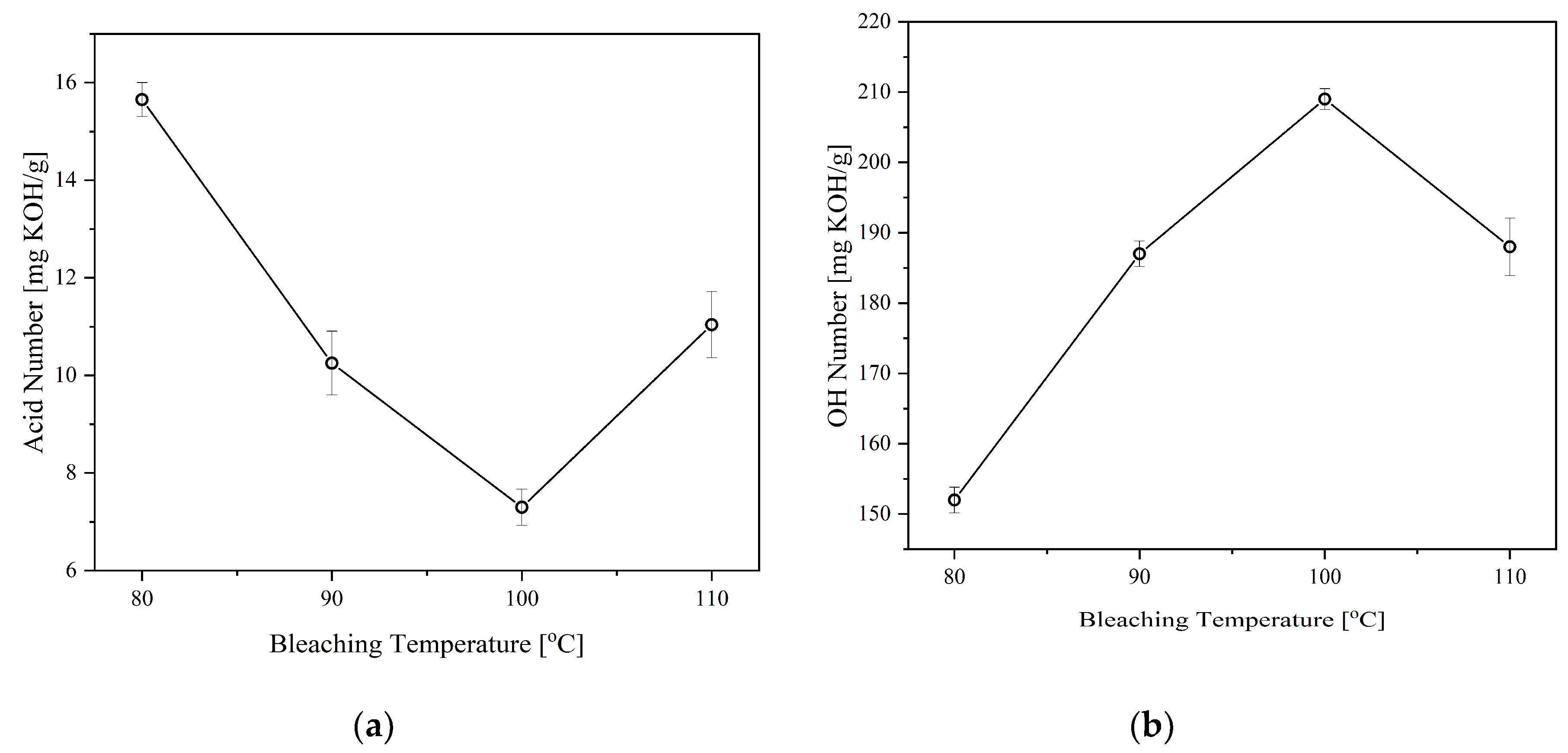1. Introduction
The polyurethane (PU) industry is one of the chemical industries that remained heavily reliant on petroleum-derived products as raw materials [
1]. A fundamental component in the PU synthesis is polyol, which is still widely produced from petroleum-based materials [
2,
3]. The global production of polyols alone reached approximately 22 million metric tons in 2019, amounting to USD26.2B, placing it as the sixth most important polymer family [
4]. Its global market is expected to grow up to USD34.4B by 2024. Being this contingent on non-renewable sources, the advancement of global industrialization of the PU industry is at risk [
5]. The threat of depletion, economic volatility, and environmental impacts are the main factors that pushed the PU industry to search for new, renewable, and sustainable polyol raw materials [
6].
One of the best routes of action is to utilize environmentally friendly products derived from renewable and natural molecules [
7]. Vegetable oils (VOs) are among the most preferred substitute raw materials for polyols due to their abundant supply, low toxicity, biodegradability, inherent fluidity, and low cost [
8]. This shift from using petroleum-based to bio-based polyols offers new options to valorize biomass into technical-grade polyols in the PU industry [
4].
Most studies utilize VOs as the raw material for polyol production oils [
9,
10,
11,
12,
13,
14]. However, the direct utilization of these vegetable oils, particularly edible oils, in the PU industry is disadvantageous as it directly competes with the food and biofuel industries. In consequence, there is a need to develop more sustainable initiatives. One of the propitious options to address this concern is the direct utilization of vegetable oil byproducts from the oil refineries. This action will lead to value-added products and advance the utilization of low-cost waste materials through a facile process.
One of the byproducts of the vegetable oil refining industry is fatty acid distillate (FAD). This material is a byproduct produced from the deodorization step, which is the final stage of the chemical refining of vegetable oils [
15,
16]. FAD is considered as a low-value waste and is usually disposed of. This type of waste accounts for around 4% of the total composition of unrefined oil, thus, disposing it is wasting potentially valuable material [
16]. Although there are multiple uses for FAD, according to the literature, they are either expensive to execute or not well-established. Coconut oil is one of such vegetable oil that can be a good source of coconut fatty acid distillate (CFAD). This material is not as widely used in the PU industry compared to other vegetable oils such as palm, soybean, rapeseed, and castor oils [
9,
10,
11,
12,
13,
14]. This distillate contains a high amount of lauric acid and is usually along with a mixture of volatile organic compounds and impurities. This kind of fatty acid distillate has not been fully utilized yet in the PU industry as it has limited application due to the disadvantages of free fatty acids in polyols.
Another waste material from vegetable oils, particularly in the biodiesel industry, is crude glycerol (CG). CG is a major byproduct in biodiesel production teeming with impurities that hinders its direct industrial usage [
17]. With this byproduct constituting 10% of the total biodiesel production, its direct utilization would be highly advantageous. Like FAD, CG’s existing applications mostly require high purity, thus necessitating a purification process. This limits the direct usage of CG in the PU industry since the presence of its impurities may compromise the properties of PU products.
The use of these two waste materials, CFAD and CG, diversifies the applications of vegetable oils without trading off their value in the food and biofuel industries. Thus, this study aims to devise a process centered around the glycerolysis reaction that will directly utilize CFAD and CG as starting materials for the synthesis of a CFAD-based polyol for PU foam applications. This study will also demonstrate the compatibility of the CFAD-based polyol as a precursor in producing a rigid thermal insulation PU foam.
Therefore, the current study directly utilizes CFAD and crude glycerol, both are waste materials from agricultural products, in the production of polyol for rigid PU foam by integrating CFAD into the polymerization of crude glycerol, thereby yielding a fully bio-based polyol suitable for the targeted application. The chemical properties of the polyol and the physical, thermal, and mechanical properties of the foam were investigated.
2. Materials and Methods
2.1 Materials
Coconut fatty acid distillate (CFAD) and crude glycerol (CG) were obtained from Chemrez Technologies, Inc. Their properties are shown in
Table 1 and
Table 2, respectively. VORANOLⓇ 490, DABCOⓇ 33-LV, POLYCATⓇ 8, and DABCOⓇ DC 2585 were also obtained from the same facility. Potassium hydroxide, absolute ethanol, and hydrogen peroxide were purchased from Sigma-Aldrich.
2.2. Crude CFAD-based Polyol Synthesis
CFAD-based polyol was synthesized through glycerolysis with CFAD and CG. A 500 mL three-necked flask on a heating mantle equipped with a magnetic stirrer, thermometer, and condenser was used as the reacting vessel. The glycerolysis reaction was allowed to proceed at different CG loading (0.5-3 molar ratio), reaction temperature (170-190°C), and reaction time (1-4 hrs). The materials with their designed ratio were loaded into the reaction vessel with 2 wt. % KOH catalyst and reacted with 600 rpm constant stirring.
2.3 CFAD-based Polyol in situ Treatment
The crude polyol produced from the glycerolysis of CFAD and CG was subjected to a two-step in situ treatment. The first step was neutralization, wherein a varying loading (8-14 wt. %) of an alkali-alcohol neutralizing agent was added to the reactor. The alkali-alcohol solution was produced by dissolving 8g KOH in 100g absolute ethanol. The neutralization reaction was conducted at 180°C for 90 minutes producing the neutralized polyol. The neutralized polyol was then subjected to the second part of the treatment process, bleaching, wherein hydrogen peroxide was loaded into the reactor vessel at 60 wt. % loading. The bleaching process was allowed to proceed at different bleaching temperatures (80-110°C) and bleaching times (20-80 mins). The final product, CFAD-based polyol, was then transferred to a separatory funnel to remove some unreacted compounds.
2.4 PU Foam Preparation
A standard laboratory mixing and pouring procedure for making water-blown PU foams was used in this study. The rigid foam formulation used is summarized in
Table 3. The B-side components were added into a 500 mL disposable plastic cup and mixed at 3450 rpm for 10-15 s. The mixture was allowed to degas for 120 s.
