1. Introduction
Better predictive biomarkers of immune checkpoint inhibitor (ICI) based treatment benefit are clinically needed. Tissue only biomarkers can be limited by tissue acquisition and sampling heterogeneity. Tissue is also limited to a one-time static assessment missing potential dynamic changes reflected in the evolving tumor biology of the cancer. A plasma-based predictive immune biomarker would extend immune biomarker testing to patients limited by tissue acquisition and could overcome tissue testing heterogeneity constraints. It would also be able to assess dynamic changes of an immune biomarker with treatment, recurrence, and upon progression guiding immune treatment decisions across the cancer treatment spectrum [
1].
Tissue programmed death-ligand 1 (PD-L1) protein expression, microsatellite instability-high (MSI-H), and tissue tumor mutational burden (TMB) are the recognized predictive immune biomarkers of ICI treatment benefit. MSI-H is a tumor agnostic biomarker and the most powerful predictive immune biomarker with 60-70% 4-year survivals with ICI treatment even in metastatic disease [
2,
3]. Plasma MSI-H testing with ctDNA has been correlated with tissue-based MSI testing and has been predictive of ICI benefit when the circulating tumor DNA (ctDNA) mutant allele fraction is > 1% [
4]. However, MSI-H is only rarely present in non-small cell lung cancer (NSCLC).
Tissue PD-L1 and TMB have not been as powerful predictive immune biomarkers as MSI-H and have not been shown to be predictive with plasma-based testing. Tissue TMB is also a tumor agnostic immune biomarker. However, in advanced or metastatic NSCLC, blood TMB (bTMB) failed to meet protocol primary endpoints of predicting improved ICI treatment benefit compared to chemotherapy in two phase 3 trials. The BFAST cohort C trial failed to meet the progression-free survival (PFS) endpoint in patients with bTMB ≥ 16 mut/Mb and in the NEPTUNE trial bTMB ≥ 20 mut/Mb failed to demonstrate a significantly better OS with ICI compared to chemotherapy alone [
5,
6]. The complexity of tumor burden impact on ctDNA shedding, ethnic differences, tumor-only panels over-estimation, as well as the need for harmonization and determination of precise predictive cut-offs, are all potential limiting factors of the clinical utility of bTMB [
7,
8,
9,
10,
11].
Prior plasma-based PD-L1 assays have also not consistently been predictive of ICI treatment benefit. What is being tested by the plasma PD-L1 assay makes a difference. Plasma PD-L1 protein testing by enzyme-linked immunosorbent assays has not been predictive. Elevated levels of soluble protein PD-L1 were associated with poorer survival with ICI treatment in NSCLC and a meta-analysis of eight studies with over 1,000 patients across a variety of solid tumors [
12,
13]. Secreted PD-L1 proteins have also been shown to contain decoy PD-L1 variants as a mediator of ICI treatment resistance [
14]. Circulating tumor cell (CTC) based PD-L1 assays have shown an overall poor correlation with tissue PD-L1 expression and have also not been associated with a predictive ICI treatment benefit [
15,
16].
PD-L1 gene amplification and mRNA expression are other potential PD-L1 testing options. Both have been associated with ICI treatment benefit. PD-L1 (CD274) copy number gains have been associated with significantly improved ICI treatment response rates of 67%-80% and potential durable OS benefit. However, the very low frequency of PD-L1 gene amplification of only 0.7-2.6% greatly underestimates the potential benefit of ICI treatment limiting its clinical utility [
17,
18]. PD-L1 polysomy is more frequent but was not predictive of ICI treatment benefit compared to patients without PD-L1 polysomy copy number gain [
18]. Tissue PD-L1 mRNA is far more frequent than PD-L1 gene amplification with 43-50% rates of expression [
19,
20]. Conroy et al concluded PD-L1 mRNA expression is comparable to PD-L1 protein expression by immunohistochemistry (IHC) both analytically and clinically in predicting ICI response rates in NSCLC [
21]. Fernandez et al also reported a statistically significantly improved OS and long-term ICI benefit in chemo-immune treated metastatic NSCLC patients with high mRNA PD-L1 expression compared to low expression. Conversely, low PD-L1 mRNA expression had a high negative predictive value for absence of long-term ICI treatment benefit [
22].
