Introduction:
Severe cerebral edema or malignant stroke is the most feared and leading cause of poor outcome following ischemic stroke [
2]. Large space occupying middle cerebral artery or hemispheric infarction associated with massive cerebral edema, which may lead to herniation and death, has been described as malignant middle cerebral artery infarction or stroke [
1,
3]. Malignant stroke has been associated with 80% mortality within the first week of stroke due to herniation [
1,
3,
4,
5,
6]. Prolonged ischemia, large parenchymal hypoattenuation on CT (Low ASPECT score), Initial high NIHSS, poor collaterals and younger age have been shown to be associated with malignant stroke [
2,
6].
Endovascular therapy for successful recanalization is the mainstay approach for the stroke with large vessel occlusion [
7]. Successful revascularization reduces the risk of developing malignant stroke[
2,
8]. However, Studies have shown that despite successful recanalization, a substantial proportion of patients do not have good clinical and functional outcomes [
8,
9]. Studies have also shown that the risk of poor outcomes increases with the number of passes during thrombectomy[
8]. Zaidat et al[
10] described the first pass effect (one pass mTICI 3) as a strong predictor of good clinical outcome. Thus, even substantial recanalization (TICI 2b-3) with higher number of passes can substantially increase the risk of poor outcome [
8,
10]. The theoretical mechanisms by which additional passes could induce worse outcomes could be increased clot fragmentation with distal embolization or accumulated endothelial damage [
8]. It has therefore been recommended to attempt for substantial recanalization (TICI2b-3) to upto 4 Passes [
8].
In our clinical experience at our stroke center, we also experienced poor clinical outcomes despite successful recanalization of MCA. Majority of poor outcomes were attributed to malignant stroke and hemorrhagic transformation with severe swelling. We also included hemorrhagic transformation with severe edema in our malignant stroke definition for our study (in the method section) as both play a role in each other's pathophysiology [
11,
12], by worsening edema through the affected blood brain barrier(BBB) and endothelial injury[
12].
Purpose of our study was to predict the risk for malignant stroke even after successful recanalization (TICI≽ 2b) post thrombectomy. Although there are multiple factors associated with malignant stroke, we only used two variables ASPECT score and number of passes to design a novel scoring scale
ASPECTS-PASS score(A-P score) to determine the likelihood of malignant stroke after mechanical thrombectomy. By virtue of predicting the risk of developing malignant stroke, this scoring system will enable the clinicians to identify the high risk patients, this way extra care and attention could be delivered to those at high risk, such as more frequent neuroimaging, neuro checks and more strict blood pressure goals. Thereby, early detection and timely therapeutic interventions could be possible.
