Submitted:
16 June 2023
Posted:
19 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
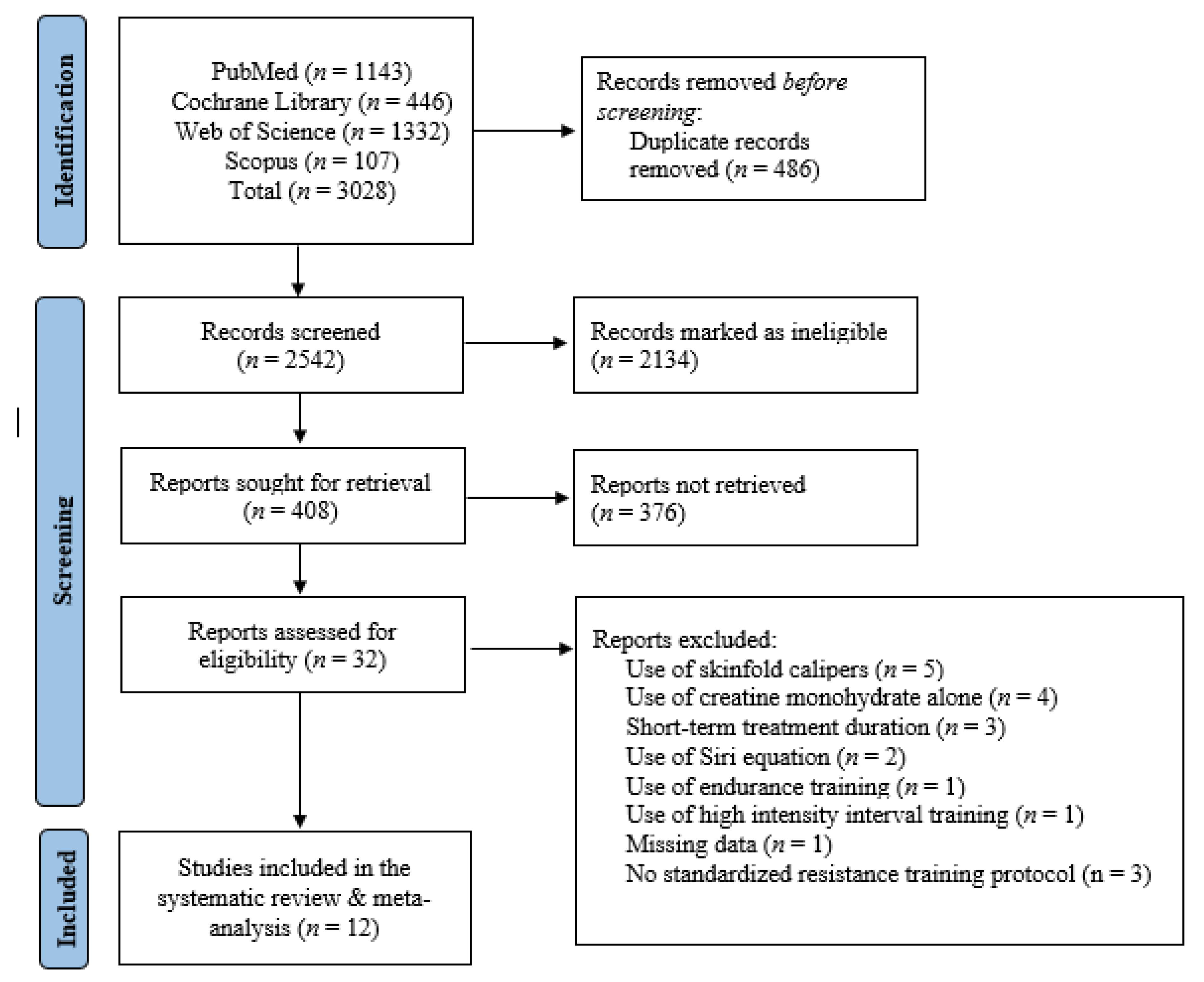
2.1. Search Strategy
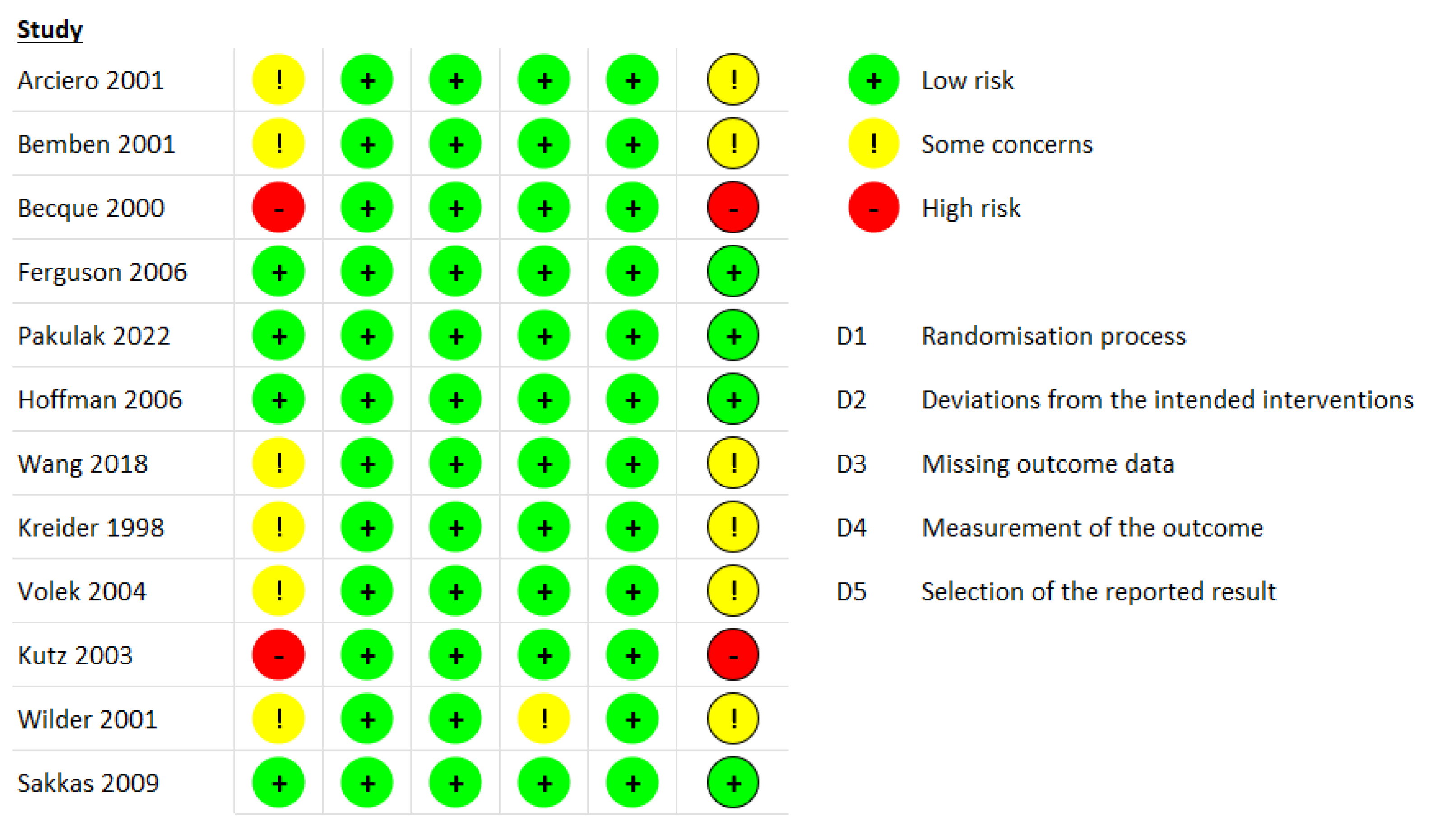
2.2. Data Extraction and Risk of Bias
2.3. Statistical Analysis
3. Results
3.1. Literature Search
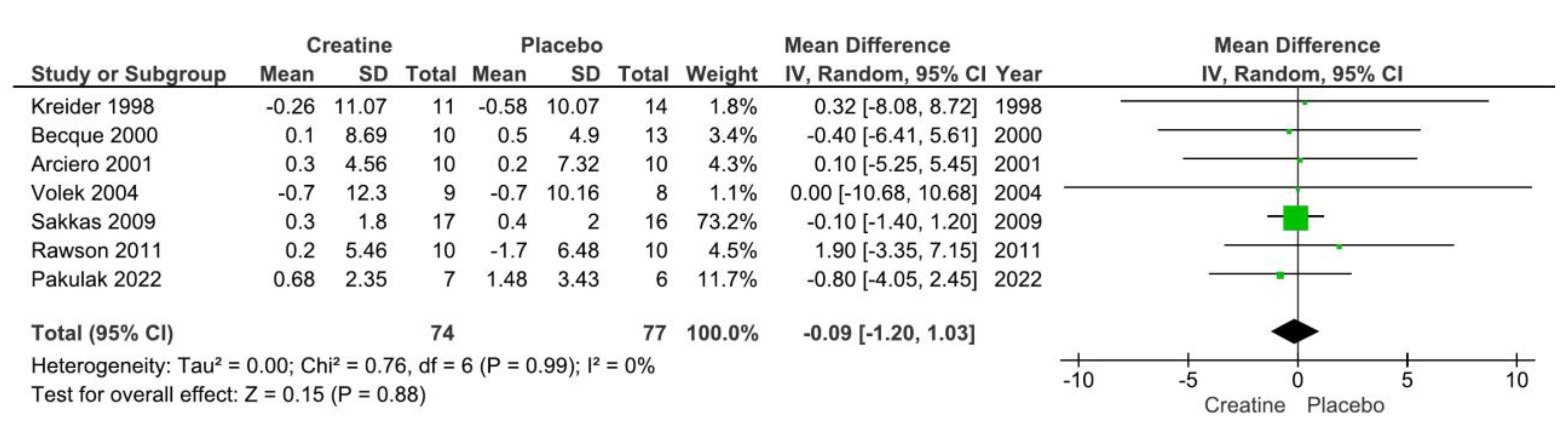
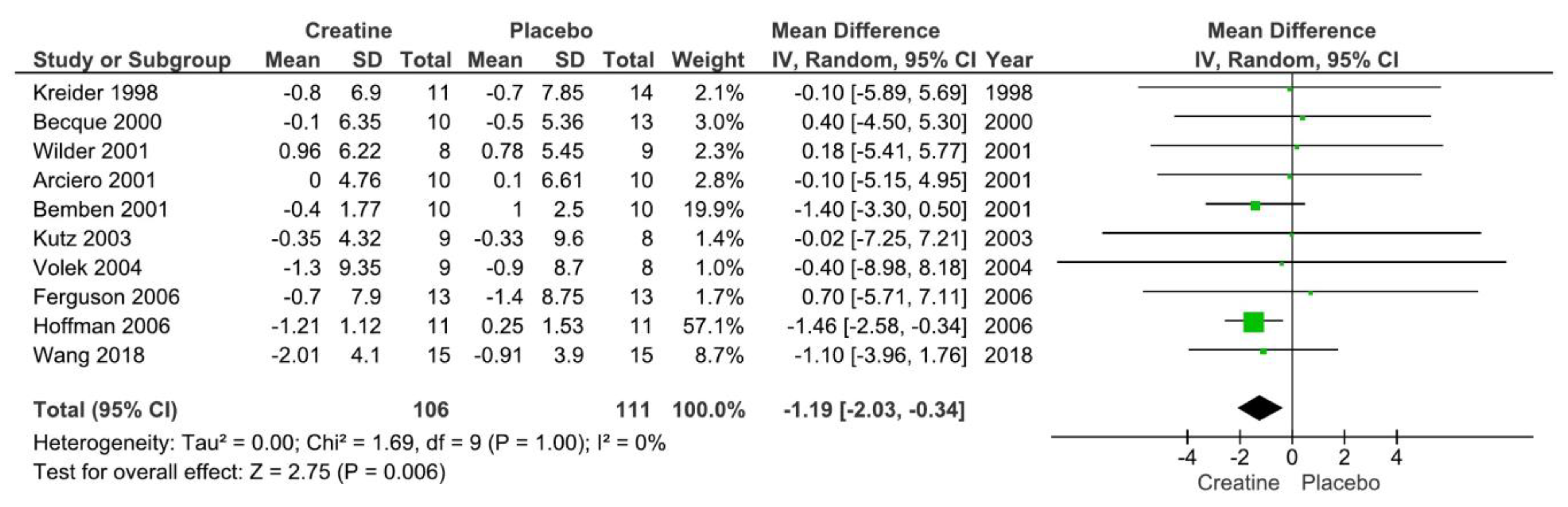
3.2. Creatine Supplementation and Body Fat Changes
3.3. Risk of Bias Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Souza, N.A.B.; Rimes-Dias, K.A.; Costa, J.C.; Canella, D.S. Weight Gain and Change in Body Mass Index after Age 20 in the Brazilian Population and Associated Sociodemographic Factors: Data from the National Health Survey. Int. J. Environ. Res. Public Health 2022, 19. [Google Scholar] [CrossRef]
- Frank, A.P.; De Souza Santos, R.; Palmer, B.F.; Clegg, D.J. Determinants of body fat distribution in humans may provide insight about obesity-related health risks. J. Lipid Res. 2019, 60, 1710–1719. [Google Scholar] [CrossRef]
- Simmonds, M.; Llewellyn, A.; Owen, C.G.; Woolacott, N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes. Rev. 2016, 17, 95–107. [Google Scholar] [CrossRef]
- Jakicic, J.M.; Powell, K.E.; Campbell, W.W.; Dipietro, L.; Pate, R.R.; Pescatello, L.S.; Collins, K.A.; Bloodgood, B.; Piercy, K.L. Physical Activity and the Prevention of Weight Gain in Adults: A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1262–1269. [Google Scholar] [CrossRef]
