(10151), 929–939. [CrossRef]
- Ordovas, K. G., Baldassarre, L. A., Bucciarelli-Ducci, C., Carr, J., Fernandes, J. L., Ferreira, V. M., Frank, L., Mavrogeni, S., Ntusi, N., Ostenfeld, E., Parwani, P., Pepe, A., Raman, S. V., Sakuma, H., Schulz-Menger, J., Sierra-Galan, L. M., Valente, A. M., & Srichai, M. B. (2021). Cardiovascular magnetic resonance in women with cardiovascular disease: position statement from the Society for Cardiovascular Magnetic Resonance (SCMR). Journal of Cardiovascular Magnetic Resonance, 23(1), 52. [CrossRef]
- Pahwa, S., Liu, H., Chen, Y., Dastmalchian, S., O’Connor, G., Lu, Z., Badve, C., Yu, A., Wright, K., Chalian, H., Rao, S., Fu, C., Vallines, I., Griswold, M., Seiberlich, N., Zeng, M., & Gulani, V. (2018). Quantitative perfusion imaging of neoplastic liver lesions: A multi-institution study. Scientific Reports, 8(1), 4990. [CrossRef]
- Patel, B. N., Rosenberg, M., Vernuccio, F., Ramirez-Giraldo, J. C., Nelson, R., Farjat, A., & Marin, D. (2018). Characterization of Small Incidental Indeterminate Hypoattenuating Hepatic Lesions: Added Value of Single-Phase Contrast-Enhanced Dual-Energy CT Material Attenuation Analysis. American Journal of Roentgenology, 211(3), 571–579. [CrossRef]
- Pepe, A., Pistoia, L., Gamberini, M. R., Cuccia, L., Lisi, R., Cecinati, V., Maggio, A., Sorrentino, F., Filosa, A., Rosso, R., Messina, G., Missere, M., Righi, R., Renne, S., Vallone, A., Dalmiani, S., Positano, V., Midiri, M., & Meloni, A. (2022). National networking in rare diseases and reduction of cardiac burden in thalassemia major. European Heart Journal, 43(26), 2482–2492. [CrossRef]
- Pomerri, F., Crimì, F., Veronese, N., Perin, A., Lacognata, C., Bergamo, F., Boso, C., & Maretto, I. (2017). Prediction of N0 Irradiated Rectal Cancer Comparing MRI Before and After Preoperative Chemoradiotherapy. Diseases of the Colon & Rectum, 60(11), 1184–1191. [CrossRef]
- Positano, V., Meloni, A., Santarelli, M. F., Pistoia, L., Spasiano, A., Cuccia, L., Casini, T., Gamberini, M. R., Allò, M., Bitti, P. P., Pepe, A., & Cademartiri, F. (2023). Deep Learning Staging of Liver Iron Content From Multiecho MR Images. Journal of Magnetic Resonance Imaging, 57(2), 472–484. [CrossRef]
- Positano, V., Salani, B., Pepe, A., Santarelli, M. F., De Marchi, D., Ramazzotti, A., Favilli, B., Cracolici, E., Midiri, M., Cianciulli, P., Lombardi, M., & Landini, L. (2009). Improved T2* assessment in liver iron overload by magnetic resonance imaging. Magnetic Resonance Imaging, 27(2), 188–197. [CrossRef]
- Pucciarelli, S., Giandomenico, F., De Paoli, A., Gavaruzzi, T., Lotto, L., Mantello, G., Barba, C., Zotti, P., Flora, S., & Del Bianco, P. (2016). Bowel function and quality of life after local excision or total mesorectal excision following chemoradiotherapy for rectal cancer. British Journal of Surgery, 104(1), 138–147. [CrossRef]
- Pushparajah, K., Duong, P., Mathur, S., & Babu-Narayan, S. V. (2019). Cardiovascular MRI and CT in congenital heart disease. Echo Research & Practice, 6(4), R121–R138. [CrossRef]
- Qin, J. J., Obeidy, P., Gok, M., Gholipour, A., & Grieve, S. M. (2023). 4D-flow MRI derived wall shear stress for the risk stratification of bicuspid aortic valve aortopathy: A systematic review. Frontiers in Cardiovascular Medicine, 9. [CrossRef]
- Quinaglia, T., Jerosch-Herold, M., & Coelho-Filho, O. R. (2019). State-of-the-Art Quantitative Assessment of Myocardial Ischemia by Stress Perfusion Cardiac Magnetic Resonance. Magnetic Resonance Imaging Clinics of North America, 27(3), 491–505. [CrossRef]
