Submitted:
21 June 2023
Posted:
22 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Locations
2.2. Experimental Mango cultivars
- Dashehari: Midseason, heavy floral induction, alternate bearing
- Langra: Mid-late bearing, heavy floral induction, strictly alternate bearing
- Chausa: Late season, shy floral induction, erratic bearer
- Amrapali: Late season, prolific floral induction, regular bearing
2.3. Collection of Weather Data
2.4. Observation of flowering and fruiting attributes
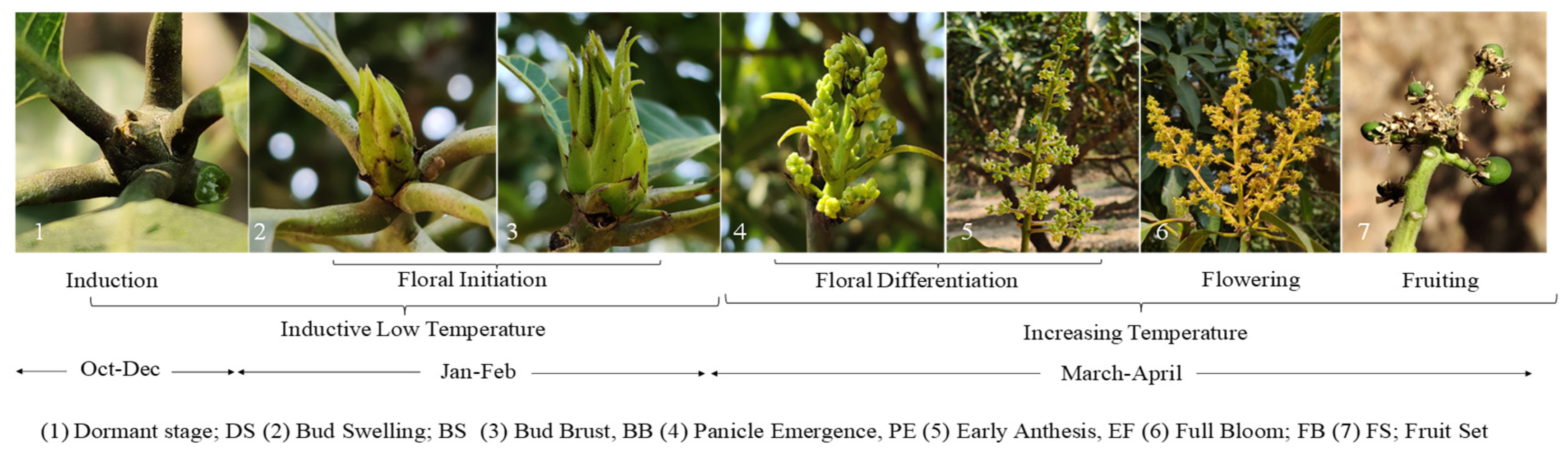
2.5. Phenological observations
2.6. Statistical analysis
3. Results
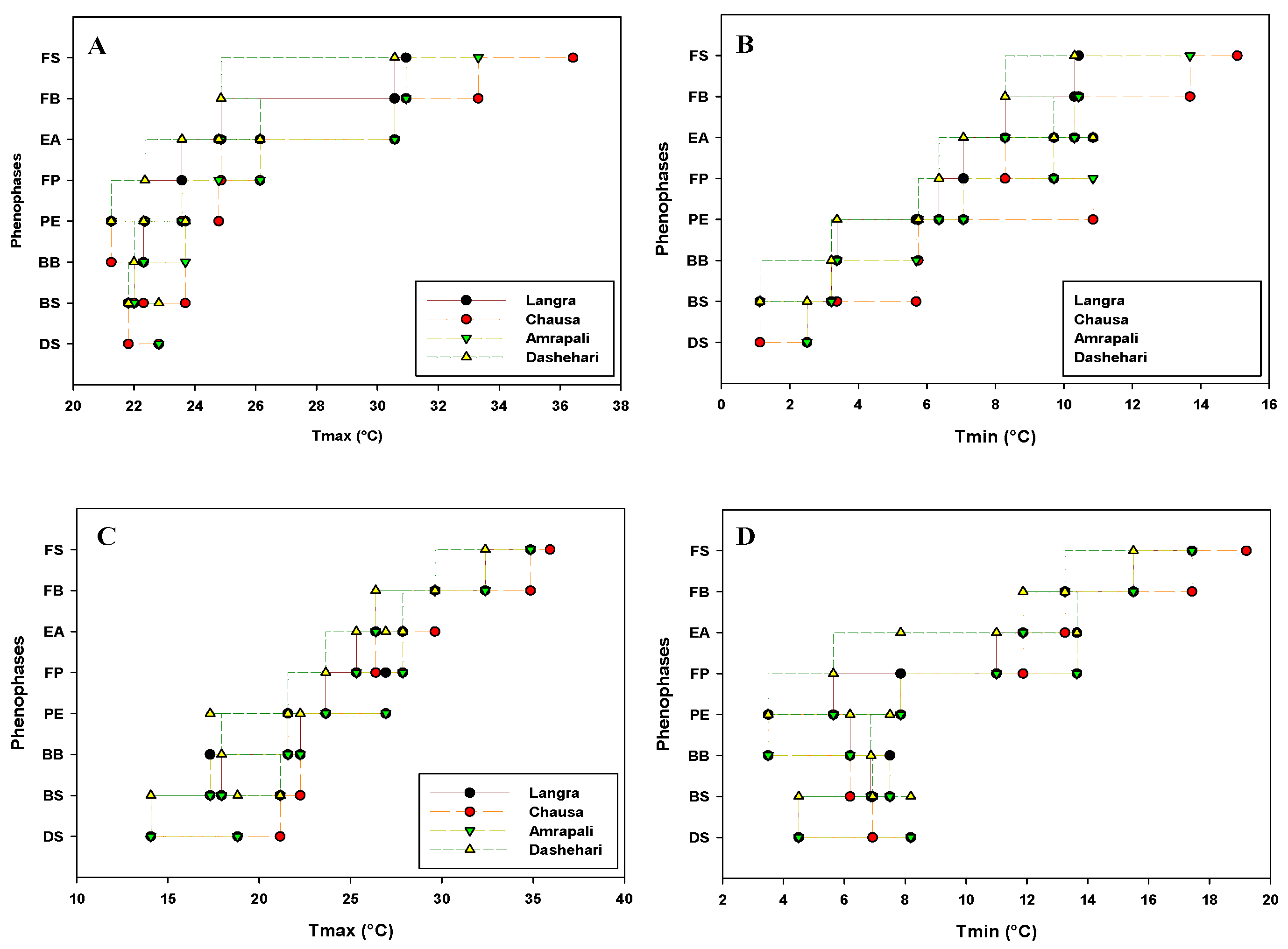
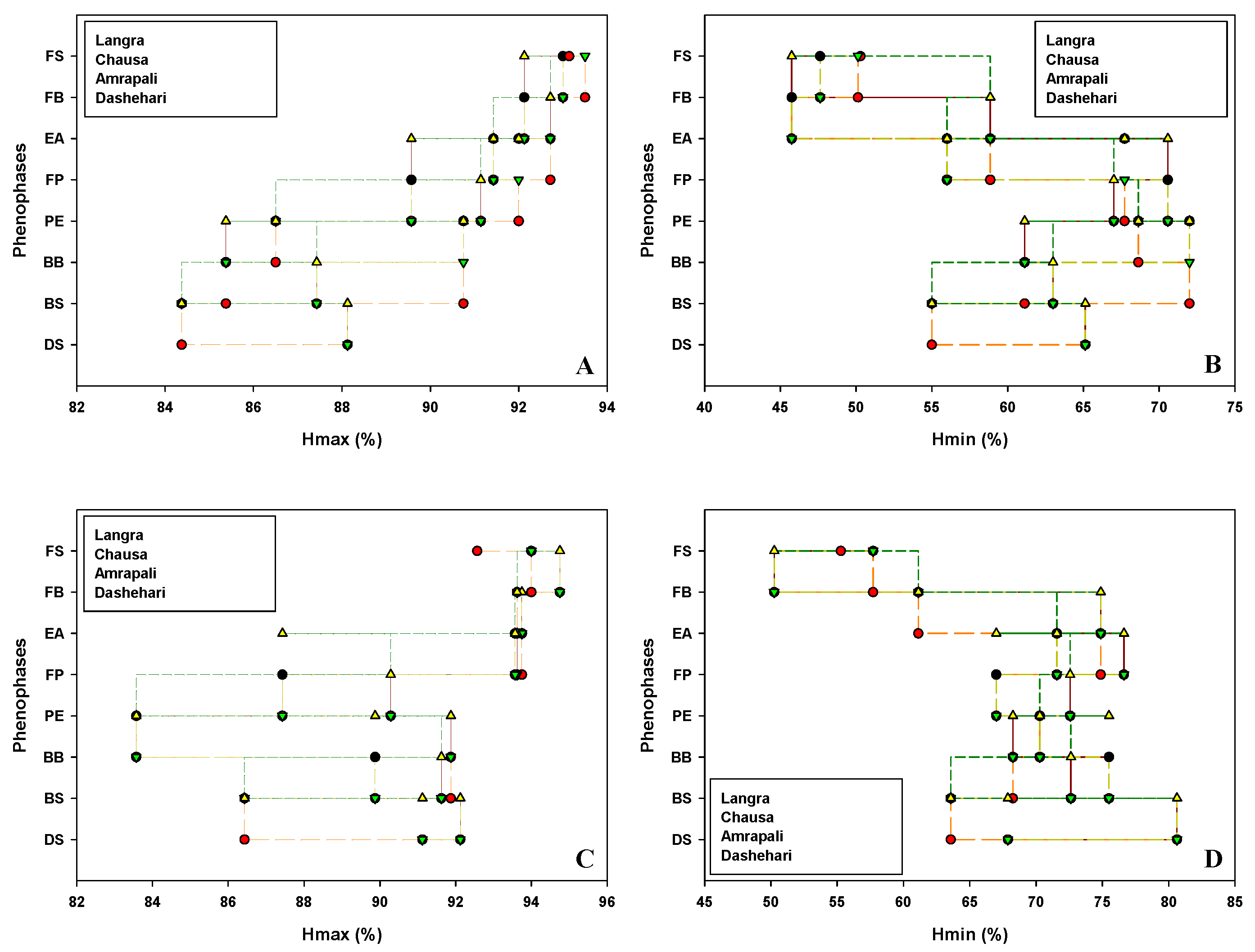
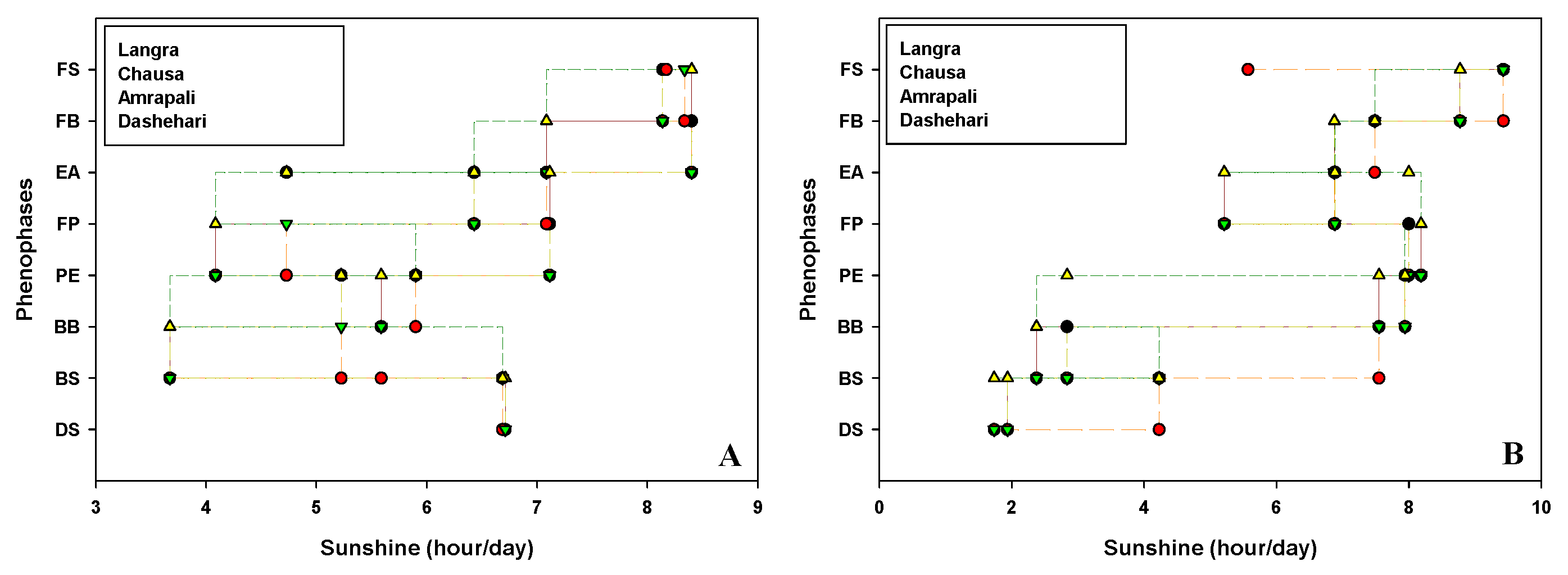
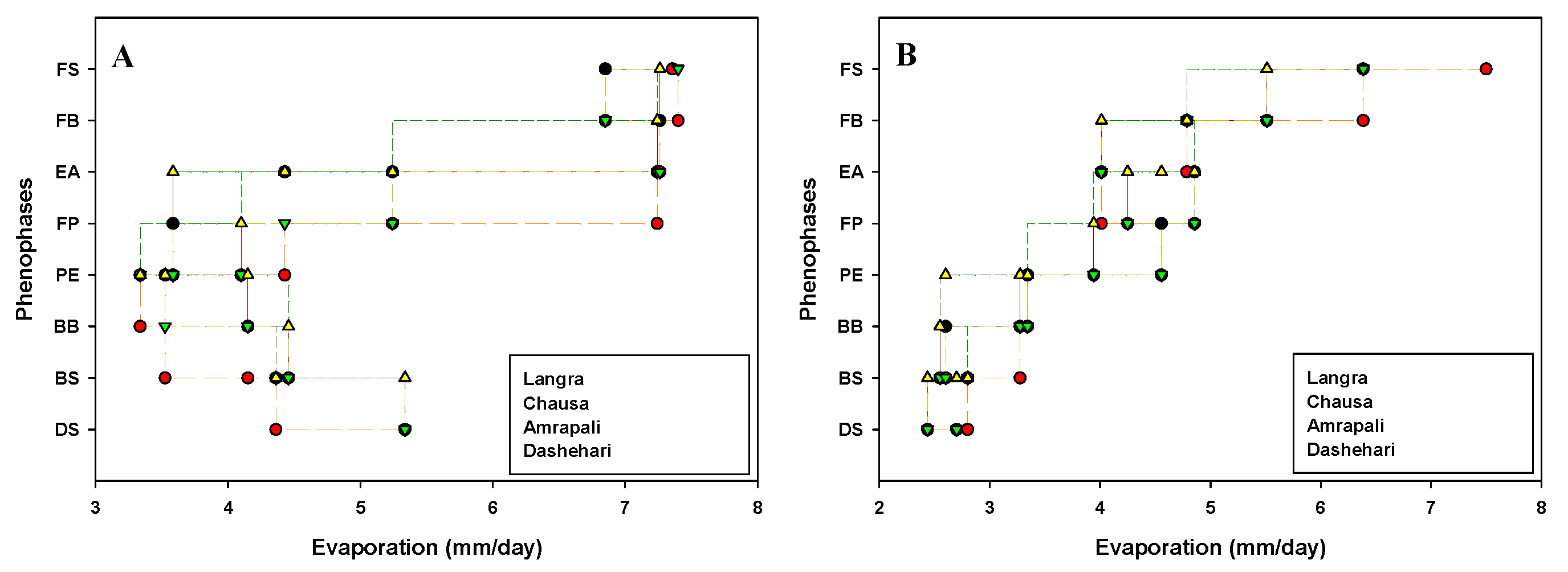
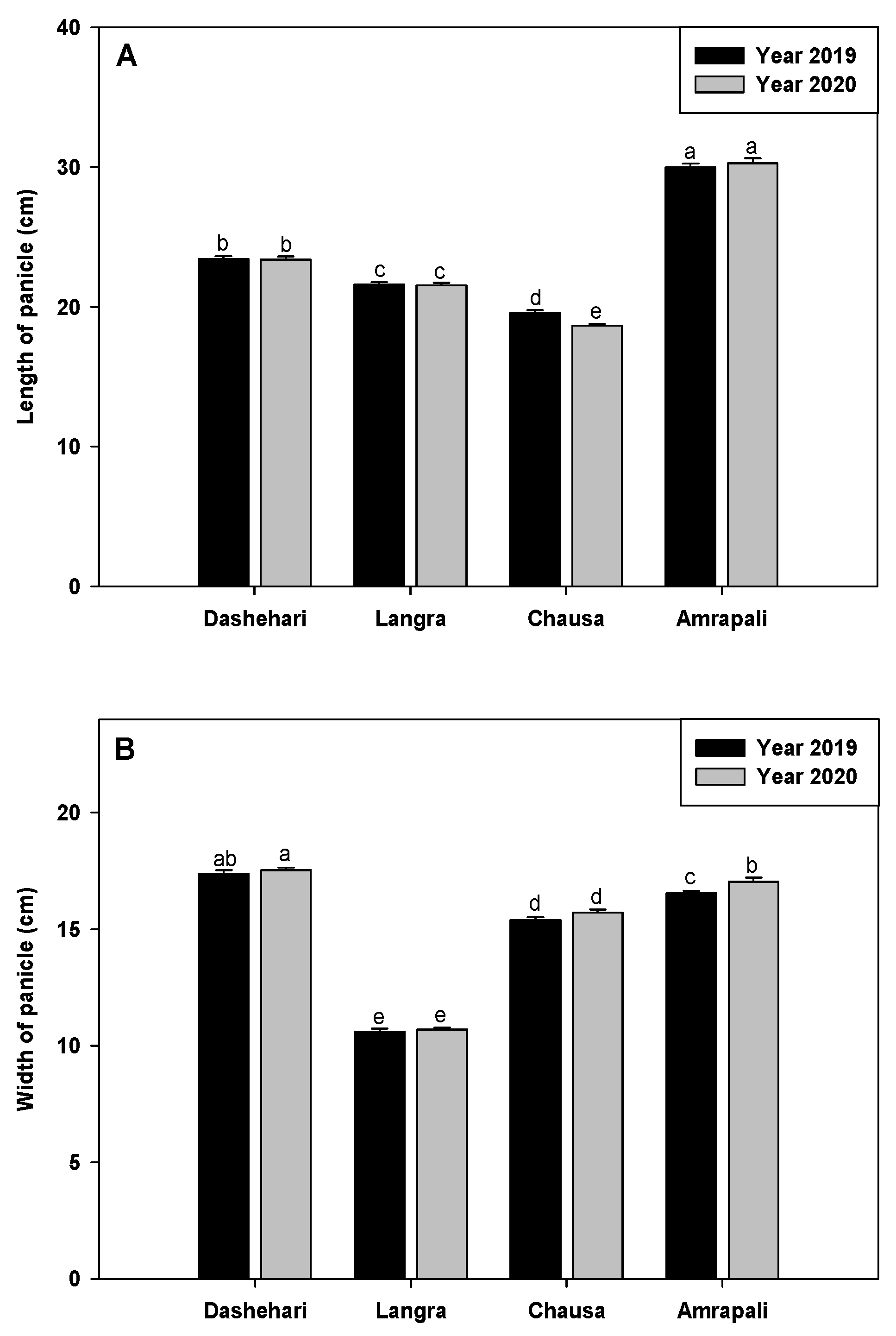
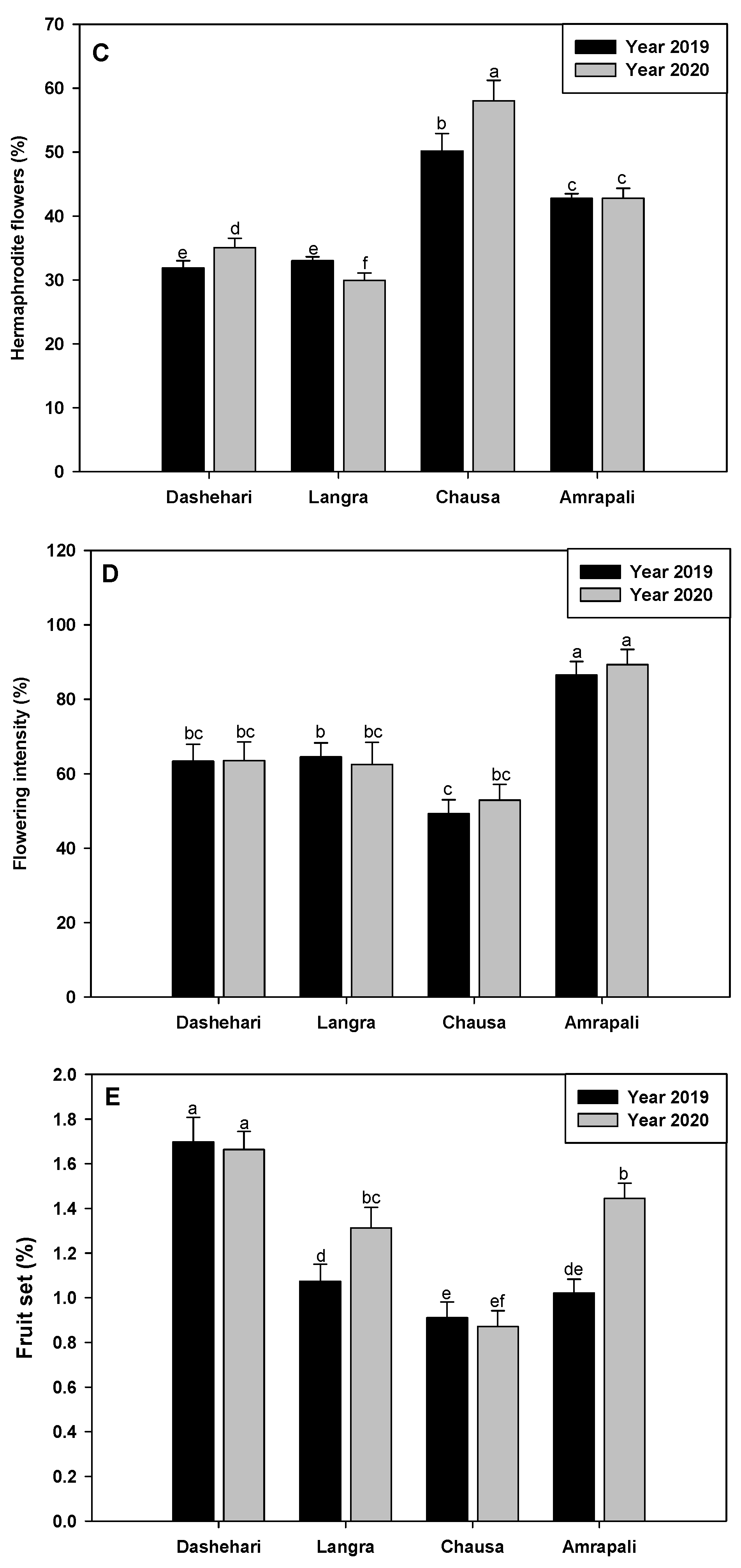
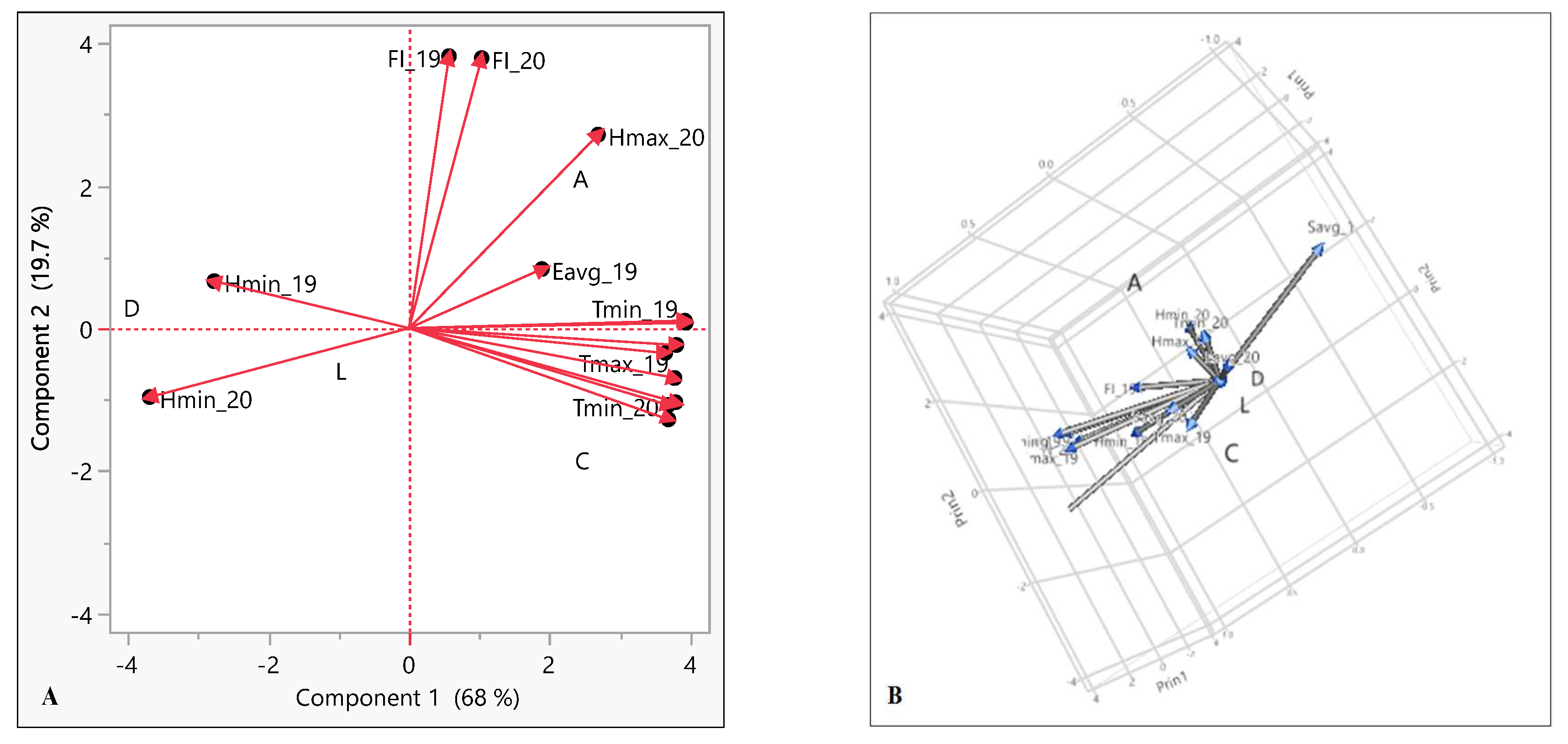
3.1. Impact of weather parameters on floral morphogenesis in different cultivars of mango
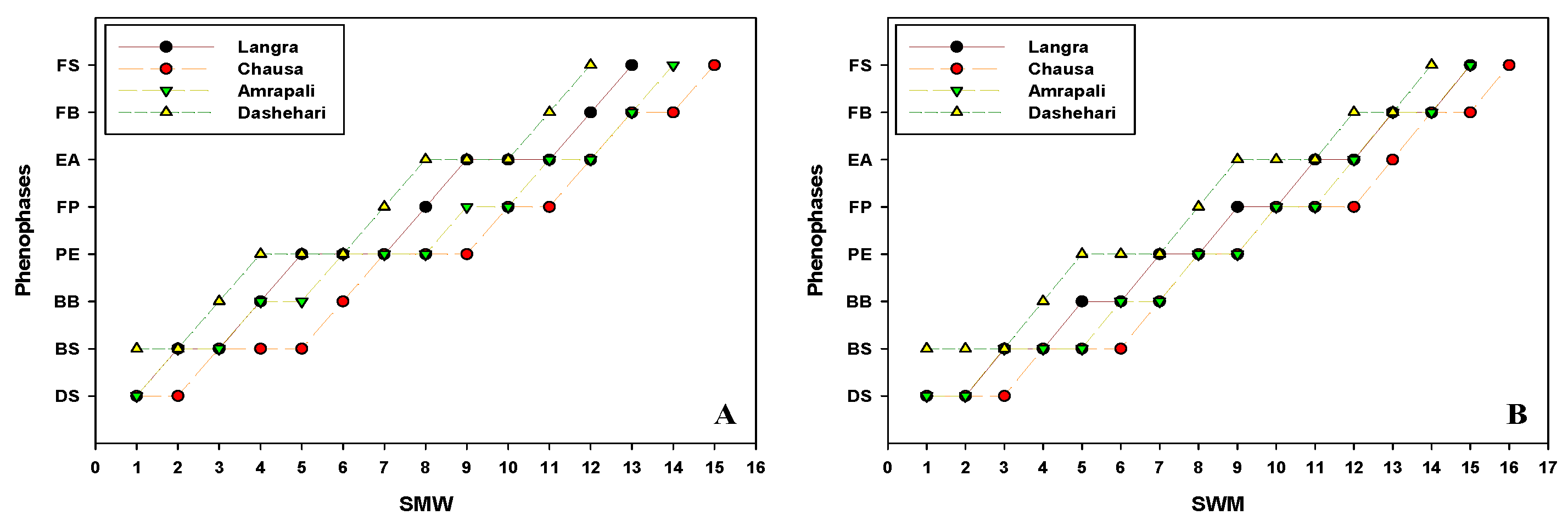
3.2. Variation of Main Phenophases in Phenological Calendar
3.3. Pattern of flowering and fruiting in response to climate
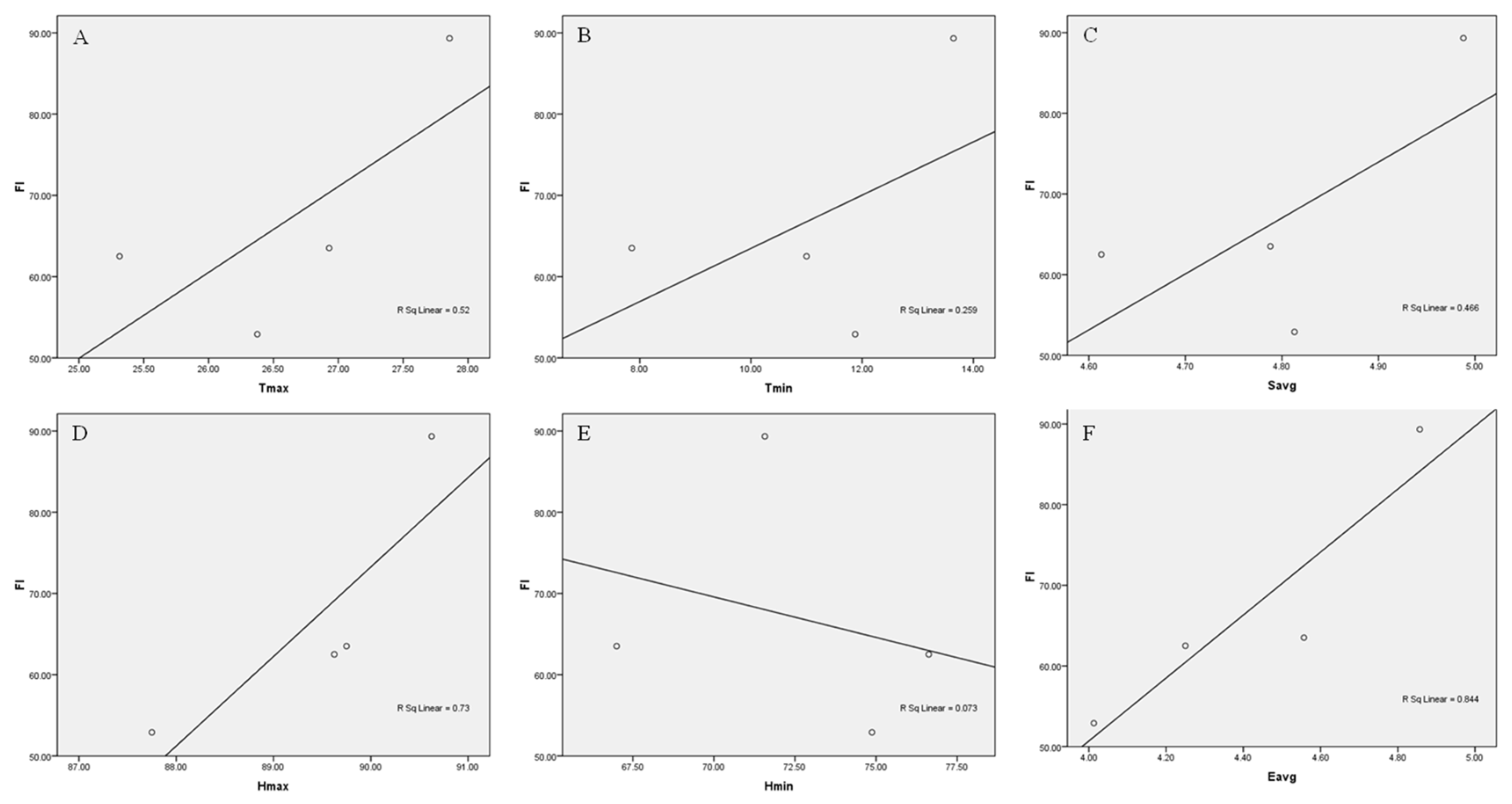
3.4. Correlation studies of flowering intensity with weather parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Consent for publication
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rastegar, S.; Khankahdani, H.H.; Rahimzadeh, M. Effects of melatonin treatment on the biochemical changes and antioxidant enzyme activity of mango fruit during storage. Sci. Hortic. 2020, 259, 108835. