Submitted:
19 June 2023
Posted:
19 June 2023
You are already at the latest version
Abstract
Keywords:
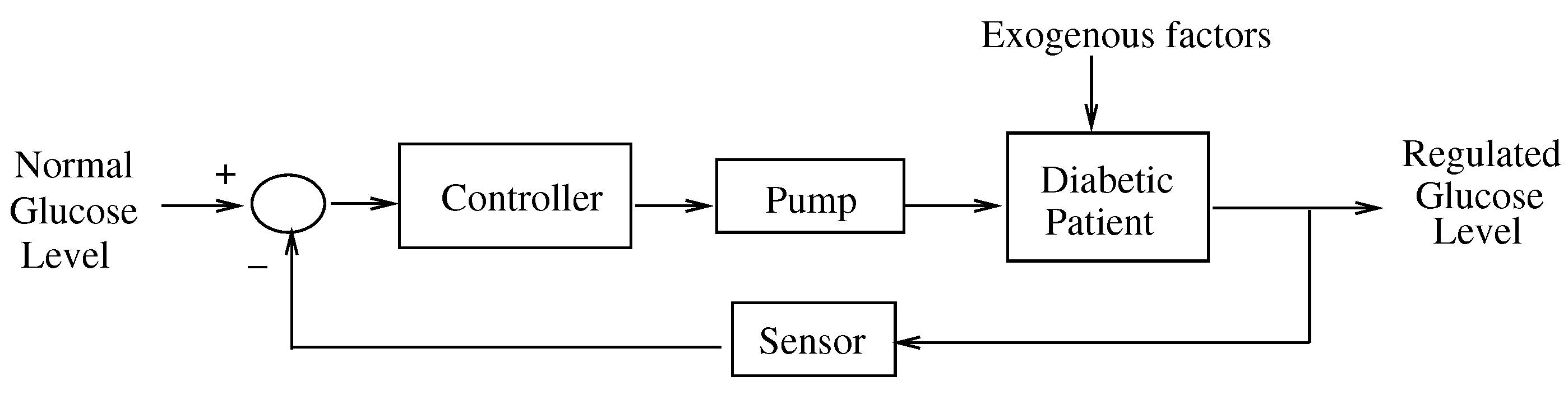
1. Introduction
2. Materials and Methods
2.1. Dynamical Model of the Glucose-Insulin Regulatory System
2.2. Modified Mathematical Model
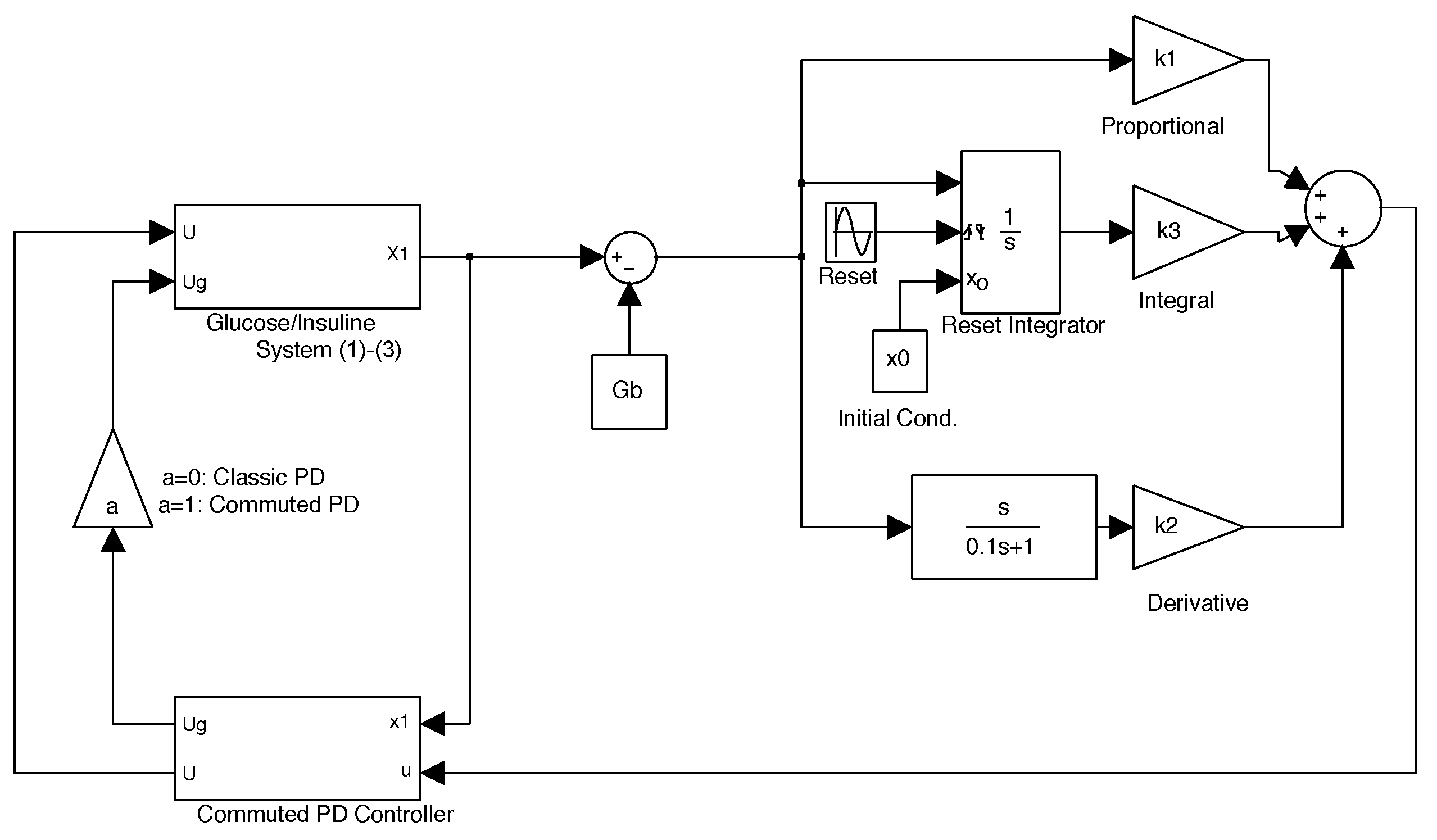
2.3. Commuted PD Control
-
