Submitted:
24 June 2023
Posted:
25 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Metabolic features of mammary cancer
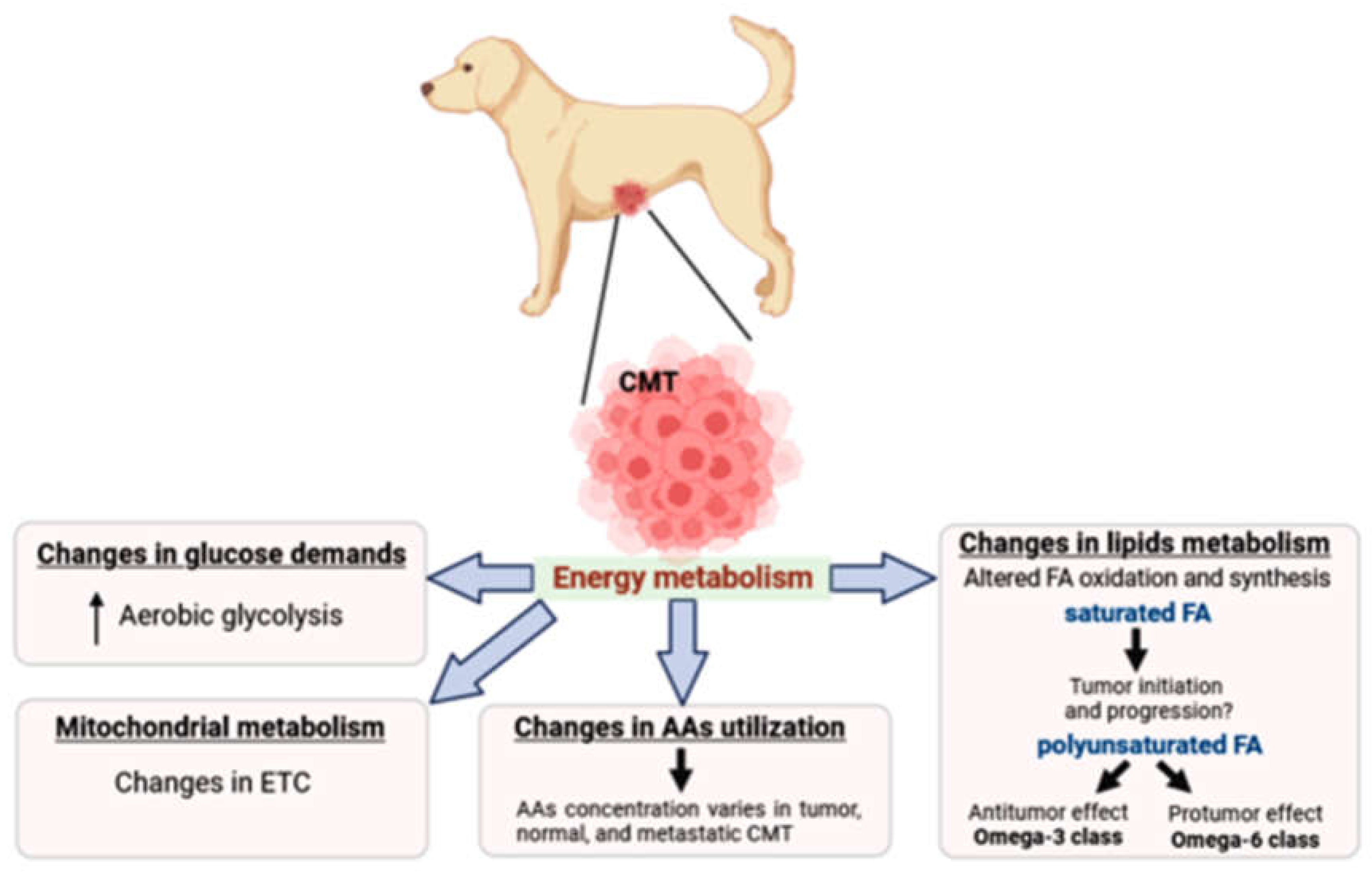
3. Metabolic reprogramming in canine mammary tumors
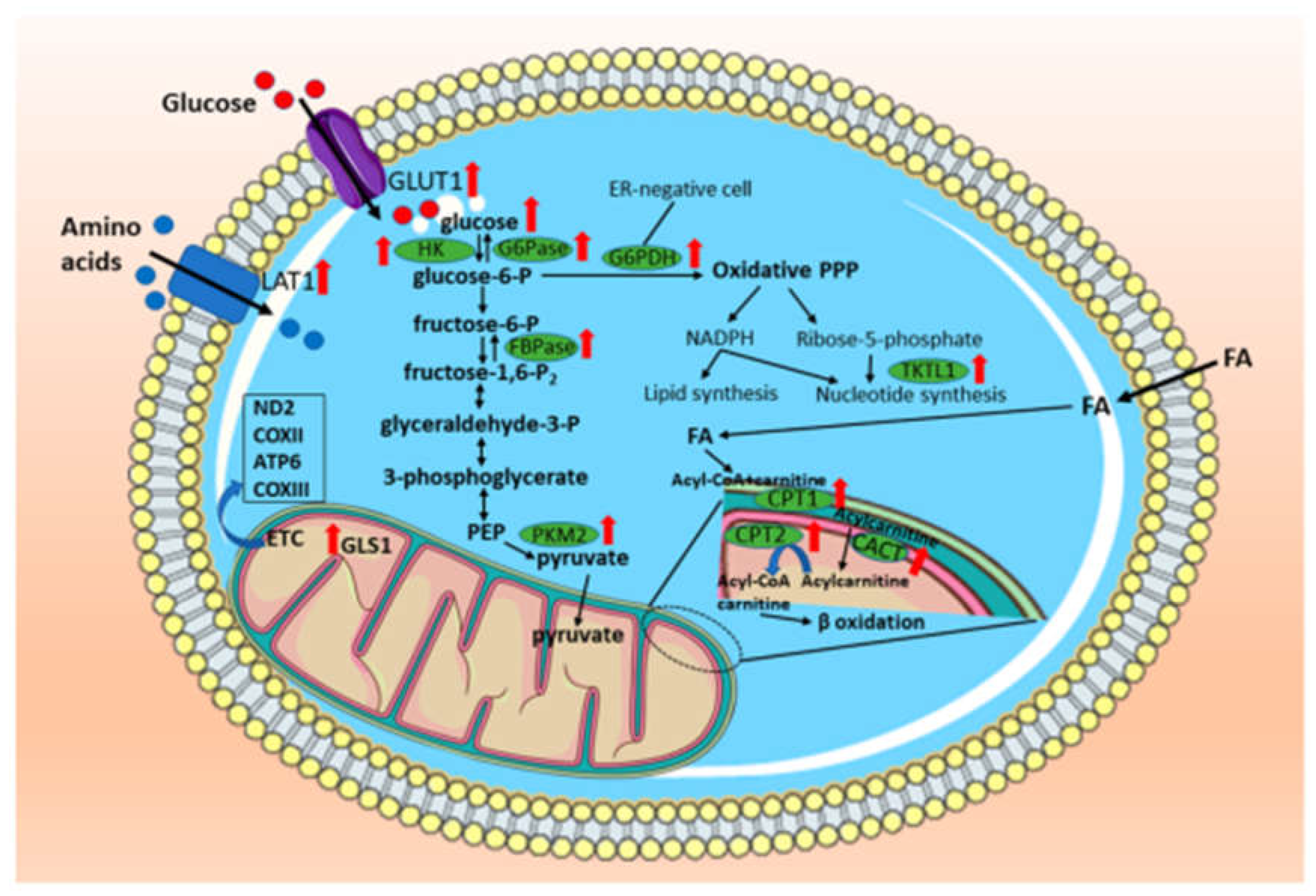
3.1. Carbohydrates
4. Lipids
5. Amino acids
6. Mitochondria
7. Concluding Remarks and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Gray, M.; Meehan, J.; Martínez-Pérez, C.; Kay, C.; Turnbull, A.K.; Morrison, L.R.; Pang, L.Y.; Argyle, D. Naturally-Occurring Canine Mammary Tumors as a Translational Model for Human Breast Cancer. Front. Oncol. 2020, 10, 617. [Google Scholar] [CrossRef] [PubMed]
- Burrai, G.P.; Gabrieli, A.; Moccia, V.; Zappulli, V.; Porcellato, I.; Brachelente, C.; Pirino, S.; Polinas, M.; Antuofermo, E. A statistical analysis of risk factors and biological behavior in canine mammary tumors: A multicenter study. Animals (Basel) 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Cassali, G.; Jark, P.; Gamba, C.; Damasceno, K.; Estrela-Lima, A.; Nardi, A.; Ferreira, E.; Horta, R.; Firmo, B.; Sueiro, F.; Rodrigues, L.; Nakagaki, K. Consensus Regarding the Diagnosis, Prognosis and Treatment of Canine and Feline Mammary Tumors - 2019. Braz. J. Vet. Pathol. 2020, 13, 555–574. [Google Scholar] [CrossRef]
- Rasotto, R.; Berlato, D.; Goldschmidt, M.H.; Zappulli, V. Prognostic significance of canine mammary tumor histologic subtypes: an observational cohort study of 229 cases. Vet. Pathol. 2017, 54, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Peña, L.; Gama, A.; Goldschmidt, M.H.; Abadie, J.; Benazzi, C.; Castagnaro, M.; Díez, L.; Gärtner, F.; Hellmén, E.; Kiupel, M.; Millán, Y.; Miller, M.A.; Nguyen, F.; Poli, A.; Sarli, G.; Zappulli, V.; de las Mulas, J.M. Canine mammary tumors: a review and consensus of standard guidelines on epithelial and myoepithelial phenotype markers, HER2, and hormone receptor assessment using immunohistochemistry. Vet. Pathol. 2014, 51, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Perez Alenza, M.D.; Peña, L.; del Castillo, N.; Nieto, A.I. Factors influencing the incidence and prognosis of canine mammary tumours. J. Small Anim. Pract. 2000, 41, 287–291. [Google Scholar] [CrossRef]
- Nicolini, A.; Carpi, A.; Rossi, G. Cytokines in breast cancer. Cytokine Growth Factor Rev. 2006, 17, 325–337. [Google Scholar] [CrossRef]
- Machado, V.S.; Crivellenti, L.Z.; Bottari, N.B.; Tonin, A.A.; Pelinson, L.P.; Borin-Crivellenti, S.; Santana, A.E.; Torbitz, V.D.; Moresco, R.N.; Duarte, T.; Duarte, M.M.M.F.; Schetinger, M.R.C.; Morsch, V.M.; Jaques, J.A.; Tinucci-Costa, M.; Da Silva, A.S. Oxidative stress and inflammatory response biomarkers in dogs with mammary carcinoma. Pathol. Res. Pract. 2015, 211, 677–681. [Google Scholar] [CrossRef]
- Benavente, M.A.; Bianchi, C.P.; Aba, M.A. Canine mammary tumors: risk factors, prognosis and treatments. J. Vet. Adv., 2016, 6, 1291–1300. [Google Scholar] [CrossRef]
- Sorenmo, K. Canine mammary gland tumors. Vet. Clin. North Am. Small Anim. Pract. 2003, 33, 573–596. [Google Scholar] [CrossRef]
- Salas, Y.; Márquez, A.; Diaz, D.; Romero, L. Epidemiological Study of Mammary Tumors in Female Dogs Diagnosed during the Period 2002-2012: A Growing Animal Health Problem. PLoS ONE 2015, 10, e0127381. [Google Scholar] [CrossRef] [PubMed]
- Kaszak, I.; Ruszczak, A.; Kanafa, S.; Kacprzak, K.; Król, M.; Jurka, P. Current biomarkers of canine mammary tumors. Acta Vet. Scand. 2018, 60, 66. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J.M.; Samuel, S.; Milstein, H.; Rogers, K.; Wood, J.L.N. Canine neoplasia in the UK: estimates of incidence rates from a population of insured dogs. J. Small Anim. Pract. 2002, 43, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Moe, L. Population-based incidence of mammary tumours in some dog breeds. J. Reprod. Fertil. Suppl. 2001, 57, 439–443. [Google Scholar] [PubMed]
- Abdelmegeed, S.M.; Mohammed, S. Canine mammary tumors as a model for human disease. Oncol. Lett. 2018, 15, 8195–8205. [Google Scholar] [CrossRef]
- Queiroga, F.L.; Raposo, T.; Carvalho, M.I.; Prada, J.; Pires, I. Canine mammary tumours as a model to study human breast cancer: most recent findings. In Vivo 2011, 25, 455–465. [Google Scholar]
- Schiliro, C.; Firestein, B.L. Mechanisms of metabolic reprogramming in cancer cells supporting enhanced growth and proliferation. Cells 2021, 10. [Google Scholar] [CrossRef]
- Sun, L.; Suo, C.; Li, S.-T.; Zhang, H.; Gao, P. Metabolic reprogramming for cancer cells and their microenvironment: Beyond the Warburg Effect. Biochim. Biophys. Acta Rev. Cancer 2018, 1870, 51–66. [Google Scholar] [CrossRef]