- Fruh, S.; Williams, S.; Hayes, K.; Hauff, C.; Hudson, G.M.; Sittig, S.; Graves, R.J.; Hall, H.; Barinas, J. A practical approach to obesity prevention: Healthy home habits. J. Am. Assoc. Nurse Pract. 2021, 33, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Wewege, M.A.; Desai, I.; Honey, C.; Coorie, B.; Jones, M.D.; Clifford, B.K.; Leake, H.B.; Hagstrom, A.D. The Effect of Resistance Training in Healthy Adults on Body Fat Percentage, Fat Mass and Visceral Fat: A Systematic Review and Meta-Analysis. Sports Med. 2022, 52, 287–300. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie-Shalders, K.; Kelly, J.T.; So, D.; Coffey, V.G.; Byrne, N.M. The effect of exercise interventions on resting metabolic rate: A systematic review and meta-analysis. J. Sports Sci. 2020, 38, 1635–1649. [Google Scholar] [CrossRef] [PubMed]
- Schuenke, M.D.; Mikat, R.P.; McBride, J.M. Effect of an acute period of resistance exercise on excess post-exercise oxygen consumption: implications for body mass management. Eur. J. Appl. Physiol. 2002, 86, 411–417. [Google Scholar] [CrossRef]
- Vechetti, I.J.; Peck, B.D.; Wen, Y.; Walton, R.G.; Valentino, T.R.; Alimov, A.P.; Dungan, C.M.; Van Pelt, D.W.; von Walden, F.; Alkner, B.; et al. Mechanical overload-induced muscle-derived extracellular vesicles promote adipose tissue lipolysis. FASEB J. 2021, 35. [Google Scholar] [CrossRef]
- Forbes, S.; Candow, D.; Krentz, J.; Roberts, M.; Young, K. Changes in Fat Mass Following Creatine Supplementation and Resistance Training in Adults ≥50 Years of Age: A Meta-Analysis. J. Funct. Morphol. Kinesiol. 2019, 4. [Google Scholar] [CrossRef]