Method:
We retrospectively reviewed cases of acute ischemic stroke due to large vessel MCA (M1/M2) occlusion who underwent endovascular therapy between 2016 and 2021 at Einstein Medical Center, Philadelphia. We followed STROBE guidelines for our observational study. Our study design was a retrospective cohort analysis. Our study was approved by Einstein Medical Center IRB department.
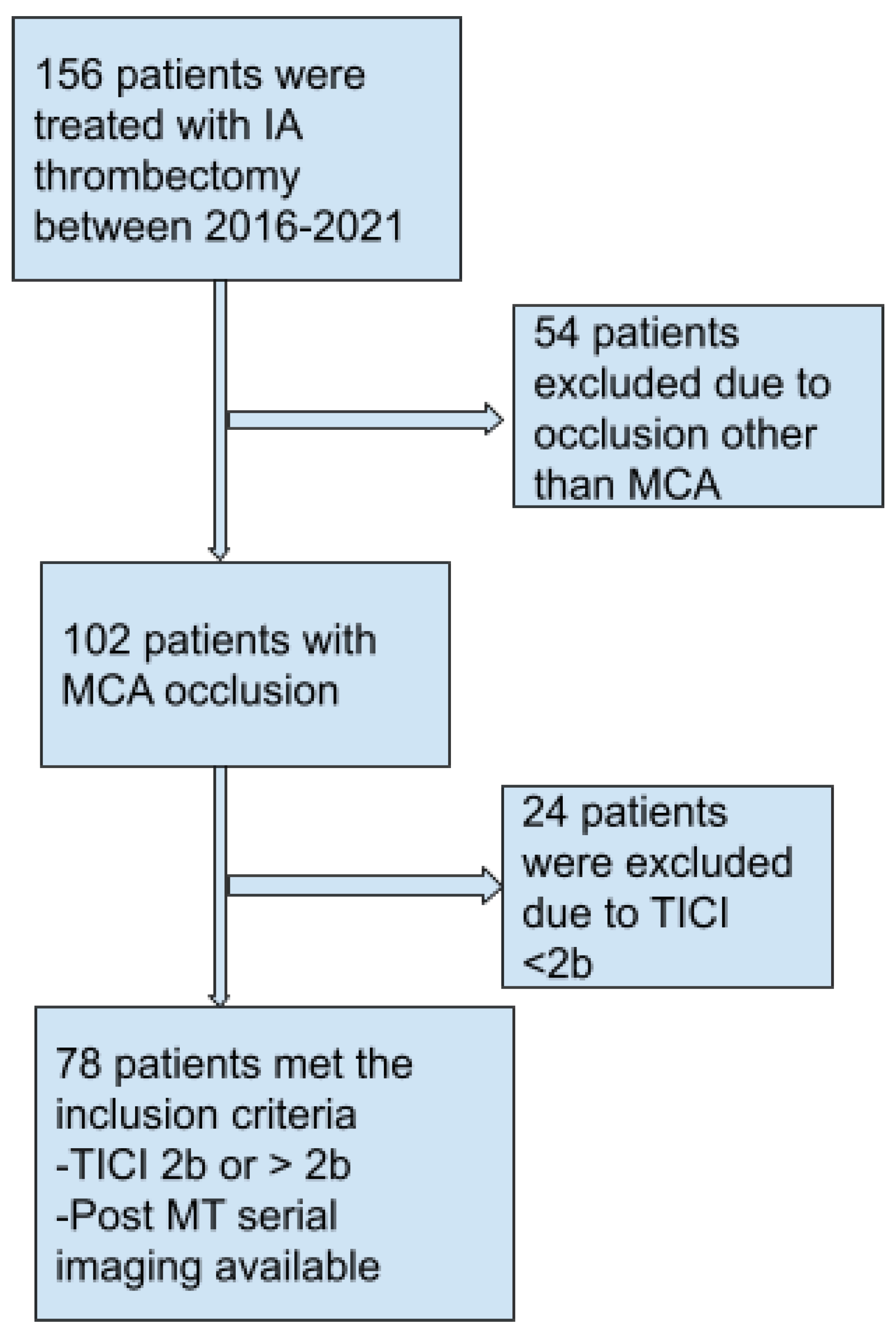
Figure 1 shows the selection process of our study. A total of 78 patients who met the inclusion criteria were finally selected for our study and analysis. Our Inclusion criteria entailed: (a) age > 18 yrs (b) ASPECT score ≽ 6 (c) TICI ≽ 2b (d) presentation within 24 hrs of symptoms onset and (e) Occlusion of other vascular territories than MCA. Intravenous thrombolysis was not an exclusion criterion.
All patients had CT Head and CT angiogram. Patients with large vessel occlusion underwent endovascular therapy. All thrombectomies performed at Einstein Medical Center using the same approach of thrombectomy, direct aspiration first pass technique (ADAPT),with a subsequent stent retriever was used if aspiration alone was not able to achieve successful recanalization TICI ≽ 2b . CT images were reviewed and analyzed for ASPECT score before the endovascular therapy. To eliminate the Bias, ASPECT scores were reviewed blindly for our study by only one reader.
We defined malignant stroke as (a) Worsening of neurological deficits (increase in NIHSS ≽ 2) with severe cerebral edema (b) New or Worsening of neurological symptoms such as headache, confusion, vision changes and vomiting etc associated with severe cerebral edema (c) No clinical improvement with severe deficit (NIHSS ≽ 14) associated with severe cerebral edema and (d)Hemorrhagic conversion with severe cerebral edema. Severe cerebral edema was identified radiologically thorough repeat or serial Neuroimaging (immediate and 24 hours post thrombectomy), defined as edema with evidence of engorgement of cisterns, transcortical herniation, midline shift and effacement of ventricles. We used two main variables: ASPECT score and total number of Passes during thrombectomy to calculate
ASPECT-PASS score (A-P Score), by subtracting number of Passes from the initial ASPECT score. A-P score ranged from 0-9. We reviewed the subsequent patients' hospital courses including any complications, clinical exams and Neuro-imagings for 5 days post thrombectomy, to identify the patients who met our malignant stroke criteria. By standard stroke protocol, we reviewed immediate and 24 hours post thrombectomy CT Head. However, more frequent serial CT heads were done in case of worsening of the neurological exam and symptoms. We also added other variables to our study including demographics, NIHSS, various timings such as door to needle, door to groin, door to first pass, and door to recanalization. The baseline characteristics and mean times are summarized in
Table 1.
