Submitted:
19 June 2023
Posted:
21 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methodology
3. Results and Discussions
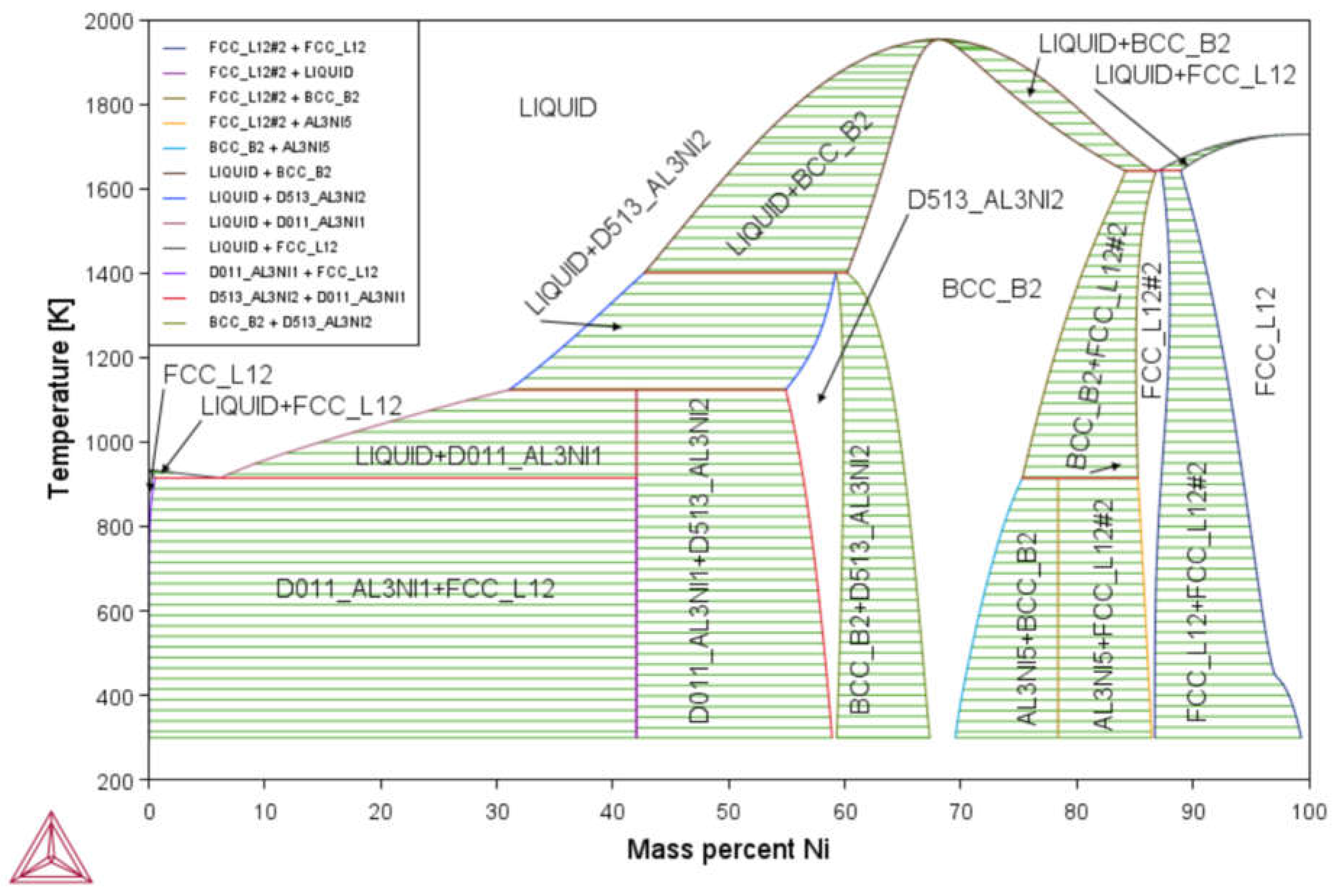
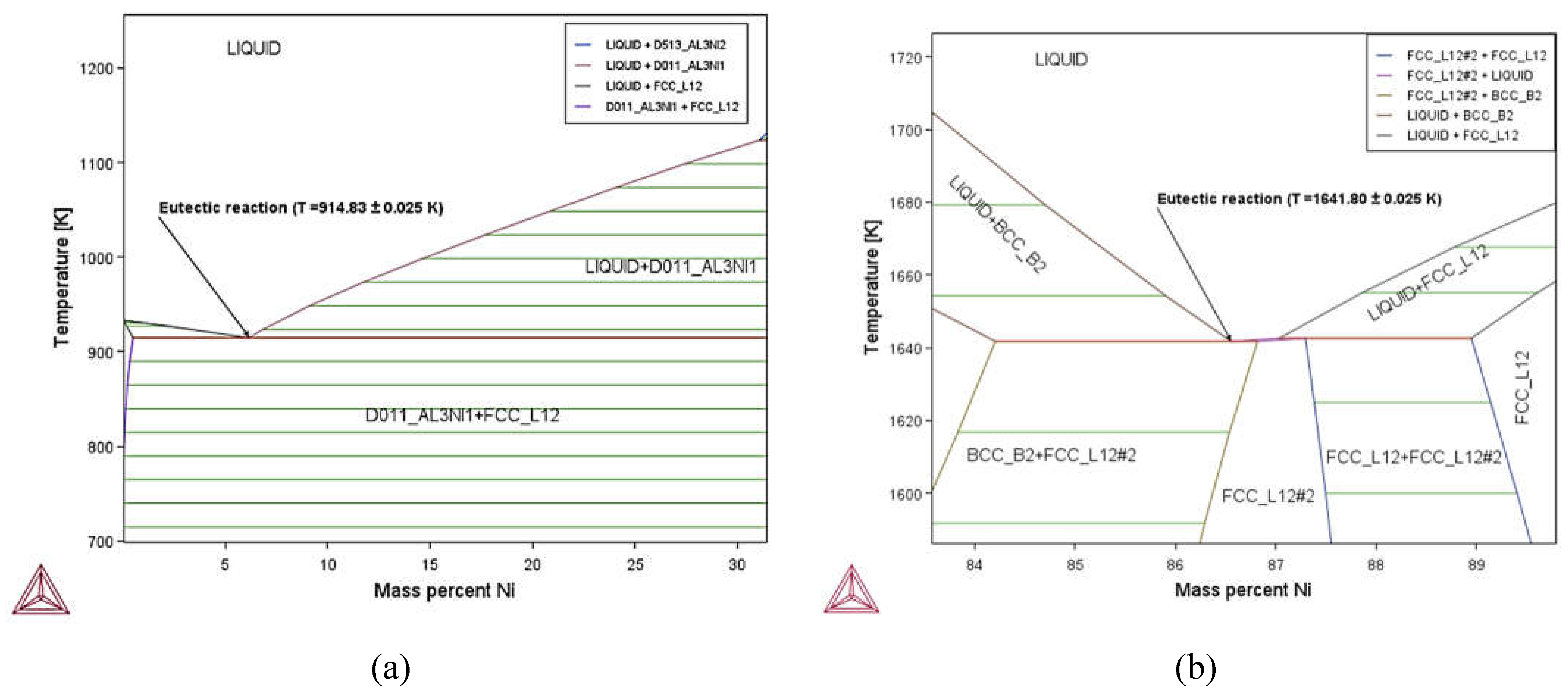
