Submitted:
19 June 2023
Posted:
19 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Work environment
2.2. Study Designs
2.3. Supporting examinations and tests performed for confirmation
- 2. Oxygen: Non-invasive ventilation procedures, such as supplemental oxygen in patients with signs of severe respiratory distress, or hypoxemia (i.e., SpO2 < 90%) delivered by ventilation. Initial oxygen therapy at 5 L/min and titrated to SpO2 ≥ 90%. High oxygen flows (10–15 or 50–60 L/min) is usually delivered through a face mask with a reservoir bag to reach a higher concentration of oxygen according to Borghes and Maroldi [26]66 Nava et al., 2011 [27]67 and Keenan et al., 2011 [28]68. All these protocols usually from simple to aggressive, as follows: First, a nasal canula (~4 L), then a simple facemask (~10 L), followed by a non-Rebreather mask (~15 L). If necessary, these are then followed by noninvasive medical ventilations for high flow, such as high-flow nasal canula (100 L) or Bilevel positive airway pressure (BiPAP). Often, tracheal intubation for high oxygen is required.
- Intubation: Mechanical ventilations increased difficulties with breathing or hypoxemia after using non-invasive ventilation. Invasive mechanical ventilation through an endotracheal tube or tracheostomy by an ICU expert according to the NIH NHLBI ARDS Clinical Network’s mechanical ventilation protocol card, available at: http://www.ardsnet.org/system/files/ Ventilator%20Protocol%20Card.pd (accessed on 5 December 2021).
- Records of Microbial Co-Infection or Superinfection and their antimicrobial Susceptibility Data during SRDS co-infections
2.4. Statistical Analysis of the data
3. Results
4. Discussion
5. Conclusion
Author Contributions
Funding
Ethical approval and Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashbaugh, D.G.; Bigelow, D.B.; Petty, T.L.; Levine, B.E. ACUTE RESPIRATORY DISTRESS IN ADULTS. The Lancet 1967, 290, 319–323. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Gajic, O.; Dabbagh, O.; Park, P.K.; Adesanya, A.; Chang, S.Y.; Hou, P.; Anderson, H.; Hoth, J.J.; Mikkelsen, M.E.; Gentile, N.T.; et al. Early Identification of Patients at Risk of Acute Lung Injury: Evaluation of Lung Injury Prediction Score in a Multicenter Cohort Study. Am J Respir Crit Care Med 2011, 183, 462–470. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Xi, X.; Zhou, J.X. The Association Between Etiologies and Mortality in Acute Respiratory Distress Syndrome: A Multicenter Observational Cohort Study. Front Med (Lausanne) 2021, 8. [Google Scholar] [CrossRef]
- Gattinoni, L.; Pelosi, P.; Suter, P.M.; Pedoto, A.; Vercesi, P.; Lissoni, A. Acute Respiratory Distress Syndrome Caused by Pulmonary and Extrapulmonary Disease. Different Syndromes? Am J Respir Crit Care Med 1998, 158, 3–11. [Google Scholar] [CrossRef]
- Lim, C.M.; Jung, H.; Koh, Y.; Lee, J.S.; Shim, T.S.; Lee, S. do; Kim, W.S.; Kim, D.S.; Kim, W.D. Effect of Alveolar Recruitment Maneuver in Early Acute Respiratory Distress Syndrome According to Antiderecruitment Strategy, Etiological Category of Diffuse Lung Injury, and Body Position of the Patient. Crit Care Med 2003, 31, 411–418. [Google Scholar] [CrossRef]
- Tugrul, S.; Akinci, O.; Ozcan, P.E.; Ince, S.; Esen, F.; Telci, L.; Akpir, K.; Cakar, N. Effects of Sustained Inflation and Postinflation Positive End-Expiratory Pressure in Acute Respiratory Distress Syndrome: Focusing on Pulmonary and Extrapulmonary Forms. Crit Care Med 2003, 31, 738–744. [Google Scholar] [CrossRef]
- Brun-Buisson, C.; Minelli, C.; Bertolini, G.; Brazzi, L.; Pimentel, J.; Lewandowski, K.; Bion, J.; Romand, J.A.; Villar, J.; Thorsteinsson, A.; et al. Epidemiology and Outcome of Acute Lung Injury in European Intensive Care Units. Results from the ALIVE Study. Intensive Care Med 2004, 30, 51–61. [Google Scholar] [CrossRef]
- Villar, J.; Blanco, J.; Añón, J.M.; Santos-Bouza, A.; Blanch, L.; Ambrós, A.; Gandía, F.; Carriedo, D.; Mosteiro, F.; Basaldúa, S.; et al. The ALIEN Study: Incidence and Outcome of Acute Respiratory Distress Syndrome in the Era of Lung Protective Ventilation. Intensive Care Med 2011, 37, 1932–1941. [Google Scholar] [CrossRef]
- Rubenfeld, G.D.; Caldwell, E.; Peabody, E.; Weaver, J.; Martin, D.P.; Neff, M.; Stern, E.J.; Hudson, L.D. Incidence and Outcomes of Acute Lung Injury. N Engl J Med 2005, 353, 1685–1693. [Google Scholar] [CrossRef]
- Brun-Buisson, C.; Minelli, C.; Bertolini, G.; Brazzi, L.; Pimentel, J.; Lewandowski, K.; Bion, J.; Romand, J.A.; Villar, J.; Thorsteinsson, A.; et al. Epidemiology and Outcome of Acute Lung Injury in European Intensive Care Units. Results from the ALIVE Study. Intensive Care Med 2004, 30, 51–61. [Google Scholar] [CrossRef]
- Villar, J.; Blanco, J.; Kacmarek, R.M. Current Incidence and Outcome of the Acute Respiratory Distress Syndrome. Curr Opin Crit Care 2016, 22, 1–6. [Google Scholar] [CrossRef]