For statistical analysis, we used Microsoft Excel and SPSS software. All the independent variables were continuous and dependent variables were categorical. Therefore, we used logistic regression to determine the probability and odds. Coefficient, log odds, P value, 95% confidence interval and probability was determined. We also performed descriptive statistical analysis to determine the central tendencies of different variables (mean, median and mode). The primary endpoint of the study was the development of malignant stroke by our definition. Secondary endpoints were the therapies used for the malignant stroke; Decompressive hemicraniectomy or medical/osmotic therapy. Other secondary endpoints included functional outcome upon hospital discharge. We categorized functional outcomes into favorable and unfavorable based on their disposition upon discharge and mortality for example; Acute inpatient rehabilitation & Home discharge were considered favorable. Skilled Nursing home care, Long term assisted care, Hospice and death were regarded as unfavorable outcomes.
Results:
78 patients with acute stroke from MCA occlusion who achieved successful recanalization (TICI ≽ 2b) after endovascular therapy were included in the study group. Our study showed a statistically significant inverse relationship of A-P score and risk of malignant stroke. In the study group, 17 patients developed malignant stroke. The coefficient of -1.2 and probability of 23% (p-value <.001) implies 23 % increase in risk of malignant stroke with every point decrease in A-P score. Approximately 90% of patients with an A-P score < 5 developed malignant stroke. 15 of the 17 malignant stroke patients had A-P score of ≤ 6, other two patients had A-P score of 7. Other variables were adjusted including timings for door to needle, door to groin, door to first pass, and door to recanalization. Individual ASPECTS and number of passes were also tested and demonstrated a statistically significant relationship with malignant stroke with a slope coefficient of -1.12 and 1.06 respectively when compared to the A-P score. Thus, the A-P score is a better predictor for malignant stroke. Total number of hemorrhagic transformations were also reported. There were a total of 7 hemorrhagic transformations; notably, only 4 out of 7 received IV thrombolytics prior to endovascular therapy.
With regard to the secondary endpoints, All 17 malignant strokes required treatment. 6 of the 17 required decompressive hemicraniectomy; others were managed medically with hypertonic saline or mannitol. The total number of unfavorable outcomes were 16, including 5 deaths. 4 of the 5 deaths developed malignant stroke, with an A-P score of 2, 4, 5, and 6. A total of 12 patients of the 16 with unfavorable outcomes developed malignant stroke.
Discussion:
Malignant MCA stroke is the most serious and leading cause of adverse outcome within the first week of MCA stroke[
2]. Malignant MCA stroke has been described as a large space occupying middle cerebral artery or hemispheric infarction associated with massive cerebral edema, which may lead to herniation and death [
1,
3]. Malignant stroke can manifest itself with signs and symptoms related to raised intracranial pressure besides the neuro-deficits corresponding to the stroke, such as blurry vision, gaze deviation, severe headache, altered consciousness, nausea, and vomiting. Thus,It is prudent to watch out for these manifestations for early recognition of malignant stroke.
There are several risk factors associated with malignant stroke including prolonged ischemia, large parenchymal hypoattenuation on CT (Low ASPECT score < 7), initial high NIHSS, poor collaterals and younger age[
2,
6]. The exact pathophysiology of development of malignant stroke is not clearly understood. However, severe ischemic cerebral edema could be pathophysiologically categorized into early or acute cytotoxic and subacute or late vasogenic edema. Cytotoxic edema starts developing within the first few minutes of the acute phase of ischemia after critically reduced blood supply[
13] . Reduced regional cerebral blood flow (r CBF < 20 %) starts causing irreversible cell damage by electrical and biological alteration[
13,
14]. This ischemia-induced energy failure causes Na+/K+ pump failure, Ca+ ion influx, membrane depolarization and increase in cell osmolality via anaerobic metabolism. These
electro-dynamic disturbances cause influx of water and Na+ inside the cells, causing cell swelling or cytotoxic edema[
15]. This intracellular shift further causes shrinkage of the extracellular compartment as cell swelling occurs at the expense of extracellular volume[
16].