- Kazak, L.; Chouchani, E.T.; Jedrychowski, M.P.; Erickson, B.K.; Shinoda, K.; Cohen, P.; Vetrivelan, R.; Lu, G.Z.; Laznik-Bogoslavski, D.; Hasenfuss, S.C.; et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 2015, 163, 643–655. [Google Scholar] [CrossRef]
- Kazak, L.; Cohen, P. Creatine metabolism: energy homeostasis, immunity and cancer biology. Nat. Rev. Endocrinol. 2020, 16, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Kazak, L.; Chouchani, E.T.; Lu, G.Z.; Jedrychowski, M.P.; Bare, C.J.; Mina, A.I.; Kumari, M.; Zhang, S.; Vuckovic, I.; Laznik-Bogoslavski, D.; et al. Genetic Depletion of Adipocyte Creatine Metabolism Inhibits Diet-Induced Thermogenesis and Drives Obesity. Cell Metab. 2017, 26, 660–671.e3. [Google Scholar] [CrossRef] [PubMed]
- Kazak, L.; Rahbani, J.F.; Samborska, B.; Lu, G.Z.; Jedrychowski, M.P.; Lajoie, M.; Zhang, S.; Ramsay, L.A.; Dou, F.Y.; Tenen, D.; et al. Ablation of adipocyte creatine transport impairs thermogenesis and causes diet-induced obesity. Nat. Metab. 2019, 1, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Candow, D.G.; Forbes, S.C.; Gualano, B.; Jagim, A.R.; Kreider, R.B.; Rawson, E.S.; Smith-Ryan, A.E.; VanDusseldorp, T.A.; Willoughby, D.S.; et al. Common questions and misconceptions about creatine supplementation: what does the scientific evidence really show? J. Int. Soc. Sports Nutr. 2021, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, T.; Syrotuik, D. Effects of creatine monohydrate supplementation on body composition and strength indices in experienced resistance trained women. J. strength Cond. Res. 2006, 20, 939–946. [Google Scholar] [CrossRef]