- Ohshima, K.; Morii, E. Metabolic Reprogramming of Cancer Cells during Tumor Progression and Metastasis. Metabolites 2021, 11. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. We need to talk about the Warburg effect. Nat. Metab. 2020, 2, 127–129. [Google Scholar] [CrossRef]
- Devic, S. Warburg Effect - a Consequence or the Cause of Carcinogenesis? J. Cancer 2016, 7, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat. Rev. Cancer 2016, 16, 619–634. [Google Scholar] [CrossRef]
- Zacharias, N.M.; McCullough, C.; Shanmugavelandy, S.; Lee, J.; Lee, Y.; Dutta, P.; McHenry, J.; Nguyen, L.; Norton, W.; Jones, L.W.; Bhattacharya, P.K. Metabolic differences in glutamine utilization lead to metabolic vulnerabilities in prostate cancer. Sci. Rep. 2017, 7, 16159. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.M.; Adamoski, D.; Dos Reis, L.M.; Ascenção, C.F.R.; de Oliveira, K.R.S.; Mafra, A.C.P.; da Silva Bastos, A.C.; Quintero, M.; de G Cassago, C.; Ferreira, I.M.; Fidelis, C.H.V.; Rocco, S.A.; Bajgelman, M.C.; Stine, Z.; Berindan-Neagoe, I.; Calin, G.A.; Ambrosio, A.L.B.; Dias, S.M.G. GLS2 is protumorigenic in breast cancers. Oncogene 2020, 39, 690–702. [Google Scholar] [CrossRef]
- Deep, G.; Schlaepfer, I.R. Aberrant lipid metabolism promotes prostate cancer: role in cell survival under hypoxia and extracellular vesicles biogenesis. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Mullen, A.R.; Wheaton, W.W.; Jin, E.S.; Chen, P.-H.; Sullivan, L.B.; Cheng, T.; Yang, Y.; Linehan, W.M.; Chandel, N.S.; DeBerardinis, R.J. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 2011, 481, 385–388. [Google Scholar] [CrossRef]
- Scaglia, N.; Tyekucheva, S.; Zadra, G.; Photopoulos, C.; Loda, M. De novo fatty acid synthesis at the mitotic exit is required to complete cellular division. Cell Cycle 2014, 13, 859–868. [Google Scholar] [CrossRef]
- Ferraro, G.B.; Ali, A.; Luengo, A.; Kodack, D.P.; Deik, A.; Abbott, K.L.; Bezwada, D.; Blanc, L.; Prideaux, B.; Jin, X.; Posada, J.M.; Chen, J.; Chin, C.R.; Amoozgar, Z.; Ferreira, R.; Chen, I.X.; Naxerova, K.; Ng, C.; Westermark, A.M.; Duquette, M.; Roberge, S.; Lindeman, N.I.; Lyssiotis, C.A.; Nielsen, J.; Housman, D.E.; Duda, D.G.; Brachtel, E.; Golub, T.R.; Cantley, L.C.; Asara, J.M.; Davidson, S.M.; Fukumura, D.; Dartois, V.A.; Clish, C.B.; Jain, R.K.; Vander Heiden, M.G. FATTY ACID SYNTHESIS IS REQUIRED FOR BREAST CANCER BRAIN METASTASIS. Nat. Cancer 2021, 2, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Migita, T.; Ruiz, S.; Fornari, A.; Fiorentino, M.; Priolo, C.; Zadra, G.; Inazuka, F.; Grisanzio, C.; Palescandolo, E.; Shin, E.; Fiore, C.; Xie, W.; Kung, A.L.; Febbo, P.G.; Subramanian, A.; Mucci, L.; Ma, J.; Signoretti, S.; Stampfer, M.; Hahn, W.C.; Finn, S.; Loda, M. Fatty acid synthase: a metabolic enzyme and candidate oncogene in prostate cancer. J Natl Cancer Inst 2009, 101, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Zadra, G.; Ribeiro, C.F.; Chetta, P.; Ho, Y.; Cacciatore, S.; Gao, X.; Syamala, S.; Bango, C.; Photopoulos, C.; Huang, Y.; Tyekucheva, S.; Bastos, D.C.; Tchaicha, J.; Lawney, B.; Uo, T.; D’Anello, L.; Csibi, A.; Kalekar, R.; Larimer, B.; Ellis, L.; Butler, L.M.; Morrissey, C.; McGovern, K.; Palombella, V.J.; Kutok, J.L.; Mahmood, U.; Bosari, S.; Adams, J.; Peluso, S.; Dehm, S.M.; Plymate, S.R.; Loda, M. Inhibition of de novo lipogenesis targets androgen receptor signaling in castration-resistant prostate cancer. Proc Natl Acad Sci USA 2019, 116, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis. 2006, 9, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Amaro, G.M.; da Silva, A.D.T.; Tamarindo, G.H.; Lamas, C. de A.; Taboga, S.R.; Cagnon, V.H.A.; Góes, R.M. Differential effects of omega-3 PUFAS on tumor progression at early and advanced stages in TRAMP mice. Prostate 2022. [CrossRef]
- Tamarindo, G.H.; Góes, R.M. Docosahexaenoic acid differentially modulates the cell cycle and metabolism- related genes in tumor and pre-malignant prostate cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158766. [Google Scholar] [CrossRef]
- Shin, E.; Koo, J.S. Glucose metabolism and glucose transporters in breast cancer. Front. Cell Dev. Biol. 2021, 9, 728759. [Google Scholar] [CrossRef]