- Caser, E.B.; Zandonade, E.; Pereira, E.; Gama, A.M.C.; Barbas, C.S.V. Impact of Distinct Definitions of Acute Lung Injury on Its Incidence and Outcomes in Brazilian ICUs: Prospective Evaluation of 7,133 Patients*. Crit Care Med 2014, 42, 574–582. [Google Scholar] [CrossRef]
- Azevedo, L.C.P.; Park, M.; Salluh, J.I.F.; Rea-Neto, A.; Souza-Dantas, V.C.; Varaschin, P.; Oliveira, M.C.; Tierno, P.F.G.M.M.; dal-Pizzol, F.; Silva, U.V.A.; et al. Clinical Outcomes of Patients Requiring Ventilatory Support in Brazilian Intensive Care Units: A Multicenter, Prospective, Cohort Study. Crit Care 2013, 17. [Google Scholar] [CrossRef]
- Cochi, S.E.; Kempker, J.A.; Annangi, S.; Kramer, M.R.; Martin, G.S. Mortality Trends of Acute Respiratory Distress Syndrome in the United States from 1999 to 2013. Ann Am Thorac Soc 2016, 13, 1742–1751. [Google Scholar] [CrossRef]
- Erickson, S.E.; Martin, G.S.; Davis, J.L.; Matthay, M.A.; Eisner, M.D. Recent Trends in Acute Lung Injury Mortality: 1996-2005. Crit Care Med 2009, 37, 1574–1579. [Google Scholar] [CrossRef]
- Sigurdsson, M.I.; Sigvaldason, K.; Gunnarsson, T.S.; Moller, A.; Sigurdsson, G.H. Acute Respiratory Distress Syndrome: Nationwide Changes in Incidence, Treatment and Mortality over 23 Years. Acta Anaesthesiol Scand 2013, 57, 37–45. [Google Scholar] [CrossRef]
- Rubenfeld, G.D.; Caldwell, E.; Peabody, E.; Weaver, J.; Martin, D.P.; Neff, M.; Stern, E.J.; Hudson, L.D. Incidence and Outcomes of Acute Lung Injury. N Engl J Med 2005, 353, 1685–1693. [Google Scholar] [CrossRef]
- Sjoding, M.W.; Cooke, C.R.; Iwashyna, T.J.; Hofer, T.P. Acute Respiratory Distress Syndrome Measurement Error Potential Effect on Clinical Study Results. Ann Am Thorac Soc 2016, 13, 1123–1128. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.M.P.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef]
- Clinics, B.W.-E. M. ; 2014, undefined. Lung-Protective Ventilation Strategies and Adjunctive Treatments for the Emergency Medicine Patient with Acute Respiratory Failure. emed.theclinics.com. [CrossRef]
- Baron, R.; F1000Research, B.L.-. ; 2016, undefined. Recent Advances in Understanding and Treating ARDS. ncbi.nlm.nih.gov.
- Villar, J.; Schultz, M.J.; Kacmarek, R.M. The LUNG SAFE: A Biased Presentation of the Prevalence of ARDS! Crit Care 2016, 20. [Google Scholar] [CrossRef]
- Zaccardelli, D.S.; Pattishall, E.N. Clinical Diagnostic Criteria of the Adult Respiratory Distress Syndrome in the Intensive Care Unit. Crit Care Med 1996, 24, 247–251. [Google Scholar] [CrossRef]
- Pepe, P.E.; Potkin, R.T.; Reus, D.H.; Hudson, L.D.; Carrico, C.J. Clinical Predictors of the Adult Respiratory Distress Syndrome. Am J Surg 1982, 144, 124–130. [Google Scholar] [CrossRef]
- Hudson, L.D.; Milberg, J.A.; Anardi, D.; Maunder, R.J. Clinical Risks for Development of the Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 1995, 151 Pt 1, 293–301. [Google Scholar] [CrossRef]
- Fowler, A.A.; Hamman, R.F.; Good, J.T.; Benson, K.N.; Baird, M.; Eberle, D.J.; Petty, T.L.; Hyers, T.M. Adult Respiratory Distress Syndrome: Risk with Common Predispositions. Ann Intern Med 1983, 98 Pt 1, 593–597. [Google Scholar] [CrossRef]
- Villar, J.; Blanco, J.; Añón, J.M.; Santos-Bouza, A.; Blanch, L.; Ambrós, A.; Gandía, F.; Carriedo, D.; Mosteiro, F.; Basaldúa, S.; et al. The ALIEN Study: Incidence and Outcome of Acute Respiratory Distress Syndrome in the Era of Lung Protective Ventilation. Intensive Care Med 2011, 37, 1932–1941. [Google Scholar] [CrossRef]
- Zilberberg, M.D.; Epstein, S.K. Acute Lung Injury in the Medical ICU: Comorbid Conditions, Age, Etiology, and Hospital Outcome. Am J Respir Crit Care Med 1998, 157 Pt 1, 1159–1164. [Google Scholar] [CrossRef]
- Baumann, W.R.; Jung, R.C.; Koss, M.; Boylen, C.T.; Navarro, L.; Sharma, O.P. Incidence and Mortality of Adult Respiratory Distress Syndrome: A Prospective Analysis from a Large Metropolitan Hospital. Crit Care Med 1986, 14, 1–4. [Google Scholar] [CrossRef]
- Mannes, G.P.; Boersma, W.G.; Baur, C.H.; Postmus, P.E. Adult Respiratory Distress Syndrome (ARDS) Due to Bacteraemic Pneumococcal Pneumonia. Eur Respir J 1991, 4, 503–504. [Google Scholar] [CrossRef]
- Pachon, J.; Prados, M.D.; Capote, F.; Cuello, J.A.; Garnacho, J.; Verano, A. Severe Community-Acquired Pneumonia. Etiology, Prognosis, and Treatment. Am Rev Respir Dis 1990, 142, 369–373. [Google Scholar] [CrossRef]
- Torres, A.; Serra-Batlles, J.; Ferrer, A.; Jimenez, P.; Celis, R.; Cobo, E.; Rodriguez-Roisin, R. Severe Community-Acquired Pneumonia. Epidemiology and Prognostic Factors. Am Rev Respir Dis 1991, 144, 312–318. [Google Scholar] [CrossRef]