- Pakulak, A.; Candow, D.G.; Totosy de Zepetnek, J.; Forbes, S.C.; Basta, D. Effects of Creatine and Caffeine Supplementation During Resistance Training on Body Composition, Strength, Endurance, Rating of Perceived Exertion and Fatigue in Trained Young Adults. J. Diet. Suppl. 2022, 19, 587–602. [Google Scholar] [CrossRef]
- Hoffman, J.; Ratamess, N.; Kang, J.; Mangine, G.; Faigenbaum, A.; Stout, J. Effect of creatine and beta-alanine supplementation on performance and endocrine responses in strength/power athletes. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 430–446. [Google Scholar] [CrossRef]
- Sakkas, G.K.; Mulligan, K.; DaSilva, M.; Doyle, J.W.; Khatami, H.; Schleich, T.; Kent-Braun, J.A.; Schambelan, M. Creatine fails to augment the benefits from resistance training in patients with HIV infection: a randomized, double-blind, placebo-controlled study. PLoS One 2009, 4. [Google Scholar] [CrossRef]
- Arciero, P.J.; Hannibal, N.S.; Nindl, B.C.; Gentile, C.L.; Hamed, J.; Vukovich, M.D. Comparison of creatine ingestion and resistance training on energy expenditure and limb blood flow. Metab. - Clin. Exp. 2001, 50, 1429–1434. [Google Scholar] [CrossRef]
- Bemben, M.G.; Bemben, D.A.; Loftiss, D.D.; Knehans, A.W. Creatine supplementation during resistance training in college football athletes. Med. Sci. Sports Exerc. 2001, 33, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Fang, C.C.; Lee, Y.H.; Yang, M.T.; Chan, K.H. Effects of 4-Week Creatine Supplementation Combined with Complex Training on Muscle Damage and Sport Performance. