Submitted:
19 June 2023
Posted:
20 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
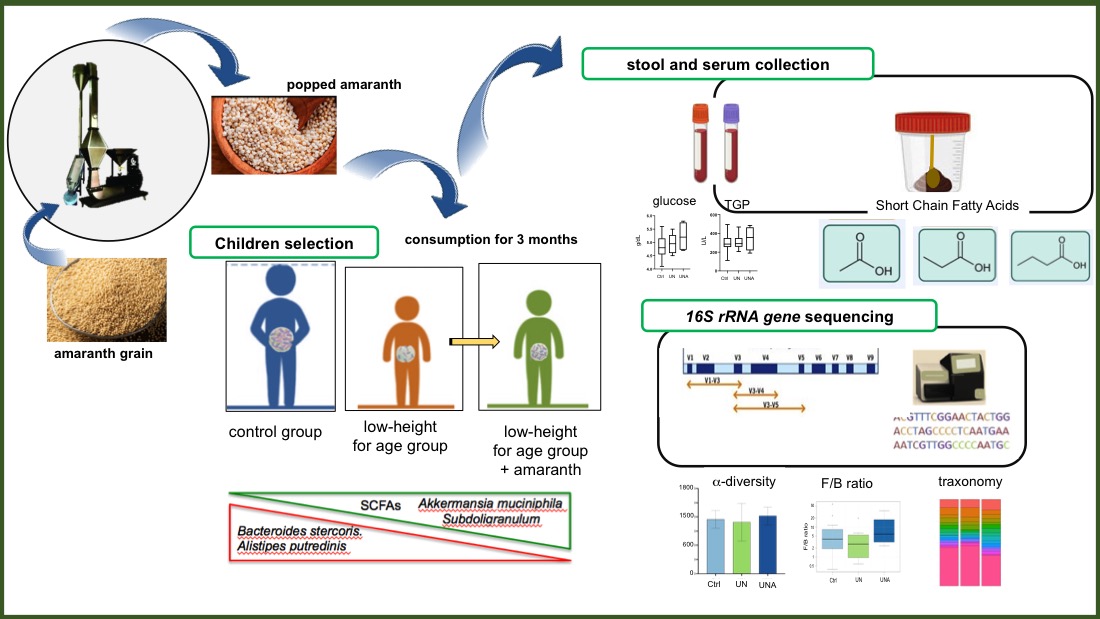
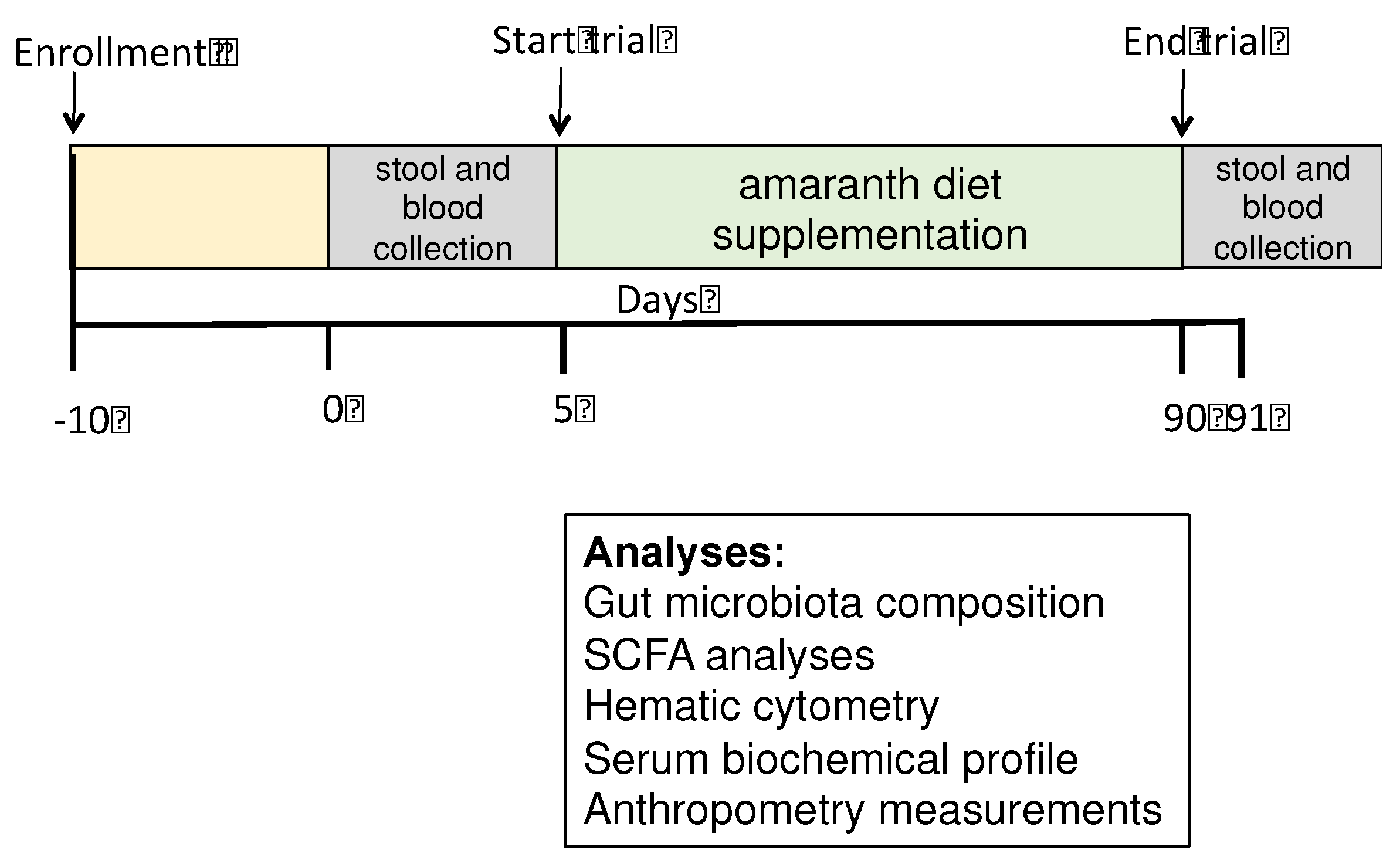
2.1. Recruitment of participants
2.2. Research design
2.3. Preparation of amaranth popped grain
2.4. Research outcome
2.5. Serum collection and biochemical profile analysis
2.6. Stool sample collection
2.6.1. Measurement of fecal Short-Chain Fatty Acids (SCFAs)