- Self, W.H.; Wunderink, R.G.; Williams, D.J.; Zhu, Y.; Anderson, E.J.; Balk, R.A.; Fakhran, S.S.; Chappell, J.D.; Casimir, G.; Courtney, D.M.; et al. Staphylococcus Aureus Community-Acquired Pneumonia: Prevalence, Clinical Characteristics, and Outcomes. Clin Infect Dis 2016, 63, 300–309. [Google Scholar] [CrossRef]
- David, M.Z.; Daum, R.S. Community-Associated Methicillin-Resistant Staphylococcus Aureus: Epidemiology and Clinical Consequences of an Emerging Epidemic. Clin Microbiol Rev 2010, 23, 616–687. [Google Scholar] [CrossRef]
- Mandell, L.A.; Wunderink, R. Methicillin-Resistant Staphylococcus Aureus and Community-Acquired Pneumonia: An Evolving Relationship. Clin Infect Dis 2012, 54, 1134–1136. [Google Scholar] [CrossRef]
- Bhatta, D.R.; Cavaco, L.M.; Nath, G.; Kumar, K.; Gaur, A.; Gokhale, S.; Bhatta, D.R. Association of Panton Valentine Leukocidin (PVL) Genes with Methicillin Resistant Staphylococcus Aureus (MRSA) in Western Nepal: A Matter of Concern for Community Infections (a Hospital Based Prospective Study). BMC Infect Dis 2016, 16. [Google Scholar] [CrossRef]
- Francis, J.S.; Doherty, M.C.; Lopatin, U.; Johnston, C.P.; Sinha, G.; Ross, T.; Cai, M.; Hansel, N.N.; Perl, T.; Ticehurst, J.R.; et al. Severe Community-Onset Pneumonia in Healthy Adults Caused by Methicillin-Resistant Staphylococcus Aureus Carrying the Panton-Valentine Leukocidin Genes. Clin Infect Dis 2005, 40, 100–107. [Google Scholar] [CrossRef]
- Gillet, Y.; Issartel, B.; Vanhems, P.; Fournet, J.C.; Lina, G.; Bes, M.; Vandenesch, F.; Piémont, Y.; Brousse, N.; Floret, D.; et al. Association between Staphylococcus Aureus Strains Carrying Gene for Panton-Valentine Leukocidin and Highly Lethal Necrotising Pneumonia in Young Immunocompetent Patients. Lancet 2002, 359, 753–759. [Google Scholar] [CrossRef]
- Pepe, P.E.; Potkin, R.T.; Reus, D.H.; Hudson, L.D.; Carrico, C.J. Clinical Predictors of the Adult Respiratory Distress Syndrome. Am J Surg 1982, 144, 124–130. [Google Scholar] [CrossRef]
- Hudson, L.D.; Milberg, J.A.; Anardi, D.; Maunder, R.J. Clinical Risks for Development of the Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 1995, 151 Pt 1, 293–301. [Google Scholar] [CrossRef]
- Doyle, R.L.; Szaflarski, N.; Modin, G.W.; Wiener-Kronish, J.P.; Matthay, M.A. Identification of Patients with Acute Lung Injury. Predictors of Mortality. Am J Respir Crit Care Med 1995, 152 Pt 1, 1818–1824. [Google Scholar] [CrossRef]
- Fein, A.M.; Lippmann, M.; Holtzman, H.; Eliraz, A.; Goldberg, S.K. The Risk Factors, Incidence, and Prognosis of ARDS Following Septicemia. Chest 1983, 83, 40–42. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Reyes, L.F.; Aliberti, S.; Restrepo, M.I. Empirical Coverage of Methicillin-Resistant Staphylococcus Aureus in Community-Acquired Pneumonia: Those Who Do Not Remember the Past Are Doomed to Repeat It. Clinical Infectious Diseases 2016, 63, 1145–1146. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Reyes, L.F.; Aliberti, S.; Restrepo, M.I. Empirical Coverage of Methicillin-Resistant Staphylococcus Aureus in Community-Acquired Pneumonia: Those Who Do Not Remember the Past Are Doomed to Repeat It. Clin Infect Dis 2016, 63, 1145–1146. [Google Scholar] [CrossRef]
- Self, W.H.; Wunderink, R.G.; Williams, D.J.; Zhu, Y.; Anderson, E.J.; Balk, R.A.; Fakhran, S.S.; Chappell, J.D.; Casimir, G.; Courtney, D.M.; et al. Staphylococcus Aureus Community-Acquired Pneumonia: Prevalence, Clinical Characteristics, and Outcomes. Clin Infect Dis 2016, 63, 300–309. [Google Scholar] [CrossRef]
- Peyrani, P.; Mandell, L.; Torres, A.; Tillotson, G.S. The Burden of Community-Acquired Bacterial Pneumonia in the Era of Antibiotic Resistance. Expert Rev Respir Med 2019, 13, 139–152. [Google Scholar] [CrossRef]
- Wang, L.; Letsiou, E.; Wang, H.; Belvitch, P.; Meliton, L.N.; Brown, M.E.; Bandela, M.; Chen, J.; Garcia, J.G.N.; Dudek, S.M. MRSA-Induced Endothelial Permeability and Acute Lung Injury Are Attenuated by FTY720 S-Phosphonate. Am J Physiol Lung Cell Mol Physiol 2022, 322, L149–L161. [Google Scholar] [CrossRef]
- Dugani, S.; Veillard, J.; Kissoon, N. Reducing the Global Burden of Sepsis. CMAJ 2017, 189, E2–E3. [Google Scholar] [CrossRef]
- Troeger, C.; Blacker, B.; Khalil, I.A.; Rao, P.C.; Cao, J.; Zimsen, S.R.M.; Albertson, S.B.; Deshpande, A.; Farag, T.; Abebe, Z.; et al. Estimates of the Global, Regional, and National Morbidity, Mortality, and Aetiologies of Lower Respiratory Infections in 195 Countries, 1990-2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 2018, 18, 1191–1210. [Google Scholar] [CrossRef]
- Naghavi, M.; Wang, H.; Lozano, R.; Davis, A.; Liang, X.; Zhou, M.; Vollset, S.E.; Abbasoglu Ozgoren, A.; Abdalla, S.; Abd-Allah, F.; et al. Global, Regional, and National Age-Sex Specific All-Cause and Cause-Specific Mortality for 240 Causes of Death, 1990-2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- McLaughlin, J.M.; Johnson, M.H.; Kagan, S.A.; Baer, S.L. Clinical and Economic Burden of Community-Acquired Pneumonia in the Veterans Health Administration, 2011: A Retrospective Cohort Study. Infection 2015, 43, 671–680. [Google Scholar] [CrossRef]
