1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a major cause of cancer-related death worldwide, with most patients presenting with either unresectable or metastatic disease at the time of diagnosis. [
1,
2] Despite the increasing incidence and advances in treatment, the survival rate of PDAC remains low. [
3] To date, only surgery remains the curative treatment modality, and the 5-year survival rate is less than 5% when curative resection is not possible. [
4] Combination chemotherapy is a mainstay treatment for patients with unresectable or metastatic PDAC. The development of combination regimens, including gemcitabine with albumin-bound paclitaxel (nab-paclitaxel) and FOLFIRINOX (a combination of oxaliplatin, irinotecan, folinic acid, and fluorouracil), has improved the survival outcomes of patients with metastatic PDAC. [
5,
6].
With respect to targeted therapy for pancreatic cancer, erlotinib, which slightly prolongs survival, has been approved as a targeted therapeutic agent for PDAC. [
7] In the phase III POLO trial, olaparib was found to be an effective maintenance therapy in patients with pancreatic cancer and germline BRCA mutations. [
8] In addition, larotrectinib or entrectinib is feasible for patients with pancreatic cancer harboring NTRK fusion. [
9,
10] However, these targeted agents could only be used for a small percentage of patients with the above genetic alterations. Thus, studies exploring new targeted agents are urgently required.
Claudins are a family of transmembrane tight junction proteins comprising at least 27 members. [
11] They are mainly located in the apical region of the cellular membrane and establish a paracellular barrier that controls molecular flow between cells. They are also associated with the transduction of cell signaling pathways and regulation of proliferation and differentiation. [
12,
13] Various claudins are expressed in different normal tissues and can be modified during carcinogenesis. Claudin-18 (CLDN18) is a member of the claudin family that is specifically expressed in stomach and lung tissues. Among the two CLDN18 variants produced by alternative splicing, claudin-18.2 (CLDN18.2) is specifically expressed in the gastric mucosa, while claudin-18.1 is expressed in lung tissue. [
14] CLDN18.2 is aberrantly expressed not only in pancreatic cystic tumors (e.g., mucinous cystic tumors and intraductal papillary mucinous neoplasms), but also in 60–90% of PDAC. [
15,
16,
17] Given that CLDN18.2 is not expressed in normal pancreatic tissue but is strongly activated during carcinogenesis, it could be an attractive therapeutic target for PDAC. [
16]
The recently developed zolbetuximab is a selective monoclonal antibody to CLDN18.2 for the treatment of gastric cancer. Zolbetuximab binds to malignant tissues that highly express CLDN18.2 without affecting healthy tissues that do not express CLDN18.2. This unique cancer-specific feature of zolbetuximab allows for maximum anticancer effects and lower toxicity. [
18] In a phase II randomized trial (FAST) for advanced gastric, gastric-esophageal junction, and esophageal cancers expressing CLD18.2, the combination of zolbetuximab with cytotoxic chemotherapy achieved longer progression-free survival and overall survival (OS) than did conventional chemotherapy alone. [
19] The phase II trial of patients with CLDN18.2-expressing metastatic PDAC is currently ongoing and is aimed to assess the efficacy and safety of zolbetuximab in combination with gemcitabine and nab-paclitaxel as first-line treatment (ClinicalTrials.gov NCT 03816163).
This study aimed to investigate the clinicopathological features of patients with CLDN18.2-expressing PDAC and to evaluate the usefulness of CLND18.2 expression as a potential prognostic biomarker. Towards this goal, CLDN18.2 expression was analyzed immunohistochemically in PDAC patients.
2. Materials and Methods
2.1. Patients
This study evaluated 130 PDAC patients who underwent curative intent surgical resection between January 2010 and December 2018 at the Catholic University of Korea, Seoul St. Mary’s Hospital. Patients with available electronic medical records were eligible according to the following criteria: (1) histologically confirmed PDAC; (2) pathological stage I–IV disease according to the American Joint Committee of Cancer Staging, 8
th edition; [
20] and (3) recurrence and survival could be confirmed at the time of data collection.
2.2. Immunohistochemistry
Formalin-fixed and paraffin-embedded primary tumor samples were obtained and cut into 4-μm-thick sections. The rabbit monoclonal antibody anti-claudin 18 (34H14L15; 1 × 1000; Invitrogen, Carlsbad, CA, USA) was used to detect the expression and localization of CLDN18. Immunohistochemistry was performed according to established protocols. Briefly, human PDAC tissue sections were deparaffinized and rehydrated. The sections were then incubated with 3.0% H2O2 in methanol for 10 min to inhibit endogenous peroxidase activity. Antigen retrieval was performed by microwaving for 15 minutes. After blocking with normal goat serum for 30 min, the tissue sections were incubated with the primary antibody against claudin 18 in a humidified chamber at 4°C overnight. Biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlington, ON, Canada) was used at a 1:250 dilution as the secondary antibody. The slides were then incubated with peroxidase solution (Vectastain Elite ABC Kit, Vector Laboratories, Burlington, ON, Canada) and diaminobenzidine substrate (Vector Laboratories, Burlington, ON, Canada). Finally, the sections were counterstained with hematoxylin.