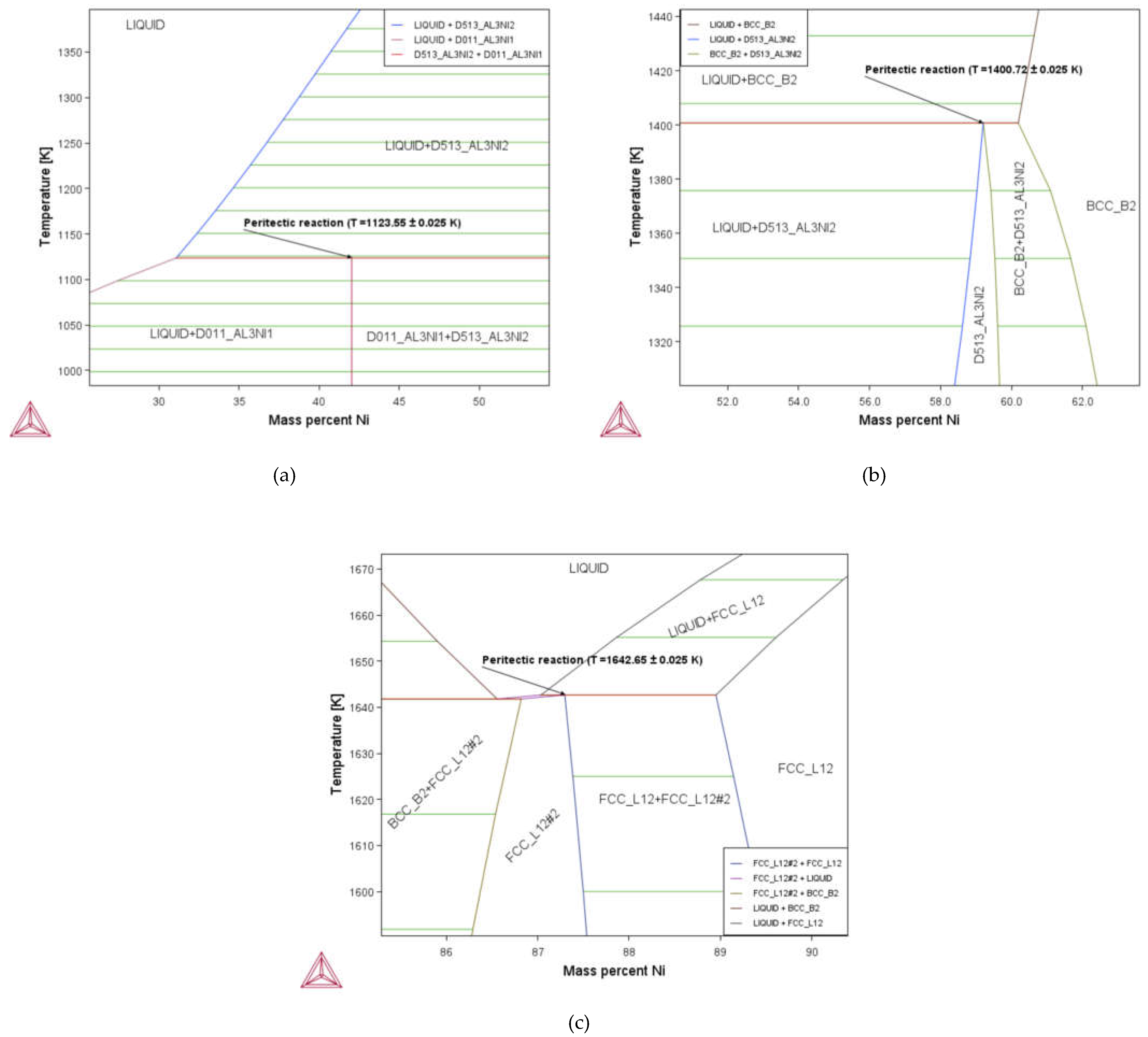
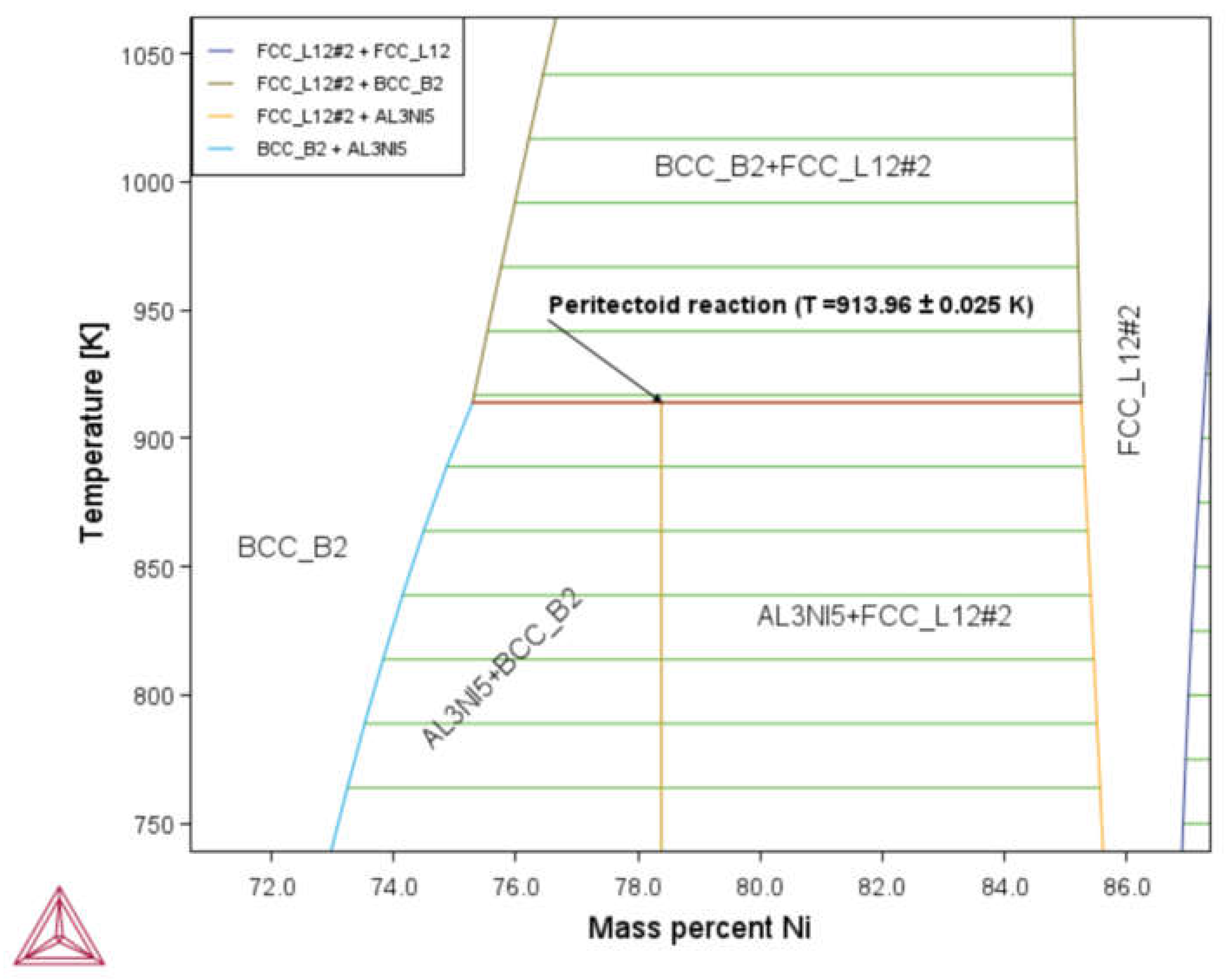
3.1. Phase Diagram Properties
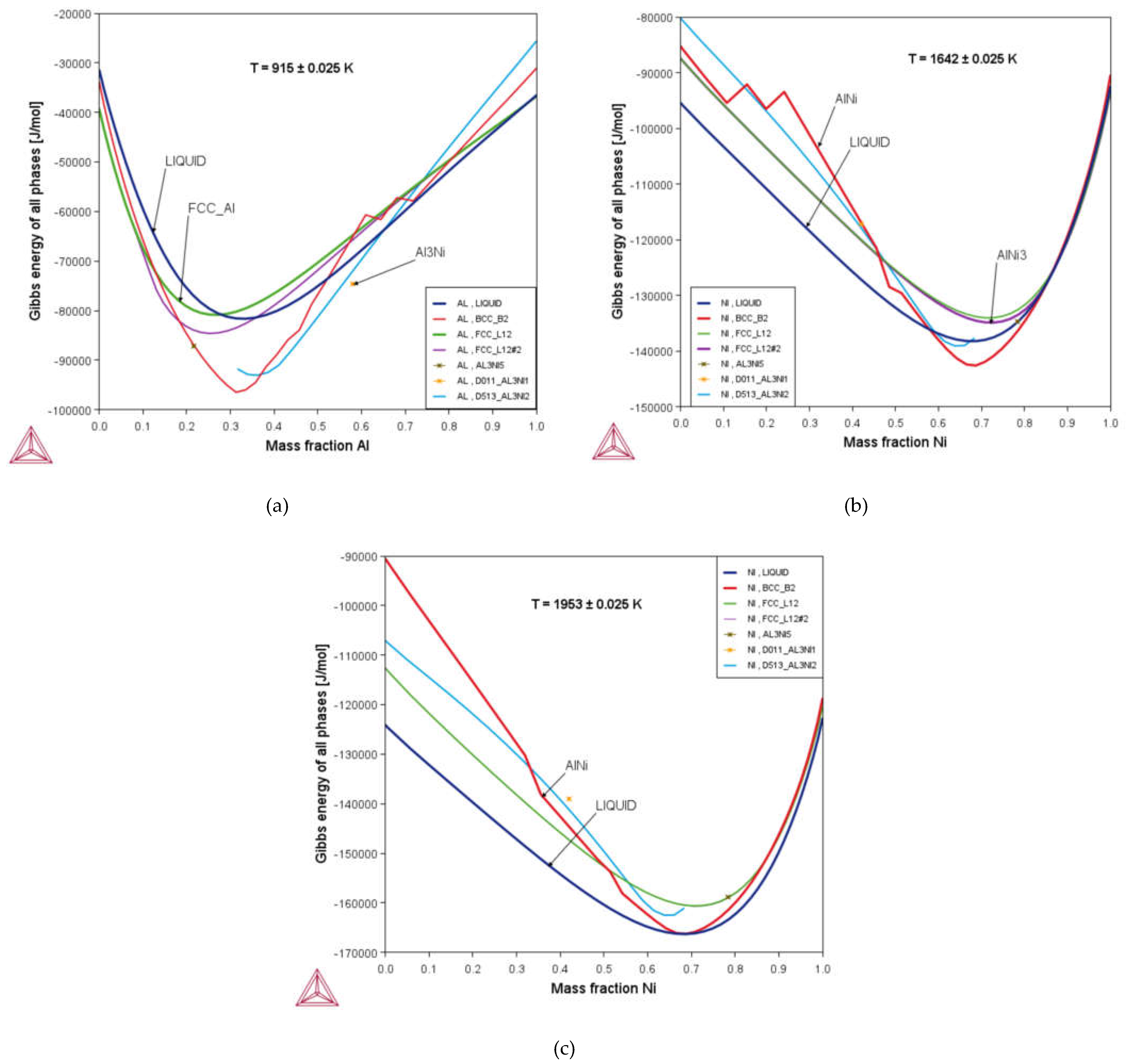
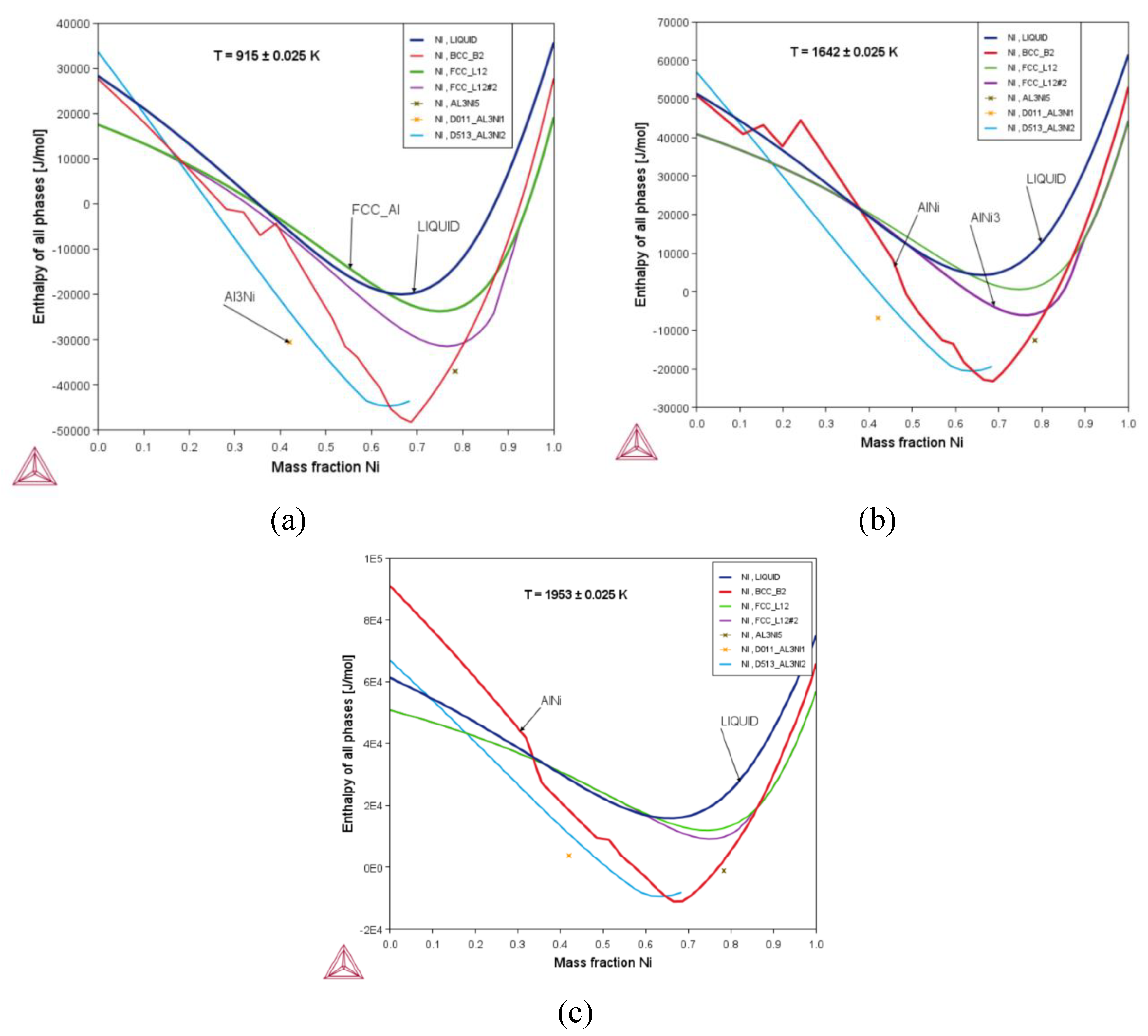