- Fong, M.Y.; Zhou, W.; Liu, L.; Alontaga, A.Y.; Chandra, M.; Ashby, J.; Chow, A.; O’Connor, S.T.F.; Li, S.; Chin, A.R.; Somlo, G.; Palomares, M.; Li, Z.; Tremblay, J.R.; Tsuyada, A.; Sun, G.; Reid, M.A.; Wu, X.; Swiderski, P.; Ren, X.; Shi, Y.; Kong, M.; Zhong, W.; Chen, Y.; Wang, S.E. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 2015, 17, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, P.; Yarani, R.; Valipour, E.; Kiani, S.; Hoseinkhani, Z.; Mansouri, K. Cell line-directed breast cancer research based on glucose metabolism status. Biomed. Pharmacother. 2022, 146, 112526. [Google Scholar] [CrossRef]
- Medina, R.A.; Owen, G.I. Glucose transporters: expression, regulation and cancer. Biol. Res. 2002, 35, 9–26. [Google Scholar] [CrossRef]
- Guan, M.; Tong, Y.; Guan, M.; Liu, X.; Wang, M.; Niu, R.; Zhang, F.; Dong, D.; Shao, J.; Zhou, Y. Lapatinib inhibits breast cancer cell proliferation by influencing PKM2 expression. Technol. Cancer Res. Treat. 2018, 17, 1533034617749418. [Google Scholar] [CrossRef]
- O’Neal, J.; Clem, A.; Reynolds, L.; Dougherty, S.; Imbert-Fernandez, Y.; Telang, S.; Chesney, J.; Clem, B.F. Inhibition of 6-phosphofructo-2-kinase (PFKFB3) suppresses glucose metabolism and the growth of HER2+ breast cancer. Breast Cancer Res. Treat. 2016, 160, 29–40. [Google Scholar] [CrossRef]
- Peng, F.; Li, Q.; Sun, J.-Y.; Luo, Y.; Chen, M.; Bao, Y. PFKFB3 is involved in breast cancer proliferation, migration, invasion and angiogenesis. Int. J. Oncol. 2018, 52, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Ren, C.; Qiao, P.; Han, X.; Wang, L.; Lv, S.; Sun, Y.; Liu, Z.; Du, Y.; Yu, Z. PIM2-mediated phosphorylation of hexokinase 2 is critical for tumor growth and paclitaxel resistance in breast cancer. Oncogene 2018, 37, 5997–6009. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.S.; Goodman, T.M.; Zasadny, K.R.; Greenson, J.K.; Wahl, R.L. Expression of hexokinase II and Glut-1 in untreated human breast cancer. Nucl. Med. Biol. 2002, 29, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Wei, L.; Liu, Y.; Ding, Y.; Liu, X.; Zhang, X.; Wang, X.; Yao, Y.; Lu, J.; Wang, Q.; Hu, R. Gen-27, a newly synthesized flavonoid, inhibits glycolysis and induces cell apoptosis via suppression of hexokinase II in human breast cancer cells. Biochem. Pharmacol. 2017, 125, 12–25. [Google Scholar] [CrossRef]
- Doyen, J.; Trastour, C.; Ettore, F.; Peyrottes, I.; Toussant, N.; Gal, J.; Ilc, K.; Roux, D.; Parks, S.K.; Ferrero, J.M.; Pouysségur, J. Expression of the hypoxia-inducible monocarboxylate transporter MCT4 is increased in triple negative breast cancer and correlates independently with clinical outcome. Biochem. Biophys. Res. Commun. 2014, 451, 54–61. [Google Scholar] [CrossRef] [PubMed]
- McCleland, M.L.; Adler, A.S.; Shang, Y.; Hunsaker, T.; Truong, T.; Peterson, D.; Torres, E.; Li, L.; Haley, B.; Stephan, J.-P.; Belvin, M.; Hatzivassiliou, G.; Blackwood, E.M.; Corson, L.; Evangelista, M.; Zha, J.; Firestein, R. An integrated genomic screen identifies LDHB as an essential gene for triple-negative breast cancer. Cancer Res. 2012, 72, 5812–5823. [Google Scholar] [CrossRef]
- Morais-Santos, F.; Granja, S.; Miranda-Gonçalves, V.; Moreira, A.H.J.; Queirós, S.; Vilaça, J.L.; Schmitt, F.C.; Longatto-Filho, A.; Paredes, J.; Baltazar, F.; Pinheiro, C. Targeting lactate transport suppresses in vivo breast tumour growth. Oncotarget 2015, 6, 19177–19189. [Google Scholar] [CrossRef]
- Nagata, C.; Wada, K.; Tsuji, M.; Hayashi, M.; Takeda, N.; Yasuda, K. Plasma amino acid profiles are associated with biomarkers of breast cancer risk in premenopausal Japanese women. Cancer Causes Control 2014, 25, 143–149. [Google Scholar] [CrossRef]
- Park, J.; Shin, Y.; Kim, T.H.; Kim, D.-H.; Lee, A. Plasma metabolites as possible biomarkers for diagnosis of breast cancer. PLoS ONE 2019, 14, e0225129. [Google Scholar] [CrossRef]
- Barnes, T.; Bell, K.; DiSebastiano, K.M.; Vance, V.; Hanning, R.; Russell, C.; Dubin, J.A.; Bahl, M.; Califaretti, N.; Campbell, C.; Mourtzakis, M. Plasma amino acid profiles of breast cancer patients early in the trajectory of the disease differ from healthy comparison groups. Appl. Physiol. Nutr. Metab. 2014, 39, 740–744. [Google Scholar] [CrossRef]
- Masisi, B.K.; El Ansari, R.; Alfarsi, L.; Rakha, E.A.; Green, A.R.; Craze, M.L. The role of glutaminase in cancer. Histopathology 2020, 76, 498–508. [Google Scholar] [CrossRef]
- Wang, J.-B.; Erickson, J.W.; Fuji, R.; Ramachandran, S.; Gao, P.; Dinavahi, R.; Wilson, K.F.; Ambrosio, A.L.B.; Dias, S.M.G.; Dang, C.V.; Cerione, R.A. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell 2010, 18, 207–219. [Google Scholar] [CrossRef]