2.6.2. DNA extraction and integrity verification
2.7. 16S rRNA gene sequencing and bioinformatics analyses
2.7.1. Amplicon Sequence Variant inference
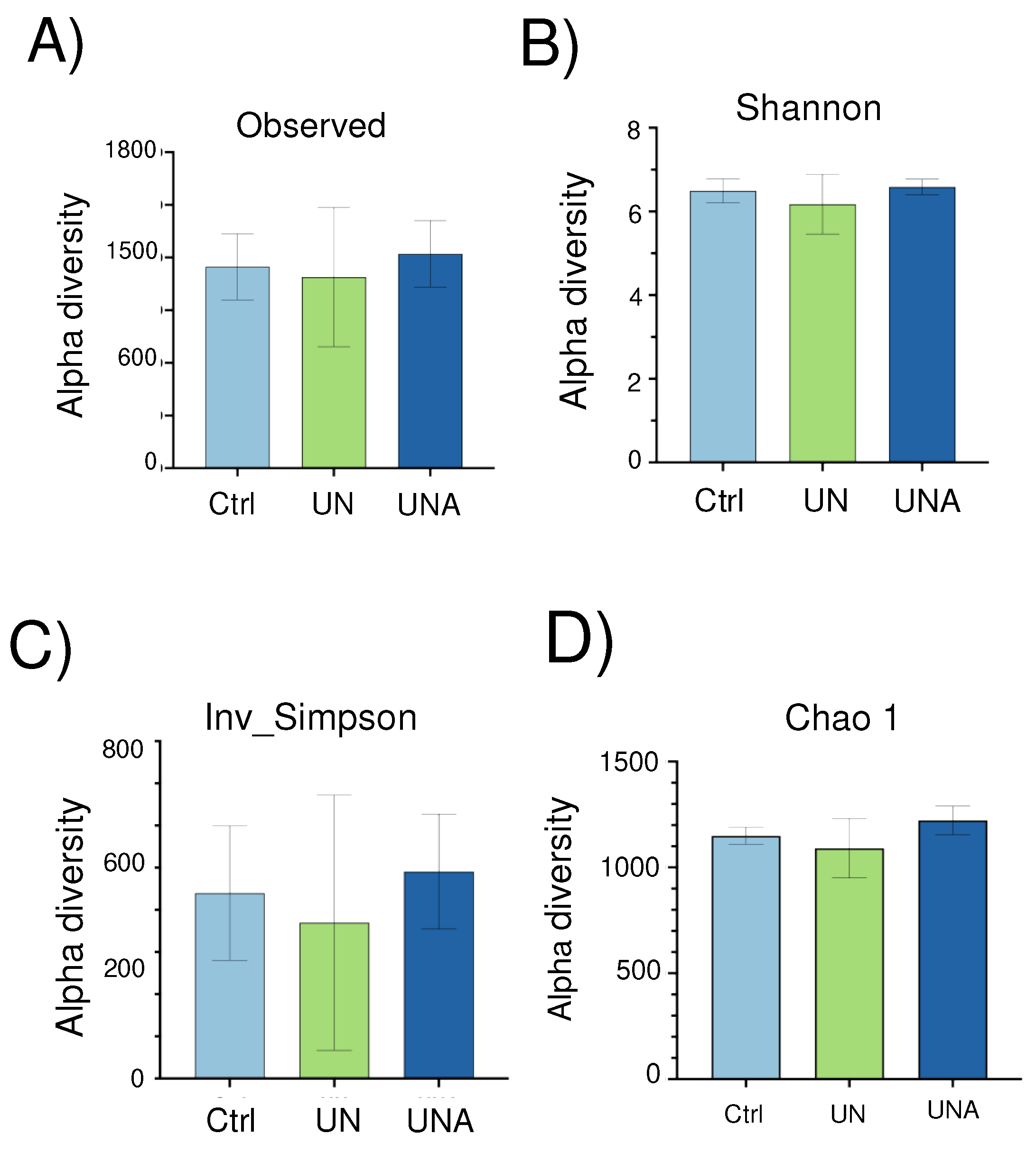
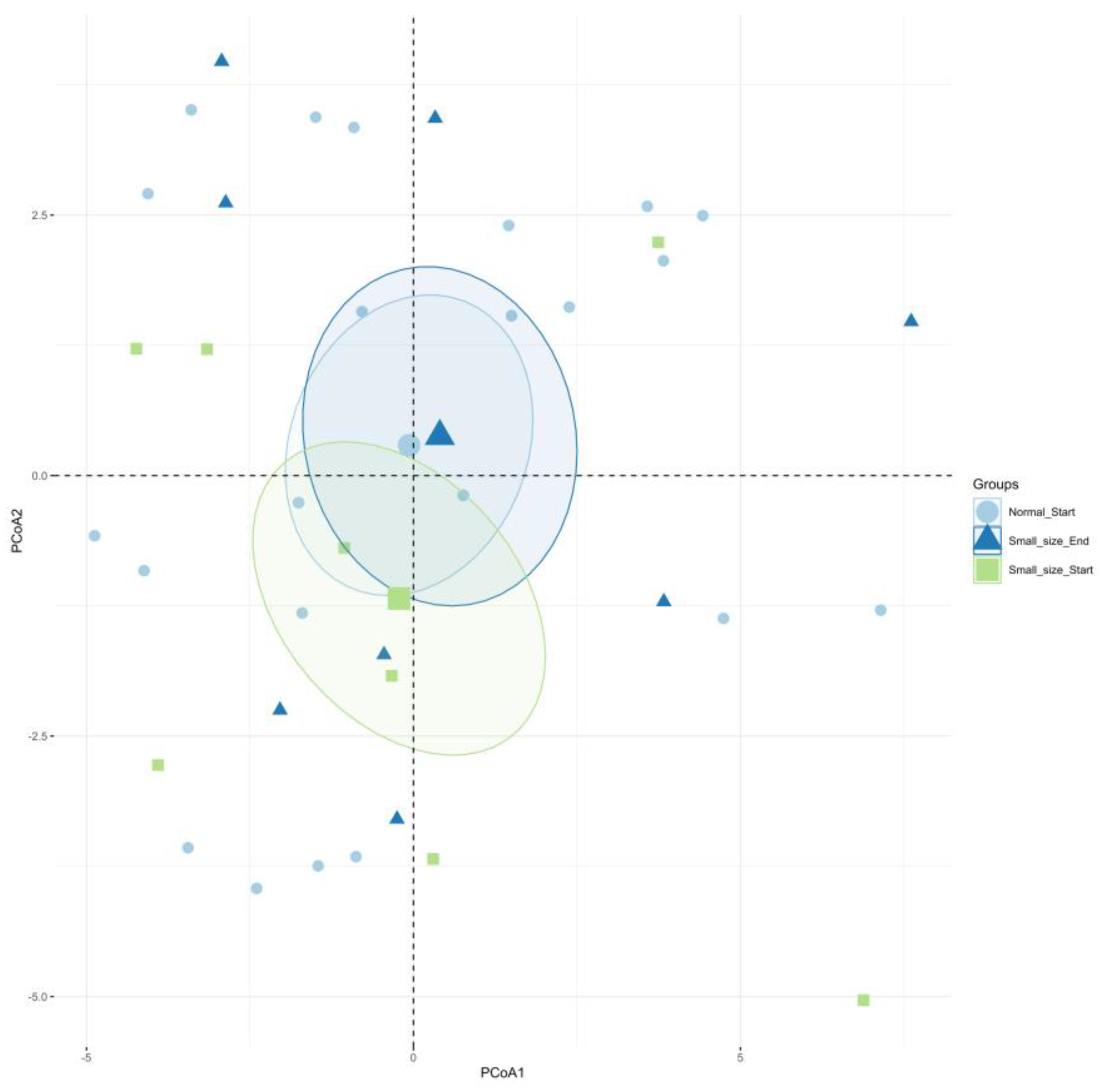
2.7.2. Diversity quantification and functional prediction
2.8. Statistical analyses
3. Results and Discussion
3.1. Popped amaranth as minimally processed food
3.2. Children´s selection and serum biochemical analysis
