1. Introduction
During the course of heart failure (HF) the left atrium (LA) undergo profound structural and electrophysiological changes ultimately leading to LA dysfunction and dilation [
1]. These changes occurr in HF patients with both reduced ejection fraction (HrEF) as well as in those with preserved EF (HFpEF) and define the so called atrial cardiomyopathy [
2,
3,
4,
5,
6,
7]. LA dysfunction is part of the pathophysiological processes that characterize HF and it contributes to the onset of symptoms in these patients [
8,
9]. LA function can be assessed through two-dimension speckle-tracking echocardiography that study the deformation of atrial walls during the cardiac cycle. This technique permits to distinguish three atrial phases: reservoir strain, a phase of LA expansion that occurs during left ventricular (LV) systole; conduit and contraction strain, corresponding respectively to the early and late phases of LV diastole [
10]. Under physiological conditions, LA function increases during exercise contributing to the enhancement of LV performance by Frank-Starling law and ultimately to the increase of cardiac output (CO) [
11]. However the relative LA contribution to CO decreases at high level of physical efforts when further increases of CO rely mostly on heart rate (HR) that increases linearly with exercise loads. According to the limited currently available literature, in patients with HFrEF and HFpEF the capacity to enhance LA function in response to increasing external loads is severely impaired [
12,
13] and the compromission of LA function appears to be a major factor limiting exercise tolerance in these patients [
14,
15,
16]. Since 2016 a third category of HF has been introduced by European guidelines and it includes patients with HF with midly reduced EF (HFmrEF) [
17]. This category of HF has been largely unexplored compared with HFrEF and HFpEF. In particular, data on LA function in HFmrEF are scant and inconclusive. A reduced LA reservoir strain has been described in HFmrEF patients and it was greater then that observed in HFpEF [
18]. It has been also suggested that a reduced LA reservoir strain could be a marker of decreased peak exercise capacity also in HFmrEF [
19]. Recently, our group showed that in,HFmrEF LA function, despite being reduce compared to normal values, properly increases in response to eccentric isometric resistance exercises. Moreover there are no studies evaluating the LA response to a progressive exercise in HFmrEF [
20]. The purpose of the present study was to assess the LA response to an incremental symptom-limited exercise, performed on a cycle-ergometer, in patients with post-ischemic HFmrEF in comparison to of healthy subjects.
2. Materials and Methods
Population. The study enrolled twenty stable patients with HFmrEF, secondary to CHD, and ten healthy controls (CT). Patients with HFmrEF were recruited during outpatients visits at our rehabilitation facility of San Raffaele IRCCS of Rome. These visits were made before starting a cardiac rehabilitation program and patients were sent to us by their cardiologist or primary care physicians. During these visits each patient underwent anthropometric assessment, HR and arterial blood pressure (BP) measurement at rest, an echocardiography and a symptom-limited ergometric test. The following inclusion criteria were adopted: age between 35 and 75 years; previous diagnosis of coronary heart disease; LV ejection fraction between 40 and 49%; NYHA class I. LA volume index lower than 34 mL/m2; stable synus rhythm; stable clinical conditions (having no hospitalizations in the last six months; having no made changes on drugs therapy in the last three months). The following exclusion criteria were adopted: high blood pressure at rest (after two repeated measurements); arrhythmyas at rest; significant heart valve diseases; hypertrophic obstructive cardiomyopathy; uncontrolled arrhythmia; neurological and or orthopedic conditions contraindicating or limiting exercise; significant concomitant chronic obstructive pulmonary disease (FEV1 <50%), or symptomatic peripheral arterial disease. Patients judged as having a poor acustic window, those with ischemic ECG pattern and/or symptoms suitable for cardiac ischemia during exercise, those with frequent uncontrolled arrhythmias and poor exercise performance (below 4 metabolic equivalents (METs) were ruled out. Healthy subjects were chosen between workers of S.Raffaele IRCCS, if they fulfil the following criteria: having an age between 35 and 75 years; no history of cardiac diseases; being at low risk of cardiac events; not being engaged in exercise training program; having a good acustic window.
The study complied with the Declaration of Helsinki and was approved by the local Ethics Committee of S. Raffaele IRCCS (protocol number 27/2021). All patients gave written informed consent before entering the study. Patients who meet the inclusion/exclusion criteria, after signing the informed consent and within a week from the initial visit performed the experimental session.