- Reeder, S. B., Yokoo, T., França, M., Hernando, D., Alberich-Bayarri, Á., Alústiza, J. M., Gandon, Y., Henninger, B., Hillenbrand, C., Jhaveri, K., Karçaaltıncaba, M., Kühn, J.-P., Mojtahed, A., Serai, S. D., Ward, R., Wood, J. C., Yamamura, J., & Martí-Bonmatí, L. (2023). Quantification of Liver Iron Overload with MRI: Review and Guidelines from the ESGAR and SAR. Radiology, 307(1). [CrossRef]
- Reeves, R. A., Halpern, E. J., & Rao, V. M. (2021). Cardiac Imaging Trends from 2010 to 2019 in the Medicare Population. Radiology: Cardiothoracic Imaging, 3(5). [CrossRef]
- Reginelli, A., Del Canto, M., Clemente, A., Gragnano, E., Cioce, F., Urraro, F., Martinelli, E., & Cappabianca, S. (2023). The Role of Dual-Energy CT for the Assessment of Liver Metastasis Response to Treatment: Above the RECIST 1.1 Criteria. Journal of Clinical Medicine, 12(3), 879. [CrossRef]
- Ren, L., Huber, N., Rajendran, K., Fletcher, J. G., McCollough, C. H., & Yu, L. (2022). Dual-Contrast Biphasic Liver Imaging With Iodine and Gadolinium Using Photon-Counting Detector Computed Tomography. Investigative Radiology, 57(2), 122–129. [CrossRef]
- Santos, M. K., Ferreira Júnior, J. R., Wada, D. T., Tenório, A. P. M., Nogueira-Barbosa, M. H., & Marques, P. M. de A. (2019). Artificial intelligence, machine learning, computer-aided diagnosis, and radiomics: advances in imaging towards to precision medicine. Radiologia Brasileira, 52(6), 387–396. [CrossRef]
- Sartoretti, T., Mergen, V., Jungblut, L., Alkadhi, H., & Euler, A. (2023). Liver Iodine Quantification With Photon-Counting Detector CT: Accuracy in an Abdominal Phantom and Feasibility in Patients. Academic Radiology, 30(3), 461–469. [CrossRef]
- Schwartz, F. R., Ashton, J., Wildman-Tobriner, B., Molvin, L., Ramirez-Giraldo, J. C., Samei, E., Bashir, M. R., & Marin, D. (2023). Liver fat quantification in photon counting CT in head to head comparison with clinical MRI – First experience. European Journal of Radiology, 161, 110734. [CrossRef]
- Seo, J. Y., Joo, I., Yoon, J. H., Kang, H. J., Kim, S., Kim, J. H., Ahn, C., & Lee, J. M. (2022). Deep learning-based reconstruction of virtual monoenergetic images of kVp-switching dual energy CT for evaluation of hypervascular liver lesions: Comparison with standard reconstruction technique. European Journal of Radiology, 154, 110390. [CrossRef]
- Si-Mohamed, S. A., Boccalini, S., Lacombe, H., Diaw, A., Varasteh, M., Rodesch, P.-A., Dessouky, R., Villien, M., Tatard-Leitman, V., Bochaton, T., Coulon, P., Yagil, Y., Lahoud, E., Erhard, K., Riche, B., Bonnefoy, E., Rioufol, G., Finet, G., Bergerot, C., … Douek, P. C. (2022). Coronary CT Angiography with Photon-counting CT: First-In-Human Results. Radiology, 303(2), 303–313. [CrossRef]
- So, A., Hsieh, J., Narayanan, S., Thibault, J.-B., Imai, Y., Dutta, S., Leipsic, J., Min, J., LaBounty, T., & Lee, T.-Y. (2012). Dual-energy CT and its potential use for quantitative myocardial CT perfusion. Journal of Cardiovascular Computed Tomography, 6(5), 308–317. [CrossRef]
- Stanzione, A., Verde, F., Romeo, V., Boccadifuoco, F., Mainenti, P. P., & Maurea, S. (2021). Radiomics and machine learning applications in rectal cancer: Current update and future perspectives. World Journal of Gastroenterology, 27(32), 5306–5321. [CrossRef]