2.3. Histological assessment
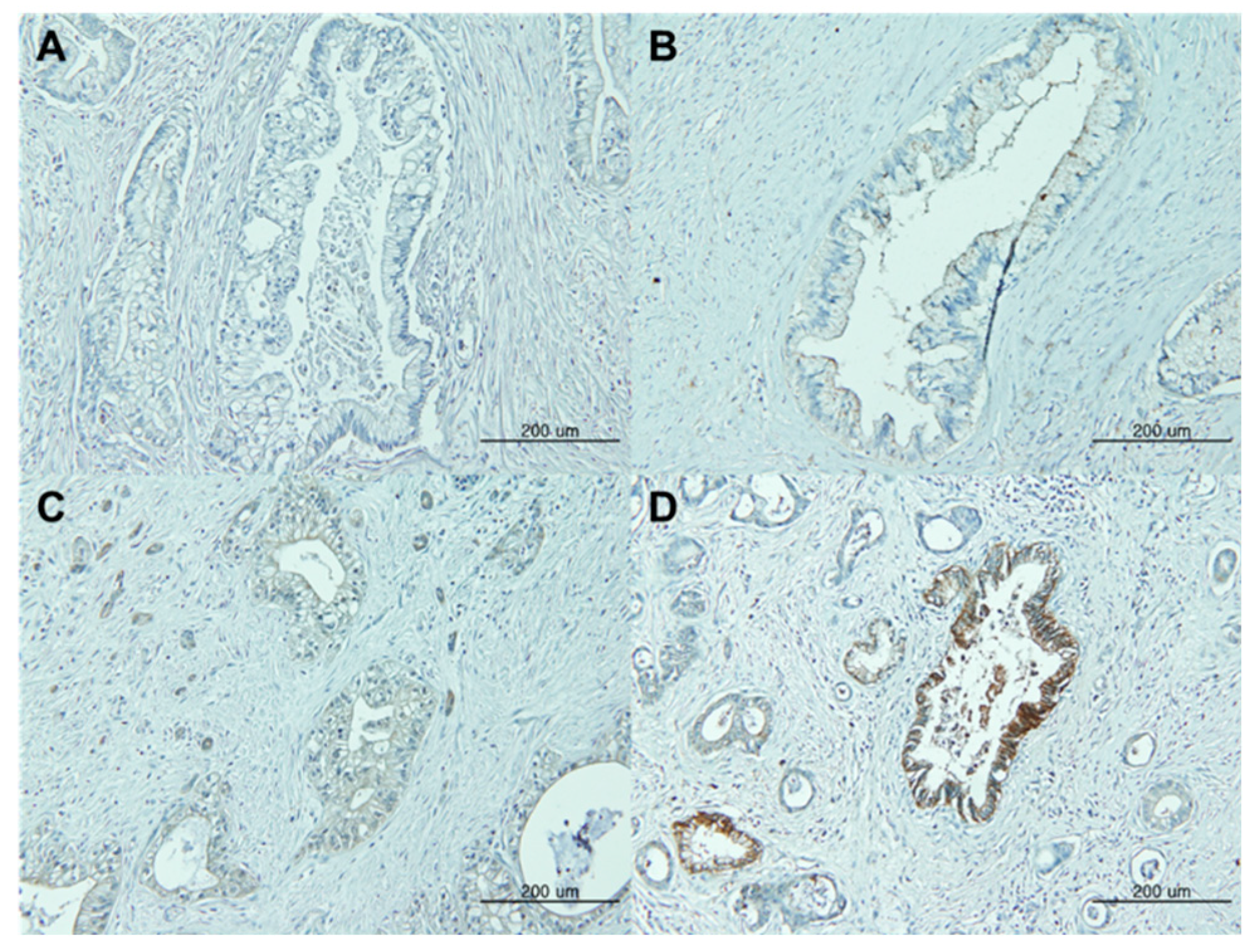
CLDN18 expression was assessed semi-quantitatively based on the intensity of membrane staining and percentage of tumor cells expressing CLDN18. Staining intensity was scored by two pathologists as follows: 0, +1 (weak intensity), +2 (moderate intensity), or +3 (strong intensity) (
Figure 1). Positive CLDN18 expression was defined as membranous staining visible in ≥80% of the tumor cells with moderate to strong staining intensity (+2 or +3). Disagreements in the assessments were resolved through a discussion to reach consensus.
2.4. Ethics
All methods were performed in accordance with Korean regulations and the Declaration of Helsinki. This study was approved by the Institutional Review Board (IRB) of The Catholic University of Korea, Seoul St. Mary’s Hospital (approval ID: KC21SISI0074) with a waiver of informed consent due to the retrospective nature of the analysis.