Experimental sessions were conducted in the ergometry room of the rehabilitation facility of S.Raffaele IRCCS, Rome. The room temperature was set at 24 °C. All subjects performed an incremental stepwise test on a cycle-ergometer (Mortara Instrument, Casalecchio Di Reno, Italy).
The exercise was initiated at a load of 20 W, and the load was increased every three minutes by 20 W. The pedaling frequency was set at around 60 rev per minute during the entire exercise; the exercise was interrupted when volitional exhaustion occurred. During the exercise, HR was continuously monitored; BP was measured at rest, at every stage of the incremental exercise, at first and fifth minute of recovery. Within the experimental session, echocardiography assessments were performed: 1) at rest; 2) at submaximal effort (50-60% HR max); 3) at peak exercise; 4) after five minutes of recovery. Rate of pressure product was calculated as index of myocardial oxygen consumption according to the formula: RPP = (HR × SBP)/100. All experimental sessions were performed in the morning, between 10:00 and 12:00 AM. Subjects were asked to not perform significant physical activities (running, swimming, cycling) during the 24-hours before the experimental session. Subjects were also asked not to smoke or drink wine or other alcoholic beverages from the day before. They were allowed to have a light breakfast the same day, at least two hours before the start of the experimental session. The research team attending each experimental session was composed by a cardiologist, a nurse and cardiac sonographer.
Echocardiography: All echocardiographic examinations were made by one experienced sonographer with subjects in sitting position. A cardiovascular ultrasound Vivid E95® (GE Healthcare, Chicago, IL) with a 4.0-MHz transducer was used for all examinations. All the echocardiographic images were first digitally stored and then reviewed offline. During the review process, an experienced technician performed deformation measures using a proprietary software (version 10.8, EchoPAC; GE Vingmed Ultrasound, Norway).
Left ventricular end-diastolic volume (LVEDV) and end-systolic volume (LVESV) were calculated from the apical two and four chamber windows using modified Simpson’s method; stroke volume (SV) was calculated as EDV−ESV; cardiac output (CO) as HR × SV, and ejection fraction (EF) as EF = (EDV−ESV)/EDV). LA volume was measured from standard apical 4-chamber views at end systole directly before mitral valve opening. The biplane method of disks was used to calculate LA volume. LA volume index (LAVI) was calculated by dividing LA volume by body surface area of the subjects. The reported E/A ratio represents the ratio of peak left ventricle filling velocity in early diastole (E wave) to that in late diastole during atrial contraction (A wave). LV E/e’ ratio was calculated as the ratio between E wave velocity and mean lateral and septal LV e’ wave velocities.
Color tissue Doppler tracings were obtained with the range gate placed at the lateral mitral annular segments in the 4-chamber view. Peak systolic LV longitudinal strain and strain rates were assessed using standard 2D apical four chambers and two chambers view using speckle-tracking analysis.
LV global longitudinal strain (GLS) was measured through two-, three-, and four-chamber views.
The detection of the LV endocardial boundary was automatically provided by the software; however, whether appropriate, it was edited, to conform to the visualized LV boundaries. The maximum negative value of strain during systole, represented the maximum contractility for each segment. Longitudinal strain measurements were subdivided into LA reservoir strain, conduit strain and contractile strain. The average of these values from each segment was used to determine LV GLS. The software program generated the longitudinal strain curves for each segment and a mean curve of all segments [
10]. The reservoir phase was expressed as peak atrial longitudinal strain (PALS) that was measured at the end of the reservoir phase (positive peak during LV systole); the contraction phase was expressed as peak atrial contraction strain (PACS) was measured before the beginning of the active contractile phase (positive peak during early diastole).
Statistical analysis
Data are expressed as mean ± SD. The assumption of normality was checked using Shapiro–Wilks hypothesis test. Pre- and post-exercise data of normally distributed variables were assessed using repeated measures two-way ANOVA, with Bonferroni corrections for post hoc testing. Not normally distributed variables were assessed using Kruskal–Wallis test and Bonferroni corrections for post hoc testing. The level of significance was set at p < 0.05. Data were analyzed using SPSS software (version 20.0 IBM Corp, Amonk, New York, NY, USA).