- Tharmaseelan, H., Froelich, M. F., Nörenberg, D., Overhoff, D., Rotkopf, L. T., Riffel, P., Schoenberg, S. O., & Ayx, I. (2022). Influence of local aortic calcification on periaortic adipose tissue radiomics texture features—a primary analysis on PCCT. International Journal of Cardiovascular Imaging. [CrossRef]
- van Heeswijk, M. M., Lambregts, D. M. J., Palm, W. M., Hendriks, B. M. F., Maas, M., Beets, G. L., & Beets-Tan, R. G. H. (2017). DWI for Assessment of Rectal Cancer Nodes After Chemoradiotherapy: Is the Absence of Nodes at DWI Proof of a Negative Nodal Status? American Journal of Roentgenology, 208(3), W79–W84. [CrossRef]
- Vernuccio, F., Cannella, R., Bartolotta, T. V., Galia, M., Tang, A., & Brancatelli, G. (2021). Advances in liver US, CT, and MRI: moving toward the future. European Radiology Experimental, 5(1), 52. [CrossRef]
- Vernuccio, F., Porrello, G., Cannella, R., Vernuccio, L., Midiri, M., Giannitrapani, L., Soresi, M., & Brancatelli, G. (2021). Benign and malignant mimickers of infiltrative hepatocellular carcinoma: tips and tricks for differential diagnosis on CT and MRI. Clinical Imaging, 70, 33–45. [CrossRef]
- Vernuccio, F., Ronot, M., Dioguardi Burgio, M., Cauchy, F., Choudhury, K. R., Dokmak, S., Soubrane, O., Valla, D., Zucman-Rossi, J., Paradis, V., & Vilgrain, V. (2020). Long-term Evolution of Hepatocellular Adenomas at MRI Follow-up. Radiology, 295(2), 361–372. [CrossRef]
- Vernuccio, F., Ronot, M., Dioguardi Burgio, M., Lebigot, J., Allaham, W., Aubé, C., Brancatelli, G., & Vilgrain, V. (2018). Uncommon evolutions and complications of common benign liver lesions. Abdominal Radiology, 43(8), 2075–2096. [CrossRef]
- Wang, Y., Xiong, F., Leach, J., Kao, E., Tian, B., Zhu, C., Zhang, Y., Hope, M., Saloner, D., & Mitsouras, D. (2023). Contrast-enhanced CT radiomics improves the prediction of abdominal aortic aneurysm progression. European Radiology, 33(5), 3444–3454. [CrossRef]
- Wei, M.-Z., Zhao, Z.-H., & Wang, J.-Y. (2020). The Diagnostic Accuracy of Magnetic Resonance Imaging in Restaging of Rectal Cancer After Preoperative Chemoradiotherapy. Journal of Computer Assisted Tomography, 44(1), 102–110. [CrossRef]
- Wielandner, A., Beitzke, D., Schernthaner, R., Wolf, F., Langenberger, C., Stadler, A., & Loewe, C. (2016). Is ECG triggering for motion artefact reduction in dual-source CT angiography of the ascending aorta still required with high-pitch scanning? The role of ECG-gating in high-pitch dual-source CT of the ascending aorta. The British Journal of Radiology, 89(1064), 20160174. [CrossRef]
- Wilson, A., & Lim, A. K. P. (2022). Microvascular imaging: new Doppler technology for assessing focal liver lesions. Is it useful? Clinical Radiology, 77(12), e807–e820. [CrossRef]
- Xu, J., Yang, W., Zhao, S., & Lu, M. (2022). State-of-the-art myocardial strain by CMR feature tracking: clinical applications and future perspectives. European Radiology, 32(8), 5424–5435. [CrossRef]
- Xu, Q., Xu, Y., Sun, H., Jiang, T., Xie, S., Ooi, B. Y., & Ding, Y. (2021). MRI Evaluation of Complete Response of Locally Advanced Rectal Cancer After Neoadjuvant Therapy: Current Status and Future Trends. Cancer Management and Research, Volume 13, 4317–4328. [CrossRef]
- Zhao, L., Zhou, S., Fan, T., Li, B., Liang, W., & Dong, H. (2018). Three-dimensional printing enhances preparation for repair of double outlet right ventricular surgery. Journal of Cardiac Surgery, 33(1), 24–27. [CrossRef]
Figure 1.