2.5. Statistical analysis
Descriptive statistics were reported as proportions and medians with ranges. The association between CLDN18 expression and clinicopathological features was evaluated using Chi-squared and Fisher’s exact tests and displayed using cross-tables. Recurrence-free survival (RFS) was defined as the interval between curative surgery and either recurrence or any-cause death. OS was estimated from the date of surgery to the time of the last follow-up or death. Survival curves were generated using the Kaplan-Meier method and analyzed using a two-tailed log-rank test. Cox proportional hazards regression models were used to identify the effects of clinical factors on recurrence and survival. Hazard ratios (HR) and 95% confidence intervals (CIs) were estimated for each factor. All statistical analyses were performed using SPSS for Windows (version 24.0; IBM SPSS Inc., Armonk, New York, USA) and GraphPad Prism version 8.0 (GraphPad Software Inc., San Diego, CA, USA). All tests were two-sided, and p-values <0.05 were considered statistically significant.
3. Results
3.1. Demographic and clinicopathological patient characteristics
The demographic and clinicopathological features of the 130 patients are shown in
Table 1. The median patient age was 68 years (range, 36–81 years), and the male-to-female ratio was 1.06. Approximately three-quarters (74.6%) of the patients had pancreatic head cancer, and the majority (76.2%) had moderate or poorly differentiated cancer. With respect to tumor stage, 33 patients (25.4%) had stage I disease (IA=5, IB=28); 65 patients (50.0%), stage II (IIA=7, IIB=58); 25 patients (19.2%), stage III; and 7 patients (5.4%), stage IV. Serum carbohydrate antigen 19-9 (CA 19-9) levels were elevated in 76 patients (58.5%) at the time of diagnosis.
3.2. CLND18 expression in PDAC
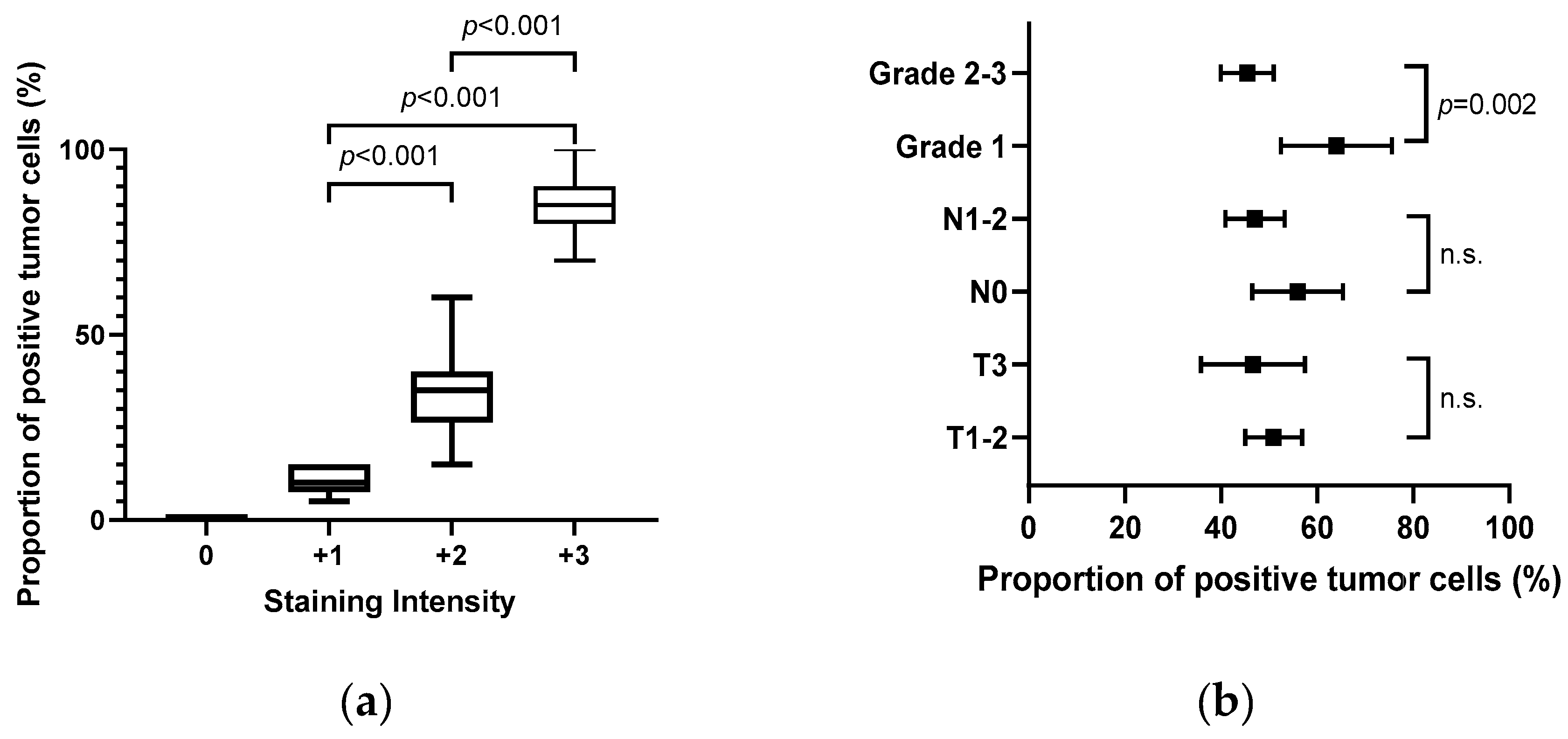
When comparing normal and reactive ducts, CLDN18 was expressed, at least focally, in 125/130 (96.2%) evaluable samples. Overall, 116 (89.2%) patients showed CLDN18 expression with a staining intensity ≥2+, and a considerable portion of patients (36.9%, n=48) showed a strong staining intensity (+3). Interestingly, the staining intensity and the proportion of positive tumor cells were significantly correlated (Rs=0.813; 95% CI, 0.86-0.93;
p<0.001,
Figure 2a). In addition, although tumor and node stages were not associated with the proportion of positive tumor cells, tumor differentiation was significantly correlated with the proportion of CLDN18-expressing tumor cells (grade 1 vs. grades 2–3,
p=0.002,
Figure 2b).