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Kreider, R.; Ferreira, M.; Wilson, M.; Grindstaff, P.; Plisk, S.; Reinardy, J.; Cantler, E.; Almada, A. Effects of creatine supplementation on body composition, strength, and sprint performance. Med. Sci. Sports Exerc. 1998, 30, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Ratamess, N.A.; Rubin, M.R.; Gómez, A.L.; French, D.N.; McGuigan, M.M.; Scheett, T.P.; Sharman, M.J.; Häkkinen, K.; Kraemer, W.J. The effects of creatine supplementation on muscular performance and body composition responses to short-term resistance training overreaching. Eur. J. Appl. Physiol. 2004, 91, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Wilder, N.; Deivert, R.G.; Hagerman, F.; Gilders, R. The Effects of Low-Dose Creatine Supplementation Versus Creatine Loading in Collegiate Football Players. J. Athl. Train. 2001, 36, 124. [Google Scholar]
- Becque, M.D.; Lochmann, J.D.; Melrose, D.R. Effects of oral creatine supplementation on muscular strength and body composition. Med. Sci. Sports Exerc. 2000, 32, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Kutz, M.; Gunter, M. Creatine monohydrate supplementation on body weight and percent body fat. J. Strength Cond. Res. 2003, 17, 817–821. [Google Scholar]
- WOF Obesity Atlas 2023 | World Obesity Federation Global Obesity Observatory. Available online: https://data.worldobesity.org/publications/?cat=19 (accessed on May 24, 2023).
