Submitted:
22 June 2023
Posted:
22 June 2023
You are already at the latest version
Abstract
Keywords:
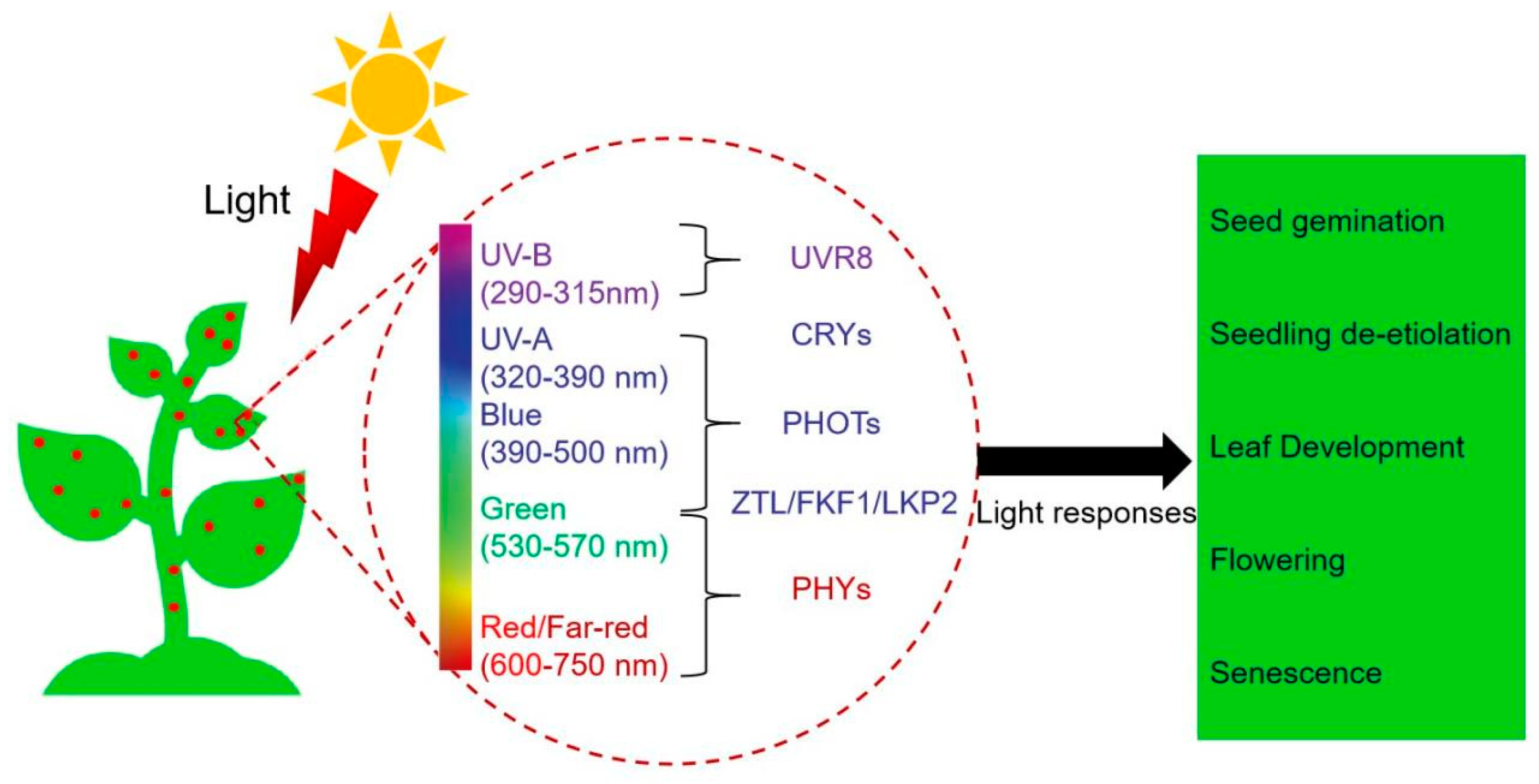
1. Introduction
2. The light signaling pathway
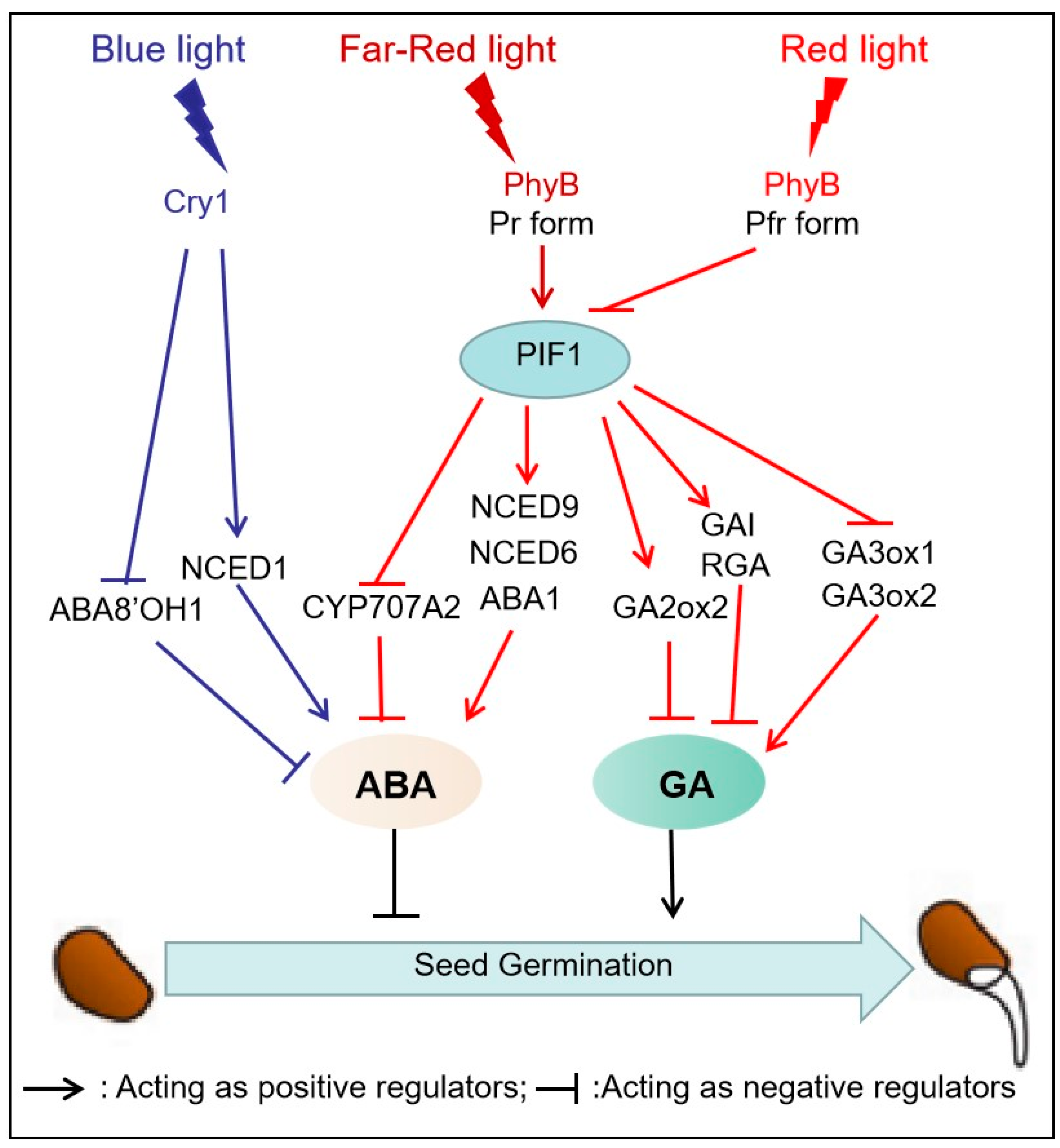
3. Roles of light quality in the regulation of seed germination
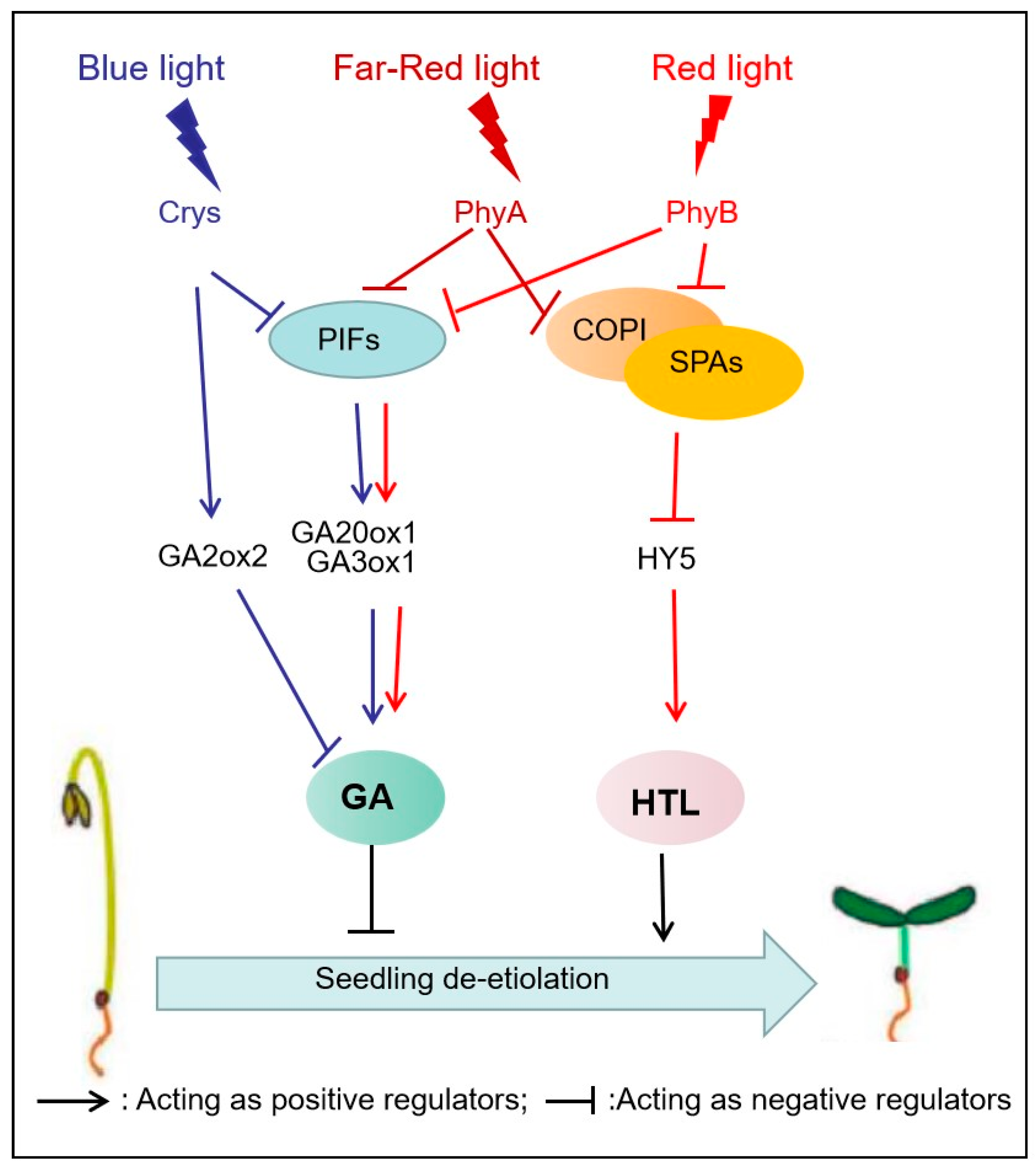
4. Roles of light quality in the regulation of photomorphogenesis
4.1. Seedling de-etiolation
4.2. Shoot-root development
4.3. Leaf development
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Devlin, P. Photocontrol of Flowering; Blackwell Publ.: Oxford, UK, 2008; pp. 185–210. [Google Scholar]
- Kaiser, E.; Correa Galvis, V.; Armbruster, U. Efficient Photosynthesis in Dynamic Light Environments: A Chloroplast’s Perspective. Biochem. J. 2019, 476, 2725–2741. [Google Scholar] [CrossRef]
- Liu, J.; van Iersel, M.W. Photosynthetic Physiology of Blue, Green, and Red Light: Light Intensity Effects and Underlying Mechanisms. Front. Plant Sci. 2021, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- Trivellini, A.; Toscano, S.; Romano, D.; Ferrante, A. The Role of Blue and Red Light in the Orchestration of Secondary Metabolites, Nutrient Transport and Plant Quality. Plants 2023, 12, 2026. [Google Scholar] [CrossRef]
- Cerdán, P.D.; Chory, J. Regulation of Flowering Time by Light Quality. Nature 2003, 423, 881–885. [Google Scholar] [CrossRef]
- Chen, C.; Huang, M.; Lin, K.; Wong, S.; Huang, W.; Yang, C. Effects of Light Quality on the Growth, Development and Metabolism of Rice Seedlings (Oryza Sativa L.). Res. J. Biotechnol. 2014, 9, 15–24. [Google Scholar]
- Zhao, J.; Bo, K.; Pan, Y.; Li, Y.; Yu, D.; Li, C.; Chang, J.; Wu, S.; Wang, Z.; Zhang, X.; Gu, F.; Weng, Y. Phytochrome-interacting factor PIF3 integrates phytochrome B and UVB signaling pathways to regulate gibberellin-and auxin-dependent growth in cucumber hypocotyls. J. Exp. Bot. 2023, erad181. [Google Scholar] [CrossRef]
- Jiang, H.; Li, Y.; He, R.; Tan, J.; Liu, K.; Chen, Y.; Liu, H. Effect of Supplemental UV-A Intensity on Growth and Quality of Kale under Red and Blue Light. Int. J. Mol. Sci. 2022, 23, 6819. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, G.; Jing, Y.; Tang, W.; Lin, R. Phytochrome B and REVEILLE1/2-Mediated Signalling Controls Seed Dormancy and Germination in Arabidopsis. Nat. Commun. 2016, 7, 12377. [Google Scholar] [CrossRef]
- Yang, L.; Wang, L.; Ma, J.; Ma, E.; Li, J.; Gong, M. Effects of Light Quality on Growth and Development, Photosynthetic Characteristics and Content of Carbohydrates in Tobacco (Nicotiana Tabacum L.) Plants. Photosynthetica 2016, 55. [Google Scholar] [CrossRef]
- Yavari, N.; Tripathi, R.; Wu, B.-S.; MacPherson, S.; Singh, J.; Lefsrud, M. The Effect of Light Quality on Plant Physiology, Photosynthetic, and Stress Response in Arabidopsis Thaliana Leaves. PloS One 2021, 16, e0247380. [Google Scholar] [CrossRef]
- Batista, D.S.; Felipe, S.H.S.; Silva, T.D.; de Castro, K.M.; Mamedes-Rodrigues, T.C.; Miranda, N.A.; Ríos-Ríos, A.M.; Faria, D.V.; Fortini, E.A.; Chagas, K.; et al. Light Quality in Plant Tissue Culture: Does It Matter? Vitro Cell. Dev. Biol. - Plant 2018, 54, 195–215. [Google Scholar] [CrossRef]