- Fernando, S.M.; Rochwerg, B.; Seely, A.J.E. Clinical Implications of the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). CMAJ 2018, 190, E1058–E1059. [Google Scholar] [CrossRef]
- Li, T.; Qian, Y.; Miao, Z.; Zheng, P.; Shi, T.; Jiang, X.; Pan, L.; Qian, F.; Yang, G.; An, H.; et al. Xuebijing Injection Alleviates Pam3CSK4-Induced Inflammatory Response and Protects Mice From Sepsis Caused by Methicillin-Resistant Staphylococcus Aureus. Front Pharmacol 2020, 11. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial Cell Infection and Endotheliitis in COVID-19. The Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. The Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Herold, S.; Becker, C.; Ridge, K.M.; Budinger, G.R.S. Influenza Virus-Induced Lung Injury: Pathogenesis and Implications for Treatment. European Respiratory Journal 2015, 45, 1463–1478. [Google Scholar] [CrossRef]
- Bernard, G.R.; Artigas, A.; Brigham, K.L.; Carlet, J.; Falke, K.; Hudson, L.; Lamy, M.; Legall, J.R.; Morris, A.; Spragg, R.; et al. The American-European Consensus Conference on ARDS. Definitions, Mechanisms, Relevant Outcomes, and Clinical Trial Coordination. https://doi.org/10.1164/ajrccm.149.3.7509706 2012, 149 (3 I), 818–824. [CrossRef]
- Bai, H.X.; Hsieh, B.; Xiong, Z.; Halsey, K.; Choi, J.W.; Tran, T.M.L.; Pan, I.; Shi, L.B.; Wang, D.C.; Mei, J.; et al. Performance of Radiologists in Differentiating COVID-19 from Viral Pneumonia on Chest CT. Radiology 2020, 296, E46–E54. [Google Scholar] [CrossRef]
- Tian, S.; Xiong, Y.; Liu, H.; Niu, L.; Guo, J.; Liao, M.; Xiao, S.Y. Pathological Study of the 2019 Novel Coronavirus Disease (COVID-19) through Postmortem Core Biopsies. Modern Pathology 2020, 33, 1007. [Google Scholar] [CrossRef]
- Hani, C.; Trieu, N.H.; Saab, I.; Dangeard, S.; Bennani, S.; Chassagnon, G.; Revel, M.P. COVID-19 Pneumonia: A Review of Typical CT Findings and Differential Diagnosis. Diagn Interv Imaging 2020, 101, 263. [Google Scholar] [CrossRef]
- Schwarz, M.I.; Albert, R.K. “Imitators” of the ARDS: Implications for Diagnosis and Treatment. Chest 2004, 125, 1530–1535. [Google Scholar] [CrossRef]
- Putman, R.K.; Hunninghake, G.M.; Dieffenbach, P.B.; Barragan-Bradford, D.; Serhan, K.; Adams, U.; Hatabu, H.; Nishino, M.; Padera, R.F.; Fredenburgh, L.E.; et al. Interstitial Lung Abnormalities Are Associated with Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2017, 195, 138–141. [Google Scholar] [CrossRef]
- Sekiguchi, H.; Schenck, L.A.; Horie, R.; Suzuki, J.; Lee, E.H.; McMenomy, B.P.; Chen, T.E.; Lekah, A.; Mankad, S. v.; Gajic, O. Critical Care Ultrasonography Differentiates ARDS, Pulmonary Edema, and Other Causes in the Early Course of Acute Hypoxemic Respiratory Failure. Chest 2015, 148, 912–918. [Google Scholar] [CrossRef]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International Evidence-Based Recommendations for Point-of-Care Lung Ultrasound. Intensive Care Med 2012, 38, 577–591. [Google Scholar] [CrossRef]
- Borghesi, A.; Maroldi, R. COVID-19 Outbreak in Italy: Experimental Chest X-Ray Scoring System for Quantifying and Monitoring Disease Progression. Radiol Med 2020, 125, 509–513. [Google Scholar] [CrossRef]
- Nava, S.; Schreiber, A.; Domenighetti, G. Noninvasive Ventilation for Patients with Acute Lung Injury or Acute Respiratory Distress Syndrome. Respir Care 2011, 56, 1583–1588. [Google Scholar] [CrossRef]
- Keenan, S.P.; Sinuff, T.; Burns, K.E.A.; Muscedere, J.; Kutsogiannis, J.; Mehta, S.; Cook, D.J.; Ayas, N.; Adhikari, N.K.J.; Hand, L.; et al. Clinical Practice Guidelines for the Use of Noninvasive Positive-Pressure Ventilation and Noninvasive Continuous Positive Airway Pressure in the Acute Care Setting. CMAJ 2011, 183. [Google Scholar] [CrossRef]
- Fan, B.E.; Chong, V.C.L.; Chan, S.S.W.; Lim, G.H.; Lim, K.G.E.; Tan, G.B.; Mucheli, S.S.; Kuperan, P.; Ong, K.H. Hematologic Parameters in Patients with COVID-19 Infection. Am J Hematol 2020, 95, E131–E134. [Google Scholar] [CrossRef]
- Kaushansky. Williams Hematology, 9e | AccessMedicine | McGraw Hill Medical. Williams Hematology, 9e Eds. Kenneth Kaushansky, et al. McGraw Hill, 2015. Available online: https://accessmedicine.mhmedical.com/book.aspx?bookid=1581&isMissingChapter=true (accessed on 5 December 2021).
- Patel, J. ; C., F.; I., E.G. ; J., S. ; L., J. S. ; B., L. ; M., A. J. ; M., T. ; P., R. ; R., S. S. ; et al. Performance Standards for Antimicrobial Susceptibility Testing An Informational Supplement for Global Application Developed through the Clinical and Laboratory Standards Institute Consensus Process. 26th Edition.