This sets up the platform for vasogenic edema, the major culprit for the development of malignant stroke, by creating a new gradient between the extracellular matrix and neurovascular unit[
16]. Ischemic injury causes disruption of the blood brain barrier(BBB) through degradation of basal lamina and endothelial tight junction [
17,
18]. Vasogenic edema starts appearing in 4-6 hours and peaks at one to several days after acute ischemic injury[
15]. Vasogenic edema increases total tissue water content in contrast to cytotoxic edema. This progressive cerebral edema exerts a mechanical force to the surrounding tissue structures, causing raised total intracranial pressure(ICP), which in turn causes reduced cerebral perfusion pressure (CPP), contributing to further reduction in CBF and worsening of ischemic injury[
16].
Successful recanalization is crucial to avoid this viscous cascade of ischemic injury and subsequent malignant stroke, although it may contribute to vasogenic edema through increased blood flow in micro-vasculature against ruptured BBB[
19]. Post-ischemic hyperperfusion or luxury perfusion is the known phenomena after recanalization, a state of overabundant CBF in the infarct area relative to
metabolic needs of the infarct tissue[
20]. This luxury perfusion is evident by 48 hours after recanalization and can last for 1-2 weeks[
21,
22,
23]. It has been correlated to severe neuronal damage and worse outcome, mediated, in part, by the overproduction and release of toxic free radicals, also termed as reperfusion injury[
24,25]. Nonetheless, it is worth mentioning again that timely successful recanalization is not only associated with better outcomes but also outweighs the risk of reperfusion injury.
We discussed earlier the importance of timely endovascular therapy with successful recanalization in acute stroke with large vessel occlusion.However, the question remains that despite the successful recanalization, a substantial proportion of patients don't achieve good clinical and functional outcomes. Majority of poor outcomes were attributed to malignant stroke and hemorrhagic transformation with severe swelling. Therefore, we tried to answer this question through predicting the risk of this feared complication after successful recanalization.
Our study was focused on risk prediction for malignant MCA stroke after a successful recanalization (TICI≽ 2b) post thrombectomy using ASPECTS-PASS score(A-P score). Although there are multiple factors associated with malignant stroke as mentioned above. ASPECTS-PASS score (A-P Score) was calculated, by subtracting the number of Passes from the initial ASPECT score. A-P score ranged from 0-9. Our study was a retrospective analysis on 78 post-thrombectomy patients. The primary endpoint of the study was the development of malignant stroke by our definition as above. Secondary endpoints were the therapies used for the malignant stroke; Decompressive hemicraniectomy or medical/osmotic therapy. Other secondary endpoints included functional outcome upon hospital discharge. We categorized functional outcomes into favorable and unfavorable based on their disposition upon discharge and mortality for example; Acute inpatient rehabilitation & Home discharge were considered favorable.
Skilled Nursing home care, Long term assisted care, Hospice and death were regarded as unfavorable outcomes.
We found an inverse relationship of A-P score and risk of malignant stroke. In the study group, 17 patients developed malignant stroke. The coefficient of -1.2 and probability of 23% (p-value <.001) implies 23 % increase in risk of malignant stroke with every point decrease in A-P score. Approximately 90% of patients with an A-P score < 5 developed malignant stroke. All 17 malignant strokes required treatment. 15 of the 17 malignant stroke patients had A-P score of ≤ 6, other two had A-P score of 7. 6 of the 17 required decompressive hemicraniectomy; others were managed medically with hypertonic saline or mannitol. The total number of unfavorable outcomes were 16, including 5 deaths. 4 of the 5 deaths developed malignant stroke, with an A-P score of 2, 4, 5, and 6. A total of 12 patients of the 16 with unfavorable outcomes developed malignant stroke.