Hyperglycemia: to decrease the glucose blood concentration, an insulin injection is needed, in terms of the PD controller. In system equation (), the control is:This administration has to be always positive. The control designer has to decide when this control is activated, in terms of critical glucose blood concentration value .
-
Hypoglycemia: to increment the glucose blood concentration, ingestion is necessary. In system equation (5), the control is:Due to the dynamic of glucose blood concentration (5), the control law tries to increment the velocity of glucose blood absorption. The control designer has to decide when this control is activated, in terms of critical glucose blood concentration value .
2.4. Robustness and Stability
- (i)
- : ,
- (ii)
- : .
3. Results
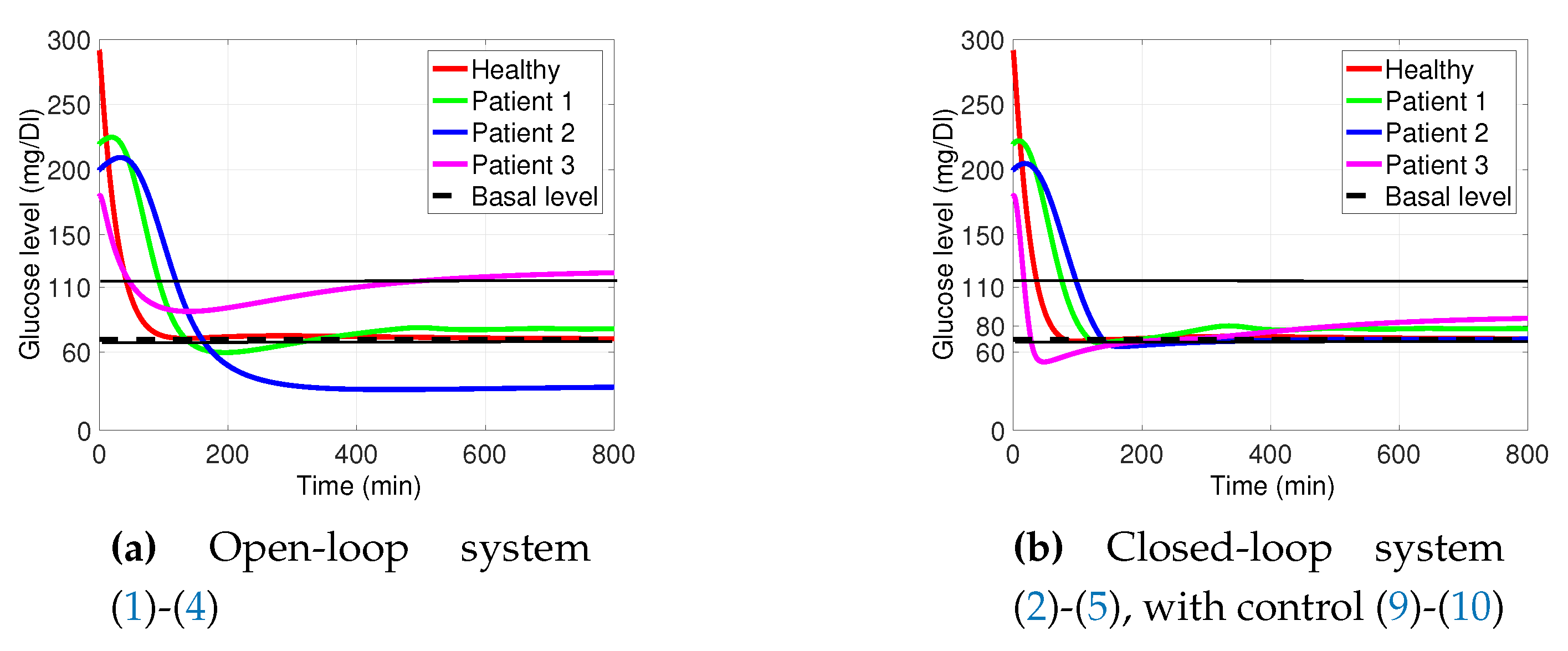
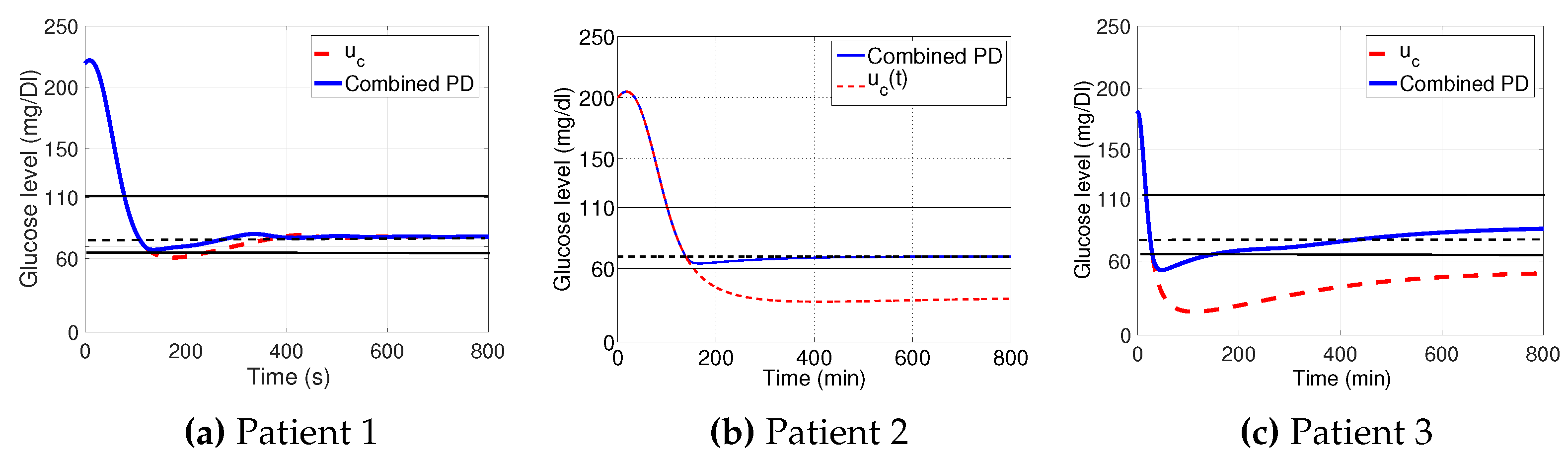
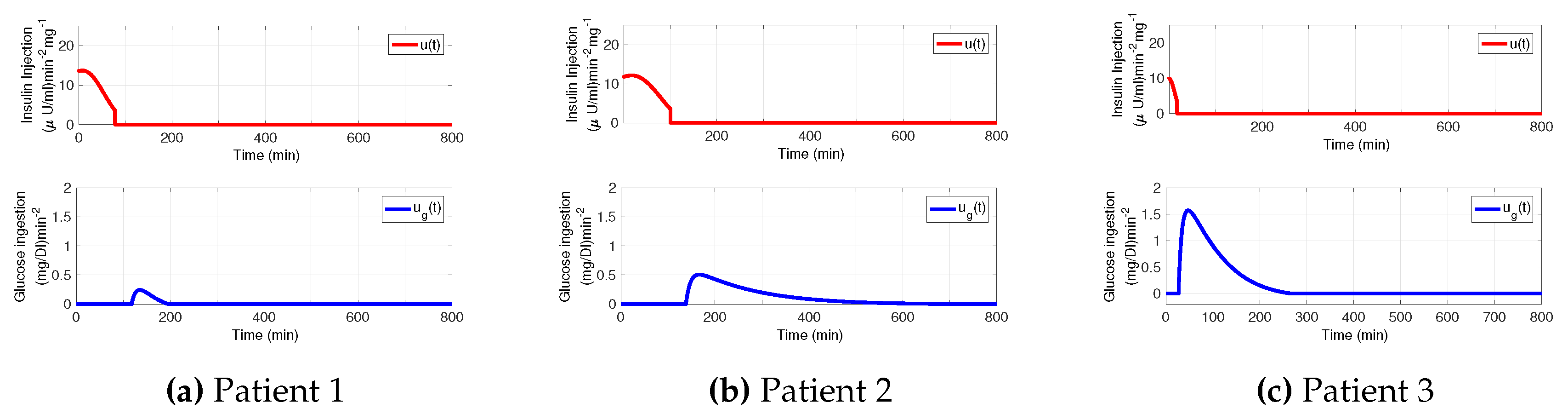
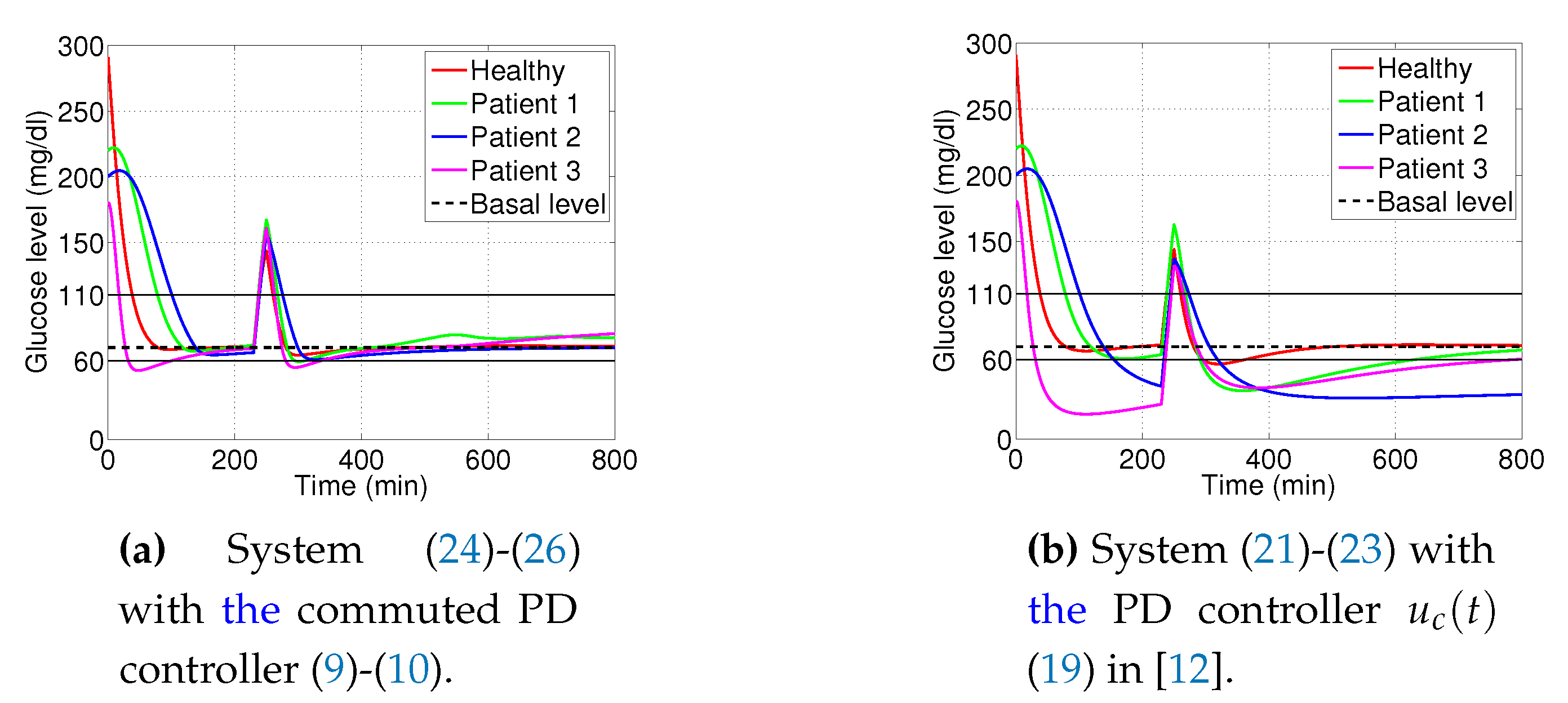
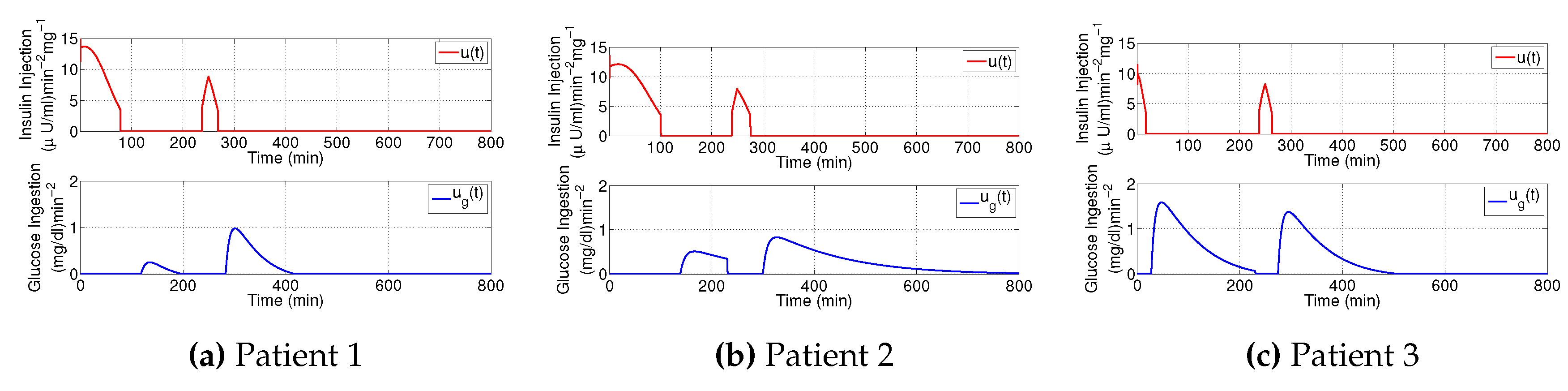
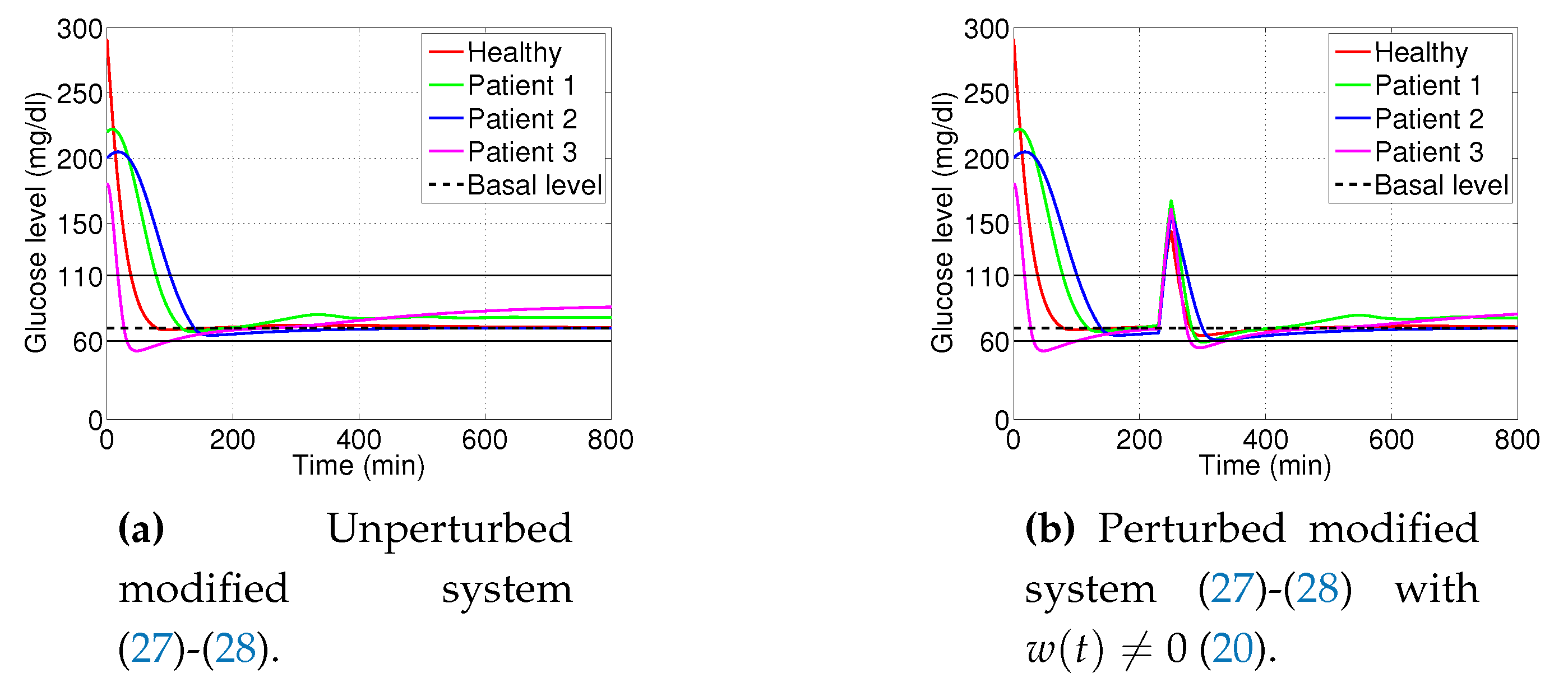
3.1. Decaying Exponential Disturbance Simulations
3.2. External Noise Perturbation