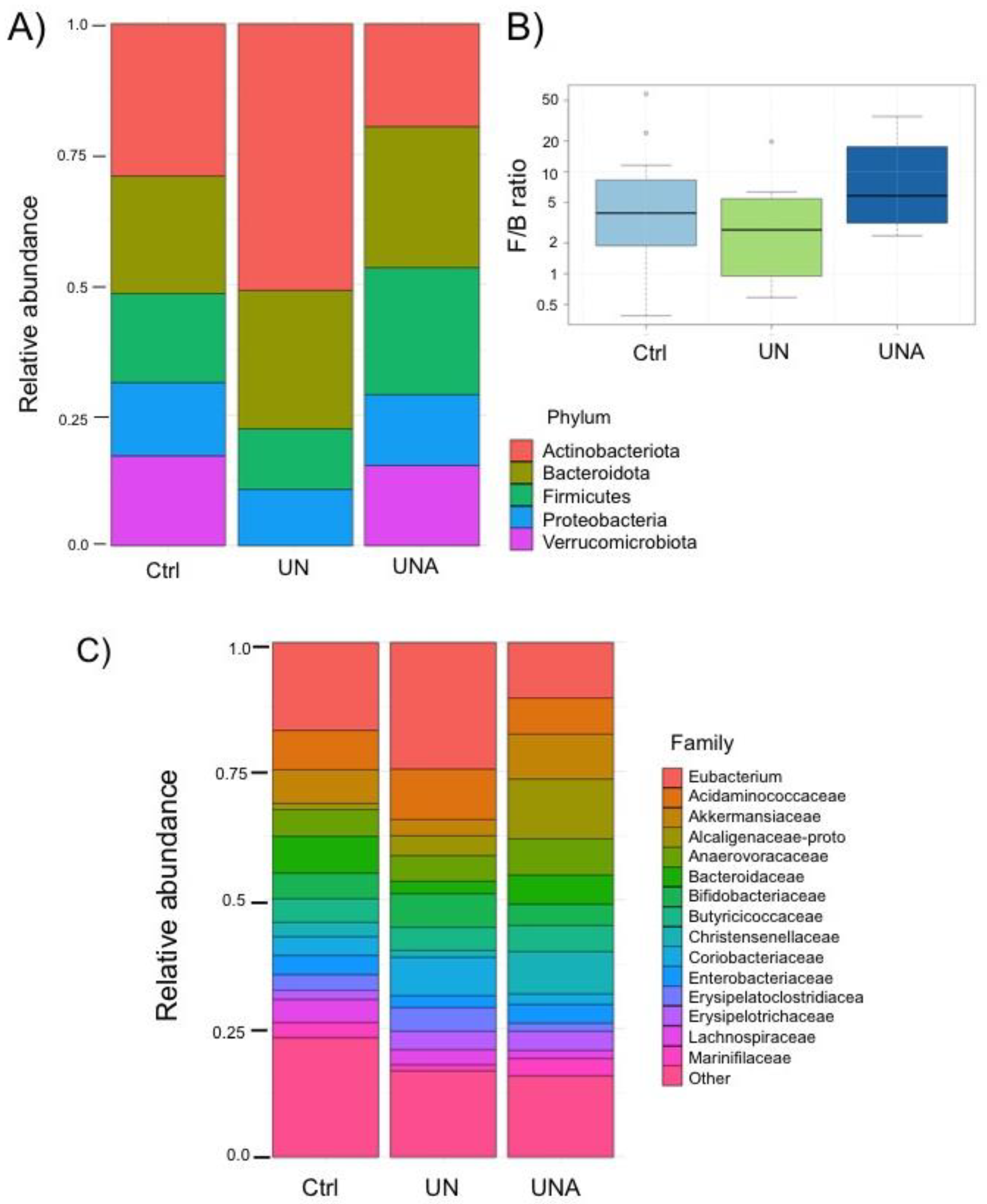
3.3. Modulation of gut microbiota in undernutrition children group after amaranth consumption
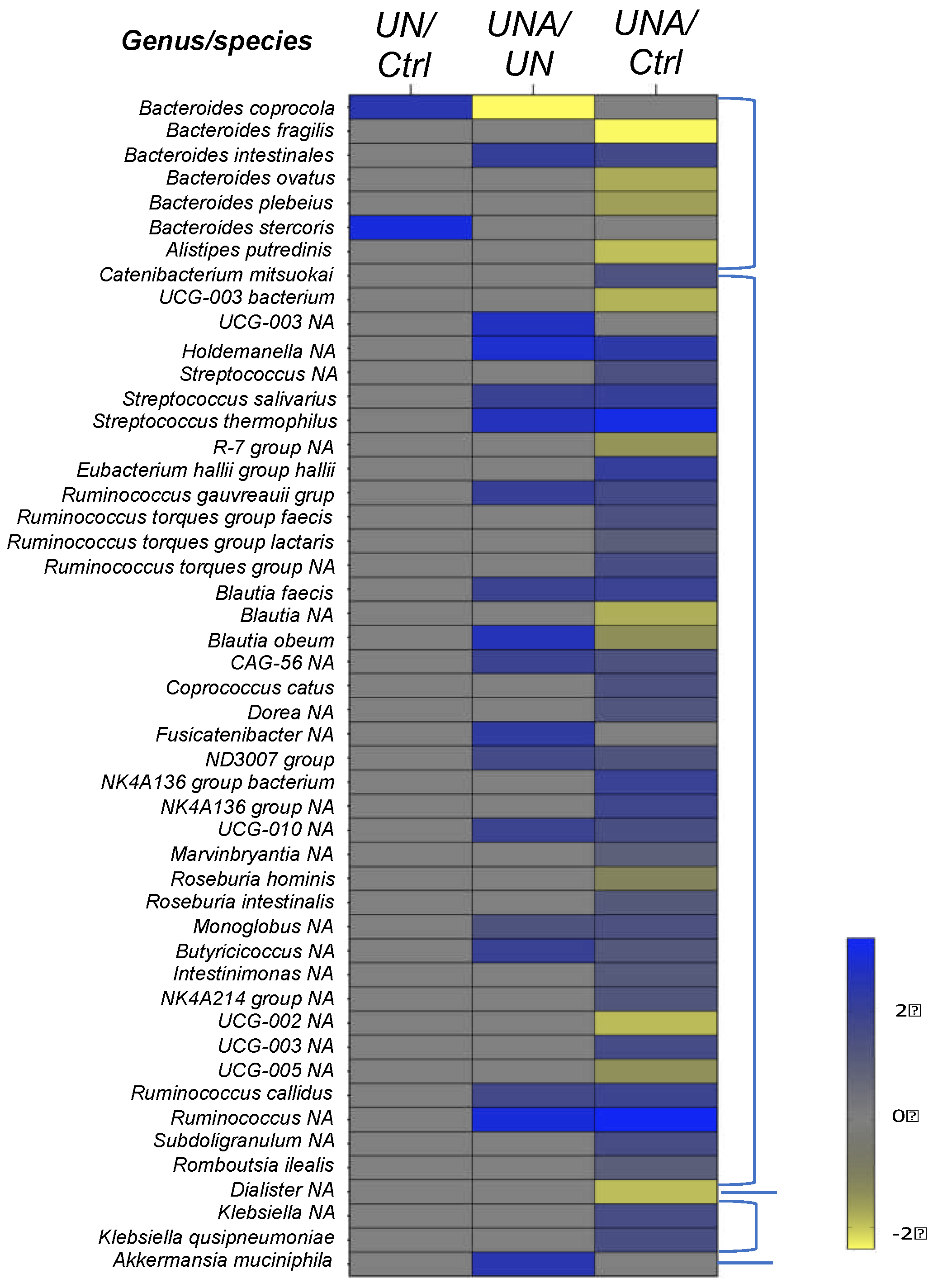
3.4. Modulation of gut microbiota at family-genus level was observed in undernourished children after amaranth consumption
3.4.1. Changes in Firmicutes phylum at genus/species level
3.4.2. Changes in Bacteroidetes phylum at genus/species level
3.4.3. Changes in Verrucomicrobiota phylum