- Tripathi, S.; Hoang, Q.; Han, Y.; Kim, J. Regulation of Photomorphogenic Development by Plant Phytochromes. Int. J. Mol. Sci. 2019, 20, 6165. [Google Scholar] [CrossRef] [PubMed]
- Mohr, H. Lectures on Photomorphogenesis; Springer Science & Business Media, 2012; ISBN 978-3-642-65418-3. [Google Scholar]
- Borthwick, H.A.; Hendricks, S.B.; Parker, M.W.; Toole, E.H.; Toole, V.K. A Reversible Photoreaction Controlling Seed Germination. Proc. Natl. Acad. Sci. U. S. A. 1952, 38, 662–666. [Google Scholar] [CrossRef]
- de Wit, M.; Galvão, V.C.; Fankhauser, C. Light-Mediated Hormonal Regulation of Plant Growth and Development. Annu. Rev. Plant Biol. 2016, 67, 513–537. [Google Scholar] [CrossRef]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-Regulated Plant Growth and Development. Current Topics in Developmental Biology 2010, 29–66. [Google Scholar]
- Heijde, M.; Ulm, R. UV-B Photoreceptor-Mediated Signalling in Plants. Trends Plant Sci. 2012, 17, 230–237. [Google Scholar] [CrossRef]
- Pedmale, U.; Huang, S.; Zander, M.; Cole, B.; Hetzel, J.; Ljung, K.; Reis, P.; Sridevi, P.; Nito, K.; Nery, J.; et al. Cryptochromes Interact Directly with PIFs to Control Plant Growth in Limiting Blue Light. Cell 2016, 164, 233–245. [Google Scholar] [CrossRef]
- Xu, P.; Chen, H.; Li, T.; Xu, F.; Mao, Z.; Cao, X.; Miao, L.; Du, S.; Hua, J.; Zhao, J.; et al. Blue Light-Dependent Interactions of CRY1 with GID1 and DELLA Proteins Regulate Gibberellin Signaling and Photomorphogenesis in Arabidopsis. Plant Cell 2021, 33. [Google Scholar] [CrossRef]
- Farooq, M.A.; Ma, W.; Shen, S.; Gu, A. Underlying Biochemical and Molecular Mechanisms for Seed Germination. Int. J. Mol. Sci. 2022, 23, 8502. [Google Scholar] [CrossRef]
- Yang, L.; Liu, S.; Lin, R. The Role of Light in Regulating Seed Dormancy and Germination. J. Integr. Plant Biol. 2020, 62, 1310–1326. [Google Scholar] [CrossRef]
- Cashmore, A. Cryptochromes: Enabling Plants and Animals to Determine Circadian Time. Cell 2003, 114, 537–543. [Google Scholar]
- Ito, S.; Song, Y.H.; Imaizumi, T. LOV Domain-Containing F-Box Proteins: Light-Dependent Protein Degradation Modules in Arabidopsis. Mol. Plant 2012, 5, 573–582. [Google Scholar] [CrossRef]
- Christie, J. Phototropin Blue-Light Receptors. Annu. Rev. Plant Biol. 2007, 58, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Rizzini, L.; Favory, J.; Cloix, C.; Faggionato, D.; O’Hara, A.; Kaiserli, E.; Baumeister, R.; Schäfer, E.; Nagy, F.; Jenkins, G.I.; et al. Perception of UV-B by the Arabidopsis UVR8 Protein. Science 2011, 332, 103–106. [Google Scholar] [CrossRef]
- Balliu, A.; Kacjan Marsic, N.; Gruda, N. Seedling Production. 2017; pp. 189–206.
- Yadav, A.; Singh, D.; Lingwan, M.; Yadukrishnan, P.; Masakapalli, S.K.; Datta, S. Light Signaling and UV-B-mediated Plant Growth Regulation. J. Integr. Plant Biol. 2020, 62, 1270–1292. [Google Scholar] [CrossRef] [PubMed]
- Bantis, F. Light Emitting Diodes (LEDs) Affect Morphological, Physiological and Phytochemical Characteristics of Pomegranate Seedlings. Sci. Hortic. 2018, 234, 267–274. [Google Scholar] [CrossRef]
- Cavallaro, V.; Muleo, R. The Effects of LED Light Spectra and Intensities on Plant Growth. Plants 2022, 11, 1911. [Google Scholar] [CrossRef]