3.3. Changes on Glucose Assimilation
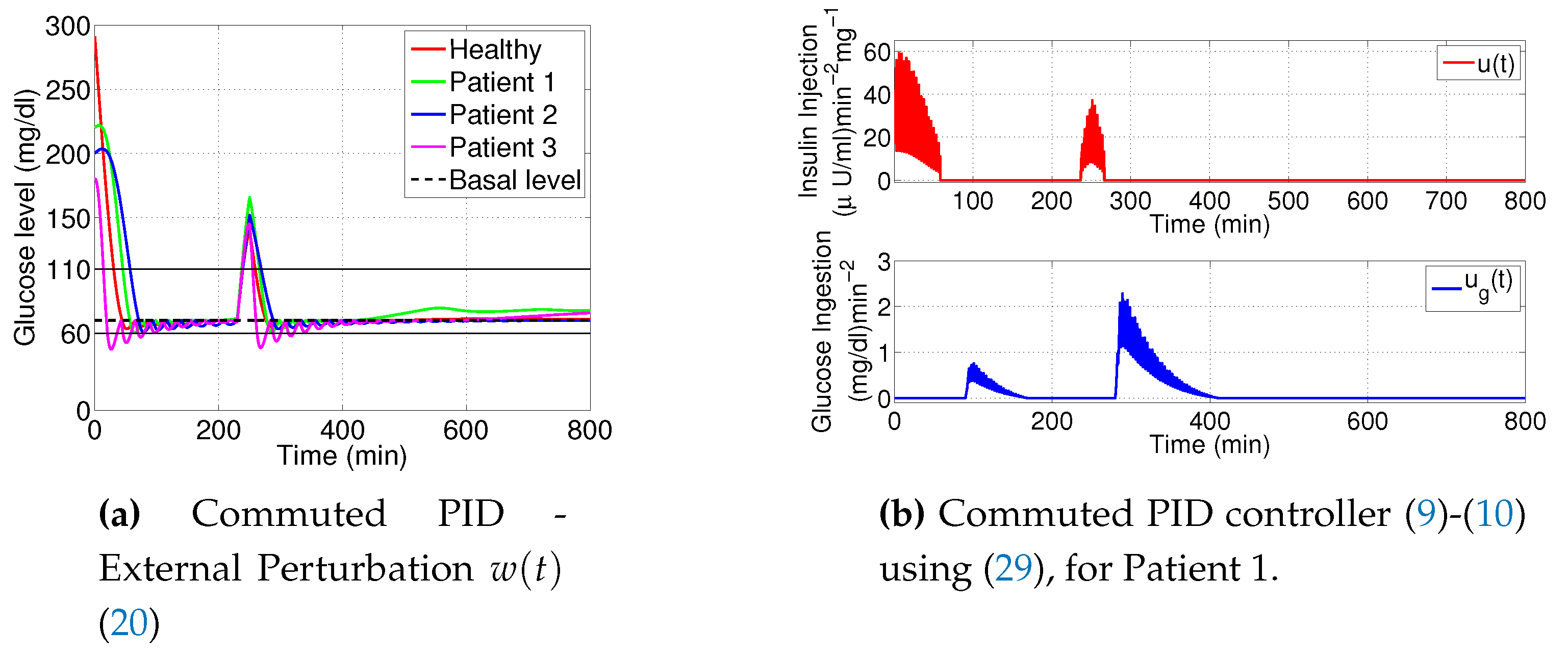
3.4. PID Using Reset Integrator
- Clegg integrator Input. The error between glucose blood concentration and the nominal value .
- Clegg integrator Initial condition. After each reset to the Clegg integrator, an initial condition is needed to integrate at each resetting action. We use the zero initial condition setting.
- Clegg integrator Resetting actions. The block can reset its state to the specified initial condition based on an external signal. We choose to reset the integrator when the sinus function changes its sign.
- Clegg integrator gain. The parameter was found by the trial and error method.
4. Discussion of Results
Appendix A
- (i)
- : ,
- (ii)
- : .