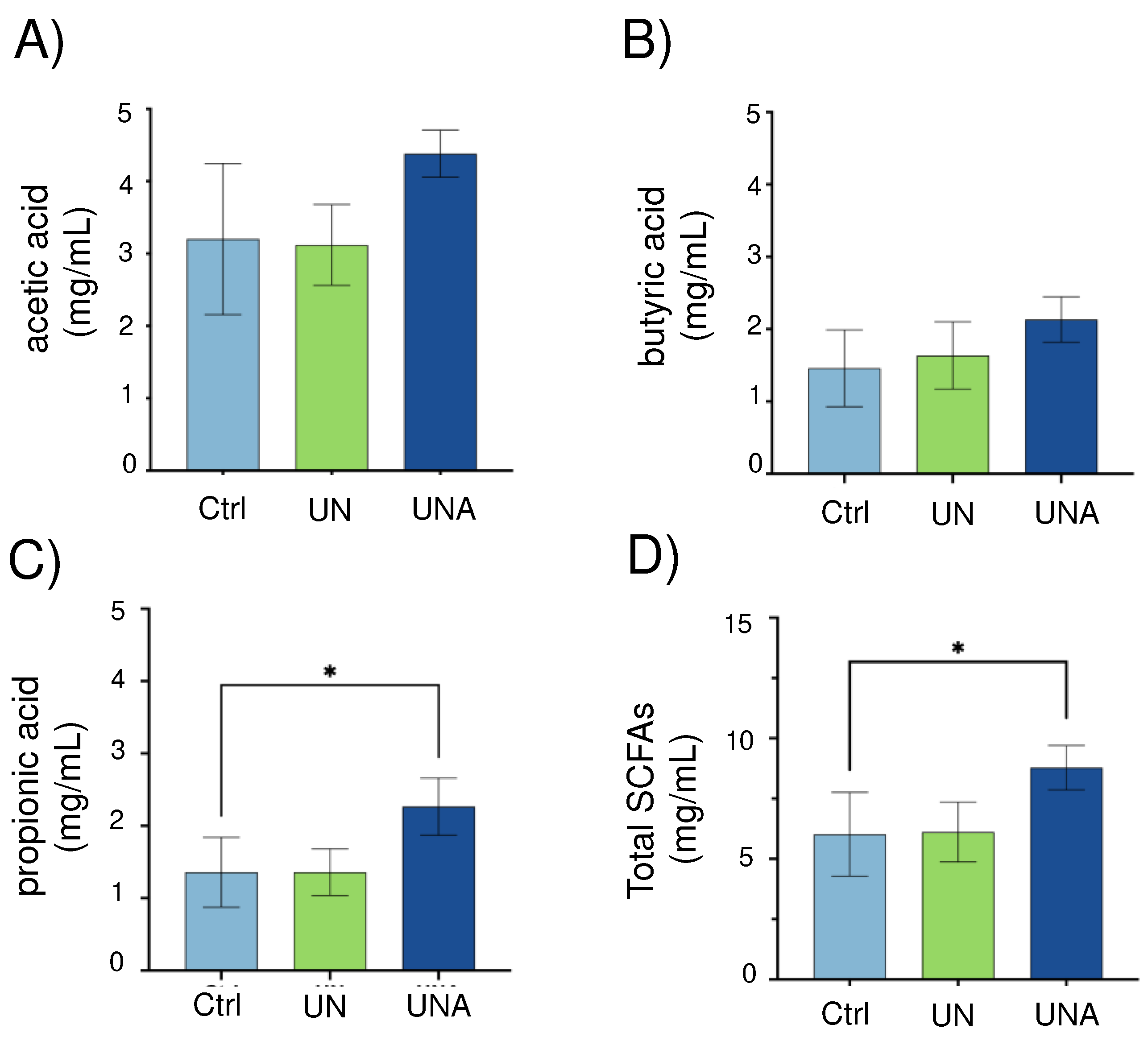
3.5. Amaranth consumption promotes gut microbiota-dependent metabolites
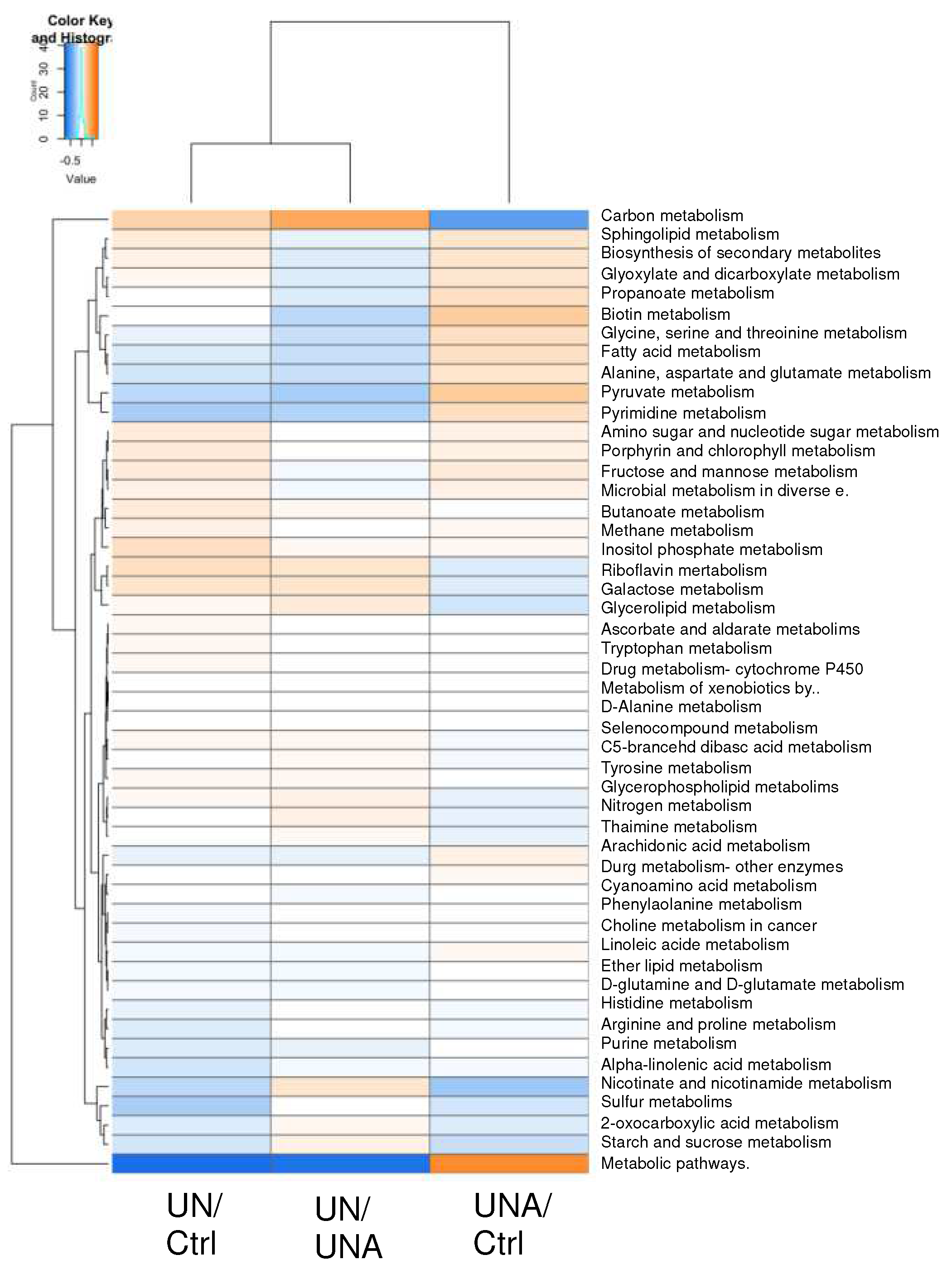
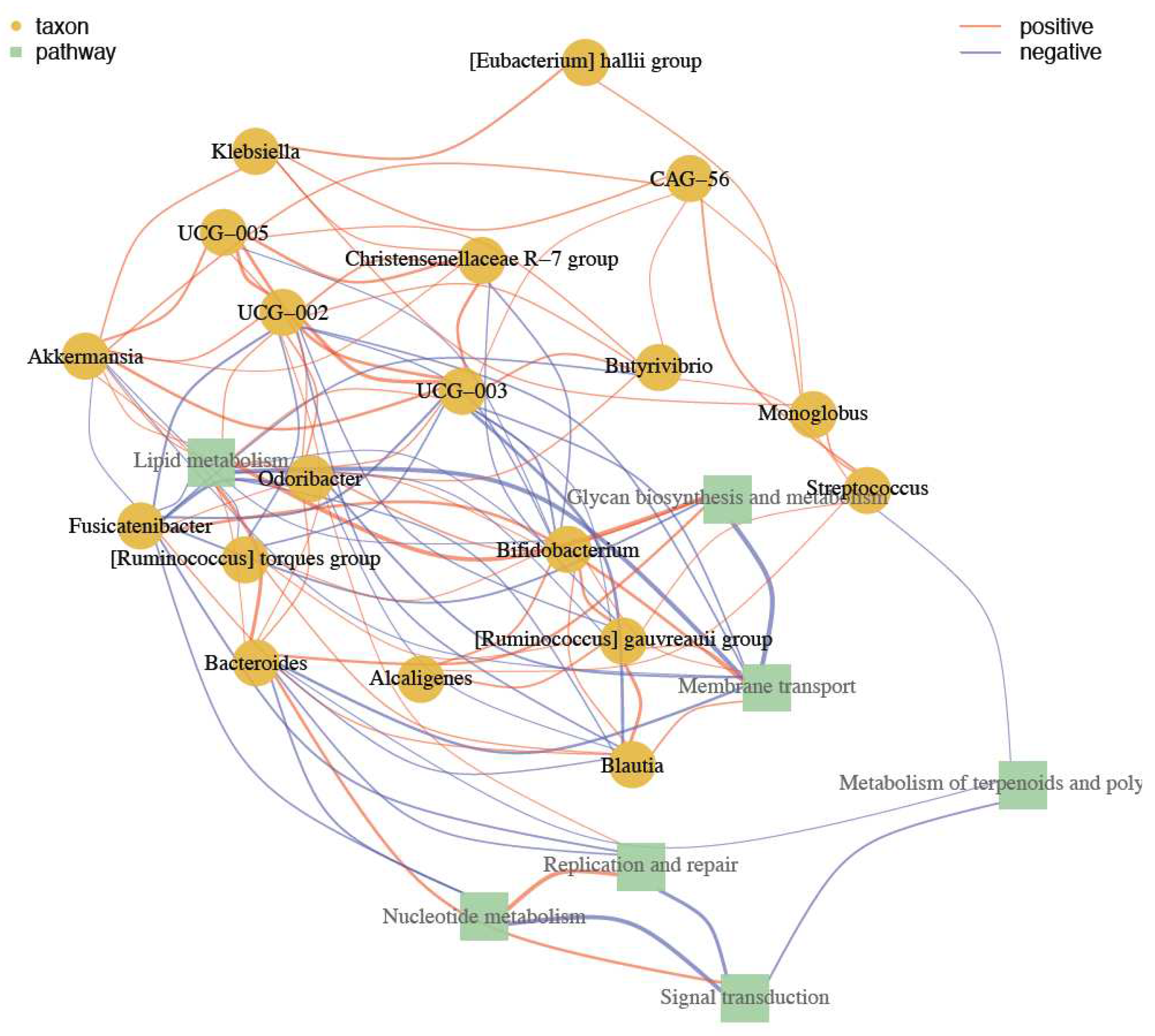
3.8. Functional prediction of bacterial taxa
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gordon, J.I.; Dewey, K.G.; Mills, D.A.; Medzhitov, R.M. The human gut microbiota and undernutrition. Sci. Transl. Med. 2012, 4, 1–12. [Google Scholar] [CrossRef]
- Blanton, L.V.; Barratt, M.J.; Charbonneau, M.R.; Ahmed, T.; Gordon, J. Childhood undernutrition, the gut microbiota, and microbiota-directed therapeutics. Science 2016, 352(6293), 1533. [Google Scholar] [CrossRef]
- WHO/UNICEF. Levels and trends in child malnutrition: UNICEF/WHO/The World Bank Group joint child malnutrition estimates: key findings of the 2021 edition. Retrieved October 2022. https://www.who.int/publications/i/item/9789240025257.