- Lazzarin, M.; Meisenburg, M.; Meijer, D.; van Ieperen, W.; Marcelis, L.F.M.; Kappers, I.F.; van der Krol, A.R.; van Loon, J.J.A.; Dicke, M. LEDs Make It Resilient: Effects on Plant Growth and Defense. Trends Plant Sci. 2021, 26, 496–508. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, M.; Cheng, F.; Liu, S.; Liang, Y. Effects of LED Photoperiods and Light Qualities on in Vitro Growth and Chlorophyll Fluorescence of Cunninghamia Lanceolata. BMC Plant Biol. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Chen, M.; Chory, J.; Fankhauser, C. Light Signal Transduction in Higher Plants. Annu. Rev. Genet. 2004, 38, 87–117. [Google Scholar] [CrossRef]
- Möglich, A.; Yang, X.; Ayers, R.A.; Moffat, K. Structure and Function of Plant Photoreceptors. Annu. Rev. Plant Biol. 2010, 61, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Shu, K.; Liu, X.-D.; Xie, Q.; He, Z.-H. Two Faces of One Seed: Hormonal Regulation of Dormancy and Germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chory, J. Phytochrome Signaling Mechanisms and the Control of Plant Development. Trends Cell Biol. 2011, 21, 664–671. [Google Scholar] [CrossRef]
- Cheng, M.; Kathare, P.; Paik, I.; Huq, E. Phytochrome Signaling Networks. Annu. Rev. Plant Biol. 2021, 72, 217–244. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Samtani, H.; Sahu, K.; Sharma, A.K.; Khurana, J.P.; Khurana, P. International Journal of Biological Macromolecules Functions of Phytochrome-Interacting Factors (PIFs) in the regulation of plant growth and development: A comprehensive review. Int. J. Biol. Macromol. 2023, 244, 125234. [Google Scholar] [CrossRef]
- Pham, V.N.; Kathare, P.K.; Huq, E. Phytochromes and Phytochrome Interacting Factors. Plant Physiol. 2018, 176, 1025–1038. [Google Scholar] [CrossRef]
- Paik, I.; Huq, E. Plant Photoreceptors: Multi-Functional Sensory Proteins and Their Signaling Networks. Semin. Cell Dev. Biol. 2019, 92, 114–121. [Google Scholar] [CrossRef]
- Rockwell, N.C.; Su, Y.-S.; Lagarias, J.C. Phytochrome Structure and Signaling Mechanisms. Annu. Rev. Plant Biol. 2006, 57, 837–858. [Google Scholar] [CrossRef]
- Leivar, P.; Monte, E. PIFs: Systems Integrators in Plant Development. Plant Cell 2014, 26, 56–78. [Google Scholar] [CrossRef]
- Wang, P.; Abid, M.A.; Qanmber, G.; Askari, M.; Zhou, L.; Song, Y.; Liang, C.; Meng, Z.; Malik, W.; Wei, Y.; et al. Photomorphogenesis in plants: The central role of phytochrome interacting factors (PIFs). Environ. Exp. Bot. 2022, 194, 104704. [Google Scholar] [CrossRef]
- Lee, K.P.; Piskurewicz, U.; Turečková, V.; Carat, S.; Chappuis, R.; Strnad, M.; Fankhauser, C.; Lopez-Molina, L. Spatially and Genetically Distinct Control of Seed Germination by Phytochromes A and B. Genes Dev. 2012, 26, 1984–1996. [Google Scholar] [CrossRef]
- Bian, Y.; Chu, L.; Lin, H.; Qi, Y.; Fang, Z.; Xu, D. PIFs- and COP1-HY5-Mediated Temperature Signaling in Higher Plants. Stress Biol. 2022, 2, 35. [Google Scholar] [CrossRef]
- Kim, J.; Song, J.; Seo, H. COP1 Regulates Plant Growth and Development in Response to Light at the Post-Translational Level. J. Exp. Bot. 2017, 68, 4737–4748. [Google Scholar] [CrossRef]