Studies however have shown significant discordance between tissue mRNA PD-L1 and PD-L1 protein. There are a striking number of patients without identifiable PD-L1 protein expression yet mRNA PD-L1 expression by PCR can be demonstrated. Venina et al compared tissue PD-L1 RNA by PCR in comparison to the Dako 22C3, Ventana SP263, and Ventana SP142 antibodies IHC staining in 167 NSCLC patients. When each antibody tumor proportion score (TPS) was < 1%, PD-L1 mRNA still demonstrated expression in 50-56% of patients. At the other end of the spectrum with TPS ≥ 50%, higher PD-L1 mRNA expression was demonstrated in 69-87% of patients [
23]. In another similar comparison to PD-L1 protein assays, Tsimafeyeu et al found PD-L1 mRNA expression in 43% of 473 biobank tissue samples of NSCLC. Among those patients only half tested positive by these same three IHC antibodies with the authors concluding PD-L1 mRNA expression has potential clinical utility in identifying PD-L1 when PD-L1 IHC protein is negative. [
20]. In both studies, lack of PD-L1 mRNA expression demonstrated a high negative predictive value of 92-99% with lack of PD-L1 protein expression. Tissue sampling heterogeneity missing PD-L1 protein IHC positive cells is the assumed explanation [
24]. This suggests the utility of mRNA PD-L1 expression as a potential better and certainly complementary predictive immune biomarker with PD-L1 protein. However, what potential advantages of tissue mRNA PD-L1, the limitations of any tissue testing remain.
Extracellular vesicle (EV) PD-L1 expression has been an effective blood-based immune biomarker. EV PD-L1 research assays have demonstrated that dynamic changes in EV PD-L1 were predictive of ICI treatment durability. Decreasing PD-L1 mRNA expression by droplet digital PCR in plasma-derived exosomes was associated with an ICI response whereas an increase was seen in non-responders [
25]. Another study identified the same response dynamics of EV PD-L1 expression and ICI treatment survival benefit but not in chemotherapy treated patients [
26]. This emphasizes the potential utility of a plasma-based PD-L1 assay in assessing ICI treatment response, however neither EV PD-L1 assay was evaluated as a pre-treatment predictor of ICI benefit.
Plasma cell free mRNA (cfRNA) PD-L1 testing by real-time polymerase chain reaction (RT-PCR) assays have also been shown to be associated with ICI response. Ishiba et al report cfRNA PD-L1 by RT-PCR associated with ICI response in twelve patients [
27]. Raez et al reported both pre-treatment and dynamic changes of decreasing plasma cfRNA PD-L1 by RT-PCR were associated with ICI treatment response rate in 52 NSCLC patients [
28]. However, OS outcomes were not reported nor were outcomes of ICI treated patients comparatively assessed with patients lacking plasma cfRNA PD-L1 expression. A previous retrospective real-word patient experience demonstrated plasma cfRNA PD-L1 by RT-PCR was associated with a statistically significant and clinically meaningful improved OS outcomes with ICI-based treatment compared to positive plasma cfRNA PD-L1 patients treated with chemotherapy alone in advanced NSCLC [
29].
Our aim in this retrospective observational cohort study was to further evaluate the association of plasma cfRNA PD-L1 expression by RT-PCR and first-line ICI-based OS clinical outcomes in metastatic NSCLC. We report the comparative median and a landmark 3-year OS of patient cohorts with positive plasma cfRNA PD-L1 expression compared to positive tissue PD-L1 protein expression and to patients lacking PD-L1 expression. Median and 3-year OS outcomes were identical whether plasma cfRNA PD-L1 was positive or tissue PD-L1 protein was positive. Patients with positive plasma cfRNA PD-L1 expression demonstrated longer median and higher 3-year OS outcomes compared to patients lacking any plasma or tissue PD-L1 expression.
2. Patients and Methods
This is a single-institution, retrospective observational study performed at the Brody School of Medicine at East Carolina University (Greenville, NC, USA) with patients treated at the Vidant Medical Center (now ECU Health Medical Center). Patients with pathologically confirmed metastatic NSCLC treated with first-line ICI-based treatment and with available plasma cfRNA PD-L1 and tissue PD-L1 protein results with the Dako 22C3 monoclonal antibody were identified through the institutional thoracic oncology program database from November 2018 through July 2019 allowing sufficient follow-up to assess a landmark 3-year OS. Patients with plasma and/or tissue PD-L1 status unknown, stage I/II/III NSCLC, stage unknown, or with the presence of a targetable oncogenic driver mutation/fusion were excluded. There were no other clinical or laboratory exclusion criteria. No patients received definitive concurrent chemoradiation therapy or thoracic radiation therapy (RT). Palliative RT with either whole brain RT or Gamma Knife radiosurgery, or palliative stereotactic body RT were undertaken as indicated upon the recommendation of the treating oncologist. Patients were treated based upon the current available standard of care during that time period with the local treating oncologist making the final treatment decision. The study was approved by the Brody School of Medicine at East Carolina University Institutional Review Board.
53 metastatic NSCLC patients treated with first-line ICI-based treatment fulfilled the inclusion criteria with PD-L1 results available and were included in the three patient cohorts. Patients were comparatively assessed within three cohorts: (i) Plasma cfRNA PD-L1 positive patients; (ii) tissue PD-L1 protein positive patients; (iii) PD-L1 negative patients by both plasma cfRNA and tissue protein.