- Prendergast, A.J.; Humphrey, J.H. The stunting syndrome in developing countries. Paediatr. Int. Child Health 2014, 34, 250–265. [Google Scholar] [CrossRef] [PubMed]
- Iddrisu, I.; Monteagudo-Mera, A.; Poveda, C.; Pyle, S.; Shahzad, M.; Andrews, S.; Walton, G.E. Malnutrition and gut microbiota in children. Nutrients 2021, 13, 2727. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gallego, C.; García-Mantrana, I.; Martínez-Costa, C.; Salmien, S.; Isolauri, E.; Collado, M.C. The microbiota and malnutrition: Impact of nutritional status during early life. Annu. Rev. Nutr. 2019, 39, 267–290. [Google Scholar] [CrossRef]
- Calder, N.; Walsh, K.; Olupot-Olupt, P.; Ssenyondo, T.; Muhindo, R.; Mpoya, A.; Brignardello, J.; Wang, X.; McKay, E.; Morrison, D.; Holmes, E.; Frost, G.; Maitland, K. Modifying gut integrity and microbiome in children with severe acute malnutrition using legume-based feeds (MIMBLE): A pilot trial. Cell Reports Medicine 2021, 2, 100280. [Google Scholar] [CrossRef]
- Nova, E.; Gómez-Martinez, S.; González-Soltero, R. The influence of dietary factors on the gut microbiota. Microorganisms 2022, 10, 1368. [Google Scholar] [CrossRef]
- Wu, S.; Bhat, Z. F.; Goudner, R.C.; Mohamed Ahmed, I.A.; Al-Juhaimi, F.Y.; Ding, Y.; Bekhit, A.E-D. Effect of dietary protein and processing on gut microbiota- A systematic review. Nutrients 2022, 14, 453. [Google Scholar] [CrossRef]
- Berding, K.; Vlckova, K.; Marx, W.; Schellekens, H.; Stanton, C.; Clarke, G.; Jacka, F.; Dinan, T.G.; Cryan, J.F. Diet and the microbiota-gut-brain-axis: sowing the seeds of good mental health. Adv. Nutr. 2021, 12, 1239–1285. [Google Scholar] [CrossRef]
- Aloo, S.O.; Oh, D.-H. The functional interplay between gut microbiota, protein hydrolysates/bioactive peptides, and obesity: A critical review on the study advances. Antioxidants 2022, 11, 333. [Google Scholar] [CrossRef]
- Gatya, M.; Nur Fibri, D.L.; Utami, T.; Suroto, D.A.; Rahayu, E.S. Gut microbiota composition in undernourished children associated with diet and sociodemographic factors: A case-control study in Indonesia. Microorganisms 2022, 10, 1748. [Google Scholar] [CrossRef]
- Marques Coelho, L.; Silva, P.M.; Martins, J.T.; Pinheiro, A.C.; Vicente, A.A. Emerging opportunities in exploring the nutritional/functional value of amaranth. Food Funct. 2018, 9, 5499. [Google Scholar] [CrossRef]
- Silva-Sánchez, C.; Barba de la Rosa, A.P.; León-Galván, M.F.; de Lumen, B.O.; De León-Rodríguez, A.; González de Mejía, E. Bioactive peptides in amaranth (Amaranthus hypochondriacus) seed. J. Agric. Food Chem. 2008, 56, 1233–1240. [Google Scholar] [CrossRef]
- Kaufam, C.S.; Weber, L.E. Grain amaranth. In J. Janick, and J.E. Simon (Eds). Advances in new crops 1990 pp. 127–139. Portland: Timber Press.
- Solanki, Ch.; Indore, N.; Saha, D.; Kudos, S.K.A. Effect of popping methods on popping characteristic of amaranth grain. Int. J. Chem. Stud. 2018, 6(2), 2779–2782. [Google Scholar]
- Valadez-Vega, C.; Lugo-Magaña, O.; Figueroa-Hernández, C.; Bautista, M.; Betanzos-Cabrera, G.; Bernardino-Nicanor, A.; González-Amaro, R.M.; Alonso-Villegas, R.; Morales-González, J.A.; González-Cruz, L. Effect of germination and popping on the anti-nutritional compounds and the digestibility of Amaranthus hypochondriacus seeds. Foods 2022, 11, 2075. [Google Scholar] [CrossRef]
- Paško, P.; Bartoń, H.; Folta, M.; Gwizdz, J. Evaluation of antioxidant activity of amaranth (Amaranthus cruentus) grain and by-products (flour, popping, cereal). Rocz. Panstw. Zakl. Hig. 2007, 58(1), 35–40. [Google Scholar] [PubMed]
- Muyonga, J.H.; Andabati, B.; Seepuuya, G. Effect of heat processing on selected grain amaranth physicochemical properties. Food Sci. Nutri. 2014, 2, 9–16. [Google Scholar] [CrossRef]
- Aderibigbe, O.R.; Ezekiel, O.O.; Owolade, S.O.; Korese, J.K.; Sturm, B.; Hensel, O. Exploring the potentials of underutilized grain amaranth (Amaranthus spp.) along the value chain for food and nutrition security: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Grundy, M.M.L.; Momanyi, D.K.; Holland, C.; Kawaka, F.; Tan, S.; Salim, M.; Boyd, B.J.; Bajka, B.; Mulet-Cabero, A-I; Bishop, J.; Owino, W,O. Effects of grain source and processing methods on the nutritional profile and digestibility of grain amaranth. J. Funct. Foods 2020, 72, 104065. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis. 18th ed. Association of Official Analytical Chemists, 2007, Washington, D.C.
- Gao, L.; Zhang, L.; Liu, H.; Hu, J. In vitro gastrointestinal digestion of whole grain noodles supplemented with soluble dietary fiber and their effects on children fecal microbiota. Food Biosci. 2023, 53, 102600. [Google Scholar] [CrossRef]
- Comeau, A.M.; Li, W.K.W.; Tremblay, J.E.; Carmack, E.C.; Lovejoy, C. Arctic ocean microbial community structure before and after the 2007 record sea ice minimum. PLoS ONE 2011, 6, e27492. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O´Hara, R.B.; Solymos, P., ... Weedon, J. Vegan: Community ecology package. R Package 2015, Version 2.2-1 2 1-2.