Which implies that both low ASPECT score and high number of passes are associated with malignant stroke or poor outcomes. However, when combined together in the equation A-P score, which becomes a much stronger predictor of malignant stroke. Lesser the A-P score, higher the chances of malignant stroke. For example even with the high ASPECT score, if it takes multiple passes to achieve the successful recanalization, then the final A-P score will be low. That can explain the risk of development of malignant stroke even after successful recanalization. Based on our study results, we can categorize the risk of malignant stroke in the three groups; Low risk (A-P score 9-8), Moderate risk (A-P score 7-6), High risk
(A-P score≤ 5 ). We recommend that the patients who are in the high risk group ( A-P score≤ 5) should require extra attention and care for example more frequent neuro-checks, vitals monitoring and serial neuro-imagings for timely identification and therapeutic interventions of malignant stroke.
we also have some limitations to our study for example; sample size of our study group was not large; n=78 with 17 developed malignant stroke, no exclusion based on IV thrombolytics, amount of cerebral atrophy & the degree of collateralization on CTA were not included and the wide window of the time of onset of symptoms and hospital arrival(24 hours). These factors have the ability to confound the study. We tried to minimize the biases by adjusting for some other variables including timings for door to needle, door to groin, door to first pass, and door to recanalization.
Conclusion:
In conclusion, ASPECTS-PASS score(A-P score) has been shown to be a strong individual predictor for malignant MCA stroke after successful recanalization post thrombectomy. Thus, A-P score of ≤ 5 suggests high risk. By virtue of predicting the risk of developing malignant stroke, this scoring system will enable the clinicians to identify the high risk patients. Thereby, early detection and timely therapeutic interventions could be possible. Of note, our study does not intend to suggest the optimal ASPECT score or number of passes for and during the thrombectomy. A-P score should only be used for the prediction of malignant stroke after successful recanalization following thrombectomy.
Authors contribution
Avirag goswami: Drafting/revision of the manuscript for content, including medical writing for content; Major role in the acquisition of data; Study concept or design; Analysis or interpretation of data. Naraharisetty Anita Rau: Drafting/revision of the manuscript for content, including medical writing for content; Major role in the acquisition of data. Jonathan Dissin: Drafting/revision of the manuscript for content, including medical writing for content. Patricia Hushen: Drafting/revision of the manuscript for content, including medical writing for content
Acknowledgement
Special thanks to Dr. George C. Newman for his support
References
- Jüttler, E.; Unterberg, A.; Woitzik, J.; Bösel, J.; Amiri, H.; Sakowitz, O.W.; Gondan, M.; Schiller, P.; Limprecht, R.; Luntz, S.; Schneider, H. Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. New England Journal of Medicine. 2014, 370, 1091–100. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yuan, R.; Wang, Y.; Wei, C.; Zhang, S.; Yang, X.; Wu, B.; Liu, M. Early prediction of malignant brain edema after ischemic stroke: a systematic review and meta-analysis. Stroke. 2018, 49, 2918–27. [Google Scholar] [CrossRef] [PubMed]
- Ropper, A.H. Brain edema after stroke: clinical syndrome and intracranial pressure. Archives of neurology. 1984, 41, 26–9. [Google Scholar] [CrossRef] [PubMed]
- Hacke, W.; Schwab, S.; Horn, M.; Spranger, M.; De Georgia, M.; von Kummer, R. 'Malignant Middle cerebral artery territory infarction: clinical course and prognostic signs. Archives of neurology. 1996, 53, 309–15. [Google Scholar] [CrossRef]
- Silver, F.L.; Norris, J.W.; Lewis, A.J.; Hachinski, V.C. Early mortality following stroke: a prospective review. Stroke. 1984, 15, 492–6. [Google Scholar] [CrossRef] [PubMed]