3. Results
Anthropometric and clinical features of patients a are summarized in
Table 1. HFmrEF patients and HS were matched regarding age, body mass index, and waist circunference, All patient with HFpEF were taking betablockers and ACE-I or ARBs. 85% of them had a previous myocardial infarction; 56% of them had a previous coronary artery bypass graft surgery. Among HS subjects, two had a diagnosis of pre–hypertension but they were not taking any medication at the time of the study.
At rest: PALS, LVGLS, SV and CO values were significantly lower in HFmrEF compared to HS (
Table 1). HS subjects presented higher resting HR and systolic BP than HFmrEF.
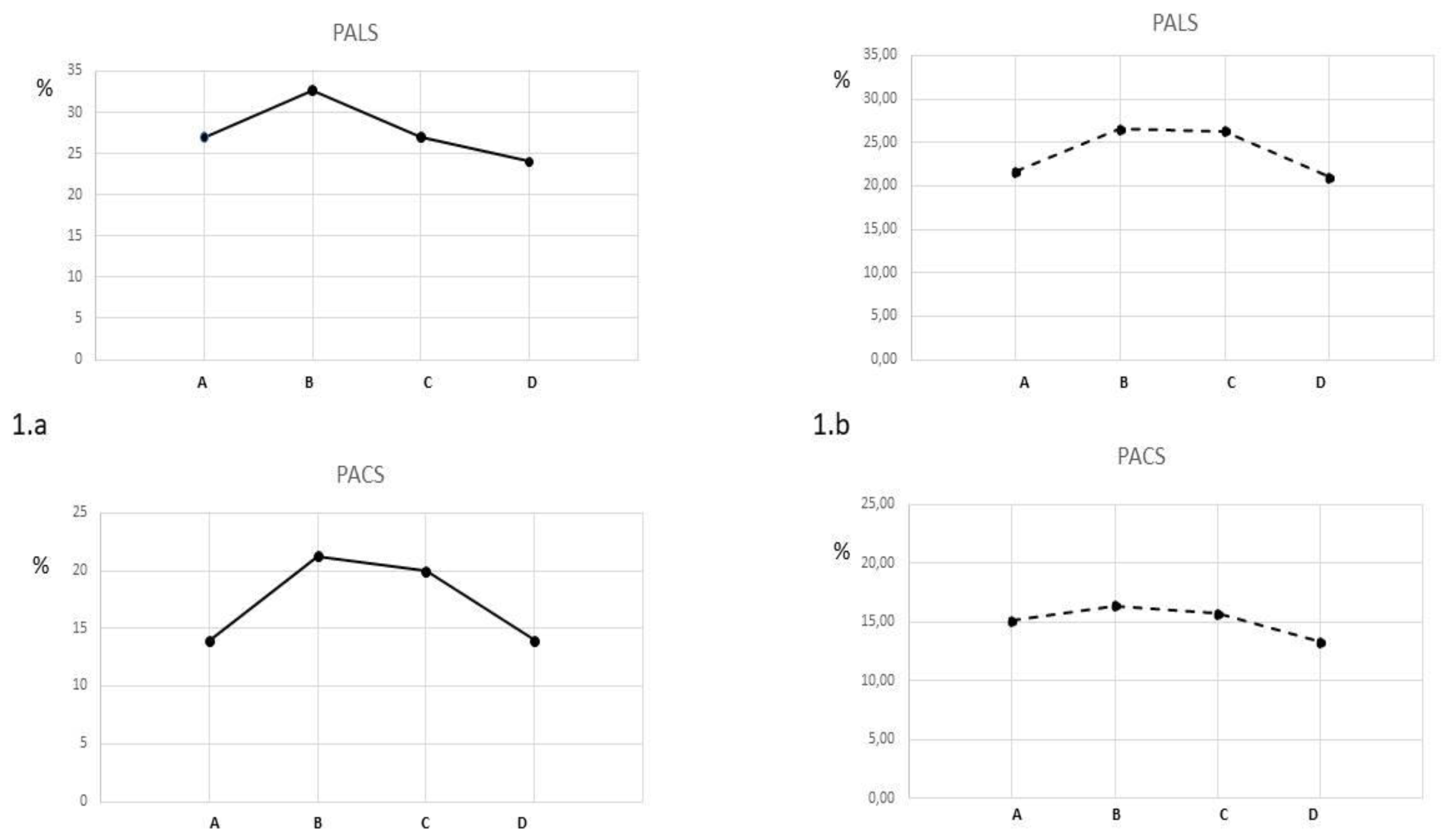
Submaximal exercise: compared to resting values, PALS increased significantly in both groups without between group differences (HS=+21.3%; HFmrEF=+23.8%; between groups p 0.354) (
Figure 1). PACS presented a greater increase HS compared to HFmrEF (+50.7% vs +13.0% respectively; between groups p 0.001). Conduit strain increased in HFmrEF while it decreased in HS. LVGLS presented small increases in both group without between group differences (HS+6%; HFmrEF +6.5%; between groups p 0.234) . SV increased significantly in both HFmrEF (+10.6%) and HS (+11.4%) groups without between-group differences. LVEDV increased in HFmrEF and HS. HR and CO increased in both groups with a significant greater increase in the HS group than in HFmrEF.