- Cassago, A.; Ferreira, A.P.S.; Ferreira, I.M.; Fornezari, C.; Gomes, E.R.M.; Greene, K.S.; Pereira, H.M.; Garratt, R.C.; Dias, S.M.G.; Ambrosio, A.L.B. Mitochondrial localization and structure-based phosphate activation mechanism of Glutaminase C with implications for cancer metabolism. Proc Natl Acad Sci USA 2012, 109, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Kim, S.-H.; Im, C.Y.; Hwang, H.-J. Recent development of small molecule glutaminase inhibitors. Curr. Top. Med. Chem. 2018, 18, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Best, S.A.; Gubser, P.M.; Sethumadhavan, S.; Kersbergen, A.; Negrón Abril, Y.L.; Goldford, J.; Sellers, K.; Abeysekera, W.; Garnham, A.L.; McDonald, J.A.; Weeden, C.E.; Anderson, D.; Pirman, D.; Roddy, T.P.; Creek, D.J.; Kallies, A.; Kingsbury, G.; Sutherland, K.D. Glutaminase inhibition impairs CD8 T cell activation in STK11-/Lkb1-deficient lung cancer. Cell Metab. 2022, 34, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, X.; Gong, G.; Lu, Y.; Guo, Z.; Chen, R.; Huang, H.; Li, Z.; Bian, J. An updated patent review of glutaminase inhibitors (2019-2022). Expert Opin. Ther. Pat. 2023, 33, 17–28. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Q.; Dong, C. Metabolic reprogramming in triple-negative breast cancer. Cancer Biol. Med. 2020, 17, 44–59. [Google Scholar] [CrossRef]
- Wang, W.; Bai, L.; Li, W.; Cui, J. The lipid metabolic landscape of cancers and new therapeutic perspectives. Front. Oncol. 2020, 10, 605154. [Google Scholar] [CrossRef]
- Giudetti, A.M.; De Domenico, S.; Ragusa, A.; Lunetti, P.; Gaballo, A.; Franck, J.; Simeone, P.; Nicolardi, G.; De Nuccio, F.; Santino, A.; Capobianco, L.; Lanuti, P.; Fournier, I.; Salzet, M.; Maffia, M.; Vergara, D. A specific lipid metabolic profile is associated with the epithelial mesenchymal transition program. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 344–357. [Google Scholar] [CrossRef]
- Simeone, P.; Tacconi, S.; Longo, S.; Lanuti, P.; Bravaccini, S.; Pirini, F.; Ravaioli, S.; Dini, L.; Giudetti, A.M. Expanding roles of de novo lipogenesis in breast cancer. Int. J. Environ. Res. Public Health 2021, 18. [Google Scholar] [CrossRef]
- German, N.J.; Yoon, H.; Yusuf, R.Z.; Murphy, J.P.; Finley, L.W.S.; Laurent, G.; Haas, W.; Satterstrom, F.K.; Guarnerio, J.; Zaganjor, E.; Santos, D.; Pandolfi, P.P.; Beck, A.H.; Gygi, S.P.; Scadden, D.T.; Kaelin, W.G.; Haigis, M.C. PHD3 loss in cancer enables metabolic reliance on fatty acid oxidation via deactivation of ACC2. Mol. Cell 2016, 63, 1006–1020. [Google Scholar] [CrossRef] [PubMed]
- Giró-Perafita, A.; Palomeras, S.; Lum, D.H.; Blancafort, A.; Viñas, G.; Oliveras, G.; Pérez-Bueno, F.; Sarrats, A.; Welm, A.L.; Puig, T. Preclinical Evaluation of Fatty Acid Synthase and EGFR Inhibition in Triple-Negative Breast Cancer. Clin. Cancer Res. 2016, 22, 4687–4697. [Google Scholar] [CrossRef] [PubMed]
- Wahdan-Alaswad, R.S.; Cochrane, D.R.; Spoelstra, N.S.; Howe, E.N.; Edgerton, S.M.; Anderson, S.M.; Thor, A.D.; Richer, J.K. Metformin-induced killing of triple-negative breast cancer cells is mediated by reduction in fatty acid synthase via miRNA-193b. Horm. Cancer 2014, 5, 374–389. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wu, J.; Zhao, Q.; Fu, S.; Jin, J. Emerging roles of aerobic glycolysis in breast cancer. Clin. Transl. Oncol. 2020, 22, 631–646. [Google Scholar] [CrossRef]
- Ogilvie, G.K.; Walters, L.; Salman, M.D.; Fettman, M.J.; Johnston, S.D.; Hegstad, R.L. Alterations in carbohydrate metabolism in dogs with nonhematopoietic malignancies. Am. J. Vet. Res. 1997, 58, 277–281. [Google Scholar]
- Rodigheri, S.M.; Paiva, F.N. de; Firmo, B.F.; Fuchs, T.; Mani, C.B.; Nardi, A.B. de Parameters of metabolic response to surgical trauma induced via unilateral total mastectomy associated or not to ovariohysterectomy in dogs. Animals (Basel) 2023, 13. [Google Scholar] [CrossRef]
- Jayasri, K.; Padmaja, K.; Saibaba, M. Altered oxidative stress and carbohydrate metabolism in canine mammary tumors. Vet. World 2016, 9, 1489–1492. [Google Scholar] [CrossRef]
- Lee, H.-J.; Han, H.-J.; Lee, J.-Y.; Son, W.-C. PKM2 in canine mammary tumors: parallels to human breast cancer. Comp. Med. 2020, 70, 349–354. [Google Scholar] [CrossRef]
- Arai, T.; Ogino, T.; Gunji, M.; Washizu, T.; Komori, S.; Washizu, M. Changes in glucose transport activities in mammary adenocarcinoma of dogs. Res. Vet. Sci. 1997, 62, 85–86. [Google Scholar] [CrossRef]
- Freeman, A.; Hetzel, U.; Cripps, P.; Mobasheri, A. Expression of the plasma membrane markers aquaporin 1 (AQP1), glucose transporter 1 (GLUT1) and Na, K-ATPase in canine mammary glands and mammary tumours. Vet. J. 2010, 185, 90–93. [Google Scholar] [CrossRef]