References
- M. Nwokolo, R. Hovorka, The Artificial Pancreas and Type 1 Diabetes, The Journal of Clinical Endocrinology and Metabolism (2023). [CrossRef]
- E. R. Carson, T. Deutsch, A Spectrum of approaches for controlling diabetes, IEEE Control System Magazine, 12(12) (1992) 25-31. [CrossRef]
- C. Cobelli, C. D. Man, G. Sparacino, L. Magni, G. De Nicolao, B. P. Kovatchev, Diabetes: Models, Signals, and Control, IEEE Rev. in Biomedical Eng., 2 (2009) 54-96. [CrossRef]
- P. Dua, F. J. Doyle-III, E. N. Pistikopoulos, Model-based glucose control for Type-1 diabetes via parametric programming, IEEE Trans. on Biomedical Eng., 53(8)(2006) 1478-1491. [CrossRef]
- F. Chee, A V. Savkin, T. L. Fernando, S. Nahavandi, Optimal H∞ insulin injection control for blood glucose regulation in diabetic patients, IEEE Trans. on Biomedical Eng., 52(10)(2005) 1625-1631. [CrossRef]
- P. Kaveh, Y. B. Shtessel, Blood glucose regulation using higher-order sliding mode control, Int. J. of Robust and Nonlinear Control, 18 (2008) 557-569. [CrossRef]
- P. Kaveh, Y. B. Shtessel, Blood Glucose Regulation in Diabetics Using Sliding Mode Control Techniques, Proc. of the 38th Southeastern Symposium on System Theory Tennessee Technological University Cookeville, TN, USA, (2006), 5-7. [CrossRef]
- B. S. Leon, A. Y. Alanis, E. N. Sanchez, F. Ornelas-Tellez, E. Ruiz-Velazquez, Inverse optimal neural control of blood glucose level for type 1 diabetes mellitus patients, J. of the Franklin Institute, 349 (2012) 1851-1870. [CrossRef]
- Anas El Fathi; Mohamed Raef Smaoui; Veronique Gingras; Benoit Boulet; Ahmad Haidar, The Artificial Pancreas and Meal Control: An Overview of Postprandial Glucose Regulation in Type 1 Diabetes, IEEE Control Systems, 38(1) ( 2018), 67 - 85. [CrossRef]
- A. Makroglou, J. Li, Y. Kuang, Mathematical models and software tools for the glucose-insulin regulatory system and diabetes: an overview, Applied Numerical Mathematics, 56 (2006) 559-573. [CrossRef]
- N. Hernjak, F. J. Doyle-III, Glucose control design using nonlinearity assessment techniques, AIChE Journal, 51(2) (2005) 544-554. [CrossRef]
- C. Li, R. Hu, Simulation study on blood glucose control in diabetics, Int. Conf. on Bioinformatics and Biomedical Engineering, (2007). [CrossRef]
- S. Amuthameena, A novel strategy for blood glucose control in human body using PID-Fuzzy logic controller, Journal of Chemical and Pharmaceutical Sciences, 8 (2016) 88-92.
- G. Marchetti, M. Barolo, L. Jovanovic, H. Zisser, D.E. Seborg, An improved PID switching control strategy for Type 1 Diabetes, IEEE Trans. on Biomedical Engineering, 55(3) (2008) 857-865. [CrossRef]
- J. D. Hoyos, F. Bolanos, M. Vallejo, and P. S. Rivadeneira, ”Population-based incremental learning algorithm for identification of blood glucose dynamics model for type-1 diabetic patients, in Proceedings of the International Conference on Artificial Intelligence,(2018), 29-35.