MDCT Curved multiplanar reformat reconstruction of left anterior descending artery (left) and respective coronary angiography (right) in a 52-year-old male with exercise stress test positive for inducible ischemia, showing diffuse calcific and non-calcific plaques with mild stenosis (orange arrow) and severe stenosis with markers of instability (yellow arrow), scheduled for revascularization.
Figure 1.
MDCT Curved multiplanar reformat reconstruction of left anterior descending artery (left) and respective coronary angiography (right) in a 52-year-old male with exercise stress test positive for inducible ischemia, showing diffuse calcific and non-calcific plaques with mild stenosis (orange arrow) and severe stenosis with markers of instability (yellow arrow), scheduled for revascularization.
Figure 2.
Curved multiplanar reformat reconstruction of left anterior descending artery (left), left circumflex (middle) and right coronary artery (right) showing patent coronary arteries without stenosis in a 46-year-old female with hypertension and familiarity for coronary artery disease complaining of chest pain with an EKG doubtful for ischemia in emergency room.
Figure 2.
Curved multiplanar reformat reconstruction of left anterior descending artery (left), left circumflex (middle) and right coronary artery (right) showing patent coronary arteries without stenosis in a 46-year-old female with hypertension and familiarity for coronary artery disease complaining of chest pain with an EKG doubtful for ischemia in emergency room.
Figure 3.
M, 51 years old, with negative cardiovascular history, underwent CMR 3 months after COVID19 pneumonia. T1 (A) and T2 (B) mapping global values turned out to be high considering our ranges, but normal according to literature. The diagnosis of acute/subacute inflammation was therefore possible, and confirmed also at the segmental evaluation (the green and red circles identify pathological segmental values considering respectively the ranges of our Institute and literature ones).
Figure 3.
M, 51 years old, with negative cardiovascular history, underwent CMR 3 months after COVID19 pneumonia. T1 (A) and T2 (B) mapping global values turned out to be high considering our ranges, but normal according to literature. The diagnosis of acute/subacute inflammation was therefore possible, and confirmed also at the segmental evaluation (the green and red circles identify pathological segmental values considering respectively the ranges of our Institute and literature ones).
Figure 4.
Patient with normal Ejection Fraction (quantitative evaluation 60%) and altered global strain values, compared to references obtained in our center from 50 healthy volunteers. Representation of Longitudinal strain in A (-19.0%, [n.v. -15.53 ±1.5]), Circumferential strain on middle short axis view in B (-19.6% [n.v. -16.3±1.8]), Radial long axis strain in C (37.6% [n.v. 26.53±3.8]), and Radial short axis strain on middle short axis view in D (35.2% [n.v. 25.75±4.1]), all in systolic phase.
Figure 4.