3.3. CLDN18 expression and clinicopathological features
Overall, 41 patients (31.5%) showed positive CLDN18 expression (
Table 1). Positive CLDN18 expression was more common in well-differentiated carcinomas, whereas focal or negative CLDN18 expression was more common in higher-grade carcinomas. In total, 18 (58.1%) patients with grade 1 tumor differentiation showed positive CLDN18 expression, whereas only 23 (23.2%) patients with grade 2–3 tumor differentiation showed positive CLDN18 expression (
p<0.001). Positive CLDN18 expression was associated with node stage (N0 vs. N1–2), with a higher prevalence of positive expression among N0 stage (
p=0.045), but not with tumor stage. A more advanced tumor, node, metastasis (TNM) stage was correlated with a low prevalence of positive CLDN18 expression (stage I vs. stage II–IV,
p=0.015). Positive CLDN18 expression was also associated with microscopic vascular invasion, with a higher prevalence of positive expression in patients without vascular invasion (
p=0.031). Meanwhile, positive CLDN18 expression was not correlated with age, sex, tumor location, lymphatic and perineural invasion, and preoperative CA 19-9 level.
3.4. CLDN18 expression and survival outcomes
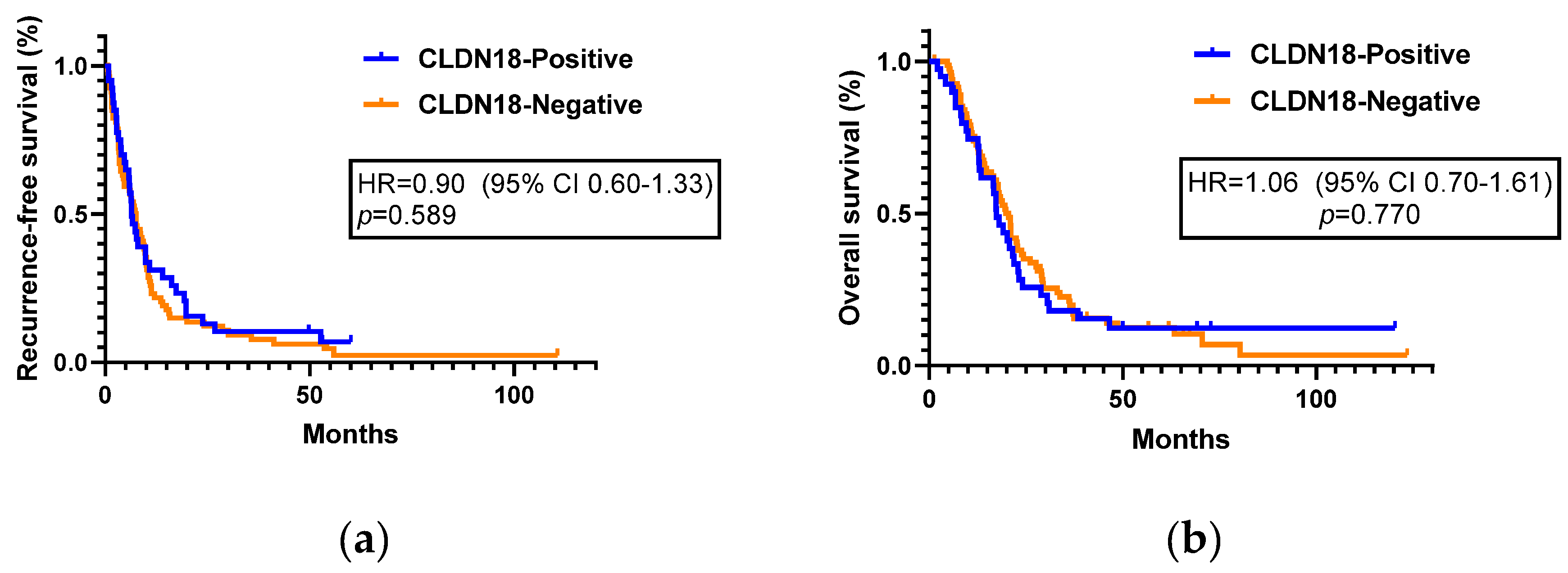
Among the 130 patients, 123 patients with stage I–III pancreatic cancer were included in the recurrence and survival analysis. Stage IV patients who already had distant metastatic lesions at the time of surgery were excluded from the analysis. Within a median follow-up of 17.6 months, 109 patients (88.6%) experienced recurrence and 103 patients (83.7%) died. Positive CLDN18 expression was not associated with prolonged or shortened RFS. The median RFS was not significantly differ between the positive and negative CLDN18 expression groups (6.5 months (95% CI, 4.9–8.0) vs. 7.5 months (95% CI, 6.1–8.9) (HR=0.90; 95% CI, 0.60–1.33;
p=0.589,
Figure 3a,
