Submitted:
21 June 2023
Posted:
21 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
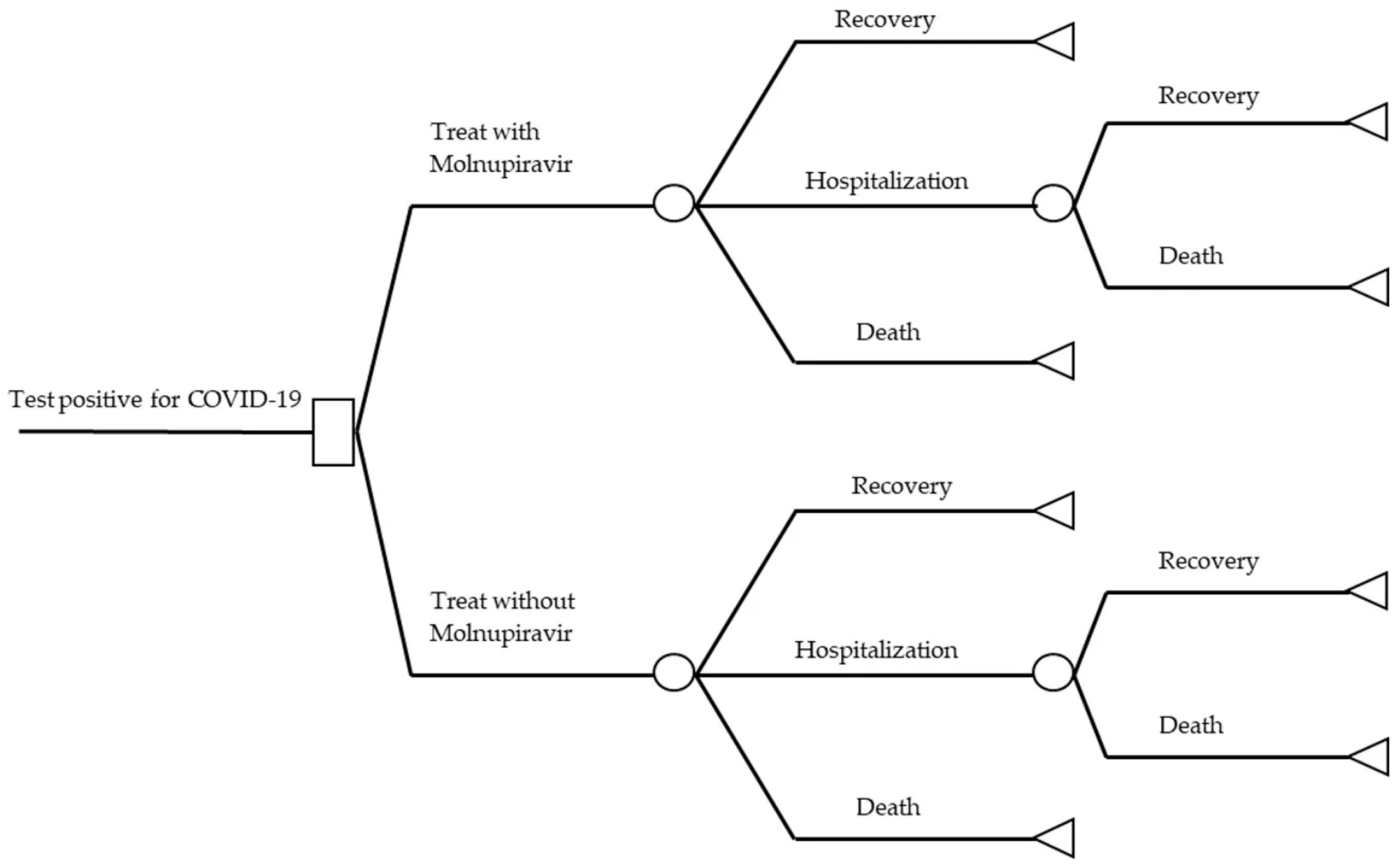
2.1. Study Design
2.2. Economic Model
2.3. Cost Variables
2.4. Clinical Variables
2.5. Health Outcomes
2.6. Uncertainty Analysis
| Parameter description | Value | Distribution | Mean | SE | Reference |
|---|---|---|---|---|---|
| Transitional probability abilities | |||||
| Probability patients received molnupiravir treatment | 0.5036 | Beta | Fischer et al., 2021, Mahase et al., 2021, Jayk Bernal et al., 2021 [24,27,28] | ||
| Probability patients received non-molnupiravir treatment | 0.4964 | Beta | |||
| Probability of recovery using molnupiravir | 0.9323 | Beta | |||
| Probability of hospitalization using molnupiravir | 0.0663 | Beta | |||
| Probability of death using molnupiravir | 0.0014 | Beta | |||
| Probability of hospitalization and recovery using molnupiravir | 0.9444 | Beta | Arribas et al., 2021 [25] | ||
| Probability of hospitalization and death using molnupiravir | 0.0556 | Beta | |||
| Probability of recovery using non-molnupiravir | 0.9027 | Beta | Fischer et al., 2021, Mahase et al., 2021, Jayk Bernal et al., 2021 [24,27,28] | ||
| Probability of hospitalization using non-molnupiravir | 0.0844 | Beta | |||