- Lee, J.; Jung, J.; Park, C. Light Inhibits COP1-Mediated Degradation of ICE Transcription Factors to Induce Stomatal Development in Arabidopsis. Plant Cell 2017, 29, 2817–2830. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chu, L.; Zhang, Y.; Bian, Y.; Xiao, J.; Xu, D. HY5: A Pivotal Regulator of Light-Dependent Development in Higher Plants. Front. Plant Sci. 2022, 12, 3294. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.E.; Gardner, K.H. Lighting the way: Recent insights into the structure and regulation of phototropin blue light receptors. J. Biol. Chem. 2021, 296, 100594. [Google Scholar] [CrossRef] [PubMed]
- Paik, I.; Huq, E. Plant Photoreceptors: Multi-Functional Sensory Proteins and Their Signaling Networks. Semin. Cell Dev. Biol. 2019, 92, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Banaś, A.K.; Aggarwal, C.; Łabuz, J.; Sztatelman, O.; Gabryś, H. Blue Light Signalling in Chloroplast Movements. J. Exp. Bot. 2012, 63, 1559–1574. [Google Scholar] [CrossRef]
- Pipitone, R.; Eicke, S.; Pfister, B.; Glauser, G.; Falconet, D.; Uwizeye, C.; Pralon, T.; Zeeman, S.C.; Kessler, F.; Demarsy, E. A multifaceted analysis reveals two distinct phases of chloroplast biogenesis during de-etiolation in arabidopsis. Elife 2021, 10, 1–32. [Google Scholar] [CrossRef]
- Barrero, J.M.; Downie, A.B.; Xu, Q.; Gubler, F. A Role for Barley CRYPTOCHROME1 in Light Regulation of Grain Dormancy and Germination. Plant Cell 2014, 26, 1094–1104. [Google Scholar] [CrossRef]
- Mao, Z.; He, S.; Xu, F.; Wei, X.; Jiang, L.; Liu, Y.; Wang, W.; Li, T.; Xu, P.; Du, S.; et al. Photoexcited CRY1 and PhyB Interact Directly with ARF6 and ARF8 to Regulate Their DNA-Binding Activity and Auxin-Induced Hypocotyl Elongation in Arabidopsis. New Phytol. 2020, 225, 848–865. [Google Scholar] [CrossRef]
- Gao, L.; Liu, Q.; Zhong, M.; Zeng, N.; Deng, W.; Li, Y.; Wang, D.; Liu, S.; Wang, Q. Blue light-induced phosphorylation of Arabidopsis cryptochrome 1 is essential for its photosensitivity. J. Integr. Plant Biol. 2022, 64, 1724–1738. [Google Scholar] [CrossRef] [PubMed]
- Martínez, C.; Nieto, C.; Prat, S. Convergent Regulation of PIFs and the E3 Ligase COP1/SPA1 Mediates Thermosensory Hypocotyl Elongation by Plant Phytochromes. Curr. Opin. Plant Biol. 2018, 45, 188–203. [Google Scholar] [CrossRef]
- Stawska, M.; Oracz, K. PhyB and HY5 Are Involved in the Blue Light-Mediated Alleviation of Dormancy of Arabidopsis Seeds Possibly via the Modulation of Expression of Genes Related to Light, GA, and ABA. Int. J. Mol. Sci. 2019, 20, 5882. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, L.; Chen, P.; Liang, T.; Li, X.; Liu, H. UV-B Photoreceptor UVR8 Interacts with MYB73/MYB77 to Regulate Auxin Responses and Lateral Root Development. EMBO J. 2020, 39, e101928. [Google Scholar] [CrossRef] [PubMed]
- Biever, J.J.; Gardner, G. The Relationship between Multiple UV-B Perception Mechanisms and DNA Repair Pathways in Plants. Environ. Exp. Bot. 2016, 124, 89–99. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, D.; Lingwan, M.; Yadukrishnan, P.; Masakapalli, S.K.; Datta, S. Light Signaling and UV-B-Mediated Plant Growth Regulation. J. Integr. Plant Biol. 2020, 62, 1270–1292. [Google Scholar] [CrossRef]
- Huché-Thélier, L.; Crespel, L.; Gourrierec, J.L.; Morel, P.; Sakr, S.; Leduc, N. Light Signaling and Plant Responses to Blue and UV Radiations—Perspectives for Applications in Horticulture. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Ravindran, P.; Kumar, P.P. Regulation of Seed Germination: The Involvement of Multiple Forces Exerted via Gibberellic Acid Signaling. Mol. Plant 2019, 12, 24–26. [Google Scholar] [CrossRef]