Table 2). Univariate analysis showed that histologic grade, adjuvant chemotherapy administration, cancer stage, vascular and perineural invasion, and lymph node ratio (LNR) status were risk factors for pancreatic cancer recurrence. However, only higher histologic grade (grade 2–3 vs. grade 1, HR=2.08; 95% CI, 1.30–3.33;
p=0.002), adjuvant chemotherapy (HR=0.29; 95% CI, 0.18–0.46;
p<0.001), and perineural invasion (HR=2.56; 95% CI, 1.32–4.95;
p=0.005) were associated with RFS after multivariate analysis (
Table 2).
Positive CLDN18 expression was not associated with survival outcomes. The median OS was 17.4 months (95% CI, 14.3–20.4) in the CLDN18-positive group and 20.6 months (95% CI, 17.5–23.6) in the CLDN18-negative group (HR=1.06; 95% CI, 0.70–1.61;
p=0.770,
Figure 3b,
Table 3). The results of the univariate and multivariate analyses for OS are shown in
Table 3, with subgroups according to clinicopathological parameters. On multivariate analysis, higher histologic grade (grade 2–3 vs. grade 1, HR=1.75; 95% CI, 1.07–2.84;
p=0.025) and vascular invasion (HR=1.78; 95% CI, 1.15–2.77;
p=0.010) were significantly associated with worse OS outcomes. In addition, adjuvant chemotherapy was associated with longer OS (HR=0.62; 95% CI, 0.40–0.98;
p=0.040) (
Table 3).
3.5. CLDN18 expression and patterns of recurrence or metastasis
Recurrence was confirmed in 109/123 patients (88.6%), and 7 patients had metastatic lesions at the time of surgery. The patterns of recurrence and metastatic sites are presented in
Table 4. Local recurrence occurred in 26/116 patients (22.4%); distant-only recurrence, 63 patients (54.3%); and both local and distant recurrence, 27 patients (23.3%). CLDN18 expression was not associated with recurrence patterns. With respect to the number of metastatic sites and metastatic burden, a similar distribution was observed between the negative and positive CLDN18 expression groups. Interestingly, the positive CLDN18 expression group had more frequent distant nodal metastases than did the negative CLDN18 expression group (
p=0.011). No significant differences were observed in the other sites of metastasis (liver, lung, and peritoneum) (
Table 4).