The ‘plasma PD-L1 positive’ cohort consisted of sixteen patients with metastatic NSCLC who demonstrated plasma cfRNA PD-L1 expression and were treated with first-line ICI-based therapies. Thirteen patients received combination anti-PD-1/L1 ICI plus chemotherapy regimens and three patients anti-PD-1/-L1 ICI alone. No patients received anti-CTLA-4 agents. Tissue PD-L1 TPS was reported as ≥ 50% in six patients and ≥ 1% in four patients. Six of the total IO cohort of sixteen patients (37%) were either tissue PD-L1 negative or unknown due to tissue quantity not sufficient (QNS) for testing. The ‘tissue PD-L1 positive’ cohort consisted of sixteen contemporaneously identified metastatic NSCLC patients receiving first-line ICI treatment who were tissue PD-L1 protein positive. Eleven patients received combination anti-PD-1/L1 plus chemotherapy. Five received anti-PD-1/L1 alone. No patients received anti-CTLA-4 agents. PD-L1 TPS was 1-49% in six and ≥ 50% in ten, with five of those ten ≥ 90%. All of these patients were plasma PD-L1 negative. The ‘PD-L1 negative’ cohort consisted of 21 contemporaneously identified metastatic NSCLC patients receiving first-line ICI treatment who were PD-L1 negative by both plasma cfRNA and tissue protein. All patients received anti-PD-1/L1 plus chemotherapy regimens. No patients received anti-CTLA-4 agents.
Table 1 summarizes the three patient cohorts.
Plasma for testing was collected at a single point in time before any treatment. Blood was collected in a single 10-ml EDTA tube. The cfRNA PD-L1 expression testing was performed at the Circulogene CLIA/CAP accredited laboratory (Birmingham, AL – Pensacola, FL, USA). Circulogene is a commercial liquid biopsy vendor with a proprietary patented pre-analytical linear-in-situ-amplification technology. The cfRNA PD-L1 Gene Expression assay is an exosome-free real-time PCR assay using beta-actin as a reference gene. The demonstrated limit of detection for cfRNA PD-L1 was 1.0 copy/uL [
30].