- Tuan, P.A.; Kumar, R.; Rehal, P.K.; Toora, P.K.; Ayele, B.T. Molecular Mechanisms Underlying Abscisic Acid/Gibberellin Balance in the Control of Seed Dormancy and Germination in Cereals. Front. Plant Sci. 2018, 9, 668. [Google Scholar] [CrossRef]
- de Wit, M.; Galvão, V.C.; Fankhauser, C. Light-Mediated Hormonal Regulation of Plant Growth and Development. Annu. Rev. Plant Biol. 2016, 67, 513–537. [Google Scholar] [CrossRef]
- Née, G.; Xiang, Y.; Soppe, W. The Release of Dormancy, a Wake-up Call for Seeds to Germinate. Curr. Opin. Plant Biol. 2017, 35, 8–14. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Y.; Zheng, Y. Integration of ABA, GA, and light signaling in seed germination through the regulation of ABI5. Front. Plant Sci. 2022, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fantini, E.; Facella, P. Cryptochromes in the Field: How Blue Light Influences Crop Development. Physiol. Plant. 2020, 169, 336–346. [Google Scholar] [CrossRef] [PubMed]
- El-Keblawy, A.; Elgabra, M.; Mosa, K.A.; Fakhry, A.; Soliman, S. Roles of hardened husks and membranes surrounding Brachypodium hybridum grains on germination and seedling growth. Plants 2019, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Goggin, D.E.; Steadman, K.J.; Powles, S.B. Green and Blue Light Photoreceptors Are Involved in Maintenance of Dormancy in Imbibed Annual Ryegrass (Lolium Rigidum) Seeds. New Phytol. 2008, 180, 81–89. [Google Scholar] [CrossRef]
- Yang, L.; Liu, S.; Lin, R. The role of light in regulating seed dormancy and germination. J. Integr. Plant Biol. 2020, 62, 1310–1326. [Google Scholar] [CrossRef]
- Jacobsen, J.V.; Barrero, J.M.; Hughes, T.; Julkowska, M.; Taylor, J.M.; Xu, Q.; Gubler, F. Roles for Blue Light, Jasmonate and Nitric Oxide in the Regulation of Dormancy and Germination in Wheat Grain (Triticum Aestivum L.). Planta 2013, 238, 121–138. [Google Scholar] [CrossRef]
- Simlat, M.; Ślęzak, P.; Moś, M.; Warchoł, M.; Skrzypek, E.; Ptak, A. The Effect of Light Quality on Seed Germination, Seedling Growth and Selected Biochemical Properties of Stevia Rebaudiana Bertoni. Sci. Hortic. 2016, 211, 295–304. [Google Scholar] [CrossRef]
- Lymperopoulos, P.; Msanne, J.; Rabara, R. Phytochrome and Phytohormones: Working in Tandem for Plant Growth and Development. Front. Plant Sci. 2018, 9, 1037. [Google Scholar] [CrossRef]
- Arana, M.V.; Sánchez-Lamas, M.; Strasser, B.; Ibarra, S.E.; Cerdán, P.D.; Botto, J.F.; Sánchez, R.A. Functional Diversity of Phytochrome Family in the Control of Light and Gibberellin-Mediated Germination in Arabidopsis. Plant Cell Environ. 2014, 37, 2014–2023. [Google Scholar] [CrossRef]