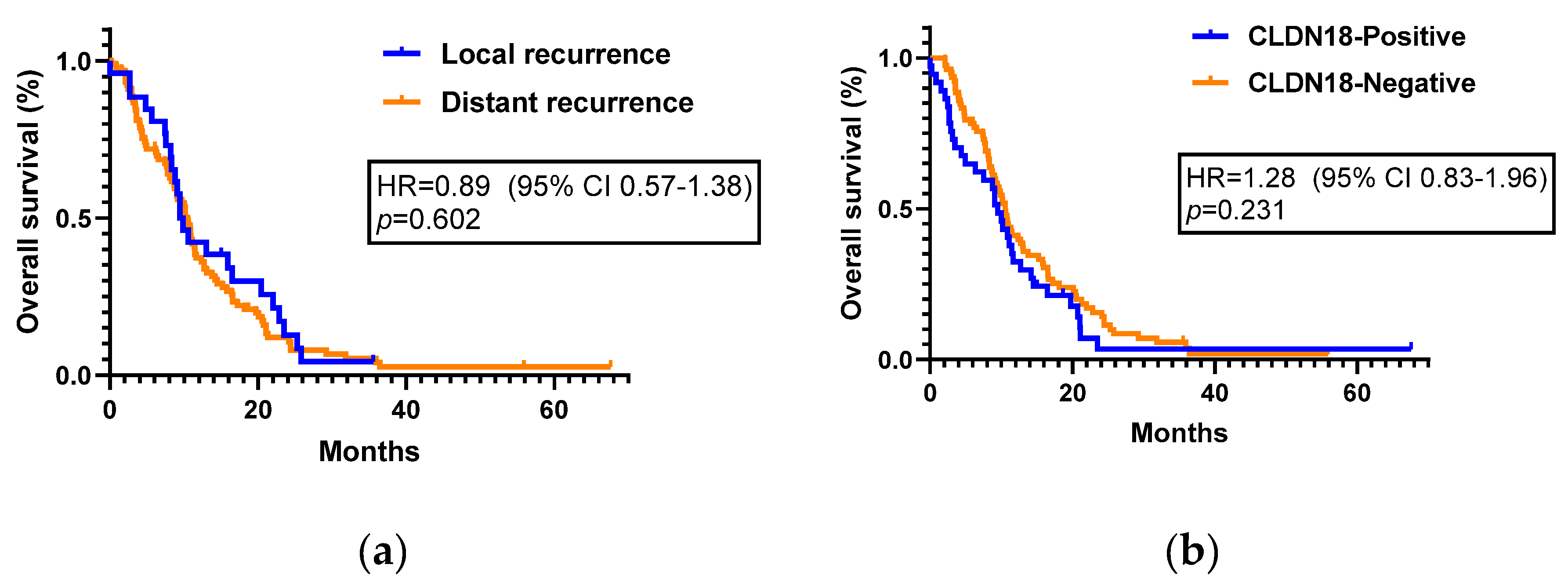
The median post-recurrence survival was 9.48 months (95% CI, 7.59–11.36) for patients with local recurrence and 10.53 months (95% CI, 8.99–12.6) for those with distant recurrence (HR=0.89; 95%CI, 0.57–1.38;
p=0.602,
Figure 4a). The median survival from the time of recurrence to the time of any-cause death was 9.48 months (95% CI, 7.76–11.20) in the CLDN18 positive group and 10.56 months (95% CI, 9.39–11.73) in the CLDN18 negative group (HR=1.28; 95% CI, 0.83-1.96;
p=0.231,
Figure 4b).
4. Discussion
Advanced pancreatic cancer has a dismal prognosis, and no effective targeted agents have been developed for pancreatic cancer. CLDN18.2 is known to be specifically expressed in PDAC, and several clinical trials using therapeutic agents against CLDN18.2 have been conducted. [
21,
22]
CLDN18 is frequently overexpressed in infiltrating PDAC and is overexpressed in pancreatic intraepithelial neoplasia, known as a precancerous lesion. Therefore, CLDN18 may be an early-stage marker of pancreatic carcinogenesis. [
23] Previous studies have reported that CLDN18 is expressed in 60–80% of PDAC cases. [
16,
17,
23] Our results are consistent with previous findings, with most patients (89.2%) having ≥2+ intensity of CLDN18 expression, and positive CLDN18 expression (defined as staining intensity ≥2+ and ≥80% of neoplastic cells) was observed in 41 (31.5%) of 130 patients. Additionally, CLDN18 staining intensity and the fraction of stained tumor cells were significantly correlated, consistent with previous findings. [
16]
We found a significant association between CLDN18 expression and tumor differentiation, with well-differentiated tumors having a higher prevalence of CLDN18-positive cases. Histologic grading is determined based on glandular differentiation, mitosis, mucin production, and nuclear pleomorphism. [
20] Heterogeneity of these features within the same tumor is common; therefore, the highest grade is reported. Despite the possibility of statistical errors, a significant association was observed between tumor grade and CLDN18 expression. Additionally, in moderately or poorly differentiated tumors, the focal well-differentiated portion showed CLDN18 overexpression. In contrast, as the grade of differentiation deteriorated, CLDN18 expression decreased. This association is supported by several previous reports demonstrating that CLDN18 is overexpressed in well-differentiated pancreatic cancer and its precursors. [
15,
16,
17,
23,
24] These results suggest that CLDN18 may be a marker of the early carcinogenetic process of PDAC.
Correlation analysis revealed that the proportion of tumors with positive CLDN18 expression was significantly higher in lymph node-negative tumors, in contrast to previously reported data. [
16] However, some prior studies have reported that no association was observed between node stage and CLDN18 expression, and thus, the association remains controversial. [
17] We also observed significantly higher CLDN18 expression in early TNM stage and low LNR-status tumors, possibly because CLDN18 positivity was correlated with node-negative tumors. These findings could be explained by increased features of tumor stemness when CLDN18 expression was downregulated. [
24] A previous study suggested that the downregulated CLDN18 expression in gastric cancer cells is associated with increased cellular proliferation and epithelial-to-mesenchymal transition, which are related to invasion and metastasis. [
25] In addition, loss of CLDN18 expression can lead to yes-associated protein activation, which can promote proliferative potential by activating the phosphoinositide 3-kinase-AKT signaling pathway. [
26]
We hypothesized that CLDN18 expression might be associated with prognosis based on the association between CLDN18 expression and the aforementioned prognostic factors. However, there was no relationship between survival and CLDN18 expression. Only histologic grading and adjuvant chemotherapy were correlated with RFS and OS outcomes. There was no significant difference in the rate of adjuvant chemotherapy according to CLDN18 expression (25/40 patients (62.5%) in the CLDN18 positive group and 59/83 patients (71.1%) in the CLDN negative group. A previous study showed an association between CLDN18 expression and better survival outcomes, [
17] whereas another study showed no correlation. [
24] Thus, the prognostic value of CLDN18 expression remains unclear and requires further investigation.