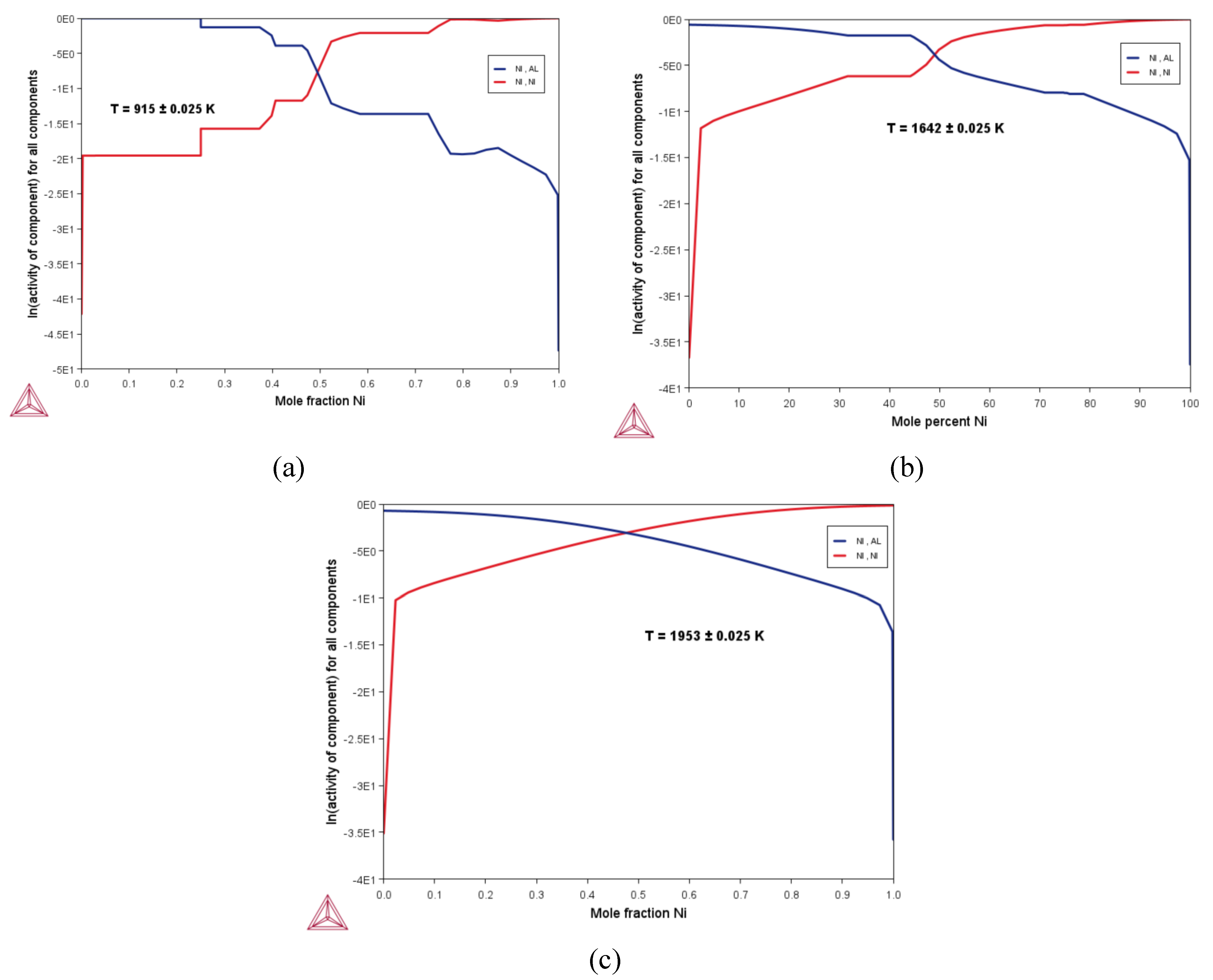
3.2. Thermodynamic Properties
4. Conclusions
Conflict of Interest
References
- T. Turpin, J. Dulcy, M. Gantois, Carbon diffusion and phase transformations during gas carburizing of high-alloyed stainless steels: Experimental study and theoretical modeling, Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2005, 36, 2751–2760. [CrossRef]
- H.L. Chen, Q. H.L. Chen, Q. Chen, A. Engström, Development and applications of the TCAL aluminum alloy database, Calphad Comput. Coupling Phase Diagrams Thermochem. 62 (2018) 154–171. [CrossRef]
- Thermo-Calc, The CALPHAD Methodology - Thermo-Calc Software, (2016). https://thermocalc.com/about-us/methodology/the-calphad-methodology/ (accessed on 28 August 2022).