The A-side component was added rapidly, stirring the mixture for 10-15 s at 3450 rpm. The mixture was poured immediately into a wooden mold (11.4×11.4×21.6 cm) with aluminum foil lining, and the foam was left to rise and cure at ambient conditions (23°C).
2.5 Polyol and Foam Characterizations
The CFAD-based polyols were characterized for their acid and OH numbers according to ASTM D1980 [
18] and ASTM D4274 [
19], respectively. A structural analysis was also done using a Shimadzu IRTracer-100 FTIR spectrometer with QATR-10 accessory (Shimadzu Corp., Kyoto, Japan) at 4000-500 cm-1 wavenumber range and 4 cm-1 resolution. The rigid PU foam was characterized for its mechanical and physical properties. The foam’s compressive strength was analyzed according to ASTM D1621 [
20] using AGS-X series universal testing machine (UTM) (Shimadzu Corp., Kyoto, Japan), its density was measured according to ASTM D1622 [
21], thermal conductivity testing according to ASTM C518 [
22] using FOX 200 heat flow meter (Laser-Comp, Wakefield, MA) with a sample size of 150 x 150 x 20 mm, its open/close cell content according to ASTM D6226 [
23] using Quantachrome Ultrapyc 1200e automatic gas pycnometer (Germany), and its Gardner color index according to ASTM D1544 [
24]. The foam’s morphology was examined using an analytical scanning electron microscope (SEM) JSM-6510LA from JEOL (Tokyo, Japan), and the molecular structure of the foam was also studied using FTIR. The foam's thermal stability and degradation profile were studied using Shimadzu DTG-60H (Shimadzu Corp., Kyoto, Japan) at a temperature range of 50-750°C, a heating rate of 10⁰C/min, and a nitrogen flow rate of 40 ml/min.
3. Results and Discussions
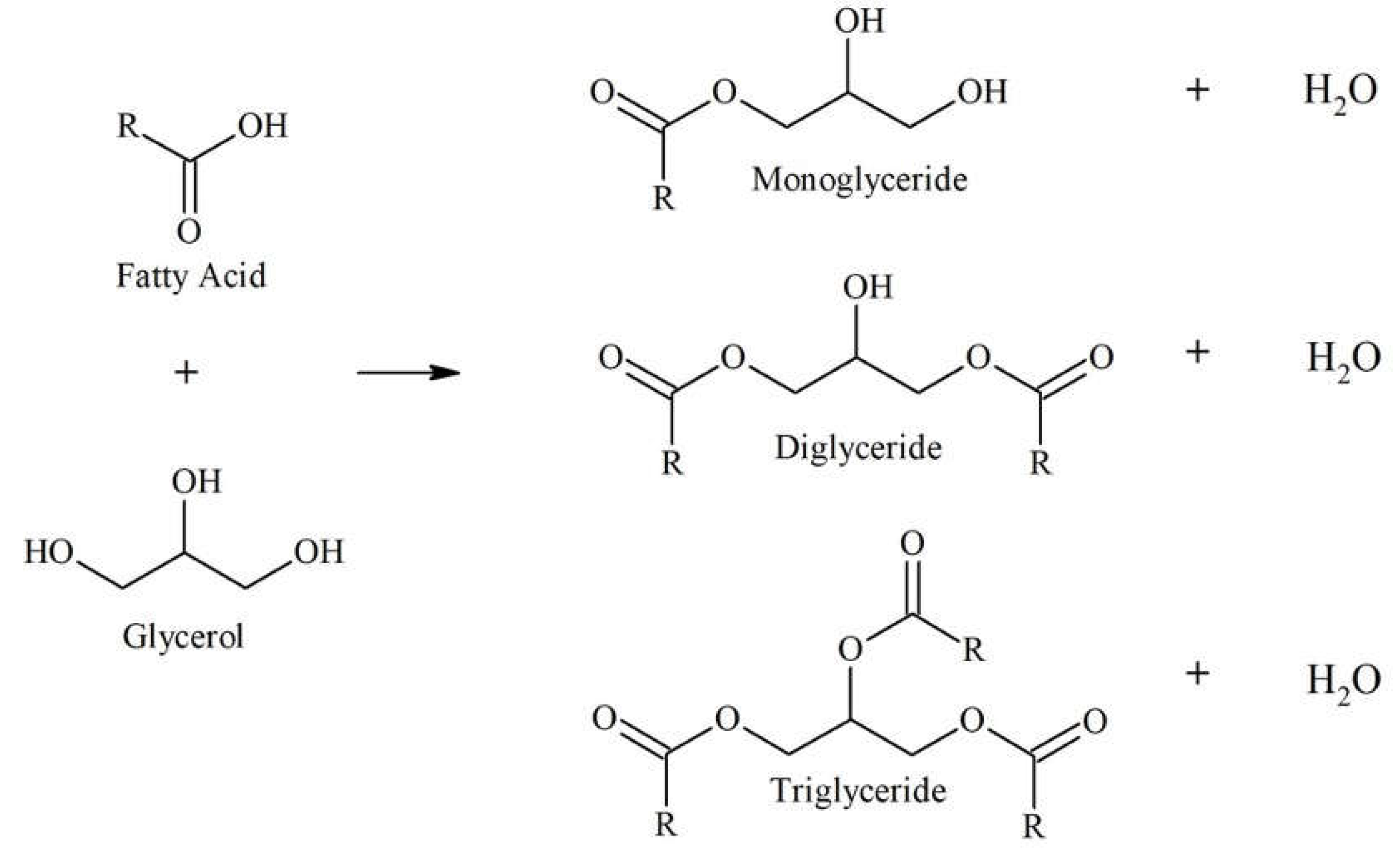
This work reports on the conversion of coconut fatty acid distillate (CFAD) and crude glycerol (CG) to a polyol for rigid PU foam application through a three-step one-pot process: glycerolysis, neutralization, and bleaching. The glycerolysis of CFAD with crude glycerol (CG) converts the free fatty acids (FFA) in CFAD into mono-, di-, and triglycerides through an esterification reaction [
25,
26].
CG acts as the alcohol providing the necessary hydroxyl groups for the reaction.
Figure 1 shows the glycerolysis reaction. Only the monoglyceride and diglyceride have the hydroxyl functionalities needed during foam synthesis. Thus, it is essential to favor the OH-containing particles in the synthesis process.
3.1. Effect of Glycerolysis Conditions on Chemical Properties of Polyol
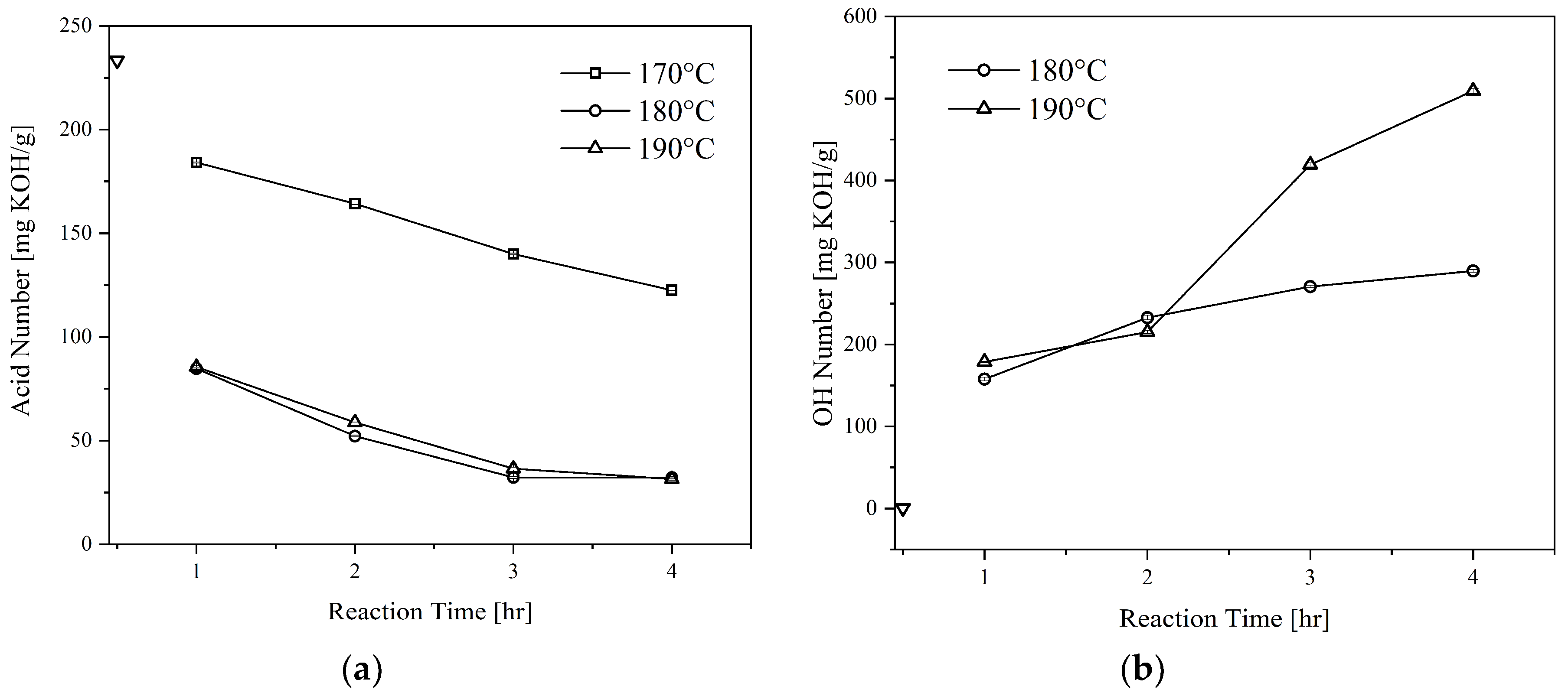
Figure 2 illustrates the effect of glycerolysis reaction time and temperature on the acid and OH numbers of the CFAD-based polyols. The results shown are for the polyols produced with CFAD:CG ratio of 1:1. In addition, the data presented are with respect to the initial acid and OH number of CFAD.