The current study found no significant difference in patten of recurrence according to the CLDN18 expression. We initially hypothesized that patients with positive CLDN18 expression would have a less aggressive tumor biology than those with negative CLDN18 expression. However, there was no difference was observed in the pattern of recurrence, number of metastatic sites, or metastatic burden according to the CLDN18 expression. With respect to organotropism, distant nodal metastasis was observed at a significantly higher prevalence in the CLDN18-positive group than in the CLDN18-negative group. Additionally, lung metastasis tended to be more frequent in the CLDN18-negative group, although the difference was not significant. Metastatic organotropism varies among host organs with different abilities to colonize cancer cells. [
27] Further studies are needed to identify the host factors associated with distant nodal metastasis in patients with CLDN18-expressing PDAC.
Similar to recurrence, survival outcomes also did not differ according to CLDN18-expression. However, these results may not be sufficient to evaluate the prognostic impact of CLDN18 expression in palliative settings because we did not assess CLDN18 expression in recurrent or metastatic samples. A previous study investigated CLDN18 expression in matched samples of primary and metastatic lesions, and the majority of patients had comparable CLDN18 expression in both lesions. [
16] However, the study had a small sample size, and thus, further studies are needed to validate previous results.
This study systematically investigated CLDN18 expression in surgically treated patients with PDAC. To the best of our knowledge, this study is the first to evaluate the clinicopathological features and patterns of recurrence in patients with CLDN18-positive pancreatic cancer. Considering CLDN18 as a potential therapeutic target for pancreatic cancer, our results may be beneficial in selecting subjects for CLDN18-targeted treatment in the future. However, this study also had some limitations. First, CLDN18 positivity was only determined according to our own criteria, but the standard criteria for CLDN18 positivity have not yet been established. Clinically meaningful criteria need to be established according to the results of ongoing clinical trials of zolbetuximab and accompanying diagnostic modalities and cutoff values also need to be determined. Second, treatment differed among patients, and this could not be controlled owing to the retrospective nature of the study. Third, the relatively small sample size limited the interpretation of the subgroup analysis according to CLDN18 expression. Further studies are warranted to identify patients who may benefit from CLDN18-targeted treatments.
In conclusion, CLDN18 overexpression is correlated with several clinicopathological features, but not with prognosis in pancreatic cancer. Patients with high proportion of well-differentiated histologic pancreatic cancer could be evaluated for CLND18 expression for possible targeted therapy in future.
Author Contributions
Park SJ was involved in manuscript writing, drafting conception and design, acquisition of data, performing procedures, and data analysis; Shin KS, Kim IH, Hong TH, and Suh JH contributed to writing the manuscript; Lee MA contributed to writing the manuscript, drafting, conception and design, performing procedures, and data analysis. All authors helped to perform the research and have read and approved the final manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Institutional Review Board (IRB) of The Catholic University of Korea, Seoul St. Mary’s Hospital (approval ID: KC21SISI0074. All methods were performed in accordance with Korean regulations and the Declaration of Helsinki. The need for informed consent was waived owing to the retrospective nature of the analysis.
Informed Consent Statement
Informed consent waived for this study due to the retrospective nature of the analysis.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Ilic, M.; Ilic, I. Epidemiology of pancreatic cancer. World J. Gastroenterol. 2016, 22, 9694–9705. [Google Scholar] [CrossRef]
- Hidalgo, M. Pancreatic cancer. New England Journal of Medicine. 2010;362(17):1605-1617. [CrossRef]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Cokkinides V, Albano J, Samuels A, et al. American cancer society: Cancer facts and figures. Atlanta: American Cancer Society. 2005.