- S.M. Shah, N. Ullah, B. Ullah, M. Shehzad, T. Usman, Thermodynamic Analysis and Calculations of ( Fe-Co ) Alloy by Modeling and Simulation using Thermo-Calc Software, 1 (2018) 35–38.
- S.J. Ikhmayies, Thermo-Calc Determination of Phase Diagram of Si-B Binary System, Jounral Miner. Met. Mater. Soc. 73 (2020) 253–259. [CrossRef]
- J O, H. T, H. L, S. P, S. B., Thermo-Calc & DICTRA, computational tools for materials science, Calphad. 26 (2002) 273. [CrossRef]
- F. Habashi, Nickel, Physical and Chemical Properties, Encycl. Met. (2013) 1520–1520. [CrossRef]
- Lenntech, Nickel (Ni) - Chemical properties, Health and Environmental effects, (n.d.). https://www.lenntech.com/periodic/elements/ni.htm (accessed 28 September 2022).
- Nickel Element Facts and Properties, (n.d.). https://www.thoughtco.com/nickel-facts-606565 (accessed 28 September 2022).
- Azom, Aluminium: Specifications, Properties, Classifications and Classes, (2005). https://www.azom.com/article.aspx?ArticleID=2863 (accessed 28 September 2022).
- Azom, Aluminium and Aluminium Alloys - Characteristic Advantages and Beneficial Properties of Aluminium Extrusions, (2008). https://www.azom.com/article.aspx?ArticleID=4192 (accessed 28 September 2022).
- Aluminium Alloys in the Aerospace Industry: Past, Present and Future - Jindal Aluminium Limited, (n.d.). https://jindalaluminium.com/aluminium-alloys-in-the-aerospace-industry-past-present-and-future/ (accessed 11 September 2022).
- V. V. Kurbatkina, Nickel Aluminides, Concise Encycl. Self-Propagating High-Temperature Synth. (2017) 212–213. [CrossRef]
- J.H. Wood and E.H. Goldman, Superalloys II, John Wiley, New York, 1987.
- K. Hilpert, D. Kobertz, V. Venugopal, M. Miller, H. Gerads, F.J. Bremer, H. Nickel, Phase Diagram Studies on the Al-Ni System, Zeitschrift Fur Naturforsch. - Sect. A J. Phys. Sci. 42 (1987) 1327–1332. [CrossRef]
- F.J. Bremer, M. F.J. Bremer, M. Beyss, E. Karthaus, A. Hellwig ~, T. Schober, J.-M. Welter, H. Wenzl, EXPERIMENTAL ANALYSIS OF THE Ni-Al PHASE DIAGRAM, J. Cryst. Growth. 87 (1988) 185–192.
- Pasturel, C. Colinet, A.T. Paxton, M. van Schilfgaarde, First-principles determination of the Ni-Al phase diagram, J. Phys. Condens. Matter. 4 (1992) 945–959. [CrossRef]
- J. Schramm, Pha, Z. Met. 33 (1941) 347.
- M. Hansen, K. Anderko, Constitution of Binary Alloys, McGraw-Hill, New York, 1958.
- R.P. Elliott, Constitution of Binary Alloys, First Supplement, McGraw-Hill, New York, 48AD.
- F.A. Shunk, Constitution of Binary Alloys, Second Supplement, McGraw-Hill, New York, 1969.
- W.O. Alexander, N.B. Vaughan, Phase Diagram Updates: Section HI Phase Diagram Updates AI-Ni (Aluminum-Nickel), J. Phase Equilibria. 37 (1937) 257.
- T.B. Massalski, Binary Alloy Phase Diagrams, Am. Soc. Met. 1 (1986) 140.
- P.D. Desai, Ref. Data, J. Phys. Chem. 16 (1987) 109.
- Y. Du, N. Y. Du, N. Clavaguera, Thermodynamic assessment of the Al-Ni system, J. Alloys Compd. 237 (1996) 20–32. [CrossRef]
- Ansara, N. Dupin, H.L. Lukas, B. Sundman, Thermodynamic assessment of the Al-Ni system, J. Alloys Compd. 247 (1997) 20–30. [CrossRef]
- L. Kaufman, H. L. Kaufman, H. Nesor, Coupled phase diagrams and thermochemical data for transition metal binary systems — V, Calphad. 2 (1978) 325–348. [CrossRef]
- A.K. Mallik, Computer calculations of phase diagrams, Buff. Mater. Sci. 8 (1986) 107–121.
- Thermodynamics Software - Thermo-Calc Software, (n.d.). https://thermocalc.com/products/thermo-calc/ (accessed 24 September 2022).