OS was assessed from the date of diagnosis and either death or censored follow-up with a data cut-off of March 9, 2022. Median follow-up was 34 months. OS analysis was performed by AnalystSoft StatPlus Kaplan-Meier and hazard ratio (HR) survival analysis. The pre-specified endpoint was median and 3-year OS.
3. Results
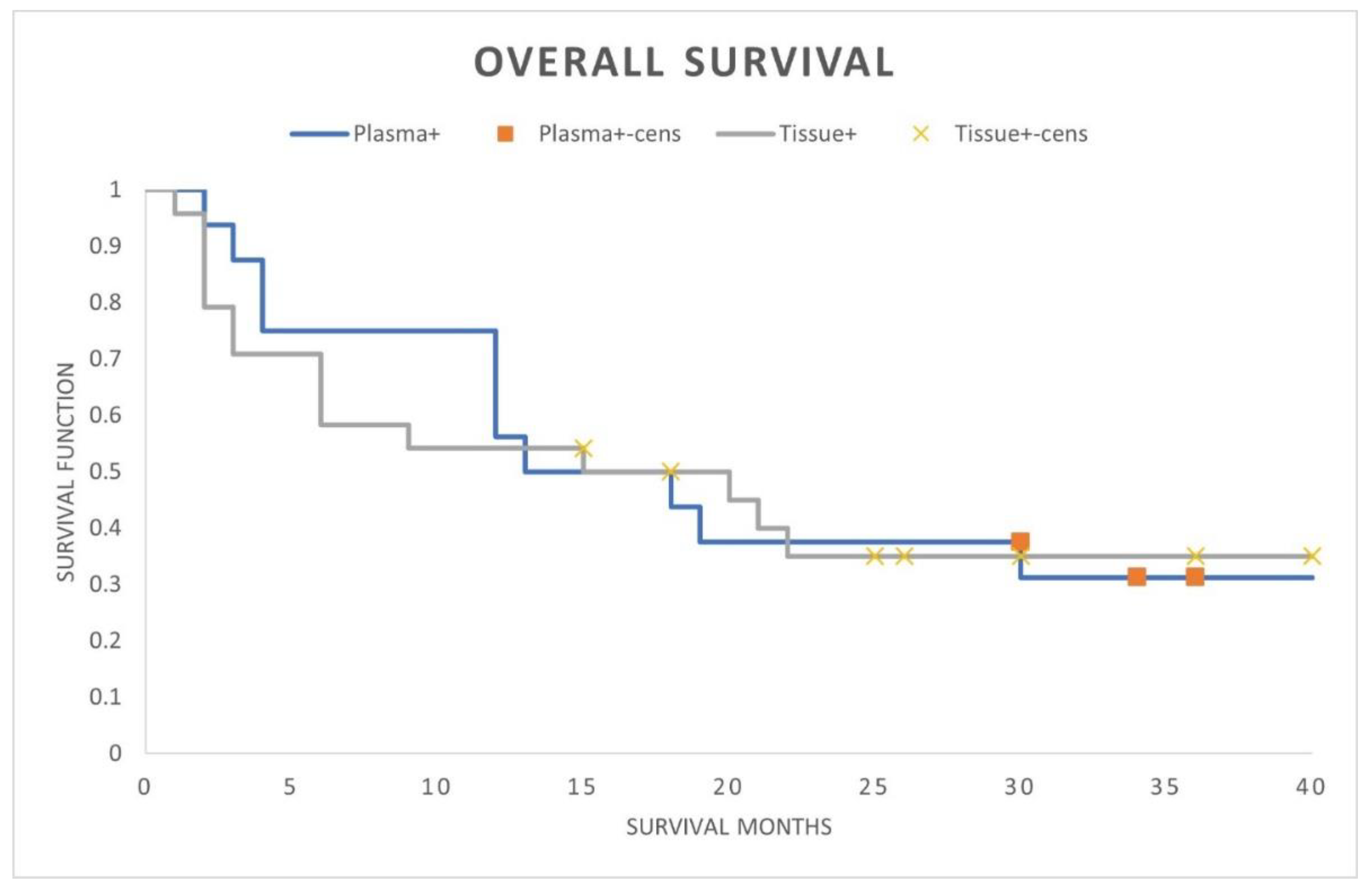
A comparison of the plasma cfRNA PD-L1 positive with tissue PD-L1 protein positive patients demonstrated identical OS outcomes (median OS 15 months; landmark 3-year OS 30%; HR 0.97; 95% CI, 0.44-2.10; p-value = 0.93) (
Figure 1). Within the plasma PD-L1 positive cohort there were no differing OS outcomes whether tissue PD-L1 positive, negative, or unknown (median OS 13-16 months; 3-year landmark OS 30%; HR 1.15; 95% CI, 0.32-4.05).
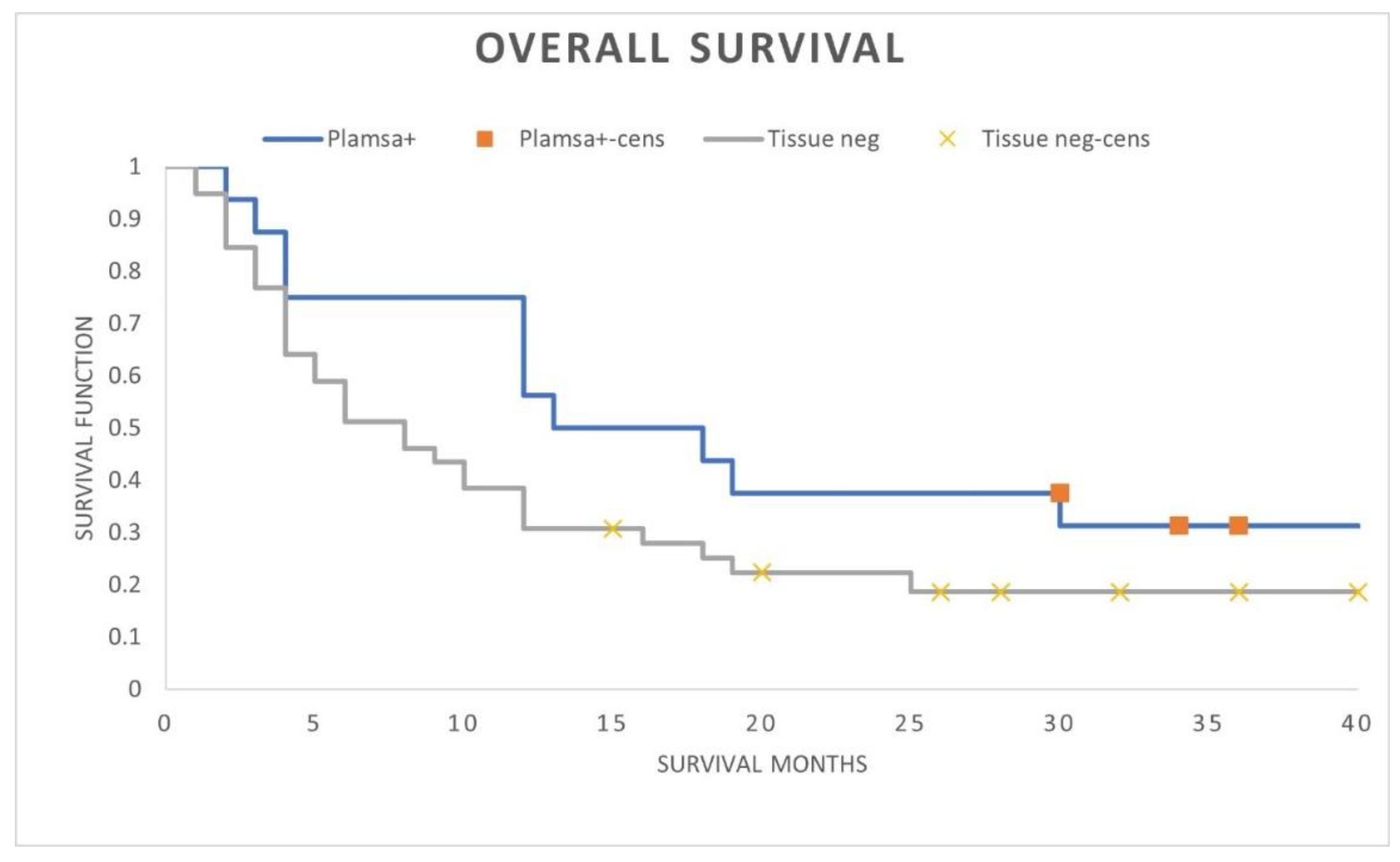
A comparison of positive plasma PD-L1 patients with patients lacking PD-L1 expression demonstrated numerically superior ICI treated OS outcomes when plasma cfRNA PD-L1 was expressed (median OS 15 months versus 8 months; landmark 3-year OS 30% versus 15%; HR 0.56; 95% CI, 0.27-1.17; p-value = 0.11) (
Figure 2). Although not stastically significant, the OS curves separated early with retained OS separation throughout the follow-up period suggesting the modest patient numbers in each cohort limiting statistical significance.
4. Discussion
Improving predictive immune biomarker assays is clinically important to better predict ICI treatment benefit and extend that benefit to more patients. A plasma-based PD-L1 assay would not be limited by tissue acquisition or sampling heterogeneity. It would also allow dynamic monitoring of ICI response and easily reassess PD-L1 expression upon cancer recurrence and/or progression. The International Association for the Study of Lung Cancer (IASLC) supports complementary tissue and plasma molecular testing for a full molecular assessment of driver mutations and fusions. The IASLC consensus statement went further advocating a ‘plasma first’ approach for this molecular testing [
31]. A similar complementary testing paradigm of PD-L1 testing could be undertaken with a predictive plasma PD-L1 assay.