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; De La Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Moore, M.J.; Goldstein, D.; Hamm, J.; Figer, A.; Hecht, J.R.; Gallinger, S.; Au, H.J.; Murawa, P.; Walde, D.; Wolff, R.A.; et al. Erlotinib Plus Gemcitabine Compared With Gemcitabine Alone in Patients With Advanced Pancreatic Cancer: A Phase III Trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2007, 25, 1960–1966. [Google Scholar] [CrossRef]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.-O.; Hochhauser, D.; Arnold, D.; Oh, D.-Y.; et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef]
- Hong DS, DuBois SG, Kummar S, et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. The Lancet Oncology. 2020;21(4):531-540. [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Morin, P.J. Claudin Proteins in Human Cancer: Promising New Targets for Diagnosis and Therapy. Cancer Res 2005, 65, 9603–9606. [Google Scholar] [CrossRef]
- Angelow S, Ahlstrom R, Yu AS. Biology of claudins. American Journal of Physiology-Renal Physiology. 2008;295(4):F867-F876. [CrossRef]
- Swisshelm, K.; Macek, R.; Kubbies, M. Role of claudins in tumorigenesis. Adv. Drug Deliv. Rev. 2005, 57, 919–928. [Google Scholar] [CrossRef]
- Niimi T, Nagashima K, Ward JM, et al. Claudin-18, a novel downstream target gene for the T/EBP/NKX2. 1 homeodomain transcription factor, encodes lung-and stomach-specific isoforms through alternative splicing. Molecular and cellular biology. 2001;21(21):7380-7390. [CrossRef]
- Lee, J.H.; Kim, K.S.; Kim, T.-J.; Hong, S.P.; Song, S.Y.; Chung, J.B.; Park, S.W. Immunohistochemical analysis of claudin expression in pancreatic cystic tumors. Oncol. Rep. 2011, 25, 971–978. [Google Scholar] [CrossRef]
- Wöll, S.; Schlitter, A.M.; Dhaene, K.; Roller, M.; Esposito, I.; Sahin, U.; Türeci, O. Claudin 18.2 is a target for IMAB362 antibody in pancreatic neoplasms. Int. J. Cancer 2014, 134, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Karanjawala, Z.E.; Illei, P.B.; Ashfaq, R.; Infante, J.R.; Murphy, K.; Pandey, A.; Schulick, R.; Winter, J.; Sharma, R.; Maitra, A.M.; et al. New Markers of Pancreatic Cancer Identified Through Differential Gene Expression Analyses: Claudin 18 and Annexin A8. Am. J. Surg. Pathol. 2008, 32, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Toom, S.; Huang, Y. Anti-claudin 18.2 antibody as new targeted therapy for advanced gastric cancer. J. Hematol. Oncol. 2017, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Türeci, O.; Manikhas, G.; Lordick, F.; Rusyn, A.; Vynnychenko, I.; Dudov, A.; Bazin, I.; Bondarenko, I.; Melichar, B.; et al. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann. Oncol. 2021, 32, 609–619. [Google Scholar] [CrossRef]
- Chun, Y.S.; Pawlik, T.M.; Vauthey, J.N. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann. Surg. Oncol. 2018, 25, 845–847. [Google Scholar] [CrossRef]
- Park, W.; O'Reilly, E.M.; Furuse, J.; Kunieda, F.; Jie, F.; Kindler, H.L. Phase II, open-label, randomized study of first-line zolbetuximab plus gemcitabine and nab-paclitaxel (GN) in Claudin 18.2–positive metastatic pancreatic cancer (mPC). J. Clin. Oncol. 2020, 38, TPS4667–TPS4667. [Google Scholar] [CrossRef]
- Qi, C.; Gong, J.; Li, J.; Liu, D.; Qin, Y.; Ge, S.; Zhang, M.; Peng, Z.; Zhou, J.; Cao, Y.; et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat. Med. 2022, 28, 1189–1198. [Google Scholar] [CrossRef]
- Tanaka, M.; Shibahara, J.; Fukushima, N.; Shinozaki, A.; Umeda, M.; Ishikawa, S.; Kokudo, N.; Fukayama, M. Claudin-18 Is an Early-Stage Marker of Pancreatic Carcinogenesis. J. Histochem. Cytochem. 2011, 59, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Soini, Y.; Takasawa, A.; Eskelinen, M.; Juvonen, P.; Kärjä, V.; Hasegawa, T.; Murata, M.; Tanaka, S.; Kojima, T.; Sawada, N. Expression of claudins 7 and 18 in pancreatic ductal adenocarcinoma: association with features of differentiation. J. Clin. Pathol. 2012, 65, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Oshima, T.; Shan, J.; Okugawa, T.; Chen, X.; Hori, K.; Tomita, T.; Fukui, H.; Watari, J.; Miwa, H. Down-Regulation of Claudin-18 Is Associated with the Proliferative and Invasive Potential of Gastric Cancer at the Invasive Front. PLOS ONE 2013, 8, e74757. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, D.-D.; Yuan, T.; Yan, F.-J.; Zeng, C.-M.; Dai, X.-Y.; Chen, Z.-B.; Chen, Y.; Zhou, T.; Fan, G.-H.; et al. Multikinase Inhibitor CT-707 Targets Liver Cancer by Interrupting the Hypoxia-Activated IGF-1R–YAP Axis. Cancer Res 2018, 78, 3995–4006. [Google Scholar] [CrossRef]
- Psaila, B.; Lyden, D. The metastatic niche: adapting the foreign soil. Nat. Rev. Cancer 2009, 9, 285–293. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).