- Asselah, T.; Durantel, D.; Pasmant, E.; Lau, G.; Schinazi, R.F. COVID-19: Discovery, Diagnostics and Drug Development. J Hepatol 2021, 74, 168–184. [Google Scholar] [CrossRef]
- Killien, E.Y.; Mills, B.; Vavilala, M.S.; Watson, R.S.; O’Keefe, G.E.; Rivara, F.P. Association between Age and Acute Respiratory Distress Syndrome Development and Mortality Following Trauma. J Trauma Acute Care Surg 2019, 86, 844–852. [Google Scholar] [CrossRef]
- Fabião, J.; Sassi, B.; Pedrollo, E.F.; Gerchman, F.; Kramer, C.K.; Leitão, C.B.; Pinto, L.C. Why Do Men Have Worse COVID-19-Related Outcomes? A Systematic review and Meta-Analysis with Sex Adjusted for Age. Brazilian Journal of Medical and Biological Research 2022, 55. [Google Scholar] [CrossRef]
- Pachpande, V.; Senapathi, S.H.V.; Williams, K.; Chai, S.; Mandal, S.; Prabhu, S. Demographics, Comorbidities, and Laboratory Parameters in Hospitalized Patients with SARS-CoV2 Infection at a Community Hospital in Rural Pennsylvania. PLoS One 2022, 17. [Google Scholar] [CrossRef]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; Genomics, C.-19; Consortium, U.K.; Peacock, S.J.; Barclay, W.S.; De Silva, T.I.; et al. Nature Reviews Microbiology SARS-CoV-2 Variant Biology: Immune Escape, Transmission and Fitness. Nature Reviews Microbiology | 2023, 21, 162–177. [Google Scholar] [CrossRef]
- Cao, Z.; Xu, C.; Zhang, P.; Wang, Y. Associations of Sedentary Time and Physical Activity with Adverse Health Conditions: Outcome-Wide Analyses Using Isotemporal Substitution Model. EClinicalMedicine 2022, 48. [Google Scholar] [CrossRef]
- Said, K.B.; Alsolami, A.; Alshammari, F.; Alreshidi, F.S.; Fathuldeen, A.; Alrashid, F.; Bashir, A.I.; Osman, S.; Aboras, R.; Alshammari, A.; et al. Selective COVID-19 Coinfections in Diabetic Patients with Concomitant Cardiovascular Comorbidities Are Associated with Increased Mortality. Pathogens 2022, 11, 508. [Google Scholar] [CrossRef]