Peak exercise: compared to submaximal values, PALS and conduit strain decreased significantly in HS (-17.5% and -31.2% respectively) while they remained unchanged in HFmrEF ( between groups p 0.025 for PALS and 0.004 for conduit strain) PACS showed a small not significant decrease in HS (-7%) and was unchanged in HFmrEF (between-groups p 0.078). LVGLS significantly increased in the HFmrEF group (+14.0%) while it decreased in the HS (-8.6%). HR and CO increased in both groups with a significant greater increase in the HS group than in HFmrEF. LVEDV and SV decreasaed in HS while they remained unchanged in HFmrEF.
HS obtained an higher value of METs at peak exercise than HFmrEF (7.4 vs 5.6; between group p 0.002)
3.2.
4. Discussion
In this study we assessed acute changes occurring to LA function during an incremental symptom-limited exercise in patients with HFmrEF, and compared them with LA changes observed in healthy subjects performing the same type of exercise. We found that, in HFmrEF, PALS increased significantly during the exercise; that increase reached its peak already at submaximal level and it was then maintained at peak exercise. Conversely, in healthy subjects, PALS increased at submaximal exercise but then decreased at peak exercise compared to submaximal values. Our results differ from similar researches showing that patients with HF are unable to enhance LA function in response to incremental exercise [
12,
13]. In the study of Sugimoto et al. [
12] patients with HFpEF presented only small increases of LA resevoir strain during incremental exercise and not increases at all were detected in patients with HFrEF. Tan et al. [
13], compared the acute LA response to exercise of hypertensive subjects and of patients with HFpEF. They observed that the capacity of enhancing atrial function in response to incremental exercise was preserved in hypertensive subjects, while it was lost in patients with HFpEF. Interestingly our data comply with the results of our previous research, conducted in patients with HFmrEF, in which we assessed the LA acute response to two different intensities of eccentric resistance exercise. In that study PALS increased at the end of both exercise protocols in comparison to rest values while no significant exercise-related increases of E/e’ ratio were observed [
20]. In this study the values of PALS at rest was significantly lower in HFmrEF, compared to HS as well as to reference values present in the literature [
22]. This was an expected result since it is known that LV chronic ischemia increases LA stiffness and impairs LA reservoir function [
23]. It should be noted however that E/e’ ratio at rest was within the normal range and, similarly to what happened in HS, it did not increase during exercise. Considering also that LAVI was normal in the present study, our results suggest that the impairment of LA resevoir strain starts at a very early stage during HFmrEF and that the LA dysfunction precedes the rise of LV filling pressure detectable through E/e’ ratio. Our data comply with other researches underlying that a reduced LA reservoir strain is an early marker of elevated LV filling pressure preceding the onset of other echocardiographic signs of LV diastolic dysfuction [
24,
25,
26,
27]. We observed that during the exercise PACS increased significantly only in HS while in HFmrEF the trend of PACS during the exercise was represented by a flat curve. The failure to increase PACS in HFmrEF could have different explanations: the inhibition of the sympathetic drive via betablockers seems to be a reasonable cause since recent studies performed in physiological conditions suggest that contraction strain is modulated mostly by autonomic system activity while it is independent by preload conditions [
28]. Alternatively it could be related to the loss of the elastic properties of the already overstretched atrial myocytes and, together with the reduced PALS values at rest, being an early sign of the LV diastolic dysfunction [