- Mees, G.; Vangestel, C.; Dierckx, R.; Loomans, S.; Van Damme, N.; Peremans, K.; De Rooster, H.; Van Goethem, B.; Pauwels, P.; Ducatelle, R.; Van de Wiele, C. Metabolic correlates of tumour hypoxia in malignant canine mammary carcinoma. Res. Vet. Sci. 2011, 91, e125–8. [Google Scholar] [CrossRef]
- Beirão, B.C.B.; Raposo, T.; Pang, L.Y.; Argyle, D.J. Canine mammary cancer cells direct macrophages toward an intermediate activation state between M1/M2. BMC Vet. Res. 2015, 11, 151. [Google Scholar] [CrossRef]
- Bao, X.; Shi, R.; Zhao, T.; Wang, Y.; Anastasov, N.; Rosemann, M.; Fang, W. Integrated analysis of single-cell RNA-seq and bulk RNA-seq unravels tumour heterogeneity plus M2-like tumour-associated macrophage infiltration and aggressiveness in TNBC. Cancer Immunol. Immunother. 2021, 70, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Shi, W.; Xu, Y.; Xu, C.; Zhao, T.; Geng, B.; Yang, J.; Pan, J.; Hu, S.; Zhang, C.; Zhang, J.; Wang, C.; Shen, J.; Che, Y.; Liu, Z.; Lv, Y.; Wen, H.; You, Q. Tumor-derived lactate induces M2 macrophage polarization via the activation of the ERK/STAT3 signaling pathway in breast cancer. Cell Cycle 2018, 17, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Burrai, G.P.; Tanca, A.; Cubeddu, T.; Abbondio, M.; Polinas, M.; Addis, M.F.; Antuofermo, E. A first immunohistochemistry study of transketolase and transketolase-like 1 expression in canine hyperplastic and neoplastic mammary lesions. BMC Vet. Res. 2017, 13, 38. [Google Scholar] [CrossRef]
- Nerurkar, V.R.; Ishwad, C.S.; Seshadri, R.; Naik, S.N.; Lalitha, V.S. Glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase activities in normal canine mammary gland and in mammary tumours and their correlation with oestrogen receptors. J. Comp. Pathol. 1990, 102, 191–195. [Google Scholar] [CrossRef]
- Sánchez, D.; Romero, L.; López, S.; Campuzano, M.; Ortega, R.; Morales, A.; Guadarrama, M.; Cesarman-Maus, G.; García-Pérez, O.; Lizano, M. 18F-FDG-PET/CT in Canine Mammary Gland Tumors. Front. Vet. Sci. 2019, 6, 280. [Google Scholar] [CrossRef] [PubMed]
- Seiler, S.M.F.; Baumgartner, C.; Hirschberger, J.; Beer, A.J.; Brühschwein, A.; Kreutzmann, N.; Laberke, S.; Wergin, M.C.; Meyer-Lindenberg, A.; Brandl, J.; von Thaden, A.-K.; Farrell, E.; Schwaiger, M. Comparative Oncology: Evaluation of 2-Deoxy-2-[18F]fluoro-D-glucose (FDG) Positron Emission Tomography/Computed Tomography (PET/CT) for the Staging of Dogs with Malignant Tumors. PLoS ONE 2015, 10, e0127800. [Google Scholar] [CrossRef]
- Borgatti, A.; Winter, A.L.; Stuebner, K.; Scott, R.; Ober, C.P.; Anderson, K.L.; Feeney, D.A.; Vallera, D.A.; Koopmeiners, J.S.; Modiano, J.F.; Froelich, J. Evaluation of 18-F-fluoro-2-deoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) as a staging and monitoring tool for dogs with stage-2 splenic hemangiosarcoma - A pilot study. PLoS ONE 2017, 12, e0172651. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.; Yun, T.; Chae, Y.; Lee, D.; Son, M.; Ku, D.; Kim, H.; Yang, M.; Kang, B. Evaluation of a dog with inflammatory mammary carcinoma using18 F-2-deoxy-2-fluoro-d -glucose positron emission tomography/computed tomography. Vet. Med. Sci. 2022, 8, 1361–1365. [Google Scholar] [CrossRef]
- Barbieri, F.; Thellung, S.; Ratto, A.; Carra, E.; Marini, V.; Fucile, C.; Bajetto, A.; Pattarozzi, A.; Würth, R.; Gatti, M.; Campanella, C.; Vito, G.; Mattioli, F.; Pagano, A.; Daga, A.; Ferrari, A.; Florio, T. In vitro and in vivo antiproliferative activity of metformin on stem-like cells isolated from spontaneous canine mammary carcinomas: translational implications for human tumors. BMC Cancer 2015, 15, 228. [Google Scholar] [CrossRef] [PubMed]
- Cortese, L.; Terrazzano, G.; Pelagalli, A. Leptin and immunological profile in obesity and its associated diseases in dogs. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.Y.; Im, K.S.; Kim, N.H.; Kim, H.W.; Shin, J.I.; Yhee, J.Y.; Sur, J.H. Effects of Obesity and Obesity-Related Molecules on Canine Mammary Gland Tumors. Vet. Pathol. 2015, 52, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Costa-Santos, K.; Damasceno, K.; Portela, R.D.; Santos, F.L.; Araújo, G.C.; Martins-Filho, E.F.; Silva, L.P.; Barral, T.D.; Santos, S.A.; Estrela-Lima, A. Lipid and metabolic profiles in female dogs with mammary carcinoma receiving dietary fish oil supplementation. BMC Vet. Res. 2019, 15, 401. [Google Scholar] [CrossRef]
- Lim, H.-Y.; Seung, B.-J.; Cho, S.-H.; Kim, S.-H.; Bae, M.-K.; Sur, J.-H. Canine mammary cancer in overweight or obese female dogs is associated with intratumoral microvessel density and macrophage counts. Vet. Pathol. 2022, 59, 39–45. [Google Scholar] [CrossRef]