- Vaistij, F.E.; Barros-Galvão, T.; Cole, A.F.; Gilday, A.D.; He, Z.; Li, Y.; Harvey, D.; Larson, T.R.; Graham, I.A. MOTHER-OF-FT-AND-TFL1 Represses Seed Germination under Far-Red Light by Modulating Phytohormone Responses in Arabidopsis Thaliana. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, 8442–8447. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Ryu, J.; Jeong, Y.; Park, J.; Song, J.; Amasino, R.M.; Noh, B.; Noh, Y. Control of Seed Germination by Light-Induced Histone Arginine Demethylation Activity. Dev. Cell 2012, 22, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Albertos, P.; Wlk, T.; Griffiths, J.; Pimenta Lange, M.J.; Unterholzner, S.J.; Rozhon, W.; Lange, T.; Jones, A.M.; Poppenberger, B. Brassinosteroid-regulated bHLH transcription factor CESTA induces the gibberellin 2-oxidase GA2ox7. Plant Physiol. 2022, 188, 2012–2025. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, G.; Jing, Y.; Tang, W.; Lin, R. Phytochrome B and REVEILLE1/2-Mediated Signalling Controls Seed Dormancy and Germination in Arabidopsis. Nat. Commun. 2016, 7, 12377. [Google Scholar] [CrossRef]
- Ahmad, M.; Cashmore, A.R. HY4 Gene of A. Thaliana Encodes a Protein with Characteristics of a Blue-Light Photoreceptor. Nature 1993, 366, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Cao, K.; Hao, Y.; Song, S.; Su, W.; Liu, H. Hypocotyl Elongation Is Regulated by Supplemental Blue and Red Light in Cucumber Seedling. Gene 2019, 707, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Zeng, B.; Tang, D.; Yang, J.; Qu, L.; Yan, J.; Wang, X.; Li, X.; Liu, X.; Zhao, X. The blue light receptor CRY1 interacts with GID1 and DELLA proteins to repress GA signaling during photomorphogenesis in Arabidopsis. Mol. Plant 2021, 14, 1328–1342. [Google Scholar] [CrossRef] [PubMed]
- Ponnu, J.; Hoecker, U. Illuminating the COP1/SPA Ubiquitin Ligase: Fresh Insights into Its Structure and Functions During Plant Photomorphogenesis. Front. Plant Sci. 2021, 12, 1–19. [Google Scholar] [CrossRef]
- Dong, J.; Tang, D.; Gao, Z.; Yu, R.; Li, K.; He, H.; Terzaghi, W.; Deng, X.W.; Chen, H. Arabidopsis DE-ETIOLATED1 Represses Photomorphogenesis by Positively Regulating Phytochrome-Interacting Factors in the Dark. Plant Cell 2014, 26, 3630–3645. [Google Scholar]
- Sun, X.; Ni, M. HYPOSENSITIVE TO LIGHT, an Alpha/Beta Fold Protein, Acts Downstream of ELONGATED HYPOCOTYL 5 to Regulate Seedling De-Etiolation. Mol. Plant 2011, 4, 116–126. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, D.; Lingwan, M.; Yadukrishnan, P.; Masakapalli, S.K.; Datta, S. Light signaling and UV-B-mediated plant growth regulation. J. Integr. Plant Biol. 2020, 62, 1270–1292. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Q.; Zhang, F.; Wang, Y.; Zhang, S.; Cheng, H.; Yan, L.; Li, L.; Chen, F.; Xie, X. Overexpression of OsPIL15, a Phytochrome-Interacting Factor-like Protein Gene, Represses Etiolated Seedling Growth in Rice. J. Integr. Plant Biol. 2014, 56, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, H. Coordinated Shoot and Root Responses to Light Signaling in Arabidopsis. Plant Commun. 2020, 1, 100026. [Google Scholar] [CrossRef] [PubMed]
- Delker, C.; Sonntag, L.; James, G.V.; Janitza, P.; Ibañez, C.; Ziermann, H.; Peterson, T.; Denk, K.; Mull, S.; Ziegler, J.; et al. The DET1-COP1-HY5 Pathway Constitutes a Multipurpose Signaling Module Regulating Plant Photomorphogenesis and Thermomorphogenesis. Cell Rep. 2014, 9, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Hoecker, U. The Activities of the E3 Ubiquitin Ligase COP1/SPA, a Key Repressor in Light Signaling. Curr. Opin. Plant Biol. 2017, 37, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Ulm, R. How Plants Cope with UV-B: From Perception to Response. Curr. Opin. Plant Biol. 2017, 37, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Sassi, M.; Lu, Y.; Zhang, Y.; Wang, J.; Dhonukshe, P.; Blilou, I.; Dai, M.; Li, J.; Gong, X.; Jaillais, Y.; et al. COP1 Mediates the Coordination of Root and Shoot Growth by Light through Modulation of PIN1- and PIN2-Dependent Auxin Transport in Arabidopsis. Development 2012, 139, 3402–3412. [Google Scholar] [CrossRef]