Figure 2(a) exhibits a downward trend in the acid number as the reaction proceeds showing the consumption of CFAD. It can be deduced that a higher temperature favors the reaction, in which this kind of observation has similarities to the findings of Felizardo et al. (2011) [
25]. A drastic decrease in the acid number of the polyols at temperatures 180°C and 190°C can be noted until a reaction time of 3 hrs. After which, the difference in acid number at the end of 4 hrs becomes negligible. In contrast, the reaction temperature of 170°C shows a gradual decrease in the polyol’s acid number as the reaction proceeds. At the end of the reaction, the polyol still has an acid number of >100 mg KOH/g, which means that the reaction temperature is insufficient to promote glycerolysis. In consequence, this level has not been included in further sections of the study. The observations also agree with the literature stating that the reaction between carboxyl and hydroxyl groups occurs at elevated temperatures, 180-200°C [
27].
Additionally, the effects of glycerolysis time and temperature on polyol OH number were also investigated, as shown in
Figure 2(b). Evidently, both time and temperature influenced the polyol's OH number. The initial OH number of CG at 497.50 mg KOH/g was lowered to approximately <200 mg KOH/g after the first hour of glycerolysis for both temperature levels. This may be attributed to the presence of primary hydroxyl groups in CG that are highly reactive. As the reaction proceeded, however, a slight increase in OH number can be observed. After the 2-hour mark, a sharp increase was recorded for 190°C while that of 180°C maintained a steady incline upwards. This suggests the start of fatty acid cleavage from the polyol [
28]. As such, this phenomenon is more drastic at higher temperatures. Thus, the conditions determined to be the most appropriate for this application is glycerolysis at 180°C for 2 hours.
3.2. Effect of Crude Glycerol Loading
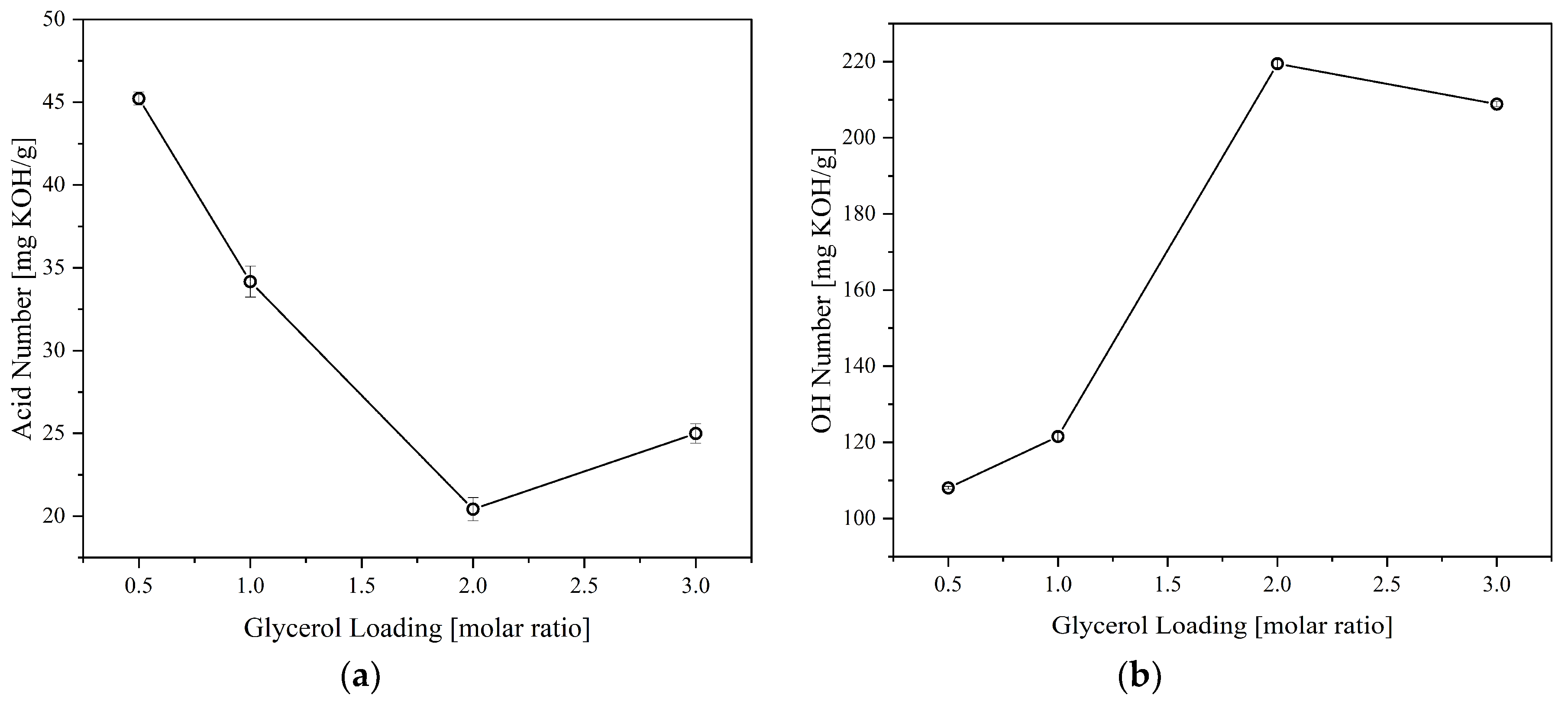
The effect of crude glycerol (CG) loading was also investigated to determine the most suitable polyol formulation.
Figure 3 shows the acid and OH numbers behavior of the polyol synthesized at 180°C for 2 hours at different CG loadings. Increasing the CG loading tends to decrease the product's acid number due to increased available OH that can react with CFAD. In addition, increasing the CG loading increases the OH number of the polyol due to an increase in excess OH present in the mixture. These trends imply that there is a successful esterification between CFAD and CG, forming a combination of the three products listed in
Figure 1. However, a slight increase in acid number was recorded at a CG loading of 3 molars. A slight decrease in OH number was also observed at this point. This phenomenon may be due to the occurrence of an oxidation reaction of the excess OH groups in CG, wherein OH groups are oxidized to form carboxyl groups leading to an increase in acid and a decrease in OH numbers [
29,
30,
31].
3.3. Effect of Alkali-Alcohol Loading
The primary goal of neutralization, the first step of the in situ treatment, is the removal of excess free fatty acids (FFA) after the glycerolysis reaction. Excess FFA contributes to the residual acidity of the polyol, which is detrimental to the polyol’s reactivity during the PU foaming process [
32]. In addition, the presence of these FFA in the polyol poses negative effects on its storage stability resulting in poor color and odor. To compensate, a neutralization reaction is essential to improve the polyol’s quality and application. The neutralization step uses an alkali-alcohol solution composed of KOH and ethanol wherein FFAs react with the base and forms the soap stock, as shown in Eq. 1 [
15]. This reaction lowers the acid number of the polyol through the consumption of the FFAs. The produced soap is generally insoluble in the oil-based polyol and can be easily removed via dissolution in a solvent and subsequent mechanical separation based on the difference in specific gravity [
33]. The alcohol component in the alkali-alcohol solution serves as the solvent that dissolves the soap stock and facilitates the separation of the polyol.