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Kato, M.; Ma, G.; Zhang, L.; Uthairatanakij, A.; Srilaong, V.; Laohakunjit, N. and Jitareerat, P. Electron beam radiation delayed the disassembly of cell wall polysaccharides in harvested mangoes. Postharvest Biol. Technol. 2021, 178, 111544. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Karoly, D.; Vicarelli, M.; Neofotis, P.; Wu, Q.; Casassa, G.; Menzel, A.; Root, T.L.; Estrella, N.; Seguin, B.; et al. Attributing physical and biological impacts to anthropogenic climate change. Nature 2008, 453, 353–357. [Google Scholar] [CrossRef]
- Menzel, A.; Fabian, P. Growing season extended in Europe. Nature 1999, 397, 659. [Google Scholar] [CrossRef]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kübler, K.; Bissolli, P.; Braslavská, O.G.; Briede, A.; et al. European phenological response to climate change matches the warming pattern. Glob. Chang. Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Chuine, I.; Bonhomme, M.; Legave, J.M.; García de Cortázar-Atauri, I.; Charrier, G.; Lacointe, A.; Améglio, T. Can phenological models predict tree phenology accurately in the future? The unrevealed hurdle of endodormancy break. Glob. Chang. Biol. 2016, 22, 3444–3460. [Google Scholar] [CrossRef]
- Tiwari, D.K.; Patel, V.B.; Pandey, A.K. Floral induction in mango: Physiological, biochemical, and molecular basis. Int. J. Chem. Stud. 2018, 6, 252–259. [Google Scholar]