A previous real-world patient experience in advanced NSCLC demonstrated a significantly improved median and landmark 3-year OS with ICI-based treatment compared to chemotherapy alone in patients with positive plasma cfRNA PD-L1 expression [
29]. Those clinical outcomes mirror the same predictive ICI treatment benefit with tissue PD-L1 compared to chemotherapy in seminal ICI clinical trials [
32,
33]. This expanded patient cohort experience of first-line ICI treated metastatic NSCLC patients further demonstrates that plasma cfRNA PD-L1 expression by PCR is associated with favorable ICI treatment outcomes. The OS was identical whether patients were positive with plasma cfRNA PD-L1 or tissue PD-L1 protein. Both plasma and tissue PD-L1 assays were associated with a 30% landmark 3-year OS. When positive plasma cfRNA PD-L1 expression was present, the OS benefit of ICI treatment was the same, whether tissue PD-L1 protein was positive, negative, or tissue QNS for testing. Positive plasma cfRNA PD-L1 patients also achieved an early and sustained separation of OS benefit resulting in a numerically improved median and landmark 3-year OS survival benefit with ICI treatment compared to patients who lacked PD-L1 expression by both plasma cfRNA and tissue protein assays.
All three patient cohorts represented a comparative real-world patient experience with consistent PD-L1 testing and ICI treatment at a single institution and reflected a true landmark 3-year OS with the prolonged median follow-up. There is now an evolving understanding that imaging-based response rates and progression free survival (PFS) are not consistent surrogates of true ICI treatment OS benefit. A pooled analysis of first-line ICI randomized trials failed to show a strong correlation between PFS or response rates with OS. Mature OS data is the gold standard endpoint for first-line ICI trials [
34,
35]. Given this, it was felt that only ICI treatment OS outcomes would reflect the potential predictive immune biomarker benefit of plasma cfRNA PD-L1 expression.