29]. However our results does not clarify the mechanisms underlying the failure to increase PACS during exercise in HFmrEF and, this aspect of the present research deserves further more focused investigations. In this study we observed that, during the exercise, HR increased in the HS group more than in HFmrEF both at submaximal and at peak exercise. This is also an expected result since all HFmrEF were treated with betablockers that curbed their exercise-related increase of HR. Differences in HR during exercise could explain differences in LA performance between HFmrEF and HS observed at peak exercise in this study. The higher HR values observed in HS, by shortening the diastolic LV filling time, may have reduced LA emptying as it is suggested by the decrease of conduit strain that we observed at peak exercise in such patients. An increased residual blood volume at atrial level reducing the ability of LA to receive further blood from pulmonary veins determining a less effective expansion of LA during LV systole. This could explain the reduction in PALS, LVEDV, LVGLS and SV that we registered at peak exercise in comparison to submaximal exercise in HS. This result is in agreement with other researches assessing LA and LV dynamics during exercise [30,31]. To the contrary, in HFmrEF, given their milder increase of HR, diastolic LV filling time remained long enough to guarantee a costant diastolic LV filling. This seems to be confirmed by the observation that conduit strain, LVEDV and SV and LV GLS increased at submaximal exercise and remained unchanged at peak exercise. This indicates that LA/LV system was working according to the Frank-Starling law during the entire duration of the exercise in HFmrEF. Therefore the relative contribution of LA to increase CO in HFmrEF was higher than in HS at peak exercise. The increase of LA function parameters that we observed in this study can be interpreted as a sign that, despite presenting low resting values of PALS, patients with HFmrEF were still able to mobilize LA functional reserve during exercise. Clearly, further studies with larger sample size are needed in order to confirm our findings. We think that results of the present study contribute to the knowledge of cardiac physiology of HFrEF patients and can be useful in order to develop increasingly individualized exercise training program for these patients.
Limitations. This study has several limitations, the most important being the small sample size that makes it necessary for our results to be verified in further larger studies. The HFmrEF group was composed mainly by males, therefore we think that our results cannot be generalized to female gender. Similarly, all HFmrEF patients had an underlying diagnosis of CHD and our results could not be valid for patients with non-ischemic cardiomyopathy. We enrolled asymptomatic patients at an early stage of LA dysfunction with normal LA size and normal E/e’ ratio; therefore our results should be applied only to HFmrEF patients with the aforementioned characteristics.
5. Conclusions
In this study patients with HFmrEF, presented a proper increase of LA function during an incremental, symptom-limited exercise, without significant rise in LV filling pressure. The enhanced LA performance contributed to the increase SV and CO during the exercise. Further researches are needed in order to confirm and extend results of the present study.
Author Contributions
Conceptualization, C.G. and F.I.; Data curation, V.D.A., S.V., A.G.; Formal analysis, G.C., M.A.P. and V.M.; Investigation, G.C. and M.A.P.; Supervision, G.M. and F.I. Writing—review & editing, M.V., G.C., V.M., M.A.P. and F.I. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funding from the Italian Ministry of Health (Ricerca corrente 2021–2022).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of IRCCS San Raffaele Pisana (protocol code 33/2021).