- Hewitt, S.L.; Hendrickson, C.A.; Dhingra, A. Evidence for the Involvement of Vernalization–related Genes in the Regulation of Cold–induced Ripening in ‘D’Anjou’ and ‘Bartlett’ Pear Fruit . Sci. Rep. 2020, 10, 8478. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Körner, C.; Basler, D. Phenology under global warming. Science 2010, 327, 1461–1462. [Google Scholar] [CrossRef]
- Sravani, V. Effect of climate change on major tropical and sub–tropical fruit crops. J. Pharmacogn. Phytochem. 2020, 9, 1710–1712. [Google Scholar]
- Wang, J.; Czech, B.; Weigel, D. miR156–regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 2009, 138, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W. Regulation of flowering time by the miR156–mediated age pathway. J. Exp. Bot. 2014, 65, 4723–4730. [Google Scholar] [CrossRef]
- Wang, J.; Ding, J. Molecular mechanisms of flowering phenology in trees. J. For. Res. 2023, 3, 2. [Google Scholar] [CrossRef]
- Malhotra, S.K.; Singh, S.K.; Nath, V. Physiology of flowering in litchi (Litchi chinensis): A review. Indian J. Agric. Sci. 2018, 88, 1319–1330. [Google Scholar] [CrossRef]
- Kulkarni, V.J. Further studies on graft–induced off season flowering and fruiting in mango (Mangiferaindica L.). J. Hortic. Sci. 1986, 63, 361–367. [Google Scholar] [CrossRef]
- Hudson, I.L.; Kim, S.W.; Keatley, M.R. Climatic influences on the flowering phenology of four Eucalypts: a GAMLSS approach. In In Phenological Research; Springer Netherlands, 2010; pp. 209–228. [Google Scholar]
- Pau, S.; Wolkovich, E.M.; Cook, B.I.; Nytch, C.J.; Regetz, J.; Zimmerman, J.K.; Joseph Wright, S. Clouds and temperature drive dynamic changes in tropical flower production. Nat. Clim. Change 2013, 3, 838–842. [Google Scholar] [CrossRef]