- Xiao, L.; Shibuya, T.; Kato, K.; Nishiyama, M.; Kanayama, Y. Effects of Light Quality on Plant Development and Fruit Metabolism and Their Regulation by Plant Growth Regulators in Tomato. Sci. Hortic. 2022, 300, 111076. [Google Scholar] [CrossRef]
- Gelderen, K.; Kang, C.; Paalman, R.; Keuskamp, D.; Hayes, S.; Pierik, R. Far-Red Light Detection in the Shoot Regulates Lateral Root Development through the HY5 Transcription Factor. Plant Cell 2018, 30, 101–116. [Google Scholar] [CrossRef]
- Xiong, J.; Yang, F.; Wei, F.; Yang, F.; Lin, H.; Zhang, D. Inhibition of SIZ1-mediated SUMOylation of HOOKLESS1 promotes light-induced apical hook opening in Arabidopsis. Plant Cell 2023, 35, 2027–2043. [Google Scholar] [CrossRef]
- Xiong, H.; Lu, D.; Li, Z.; Wu, J.; Ning, X.; Lin, W.; Bai, Z.; Zheng, C.; Sun, Y.; Chi, W.; et al. The DELLA-ABI4-HY5 module integrates light and gibberellin signals to regulate hypocotyl elongation. Plant Commun. 2023, 100597. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.G.; Muneer, S.; Jeong, B.R. Morphogenesis, flowering, and gene expression of Dendranthema grandiflorum in response to shift in light quality of night interruption. Int. J. Mol. Sci. 2015, 16, 16497–16513. [Google Scholar] [CrossRef] [PubMed]
- Pierik, R.; Ballaré, C.L. Control of Plant Growth and Defense by Photoreceptors: From Mechanisms to Opportunities in Agriculture. Mol. Plant 2021, 14, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.P.; Hayes, S.; Franklin, K.A. Photoreceptor Crosstalk in Shade Avoidance. Curr. Opin. Plant Biol. 2016, 33, 1–7. [Google Scholar] [CrossRef]
- Gommers, C.M.M.; Visser, E.J.W.; Onge, K.R.S.; Voesenek, L.A.C.J.; Pierik, R. Shade Tolerance: When Growing Tall Is Not an Option. Trends Plant Sci. 2013, 18, 65–71. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dwivedi, S.; Bhagavatula, L.; Datta, S. Integration of light and ABA signaling pathways to combat drought stress in plants. Plant Cell Rep. 2023, 42, 829–841. [Google Scholar] [CrossRef]
- Matthews, J.; Vialet-Chabrand, S.; Lawson, T. Role of Blue and Red Light in Stomatal Dynamic Behaviour. J. Exp. Bot. 2019, 71, 2253–2269. [Google Scholar] [CrossRef]
- Wei, H.; Kong, D.; Yang, J.; Wang, H. Light Regulation of Stomata Development and Patterning: Shifting the Paradigm from Arabidopsis to Grasses. Plant Commun. 2020, 1, 100030. [Google Scholar] [CrossRef]
- Delgado, D.; Ballesteros, I.; Torres-Contreras, J.; Mena, M.; Fenoll, C. Dynamic Analysis of Epidermal Cell Divisions Identifies Specific Roles for COP10 in Arabidopsis Stomatal Lineage Development. Planta 2012, 236, 447–461. [Google Scholar] [CrossRef]
- Kreiss, M.; Haas, F.B.; Hansen, M.; Rensing, S.A.; Hoecker, U. Co-action of COP1, SPA and cryptochrome in light signal transduction and photomorphogenesis of the moss Physcomitrium patens. Plant J. 2023, 159–175. [Google Scholar] [CrossRef]