Predictive immune biomarkers remain important to identify patients who will benefit from ICI-based treatment [
36]. These biomarkers need to continue to evolve and improve. A plasma PD-L1 assay would be a potential step in that improvement. Complementary tissue and plasma testing of genomic tumor biology testing is supported by multiple studies in NSCLC, as tissue testing only approaches will miss a significant number of alterations [
37,
38,
39]. That appears to also be true for PD-L1. Tissue PD-L1 was either negative or unknown when plasma PD-L1 was positive in 37% of this patient population. Tissue PD-L1 mRNA has been reported to be expressed in up to half of patients even when tissue PD-L1 protein TPS is negative. With tissue PD-L1 protein testing alone, a significant number of patients with PD-L1 upregulation could be missed. This real-world data provides a framework of the potential clinical utility of plasma cfRNA PD-L1 expression. Complementary tissue and plasma PD-L1 testing could have clinical impact, especially when tissue PD-L1 protein is negative or QNS for testing. Just as IASLC advocates for molecular testing, it would also allow a ‘plasma first’ approach for PD-L1 testing. Plasma PD-L1 would also be integral in the development of a potential liquid biopsy composite predictive immune biomarker assay including ctDNA/RNA ICI sensitive and resistant alterations, other immune checkpoints, immune cellular levels, as well as host inflammatory markers [
40].
There are well acknowledged limitations of this reported patient experience. It is retrospective observational outcomes data treated from a single institution and not a prospective multi-institutional randomized comparison. A biomarker comparison of the plasma cfRNA PD-L1 by Ct-value PCR expression correlation with tissue PD-L1 protein TPS by IHC or ICI treatment outcomes was not able to be undertaken. To further assess any immune tumor biology and ICI treatment benefit differences of plasma PD-L1 RNA and tissue PD-L1 protein levels of expression and insight into the full clinical utility of plasma PD-L1 testing, further research is needed and planned with a prospective clinical trial. Even with these limitations and the modest patient sample size, to our knowledge it does represent the largest patient experiences of ICI treated OS outcomes of patients with positive cfRNA PD-L1 expression compared to positive tissue PD-L1 protein expression and to patients lacking any identifiable PD-L1 expression.
5. Conclusions
In a real-world population of symptomatic metastatic NSCLC patients, positive plasma cfRNA PD-L1 expression was associated with favorable outcome findings with first-line ICI treatment. Either tissue PD-L1 protein expression by IHC or plasma cfRNA PD-L1 expression by RT-PCR was associated with an identical OS ICI treatment benefit. Patients with positive plasma cfRNA PD-L1 expression were also associated with better ICI treated OS compared to patients with a lack of cfRNA PD-L1 expression. This data lends support for needed further and expanded study of the potential clinical utility and benefit of plasma cfRNA PD-L1 as a predictive immune biomarker.
Author Contributions
Conceptualization, P.W.; methodology, P.W, S.J, M.P. and M.M.; software, P.W.; validation, P.W., S.J., M.P., and M.M.; formal analysis, P.W., S.J., and M.P.; investigation, P.W., S.J., and M.P.; resources, P.W. and M.M.; writing-original draft, P.W.; writing-review and editing, P.W., S.J., M.P., and M.M.; visualization, P.W., S.J., M.P., and M.M.; supervision, P.W. and M.M.; project administration, P.W. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Brody School of Medicine (UMCIRB 20-002795-12 March, 2021; UMCIRB 21-000046-30 July, 2021).
Informed Consent
Patient consent for this de-identified retrospective analysis was waived by the Brody School of Medicine Institutional Review Board. However, individual specific cancer treatment consent was obtained in all patients.
Data Availability
Data are available in anonymized form upon reasonable request and Brody School of Medicine IRB review.
Acknowledgments
The authors would like to thank Teresa Parent, Thoracic Program Nurse navigator, and Cindy Cherry, Thoracic Program Nurse Practitioner, for their patient care and administrative support. Circulogene provided the plasma cfRNA PD-L1 testing at no charge to patients.
Conflicts of Interest
PW is Emeritus faculty at the Brody School of Medicine at East Carolina University and a current employee of Circulogene. The other authors have no conflicts of interest to declare.