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fang, F.; Lee, A.P.-W.; Yu, C.-M. Left atrial function in heart failure with impaired and preserved ejection fraction. Curr. Opin. Cardiol. 2014, 29, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Nauta, J.F.; Hung, C.-L.; Ouwerkerk, W.; Teng, T.-H.K.; Voors, A.A.; Lam, C.S.; van Melle, J.P. Left atrial structure and function in heart failure with reduced (HFrEF) versus preserved ejection fraction (HFpEF): systematic review and meta-analysis. Hear. Fail. Rev. 2022, 27, 1933–1955. [Google Scholar] [CrossRef] [PubMed]
- Hohendanner, F.; Messroghli, D.; Bode, D.; Blaschke, F.; Parwani, A.; Boldt, L.-H.; Heinzel, F.R. Atrial remodelling in heart failure: recent developments and relevance for heart failure with preserved ejection fraction. ESC Hear. Fail. 2018, 5, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Carpenito, M.; Fanti, D.; Mega, S.; Benfari, G.; Bono, M.C.; Rossi, A.; Ribichini, F.L.; Grigioni, F. The Central Role of Left Atrium in Heart Failure. Front. Cardiovasc. Med. 2021, 8, 704762. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Kusunose, K. Linkage of Left Atrial Function to Heart Failure with Preserved Ejection Fraction. Int. Hear. J. 2023, 64, 4–9. [Google Scholar] [CrossRef]
- Kreimer, F.; Gotzmann, M. Left Atrial Cardiomyopathy – A Challenging Diagnosis. Front. Cardiovasc. Med. 2022, 9, 942385. [Google Scholar] [CrossRef]
- Melenovsky, V.; Hwang, S.-J.; Redfield, M.M.; Zakeri, R.; Lin, G.; Borlaug, B.A. Left Atrial Remodeling and Function in Advanced Heart Failure With Preserved or Reduced Ejection Fraction. Circ. Hear. Fail. 2015, 8, 295–303. [Google Scholar] [CrossRef]
- Sanchis, L.; Gabrielli, L.; Andrea, R.; Falces, C.; Duchateau, N.; Perez-Villa, F.; Bijnens, B.; Sitges, M. Left atrial dysfunction relates to symptom onset in patients with heart failure and preserved left ventricular ejection fraction. Eur. Hear. J. - Cardiovasc. Imaging 2014, 16, 62–67. [Google Scholar] [CrossRef]
- Bisbal, F.; Baranchuk, A.; Braunwald, E.; Bayés de Luna, A.; Bayés-Genís, A. Atrial Failure as a Clinical Entity: JACC Review Topic of the Week. J Am Coll Cardiol. 2020, 75, 222–232. [Google Scholar] [CrossRef]
- Gan, G.C.H.; Ferkh, A.; Boyd, A.; Thomas, L. Left atrial function: evaluation by strain analysis. Cardiovasc. Diagn. Ther. 2018, 8, 29–46. [Google Scholar] [CrossRef]
- Bhatt, A.; Flink, L.; Lu, D.-Y.; Fang, Q.; Bibby, D.; Schiller, N.B. Exercise physiology of the left atrium: quantity and timing of contribution to cardiac output. Am. J. Physiol. Circ. Physiol. 2021, 320, H575–H583. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Bandera, F.; Generati, G.; Alfonzetti, E.; Bussadori, C.; Guazzi, M. Left Atrial Function Dynamics During Exercise in Heart Failure: Pathophysiological Implications on the Right Heart and Exercise Ventilation Inefficiency. JACC Cardiovasc Imaging 2017, 10 Pt B, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.T.; Wenzelburger, F.; Lee, E.; Nightingale, P.; Heatlie, G.; Leyva, F.; Sanderson, J.E. Reduced left atrial function on exercise in patients with heart failure and normal ejection fraction. Hear. 2010, 96, 1017–1023. [Google Scholar] [CrossRef]
- Maffeis, C.; Rossi, A.; Cannata, L.; Zocco, C.; Belyavskiy, E.; Radhakrishnan, A.K.; Feuerstein, A.; Morris, D.A.; Pieske-Kraigher, E.; Pieske, B.; et al. Left atrial strain predicts exercise capacity in heart failure independently of left ventricular ejection fraction. ESC Hear. Fail. 2022, 9, 842–852. [Google Scholar] [CrossRef]
- Sun, P.; Cen, H.; Chen, S.; Chen, X.; Jiang, W.; Zhu, H.; Liu, Y.; Liu, H.; Lu, W. Left atrial dysfunction can independently predict exercise capacity in patients with chronic heart failure who use beta-blockers. BMC Cardiovasc. Disord. 2023, 23, 128. [Google Scholar] [CrossRef]