| Probability of death using non-molnupiravir | 0.0129 | Beta | |||
| Probability of hospitalization and recovery using non-molnupiravir | 0.8516 | Beta | |||
| Probability of hospitalization and death using non-molnupiravir | 0.1484 | Beta | Carta et al., 2021 [26] | ||
| Resource cost parameter | |||||
| Direct medical care cost | |||||
| Direct medical care cost of molnupiravir | 10.83 | Gamma | Sheet Additional information | ||
| Direct medical care cost of hospitalization | 1,476.36 | Gamma | Sheet Additional information Carta et al., 2021 [26] |
||
| Direct medical care cost of quick test | 5.52 | Gamma | Sheet Additional information | ||
| Direct medical care cost of PCR test | 29.53 | Gamma | 14/2019/TT-BYT [29] | ||
| Direct medical care cost of subclinical | 130.41 | Gamma | |||
| Direct non-medical care cost | |||||
| Direct non-medical care cost of eating and drinking for patient (1 day) | 2.31 | Gamma | Thuy et al., 2013 [30] | ||
| Direct non-medical care cost of eating and drinking for caregiver (1 day) | 0.92 | Gamma | |||
| Direct non-medical care cost of moving for patient (1 day) | 0.38 | Gamma | |||
| Direct non-medical care cost of moving for caregiver (1 day) | 0.57 | Gamma | |||
| Direct non-medical care cost of another thing (1 day) | 1.51 | Gamma | |||