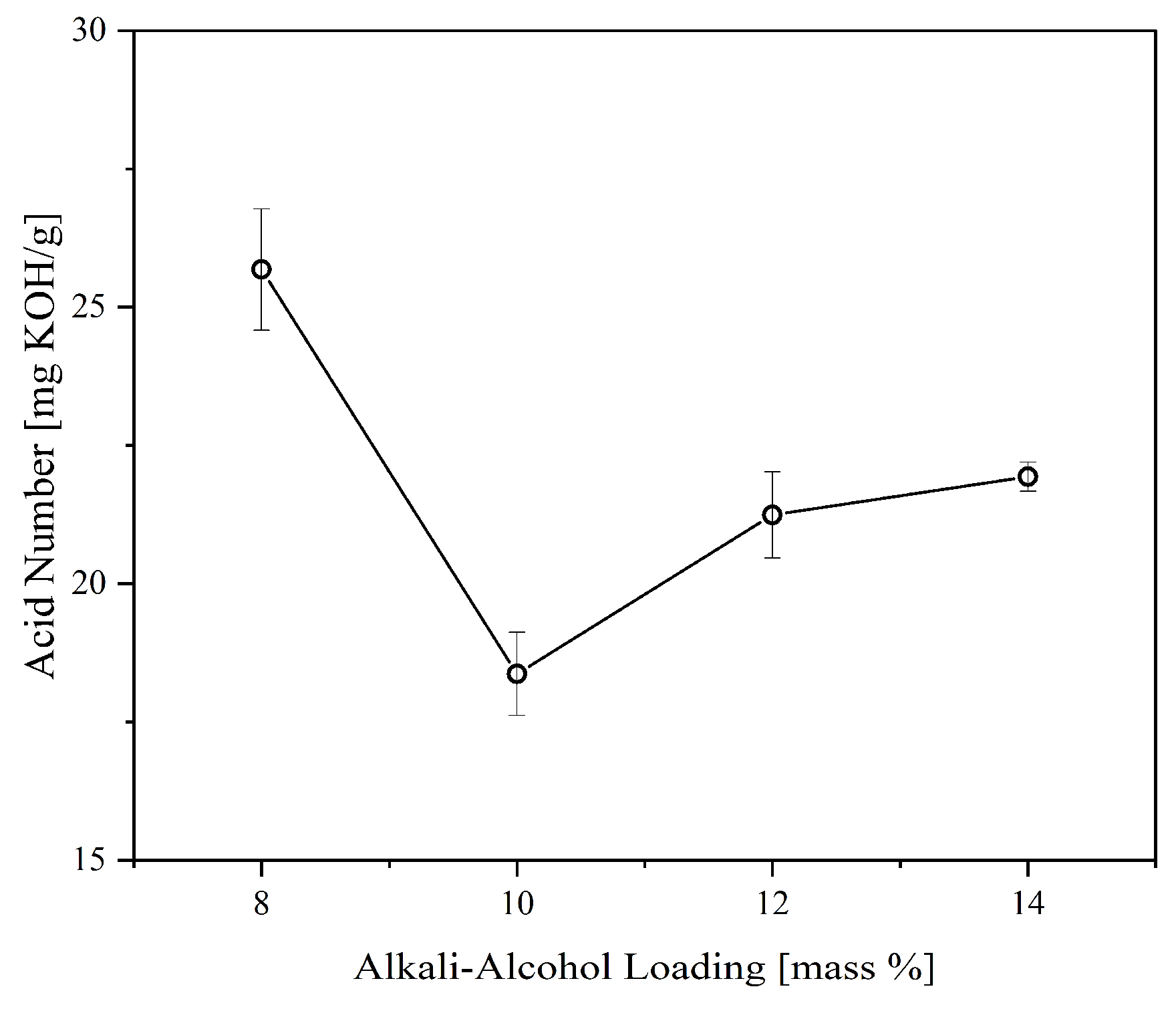
Figure 4 illustrates the effect of alkali-alcohol loading on the acid number of the CFAD-based polyol. It can be observed that there is a significant reduction in the polyol’s acid number, as predicted, due to the consumption of excess FFA. The highest acid reduction was recorded with an alkali-alcohol loading of 10%. Beyond this point, the acid number showed a significant increase. This may be due to the production of emulsified polyol or salt formation induced by the emergence of potassium carboxylate salts destabilized by ethanol, as previously determined by a related study by Xu et al. (2001) [
34]. Thus, 10% alkali-alcohol loading is employed in the subsequent areas investigated in this study.
3.4. Effect of Bleaching Conditions
The bleaching process is employed mainly to improve the color of the CFAD-based polyol. Hydrogen peroxide (6%) was used as the bleaching agent in accordance with the process performed by Frey et al. (2012) [
35]. The color change is characterized by the Gardner color index of the samples.
Table 4 lists the color indices of the products and the reactants. Evidently, the bleaching process improved the CFAD-based polyol's appearance compared to the crude polyol's color index, with the latter having an index of 35 and the former having an index of 18. With respect to its raw material, specifically CFAD, there is also a significant improvement in its color.
Aside from color improvement, bleaching can also remove other minor components in the polyol, such as FFA and non-fatty materials as well as the components that give an odor to the polyol [
36]. In consequence, the effects of bleaching temperature and time on the properties of the CFAD-based polyols were investigated using crude polyol. This is done consecutively after the neutralization step. There is a need to study these factors as bleaching using peroxide is highly subjective and requires customization for different polyols to achieve optimal decolorization and minimal degradation effects [
35].
Figure 5 shows the impact of bleaching temperature on the acid and OH numbers of the polyols, while
Figure 6 depicts the influence of bleaching time on the said polyol properties. Both the acid and OH values of the polyols were affected by the two factors in varying degrees. The acid value is significantly lowered with increasing bleaching temperature and time, as shown in
Figure 5 (a) and 6(a), respectively. In both setups, there is a decreasing trend in the acid number of the polyol as hydrogen peroxide is consumed in the bleaching reaction. However, an increase in acid number is observed at temperatures above 100°C and reaction time beyond 1 hr.
On the other hand, the OH number drastically decreased after the temperature of 100C and time of 1 hr, as shown in
Figure 5(b) and 6(b). These findings are similar to the findings of Kamairudin et al. (2021) [
37]. It can be deduced from the behavior of the polyol’s acid and OH numbers that oxidation occurred in the reaction due to the addition of peroxide as the bleaching agent.
3.5. Mechanical and Physical Analyses of Rigid PU Foams
Being a cellular material, the most common application of rigid PU foams is against compressive loadings [
38,
39]. When coupled with low thermal conductivity for insulation functionality, the use and potential of PU foams in construction become interminable [
40]. Thus, the two main areas of investigation focused on here are the mechanical property of the foam, specifically, compressive strength, and the physical properties, which include density, thermal conductivity, and cell type.
The mechanical and physical properties of the CFAD-based foam are listed in
Table 5, along with standard values of the acceptable properties of commercial materials with similar applications. The compressive strength is recorded to be 70.59 kPa, well within the range of rigid foam materials between 68-413 kPa [
41]. An important thing to note is that for a material with bio-replacement, this result is competitive. In addition, comparing to a similar study with the same polyol replacement only yielded rigid foams with compressive strength of 17-32 kPa [
42]. Another crucial property of rigid foams is their density. The CFAD-based foam recorded a density of 32.79 kg/m
3. This value agrees with the established direct correlation between density and compressive strength [
42,
43,
44,
45]. Compared with standards, the obtained result sits within the acceptable foam density of 30-45 kg/m
3 [
46].
As for the main evaluating criteria for a material's capability to be a thermal insulator, the thermal conductivity of the foam is the most straightforward indicator. Typically, the thermal conductivity values of thermal insulators at ambient conditions are between 20-60 mW⋅m
-1K
-1 [
47]. The density that was recorded for the rigid foam block in this study is 46.50 mW⋅m
-1K
-1. This is well within the given standard range and can be considered at par with other studies that yielded vegetable oil-based foam with thermal conductivities of 39-52 mW⋅m
-1K
-1 [
42,
48].
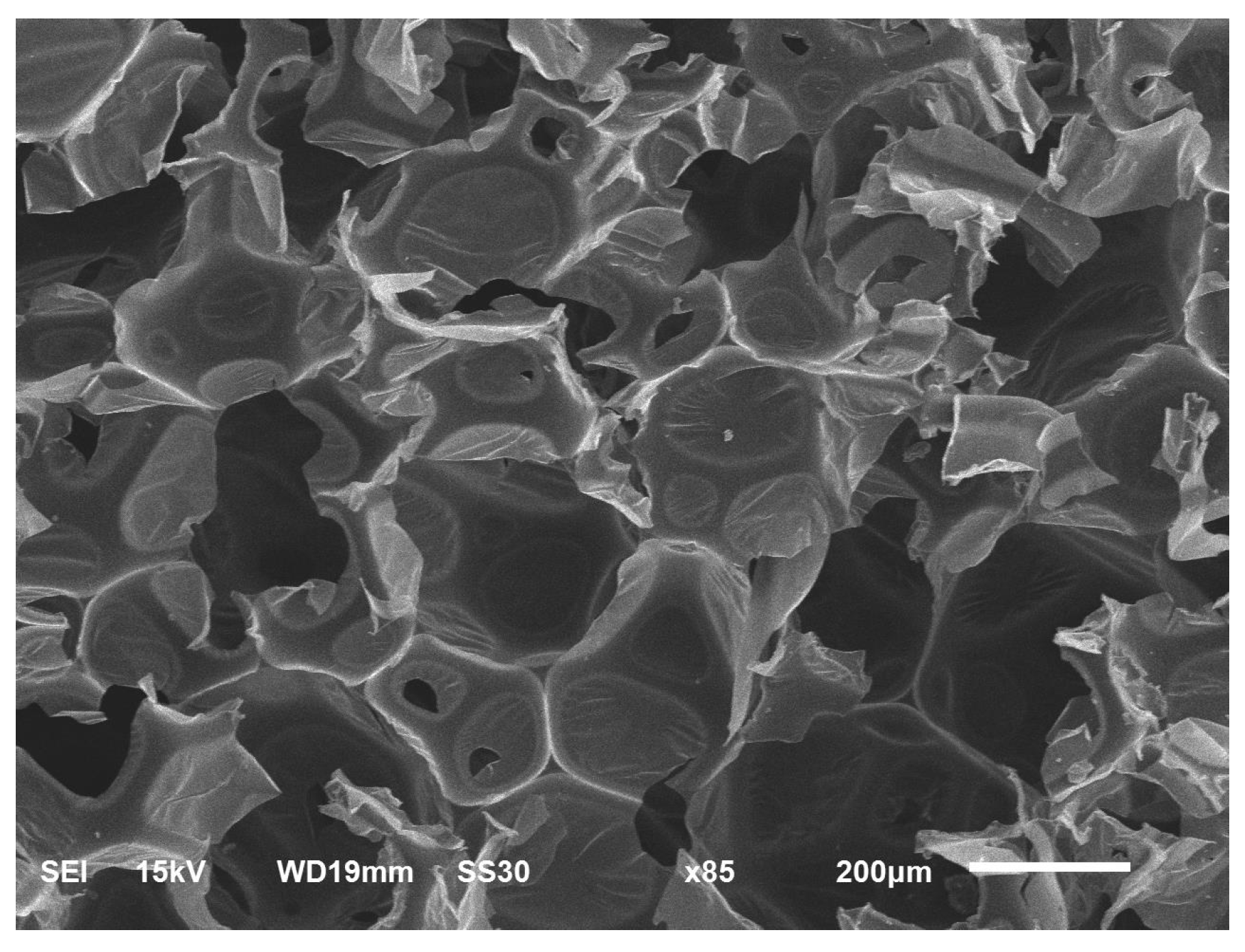
3.6. Foam Morphology and Cell Type
The morphology of the CFAD-based foam is shown in
Figure 7. An interesting observation of the scanned image is the type of cell dominating the CFAD-based foam structure as examined using the SEM. Evaluation of the foam morphology reveals the PU foam matrix's open- and closed-cell structures, with most of the structure revealing an open-celled network. This observation was confirmed by the 92.71% open-cell content of the foam tested using a gas pycnometer, as listed in
Table 5. Cell regularity is moderately observable from the morphology presented, with seemingly hexagonal cellular formation accompanied by occasional cellular breaks along the cell boundaries. Microscale level evaluation of the matrix revealed a consistent thin-film-like appearance affirming not only crosslinking of the -OH moieties with the -NCO of the isocyanate but also with the dangling chains caused by the fatty-acid tails of CFAD-based polyol. This combined cross-linking may result in an increased compressive strength of the resulting foam material, although with a lower density and high open-cell content. Given this level of open-cell content, the foam’s compressive strength can be considered impressively high since the higher the open-cell content, the lower the material’s compressive strength [
42]. Generally, open-celled foams have a compressive strength of approximately 10 kPa. Moreover, this feature constitutes the most to the insulating property of the foam as it has fewer PU linkages that increase thermal conductivity.