- Wannamethee, S.G.; Atkins, J.L. Muscle loss and obesity: the health implications of sarcopenia and sarcopenic obesity. Proc. Nutr. Soc. 2015, 74, 405–412. [Google Scholar] [CrossRef]
- Ryan, C.R.; Finch, M.S.; Dunham, T.C.; Murphy, J.E.; Roy, B.D.; Macpherson, R.E.K. Creatine Monohydrate Supplementation Increases White Adipose Tissue Mitochondrial Markers in Male and Female Rats in a Depot Specific Manner. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Chang, E.; Varghese, M.; Singer, K. Gender and Sex Differences in Adipose Tissue. Curr. Diab. Rep. 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Wakatsuki, T.; Hirata, F.; Ohno, H.; Yamamoto, M.; Sato, Y.; Ohira, Y. Thermogenic responses to high-energy phosphate contents and/or hindlimb suspension in rats. Jpn. J. Physiol. 1996, 46, 171–175. [Google Scholar] [CrossRef]
- Earnest, C.P.; Almada, A.L.; Mitchell, T.L. High-performance capillary electrophoresis-pure creatine monohydrate reduces blood lipids in men and women. Clin. Sci. (Lond). 1996, 91, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.C.; Candow, D.G.; Ostojic, S.M.; Roberts, M.D.; Chilibeck, P.D. Meta-analysis examining the importance of creatine ingestion strategies on lean tissue mass and strength in older adults. 2021. [Google Scholar] [CrossRef]
- Chilibeck, P.D.; Kaviani, M.; Candow, D.G.; Zello, G.A. Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: a meta-analysis. Open Access J. Sport. Med. 2017, 8, 226. [Google Scholar] [CrossRef] [PubMed]
- Delpino, F.M.; Figueiredo, L.M.; Forbes, S.C.; Candow, D.G.; Santos, H.O. Influence of age, sex, and type of exercise on the efficacy of creatine supplementation on lean body mass: A systematic review and meta-analysis of randomized clinical trials. Nutrition 2022, 103–104, 111791. [Google Scholar] [CrossRef]
- Candow, D.G.; Chilibeck, P.D.; Forbes, S.C. Creatine supplementation and aging musculoskeletal health. Endocrine 2014, 45, 354–361. [Google Scholar] [CrossRef]
- Devries, M.C.; Phillips, S.M. Creatine supplementation during resistance training in older adults - A meta-analysis. Med. Sci. Sports Exerc. 2014, 46, 1194–1203. [Google Scholar] [CrossRef]
- Burke, R.; Piñero, A.; Coleman, M.; Mohan, A.; Sapuppo, M.; Augustin, F.; Aragon, A.A.; Candow, D.G.; Forbes, S.C.; Swinton, P.; et al. The Effects of Creatine Supplementation Combined with Resistance Training on Regional Measures of Muscle Hypertrophy: A Systematic Review with Meta-Analysis. Nutrients 2023, 15, 2116. [Google Scholar] [CrossRef]
- Westerterp, K.R. Control of energy expenditure in humans. Eur. J. Clin. Nutr. 2017 713 2016, 71, 340–344. [Google Scholar] [CrossRef]
- Dos Santos, E.E.P.; de Araújo, R.C.; Candow, D.G.; Forbes, S.C.; Guijo, J.A.; de Almeida Santana, C.C.; Do Prado, W.L.; Botero, J.P. Efficacy of Creatine Supplementation Combined with Resistance Training on Muscle Strength and Muscle Mass in Older Females: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3757. [Google Scholar] [CrossRef] [PubMed]
| First Author, Year | Population | Supplement Dose | Fat MassTool | Duration | RT Protocol | Result | |
|---|---|---|---|---|---|---|---|