References
- Sivapalan L: Murray J, Canzoniero J, et al. Liquid biopsy approaches to capture tumor evolution and clinical outcomes during cancer immunotherapy. J. Immunother. Cancer 2023,11, e005924. [CrossRef]
- Andre T, Lonardi S, Wong K, et al. Nivolumab plus low-dose ipilimumab in previously treated patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: 4-year follow-up from CheckMate 142. Ann. Oncol. 2022, 33(10), 1052-1060. [CrossRef]
- Chao J, Fuchs C, Shitara K, et al. Assessment of Pembrolizumab Therapy for the Treatment of Microsatellite Instability-High Gastric or Gastroesophageal Junction Cancer Among Patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 Trials. JAMA Oncol. 2021, 7(6), 895-902. [CrossRef]
- Nakamura Y, Okamoto W, Denda T, et al. Clinical Validity of Plasma-Based Genotyping for Microsatellite Instability in Advanced GI Cancers: SCRUM-Japan and GOZILA Substudy. JCO Precis. Oncol. 2022, 6, e2100383. [CrossRef]
- Peters S, Dziadziuszko R, Morabito A, et al. Atezolizumab versus chemotherapy in advanced or metastatic NSCLC with high blood-based tumor mutational burden: primary analysis of BFAST cohort C randomized phase 3 trial. Nat. Med. 2022, 28, 1831-1839. [CrossRef]
- de Castro G, Rizvi N, Schmid P, et al. NEPTUNE: Phase 3 Study of First-Line Durvalumab Plus Tremelimumab in Patients With Metastatic NSCLC. J. Thorac. Oncol. 2022, 18(1), 106-119. [CrossRef]
- Fridland S, Choi J, Nam M, et al. Assessing tumor heterogeneity: integrating tissue and circulating tumor DNA (ctDNA) analysis in the era of immune-oncology-blood TMB is not the same as tissue TMB. J. Immunother. Cancer 2021, 9, e002551. [CrossRef]
- Nassar A, Adib E, Alaiwi S, et al. Ancestry-driven recalibration of tumor mutational burden and disparate clinical outcomes in response to immune checkpoint inhibitors. Cancer Cell 2022, 40, 1161-1172. [CrossRef]
- Brawley O, Luhn P, Reese-White D, et al. Disparities in Tumor Mutational Burden, Immunotherapy Use, and Outcomes Based on Genomic Ancestry in Non-Small-Cell Lung Cancer. JCO Glob. Oncol. 2021, 7, 1537-1546. [CrossRef]
- Kasi P. Liquid biopsies and tumor mutational burden: the cutoff conundrum. Nat. Med. 2022, 28, 1753-1754. [CrossRef]
- Sturgill E, Misch A, Jones C, et al. Discordance in Tumor Mutation Burden from Blood and Tissue Affects Association with Response to Immune Checkpoint Inhibition in Real-World Settings. Oncologist 2022, 27, 175-182. [CrossRef]
- Okuma Y, Wako H, Utsumi H, et al. Soluble Programmed Cell Death Ligand 1 as a Novel Biomarker for Nivolumab Therapy for Non-Small-cell Lung Cancer. Clin. Lung Cancer 2018, 19(5), 410-4. [CrossRef]
- Wei W, Xu B, Wang Y, et al. Prognostic significance of circulating soluble programmed death ligand-1 in patients with solid tumors. Med. 2018, 97, 3(e9617). [CrossRef]
- Gong B, Kiyo Tani K, Sakata S, et al. Secreted PD-L1 variants mediate resistance to PD-L1 blockade therapy in non-small cell lung cancer. J. Exp. Med. 2019, 216(4), 982-1000. [CrossRef]
- Guibert N, Delaunay M, Lacquer A, et al. PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab. Lung Cancer 2018, 120, 108-112. [CrossRef]
- Moran J, Adams D, Edelman M, et al. Monitoring PD-L1 Expression on Circulating Tumor-Associated Cells in Recurrent Metastatic Non-Small-Cell Lung Carcinoma Predicts Response to Immunotherapy with radiation Therapy. JCO Precis. Oncol. 2022, 6, e2200457. [CrossRef]
- Goodman A, Piccioni D, Kato S, et al. Prevalence of PDL1 Amplification and Preliminary Response to Immune Checkpoint Blockade in Solid Tumors. JAMA Oncol. 2018, 4(9), 1237-1244. [CrossRef]
- Inoue Y, Yoshimura K, Nishimoto K, et al. Evaluation of Programmed Death Ligand 1 (PD-L1) Gene Amplification and Response to Nivolumab Monotherapy in Non-small Cell Lung Cancer. JAMA Netw Open 2020, 3(9), e2011818. [CrossRef]
- Coppock J, Volaric A, Mills A, et al. Concordance levels of PD-L1 expression by immunohistochemistry, mRNA in situ hybridization, and outcome in lung carcinomas. Hum. Pathol. 2018, 82, 282-288. [CrossRef]
- Tsimafeyeu I, Imyanitov E, Zavalishina L, et al. Agreement between PDL1 immunohistochemistry assays and polymerase chain reaction in non-small cell lung cancer: CLOVER comparison study. Sci. Rep. 2020, 10, 3928. [CrossRef]