- Wright, S.J. Seasonal drought and the phenology of understory shrubs in a tropical moist forest. Ecology 1991, 72, 1643–1657. [Google Scholar] [CrossRef]
- Shii, Z.H. Effect of temperature on the flowering biology and fertilization of mangoes (Mangifera indica L..). J. Appl. Hort. 1999, 1, 79–83. [Google Scholar] [CrossRef]
- Davenport, T.L. Reproductive physiology. In The mango: botany, production and uses; CABI: Wallingford, UK, 2009; pp. 97–169. [Google Scholar]
- Bhatt, R.; Hossain, A. Concept and consequence of evapotranspiration for sustainable crop production in the era of climate change. Adv. Evapotranspiration Methods Appl 2019, 1, 1–13. [Google Scholar] [CrossRef]
- Zhang, R.; Lin, J.; Wang, F.; Delpierre, N.; Kramer, K.; Hänninen, H.; Wu, J. Spring phenology in subtropical trees: Developing process–based models on an experimental basis. Agric. For. Meteorol. 2022, 314, 108802. [Google Scholar] [CrossRef]
- Rajan, S.; Tiwari, D.; Singh, V.K.; Saxena, P.; Singh, S.; Reddy, Y.T.N.; Upreti, K.K.; Burondkar, M.M.; Bhagwan, A.; Kennedy, R. Application of extended BBCH scale for phenological studies in mango (Mangiferaindica L..). J. Appl. Hort. 2011, 13, 108–114. [Google Scholar]
- Rajan, S.; Ravishankar, H.; Tiwari, D.; Singh, V.K.; Saxena, P.; Singh, S.; Reddy, Y.T.N.; Upreti, K.K.; Burondkar, M.M.; Bhagwan, A.; et al. Harmonious phenological data: a basic need for understanding the impact of climate change on mango. In Climate–resilient horticulture: adaptation and mitigation strategies 2013, 53–65. [CrossRef]