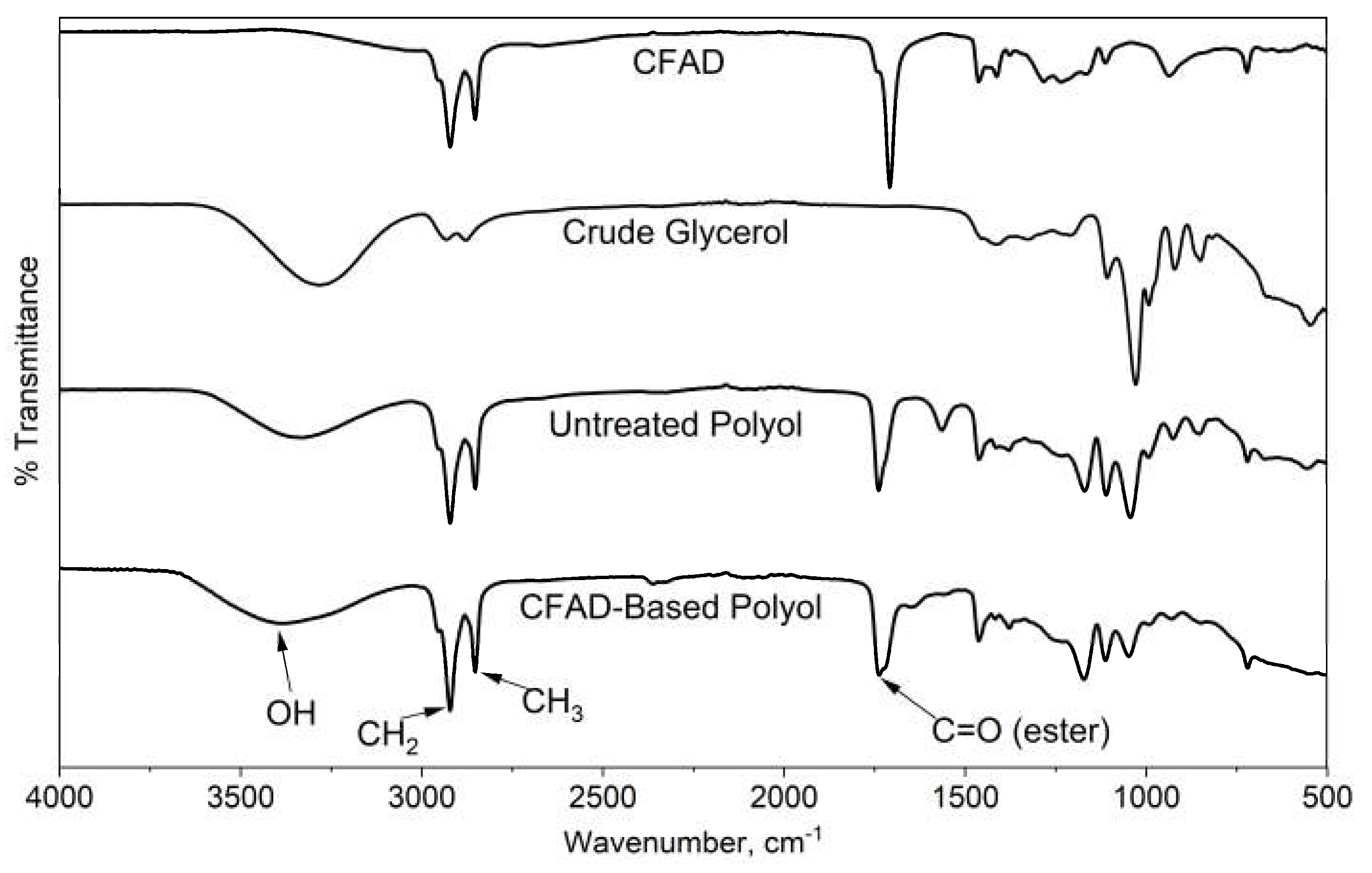
3.7. FTIR Analysis of CFAD-based Polyol and Rigid PU Foams
Figure 8 exhibits the FTIR spectra of the raw materials, CFAD and crude glycerol, and the untreated and treated polyol products. One of the main features of the raw materials is the absence of an O-H peak of CFAD between 3700 cm
-1 to 3200 cm
-1 in contrast with the broad peak observed in crude glycerol. On the polyol products, the O-H peak has decreased. This suggests the occurrence of an esterification reaction between the OH groups of crude glycerol and the carboxylic acid groups in CFAD. Between the untreated and treated CFAD-based polyols, the O-H peak is significantly pronounced in the latter. These peaks are much more discernible on the polyol products than on the reactants. This suggests the occurrence of the esterification reaction of crude glycerol with CFAD. In addition to this, the sharp peak at 1750 cm
-1 is an indication of C=O ester stretching, which further supports the proposed esterification reaction between CFAD and crude glycerol.
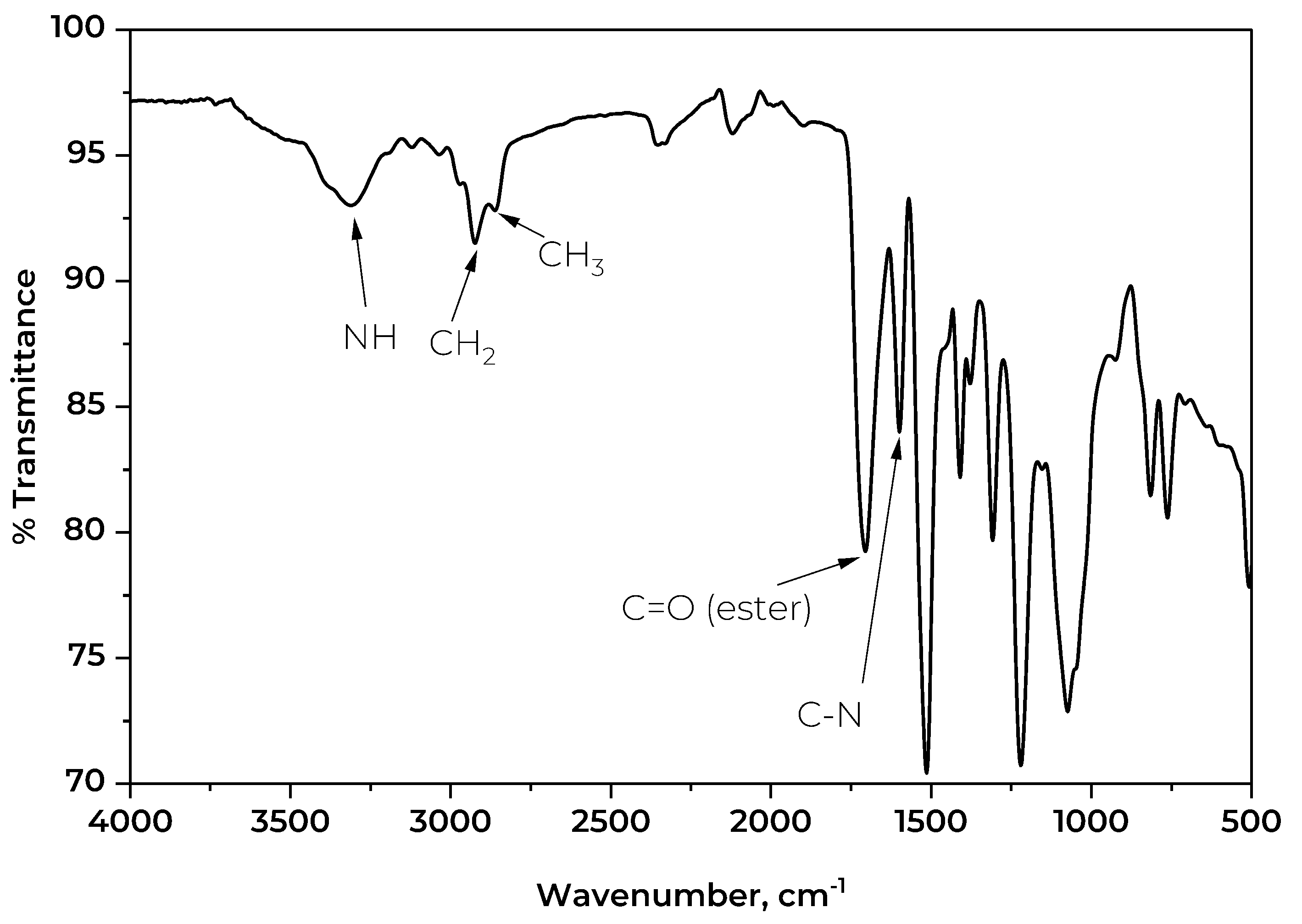
The IR spectrum of the foam was also investigated.