- Said, K.B.; Alsolami, A.; Moussa, S.; Alfouzan, F.; Bashir, A.I.; Rashidi, M.; Aborans, R.; Taha, T.E.; Almansour, H.; Alazmi, M.; et al. COVID-19 Clinical Profiles and Fatality Rates in Hospitalized Patients Reveal Case Aggravation and Selective Co-Infection by Limited Gram-Negative Bacteria. Int J Environ Res Public Health 2022, 19, 5270. [Google Scholar] [CrossRef]
- Butowt, R.; von Bartheld, C.S. Anosmia in COVID-19: Underlying Mechanisms and Assessment of an Olfactory Route to Brain Infection. Neuroscientist 2020. [Google Scholar] [CrossRef]
- Brann, D.H.; Tsukahara, T.; Weinreb, C.; Lipovsek, M.; Van Den Berge, K.; Gong, B.; Chance, R.; Macaulay, I.C.; Chou, H.J.; Fletcher, R.B.; et al. Non-Neuronal Expression of SARS-CoV-2 Entry Genes in the Olfactory System Suggests Mechanisms Underlying COVID-19-Associated Anosmia. Sci Adv 2020, 6. [Google Scholar] [CrossRef]
- Hosoda, T.; Harada, S.; Okamoto, K.; Ishino, S.; Kaneko, M.; Suzuki, M.; Ito, R.; Mizoguchi, M. COVID-19 and Fatal Sepsis Caused by Hypervirulent Klebsiella Pneumoniae, Japan, 2020. Emerg Infect Dis 2021, 27, 556. [Google Scholar] [CrossRef]
- Boschiero, M.N.; Duarte, A.; Palamim, C.V.C.; Alvarez, A.E.; Mauch, R.M.; Marson, F.A.L. Frequency of Respiratory Pathogens Other than SARS-CoV-2 Detected during COVID-19 Testing. Diagn Microbiol Infect Dis 2022, 102, undefined. [Google Scholar] [CrossRef]
- Calcagno, A.; Ghisetti, V.; Burdino, E.; Trunfio, M.; Allice, T.; Boglione, L.; Bonora, S.; Di Perri, G. Co-Infection with Other Respiratory Pathogens in COVID-19 Patients. Clinical Microbiology and Infection 2021, 27, 297–298. [Google Scholar] [CrossRef]