- Azam, K.; Mir, H.; Kumar, R. and Ahmad, F. Study on flowering behaviour of elite mango cultivars in subtropical conditions of Bihar. Int. J. Chem. Stud. 2018, 6, 2913–2917. [Google Scholar]
- Kumar, M.; Ponnuswami, V.; Kumar, P.J.; Saraswathy, S. Influence of season affecting flowering and physiological parameters in mango. Sci. Res. Essays 2014, 9, 1–6. [Google Scholar] [CrossRef]
- Sharma, D.K.; Singh, R.N. Studies on some pollination problems in mango (Mangiferaindica L..). Indian J. Hort. 1969, 26, 1–5. [Google Scholar]
- Hernández Delgado, P.M.; M. Aranguren, C.; Reig, D.; Fernandez Galvan, C.; Mesejo, A.; Martinez Fuentes, V.; Sauco, G.; Agusti, M. Phenological growth stages of mango (Mangiferaindica L..) according to the BBCH scale. Sci. Hortic. 2011, 130, 536–540. [CrossRef]
- Singh, R.; Manav, M.K.; Sharma, A. Effect of weather parameters (Abiotic factors) on flowering fruiting and quality behaviour of mango cultivars. The Ecosan. 2014, 6, 103–109. [Google Scholar]
- Prasad, S.S.; Reddy, Y.T.N.; Upreti, K.K.; Rajeshwara, A.N. Studies on changes in carbohydrate metabolism in regular bearing and “off” season bearing cultivars of mango (Mangifera indica L..) during flowering. Int. J. Fruit Sci. 2014, 14, 437–459. [Google Scholar] [CrossRef]
- Naik, K.C.; Rao, M.M. Studies on the blossom biology and pollination in mangoes (Mangifera indica L..). Indian J Hortic. 1943, 1, 107–119. [Google Scholar]
- Singh, A.R.; Singh, N.D. Studies on bloom biology and pollination in mango (Mangifera indica L.). Recent Hortic. 1996, 3, 4–7. [Google Scholar]