| Indirect cost | |||||
| Indirect cost of the patient's opportunity cost (1 day) | 9.44 | Gamma | |||
| Indirect cost of the caregiver's opportunity cost (1 day) | 9.44 | Gamma | |||
| Total cost | |||||
| Total treatment cost of recovery with using molnupiravir | 489.00 | Gamma | Calculation | ||
| Total treatment cost of hospitalization with using molnupiravir | 2,119.78 | Gamma | |||
| Total treatment cost of hospitalization and recovery with using molnupiravir | 344.19 | Gamma | |||
| Total treatment cost of recovery without using molnupiravir | 478.17 | Gamma | |||
| Total treatment cost of hospitalization without using molnupiravir | 2,108.95 | Gamma | |||
| Total treatment cost of hospitalization and recovery without using molnupiravir | 344.19 | Gamma | |||
| Utility parameter | |||||
| Utility of patients using molnupiravir with recovery (mild-moderate) | 0.8900 | ||||
| Utility of patients using molnupiravir with hospitalization and recovery | 0.8900 | Sheinson et al., 2021 [31] | |||
| Utility of patients using non-molnupiravir with recovery | 0.6200 | ||||
| Utility of patients using non-molnupiravir with hospitalization and recovery | 0.7870 | Calculation | |||
| Life-years gained of using antiviral drug | 12.9610 | Sheinson et al., 2021 [31] | |||