- Aran, D.; Beachler, D.C.; Lanes, S.; Overhage, J.M. Prior Presumed Coronavirus Infection Reduces COVID-19 Risk: A Cohort Study. Journal of Infection 2020, 81, 923–930. [Google Scholar] [CrossRef]
- Reche, P.A. Potential Cross-Reactive Immunity to SARS-CoV-2 From Common Human Pathogens and Vaccines. Front Immunol 2020, 11. [Google Scholar] [CrossRef] [PubMed]
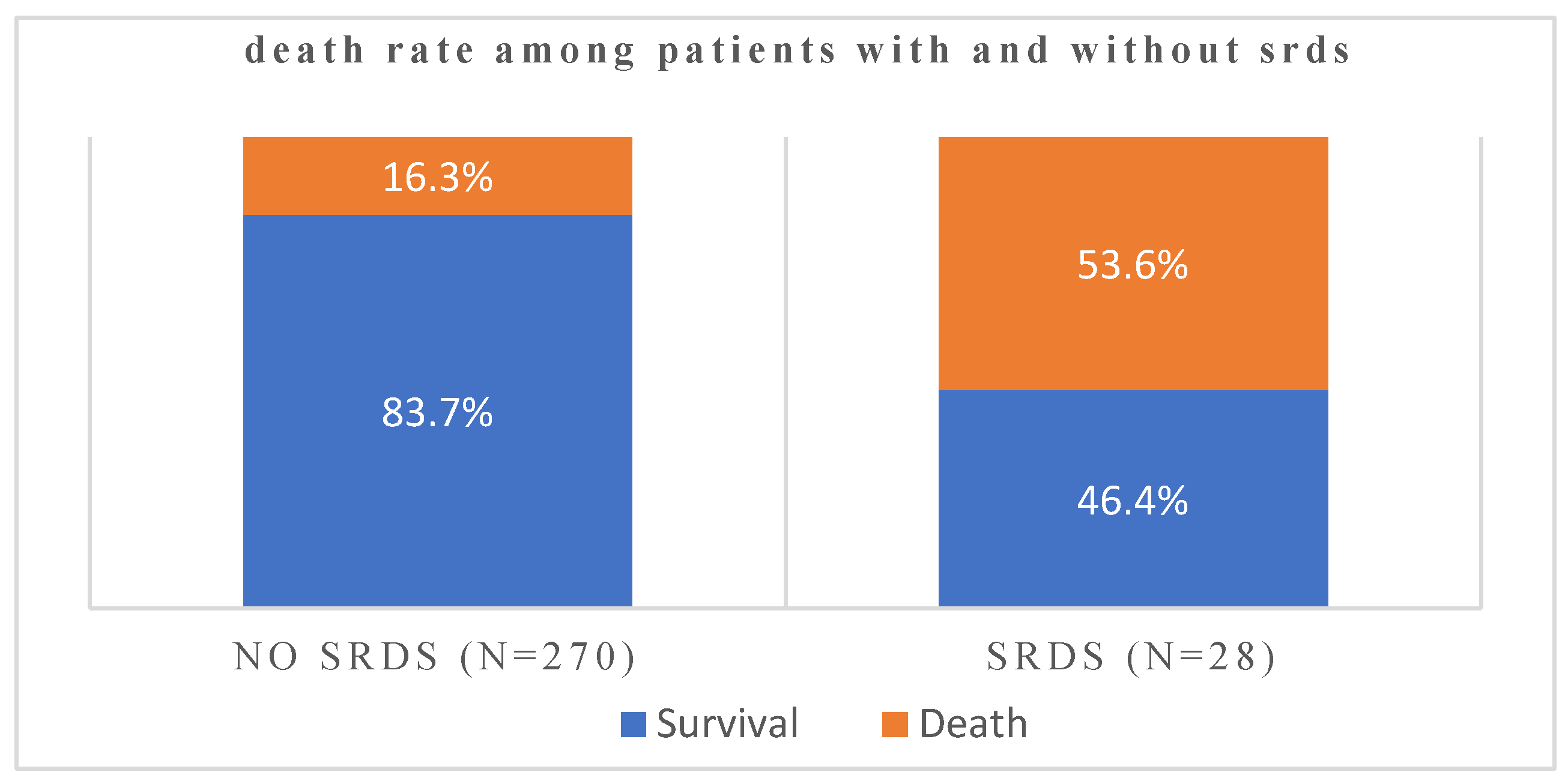
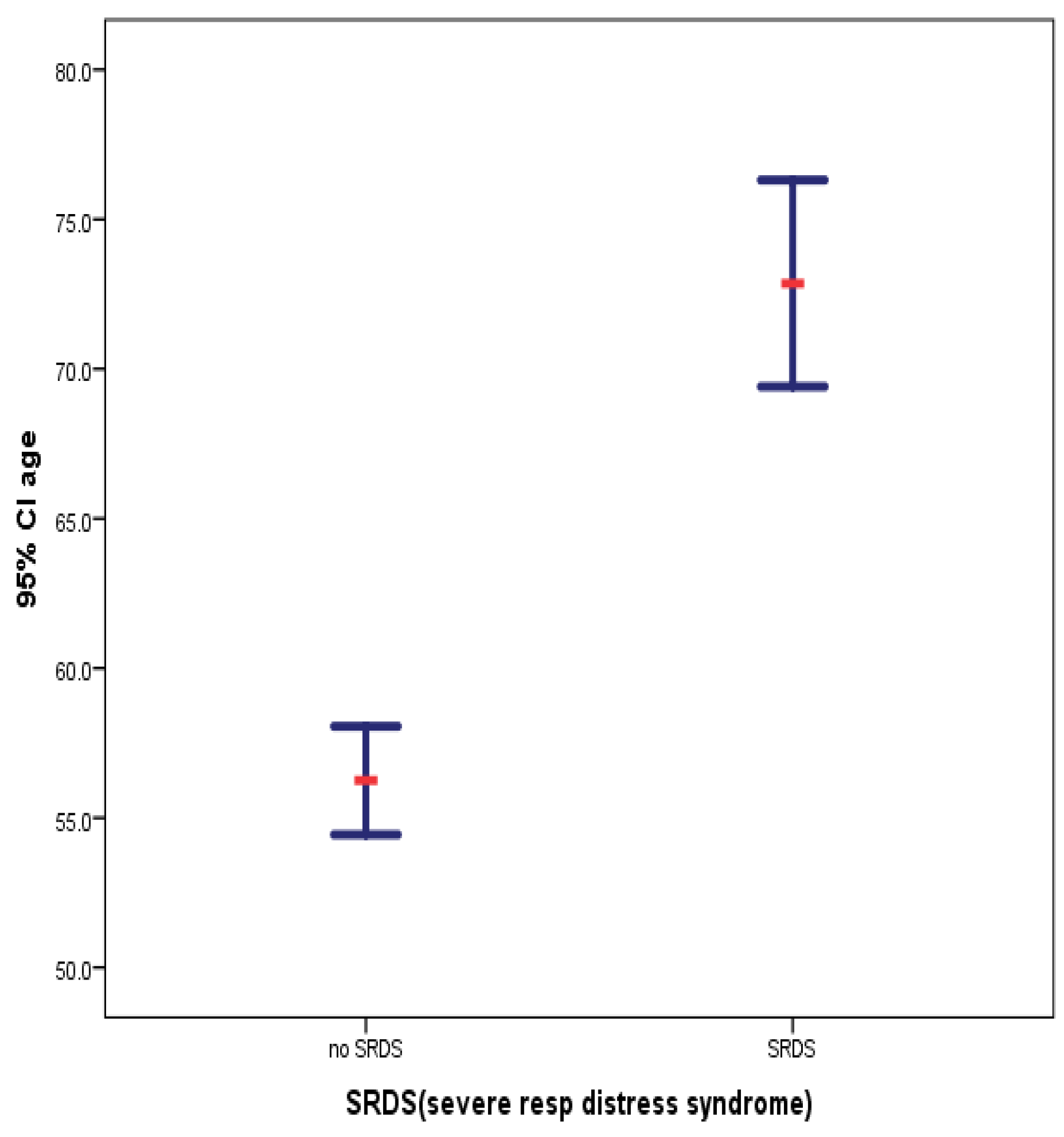
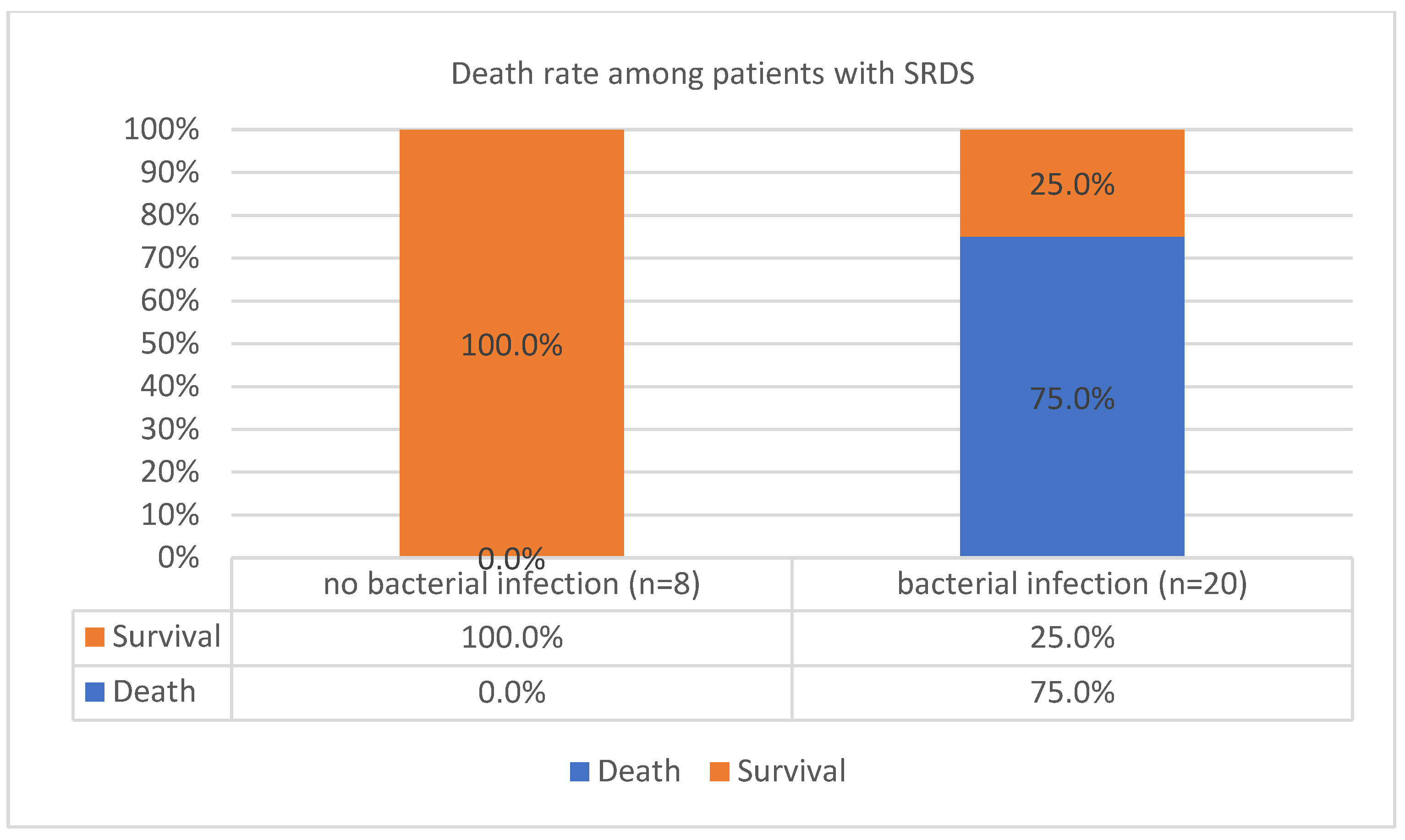
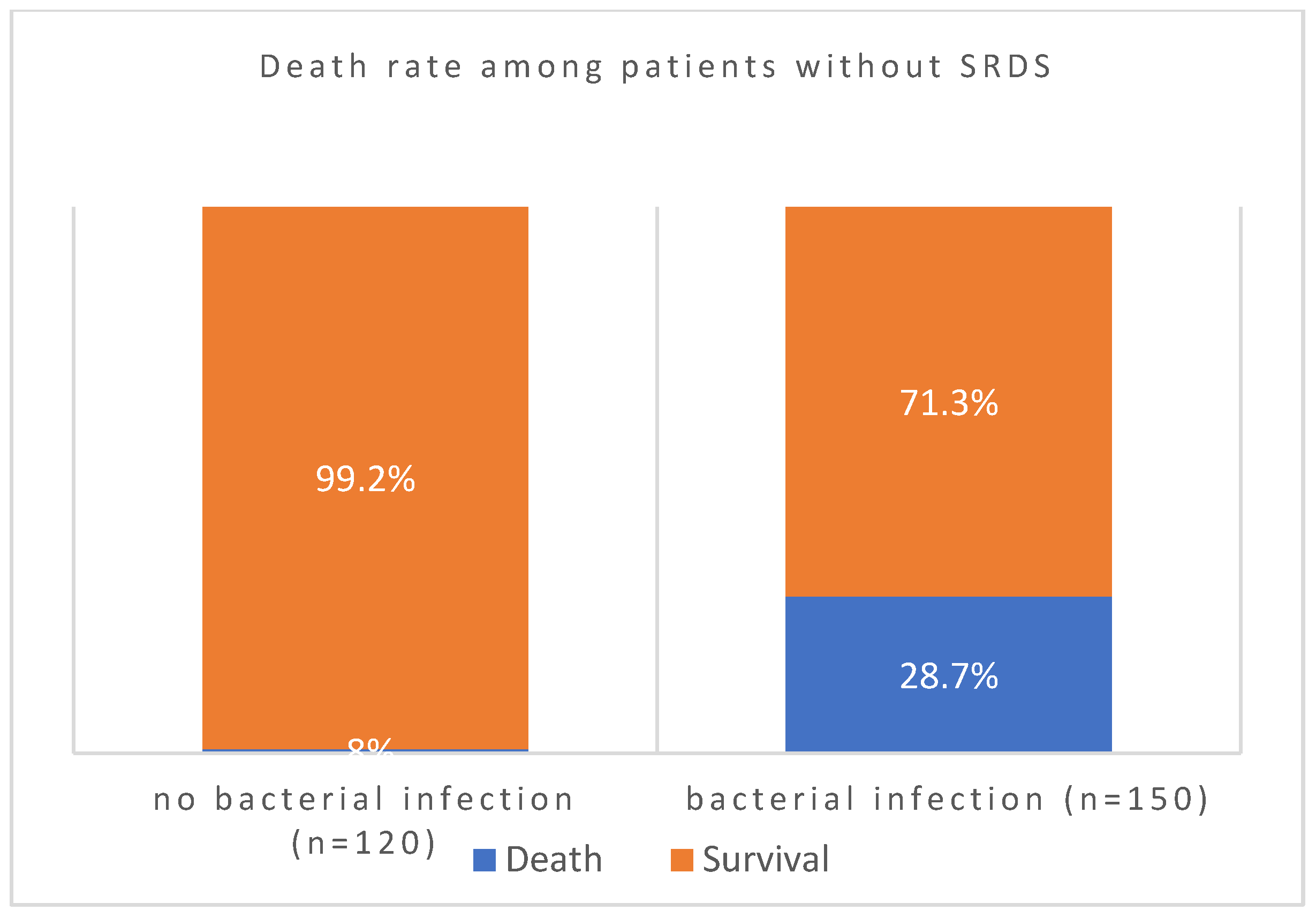
| SRDS(severe resp distress syndrome) | N | Mean | Std. Deviation | Std. Error Mean | |
|---|---|---|---|---|---|
| age | yes | 28 | 72.857 | 8.8974 | 1.6815 |
| no | 270 | 56.248 | 15.1019 | .9191 | |
| Group Statistics: Age profiles of patients before and after COVID-19 pandemic | |||||
|---|---|---|---|---|---|
| Group | N | Mean | Std. Deviation | Std. Error Mean | |
| age | During COVID-19 | 149 | 57.154 | 15.5058 | 1.2703 |
| Before COVID-19 | 149 | 58.463 | 15.3286 | 1.2558 | |
| a. Chi-Square Tests. Admitted patients’ gender profiles before and during the COVID-19 pandemic | |||||
| Value | df | Asymptotic Significance (2-sided) | Exact Sig. (2-sided) | Exact Sig. (1-sided) | |
| Pearson Chi-Square | .000a | 1 | 1.000 | ||
| Continuity Correctionb | 0.000 | 1 | 1.000 | ||
| Likelihood Ratio | 0.000 | 1 | 1.000 | ||
| Fisher’s Exact Test | 1.000 | .546 | |||
| N of Valid Cases | 298 | ||||
| a. 0 cells (.0%) have expected count less than 5. The minimum expected count is 68.00. | |||||
| b. Chi-Square Tests: Analysis of the association of admitted patients’ gender and Severe Respiratory Distress Syndrome in the study population | |||||
| Value | df | Asymptotic Significance (2-sided) | Exact Sig. (2-sided) | Exact Sig. (1-sided) | |