- Geetha, G.A.; Shivashankara, K.S.; Reddy, Y.T.N. Varietal variations in temperature response for hermaphrodite flower production and fruit set in mango (Mangifera indica L.). S. Afr. J. Bot. 2016, 106, 196–203. [Google Scholar] [CrossRef]
- Ramirez, F; Davenport, T.L. Mango (Mangifera indica L..) flowering physiology. Sci. Hortic. 2010, 126, 65–72. [Google Scholar] [CrossRef]
- Anjum, M.A.; Chattha, G. A; Sultan, A; Abbas, S. Studies on flowering behavior, fruit setting and extent of floral malformation in different cultivars of mango (Mangifera indica L.). Int. J. Agric. Biol. 1999, 1, 88–90. [Google Scholar]
- Ausin, I.; Alonso–Blanco, C.; Martinez–Zapater, J.M. Environmental regulation of flowering. Int J Dev Biol. 2005, 49, 689–705. [Google Scholar] [CrossRef] [PubMed]
- Davenport, T.L. Reproductive physiology of mango. Braz. J. Plant Physiol. 2007, 19, 363–376. [Google Scholar] [CrossRef]
- Normand, F.; Lauri, P.E.; Legave, J.M. Climate change and its probable effects on mango production and cultivation. Acta Hortic. 2015, 1075, 21–31. [Google Scholar] [CrossRef]
- Palanisamy, V.; Bhaskar Mitra, A.M.S.; Deepa Sankar, P. Studies on fruit bud differentiation in mango (Mangiferaindica L.). Res Plant Biol. 2011, 1, 55–67. [Google Scholar]
- Clonan, M.; McConchie, C.; Hall, M.; Hearnden, M.; Olesen, T.; Sarkhosh, A. Effects of ambient temperatures on floral initiation in Australian mango (Mangiferaindica L.) selections. Sci. Hortic. 2021, 276, 109767. [Google Scholar] [CrossRef]