- Lampard, G.R.; Macalister, C.A.; Bergmann, D.C. Arabidopsis Stomatal Initiation Is Controlled by MAPK-Mediated Regulation of the BHLH SPEECHLESS. Science 2008, 322, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, P.; Si, T.; Hsu, C.; Wang, L.; Zayed, O.; Yu, Z.; Zhu, Y.; Dong, J.; Tao, W.A.; et al. MAP Kinase Cascades Regulate the Cold Response by Modulating ICE1 Protein Stability. Dev. Cell 2017, 43, 618–629.e5. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.N.; Kathare, P.K.; Huq, E. Phytochromes and Phytochrome Interacting Factors. Plant Physiol. 2018, 176, 1025–1038. [Google Scholar] [CrossRef]
- Lau, O.S.; Song, Z.; Zhou, Z.; Davies, K.A.; Chang, J.; Yang, X.; Wang, S.; Lucyshyn, D.; Tay, I.H.Z.; Wigge, P.A.; et al. Direct Control of SPEECHLESS by PIF4 in the High-Temperature Response of Stomatal Development. Curr. Biol. CB 2018, 28, 1273–1280. [Google Scholar] [CrossRef]
- Klermund, C.; Ranftl, Q.L.; Diener, J.; Bastakis, E.; Richter, R.; Schwechheimer, C. LLM-Domain B-GATA Transcription Factors Promote Stomatal Development Downstream of Light Signaling Pathways in Arabidopsis Thaliana Hypocotyls. Plant Cell 2016, 28, 646–660. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, B.; Li, J.; Tang, H.; Tang, J.; Yang, Z. Formation and Change of Chloroplast-Located Plant Metabolites in Response to Light Conditions. Int. J. Mol. Sci. 2018, 19, 654. [Google Scholar] [CrossRef]
- Chory, J. Light Signals in Leaf and Chloroplast Development: Photoreceptors and Downstream Responses in Search of a Transduction Pathway. New Biol. 1991, 3, 538–548. [Google Scholar]
- Yang, E.J.; Yoo, C.Y.; Liu, J.; Wang, H.; Cao, J.; Li, F.; Pryer, K.M.; Sun, T.; Weigel, D.; Zhou, P.; et al. NCP Activates Chloroplast Transcription by Controlling Phytochrome-Dependent Dual Nuclear and Plastidial Switches. Nat. Commun. 2019, 10, 2630. [Google Scholar] [CrossRef]
- Yoo, C.Y.; Pasoreck, E.K.; Wang, H.; Cao, J.; Blaha, G.M.; Weigel, D.; Chen, M. Phytochrome Activates the Plastid-Encoded RNA Polymerase for Chloroplast Biogenesis via Nucleus-to-Plastid Signaling. Nat. Commun. 2019, 10, 2629. [Google Scholar] [CrossRef]
- Mellenthin, M.; Ellersiek, U.; Börger, A.; Baier, M. Expression of the Arabidopsis Sigma Factor SIG5 Is Photoreceptor and Photosynthesis Controlled. Plants 2014, 3, 359–391. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Y.; Liu, H.; Zhang, Y.; Hao, Y.; Song, S.; Lei, B. Effect of Supplemental Blue Light Intensity on the Growth and Quality of Chinese Kale. Hortic. Environ. Biotechnol. 2019, 60, 49–57. [Google Scholar]
- Li, Y.; Wu, L.; Jiang, H.; He, R.; Song, S.; Su, W.; Liu, H. Supplementary Far-Red and Blue Lights Influence the Biomass and Phytochemical Profiles of Two Lettuce Cultivars in Plant Factory. Molecules 2021, 26, 7405. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Batista, D.; Fortini, E.; Castro, K.; Sousa Felipe, S.; Fernandes, A.; De Jesus Sousa, R.M.; Chagas, K.; Da Silva, J.; Correia, L.; et al. Blue and Red Light Affects Morphogenesis and 20-Hydroxyecdisone Content of in Vitro Pfaffia Glomerata Accessions. J. Photochem. Photobiol. B: Biology 2019, 203, 111761. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