- Wemheuer, F.; Taylor, J.A.; Daniel, R.; Johnston, E.; Meinicke, P.; Thomas, T.; Wemheuer, B. Tax4Fun2: prediction of habitat-specific functional profiles and functional redundancy based on 16S rRNA gene sequences. Environ. Microbiome 2020, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.-A. mixOmics: An R package for “omics” feature selection and multiple data integration. PloS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef]
- Csardi, G.; Nepusz, T. The igraph software package for complex network research. Int. J. Complex Syst. 2006, 1695, 1–9. [Google Scholar]
- Morales, E.; Lembcke, J.; Graham, G.G. Nutritional value for young children of grain amaranth and maize-amaranth mixtures: Effect of processing. J. Nutr. 1988, 118, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Lozano, A.M. Los cuerpos divinos: el amarnato, comida ritual y cotidiana. Amarnato fuente de alegría Arqueología Mexicana 2016, 23, 26–33. [Google Scholar]
- Getawa, S.; Getaneh, Z.; Melku, M. Hematological abnormalities and associated factors among undernourished under-five children attending university of Gondar specialized referral hospital, Northwest Ethiopia. J. Blood Med. 2020, 11, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D. Amplicon sequence variants artificially split bacterial genomes into separate clusters. mSphere 2021, 6, e0019–21. [Google Scholar] [CrossRef]
- Qi, Ch.; Cai, Y.; Qian, K.; Li, X.; Ren, J.; Wang, P.; Fu, T.; Zhao, T.; Cheng, L; Shi, L; Zhang, X. gutMDisorder v2.0: a comprehensive database for dysbiosis of gut microbiota in phenotypes and interventions. Nucl. Acids Res. 2022, 51, D717–D722. [Google Scholar] [CrossRef]
- Monira, Sh.; Nakamura, Sh.; Gotoh, K.; Izutsu, K.; Watanabe, H.; Alam, N.H.; Endtz, H.P.; Cravioto, A.; Ali, S.I.; Nakaya, T.; Horii, T.; Lida, T.; Alam, M. Gut microbiota of healthy and malnourished children in Bangladesh. Front. Microbiol. 2011, 2, 228. [Google Scholar] [CrossRef]
- Kamil, R.Z.; Murdiati, A.; Juffrie, M.; Nakayama, J.; Rahayu, E.S. Gut microbiota and shot-chain fatty acid profile between normal and moderate malnutrition children in Yogyakarta, Indonesia. Microorganisms 2021, 9, 127. [Google Scholar] [CrossRef]
- Subramanian, S.; Hug, S.; Yatsunenko, T.; Haque, R.; Mahfuz, M.; Alam, M.A.; Benezra, A.; DeStefano, J.; Meier, M.F.; Muegge, B.D.; Barratt, M.J.; VanArendonk, L.G.; Zhang, Q.; Province, M.A.; Petri Jr, W.A.; Ahmed, T.; Gordon, J.I. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 2014, 510, 417–421. [Google Scholar] [CrossRef]
- Kim, K.; Veerappan, K.; Woo, N.; Park, B.; Natarajan, S.; Chung, H.; Kim, Ch.; Park, J. Ulmus macrocarpa Hance extract modulates intestinal microbiota in healthy adults: a randomized, placebo-controlled clinical trial. J. Microbiol. 2021, 59, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Guo, H.; Teng, X.; Yang, X.; Qin, P.; Richel, A.; Zhang, L.; Blecker, Ch.; Ren, G. Supplementation of quinoa peptides alleviates colorectal cancer and restores gut microbiota in AOM/DSS-treated mice. Food Chem. 2023, 408, 135196. [Google Scholar] [CrossRef] [PubMed]
- Kamil, R.Z.; Murdiati, A.; Juffrie, M.; Rahayu, E.S. Gut microbiota modulation of moderate undernutrition in infants through gummy Lactobacillus plantarum Dad-13 consumption: A randomized double-blind controlled trial. Nutrients 2022, 14, 1049. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Méndez-Salazar, E.O.; Ortiz-López, M.G.; Granados-Silvestre, M.A.; Palacios-González, B.; Menjivar, M. Altered gut microbiota and compositional changes in Firmicutes and Proteobacteria in Mexican undernourished and obese children. Front. Microbiol. 2018, 9, 2494. [Google Scholar] [CrossRef]
- Sutoyo, D.A.; Atmaka, D.R.; Sidabutar, L.M.G.B. Dietary factors affecting Firmicutes and Bacteroidetes ratio in solving obesity problem: A literature review. J. Media Gizi Indon. 2020, 15, 94. [Google Scholar] [CrossRef]
- Bervoets, L.; Vankerckhoven, K.V.; Kortleven, I.; Noten, C.V.; Hens, N.; Vael, C.; Goossens, H.; Desager, K.N.; Vankerckhoven, V. Differences in gut microbiota composition between obese and lean children: A cross-sectional study. Gut Pathog. 2013, 5, 10. [Google Scholar] [CrossRef]
- Kim, B.-S.; Choi, C.W.; Shin, H.; Jin, S.-P.; Bae, J.-S.; Han, M.; Seo, E.Y.; Chun, J.; Chung, J.H. Comparison of the gut microbiota of centenarians in longevity villages of South Korea with those of other age groups. J. Microbiol. Biotechnol. 2019, 29, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.W.; de Souza, R.; Kendall, C.W.C.; Emam, Z.; Jenkins, D.J.A. Colonic health: fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Udayappan, S.; Manneras-Holm, L.; Chaplin-Scott, A.; Belzer, C.; Herrera, H.; Dallinga-Thie, G.M.; Duncan, S.H.; Stroes, E.S.G.; Groen, A.K.; Flint, H.J.; Backhed, F.; de Vos, W.M.; Nieuwdorp, M. Oral treatment with Eubacterium hallii improves insulin sensitivity in db/db mice. NPJ Biofilms Microbiomes 2016, 6, 16009. [Google Scholar] [CrossRef]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, Sh.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia-a new functional genus with potential probiotic properties? Gut Microbes 2021, 13, 1–21. [Google Scholar] [CrossRef]
- Shi, Z.; Zhu, Y.; Teng, C.; Yao, Y.; Ren, G.; Richel, A. Anti-obesity effects of a-amylase inhibitor enriched-extract from white common beans (Phaseolus vulgaris L.) associated with the modulation of gut microbiota composition in high-fat diet-induced obese rats. Food Funct. 2020, 11, 1624–1634. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The influence of probiotics on the Firmicutes/Bacteroides ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Song, L.; He, M.; Sun, Q.; Wang, Y.; Zhang, J.; Fang, Y.; Liu, Sh.; Duan, L. Roseburia hominis: Increases intestinal melatonin level by activating p-CREB-AANAT pathway. Nutrients 2022, 14, 117. [Google Scholar] [CrossRef]
- Nie, K.; Ma, K.; Luio, W.; Shen, Zh.; Yang, Zh.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia intestinalis: A beneficial gut organism from the discoveries in genus species. Front. Cell. Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Alou, M.T.; Khelaifia, S.; Bachar, D.; Lagier, J.-Ch.; Dione, N.; Brah, S.; Hugon, P.; Lombard, V.; Armougom, F.; Fromonot, J.; Robert, C.; Michelle, C.; Diallo, A.; Fabre, A.; Guieu, R.; Sokhna, Ch.; Henrissat, B.; Parola, P.; Raoult, D. Increased gut redox and depletion of anaerobic and methanogenic prokaryotes in severe acute malnutrition. Sci. Rep. 2016, 6, 26051. [Google Scholar] [CrossRef] [PubMed]