Patient with normal Ejection Fraction (quantitative evaluation 60%) and altered global strain values, compared to references obtained in our center from 50 healthy volunteers. Representation of Longitudinal strain in A (-19.0%, [n.v. -15.53 ±1.5]), Circumferential strain on middle short axis view in B (-19.6% [n.v. -16.3±1.8]), Radial long axis strain in C (37.6% [n.v. 26.53±3.8]), and Radial short axis strain on middle short axis view in D (35.2% [n.v. 25.75±4.1]), all in systolic phase.
Figure 5.
Parasagittal reconstruction status post-Bentall procedure in a patient with type A aortic dissection (sec Stanford) with thrombosis of the true lumen, caudal to the superior mesenteric artery.
Figure 5.
Parasagittal reconstruction status post-Bentall procedure in a patient with type A aortic dissection (sec Stanford) with thrombosis of the true lumen, caudal to the superior mesenteric artery.
Figure 6.
Cine SSFP sequences of parasagittal thoracic aorta in a patient with aortic regurgitation in bicuspid valve with para-axial sections orthogonal to the long axis of the vessel at aortic sinuses (orange), sinus-tubular junction (blue), tubular ascending aorta (yellow), medium transverse arch in bovine trunk (green), isthmus (red) and diaphragmatic aorta (white). Flowmetric curve in aortic sinuses by 2D phase contrast (below).
Figure 6.
Cine SSFP sequences of parasagittal thoracic aorta in a patient with aortic regurgitation in bicuspid valve with para-axial sections orthogonal to the long axis of the vessel at aortic sinuses (orange), sinus-tubular junction (blue), tubular ascending aorta (yellow), medium transverse arch in bovine trunk (green), isthmus (red) and diaphragmatic aorta (white). Flowmetric curve in aortic sinuses by 2D phase contrast (below).
Figure 7.
T2-weighted axial oblique MRI of a T3b rectal cancer (A) before chemoradiotherapy. After neoadjuvant therapy a reduction of the rectal tumor can be appreciated (B) with fibrotic hypointense T2-weighted areas in the lesion but with signs of signal restriction in high b value DWI sequences (C). The MRI was scored as T2 mrTRG3(more than 50% of intra-tumoral fibrosis), and it was confirmed at histopathology.
Figure 7.
T2-weighted axial oblique MRI of a T3b rectal cancer (A) before chemoradiotherapy. After neoadjuvant therapy a reduction of the rectal tumor can be appreciated (B) with fibrotic hypointense T2-weighted areas in the lesion but with signs of signal restriction in high b value DWI sequences (C). The MRI was scored as T2 mrTRG3(more than 50% of intra-tumoral fibrosis), and it was confirmed at histopathology.
Figure 8.
Staging PET/MRI staging of a T3 rectal cancer (arrows) showing hypermetabolism in both PET image (A) and fused PET/MR image (B) and a rectal wall thickening at MR image (C). Restaging PET/MRI after chemo-radiotherapy showing no hypermetabolism on PET (D) and fused PET/MR images (E) and a T2 hypointense thickening of rectal wall on MR image (F). The lesion was scored as a complete response (ycT0N0) and confirmed at histopathology after transanal local excision.
Figure 8.
Staging PET/MRI staging of a T3 rectal cancer (arrows) showing hypermetabolism in both PET image (A) and fused PET/MR image (B) and a rectal wall thickening at MR image (C). Restaging PET/MRI after chemo-radiotherapy showing no hypermetabolism on PET (D) and fused PET/MR images (E) and a T2 hypointense thickening of rectal wall on MR image (F). The lesion was scored as a complete response (ycT0N0) and confirmed at histopathology after transanal local excision.
Figure 9.
T2* multiecho BB sequence with TE = 0,93 ms in a patient in lung transplant checklist for idiopathic lung fibrosis demonstrates mild steatosi with fat fraction of 9.58% and hyperferritinemia (>6000 mcg/ml).
Figure 9.
T2* multiecho BB sequence with TE = 0,93 ms in a patient in lung transplant checklist for idiopathic lung fibrosis demonstrates mild steatosi with fat fraction of 9.58% and hyperferritinemia (>6000 mcg/ml).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).