| Quality adjusted life years of using antiviral drug | 10.2280 | ||||
| Life-years gained of non-using antiviral drug | 12.4320 | ||||
| Quality adjusted life years of non-using antiviral drug | 9.7900 | ||||
| Discounting rate for course | 3% | WHO | |||
| Discounting rate for outcomes | 3% | ||||
3. Results
3.1. Base Case Analysis
| Content | MPV | Non-MPV |
|---|---|---|
| Utility | 0.89 | 0.62 |
| LYG | 32.00 | 32.00 |
| QALYs | 28.33 | 19.72 |
| Incremental QALYs | 8.6137 | |
| Costs | 617.96 | 634.40 |
| Incremental Costs | -16.44 | |
| ICER | -1.91 | |
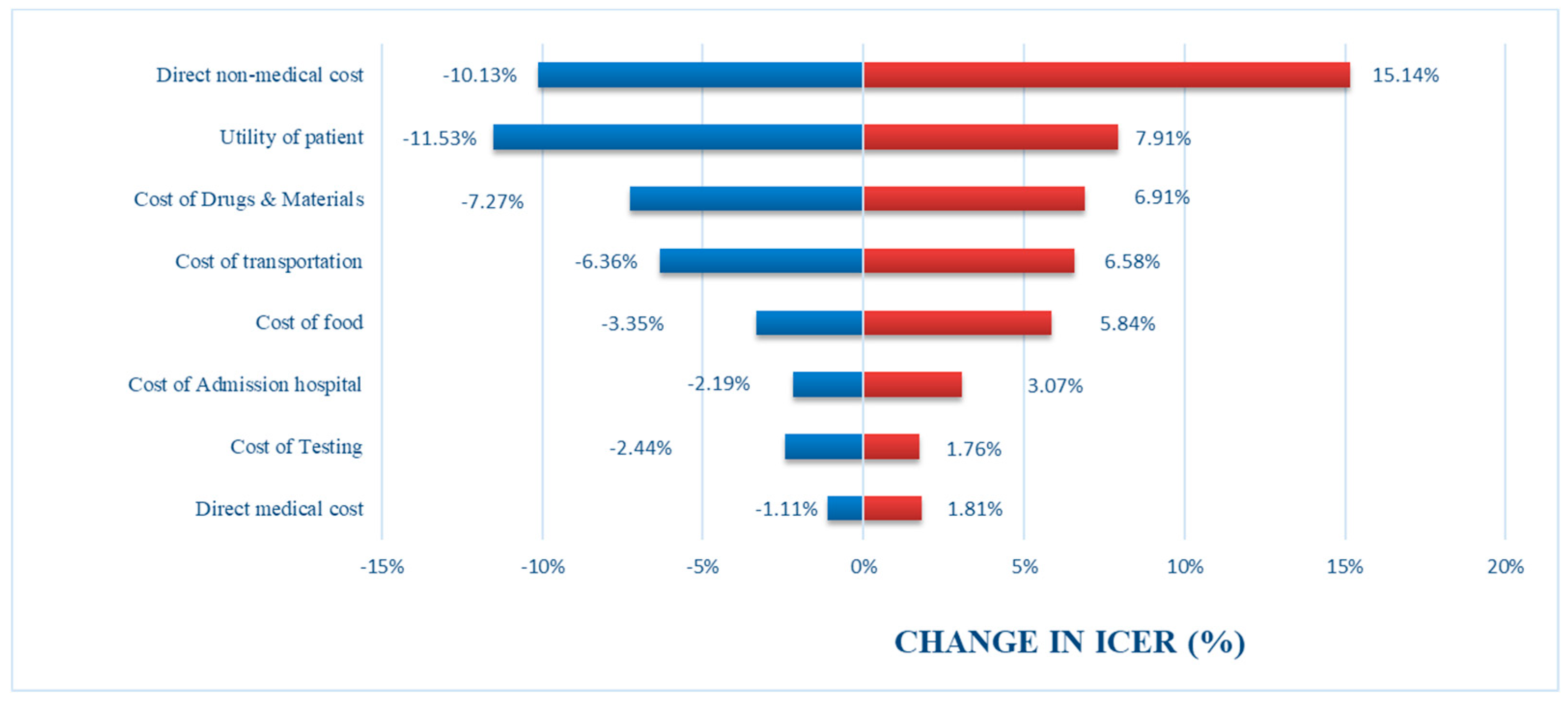
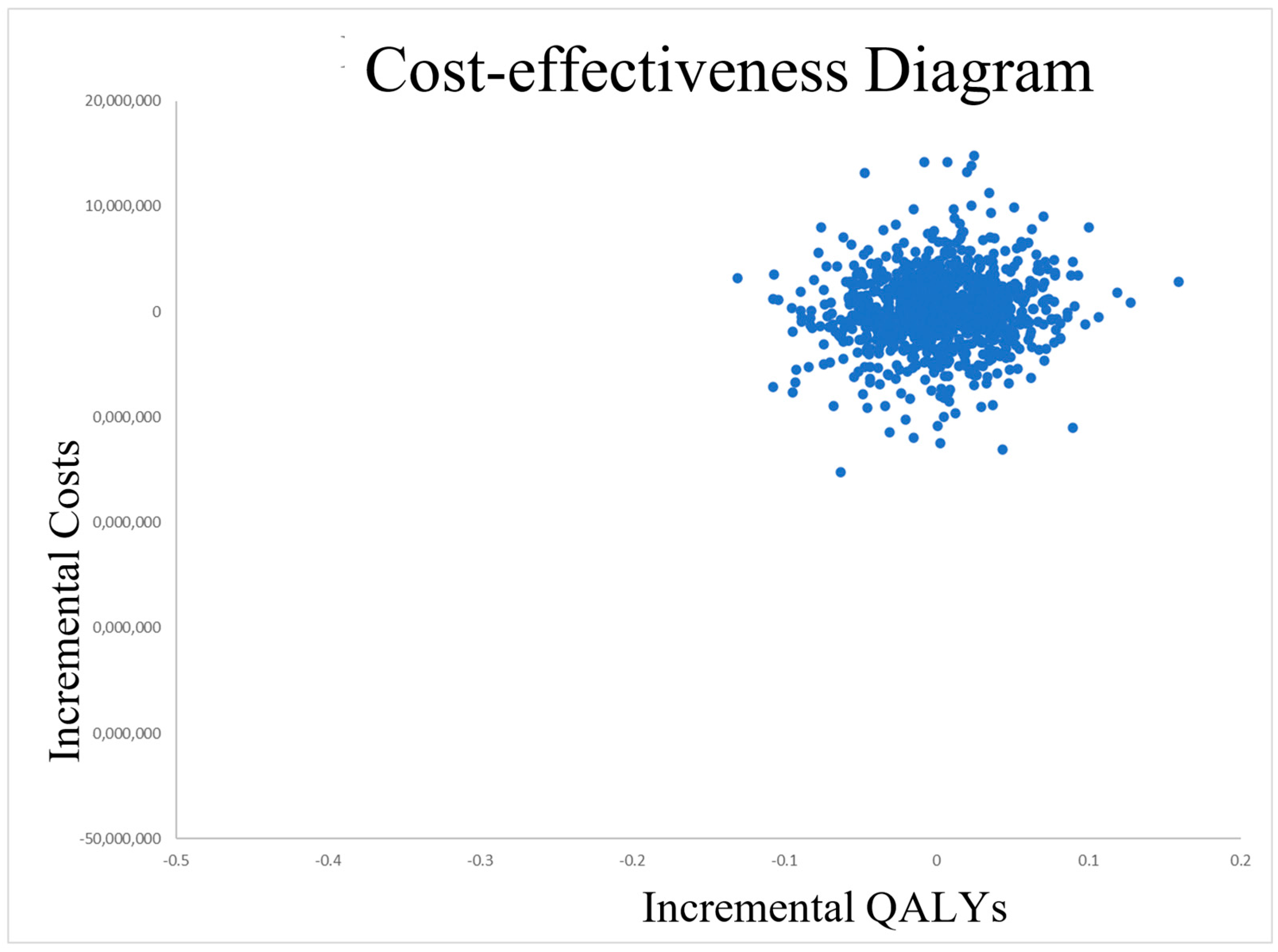
3.2. Uncertainty Analysis
4. Discussion
5. Conclusions
References
- Tu, Y.-F.; Chien, C.-S.; Yarmishyn, A.A.; Lin, Y.-Y.; Luo, Y.-H.; Lin, Y.-T.; Lai, W.-Y.; Yang, D.-M.; Chou, S.-J.; Yang, Y.-P.; et al. A Review Of SARS-Cov-2 And The Ongoing Clinical Trials. International Journal Of Molecular Sciences 2020, 21, 2657. [Google Scholar] [CrossRef] [PubMed]
- WHO. COVID-19 Situation Reports. 2019. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 7 November 2022).
- WHO. COVID-19 Weekly Epidemiological Update; 25 May 2023, 2023. 25 May.
- Vietnam, W. Viet Nam COVID-19 Situation Report #109; 2023.