- Lim, H.Y.; Im, K.S.; Kim, N.H.; Kim, H.W.; Shin, J.I.; Sur, J.H. Obesity, expression of adipocytokines, and macrophage infiltration in canine mammary tumors. Vet. J. 2015, 203, 326–331. [Google Scholar] [CrossRef]
- Sonnenschein, E.G.; Glickman, L.T.; Goldschmidt, M.H.; McKee, L.J. Body conformation, diet, and risk of breast cancer in pet dogs: a case-control study. Am. J. Epidemiol. 1991, 133, 694–703. [Google Scholar] [CrossRef]
- Shofer, F.S.; Sonnenschein, E.G.; Goldschmidt, M.H.; Laster, L.L.; Glickman, L.T. Histopathologic and dietary prognostic factors for canine mammary carcinoma. Breast Cancer Res. Treat. 1989, 13, 49–60. [Google Scholar] [CrossRef]
- Cacciola, N.A.; Sgadari, M.; Sepe, F.; Petillo, O.; Margarucci, S.; Martano, M.; Maiolino, P.; Restucci, B. Metabolic flexibility in canine mammary tumors: implications of the carnitine system. Animals (Basel) 2021, 11. [Google Scholar] [CrossRef]
- Cacciola, N.A.; Sgadari, M.; Petillo, O.; Margarucci, S.; Martano, M.; Cocchia, N.; Maiolino, P.; Restucci, B. Carnitine palmitoyltransferase 1 A expression profile in canine mammary tumors. Vet. J. 2020, 257, 105453. [Google Scholar] [CrossRef]
- Balaban, S.; Lee, L.S.; Varney, B.; Aishah, A.; Gao, Q.; Shearer, R.F.; Saunders, D.N.; Grewal, T.; Hoy, A.J. Heterogeneity of fatty acid metabolism in breast cancer cells underlies differential sensitivity to palmitate-induced apoptosis. Mol. Oncol. 2018, 12, 1623–1638. [Google Scholar] [CrossRef] [PubMed]
- Balaban, S.; Nassar, Z.D.; Zhang, A.Y.; Hosseini-Beheshti, E.; Centenera, M.M.; Schreuder, M.; Lin, H.-M.; Aishah, A.; Varney, B.; Liu-Fu, F.; Lee, L.S.; Nagarajan, S.R.; Shearer, R.F.; Hardie, R.-A.; Raftopulos, N.L.; Kakani, M.S.; Saunders, D.N.; Holst, J.; Horvath, L.G.; Butler, L.M.; Hoy, A.J. Extracellular fatty acids are the major contributor to lipid synthesis in prostate cancer. Mol. Cancer Res. 2019, 17, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Labbé, D.P.; Zadra, G.; Yang, M.; Reyes, J.M.; Lin, C.Y.; Cacciatore, S.; Ebot, E.M.; Creech, A.L.; Giunchi, F.; Fiorentino, M.; Elfandy, H.; Syamala, S.; Karoly, E.D.; Alshalalfa, M.; Erho, N.; Ross, A.; Schaeffer, E.M.; Gibb, E.A.; Takhar, M.; Den, R.B.; Lehrer, J.; Karnes, R.J.; Freedland, S.J.; Davicioni, E.; Spratt, D.E.; Ellis, L.; Jaffe, J.D.; DʼAmico, A.V.; Kantoff, P.W.; Bradner, J.E.; Mucci, L.A.; Chavarro, J.E.; Loda, M.; Brown, M. High-fat diet fuels prostate cancer progression by rewiring the metabolome and amplifying the MYC program. Nat. Commun. 2019, 10, 4358. [Google Scholar] [CrossRef] [PubMed]
- Casciano, J.C.; Perry, C.; Cohen-Nowak, A.J.; Miller, K.D.; Vande Voorde, J.; Zhang, Q.; Chalmers, S.; Sandison, M.E.; Liu, Q.; Hedley, A.; McBryan, T.; Tang, H.-Y.; Gorman, N.; Beer, T.; Speicher, D.W.; Adams, P.D.; Liu, X.; Schlegel, R.; McCarron, J.G.; Wakelam, M.J.O.; Gottlieb, E.; Kossenkov, A.V.; Schug, Z.T. MYC regulates fatty acid metabolism through a multigenic program in claudin-low triple negative breast cancer. Br. J. Cancer 2020, 122, 868–884. [Google Scholar] [CrossRef] [PubMed]
- Camarda, R.; Zhou, A.Y.; Kohnz, R.A.; Balakrishnan, S.; Mahieu, C.; Anderton, B.; Eyob, H.; Kajimura, S.; Tward, A.; Krings, G.; Nomura, D.K.; Goga, A. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat. Med. 2016, 22, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Michishita, M.; Saito, N.; Nozawa, S.; Furumoto, R.; Nakagawa, T.; Sato, T.; Ochiai, K.; Azakami, D.; Katayama, K.; Nakahira, R.; Tazaki, H.; Machida, Y.; Ishiwata, T. Metabolite profiling in sphere-forming cells from canine mammary adenocarcinoma cell lines using gas chromatography-mass spectrometry. J. Vet. Med. Sci. 2019, 81, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Pérez Alenza, D.; Rutteman, G.R.; Peña, L.; Beynen, A.C.; Cuesta, P. Relation between habitual diet and canine mammary tumors in a case-control study. J. Vet. Intern. Med. 1998, 12, 132–139. [Google Scholar] [CrossRef]
- Rahman, M.M.; Veigas, J.M.; Williams, P.J.; Fernandes, G. DHA is a more potent inhibitor of breast cancer metastasis to bone and related osteolysis than EPA. Breast Cancer Res. Treat. 2013, 141, 341–352. [Google Scholar] [CrossRef]
- Bai, X.; Shao, J.; Zhou, S.; Zhao, Z.; Li, F.; Xiang, R.; Zhao, A.Z.; Pan, J. Inhibition of lung cancer growth and metastasis by DHA and its metabolite, RvD1, through miR-138-5p/FOXC1 pathway. J. Exp. Clin. Cancer Res. 2019, 38, 479. [Google Scholar] [CrossRef]