| Arciero et al. 2001 | N=30; Healthy males (21±3 y | CR: 20 g/day (5 g 4 x daily) for 5 days and then 10 g/day (5 g 2 x daily) for the remainder; PLA: same dosing as CR (dextrose) | DEXA | 28 days | RT 3x/wk; 2 sets of 10 @ 70% 1RM and 1 set performed to failure. | CR ↑ body mass, FFM, and RMR. ↔ fat mass or %BF. | |
| Becque et al. 2000 | N=23; healthy males with at least 1 y weight training experience (CR: n=10; PLA: n=13); Age: 21.5±2.7 y | CR: 20 g/day (5 g 4 x daily) for 5 days and then 2 g/day for the remainder; PLA: same dosing as CR (sucrose). | hydrodensitometry | 6 weeks | RT 2/wk (arm flexor: preacher curl). | CR ↑ body mass, FFM. ↔ fat mass or %BF. | |
| Bemben et al. 2001 | N=25 NCAA Division 1 football athletes; (PLA: n=8; CR: n=9; Control: n=8). Age: 18-22 y | CR: 20 g/d (4 equal doses) for 5 d followed by 5 g/d; PLA: same dose (sodium phosphate) | hydrodensitometry | 9 weeks | RT 4/wk split routine. | CR ↑ LBM, body mass. ↔ on %BF. | |
| Ferguson and Syrotuik 2006 | N=26 healthy recreationally strength trained women Age: 18-35 y | CR: 0.3 g/kg/d for 7 days and then 0.03 g/kg/d for the remainder or PLA | DEXA | 10 weeks | RT 4x/wks split routine. | ↔ between groups for LBM, fat mass, %BF, or total body mass. | |
| Hoffman et al. 2006 | N=33 male strength power athletes. Age: not reported | CR: 10.5 g/day or PLA: Dextrose | DEXA | 10 weeks | RT 4x/wks split routine. | ↔ between groups for LBM, fat mass, %BF, or total body mass. | |
| Kreider et al. 1998 | N=25 NCAA Division 1 football athletes. Age: 19.9±0.3 y | CR: 15.75 g/day or PLA | DEXA | 28 days | RT: 4x/wk + agility/sprint training 3x/wk | CR ↑ body mass and LBM; ↔ fat mass or %BF | |
| Kutz and Gunter 2003 | N=17 active males. Age: 22.9±4.9 y. | CR: 30 g/day for 2 weeks followed by 15 g/day for the remainder or PLA (dextrose) | hydrodensitometry | 4 weeks | RT: 2x/wk lower body only | CR ↑ body mass and TBW; ↔ %BF | |
| Pakulak et al. 2022 | N=28 resistance trained males and females (CR: n=7; PLA: n=6). Age: 18-38 y. | CR: 0.1 g/kg/d or PLA (maltodextrin) | air displacement plethysmography | 6 weeks | RT: 5-6x/wk split routine | ↔ FFM, fat mass, body mass | |
| Sakkas et al. 2009 | N=40 HIV-positive men | CR: 20 g/day for 5 days followed by 4.8 g/d for the remainder or PLA | DEXA | 14 weeks | RT: 3x/wk | CR ↑ body mass and LBM; ↔ fat mass | |
| Volek et al. 2003 | N=17 healthy males; 21±3 y | CR: 0.3 g/kg/d for 7 days followed by 0.05 g/kg/d for the remainder or PLA (cellulose) | DEXA | 4 weeks | RT: 5x/wk | CR ↑ body mass and LBM (trend); ↔ fat mass, %BF | |
| Wang et al. 2018 | N=30 males athletes (baseball, basketball, tchoukball); age: 20±2 y. | CR: 20 g/day for 6 days followed by 2 g/d for the remainder or PLA (cellulose) | Bioelectrical impedance analysis | 4 weeks | RT: Complex training including heavy resistance training and plyometrics 3x/wk. | ↔ %BF, body mass or FFM. There was a main effect of time for %BF | |
| Wilder et al. 2001 | N=25 division 1A collegiate football players; age: 20±2 y | CR: 20 g/day for 6 days followed by 5 g/d for the remainder or 3 g/day or PLA (cellulose) | hydrodensitometry | 4 weeks | RT: Complex training including heavy resistance training and plyometrics 3x/wk. | ↔ %BF, FFM. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