- Romaní-Pérez, M.; López-Almela, I.; Bullich-Vilarrubias, C.; Rueda-Ruzafa, L.; Gómez Del Pulgar, E.M.; Benítez-Páez, A.; Liebish, G.; Lamas, J.A.; Sanz, Y. Holdemanella biformis improves glucose tolerance and regulates GLP-1 signaling in obese mice. FASEB J. 2021, 35, e21734. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, H.; Hollis, J.H.; Jacques, P.F. The associations between yogurt consumption, diet quality, and metabolic profiles in children in the USA. Eur. J. Nutr. 2015, 54, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Delorme, C.; Abraham, A.-L.; Renault, P.; Guédon, E. Genomics of Streptococcus salivarius, a major human commensal. Infec. Genet. Evol. 2015, 33, 381–392. [Google Scholar] [CrossRef]
- Van Hul, M.; Le Roy, T.; Prifti, E.; Dao, M.C.; Paquot, A.; Zucker, J.-D.; Delzenne, N.M.; Muccioli, G.; Clément, K.; Cani, P.D. From correlation to causality: the case of Subdoligranulum. Gut Microbes 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Boesmans, L.; Valles-Colomer, M.; Wang, J.; Eeckhaut, V.; Falony, G.; Ducatelle, R.; Van Immerseel, F.; Raes, J.; Verbeke, K. Butyrate producers as potential next-generation probiots: safety assessment of the administration of Butyricicoccus pullicaecarum to healthy volunteers. mSystems 2018, 3(6), e00094-18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Ch.; Huang, J.; Xie, M.; Li, X.; Fu, L. Butyricicoccus plays a key role in mediating the antagonism between probiotic and antibiotic on food allergy. Food Agric. Immunol. 2019, 30, 446–461. [Google Scholar] [CrossRef]
- Wang, L.-J.; Yang, Ch.-Y.; Chou, W.-J.; Lee, M.-J.; Chou, M.-Ch.; Kuo, H.-Ch.; Yeh, Y.-M.; Lee, Sh.Y.; Huang, L.-H.; Li, S.-Ch. Gut microbiota and dietary patterns in children with attention-deficit/hyperactivity disorder. Eur. Child Adolesc. Psychiatry 2020, 29, 287–297. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Hu, Sh.; Zhang, J.; Wu, J.; Shao, N.; Bo, X.; Ni, M.; Ying, X. Gut metagenomes of type 2 diabetic patients have characteristic single-nucleotide polymorphism distribution in Bacteroides coprocola. Microbiome 2017, 5, 15. [Google Scholar] [CrossRef]
- Calderón-Pérez, L.; Gosalbes, M.J.; Yuste, S.; Valls, R.M.; Pedret, A.; Llauradó, E.; Jimenez-Hernandez, N.; Artacho, A.; Pla-Pagà, L.; Companys, J.; Ludwig, I.; Romero, M.P.; Rubió, L.; Solà, R. Gut metagenomic and short chain fatty acids signature in hypertension: a cross-sectional study. Sci. Rep. 2020, 10, 6436. [Google Scholar] [CrossRef]
- Nomura, K.; Ishikawa, D.; Okahara, K.; Ito, Sh.; Haga, K.; Takahashi, M.; Arakawa, A.; Shibuya, T.; Osada, T.; Kuwahara-Arai, K.; Kirikae, T.; Nagahara, A. Bacteroidetes species are correlated with disease activity in ulcerative colitis. J. Clin. Med. 2021, 10, 1749. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, D.; Zeng, W.; Chen, Y.; Guo, M.; Lu, B.; Li, H.; Sun, Ch.; Yang, L.; Jiang, X.; Gao, Q. The role of intestinal dysbacteriosis induced arachidonic acid metabolism disorder in inflammaging in atherosclerosis. Front. Cell. Infect. Microbiol. 2021, 11, 618265. [Google Scholar] [CrossRef]
- Chu, H.; Khosravi, A.; Kusumawardhani, I.P.; Kwon, A.H.K.; Vasconcelos, A.C.; Cunha, L.D.; Mayer, A.E.; Shen, Y.; Wu, W.-L.; Kambla, A.; Targan, S.R.; Xavier, R.J.; Ernst, P.B.; Green, D.R.; McGovern, D.P.B.; Virgin, H.W.; Mazmanian, S.K. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science 2016, 352, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Yang, Ch.; Mogno, I.; Contijoch, E.J.; Borgerding, J.N.; Aggarwala, V.; Li, Zh.; Siu, S.; Grasset, E.K.; Helmus, D.S.; Dubinsky, M.C.; Mehandru, S.; Cerutti, A.; Faith, J.J. Fecal IgA levels are determined by strain-level differences in Bacteroides ovatus and are modifiable by gut microbiota manipulation. Cell Host Microbe 2020, 27, 467–475. [Google Scholar] [CrossRef]
- Suskun, C.; Killic, O.; Ciftdogan, D.Y.; Guven, S.; Karbuz, A.; Parlakay, A.O.; Kara, Y.; Kacmaz, E.; Sahin, A.; Boga, A.; Isancli, D.K.; Gulhan, B.; Kanik-Yuksek, S.; Kiral, E.; Bozan, G.; Arslanoglu, M.O. Kizil, M.C.; Dinleyci, M.; Us, T.; Varis, A.; Kaya, M.; Vandenplas, Y.; Dinleyici, E.C. Intestinal microbiota composition of children with infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and multisystem inflammatory syndrome (MIS-C). Eur. J. Pediatr. 2022, 181, 3175–3191. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Bakir, M.; Kitahara, M.; Sakamoto, M.; Matsumoto, M.; Benno, Y. Bacteroides intestinalis sp. Nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2006, 56(Pt1), 151–154. [Google Scholar] [CrossRef]
- Hänninen, A.; Tivonen, R.; Pöysti, S.; Belzer, C.; Plovier, H.; Ouwerkerk, J.P.; Emani, R.; Cani, P.D.; De Vos, W.M. Akkermansia mucciniphila induces gut microbiota remodeling and controls islet autoimmunity in NOD mice. Gut 2018, 67, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Del Cerro, E.; Lambea, M.; Félix, J.; Salazar, N.; Gueimonde, M.; De la Fuente, M. Daily ingestion of Akkermansia mucciniphila for one month promotes healthy aging and increases lifespan in old female mice. Biogerontology 2022, 23, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; van Baarlen, P.; Hooiveld, G.; Norin, E.; Müller, M.; de Vos, W.M. Modulation of mucosal immune response, tolerance, and proliferation in mice colonized by the mucin-degrader Akkermansia muciniphila. Front. Microbiol. 2011, 2, 166. [Google Scholar] [CrossRef]
- Han, W.; Zhuang, X.; Liu, Q.; Sun, B.; Miao, H.; Zhang, X. Fermented soy whey induced changes on intestinal microbiota and metabolic influence in mice. Food Sci. Hum. Well. 2022, 11, 41–48. [Google Scholar] [CrossRef]
- Cummings, J.H. Short chain fatty acids in the human colon. Gut 1981, 22(9), 763–779. [Google Scholar] [CrossRef]
- Sun, Y.; O’Riordan, M.X.D. Regulation of bacterial pathogenesis by intestinal short-chain fatty acids. Adv. Appl. Microbiol. 2013, 85, 93–118. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