- Conroy J, Pable S, Nesline M, et al. Next generation sequencing of PD-L1 for predicting response to immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 18. [CrossRef]
- Fernandez A, Gavrielatou N, McCann L, et al. Programmed Death-Ligand 1 and Programmed Death-Ligand 2 mRNAs Measured Using Closed-System Quantitative Real-Time Polymerase Chain Reaction Are Associated With Outcome and High Negative Predictive value In Immunotherapy-Treated NSCLC. J. Thorac. Oncol. 2022, 17(9), 1078-1085. [CrossRef]
- Venina A, Ivantsov A, Iyevleva A, et al. PCR-based analysis of PD-L1 RNA expression in lung cancer: comparison with commonly used immunohistochemical assays. Ann. Diag. Path. 2022, 59, 151968. [CrossRef]
- Hwang D, Albaqar T, Santiago R, et al. Prevalence and Heterogeneity of PD-L1 Expression by 22C3 Assay in Routine Population-Based and Reflexive Clinical Testing in Lung Cancer. J. Thorac. Oncol. 2021, 16(9), 1490-1500. [CrossRef]
- Del Re M, Marconi R, Pasquini G, et al. PD-L1 mRNA expression in plasma-derived exosomes is associated with response to anti-PD-1 antibodies in melanoma and NSCLC. Br. J. Cancer 2018, 118, 820-824. [CrossRef]
- Miguel-Perez D, Russo A, Arrieta O, et al. Extracellular vesicle PD-L1 dynamics predict durable response to immune-checkpoint inhibitors and survival in patients with non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2022, 41, 186. [CrossRef]
- Ishiba T, Hoffmann A, Usher J, et al. Frequencies and expression levels of programmed death ligand 1 (PD-L1) in circulating tumor RNA (ctRNA) in various cancer types. Biochem. Biophys. Res. Commun. 2018, 500, 621-625. [CrossRef]
- Raez L, Dannenberg K, Sumarriva D, et al. Using cfRNA as a tool to evaluate clinical treatment outcomes in patients with metastatic lung cancers and other tumors. Cancer Drug Resist. 2021, 4, 1061-1071. [CrossRef]
- Jayananda S, Muzaffar M, Namireddy P, et al. Plasma Cell-Free RNA PD-L1 Expression and Clinical Outcomes With Immunotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112(2), e9. [CrossRef]
- Yeh C. Enabling circulating cell-free mRNA theranostics from PD-L1, ALK, ROS1, NTRK to transcriptomic profiling. J Clin. Oncol. 2022, 40, 16_suppl,3033-3033. [CrossRef]
- Rolfo C, Mack P, Scagliotti G, et al. Liquid Biopsy for Advanced NSCLC: A Consensus Statement From the International Association for the Study of Lung Cancer. J. Thorac. Oncol. 2021, 16(10), 1647-1662. [CrossRef]
- Mok T, Wu L, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042) a randomized, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819-1830. [CrossRef]
- Herbst R, Giaccone G, de Marinis F, et al. Atezolizumab for First-Line Treatment of PD-L1 Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328-1339. [CrossRef]
- Kok P-S, Yoon W-H, Lord S, et al. Tumor Response End Points as Surrogates for Overall Survival in Immune Checkpoint Inhibitor Trials: A Systemic Review and Meta-Analysis. JCO Precis. Oncol. 2021, 5, 1151-1159. [CrossRef]
- Merino M, Kasamon Y, Theoret M, et al. Irreconcilable Differences: The Divorce Between Response Rates, Progression-Free Survival, and Overall Survival. J. Clin. Oncol. 2023, 41(15), 2706-2712. [CrossRef]
- Fountzilas E, Hiep H, Mueller P, et al. Correlation Between Biomarkers and Treatment Outcomes in Diverse Cancers: A Systematic Review and Meta-Analysis of Phase I and II Immunotherapy Clinical Trials. Eur. J. Cancer 2023, online 22 May 2023. [CrossRef]
- Aggarwal C, Thompson J, Black T, et al. Clinical Implications of Plasma-Based Genotyping With the Delivery of Personalized Therapy in Metastatic Non-Small Cell Lung Cancer. JAMA Oncol. 2019, 5(2), 173-180. [CrossRef]
- Leighl N, Page R, Raymond V, et al. Clinical Utility of Comprehensive Cell-free DNA Analysis to Identify Genomic Biomarkers in Patients with Newly Diagnosed Metastatic non-small Cell Lung Cancer. Clin. Cancer Res. 2019, 25(15), 4691-4700. [CrossRef]
- Palmero R, Taus A, Viteri S, et al. Biomarker Discovery and Outcomes for Comprehensive Cell-Free Circulating Tumor DNA Versus Standard-of-Care Tissue Testing in advanced Non-Small-Cell Lung Cancer. JCO Precis. Oncol. 2021, 5, 93-102. [CrossRef]
- Fountzilas E, Kurzrock R, Hiep H, et al. Wedding of Molecular Alterations and Immune Checkpoint Blockade: Genomics as a Matchmaker. J. Natl. Cancer Inst. 2021, 113(12), djabo67. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).