- Wu, Z.; Harrich, D.; Li, Z.; Hu, D.; Li, D. The Unique Features Of SARS-Cov-2 Transmission: Comparison With SARS-Cov, MERS-Cov And 2009 H1N1 Pandemic Influenza Virus. Reviews In Medical Virology 2020, 31, e2171. [Google Scholar] [CrossRef]
- Kai-Wang, K.; Sridhar, S.; Chiu, K.H.-Y.; Hung, D.L.-L.; Li, X.; Hung, I.F.-N.; Tam, A.R.; Chung, T.W.-H.; Chan, J.F.-W.; Zhang, A.J.-X.; et al. Lessons Learned 1 Year After SARS-Cov-2 Emergence Leading To COVID-19 Pandemic. Emerging Microbes & Infections 2021, 10, 507–535. [Google Scholar]
- Wang, Y.; Wang, Y.; Chen, Y.; Qin, Q. Unique Epidemiological And Clinical Features Of The Emerging 2019 Novel Coronavirus Pneumonia (COVID-19) Implicate Special Control Measures. Journal Of Medical Virology 2020, 92, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Bchetnia, M.; Girard, C.; Duchaine, C.; Laprise, C. The Outbreak Of The Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-Cov-2): A Review Of The Current Global Status. Journal Of Infection And Public Health 2020, 13, 1601–1610. [Google Scholar] [CrossRef]
- Yip, A.J.W.; Low, Z.Y.; Chow, V.T.K.; Lal, S.K. Repurposing Molnupiravir For COVID-19: The Mechanisms Of Antiviral Activity. Viruses 2022, 14, 1345. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, Y.; Lu, X.; Zhang, W.; Fang, W.; Yuan, L.; Wang, X. An Update On Inhibitors Targeting RNA-Dependent RNA Polymerase For COVID-19 Treatment: Promises And Challenges. Biochemical Pharmacology 2022, 205, 115279. [Google Scholar] [CrossRef]
- Masyeni, S.; Iqhrammullah, M.; Frediansyah, A.; Nainu, F.; Tallei, T.; Emran, T.B.; Ophinni, Y.; Dhama, K.; Harapan, H. Molnupiravir: A Lethal Mutagenic Drug Against Rapidly Mutating Severe Acute Respiratory Syndrome Coronavirus 2—A Narrative Review. Journal Of Medical Virology 2022, 94, 3006–3016. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.H.; Au, I.C.H.; Lau, K.T.K.; Lau, E.H.Y.; Cowling, B.J.; Leung, G.M. Real-World Effectiveness Of Early Molnupiravir Or Nirmatrelvir–Ritonavir In Hospitalised Patients With COVID-19 Without Supplemental Oxygen Requirement On Admission During Hong Kong's Omicron BA.2 Wave: A Retrospective Cohort Study. The Lancet Infectious Diseases 2022, 22, 1681–1693. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.H.; Au, I.C.H.; Lau, K.T.K.; Lau, E.H.Y.; Cowling, B.J.; Leung, G.M. Real-World Effectiveness Of Molnupiravir And Nirmatrelvir Plus Ritonavir Against Mortality, Hospitalisation, And In-Hospital Outcomes Among Community-Dwelling, Ambulatory Patients With Confirmed SARS-Cov-2 Infection During The Omicron Wave In Hong Kong: An Observational Study. The Lancet 2022, 400, 1213–1222. [Google Scholar]
- Vietnam, M. One more domestically generic produced drug Molnupiravir to treat COVID-19 has been granted a certificate of circulation in Vietnam. Available online: https://moh.gov.vn/thong-tin-chi-dao-dieu-hanh/-/asset_publisher/DOHhlnDN87WZ/content/them-1-thuoc-molnupiravir-ieu-tri-covid-19-san-xuat-trong-nuoc-uoc-cap-giay-ang-ky-luu-hanh-tai-viet-nam?inheritRedirect=false (accessed on 7 November 2022).
- Vietnam, M. Newsletter on COVID-19 prevention and control of the Ministry of Health on August 11th. Available online: https://moh.gov.vn/tin-tong-hop/-/asset_publisher/k206Q9qkZOqn/content/ban-tin-phong-chong-dich-covid-19-cua-bo-y-te-ngay-8-11?inheritRedirect=false&redirect=https%3A%2F%2Fmoh.gov.vn%3A443%2Ftin-tong-hop%3Fp_p_id%3D101_INSTANCE_k206Q9qkZOqn%26p_p_lifecycle%3D0%26p_p_state%3Dnormal%26p_p_mode%3Dview%26p_p_col_id%3Drow-3-column-2%26p_p_col_pos%3D1%26p_p_col_count%3D2 (accessed on 7 November 2022).