- Naphrom, D.; Sruamsiri, P.; Hegele, M.; Boonplod, N.; Bangerth, F.; Manochai, P. Hormonal changes in various tissues of mango trees during flower induction following cold temperature. Acta Hortic. 2004, 645, 453–457. [Google Scholar] [CrossRef]
- Pérez–Barraza, M.; Avitia–García, E.; Cano–Medrano, R.; Gutiérrez–Espinosa, M.A.; Osuna–Enciso, T.; Pérez–Luna, A.I. Temperature and gibberellin inhibitors in the flowering process of mango cv.' Ataulfo’. Rev. Fitotec. Mex. 2018, 41, 543–549. [Google Scholar]
- Rangare, N.R.; Bhan, M.; Pandey, S.K. Assessment of weather effect on flower morphogenesis and fruit set in mango varieties in central India. J. Agric. Meteorol. 2022, 24, 33–37. [Google Scholar] [CrossRef]
- Bajpai, Y.; Trivedi, M.; Muthukumar, M.; Bajpai, A. Novel insights into biochemical and hormonal factors regulating floral transition in mango (Mangifera indica L.). Indian J. Biotechnol. 2021, 20, 54–64. [Google Scholar]
- Chacko, K.K.; Randhawa, G.S. Towards an understanding of the factors affecting flowering in mango (Mangifera indica L.). AAJ 1971, 18, 226–236. [Google Scholar]
- Pavón, N.P.; Briones, O. Phenological patterns of nine perennial plants in an intertropical semi–arid Mexican scrub. J. Arid Environ. 2001, 49, 265–277. [Google Scholar] [CrossRef]
- Primack, D.; Imbres, C.; Primack, R.B.; Miller-Rushing, A.J.; Del Tredici, P. Herbarium specimens demonstrate earlier flowering times in response to warming in Boston. Am. J. Bot. 2004, 91, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Rajatiya, J.H.; Varu, D.K.; Halepotara, F.H.; Solanki, M.B. Correlation of Climatic Parameters with Flowering Characters of Mango. Int. J. Pure Appl. Biosci. 2018, 6, 597–601. [Google Scholar] [CrossRef]