Figure 9 depicts the key transmission bands on the spectrum of the rigid PU foam synthesized using the CFAD-based polyol. The detected sharp peak at 3312 cm
-1 corresponds to the N-H stretching of the urethane bond. The low intensity of this band is characteristic of rigid foams as it signifies little to no presence of excess OH groups [
49]. This suggests a high degree of crosslinking through the reaction of OH groups, thus increasing the foam's rigidity. Correspondingly, the weak bands observed at 2925 cm
-1 and 2860 cm
-1 represent the C-H stretching of groups. These groups represent the incorporated fatty acid chains in the glycerol backbone of the polyol. Lastly, the conspicuous peak at 1514 cm
-1 shows the C-N stretching band of the urethane linkage.
3.7. Thermal Analysis of Rigid PU Foams
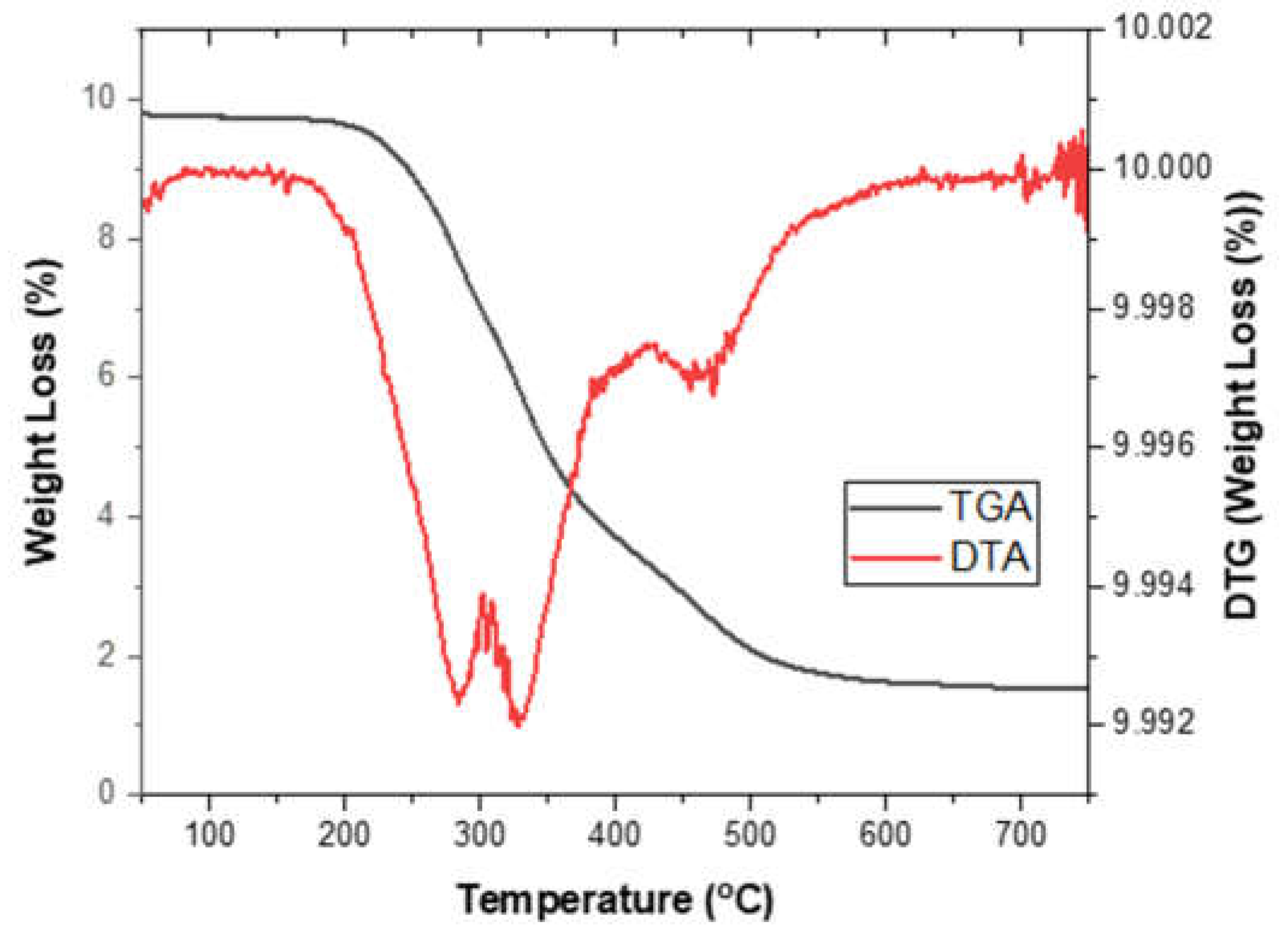
TGA/DTA was used to determine the thermal stability of the synthesized PU foam made from CFAD. The thermal decomposition of PU foam samples was done in an N2 environment at a heating rate of 10°C/min. The TGA/DTA curve of the synthesized PU foam can be seen in
Figure 10. The first stage of degradation (T
m1) is related to the degradation of PU soft segments in the case of PU foam and the decoupling and pyrolysis of fatty acid dangling chains [
50]. The second stage of decomposition (T
m2) is attributed to the degradation of the urea and carbodiimide group and the isocyanurate rings [
51]. Lastly, the third decomposition stage (T
m3) is associated with the fracture of the polyester and the crosslinked [
52]. The corresponding weight loss in % per degradation is shown in
Table 6.
4. Conclusions
This study directly used coconut fatty acid distillate (CFAD) and crude glycerol (CG) to synthesize polyol via glycerolysis for rigid PU foam. The appropriate glycerolysis conditions were determined to be with CFAD:CG ratio of 1:2, reaction temperature of 180°C, and reaction time of 2hrs. Additional steps were also employed to improve the properties of the CFAD-based polyols, alkali-alcohol neutralization, and hydrogen peroxide bleaching. Investigation of these processes showed that an alkali-alcohol loading of 10% and bleaching conditions of 100°C and 1 hr yielded a suitable CFAD-based polyol for rigid foam application. Consequently, the CFAD-based polyols were used to produce rigid PU foam having a compressive strength of 70.59 kPa, thermal conductivity of 46.50 mW⋅m-1K-1, density of 32.79 kg⋅m-3, and an open-cell content of 92.71%. The morphology of the foam supported the nature of the cell type. These mechanical and physical properties showed that the CFAD-based rigid PU foam could be used as a thermal insulating material in industrial settings. Thus, this study has demonstrated the direct utility of CFAD and CG in the PU industry.
5. Patents
A patent entitled “Coconut fatty acid distillate-based polyols,” resulting from this work with application number 1/2022/050672 has been filed and submitted to the Intellectual Property Office of the Philippines (IPOPHL).
Author Contributions
Conceptualization, M.L.D.S.; methodology, A.O.M., and A.C.T.; validation, B.J.M.A., D.M.A.A., and D.J.D.E.; investigation, D.J.E.E.; resources, G.A. and H.S.; data curation, C.J.M.O.; writing—original draft preparation, C.J.M.O., and M.L.D.S.; writing—review and editing, G.G.D. and A.A.L.; supervision, R.M.M., and A.C.A.; project administration, A.A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request.
Acknowledgments
Dr. Gerard Dumancas wishes to thank the Balik Scientist Program of the Department of Science and Technology—Philippine Council for Health, Research, and Development for the opportunity to serve the Filipino community through science, technology, and innovation. The Balik (Filipino word for “Return” or “Repatriate”) Scientist Program (BSP) seeks highly trained Filipino scientists, technologists, experts, and professionals residing abroad to return to the Philippines and transfer their expertise to the local community for the acceleration of scientific, agro-industrial, and economic development of the country.
Conflicts of Interest
The authors declare no conflict of interest.