- Thermo-Calc Documentation Set Thermo-Calc Version 2021b, in: Introd. to Thermo-Calc, 2021: p. 341. https://www.thermocalc.com/content/uploads/Documentation/Current_Static/thermo-calc-documentation-set.pdf (accessed 28 August 2022).
- S.J. Ikhmayies, Phase Diagram and Thermodynamic Properties of Cu–O Binary System, Miner. Met. Mater. Ser. 4 (2021) 139–147. [CrossRef]
- R. Baur, V.GEROLD, The Existence of a Metastable Miscibility Gap in Aluminium-Silver Alloys, Acta Metall. 10 (1962) 637–645.
- T.H. and T.S. Kozo Osamura, Takao Nakamura, Akiko Kobayashi, Chemical composition of G.P. zones in Al-Ag Alloys, Pergamon Journals. 21 (1987) 255–258.
- S.S. Lim, P.L. Rossiter, J.E. Tibballs, Assessment of the AI-Ag Binary Phase Diagram, 19 (1995) 131–141.
- K. Osamura, T. Nakamura, A. Kobayashi, T. Hashizume, T. Sakurai, An AP-FIM study on metastable phases in Al-Ag binary alloy, Acta Metall. 34 (1986) 1563–1570. [CrossRef]
- A.J. McAlister, The Ag-AI ( Silver-Aluminum ) System, Bull. Alloy Phase Diagrams. 8 (1987) 526–533.
- T.B. Massalski, H. Okamoto, Binary alloy phase diagrams, ASM Int. (1990).
- Pasturel, C. Colinet, A.T. Paxton, M. Van Schilfgaarde, First-principles determination of the Ni-Al phase diagram, J. Phys. Condens. Matter. 4 (1992) 945–959. [CrossRef]
- G.A. López, S. Sommadossi, W. Gust, E.J. Mittemeijer, P. Zieba, Phase characterization of diffusion soldered Ni/Al/Ni interconnections, Interface Sci. 10 (2002) 13–19. [CrossRef]
- M. Hansen, K. Anderko, H.W. Salzberg, Constitution of binary alloys, Nature. 116 (1925) 693. [CrossRef]
- F.J. Bremer, M. Beyss, E. Karthaus, A. Hellwig, T. Schober, J.M. Welter, H. Wenzl, Experimental analysis of the Ni-Al phase diagram, J. Cryst. Growth. 87 (1988) 185–192. [CrossRef]
- J.D. Verhoeven, J.H. Lee, F.C. Laabs, L.L. Jones, The phase equilibria of N3Al evaluated by directional solidification and diffusion couple experiments, J. Phase Equilibria 1991 121. 12 (2007) 15–23. [CrossRef]
- L. Hou, B. Li, R. Wu, L. Cui, P. Ji, R. Long, J. Zhang, X. Li, A. Dong, B. Sun, Microstructure and mechanical properties at elevated temperature of Mg-Al-Ni alloys prepared through powder metallurgy, J. Mater. Sci. Technol. 33 (2017) 947–953. [CrossRef]
- AmericanElements, Melting Point of Common Metals, Alloys and Other Materials, (2006). http://www.americanelements.com/amp.meltingpoint.html (accessed 10 November 2021).
- Nuclear-Power, Nickel – Melting Point – Boiling Point, (2021). https://www.nuclear-power.net/nickel-melting-point-boiling-point/ (accessed 30 September 2022).
- N. Institute, Key properties of nickel | Nickel Institute, (n.d.). https://nickelinstitute.org/en/about-nickel-and-its-applications/properties-of-nickel/ (accessed 30 September 2022).
- J.D. Cotton. R.D. Noebe and M.J. Kaufman, No Title, J. Phase Equil. 14 (1993) 579.
- Pasturel, C. Colinet, A.T. Paxton, M. Van Schilfgaarde, First-principles determination of the Ni-Al phase diagram, J. Phys. Condens. Matter. 4 (1992) 945. [CrossRef]
- L. Čelko, L. Klakurková, J. Švejcar, Diffusion in Al-Ni and Al-NiCr interfaces at moderate temperatures, Defect Diffus. Forum. 297–301 (2010) 771–777. [CrossRef]
- E. Copland, FORMATION OF γ′-Ni 3 Al VIA THE PERITECTOID REACTION: γ + β (+ Al 2 O 3 ) = γ′ (+ Al 2 O 3 ), (n.d.).
- D. Perkins, Activity Models, (2019). https://serc.carleton.edu/research_education/equilibria/activitymodels.html (accessed 10 January 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