- Maria, F. Villa Tamayo, Michelle A. Caicedo Alvarez, and Pablo S. Rivadeneira, Handling Parameter Variations during the Treatment of Type 1 Diabetes Mellitus: In Silico Results, Mathematical Problems in Engineering, vol. 2019, Article ID 2640405, (2019), 1- 21. [CrossRef]
- Ndakara, A.I., Essabbar, M., Saikouk, H. Blood Glucose-Insulin Dynamics in Type-1 Diabetic Patients for the Mitigation of Hyperglycemic Conditions: A PID Controller with a Step Response. In: Motahhir, S., Bossoufi, B. (eds) Digital Technologies and Applications. ICDTA 2023. Lecture Notes in Networks and Systems, vol 668 (2023). Springer, Cham. [CrossRef]
- S. Syafiie, F. AlHarbi, A. Ali Alshehri, B. Hasanain, PID and LQG controllers for diabetes system with internal delay: a comparison study, Biomed. Phys. Eng. Express 9 (2023), 035031. [CrossRef]
- A. De Gaetano, O. Arino, Mathematical modelling of the intravenous glucose tolerance test, J. of Mathematical Biology, 40 (2000), 136-168. [CrossRef]
- M. E. Fisher, A semiclosed-loop algorithm for the control of blood glucose levels in diabetics, IEEE Trans. on Biomedical Eng., 38 (1) (1991), 57-61. [CrossRef]
- Patricio, H. Colmegna; Ricardo S. Sanchez-Pena; Ravi Gondhalekar; Eyal Dassau; Frank J. Doyle, Switched LPV Glucose Control in Type 1 Diabetes, IEEE Transactions on Biomedical Engineering 63(6) (2016), 1192 - 1200. [CrossRef]
- Y. Batmani, Blood glucose concentration control for type 1 diabetic patients: a non-linear suboptimal approach, IET Systems Biology, 11(4) (2017), 119 - 125. [CrossRef]
- M. Messori, G.P. Incremona, C. Cobelli, L. Magni, Individualised model predictive control for the artificial pancreas: In silico evaluation of closed-loop glucose control, IEEE Control Systems, 38(1) (2018), 86 - 104. [CrossRef]
- N. Ankit Sharma, H.P. Singh, Computer-controlled diabetes disease diagnosis technique based on fuzzy inference structure for insulin-dependent patients. Appl Intell 53 (2023), 1945?1958. [CrossRef]
- H.Emerson, M. Guy, R. McConville, Offline reinforcement learning for safer blood glucose control in people with type 1 diabetes, Journal of Biomedical Informatics (2023), 104376. [CrossRef]
- J.C. Clegg, A nonlinear integrator for servomechanism, Trans. A.I.E.E., 77(II) (1958) 41-42. [CrossRef]
- D. Nesic, L. D. Nesic, L. Zaccarian, A.R. Teel, Stability properties of reset systems, 16th Triennial World Congress, IFAC 2005, 67-72. [CrossRef]
- A. Banos, A. A. Banos, A. Barreiro, Reset control systems, (2011) Springer Sciences and Business Media. [CrossRef]
- P. Palumbo, S. Ditlevsen, A. Bertuzzi, A. De Gaetano, Mathematical modeling of glucose-insulin system: A review, Mathematical Bioscience, 244(2) (2013), 69-81. [CrossRef]
- K.H. Ang, G. K.H. Ang, G. Chong, Y. Li, PID control system analysis, design, and technology, IEEE Trans. on Control Systems Technology, 13(4), (2005) 559-576. [CrossRef]
- Filippov, AF. Differential Equations with Discontinuous Right-hand Sides. Kluwer Academic: Boston, 1988. [CrossRef]
- H. Frankowska, Filippov’s and Filippov-Wazewski’s theorems on closed domains, Journal of Differential Equations, 161 (2000) 449-478. [CrossRef]
- L. Dieci, L. L. Dieci, L. Lopez, Sliding motion in Filippov differential systems: Theoretical results and a computational approach, SIAM Journal on Numerical Analysis, 47(3) (2009), 2023-2051.
- F. León-Vargas, F. Garelli, H. De Battista, J. Vehí, Postprandial response improvement via safety layer in closed-loop blood glucose controllers, Biomedical Signal Processing and Control, 16 (2015), 80-87, 2015. [CrossRef]
- P. Rossetti, C. Quirós, V. Moscardó, A. Comas, M. Giménez, F. J. Ampudia-Blasco, F. León, E. Montaser, I. Conget, J. Bondia, J. Vehí, Closed-Loop Control of Postprandial Glycemia Using an Insulin-on-Board Limitation Through Continuous Action on Glucose Target, Diabetes Technology and Therapeutics, 19(6) (2017). [CrossRef]
- D. Boiroux, A. K. Duun-Henriksen, S. Schmidt, K. Norgaard, N. Kjolstad Poulsen, H. Madsen, J. Bagterp Jorgensen, Adaptive control in an artificial pancreas for people with type 1 diabetes, Control Engineering Practice 58 (2017) 332-342. [CrossRef]
| Healthy | Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|---|
| 0.0317 | 0 | 0 | 0 | |
| 0.0123 | 0.02 | 0.0072 | 0.0142 | |
| 4.92 | 5.3 | 2.16 | 9.94 | |
| 0.0039 | 0.005 | 0.0038 | 0.0 | |
| n | 0.2659 | 0.3 | 0.2465 | 0.2814 |
| 79.0353 | 78 | 77.5783 | 82.9370 | |
| 70 | 70 | 70 | 70 | |
| 7 | 7 | 7 | 7 | |
| 291.2 | 220 | 200 | 180 | |
| 0 | 0 | 0 | 0 | |
| 364.8 | 50 | 55 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