References
- N. F. Enderus and S. M. Tahir, “Green waste cooking oil-based rigid polyurethane foam,” IOP Conf Ser Mater Sci Eng, vol. 271, p. 012062, Nov. 2017. [CrossRef]
- M. L. Pinto, “Formulation, Preparation, and Characterization of Polyurethane Foams,” J Chem Educ, vol. 87, no. 2, pp. 212–215, Jan. 2010. [CrossRef]
- C. Zhang, S. A. Madbouly, and M. R. Kessler, “Biobased Polyurethanes Prepared from Different Vegetable Oils,” ACS Appl Mater Interfaces, vol. 7, no. 2, pp. 1226–1233, Jan. 2015. [CrossRef]
- H. Sardon, D. Mecerreyes, A. Basterretxea, L. Avérous, and C. Jehanno, “From Lab to Market: Current Strategies for the Production of Biobased Polyols,” ACS Sustain Chem Eng, vol. 9, no. 32, pp. 10664–10677, Aug. 2021. [CrossRef]
- X. Leng et al., “A study on coconut fatty acid diethanolamide-based polyurethane foams,” RSC Adv, vol. 12, no. 21, pp. 13548–13556, 2022. [CrossRef]
- D. Abril-Milán, O. Valdés, Y. Mirabal-Gallardo, A. F. de la Torre, C. Bustamante, and J. Contreras, “Preparation of Renewable Bio-Polyols from Two Species of Colliguaja for Rigid Polyurethane Foams,” Materials, vol. 11, no. 11, p. 2244, Nov. 2018. [CrossRef]
- S. Saalah et al., “Physicochemical Properties of Jatropha Oil-Based Polyol Produced by a Two Steps Method,” Molecules, vol. 22, no. 4, p. 551, Mar. 2017. [CrossRef]
- L. Maisonneuve, G. Chollet, E. Grau, and H. Cramail, “Vegetable oils: a source of polyols for polyurethane materials,” OCL, vol. 23, no. 5, p. D508, Sep. 2016. [CrossRef]
- H. Liang et al., “Bio-based cationic waterborne polyurethanes dispersions prepared from different vegetable oils,” Ind Crops Prod, vol. 122, pp. 448–455, Oct. 2018. [CrossRef]
- L. Zhang, M. Zhang, L. Hu, and Y. Zhou, “Synthesis of rigid polyurethane foams with castor oil-based flame retardant polyols,” Ind Crops Prod, vol. 52, pp. 380–388, Jan. 2014. [CrossRef]
- S. Das, M. Dave, and G. L. Wilkes, “Characterization of flexible polyurethane foams based on soybean-based polyols,” J Appl Polym Sci, vol. 112, no. 1, pp. 299–308, Apr. 2009. [CrossRef]
- J. Jia, Z. Huang, and Y. Wang, “Thermal and Mechanical Properties of a Biobased Unsaturated Polyester Resin,” Asian Journal of Chemistry, vol. 25, no. 8, pp. 5001–5005, 2013. [CrossRef]
- S. Soloi, R. Abdul Majid, and A. R. Rahmat, “Novel Palm Oil Based Polyols with Amine Functionality Synthesis via Ring Opening Reaction of Epoxidized Palm Oil,” J Teknol, vol. 80, no. 6, Aug. 2018. [CrossRef]
- P. Rojek and A. Prociak, “Effect of different rapeseed-oil-based polyols on mechanical properties of flexible polyurethane foams,” J Appl Polym Sci, vol. 125, no. 4, pp. 2936–2945, Aug. 2012. [CrossRef]
- S. Gharby, “Refining Vegetable Oils: Chemical and Physical Refining,” The Scientific World Journal, vol. 2022, pp. 1–10, Jan. 2022. [CrossRef]
- A. Siyal, A. Low, and R. Shamsuddin, “Fatty acid distillate as an alternative boiler fuel,” Energy Reports, vol. 7, pp. 8688–8698, Nov. 2021. [CrossRef]
- S. Chozhavendhan, G. Karthiga Devi, B. Bharathiraja, R. Praveen Kumar, and S. Elavazhagan, “Assessment of crude glycerol utilization for sustainable development of biorefineries,” in Refining Biomass Residues for Sustainable Energy and Bioproducts, Elsevier, 2020, pp. 195–212. [CrossRef]
- ASTM Standard D1980, “Standard Test Method for Acid Value of Fatty Acids and Polymerized Fatty Acids.” ASTM International, West Conshohocken, PA, 1998.
- ASTM Standard D4274, “Standard Test Methods for Testing Polyurethane Raw Materials: Determination of Hydroxyl Numbers of Polyols.” ASTM International, West Conshohocken, PA, 2021. [CrossRef]
- ASTM Standard D1621, “Standard Test Method for Compressive Properties of Rigid Cellular Plastics.” ASTM International, West Conshohocken, PA, 2016. [CrossRef]
- ASTM Standard D1622, “Standard Test Method for Apparent Density of Rigid Cellular Plastics.” ASTM International, West Conshohocken, PA, 2020. [CrossRef]
- ASTM Standard C518, “Standard Test Method for Steady-State Thermal Transmission Properties by Means of the Heat Flow Meter Apparatus.” ASTM International, West Conshohocken, PA, 2021. [CrossRef]
- ASTM Standard D6226, “Standard Test Method for Open Cell Content of Rigid Cellular Plastics.” ASTM International, West Conshohocken, PA, 2015. [CrossRef]
- ASTM Standard D1544, “Standard Test Method for Color of Transparent Liquids (Gardner Color Scale).” ASTM International, West Conshohocken, PA, 2018. [CrossRef]
- P. Felizardo, J. Machado, D. Vergueiro, M. J. N. Correia, J. P. Gomes, and J. M. Bordado, “Study on the glycerolysis reaction of high free fatty acid oils for use as biodiesel feedstock,” Fuel Processing Technology, vol. 92, no. 6, pp. 1225–1229, Jun. 2011. [CrossRef]
- K. Mamtani, K. Shahbaz, and M. M. Farid, “Glycerolysis of free fatty acids: A review,” Renewable and Sustainable Energy Reviews, vol. 137, p. 110501, Mar. 2021. [CrossRef]
- T. S. Velayutham, W. H. A. Majid, A. B. Ahmad, G. Y. Kang, and S. N. Gan, “Synthesis and characterization of polyurethane coatings derived from polyols synthesized with glycerol, phthalic anhydride and oleic acid,” Prog Org Coat, vol. 66, no. 4, pp. 367–371, Dec. 2009. [CrossRef]
- T. Bánsági and A. F. Taylor, “Ester hydrolysis: Conditions for acid autocatalysis and a kinetic switch,” Tetrahedron, vol. 73, no. 33, pp. 5018–5022, Aug. 2017. [CrossRef]
- T. Yamada and H. Ono, “Characterization of the products resulting from ethylene glycol liquefaction of cellulose,” Journal of Wood Science, vol. 47, no. 6, pp. 458–464, Dec. 2001. [CrossRef]
- T. Yamada, M. Aratani, S. Kubo, and H. Ono, “Chemical analysis of the product in acid-catalyzed solvolysis of cellulose using polyethylene glycol and ethylene carbonate,” Journal of Wood Science, vol. 53, no. 6, pp. 487–493, Dec. 2007. [CrossRef]
- S.-H. Lee, M. Yoshioka, and N. Shiraishi, “Liquefaction of corn bran (CB) in the presence of alcohols and preparation of polyurethane foam from its liquefied polyol,” J Appl Polym Sci, vol. 78, no. 2, pp. 319–325, Jul. 2000. [CrossRef]
- M. Ionescu, Chemistry and Technology of Polyols for Polyurethanes, 2nd ed., vol. 1. Smithers Rapra Technology, Ltd., 2016.
- V. R. Patel, G. G. Dumancas, L. C. K. Viswanath, R. Maples, and B. J. J. Subong, “Castor Oil: Properties, Uses, and Optimization of Processing Parameters in Commercial Production,” Lipid Insights, vol. 9, p. LPI.S40233, Jan. 2016. [CrossRef]
- Q. Xu, M. Nakajima, H. Nabetani, S. Iwamoto, and X. Liu, “The effects of ethanol content and emulsifying agent concentration on the stability of vegetable oil-ethanol emulsions,” J Am Oil Chem Soc, vol. 78, no. 12, pp. 1185–1190, Dec. 2001. [CrossRef]
- G. D. Frey, T. Kreickmann, T. Weber, and H. Strutz, “Process for lightening the color of polyol esters,” 8 158 816 B12, Apr. 17, 2012.
- U. D. Palanisamy, M. Sivanathan, A. K. Radhakrishnan, N. Haleagrahara, T. Subramaniam, and G. S. Chiew, “An Effective Ostrich Oil Bleaching Technique Using Peroxide Value as an Indicator,” Molecules, vol. 16, no. 7, pp. 5709–5719, Jul. 2011. [CrossRef]
- N. Kamairudin, S. S. Hoong, L. C. Abdullah, H. Ariffin, and D. R. A. Biak, “Optimisation of Epoxide Ring-Opening Reaction for the Synthesis of Bio-Polyol from Palm Oil Derivative Using Response Surface Methodology,” Molecules, vol. 26, no. 3, p. 648, Jan. 2021. [CrossRef]
- D. Qiu, Y. He, and Z. Yu, “Investigation on Compression Mechanical Properties of Rigid Polyurethane Foam Treated under Random Vibration Condition: An Experimental and Numerical Simulation Study,” Materials, vol. 12, no. 20, p. 3385, Oct. 2019. [CrossRef]
- Z. Fan, Y. Miao, Z. Wang, B. Zhang, and H. Ma, “Effect of the cenospheres size and internally lateral constraints on dynamic compressive behavior of fly ash cenospheres polyurethane syntactic foams,” Compos B Eng, vol. 171, pp. 329–338, Aug. 2019. [CrossRef]
- M. Kirpluks, U. Cabulis, and A. Avots, “Flammability of Bio-Based Rigid Polyurethane Foam as Sustainable Thermal Insulation Material,” in Insulation Materials in Context of Sustainability, InTech, 2016. [CrossRef]
- Go Plymouth Foam, “For the record: Plymouth Foam’s EPS - Compressive Strength,” Dec. 15, 2018.
- M. Kurańska, E. Malewska, K. Polaczek, A. Prociak, and J. Kubacka, “A Pathway toward a New Era of Open-Cell Polyurethane Foams—Influence of Bio-Polyols Derived from Used Cooking Oil on Foams Properties,” Materials, vol. 13, no. 22, p. 5161, Nov. 2020. [CrossRef]
- Hejna, M. Kirpluks, P. Kosmela, U. Cabulis, J. Haponiuk, and Ł. Piszczyk, “The influence of crude glycerol and castor oil-based polyol on the structure and performance of rigid polyurethane-polyisocyanurate foams,” Ind Crops Prod, vol. 95, pp. 113–125, Jan. 2017. [CrossRef]
- Palanisamy, M. S. L. Karuna, T. Satyavani, and D. B. Rohini Kumar, “Development and Characterization of Water-Blown Polyurethane Foams from Diethanolamides of Karanja Oil,” J Am Oil Chem Soc, vol. 88, no. 4, pp. 541–549, Apr. 2011. [CrossRef]
- Prociak et al., “Effect of bio-polyols with different chemical structures on foaming of polyurethane systems and foam properties,” Ind Crops Prod, vol. 120, pp. 262–270, Sep. 2018. [CrossRef]
- M. Kapps and S. Buschkamp, The production of rigid polyurethane foam. Business Development - Insulation, 2004.