- Shekari, N.; Javadian, M.; Ghasemi, M.; Baradaran, B.; Darabi, M.; Kazemi, T. Synergistic Beneficial Effect of Docosahexaenoic Acid (DHA) and Docetaxel on the Expression Level of Matrix Metalloproteinase-2 (MMP-2) and MicroRNA-106b in Gastric Cancer. J. Gastrointest. Cancer 2020, 51, 70–75. [Google Scholar] [CrossRef]
- Abdi, J.; Garssen, J.; Faber, J.; Redegeld, F.A. Omega-3 fatty acids, EPA and DHA induce apoptosis and enhance drug sensitivity in multiple myeloma cells but not in normal peripheral mononuclear cells. J. Nutr. Biochem. 2014, 25, 1254–1262. [Google Scholar] [CrossRef]
- Wang, L.-S.; Huang, Y.-W.; Liu, S.; Chang, H.-L.; Ye, W.; Shu, S.; Sugimoto, Y.; Funk, J.A.; Smeaks, D.D.; Hill, L.N.; Lin, Y.C. Conjugated linoleic acid (CLA) modulates prostaglandin E2 (PGE2) signaling in canine mammary cells. Anticancer Res. 2006, 26, 889–898. [Google Scholar]
- Brunelle, M.; Sartin, E.A.; Wolfe, L.G.; Sirois, J.; Doré, M. Cyclooxygenase-2 expression in normal and neoplastic canine mammary cell lines. Vet. Pathol. 2006, 43, 656–666. [Google Scholar] [CrossRef]
- Da Silva, E.D.; Sandri, E.C.; Fernandes, L.A.; Carraro, P.C.; De Oliveira, D.E. Conjugated linoleic acid trans-10, cis-12 increases the expression of genes from cell cycle progression and cis-9, trans-11 stimulates apoptotic genes in different mammary tumor explants of female dogs. Mol. Biol. Rep. 2023. [Google Scholar] [CrossRef] [PubMed]
- Fabian, C.J.; Kimler, B.F.; Hursting, S.D. Omega-3 fatty acids for breast cancer prevention and survivorship. Breast Cancer Res. 2015, 17, 62. [Google Scholar] [CrossRef]
- Lee, K.H.; Seong, H.J.; Kim, G.; Jeong, G.H.; Kim, J.Y.; Park, H.; Jung, E.; Kronbichler, A.; Eisenhut, M.; Stubbs, B.; Solmi, M.; Koyanagi, A.; Hong, S.H.; Dragioti, E.; de Rezende, L.F.M.; Jacob, L.; Keum, N.; van der Vliet, H.J.; Cho, E.; Veronese, N.; Grosso, G.; Ogino, S.; Song, M.; Radua, J.; Jung, S.J.; Thompson, T.; Jackson, S.E.; Smith, L.; Yang, L.; Oh, H.; Choi, E.K.; Shin, J.I.; Giovannucci, E.L.; Gamerith, G. Consumption of Fish and ω-3 Fatty Acids and Cancer Risk: An Umbrella Review of Meta-Analyses of Observational Studies. Adv. Nutr. 2020, 11, 1134–1149. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.E. Therapeutic use of fish oils in companion animals. J. Am. Vet. Med. Assoc. 2011, 239, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Pizato, N.; Luzete, B.C.; Kiffer, L.F.M.V.; Corrêa, L.H.; de Oliveira Santos, I.; Assumpção, J.A.F.; Ito, M.K.; Magalhães, K.G. Omega-3 docosahexaenoic acid induces pyroptosis cell death in triple-negative breast cancer cells. Sci. Rep. 2018, 8, 1952. [Google Scholar] [CrossRef]
- Ogilvie, G.K.; Fettman, M.J.; Mallinckrodt, C.H.; Walton, J.A.; Hansen, R.A.; Davenport, D.J.; Gross, K.L.; Richardson, K.L.; Rogers, Q.; Hand, M.S. Effect of fish oil, arginine, and doxorubicin chemotherapy on remission and survival time for dogs with lymphoma: a double-blind, randomized placebo-controlled study. Cancer 2000, 88, 1916–1928. [Google Scholar] [CrossRef]
- Tuzlu, T.; Saribay, M.K.; KOLDAS ÜRER, E.; KÖSE, A.M.; GÖZER, A.; YAKAN, A.; ÖZSOY, Ş.Y. Evaluation of blood omega-3 and omega-6 levels in healthy female dogs and. J HELLENIC VET MED SOC 2021, 72, 2925–2934. [Google Scholar]
- Cheng, C.; Geng, F.; Cheng, X.; Guo, D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun (Lond) 2018, 38, 27. [Google Scholar] [CrossRef] [PubMed]
- Bastos, D.C.; Ribeiro, C.F.; Ahearn, T.; Nascimento, J.; Pakula, H.; Clohessy, J.; Mucci, L.; Roberts, T.; Zanata, S.M.; Zadra, G.; Loda, M. Genetic ablation of FASN attenuates the invasive potential of prostate cancer driven by Pten loss. J. Pathol. 2021, 253, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Osaki, T.; Tsuka, T.; Imagawa, T.; Minami, S.; Okamoto, Y. Plasma free amino acid profiles of canine mammary gland tumors. J. Vet. Sci. 2012, 13, 433. [Google Scholar] [CrossRef] [PubMed]
- Junior, R.P.; Sonehara, N.M.; Jardim-Perassi, B.V.; Pal, A.; Asad, Y.; Almeida Chuffa, L.G.; Chammas, R.; Raynaud, F.I.; Zuccari, D.A.P.C. Presence of human breast cancer xenograft changes the diurnal profile of amino acids in mice. Sci. Rep. 2022, 12, 1008. [Google Scholar] [CrossRef]
- Derakhshan, F.; Reis-Filho, J.S. Pathogenesis of Triple-Negative Breast Cancer. Annu. Rev. Pathol. 2022, 17, 181–204. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S.-C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef]
- Gross, M.I.; Demo, S.D.; Dennison, J.B.; Chen, L.; Chernov-Rogan, T.; Goyal, B.; Janes, J.R.; Laidig, G.J.; Lewis, E.R.; Li, J.; Mackinnon, A.L.; Parlati, F.; Rodriguez, M.L.M.; Shwonek, P.J.; Sjogren, E.B.; Stanton, T.F.; Wang, T.; Yang, J.; Zhao, F.; Bennett, M.K. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol. Cancer Ther. 2014, 13, 890–901. [Google Scholar] [CrossRef]