| Pearson Chi-Square | .503a | 1 | .478 | ||
| Continuity Correctionb | .260 | 1 | .610 | ||
| Likelihood Ratio | .507 | 1 | .476 | ||
| Fisher’s Exact Test | .553 | .307 | |||
| N of Valid Cases | 298 | ||||
| a. 0 cells (.0%) have expected count less than 5. The minimum expected count is 12.78. | |||||
| c. Chi-Square Tests: association of patients’ gender and COVID-19 fatality | |||||
| Value | df | Asymptotic Significance (2-sided) | Exact Sig. (2-sided) | Exact Sig. (1-sided) | |
| Pearson Chi-Square | .098a | 1 | .754 | ||
| Continuity Correctionb | .028 | 1 | .867 | ||
| Likelihood Ratio | .098 | 1 | .754 | ||
| Fisher’s Exact Test | .772 | .433 | |||
| N of Valid Cases | 298 | ||||
| a. 0 cells (.0%) have expected count less than 5. The minimum expected count is 26.93. | |||||
| d. Chi-Square Tests: Whether Severe respiratory distress syndrome predisposes to coinfections | |||||
| Value | df | Asymptotic Significance (2-sided) | Exact Sig. (2-sided) | Exact Sig. (1-sided) | |
| Pearson Chi-Square | 2.609a | 1 | .106 | ||
| Continuity Correctionb | 2.001 | 1 | .157 | ||
| Likelihood Ratio | 2.714 | 1 | .099 | ||
| Fisher’s Exact Test | .114 | .077 | |||
| N of Valid Cases | 298 | ||||
| a. 0 cells (.0%) have expected count less than 5. The minimum expected count is 12.03. | |||||
| Chi-Square Tests | ||||
|---|---|---|---|---|
| Value | df | Asymptotic Significance (2-sided) | Exact Sig. (2-sided) | |
| Pearson Chi-Square | 47.251a | 21 | .001 | .008 |
| Likelihood Ratio | 30.691 | 21 | .079 | .024 |
| Fisher’s Exact Test | 32.718 | .034 | ||
| N of Valid Cases | 149 | |||
| a. 38 cells (86.4%) have expected count less than 5. The minimum expected count is .11. | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