- H.-P. Ebert, “Functional materials for energy-efficient buildings,” EPJ Web Conf, vol. 98, pp. 1–14, Aug. 2015. [CrossRef]
- X. Luo, S. Hu, X. Zhang, and Y. Li, “Thermochemical conversion of crude glycerol to biopolyols for the production of polyurethane foams,” Bioresour Technol, vol. 139, pp. 323–329, Jul. 2013. [CrossRef]
- G. Trovati, E. A. Sanches, S. C. Neto, Y. P. Mascarenhas, and G. O. Chierice, “Characterization of polyurethane resins by FTIR, TGA, and XRD,” J Appl Polym Sci, vol. 115, no. 1, pp. 263–268, Jan. 2010. [CrossRef]
- M. Kirpluks, U. Cabulis, and A. Avots, “Flammability of Bio-Based Rigid Polyurethane Foam as Sustainable Thermal Insulation Material,” Insulation Materials in Context of Sustainability, 2016. [CrossRef]
- Zarzyka and D. Majda, “Thermogravimetric and qualitative analysis of thermal decomposition characteristics of polyurethane foams based on polyols with carbamide or oxamide and borate groups,” Polym Int, vol. 66, no. 11, pp. 1675–1683, 2017. [CrossRef]
- M. Kumar and R. Kaur, “Effect of Different Formulations of MDI on Rigid Polyurethane Foams based on Castor Oil,” vol. 2, no. May, pp. 29–42, 2013.
Figure 1.
Glycerolysis of free fatty acid in coconut fatty acid distillate with glycerol producing mono-, di-, and triglycerides, adapted from Felizardo et al. (2011) [
25] and Mamtani et al. (2021) [
26].
Figure 1.
Glycerolysis of free fatty acid in coconut fatty acid distillate with glycerol producing mono-, di-, and triglycerides, adapted from Felizardo et al. (2011) [
25] and Mamtani et al. (2021) [
26].
Figure 2.
Effect of reaction time and temperature on the: (a) acid number and; (b) OH number of polyols produced from coconut fatty acid distillate (CFAD) and crude glycerol (CG) at CFAD:CG ratio of 1:1. The symbol ∇ refers to the initial properties of CFAD.
Figure 2.
Effect of reaction time and temperature on the: (a) acid number and; (b) OH number of polyols produced from coconut fatty acid distillate (CFAD) and crude glycerol (CG) at CFAD:CG ratio of 1:1. The symbol ∇ refers to the initial properties of CFAD.
Figure 3.
Effect of glycerol loading on the: (a) acid number and; (b) OH number of polyols produced from coconut fatty acid distillate (CFAD) and crude glycerol (CG) at 180°C for 2 hours.
Figure 3.
Effect of glycerol loading on the: (a) acid number and; (b) OH number of polyols produced from coconut fatty acid distillate (CFAD) and crude glycerol (CG) at 180°C for 2 hours.
Figure 4.
Effect of alkali-alcohol loading on the acid number of polyol synthesized from coconut fatty acid distillate (CFAD) and crude glycerol (CG) at CFAD:CG ration of 1:2 at 180°C for 2 hours.
Figure 4.
Effect of alkali-alcohol loading on the acid number of polyol synthesized from coconut fatty acid distillate (CFAD) and crude glycerol (CG) at CFAD:CG ration of 1:2 at 180°C for 2 hours.
Figure 5.
Effect of bleaching temperature on the: (a) acid number and; (b) OH number of polyols produced from coconut fatty acid distillate (CFAD) and crude glycerol (CG) at CFAD:CG ratio 1:2, 180°C for 2 hours, neutralized with 10% alkali-alcohol loading, and bleached for 1 hour.
Figure 5.
Effect of bleaching temperature on the: (a) acid number and; (b) OH number of polyols produced from coconut fatty acid distillate (CFAD) and crude glycerol (CG) at CFAD:CG ratio 1:2, 180°C for 2 hours, neutralized with 10% alkali-alcohol loading, and bleached for 1 hour.
Figure 6.
Effect of bleaching time on the: (a) acid number and; (b) OH number of polyols produced from coconut fatty acid distillate (CFAD) and crude glycerol (CG) at CFAD:CG ratio 1:2, 180°C for 2 hours, neutralized with 10% alkali-alcohol loading, and bleached at 100°C.
Figure 6.
Effect of bleaching time on the: (a) acid number and; (b) OH number of polyols produced from coconut fatty acid distillate (CFAD) and crude glycerol (CG) at CFAD:CG ratio 1:2, 180°C for 2 hours, neutralized with 10% alkali-alcohol loading, and bleached at 100°C.
Figure 7.
Morphology of the rigid polyurethane foam from coconut fatty acid-based polyol.
Figure 7.
Morphology of the rigid polyurethane foam from coconut fatty acid-based polyol.
Figure 8.
Fourier transform infrared (FTIR) spectra of coconut fatty acid distillate (CFAD), crude glycerol (CG), crude polyol, and CFAD-based polyol.
Figure 8.
Fourier transform infrared (FTIR) spectra of coconut fatty acid distillate (CFAD), crude glycerol (CG), crude polyol, and CFAD-based polyol.
Figure 9.
Fourier transform infrared (FTIR) spectra of rigid polyurethane foam synthesized using coconut fatty acid distillate (CFAD)-based polyol.
Figure 9.
Fourier transform infrared (FTIR) spectra of rigid polyurethane foam synthesized using coconut fatty acid distillate (CFAD)-based polyol.
Figure 10.
Thermogravimetric analysis (TGA) and differential thermal analysis (DTA) graphs of rigid polyurethane foam from coconut fatty acid distillate (CFAD)-based polyol.
Figure 10.
Thermogravimetric analysis (TGA) and differential thermal analysis (DTA) graphs of rigid polyurethane foam from coconut fatty acid distillate (CFAD)-based polyol.
Table 1.
Coconut fatty acid distillate (CFAD) properties.
Table 1.
Coconut fatty acid distillate (CFAD) properties.
| Parameters |
Test Method |
CFAD Properties |
| Acid Number, mg KOH/g |
ASTM D1980 |
233.33 |
| OH Number, mg KOH/g |
ASTM D4274 |
nil. |
| Iodine Value, g I 2/100g |
AOCS Cd 1-5 |
14.6 |
| Saponification Value, mg KOH/g |
AOCS Cd 3-25 |
257.56 |
| Appearance |
Ocular Inspection |
Clear, dark yellow to brown |
Table 2.
Crude glycerol (CG) properties.
Table 2.
Crude glycerol (CG) properties.
| Components |
CAS Number |
CG Properties |
| OH Number, mg KOH/g |
56-81-5 |
497.50 |
| Water |
7732-18-5 |
12% max. |
| Methanol |
67-56-1 |
0.50% max. |
| Fatty Acid & Ester |
67762-38-3 |
0.10% max. |
| Ash |
- |
8% max. |
Table 3.
Rigid polyurethane foam formulation.
Table 3.
Rigid polyurethane foam formulation.
| Foam Components |
Parts by Weight |
| B-side Materials |
| VORANOL® 490 |
80 |
| CFAD-based Polyol |
20 |
| DABCO® 33-LV |
0.25-0.75 |
| POLYCAT® 8 |
1.0-1.5 |
| DABCO® DC 2585 |
0.5-1.0 |
| Distilled Water |
0 |
| A-side Materials |
| Polymeric MDI |
Index 110 |
Table 4.
Gardner color index of coconut fatty acid distillate (CFAD)-based polyols in comparison with raw materials and untreated crude polyol.
Table 4.
Gardner color index of coconut fatty acid distillate (CFAD)-based polyols in comparison with raw materials and untreated crude polyol.
| Sample |
Gardner Color Index |
| CFAD-based polyol |
18 |
| Crude polyol |
35 |
| CFAD |
27 |
| Crude glycerol |
6 |
Table 5.
Mechanical and physical properties of rigid polyurethane foam from coconut fatty acid distillate (CFAD)-based polyols.
Table 5.
Mechanical and physical properties of rigid polyurethane foam from coconut fatty acid distillate (CFAD)-based polyols.
| Properties |
CFAD-based PU foam |
Standard Values |
| Mechanical |
Compressive Strength, kPa |
70.59 |
68-413 [42] |
| Physical |
Density, kg⋅m-3
|
32.79 |
30-45 [47] |
| Thermal Conductivity, mW⋅ m-1 K-1
|
46.50 |
20-60 [48] |
| Open Cell Content, % |
92.71 |
- |
Table 6.
Thermal decomposition temperatures and percent weight loss of coconut fatty acid distillate (CFAD)-based polyurethane foam.
Table 6.
Thermal decomposition temperatures and percent weight loss of coconut fatty acid distillate (CFAD)-based polyurethane foam.
| Thermal Decomposition |
Tm1 (°C) |
Tm2 (°C) |
Tm3 (°C) |
| 282.32 |
327.25 |
459.70 |
| % Weight Loss |
9.72 |
51.97 |
22.08 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).