- Jo, Y.; Kim, S.B.; Radnaabaatar, M.; Huh, K.; Yoo, J.-H.; Peck, K.R.; Park, H.; Jung, J. Model-Based Cost-Effectiveness Analysis Of Oral Antivirals Against SARS-Cov-2 In Korea. Epidemiology And Health 2022, 44, e2022034. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cost-effectiveness thresholds. 2012.
- WHO. MAKING CHOICES IN HEALTH: WHO GUIDE TO COST-EFFECTIVENESS ANALYSIS. Available online: https://apps.who.int/iris/bitstream/handle/10665/42699/9241546018.pdf;jsessionid=6A5719658CC4ADCE9ECF39BFB22A260C?sequence=1.
- WHO. New cost-effectiveness updates from WHO-CHOICE. Available online: https://www.who.int/news-room/feature-stories/detail/new-cost-effectiveness-updates-from-who-choice.
- Goswami, H.; Alsumali, A.; Jiang, Y.; Schindler, M.; Duke, E.R.; Cohen, J.; Briggs, A.; Puenpatom, A. Cost-Effectiveness Analysis of Molnupiravir Versus Best Supportive Care for the Treatment of Outpatient COVID-19 in Adults in the US. Pharmacoeconomics 2022, 40, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Kim, S.B.; Radnaabaatar, M.; Huh, K.; Yoo, J.H.; Peck, K.R.; Park, H.; Jung, J. Model-based cost-effectiveness analysis of oral antivirals against SARS-CoV-2 in Korea. Epidemiol Health 2022, 44, e2022034. [Google Scholar] [CrossRef] [PubMed]
- Vietnam, T.S.B.o. Reference exchange rate at the State Bank of Vietnam 2022. Available online: https://sbv.gov.vn/TyGia/faces/TyGia.jspx?_afrLoop=4216357552103891&_afrWindowMode=0&_adf.ctrl-state=owrk6r3t8_4 (accessed on 12 November 2022).
- Bernal, A.J.; Silva, M.M.G.d.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Reyes, V.D.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med 2022, 386, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Arribas, J.R.; Bhagani, S.; Lobo, S.M.; Khaertynova, I.; Mateu, L.; Fishchuk, R.; Park, W.Y.; Hussein, K.; Kim, S.W.; Ghosn, J.; et al. Randomized Trial of Molnupiravir or Placebo in Patients Hospitalized with Covid-19. NEJM Evidence 2021, 1, 9. [Google Scholar] [CrossRef]
- Carta, A.; Conversano, C. Cost utility analysis of Remdesivir and Dexamethasone treatment for hospitalised COVID-19 patients - a hypothetical study. BMC Health Serv Res 2021, 21, 986. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.A.; Eron, J.J., Jr.; Holman, W.; Cohen, M.S.; Fang, L.; Szewczyk, L.J.; Sheahan, T.P.; Baric, R.; Mollan, K.R.; Wolfe, C.R.; et al. A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci Transl Med 2022, 14, eabl7430. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Covid-19: Molnupiravir reduces risk of hospital admission or death by 50% in patients at risk, MSD reports. BMJ 2021, 375, n2422. [Google Scholar] [CrossRef] [PubMed]
- Vietnam, M. Circular 14/2019/TT-BYT. Ha Noi, 2019.
- THUY, N.T.B.; PHU, V.X.; ANH, N.Q. Direct medical care cost and non-medical care cost of patients with diabetes in the endocrinology department, hospital Thanh Nhan – Hanoi, 2013. Y HỌC THỰC HÀNH 2013, 893, 8. [Google Scholar]
- Sheinson, D.; Dang, J.; Shah, A.; Meng, Y.; Elsea, D.; Kowal, S. A Cost-Effectiveness Framework for COVID-19 Treatments for Hospitalized Patients in the United States. Adv Ther 2021, 38, 1811–1831. [Google Scholar] [CrossRef] [PubMed]
- IBRD. GDP per capita (current US$) - Vietnam 2021. Available online: https://data.worldbank.org/country/vietnam (accessed on 11 November 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