- Von Roeder, M.; Rommel, K.P.; Kowallick, J.T.; Blazek, S.; Besler, C.; Fengler, K.; Lotz, J.; Hasenfuß, G.; Lücke, C.; Gutberlet, M.; Schuler, G.; Schuster, A.; Lurz, P. Influence of Left Atrial Function on Exercise Capacity and Left Ventricular Function in Patients with Heart Failure and Preserved Ejection Fraction. Circ Cardiovasc Imaging 2017, 10, e005467. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; Jessup, M.; Linde, C.; Nihoyannopoulos, P.; Parissis, J.T.; Pieske, B.; Riley, J.P.; Rosano, G.M.C.; Ruilope, L.M.; Ruschitzka, F.; Rutten, F.H.; Van der Meer, P. ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016, 38, 2129–2200. [Google Scholar]
- Al Saikhan, L.; Hughes, A.D.; Chung, W.-S.; Alsharqi, M.; Nihoyannopoulos, P. Left atrial function in heart failure with mid-range ejection fraction differs from that of heart failure with preserved ejection fraction: a 2D speckle-tracking echocardiographic study. Eur. Hear. J. - Cardiovasc. Imaging 2018, 20, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Maffeis, C.; Morris, D.A.; Belyavskiy, E.; Kropf, M.; Radhakrishnan, A.K.; Zach, V.; da Conceicao, C.R.; Trippel, T.D.; Pieske-Kraigher, E.; Rossi, A.; et al. Left atrial function and maximal exercise capacity in heart failure with preserved and mid-range ejection fraction. ESC Hear. Fail. 2020, 8, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, G.; Perrone, M.A.; Iellamo, F.; D’antoni, V.; Catena, M.; Franchini, A.; Volterrani, M. Acute Left Atrial Response to Different Eccentric Resistance Exercise Loads in Patients with Heart Failure with Middle Range Ejection Fraction: A Pilot Study. J. Pers. Med. 2022, 12, 689. [Google Scholar] [CrossRef] [PubMed]
- Halliday, B.P.; Senior, R.; Pennell, D.J. Assessing left ventricular systolic function: from ejection fraction to strain analysis. Eur. Hear. J. 2020, 42, 789–797. [Google Scholar] [CrossRef]
- Nielsen, A.B.; Skaarup, K.G.; Hauser, R.; Johansen, N.D.; Lassen, M.C.H.; Jensen, G.B.; Schnohr, P.; Møgelvang, R.; Biering-Sørensen, T. Normal values and reference ranges for left atrial strain by speckle-tracking echocardiography: the Copenhagen City Heart Study. Eur. Hear. J. - Cardiovasc. Imaging 2021, 23, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Sharifov, O.; Denney, T.S. Jr.; Girard, A.A.; Gupta, H.; Lloyd, S.G. Coronary artery disease is associated with impaired atrial function regardless of left ventricular filling pressure. Int J Cardiol. 2023, 29, S0167-5273(23)00744-1. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Khan, F.H.; Remme, E.W.; Ohte, N.; Garcia-Izquierdo, E.; Chetrit, M.; Monivas-Palomero, V.; Mingo-Santos, S.; Andersen, O.S.; Gude, E.; et al. Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur Heart J Cardiovasc Imaging 2021, 18, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Egbe, A.C.; Connolly, H.M.; Anderson, J.H.; Oh, J.K.; Andi, K.; Goda, A.; Abozied, O.; Ramachandran, D.; Miranda, W.R. Estimation of Left Ventricular Filling Pressure Using Left Atrial Strain in Coarctation of Aorta. J Invasive Cardiol. 2022, 34, E858–E865. [Google Scholar]
- Jarasunas, J.; Aidietis, A.; Aidietiene, S. Left atrial strain - an early marker of left ventricular diastolic dysfunction in patients with hypertension and paroxysmal atrial fibrillation. Cardiovasc. Ultrasound 2018, 16, 29. [Google Scholar] [CrossRef]
- Aga, Y.S.; Kamar, S.A.; Chin, J.F.; Berg, V.J.v.D.; Strachinaru, M.; Bowen, D.; Frowijn, R.; Akkerhuis, M.K.; Constantinescu, A.A.; Umans, V.; et al. Potential role of left atrial strain in estimation of left atrial pressure in patients with chronic heart failure. ESC Hear. Fail. 2023, 10, 2345–2353. [Google Scholar] [CrossRef]
- Stringer, W.W.; Whipp, B.J.; Wasserman, K.; Christenson, P.; French, W.J.; Pórszász, J. Non-linear cardiac output dynamics during ramp-incremental cycle ergometry. Eur. J. Appl. Physiol. 2004, 93, 634–639. [Google Scholar] [CrossRef]
- Zhou B, Conlee RK, Jensen R, Fellingham GW, George JD, Fisher AG. Stroke volume does not plateau during graded exercise in elite male distance runners. Med Sci Sports Exerc 2001, 33, 1849–1854. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).