- Kasner, S.E.; Demchuk, A.M.; Berrouschot, J.; Schmutzhard, E.; Harms, L.; Verro, P.; Chalela, J.A.; Abbur, R.; McGrade, H.; Christou, I.; Krieger, D.W. Predictors of fatal brain edema in massive hemispheric ischemic stroke. Stroke. 2001, 32, 2117–23. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; Jauch, E.C. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. stroke. 2018, 49, e46–e99. [Google Scholar] [CrossRef]
- Álvaro, G.T.; Requena, M.; Rubiera, M. When to stop. Stroke. 2019;50:1781-8. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CB, van der Lugt A, De Miquel MA, Donnan GA. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. The Lancet. 2016, 387, 1723–31. [Google Scholar]
- Zaidat, O.O.; Castonguay, A.C.; Linfante, I.; Gupta, R.; Martin, C.O.; Holloway, W.E.; Mueller-Kronast, N.; English, J.D.; Dabus, G.; Malisch, T.W.; Marden, F.A. First pass effect: a new measure for stroke thrombectomy devices. Stroke. 2018, 49, 660–6. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, Y.; Wang, H.; Yang, D.; Jiang, T.; Yuan, K.; Gong, P.; Xu, P.; Li, Y.; Chen, J.; Wu, M. Symptomatic intracranial hemorrhage after mechanical thrombectomy in Chinese ischemic stroke patients: the ASIAN score. Stroke. 2020, 51, 2690–6. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Sun, H.; Xing, Y. Hemorrhagic transformation after cerebral infarction: current concepts and challenges. Annals of translational medicine. 2014, 2. [Google Scholar]
- Astrup, J.; Siesjö, B.K.; Symon, L. Thresholds in cerebral ischemia-the ischemic penumbra. Stroke. 1981, 12, 723–5. [Google Scholar] [CrossRef] [PubMed]
- Heiss, W.D.; Rosner, G. Functional recovery of cortical neurons as related to degree and duration of ischemia. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society. 1983, 14, 294–301. [Google Scholar] [CrossRef]
- Heiss, W.D. Malignant MCA infarction: pathophysiology and imaging for early diagnosis and management decisions. Cerebrovascular Diseases. 2016, 41, 1–7. [Google Scholar] [CrossRef]
- Liang, D.; Bhatta, S.; Gerzanich, V.; Simard, J.M. Cytotoxic edema: mechanisms of pathological cell swelling. Neurosurgical focus. 2007, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.X.; Shuaib, A. Critical role of microvasculature basal lamina in ischemic brain injury. Progress in neurobiology. 2007, 83, 140–8. [Google Scholar] [CrossRef] [PubMed]
- Kniesel, U.; Wolburg, H. Tight junctions of the blood–brain barrier. Cellular and molecular neurobiology. 2000, 20, 57–76. [Google Scholar] [CrossRef]
- Nielsen, T.H.; Ståhl, N.; Schalén, W.; Reinstrup, P.; Toft, P.; Nordström, C.H. Recirculation usually precedes malignant edema in middle cerebral artery infarcts. Acta neurologica scandinavica. 2012, 126, 404–10. [Google Scholar] [CrossRef]
- Lassen, N. The luxury perfusion of the brain and its possible relation to acute metabolic acidosis localized in the brain. Lancet. 1966, 113–5. [Google Scholar]
- Hakim, A.M.; Pokrupa, R.P.; Villanueva, J.; Diksic, M.; Evans, A.C.; Thompson, C.J.; Meyer, E.; Yamamoto, Y.L.; Feindel, W.H. The effect of spontaneous reperfusion on metabolic function in early human cerebral infarcts. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society. 1987, 21, 279–89. [Google Scholar] [CrossRef] [PubMed]
- Marchal, G.; Rioux, P.A.; Petit-Tabou, M.C.; Derlon, J.M.; Baron, J.C.; Serrati, C.; Viader, F.; de la Sayette, V.; Le Doze, F.; Lochon, P.; Petit-Taboué, M.C. PET imaging of cerebral perfusion and oxygen consumption in acute ischaemic stroke: relation to outcome. The Lancet. 1993, 341, 925–7. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, G.L.; Frackowiak, R.S.; Jones, T. Cerebral oxygen metabolism and blood flow in human cerebral ischemic infarction. Journal of Cerebral Blood Flow & Metabolism. 1982, 2, 321–35. [Google Scholar]
- Soehle, M.; Heimann, A.; Kempski, O. Postischemic application of lipid peroxidation inhibitor U-101033E reduces neuronal damage after global cerebral ischemia in rats. Stroke. 1998, 29, 1240–7. [Google Scholar] [CrossRef] [PubMed]
- Heiss, W.D.; Graf, R.; Löttgen, J.; Ohta, K.; Fujita, T.; Wagner, R.; Grond, M.; Weinhard, K. Repeat positron emission tomographic studies in transient middle cerebral artery occlusion in cats: residual perfusion and efficacy of postischemic reperfusion. Journal of Cerebral Blood Flow & Metabolism. 1997, 17, 388–400. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).