- Ryu, J.E.; Park, H.K.; Choi, H.J.; Lee, H.B.; Lee, H.J.; Lee, H.; Yu, E.S.; Son, W.C. Expression of the glutamine metabolism-related proteins glutaminase 1 and glutamate dehydrogenase in canine mammary tumours. Vet. Comp. Oncol. 2018, 16, 239–245. [Google Scholar] [CrossRef]
- Wakshlag, J.J.; McNeill, C.J.; Antonyak, M.A.; Boehm, J.E.; Fuji, R.; Balkman, C.E.; Zgola, M.; Cerione, R.A.; Page, R.L. Expression and activity of transglutaminase II in spontaneous tumours of dogs and cats. J. Comp. Pathol. 2006, 134, 202–210. [Google Scholar] [CrossRef]
- Fukumoto, S.; Hanazono, K.; Komatsu, T.; Ueno, H.; Kadosawa, T.; Iwano, H.; Uchide, T. L-type amino acid transporter 1 (LAT1): a new therapeutic target for canine mammary gland tumour. Vet. J. 2013, 198, 164–169. [Google Scholar] [CrossRef]
- Cherrington, B.D.; Mohanan, S.; Diep, A.N.; Fleiss, R.; Sudilovsky, D.; Anguish, L.J.; Coonrod, S.A.; Wakshlag, J.J. Comparative analysis of peptidylarginine deiminase-2 expression in canine, feline and human mammary tumours. J. Comp. Pathol. 2012, 147, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Cherrington, B.D.; Morency, E.; Struble, A.M.; Coonrod, S.A.; Wakshlag, J.J. Potential role for peptidylarginine deiminase 2 (PAD2) in citrullination of canine mammary epithelial cell histones. PLoS ONE 2010, 5, e11768. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, S.E.; Jeong, H.W.; Ha, J.H. The complete nucleotide sequence of the domestic dog (Canis familiaris) mitochondrial genome. Mol. Phylogenet. Evol. 1998, 10, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Bertagnolli, A.C.; Soares, P.; van Asch, B.; Amorim, A.; Cirnes, L.; Máximo, V.; Cassali, G.D. An assessment of the clonality of the components of canine mixed mammary tumours by mitochondrial DNA analysis. Vet. J. 2009, 182, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Kowal, K.; Tkaczyk-Wlizło, A.; Pierzchała, M.; Gawor, J.; Ślaska, B. Molecular differences in mitochondrial DNA genomes of dogs with malignant mammary tumours. Vet. Comp. Oncol. 2022, 20, 256–264. [Google Scholar] [CrossRef]
- Karayannopoulou, M.; Fytianou, A.; Assaloumidis, N.; Psalla, D.; Constantinidis, T.C.; Kaldrymidou, E.; Koutinas, A.F. Markers of lipid peroxidation and α-tocopherol levels in the blood and neoplastic tissue of dogs with malignant mammary gland tumors. Vet. Clin. Pathol. 2013, 42, 323–328. [Google Scholar] [CrossRef]
- Szczubiał, M.; Kankofer, M.; Łopuszyński, W.; Dabrowski, R.; Lipko, J. Oxidative stress parameters in bitches with mammary gland tumours. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2004, 51, 336–340. [Google Scholar] [CrossRef]
- Kumaraguruparan, R.; Balachandran, C.; Manohar, B.M.; Nagini, S. Altered oxidant-antioxidant profile in canine mammary tumours. Vet. Res. Commun. 2005, 29, 287–296. [Google Scholar] [CrossRef]
- Surdyka, M.; Slaska, B. Defect of the mitochondrial DNA hypervariable region as a risk factor for canine mammary tumour. Vet. Comp. Oncol. 2017, 15, 820–828. [Google Scholar] [CrossRef]
- Surdyka, M.; Slaska, B. Defect in ND2, COX2, ATP6 and COX3 mitochondrial genes as a risk factor for canine mammary tumour. Vet. Comp. Oncol. 2017, 15, 1062–1072. [Google Scholar] [CrossRef]
- Yang, Y.; Mei, C.; Xian, H.; Zhang, X.; Li, J.; Liang, Z.-X.; Zhi, Y.; Ma, Y.; Wang, H.-J. Toosendanin-induced apoptosis of CMT-U27 is mediated through the mitochondrial apoptotic pathway. Vet. Comp. Oncol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.R.; Maschio, L.B.; Jardim-Perassi, B.V.; Moschetta, M.G.; Ferreira, L.C.; Martins, G.R.; Gelaleti, G.B.; De Campos Zuccari, D.A.P. Evaluation of melatonin treatment in primary culture of canine mammary tumors. Oncol. Rep. 2015, 33, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Zambrano-Estrada, X.; Landaverde-Quiroz, B.; Dueñas-Bocanegra, A.A.; De Paz-Campos, M.A.; Hernández-Alberto, G.; Solorio-Perusquia, B.; Trejo-Mandujano, M.; Pérez-Guerrero, L.; Delgado-González, E.; Anguiano, B.; Aceves, C. Molecular iodine/doxorubicin neoadjuvant treatment impair invasive capacity and attenuate side effect in canine mammary cancer. BMC Vet. Res. 2018, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Song, X.Y.; Yang, C.; Li, Q.; Tang, D.; Tian, W.R.; Liu, Y. Effect of hematoporphyrin monomethyl ether-mediated PDT on the mitochondria of canine breast cancer cells. Photodiagnosis Photodyn. Ther. 2013, 10, 414–421. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