- Pongsomboon, W.; Subhadrabandhu, S.; Stephenson, R. A.Some aspects of the ecophysiology of flowering intensity of mango (Mangifera indica L..) cv. Nam Dok Mai in a semi–tropical monsoon Asian climate. Sci. Hortic. 1997, 70, 45–56. [Google Scholar] [CrossRef]
- Mouco, M.A.D.C.; Ono, E.O.; Rodrigues, J.D. Synthesis inhibitors of gibberellins and mango 'Tommy Atkins' seedlings growth. Cienc. Rural 2010, 40, 273–279. [Google Scholar] [CrossRef]
- Das, A.; Geetha, G.A.; Ravishankar, K.V.; Shivashankara, K.S.; Roy, T.K.; Dinesh, M.R. Interrelations of growth regulators, carbohydrates and expression of flowering genes (FT, LFY, AP1) in leaf and shoot apex of regular and alternate bearing mango (Mangifera indica L.) cultivars during flowering. Sci. Hortic. 2019, 253, 263–269. [Google Scholar] [CrossRef]
- Kulkarni, V.J. Chemical control of tree vigour and the promotion of flowering and fruiting in mango (Mangiferaindica L.) using paclobutrazol. J. Horti. Sci. 1988, 63, 557–566. [Google Scholar] [CrossRef]
- Núñez–Elisea, R.; Davenport, T.L. Flowering of mango trees in containers as influenced by seasonal temperature and water stress. Sci. Hortic. 1994, 58, 57–66. [Google Scholar] [CrossRef]
- Shen, J.; Xiao, Q.; Qiu, H.; Chen, C.; Chen, H. Integrative effect of drought and low temperature on litchi (Litchi chinensis Sonn.) floral initiation revealed by dynamic genome–wide transcriptome analysis. Sci. Rep. 2016, 6, 32005. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




